Summary
This report describes an unusual case of primary cryptococcoma in the proximal thoracic spinal cord of an 11-year-old immunocompetent cat from a farm on which there were large numbers of pigeons. This animal was referred for examination with progressive paralysis and shown to be free from feline immunodeficiency virus, feline leukaemia virus, feline coronavirus and Toxoplasma gondii. It died 2 months later. At necropsy, the only lesion detected was a malacic area, 4 cm in length, in the spinal cord. Histopathological examination of the spinal cord revealed severe granulomatous inflammation associated with large numbers of encapsulated yeast cells. In addition to the granulomatous host response, necrosis, digestion chambers, Gitter cells, spheroids and lymphocytic perivascular cuffs were features of the malacic areas. Immunohistochemistry confirmed the presence of Cryptococcus neoformans var. grubii yeast cells.
Keywords: cat, cryptococcosis, Cryptococcus neoformans, spinal cord
Cryptococcus neoformans is a dimorphic fungus. It has a worldwide distribution and has been recovered from numerous environmental sources, including soil and avian excreta (Bicanic and Harrison, 2004, Shoham and Levitz, 2005). Based on genetic characteristics and capsular antigen, two varieties have been defined, namely C. neoformans var. neoformans (serotype D) and C. neoformans var. grubii (serotype A), as well as a hybrid between serotypes A and D. The closely related Cryptococcus gattii (serotypes B and C) was formerly considered a variety of C. neoformans (Franzot et al., 1999). Cryptococcosis is thought to result primarily from inhalation of infectious propagules (probably basidiospores) (Rodrigues et al., 1999) with initial localization in the respiratory tract and then dissemination to other tissues, particularly brain (Rodrigues et al., 1999, Shoham and Levitz, 2005, Caswell and Williams, 2007, Lopez, 2007). Grossly, in central nervous system (CNS) tissue, multiple small lesions with a viscous, gelatinous appearance are often seen. Histological lesions generally consist of numerous yeast cells that present the classically described “soap-bubble” appearance, because of the unstained capsule. There is a variable, often minimal, inflammatory host response, consisting of macrophages, lymphocytes and plasma cells (Summers et al., 1995, Caswell and Williams, 2007, Lopez, 2007). The severity of the inflammatory response is dependent on the balance between the yeast's virulence factors and the immunological status of the host. When the lesion is well delimited it is called a cryptococcoma.
Cryptococcosis is often fatal in man and is associated with immunosuppression. In animals, it is most common in adult intact cats and dogs, but it occurs occasionally in horses and rarely in other species (Summers et al., 1995, Mandrioli et al., 2002, Caswell and Williams, 2007, Lopez, 2007). Immunosuppression of cell-mediated immunity, caused by feline immunodeficiency virus (FIV) or feline leukaemia virus (FeLV) in cats or by Ehrlichia canis or long-term glucocorticoid therapy in dogs, may increase susceptibility to cryptococcosis (Lopez, 2007); there is, however, some disagreement about the predisposing effect of immunosuppression in animals (O'Brien et al., 2004).
CNS cryptococcosis without respiratory tract involvement has not previously been reported in animals. The present report describes an unusual case of spinal cryptococcoma in an apparently immunocompetent cat.
An 11-year-old male neutered cat was referred with a progressive paraparesis. On neurological examination, hindlimb conscious proprioceptive deficits with normal spinal reflexes were observed. A clinical diagnosis of upper motoneuron syndrome, probably with a lesion in the thoracolumbar spinal cord (T3–L3), was made. The most likely differential diagnoses for a lesion at this site were considered to be (1) a disorder of growth (e.g., a neoplasm such as lymphosarcoma), (2) an inflammatory lesion associated with an infectious disease (e.g., feline infectious peritonitis, toxoplasmosis, cryptococcosis or other mycosis), or (3) a traumatic lesion, such as that produced by disc prolapse. Lateral and dorsoventral radiography and myelography of the thoracolumbar vertebral region revealed no significant abnormalities. Complete blood count, serum biochemistry, serum protein electrophoresis, abdominal and cardiac ultrasound examination, coproscopy and FeLV antigen and FIV antibody assays gave negative results. Cerebrospinal fluid (CSF) analysis revealed a mild mixed pleocytosis with 2% of eosinophils and an increased total protein content (0.43 g/litre). A polymerase chain reaction (PCR) for feline coronavirus and Toxoplasma gondii and a C. neoformans antigen assay were performed on CSF, with negative results. Magnetic resonance imaging revealed no brain abnormality, but the cervical and thoracic regions of the spinal cord were heterogeneously hyperintense on T2-weighted-images and iso to hypointense on T1. A small syrinx could be seen in the cervical area. After contrast enhancement, an intramedullary mass with an irregular outline was detected (Fig. 1 ). These findings were interpreted as a tumour or a granuloma with secondary extensive oedema, or gliosis and syringohydromyelia. The cat died spontaneously 2 months later and a full necropsy was performed.
Fig. 1.
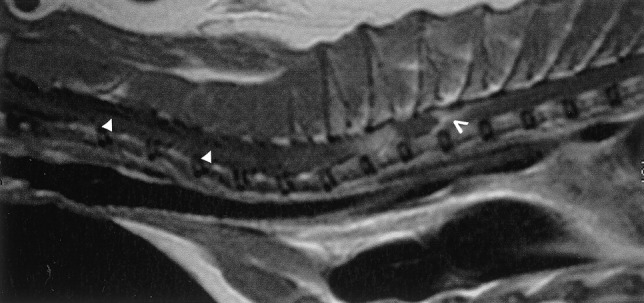
Post-contrast sagittal T1-weighted image of the cervical and thoracic spine. The cryptococcoma was observed as an isointense mass with peripheral enhancement (open arrowhead). Secondary syringohydromyelia was also noted in the cervical spine (arrowheads).
A focal brownish malacic area (4 cm in length) in the proximal thoracic spinal cord (Fig. 2 ) was the only macroscopical abnormality detected. Samples of the spinal cord were formalin-fixed, processed for histology by routine methods, sectioned (4 μm) and stained with haematoxylin and eosin (HE). In addition, some sections were stained with periodic acid-Schiff (PAS), Grocott or mucicarmine (Ganter and Jollès, 1970).
Fig. 2.
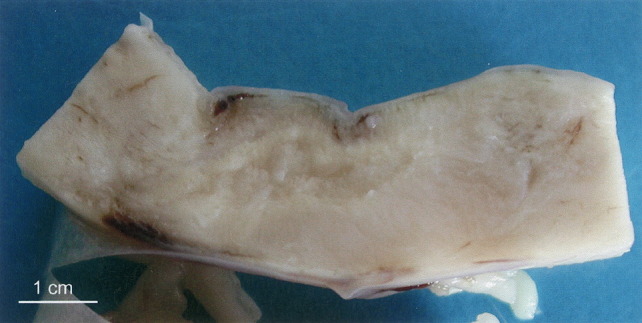
Thoracic spinal cord, sagittal section: in the grey and the white matter, a 4 cm malacic lesion is present.
Sections (4 μm) were examined by immunohistochemistry (IHC) in an Autostainer (Autostainer Plus; Dakocytomation, Carpinteria, CA, USA) at room temperature. The three primary monoclonal antibodies (mAbs) used, kindly supplied by Prof. T. Kozel, were: mAb 471, which labels all strains of C. neoformans complex; 302, which labels C. neoformans var. grubii and C. neoformans var. neoformans strains; and F10F5, which labels C. neoformans var. grubii and C. gattii strains (T. Kozel, personal communication). Tests were made in duplicate and two negative controls were included for each tissue examined, the method used being that described by Krockenberger et al. (2001). The avidin–biotin–horseradish-peroxidase (HRP) complex detection system was employed to detect antibody–antigen binding, with 3,3′-diaminobenzidine tetrahydrochloride (DAB) (Immunodiagnostics, Camperdown, NSW, Australia). Specimens were lightly counterstained with haematoxylin, dehydrated in ethanol to xylol, and “coverslipped”.
Histopathological examination of the thoracic spinal cord revealed a discrete granulomatous lesion, approximately 4 cm in length, effacing the grey and the white ventral matter (Fig. 3 ), with myriads of round, intra-cellular and extra-cellular yeasts enmeshed in abundant eosinophilic amorphous material and nuclear debris (necrosis). The yeasts were round in shape, 7 μm in diameter and rimmed by a basophilic thin cell wall, which was surrounded by a halo of variable thickness (Fig. 4 ). Narrow-necked budding forms were also present. Yeast cells were surrounded by numerous macrophages and epithelioid cells and smaller numbers of lymphocytes and plasma cells rimmed the lesion. The parenchymal remnants contained digestion chambers in which Gitter cells, spheroids and myelinic debris were present. Perivascular lymphocytic cuffs were also observed. The yeast cell walls were stained positively with PAS (Fig. 4, inset), mucicarmine (Fig. 4, inset) and black with Grocott stain. The diagnosis made was that of a focally extensive, severe, chronic granulomatous myelitis, probably caused by a cryptococcoma. This aetiology was confirmed by positive immunohistochemical labelling with antibodies 471, 302 and F10F5. The antibody specificities (see above) indicated that the strain was C. neoformans var. grubii (serotype A) (Fig. 5, Fig. 6 ).
Fig. 3.
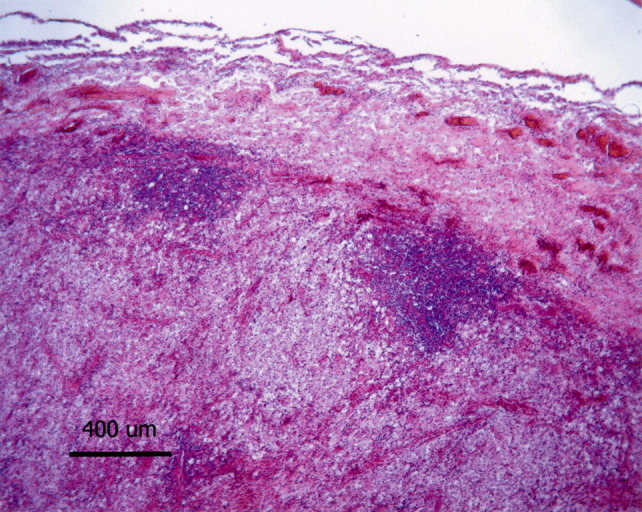
Thoracic spinal cord: effacing the spinal cord, a discrete granulomatous lesion characterized by a large number of foamy macrophages is present. It is associated with lymphocytic perivascular cuffs, localized at the edges of the lesion, and vascular hyperaemia. HE. Bar, 400 μm.
Fig. 4.
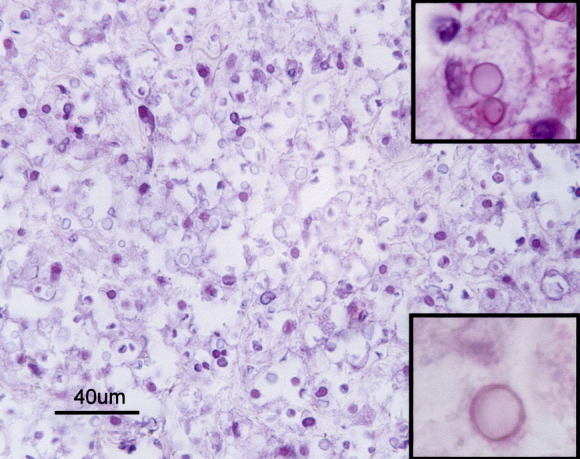
Thoracic spinal cord: granuloma composed of many foamy macrophages and myriads of round, intra- and extra-cellular yeasts. HE. Bar, 40 μm. Fungal capsule stained with PAS (top inset) and mucicarmine (bottom inset). Insets: ×100.
Fig. 5.
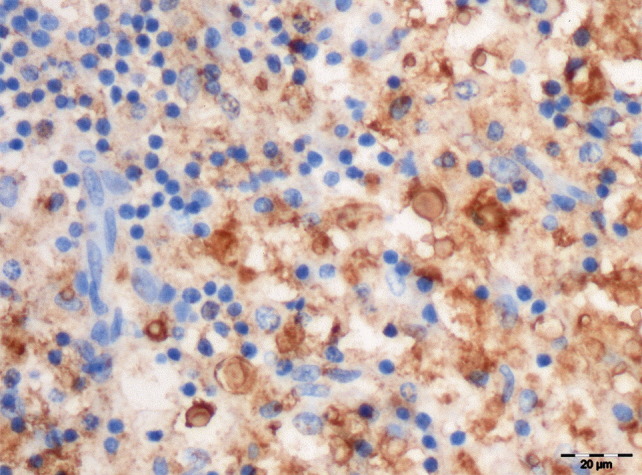
Labelling of cryptococcal yeast cells with F10F5 antibody, against var. grubii and var. neoformans capsular antigens. IHC. Bar, 20 μm.
Fig. 6.
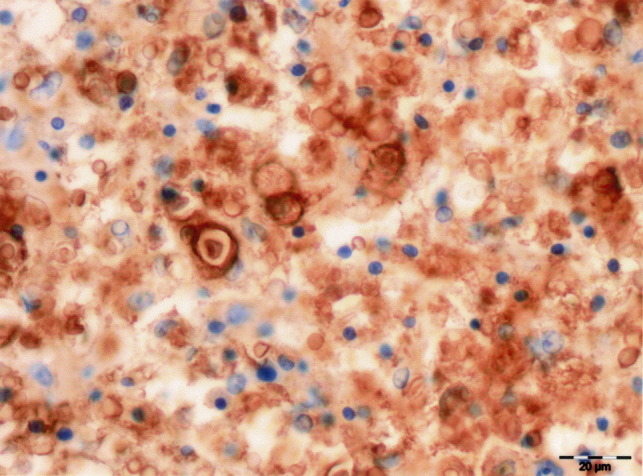
Labelling of cryptococcal yeast cells with 302 antibody, against var. grubii and var. neoformans capsular antigens. IHC. Bar, 20 μm.
To the authors' knowledge, this is the first reported case of spinal cryptococcoma in an immunocompetent cat with no apparent mycotic respiratory lesion. In animals, cryptococcosis is mainly reported in adult intact cats. Immunosuppression was formerly considered to be an important predisposing factor (Summers et al., 1995, Caswell and Williams, 2007, Lopez, 2007), but evidence to support this hypothesis would seem to be lacking (O'Brien et al., 2004, O'Brien et al., 2006). In the present case, the cat was neutered and, according to the owners, lived on a pigeon-breeding farm. Pigeons may be a natural source of cryptococcal yeasts, which are excreted in the faeces and can infect other animals by inhalation (Caswell and Williams, 2007, Lopez, 2007).
In cats, the respiratory tract is the usual site of primary infection, the nasal cavity being more often affected than the lungs (Summers et al., 1995, O'Brien et al., 2004, Malik et al., 2006, Caswell and Williams, 2007, Lopez, 2007). From a nasal or sinus infection, the yeast may spread to the leptomeninges, subarachnoid space and brain. Alternatively, following a pulmonary infection, the yeast may spread haematogenously by “leucocytic trafficking” (Garcia-Hermoso et al., 1999, Bicanic and Harrison, 2004, Caswell and Williams, 2007, Lopez, 2007). In the present case, a single malacic lesion was found in the thoracic spinal cord, with no associated lesions in the respiratory tract or brain. The intramedullary localization without any meningeal lesion excludes the possibility of extension by a neural route from the nasal cavity. Despite the absence of macroscopical signs of pulmonary infection, it is possible that primary infection occurred in the lungs or tracheobronchial lymph nodes but was limited by the immune system (Baker, 1976); infection may then, nonetheless, have spread haematogenously into the spinal cord. This hypothesis is supported by the findings of Garcia-Hermoso et al. (1999), who reported systemic cryptococcosis, in the absence of gross pulmonary findings, 13 years after exposure to the causative organism.
The metastatic medullary lesion with no other sign of systemic dissemination may have been related to the neurotropic properties of the agent and the immunological state of the cat. In the CNS, the tropism of Cryptococcus spp. is probably due, at least in part, to the lack of the alternative complement pathway necessary for phagocytosis and for the lethal effect of binding to capsular glucuronoxilomannan (GXM) (Rodrigues et al., 1999); it is perhaps also due to the presence of a high concentration of catecholamines, which are used by C. neoformans to produce a melanin-like pigment by means of the enzyme laccase. Melanins are scavengers of reactive oxygen intermediates generated by leucocytes, contributing to the resistance of C. neoformans to host immune defences (Rodrigues et al., 1999, Shoham and Levitz, 2005). For these reasons, the nervous system provides an ideal environment for the survival of Cryptococcus spp.
The cat in this report appeared to be immunocompetent, being serologically negative for FIV and FeLV and PCR negative for feline coronavirus and T. gondii. Bone marrow examination and serum protein electrophoresis showed no abnormality. Histologically, the cat showed a granulomatous lesion rich in inflammatory cells, indicative of an immune response, which may have confined the infection. In cats, cryptococcosis is sometimes associated with FIV or FeLV infection, both of which impair the CD4 T lymphocytes, which are necessary to control the infection (Rodrigues et al., 1999, Shoham and Levitz, 2005). CD4 T lymphocytes produce interferon-γ (INF-γ), which is a TH1 pathway cytokine responsible for the recruitment of macrophages in a cell-mediated immune response, forming multinucleated giant cells and releasing hydrolytic enzymes. Granuloma formation is associated with resistance to cryptococcal infection (Rodrigues et al., 1999). Moreover, nitrites produced by macrophages activated by INF-γ, tumour necrosis α and interleukin-6 are fundamental in destroying yeasts (Rodrigues et al., 1999, Bicanic and Harrison, 2004, Shoham and Levitz, 2005). Cryptococcosis has been reported in immunocompetent human patients (Krishnan and Corbett, 2004, Ecevit et al., 2006, Marroni et al., 2007). Such cases are generally associated with C. gattii infection, while infections by var. grubii generally occur in immunodeficient patients (Chen et al., 2000). However, a study based on strains of var. grubii isolated from apparently immunocompetent human patients suggested that some such infections depended on mutations conferring increased virulence, or on underlying immunosuppressive disease, or on both (D'Souza et al., 2004). Different degrees of virulence in var. grubii were demonstrated by Silva et al. (2006). The cat in the present report may have been infected by a strain of C. neoformans var grubii whose virulence was sufficient to overcome the resistance of an apparently immunocompetent animal. An alternative hypothesis is that the cat was exposed to particularly high environmental levels of C. neoformans var. grubii, which overwhelmed the immune response. The cat was kept in close proximity to large numbers of pigeons, in whose faeces the concentration of the yeast may well have been high (Bicanic and Harrison, 2004, Shoham and Levitz, 2005).
C. neoformans var. grubii (serotype A) predominates in Europe, is heat tolerant, and is generally associated with systemic disease (Martinez et al., 2001, Steen et al., 2002, Tintelnot et al., 2004). Heat tolerance and specific virulence factors are considered to be the reasons for its tendency to produce systemic lesions, in contrast to var. neoformans (serotype D) (Tintelnot et al., 2004, Bahn et al., 2005).
The C. neoformans antigen assay was negative in CSF, probably because the inflammatory reaction in the spinal cord lesion prevented the spread of the yeast cells into the subarachnoid space. The pleocytosis detected was characterized by 2% of eosinophils and increased total protein, suggestive of a fungal or parasitic infection.
In conclusion, this report describes an unusual C. neoformans var. grubii infection in the spinal cord of an immunocompetent cat, in the absence of a primary respiratory lesion.
Acknowledgments
We thank Professor Thomas Kozel for the antibodies, and Dr. Gregory Jouvion and Jerome Amiaud for their technical assistance.
References
- Bahn Y.S., Kojima K., Cox G.M., Heitman J. Specialization of the HOG pathway and its impact on differentiation and virulence of Cryptococcus neoformans. Molecular Biology of the Cell. 2005;16:2285–2300. doi: 10.1091/mbc.E04-11-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R.D. The primary pulmonary lymph node complex of crytptococcosis. American Journal of Clinical Pathology. 1976;65:83–92. doi: 10.1093/ajcp/65.1.83. [DOI] [PubMed] [Google Scholar]
- Bicanic T., Harrison T.S. Cryptococcal meningitis. British Medical Bulletin. 2004;72:99–118. doi: 10.1093/bmb/ldh043. [DOI] [PubMed] [Google Scholar]
- Caswell J.L., Williams K.J. Cryptococcosis. In: Maxie M.G., editor. vol. 2. Elsevier Saunders; Philadelphia: 2007. pp. 642–644. (Pathology of Domestic Animals). [Google Scholar]
- Chen S., Sorrell T., Nimmo G., Speed B., Currie B., Ellis D., Marriott D., Pfeiffer T., Parr D., Byth K. Epidemiology and host- and variety-dependent characteristics of infection due to Cryptococcus neoformans in Australia and New Zealand. Clinical Infectious Diseases. 2000;31:499–508. doi: 10.1086/313992. [DOI] [PubMed] [Google Scholar]
- D'Souza C.A., Hagen F., Boekhout T., Cox G.M., Heitman J. Investigation of the basis of virulence in serotype A strains of Cryptococcus neoformans from apparently immunocompetent individuals. Current Genetics. 2004;46:92–102. doi: 10.1007/s00294-004-0511-y. [DOI] [PubMed] [Google Scholar]
- Ecevit I.Z., Clancy C.J., Schmalfuss I.M., Nguyen M.H. The poor prognosis of central nervous system cryptococcosis among nonimmunosuppressed patients: a call for better disease recognition and evaluation of adjuncts to antifungal therapy. Clinical Infectious Diseases. 2006;42:1443–1447. doi: 10.1086/503570. [DOI] [PubMed] [Google Scholar]
- Franzot S.P., Salkin I.F., Casadevall A. Cryptococcus neoformans var. grubii: separate varietal status for Cryptococcus neoformans serotype A isolates. Journal of Clinical Microbiology. 1999;37:838–840. doi: 10.1128/jcm.37.3.838-840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganter P., Jollès G. Gauthier-Villars; Paris: 1970. Histochimie Normale et Pathologique. [Google Scholar]
- Garcia-Hermoso D., Janbon G., Dromer F. Epidemiological evidence for dormant Cryptococcus neoformans infection. Journal of Clinical Microbiology. 1999;37:3204–3209. doi: 10.1128/jcm.37.10.3204-3209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A.V., Corbett A. Intracranial and dermatological cryptococcal infection in an immunocompetent man. Journal of Clinical Neuroscience. 2004;11:765–767. doi: 10.1016/j.jocn.2003.10.021. [DOI] [PubMed] [Google Scholar]
- Krockenberger M.B., Canfield P.J., Kozel T.R., Shinoda T., Ikeda R., Wigney D.I., Martin P., Barnes K., Malik R. An immunohistochemical method that differentiates Cryptococcus neoformans varieties and serotypes in formalin-fixed paraffin-embedded tissues. Medical Mycology. 2001;39:523–533. doi: 10.1080/mmy.39.6.523.533. [DOI] [PubMed] [Google Scholar]
- Lopez A. Mycotic pneumonias of cats. In: McGavin M.D., Zachary J.F., editors. vol. 1. Mosby Elsevier; Phildelphia: 2007. p. 548. (Pathologic Basis of Veterinary Disease). [Google Scholar]
- Malik R., Krockenberger M., O'Brien C.R., Martin P., Wigney D., Medleau L. vol. 1. Elsevier Saunders; Philadelphia: 2006. Cryptococcosis; pp. 584–598. (Infectious Diseases of the Dog and Cat). [Google Scholar]
- Mandrioli L., Bettini G., Marcato P.S., Benazzi C., Della Salda L., Krockenberger M.B., Jensen H.E. Central nervous system cryptococcoma in a cat. Journal of Veterinary Medicine Series A: Physiology, Pathology, Clinical Medicine. 2002;49:526–530. doi: 10.1046/j.1439-0442.2002.00501.x. [DOI] [PubMed] [Google Scholar]
- Marroni M., Pericolini E., Cenci E., Bistoni F., Vecchiarelli A. Functional defect of natural immune system in an apparent immunocompetent patient with pulmonary cryptococcosis. Journal of Infection. 2007;54:5–8. doi: 10.1016/j.jinf.2006.03.018. [DOI] [PubMed] [Google Scholar]
- Martinez L.R., Garcia-Rivera J., Casadevall A. Cryptococcus neoformans var. neoformans (serotype D) strains are more susceptible to heat than C. neoformans var. grubii (serotype A) strains. Journal of Clinical Microbiology. 2001;39:3365–3367. doi: 10.1128/JCM.39.9.3365-3367.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien C.R., Krockenberger M.B., Martin P., Wigney D.I., Malik R. Long-term outcome of therapy for 59 cats and 11 dogs with cryptococcosis. Australian Veterinary Journal. 2006;84:384–392. doi: 10.1111/j.1751-0813.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- O'Brien C.R., Krockenberger M.B., Wigney D.I., Martin P., Malik R. Retrospective study of feline and canine cryptococcosis in Australia from 1981 to 2001: 195 cases. Medical Mycology. 2004;42:449–460. doi: 10.1080/13693780310001624547. [DOI] [PubMed] [Google Scholar]
- Rodrigues M.L., Alviano C.S., Travassos L.R. Pathogenicity of Cryptococcus neoformans: virulence factors and immunological mechanisms. Microbes and Infection. 1999;1:293–301. doi: 10.1016/s1286-4579(99)80025-2. [DOI] [PubMed] [Google Scholar]
- Shoham S., Levitz S.M. The immune response to fungal infections. British Journal of Haematology. 2005;129:569–582. doi: 10.1111/j.1365-2141.2005.05397.x. [DOI] [PubMed] [Google Scholar]
- Silva E.G., Baroni F. de A., Viani F.C., Ruiz L. da S., Gandra R.F., Auler M.E., Dias A.L., Gambale W., Paula C.R. Virulence profile of strains of Cryptococcus neoformans var. grubii evaluated by experimental infection in BALB/c mice and correlation with exoenzyme activity. Journal of Medical Microbiology. 2006;55:139–142. doi: 10.1099/jmm.0.46206-0. [DOI] [PubMed] [Google Scholar]
- Steen B.R., Lian T., Zuyderduyn S., MacDonald W.K., Marra M., Jones S.J., Kronstad J.W. Temperature-regulated transcription in the pathogenic fungus Cryptococcus neoformans. Genome Research. 2002;12:1386–1400. doi: 10.1101/gr.80202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers B.A., Cummings J.F., De Lahunta A. Mosby; St Louis: 1995. Veterinary Neuropathology. [Google Scholar]
- Tintelnot K., Lemmer K., Losert H., Schar G., Polak A. Follow-up of epidemiological data of cryptococcosis in Austria, Germany and Switzerland with special focus on the characterization of clinical isolates. Mycoses. 2004;47:455–464. doi: 10.1111/j.1439-0507.2004.01072.x. [DOI] [PubMed] [Google Scholar]


