Abstract
Transfusion of red blood cells (RBCs) is a balance between providing benefit for patients while avoiding risks of transfusion. Randomized, controlled trials of restrictive RBC transfusion practices have shown equivalent patient outcomes compared with liberal transfusion practices, and meta-analyses have shown improved in-hospital mortality, reduced cardiac events, and reduced bacterial infections. This body of level 1 evidence has led to substantial, improved blood utilization and reduction of inappropriate blood transfusions with implementation of clinical decision support via electronic medical records, along with accompanying educational initiatives.
Keywords: Anemia, Red blood cell transfusion, Clinical decision support, Restrictive transfusion practices
Key points
-
•
Transfusion of red blood cells (RBCs) is a balance between providing benefit for patients while avoiding risks of transfusion.
-
•
Randomized, controlled trials of restrictive RBC transfusion practices have shown equivalent patient outcomes compared with liberal transfusion practices, and meta-analyses have shown improved in-hospital mortality, reduced cardiac events, and reduced bacterial infections.
-
•
This body of level 1 evidence has led to substantial, improved blood utilization and reduction of inappropriate blood transfusions with implementation of clinical decision support via electronic medical records, along with accompanying educational initiatives.
Introduction
Blood transfusion therapy is frequently used in the supportive care for treatment of anemia. The transfusion of red blood cells (RBC) is a balance between the benefits of maintaining oxygen delivery and the inherent risks from blood transfusion. The signs and symptoms of anemia vary based on the acuity of the anemia, compensatory change in blood volume, and the compensatory change in cardiac output from the patient’s cardiovascular system. Chronic anemia is generally well tolerated due to compensatory expansion of intravascular plasma volume, increased cardiac output, vasodilatation, increased blood flow due to decreased viscosity, and not least, increased RBC 2,3 diphosphoglycerate, with a right shift of the oxygen dissociation curve, so that oxygen is unloaded to the peripheral tissues more readily. Symptoms of anemia are often nonspecific and can include fatigue, pallor, dizziness, headaches, vertigo, tinnitus, dyspnea, and inactivity. Fatigue particularly has been associated with poor quality of life.1
The traditional therapy for chronic, medically related anemia has been RBC transfusions. However, transfusion therapy has been identified as one of the most overused (and inappropriate) therapeutic interventions by national accreditation (Joint Commission) and medical societies, such as the American Board of Internal Medicine,2 the American Medical Association, the American Society of Hematology (ASH), and the American Association of Blood Banks (AABB; http://www.choosingwisely.org/doctor-patient-lists/american-society-of-hematology/). Recommendations have been published by several medical societies for RBC transfusion therapy in adult3 and pediatric4 patients.
The authors have previously reviewed blood transfusion practices,3, 5, 6 and herein they provide an updated review of RBC therapy in adult and pediatric patients. The article summarizes current blood risks and indications for RBC transfusion. Important, alternative therapies for management of anemia, such as iron therapy and erythropoietic stimulating agents (ESAs), are outside the scope of this review, but have been published elsewhere.7, 8 Where possible, the article provides evidence-based guidelines for best transfusion practices.
Risks of blood transfusion
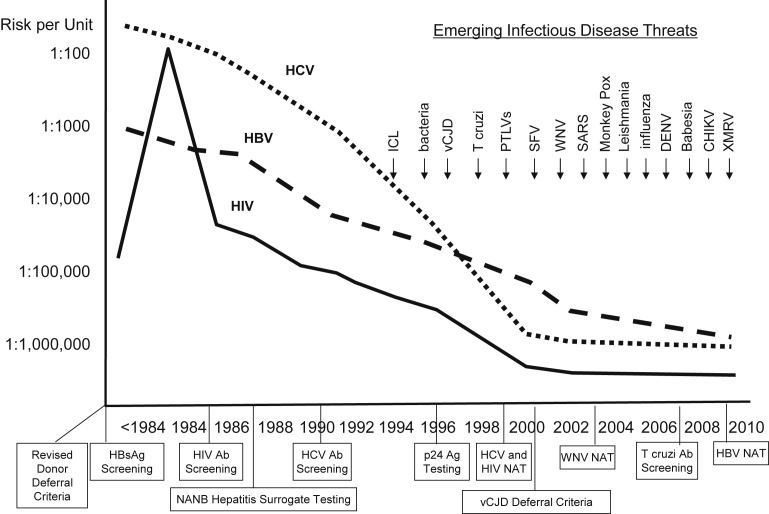
Transfusion-transmitted infections prompted concern by patients and health care providers since the 1980s, with the recognition of transfusion transmission of human immunodeficiency virus (HIV) and hepatitis C virus (HCV).9 These risks have decreased substantially, and responses to emerging pathogens transmitted by blood transfusion have been rapid (Fig. 1 ).10 Nevertheless, emerging threats of blood-transmissible pathogens is always a concern, the most recent example of which is the Zika virus, in which potential blood donors who are acutely ill and viremic may be asymptomatic and not be deferred during donor screening.11 For this reason, an experimental nucleic acid test (NAT) was implemented for universal donor testing by end of November 2016. Between 2007 and 2011, transfusion-related acute lung injury (TRALI) caused the highest percentage (43%) of fatalities reported to the US Food and Drug Administration (FDA), followed by hemolytic transfusion reactions (23%) caused by non-ABO- (13%) or ABO- (10%) incompatible blood transfusions.12
Fig. 1.
Risks of major transfusion-transmissible viruses linked to interventions, and accelerating rate of EIDs of concern to blood safety. Evolution of the risks of transmission by blood transfusion for HIV, HBV, and HCV. Major interventions to reduce risks are indicated below the time line on the x-axis. Emerging infectious disease threats over the past 20 years are indicated above in the top right quadrant of the figure. Ab, antibody; Ag, antigen; CHIKV, Chikungunya virus; DENV, dengue virus; HBsAg, hepatitis B surface antigen; ICL, idiopathic CD4+ T lymphocytopenia; PTLV, posttransplant lymphoproliferative disease; SARS, severe acute respiratory syndrome; SFV, simian foamy virus; vCJD, variant Creutzfeldt-Jakob disease; WNV, West Nile virus; XMRV, xenotropic murine leukemia virus-related virus.
(From Perkins HA, Busch MP. Transfusion-associated infections: 50 years of relentless challenges and remarkable progress. Transfusion 2010;50:2092; with permission.)
Increasing evidence suggests that a far greater number of patients now have adverse clinical outcomes (increased morbidity and mortality) associated with unnecessary blood transfusions.13, 14, 15 Table 1 lists risks that include not only known transmissible pathogens for infectious disease, transfusion reactions, TRALI, errors in blood administration, and circulatory overload but also potential, as yet undefined risks such as immunomodulation (eg, perioperative infection or tumor progression), unknown or emerging risks (such as the new variant Creutzfeldt-Jakob disease and Zika virus),10, 16 and potential risks associated with storage lesions from blood transfusions.17, 18
Table 1.
Transfusion-associated adverse events
| I. Infectious Agents | |
|---|---|
| Transfusion-transmitted disease routinely tested | |
| Hepatitis B virus (HBV; 1970 [surface antigen]; 1986–1987 [core antibody]; 2009 [nucleic acid]) | 1:1,000,000 |
| HIV (1985 [antibody]; 2000 [nucleic acid]) | 1:2,000,000 |
| HCV (1986–1987 [alanine aminotransferase]; 1990 [antibody]; 1999 [nucleic acid]) | 1:2,000,000 |
| Human T-cell lymphotropic virus (1988 [antibody]) | Very rare |
| West Nile virus (2003 [nucleic acid]) | Very rare |
| Bacteria (in platelets only; 2004) | 1:20,000 |
| Trypanosoma cruzi (2007 [antibody]) | Very rare |
| Syphilis | Very rare |
| Cytomegalovirus (for patients at risk) | Rare |
| Zika virus | Rare |
| Transfusion-transmitted disease not currently routinely tested | Very rare or unknown |
| Hepatitis A virus | |
| Parvovirus B19 | |
| Dengue fever virus | |
| Malaria | |
| Hepatitis E | |
| Babesia sp | |
| Plasmodium sp | |
| Leishmania sp | |
| Brucella sp | |
| New variant Creutzfeldt-Jakob disease prions | |
| Unknown pathogens | |
| II. Transfusion-associated adverse reactions events | |
| Estimated risk per unit infused | |
| ABO incompatible blood transfusions | 1 in 60,000 |
| Symptoms | 40% |
| Fatalities | 1 in 600,000 |
| Delayed serologic reactions | 1 in 1600 |
| Delayed hemolytic reactions | 1 in 6700 |
| TRALI | 1 in 20,000 |
| Graft-versus-host disease | Very rare |
| Posttransfusion purpura | Very rare |
| Febrile, nonhemolytic transfusion reactions | |
| RBCs | 1 in 200 |
| Platelets | 1 in 5–20 |
| Allergic reactions | 1 in 30–100 |
| Transfusion-associated circulatory overload | 1 in 12 |
| Anaphylactic reactions (Immunoglobulin A deficiency) | 1 in 150,000 |
| Iron overload | Estimated 80–100 U for adults |
| Transfusion-related immunosuppression | Unestablished |
| Storage lesions | Unestablished |
Adapted from Goodnough LT. Blood management: transfusion medicine comes of age. Lancet 2013;381:1792; with permission.
Awareness of blood risks and costs19 has led providers to develop institution-based initiatives in Patient Blood Management, including the adoption of recommendations that limit the use of blood transfusion.3 Patient Blood Management encompasses an evidence-based approach that is multidisciplinary (transfusion medicine specialists, surgeons, anesthesiologists, and critical care specialists) and multiprofessional (physicians, nurses, pump technologists, and pharmacists).20 Preventative strategies are emphasized to identify, evaluate, and manage anemia in medical6 and surgical21 patients, use of pharmacologic interventions,7, 8 and the avoidance of unnecessary diagnostic testing to minimize iatrogenic blood loss22; and to establish clinical practice recommendations for blood transfusions.3 For anemic patients being evaluated for elective procedures with the potential for blood loss, counseling on the risks of blood transfusion should be provided and steps taken to further characterize and treat anemia before surgery,21, 23 because preoperative anemia is associated with increased morbidity,24 mortality,25, 26 and hospital length of stay.27
Indications for red blood cell transfusion therapy
Pediatric Patients
A single randomized, prospective multicenter trial to evaluate a hemoglobin (Hb) “trigger” in children was published in 2007.28 In this study, more than 600 children admitted to the pediatric intensive care units (ICU) were randomized to either a restrictive-strategy group where Hb threshold was set at 7 g/dL or a liberal-strategy transfusion group where Hb threshold was set at 9 g/dL. The investigators found that the restrictive strategy resulted in a 44% decrease in the number of packed RBC transfusions without increasing rates of new or progressive multiorgan dysfunction, the primary outcome of the study. Several secondary outcomes, including sepsis, transfusion reactions, nosocomial respiratory infections, catheter-related infections, adverse events, length of stay in the ICU and hospital, and mortality were no different between the groups. The investigators recommended a restrictive RBC transfusion strategy in pediatric patients who are stable in the ICU.28
In addition, the TOTAL trial involving children aged 6 to 60 months presenting with severe anemia due to malaria or sickle cell disease revealed significant improvements in signs and symptoms of anemia after RBC transfusion to increase Hb concentrations from 3.7 to 7.1 g/dL.29 Serum lactate levels decreased from 9.1 mmol/L to less than or equal to 3 mmol/L 6 hours after transfusion in 59% of children. Similarly, cerebral tissue oxygen saturation, as measured by near-infrared spectrometry, increased by more than 5% at the completion of transfusion. Furthermore, rates of stupor or coma were reduced by half, whereas respiratory distress decreased by 60%. These findings suggest that tissue perfusion with Hb concentrations of 7 g/dL may be sufficient in this population.
Other randomized trials investigating Hb thresholds have been completed or are underway in neonates.30, 31, 32 The Prematures in Need of Transfusion study30 suggested that liberal RBC transfusions were beneficial to neurocognitive outcomes of premature infants at 18 to 22 months, in contrast to a randomized clinical trial that showed poorer neurologic outcomes at 7- to 10-year follow-up for those premature infants who were transfused liberally.31 The Transfusion of Prematures trial is underway to address these conflicting results.32 In a survey of pediatric centers from Children’s Oncology Group, 60% of centers used a transfusion trigger of Hb 8 g/dL, whereas 25% of centers used 7 g/dL.33
The notable exception in which liberal RBC transfusions have been found to be superior for improved clinical outcomes is in children with sickle cell anemia, who have overt stroke or abnormal transcranial Doppler ultrasonography and who are managed with chronic blood transfusions to keep the percentage of sickle cell hemoglobin less than 30% and the total Hb level at approximately 10 g/dL.34, 35 Interruption of such aggressive transfusion therapy when children reach the age of 18 to 20 years during transition of care to adult medical services has been described as associated with increased mortality and overt stroke events.36
Adult Patients
Symptomatic manifestations in medical anemias generally occur when the Hb is less than two-thirds of normal (ie, <9–10 g/dL), because basal cardiac output increases with anemia and is manifested by symptoms of increased cardiac work.37 The historical practice was to correct mild to moderate anemia with RBC transfusions in order to treat these signs and symptoms or to transfuse blood prophylactically. The view at that time was reflected in one publication that stated “when the concentration of hemoglobin is less than 8 to 10 g/dL, it is wise to give a blood transfusion before operation.”38
This readjustment of the transfusion trigger from an Hb of 10 g/dL to a somewhat lower threshold was triggered by concern over blood risks, particularly HIV; accompanied by the realization in populations such as Jehovah’s Witness patients, who decline blood transfusions because of religious beliefs, that morbidity and mortality do not increase until the Hb is very low.39 Data from this population indicate that the critical level of hemodilution, as defined as the point at which oxygen consumption starts to decrease because of insufficient oxygen delivery, occurs at an Hb level of approximately 4 g/dL,40 which was corroborated in a recent study of RBC transfusions in Ugandan children with SS anemia or malaria.29
For anemic patients known to have cardiovascular disease (CVD), perioperative mortality has been reported to be increased significantly, when compared with patients not known to have CVD.41 Management of anemia and the Hb threshold for RBC therapy should therefore be different for these patients. A post hoc analysis of one study42 was accompanied by an editorial observing that “survival tended to decrease for patients with pre-existing heart disease in the restrictive transfusion strategy group, suggesting that critically ill patients with heart and vascular disease may benefit from higher Hb.”43 Previously published clinical practice guidelines concluded “the presence of coronary artery disease likely constitutes an important factor in determining a patient’s tolerance to low Hb.”44 A retrospective analysis of 79,000 elderly patients (>65 years of age) hospitalized with acute myocardial infarction (MI) in the United States found that blood transfusion in patients whose admission hematocrit values were less than 33% was associated with significantly lower mortalities.45 A more aggressive use of blood transfusion in the management of anemia in elderly patients with cardiac disease might well be warranted.6, 46
There are an increasing number of randomized, controlled trials in adults providing level I evidence for blood transfusion practices. A previous systematic review of the literature to year 2000 identified 10 trials.47 The investigators concluded at that time that the existing evidence supported the use of restrictive transfusion triggers in patients who were free of serious cardiac disease. A Cochrane systematic review of prospective randomized trials to 201248 compared “high” versus “low” Hb thresholds of 19 trials involving a total of 6264 patients. The investigators found that (1) “low” Hb thresholds were well tolerated; (2) RBC transfusions were reduced by 34% (confidence interval [CI] 24%–45%) in patients randomized to the “low” Hb cohorts; and (3) the number of RBC transfusions was reduced by 1.2 units (CI 0.5–1.8 units) in the “low” Hb cohorts. A more recent meta-analysis found that a restrictive RBC transfusion strategy aiming to allow an Hb concentration as low as 7 g/dL reduced cardiac events, rebleeding, bacterial infections, and mortality.15
There are 7 key randomized, clinical trials in adult patients that compare “restrictive” versus “liberal” RBC transfusion strategies in various clinical settings (Table 2 ). The Transfusion Requirements in Critical Care (TRICC) trial49 found that intensive care patients could tolerate a restrictive transfusion strategy (Hb range 7–9 g/dL, 8.2 g/dL on average) as well as patients transfused more liberally (Hb range 10–12 g/dL, 10.5 g/dL on average), with no differences in 30-day mortalities. Similarly, in the Transfusion Requirements in Septic Shock trial50 of lower (<7 g/dL) versus higher (<9 g/dL) Hb thresholds for transfusion in patients with septic shock, equivalent 90-day mortalities (43 vs 45%, respectively) were found for patients in the 2 cohorts. However, a retrospective study of 2393 patients51 consecutively admitted to the ICU found that an admission hematocrit less than 25%, in the absence of transfusion, was associated with long-term mortality; so that there may be hematocrit levels below which the risk-to-benefit imbalance for transfusion reverses.
Table 2.
Seven key clinical trials in adults of red blood cell transfusion in adults
| Clinical Setting (Ref) | Hemoglobin Threshold (g/dL) | Mean Age (y) | Patients Transfused (%) | Deviation from Transfusion Protocol (%) | Mean Hemoglobin (g/dL)a | Participation of Eligible Patients (%) |
|---|---|---|---|---|---|---|
| Intensive care49 | 7 | 57.1 | 67 | 1.4 | 8.5 | 41 |
| 10 | 58.1 | 99 | 4.3 | 10.7 | ||
| CT surgery52 | 8 | 58.6 | 47 | 1.6 | 9.1 | 75 |
| 10 | 60.7 | 78 | 0.0 | 10.5 | ||
| Hip fracture repair53 | 8 | 81.5 | 41 | 9.0 | 7.9 | 56 |
| 10 | 81.8 | 97 | 5.6 | 9.2 | ||
| Acute upper GI bleeding54 | 7 | NA | 49 | 9.0 | 7.3 | 93 |
| 9 | NA | 86 | 3.0 | 8.0 | ||
| Symptomatic coronary artery disease55 | 8 | 74.3 | 28.3 | 1.8 | 7.9 | 12.2 |
| 10 | 67.3 | NAb | 9.1 | 9.3 | ||
| Sepsis trial50 | 7 | 67.0 | 64 | 5.9 | 7.7 | 82 |
| 9 | 67.0 | 99 | 2.2 | 9.3 | ||
| TITR56 | 7.5 | 69.9 | 53.4 | 30 | 8–9 | 98 |
| 9 | 70.8 | 92.2 | 45 | 9.2–9.8 |
Abbreviations: CAD, coronary artery disease; CT, cardiothoracic; TITR, Transfusion Indication Threshold Reduction.
Mean daily hemoglobin.
NA: Not available.
From Goodnough LT, Shah N. Is there a “magic” hemoglobin number? Clinical decision support promoting restrictive blood transfusion practices. Am J Hematol 2015;90:929; with permission.
The Transfusion Requirements after Cardiac Surgery (TRACS) trial52 was a large, single-center study of patients randomized to receive either restrictive (hematocrit >24%) or liberal (hematocrit >30%) RBC transfusions postoperatively. Thirty-day all-cause mortality was not different (10% vs 11%, respectively) between the 2 cohorts. The FOCUS trial found that elderly (mean >80 years of age) patients who underwent repair of hip fracture surgery tolerated an Hb trigger without RBC transfusions postoperatively to as low as 8 g/dL (or higher with transfusions, if symptomatic).53 Subsequently, a single-center prospective study54 of patients with upper gastrointestinal bleeding demonstrated that patients randomized to a restrictive (Hb <7 g/dL) versus a liberal (Hb <9 g/dL) Hb threshold for blood transfusions had significantly improved outcomes, including mortality at 45 days and rates of rebleeding.
The MINT trial55 was a pilot, feasibility study of liberal (Hb ≥10 g/dL) versus restrictive (Hb <8 g/dL) transfusion thresholds initiated for a planned enrollment of 200 patients with symptomatic coronary artery disease (acute coronary syndrome or stable angina undergoing cardiac catheterization), but was terminated at the end of 18 months after enrollment of only 110 patients; of eligible screened patients, only 12% were enrolled (see Table 2). The primary, composite outcome (death, MI, or revascularization) occurred in 10.9% of the liberal transfusion cohort, compared with 25.9% of the restrictive cohort (P = .054); mortality occurred in 1.8% and 13.0%, respectively (P = .032). In addition, the TITRe2 trial, which focused on postoperative coronary artery bypass graft and valve surgery patients, found no difference in primary outcomes of ischemic events (MI, stroke, bowel infarction, acute kidney injury) or infection (sepsis or wound infection) between restrictive (Hb <7.5 g/dL) and liberal (Hb <9 g/dL) transfusion triggers (35.1% vs 33.0%, P = .30). However, they observed more deaths in the restrictive group as compared with the liberal group (4.2% vs 2.6%, P = .045).56 Furthermore, a recent meta-analysis stratifying study patients into “context-specific” risk groups based on patient characteristics and clinical setting found increased risk of inadequate oxygen delivery and mortality among patients with CVD undergoing cardiac or vascular surgery as well as elderly patients undergoing orthopedic surgery.57 These trials50, 55, 57 provide evidence that a more liberal transfusion practice to maintain higher Hb thresholds may represent prudent management of high-risk patients who have symptomatic coronary artery disease or are undergoing cardiac surgery.
One of the important limitations of prospectively, randomized clinical trials is that patients who are eligible and who agree to participate in the study may not be particularly reflective of all patients in these clinical settings. Only 41% of the patients who were determined to be eligible for the TRICC trial49 and 56% of patients eligible for the FOCUS trial53 were actually enrolled in the studies, leading to concerns over selection bias; did the treating physicians accurately predict which patients would survive the study, and not enroll the others, thereby ensuring that no differences in survival outcomes would be found between treatment groups?
Another limitation is the interpretation of the “transfusion trigger” in these studies. The mean pretransfusion Hb for patients in the “restrictive” red cell transfusion arm of the TRACS trial was 9.1 g/dL (see Table 2). Similarly, the mean Hb for patients in the “restrictive” arm of the TRICC trial was 8.5 g/dL; yet many have interpreted this study to advocate that an Hb of 7 g/dL is appropriate for use as the transfusion trigger in critical care patients.
Clinical Practice Guidelines
The number of published clinical practice guidelines for RBC44, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75 transfusions attests to the increasing interest and importance of appropriate blood utilization by professional societies and health care institutions (Table 3 ). The selection of a discrete Hb as a “trigger” for RBC transfusion has been controversial.76 The guidelines generally acknowledge the necessity of considering patient covariables or other patient-specific criteria for making transfusion decisions. Among published guidelines, it is generally agreed that transfusion is not of benefit when the Hb is greater than 10 g/dL, but may be beneficial when the Hb is less than 6 to 7 g/dL.61, 62, 63, 66, 67, 68, 69, 70, 71, 72
Table 3.
Clinical practice guidelines for red blood cell transfusion
| RBC Transfusion | |||
|---|---|---|---|
| Year | Society | Recommendations | Reference |
| 1988 | National Institutes of Health Consensus Conference | <7 g/dL (acute) | JAMA 1988;260:2700.58 |
| 1992 | American College of Physicians (ACP) | No number | Ann Int Med 1992;116:393–402.59 |
| 1996/2006 | American Society of Anesthesiologists (ASA) | <6 g/dL (acute) | Anesth 1996;84:732–747.60 |
| No number | Anesth 2006;105:198–208.61 | ||
| 1997/1998 | Canadian Medical Association (CMA) | No number | Can Med Assoc J 1997;156: S1–24.44 J Emerg Med 1998;16:129–31.62 |
| 1998 | College of American Pathologists (CAP) | 6 g/dL (acute) | Arch Path Lab Med 1998;122:130–8.63 |
| 2001/2012 | British Committee for Standards in Haematology | No number | Br J Haematol 2001;113:24–31.64 |
| 7–8 g/dLa | http://www.bcshguidelines.com/documents/BCSH_Blood_Admin_-_addendum_August_2012.pdf.65 | ||
| 2001 | Australasian Society of Blood Transfusion | 7 g/dL | http://www.nhmrc.health.gov.au.66 |
| 2007/2011 | Society of Thoracic Surgeons (STS) Society of Cardiovascular Anesthesiologists (CVA) |
7 g/dL or 8 g/dLa |
Ann Thorac Surg 2007;83:S27–86.67 Ann Thorac Surg 2011;91:944–82.68 |
| 2009 | American College of Critical Care Medicine Society of Critical Care Medicine |
7 g/dL | Crit Care Med 2009;37:3124–57.69 |
| 7 g/dL | J Trauma 2009;67:1439–42.70 | ||
| 2011 | Society for the Advancement of Blood Management | 8 g/dL | Trans Med Rev 2011;232–246.71 |
| 2012 | National Blood Authority, Australia | No number | http://www.nba.gov.au/guidelines/review.html.75 |
| 2012 | AABB | 7–8 g/dL or 8 g/dLb | Ann Int Med 2012;157:49–58.72 |
| 2012 | Kidney Disease: Improving Global Outcomesc | No number | Kid Int 2012;2:311–316.73 |
| 2012 | National Cancer Center Network (NCCN) | 7–9 g/dL | JNCCN 2012;10:628–53.74 |
For patients with acute blood loss.
For patients with symptoms of end-organ ischaemia.
Acute coronary syndrome or cardiac bypass patients.
From Goodnough LT, Levy JH, Murphy MF. Concepts of blood transfusion in adults. Lancet 2013;381:1848; with permission.
An editorial77 summarized the implications of these trials and meta-analyses with a call for a target Hb level for transfusion, stating “it is no longer acceptable to recommend that we transfuse using vague approaches such as clinical judgment or in the hope of alleviating symptoms.” However, this approach would use transfusion to treat laboratory numbers, rather than patients, and would risk overinterpreting available evidence for a “transfusion trigger” and risk underestimating both the heterogeneity of anemias (eg, acute vs chronic) and the heterogeneity of patients (ie, comorbidities). Given the increasing evidence that shows blood transfusions are poorly effective and possibly harmful, the guiding principle for transfusion therapy should be that “Less is More.”. The AABB78 and the ASH79 have published recommendations from the American Board of Internal Medicine’s Choosing Wisely campaign advocating single-unit RBC transfusions for nonbleeding hospitalized patients, which nearly 25 years ago had previously been recommended by the American College of Physicians (ACP).80 Additional RBC units should be prescribed only after reassessment of the patient between transfusion decisions.
Improving Blood Utilization
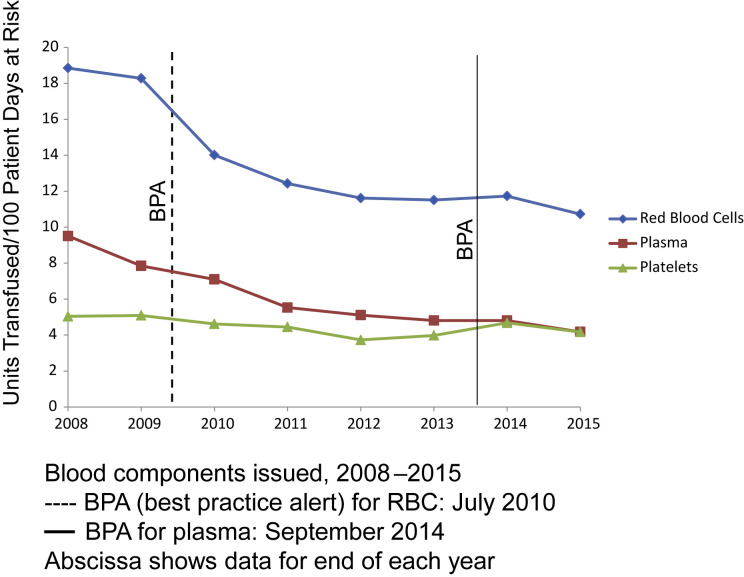
Both the pediatric81 and the adult hospital82, 83 at Stanford Health Care (SHC) have reduced blood use by using computerized physician order entry (POE) process for blood transfusions. The Hb concentration threshold for blood transfusions decreased after clinical effectiveness teams instituted physician education and clinical decision support (CDS) in July 2010, via best practices alerts (BPA) at the time of electronic POE.82, 83, 84, 85 Fig. 2 shows a subsequent analysis of trends in blood use at SHC. Overall blood component transfusions increased yearly until 2009; after the BPA was implemented in July 2010, however, RBC transfusions have decreased nearly 50% through 2015, over this same interval.84 Clinical patient outcomes (length of stay, 30-day readmission rate, mortality) showed improvement associated with implementation of CDS for restrictive transfusion practice.
Fig. 2.
Blood components issued to patients at SHC. Transfusion of RBCs per 100 days at risk, decreased by 42% from 2009 through 2015.
(Adapted from Goodnough LT, Shah N. The next chapter in patient blood management: real-time clinical decision support. Am J Clin Pathol 2014;142:743; with permission.)
Several other institutions have been able to use electronic health records to improve blood utilization, as most recently described by McKinney and colleagues.86 In another analysis of 21 medical facilities in Kaiser Permanente Northern California and nearly 400,000 inpatients from 2009 to 2013, the incidence of RBC transfusion decreased from 14% to 10.8%, with a decline in pretransfusion Hb levels from 8.1 to 7.6 g/dL; yet 30-day mortality did not change significantly over this same time interval.87
Although the improvement in patient outcomes concurrent with reduction in RBC transfusions cannot be proven to be causal, it is reassuring that there was no deleterious effect on patient outcomes after hospital-wide adoption of restrictive transfusion practices.13 A study monitoring for inappropriate undertransfusion found no evidence that cases of nonadministration of blood were unjustified.88
Additional benefits of the restrictive transfusion strategy included a significant improvement in the laboratory budget, with direct cost reductions of $1.6 million annually.82 Purchase acquisition costs represents a fraction of total costs of blood transfusion that additionally include laboratory testing, reagent costs, nursing time dedicated to transfusion, and monitoring. An activity-based cost summary of blood transfusions estimates that total costs related to transfusion are 3.2 to 4.8 times the purchase costs.19 Hence, the total transfusion-related savings potentially surpasses $30 million, over a 4-year period. In September 2014, the authors implemented a smart BPA for plasma, triggered when the last recorded international normalized ratio (INR) is less than 1.7 to guide more appropriate plasma transfusion.
This model of concurrent real-time utilization review can be supplemented by peer performance review committees, in which analysis of providers is undertaken for transfusions outside institutional-recommended guidelines. Because up to 30% of RBC transfusions continue to occur in patients whose Hb was greater than 8 g/dL at the authors’ institution, peer-performance executive committees can help reduce variability by providers within clinical services that was unchanged by the CDS, and/or help modify the CDS for known clinical exceptions. This process serves as continuous education and feedback, which is seen as vital in the success of utilization programs89 by augmenting improvements through CDS.
Other programs have been able to use electronic health records to improve blood utilization in a different manner. One center reconfigured their POE system for nonbleeding (excluding procedural units such as operating rooms, cardiac catheterization laboratories) patients to remove single-click ordering for 2-unit RBC transfusions; the provider must select from a drop-down menu if additional RBC units are desired. The proportion of 2-unit RBC transfusions decreased from 47% before to 15% after this intervention.90 A second center similarly reported a reduction in 2-unit RBC orders (48% to 33%) and an increase in 1-unit RBC transfusions (22% to 48%), before and after, respectively, implementation of a comprehensive education and audit program promoting restrictive transfusion practices.91 One review concluded that although CDS can improve RBC usage, further data are needed to assess whether CDS can improve plasma and platelet use utilization.92 The authors have been able to show a 19% reduction in plasma utilization after implementing a smart BPA for plasma orders in the context of a most recent INR result in the patient’s electronic medical record (EMR), at the authors’ institution.93
Additional opportunities to improve blood utilization are in patients undergoing surgical procedures. Because the most important predictor of the need for blood transfusion during perioperative bleeding is the patient’s preoperative RBC volume, preadmission testing to include identification and correction of anemia in patients undergoing elective surgical procedures is particularly important.21 The authors’ hospital has initiated a checklist and boarding pass “timeout” before induction of anesthesia, designed to facilitate a conversation between the surgical and anesthesiology teams for individual patients on their anticipated blood loss, cross-matched blood availability, and strategies for managing blood loss anemia; this initiative was based on a strategy described by Atul Gawande using a surgical checklist at his own institution.94
Summary
According to the Institute of Medicine, $2.5 trillion was spent on health care, consuming 17.6% of the gross domestic product. In 2009, almost one-third of this health care expenditure was estimated to be wasteful. Transfusion therapy has been identified as one of the most overused (and inappropriate) therapeutic interventions. Reducing this waste helps improve patient outcomes by reducing unnecessary blood donor exposures. The increased adoption of EMRs and features such as CDS allows the practice of prospective, real-time monitoring of transfusion therapy in an automated fashion at the critical time of POE.
Future measures include providing the prescriber with evidence-based and practical RBC ordering options,95 and distributing the CDS burden to personnel with the highest knowledge base to make decisions. Long term, these users will be engaged for further education or refinement of CDS for continuous quality improvement.96
References
- 1.Straus D.J., Testa M.A., Sarokhan B.J. Quality-of-life and health benefits of early treatment of mild anemia: a randomized trial of epoetin alfa in patients receiving chemotherapy for hematologic malignancies. Cancer. 2006;107:1909–1917. doi: 10.1002/cncr.22221. [DOI] [PubMed] [Google Scholar]
- 2.Bulger J., Nickel W., Messler J. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8:486–492. doi: 10.1002/jhm.2063. [DOI] [PubMed] [Google Scholar]
- 3.Goodnough L.T., Levy J.H., Murphy M.F. Concepts of blood transfusion in adults. Lancet. 2013;381:1845–1854. doi: 10.1016/S0140-6736(13)60650-9. [DOI] [PubMed] [Google Scholar]
- 4.Roseff S.D., Luban N.L., Manno C.S. Guidelines for assessing appropriateness of pediatric transfusion. Transfusion. 2002;42:1398–1413. doi: 10.1046/j.1537-2995.2002.00208.x. [DOI] [PubMed] [Google Scholar]
- 5.Shah N., Andrews J., Goodnough L.T. Transfusions for anemia in adult and pediatric patients with malignancies. Blood Rev. 2015;29:291–299. doi: 10.1016/j.blre.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodnough L.T., Schrier S.L. Evaluation and management of anemia in the elderly. Am J Hematol. 2014;89:88–96. doi: 10.1002/ajh.23598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodnough L.T., Shander A. Current status of pharmacologic therapies in patient blood management. Anesth Analg. 2013;116:15–34. doi: 10.1213/ANE.0b013e318273f4ae. [DOI] [PubMed] [Google Scholar]
- 8.Spahn D.R., Goodnough L.T. Alternatives to blood transfusion. Lancet. 2013;381:1855–1865. doi: 10.1016/S0140-6736(13)60808-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perkins H.A., Busch M.P. Transfusion-associated infections: 50 years of relentless challenges and remarkable progress. Transfusion. 2010;50:2080–2099. doi: 10.1111/j.1537-2995.2010.02851.x. [DOI] [PubMed] [Google Scholar]
- 10.Goodnough L.T. Blood management: transfusion medicine comes of age. Lancet. 2013;381:1791–1792. doi: 10.1016/S0140-6736(13)60673-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fauci A.S., Morens D.M. Zika virus in the Americas–yet another arbovirus threat. N Engl J Med. 2016;374:601–604. doi: 10.1056/NEJMp1600297. [DOI] [PubMed] [Google Scholar]
- 12.US Food and Drug Administration Center for Biologics Evaluation and Research. Fatalities reported to FDA following blood collection and transfusion. Annual summary for fiscal year 2011. Available at: http://www.fda.gov/downloads/BiologicsBloodVaccines/SafetyAvailability/ReportaProblem/TransfusionDonationFatalities/UCM300764.pdf. Accessed June 9, 2016.
- 13.Goodnough L.T., Murphy M.F. Do liberal blood transfusions cause more harm than good? BMJ. 2014;349:g6897. doi: 10.1136/bmj.g6897. [DOI] [PubMed] [Google Scholar]
- 14.Brunskill S.J., Millette S.L., Shokoohi A. Red blood cell transfusion for people undergoing hip fracture surgery. Cochrane Database Syst Rev. 2015;(4) doi: 10.1002/14651858.CD009699.pub2. CD009699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salpeter S.R., Buckley J.S., Chatterjee S. Impact of more restrictive blood transfusion strategies on clinical outcomes: a meta-analysis and systematic review. Am J Med. 2014;127:124–131.e3. doi: 10.1016/j.amjmed.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Bloch E.M., Simon M.S., Shaz B.H. Emerging infections and blood safety in the 21st century. Ann Intern Med. 2016 doi: 10.7326/M15-1329. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Wang D., Sun J., Solomon S.B. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52:1184–1195. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steiner M.E., Triulzi D.J., Assmann S.F. Randomized trial results: red cell storage is not associated with a significant difference in multiple-organ dysfunction score or mortality in transfused cardiac surgery patients. Transfusion. 2014;54:15A. [Google Scholar]
- 19.Shander A., Hofmann A., Ozawa S. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50:753–765. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 20.Goodnough L.T., Shander A. Patient blood management. Anesthesiology. 2012;116:1367–1376. doi: 10.1097/ALN.0b013e318254d1a3. [DOI] [PubMed] [Google Scholar]
- 21.Goodnough L.T., Maniatis A., Earnshaw P. Detection, evaluation, and management of preoperative anaemia in the elective orthopaedic surgical patient: NATA guidelines. Br J Anaesth. 2011;106:13–22. doi: 10.1093/bja/aeq361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salisbury A.C., Reid K.J., Alexander K.P. Diagnostic blood loss from phlebotomy and hospital-acquired anemia during acute myocardial infarction. Arch Intern Med. 2011;171:1646–1653. doi: 10.1001/archinternmed.2011.361. [DOI] [PubMed] [Google Scholar]
- 23.Guinn N.R., Guercio J.R., Hopkins T.J. How do we develop and implement a preoperative anemia clinic designed to improve perioperative outcomes and reduce cost? Transfusion. 2016;56:297–303. doi: 10.1111/trf.13426. [DOI] [PubMed] [Google Scholar]
- 24.Jans Ø., Jorgensen C., Kehlet H. Role of preoperative anemia for risk of transfusion and postoperative morbidity in fast-track hip and knee arthroplasty. Transfusion. 2014;54:717–726. doi: 10.1111/trf.12332. [DOI] [PubMed] [Google Scholar]
- 25.Wu W.C., Schifftner T.L., Henderson W.G. Preoperative hematocrit levels and postoperative outcomes in older patients undergoing noncardiac surgery. JAMA. 2007;297:2481–2488. doi: 10.1001/jama.297.22.2481. [DOI] [PubMed] [Google Scholar]
- 26.Beattie W.S., Karkouti K., Wijeysundera D.N. Risk associated with preoperative anemia in noncardiac surgery: a single-center cohort study. Anesthesiology. 2009;110:574–581. doi: 10.1097/ALN.0b013e31819878d3. [DOI] [PubMed] [Google Scholar]
- 27.Gruson K.I., Aharonoff G.B., Egol K.A. The relationship between admission hemoglobin level and outcome after hip fracture. J Orthop Trauma. 2002;16:39–44. doi: 10.1097/00005131-200201000-00009. [DOI] [PubMed] [Google Scholar]
- 28.Lacroix J., Hebert P.C., Hutchison J.S. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356:1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 29.Dhabangi A., Ainomugisha B., Cserti-Gazdewich C. Effect of transfusion of red blood cells with longer vs shorter storage duration on elevated blood lactate levels in children with severe anemia: the total randomized clinical trial. JAMA. 2015;314:2514–2523. doi: 10.1001/jama.2015.13977. [DOI] [PubMed] [Google Scholar]
- 30.Kirpalani H., Whyte R.K., Andersen C. The Premature Infants in Need of Transfusion (PINT) study: a randomized, controlled trial of a restrictive (low) versus liberal (high) transfusion threshold for extremely low birth weight infants. J Pediatr. 2006;149:301–307. doi: 10.1016/j.jpeds.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Bell E.F., Strauss R.G., Widness J.A. Randomized trial of liberal versus restrictive guidelines for red blood cell transfusion in preterm infants. Pediatrics. 2005;115:1685–1691. doi: 10.1542/peds.2004-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirpalani H, Bell E, D'Angio C, et al. Transfusion of Prematures (TOP) trial: does a liberal red blood cell transfusion strategy improve neurological-intact survival of extremely-low-birth-weight infants as compared to a restricitve strategy? Available at: http://www.nichd.nih.gov/about/Documents/TOP_Protocol.pdf. Accessed June 9, 2016.
- 33.Bercovitz R.S., Quinones R.R. A survey of transfusion practices in pediatric hematopoietic stem cell transplant patients. J Pediatr Hematol Oncol. 2013;35:e60–e63. doi: 10.1097/MPH.0b013e3182707ae5. [DOI] [PubMed] [Google Scholar]
- 34.Adams R.J., McKie V.C., Hsu L. Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med. 1998;339:5–11. doi: 10.1056/NEJM199807023390102. [DOI] [PubMed] [Google Scholar]
- 35.Adams R.J., Brambilla D. Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med. 2005;353:2769–2778. doi: 10.1056/NEJMoa050460. [DOI] [PubMed] [Google Scholar]
- 36.McLaughlin J.F., Ballas S.K. High mortality among children with sickle cell anemia and overt stroke who discontinue blood transfusion after transition to an adult program. Transfusion. 2016;56:1014–1021. doi: 10.1111/trf.13418. [DOI] [PubMed] [Google Scholar]
- 37.Finch C.A., Lenfant C. Oxygen transport in man. N Engl J Med. 1972;286:407–415. doi: 10.1056/NEJM197202242860806. [DOI] [PubMed] [Google Scholar]
- 38.Adams R.C., Lundy J.S. Anesthesia in cases of poor surgical risk: some suggestions for decreasing the risk. Surg Gynecol Obstet. 1941;71:1011–1014. [Google Scholar]
- 39.Carson J.L., Noveck H., Berlin J.A. Mortality and morbidity in patients with very low postoperative Hb levels who decline blood transfusion. Transfusion. 2002;42:812–818. doi: 10.1046/j.1537-2995.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 40.van Woerkens E.C., Trouwborst A., van Lanschot J.J. Profound hemodilution: what is the critical level of hemodilution at which oxygen delivery-dependent oxygen consumption starts in an anesthetized human? Anesth Analg. 1992;75:818–821. doi: 10.1213/00000539-199211000-00029. [DOI] [PubMed] [Google Scholar]
- 41.Carson J.L., Duff A., Poses R.M. Effect of anaemia and cardiovascular disease on surgical mortality and morbidity. Lancet. 1996;348:1055–1060. doi: 10.1016/S0140-6736(96)04330-9. [DOI] [PubMed] [Google Scholar]
- 42.Hebert P.C., Yetisir E., Martin C. Is a low transfusion threshold safe in critically ill patients with cardiovascular diseases? Crit Care Med. 2001;29:227–234. doi: 10.1097/00003246-200102000-00001. [DOI] [PubMed] [Google Scholar]
- 43.Parrillo J.E. Journal supplements, anemia management, and evidence-based critical care medicine. Crit Care Med. 2001;29(Supplement):S139–S140. [Google Scholar]
- 44.Expert Working Group Guidelines for red blood cell and plasma transfusion for adults and children. Can Med Assoc J. 1997;156(Suppl 11):S1–S24. [Google Scholar]
- 45.Wu W.C., Rathore S.S., Wang Y. Blood transfusion in elderly patients with acute myocardial infarction. N Engl J Med. 2001;345:1230–1236. doi: 10.1056/NEJMoa010615. [DOI] [PubMed] [Google Scholar]
- 46.Goodnough L.T., Bach R.G. Anemia, transfusion, and mortality. N Engl J Med. 2001;345:1272–1274. doi: 10.1056/NEJM200110253451711. [DOI] [PubMed] [Google Scholar]
- 47.Carson J.L., Hill S., Carless P. Transfusion triggers: a systematic review of the literature. Transfus Med Rev. 2002;16:187–199. doi: 10.1053/tmrv.2002.33461. [DOI] [PubMed] [Google Scholar]
- 48.Carson J.L., Carless P.A., Hebert P.C. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;(4) doi: 10.1002/14651858.CD002042.pub3. CD002042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hebert P.C., Wells G., Blajchman M.A. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340:409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 50.Holst L.B., Haase N., Wetterslev J. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371:1381–1391. doi: 10.1056/NEJMoa1406617. [DOI] [PubMed] [Google Scholar]
- 51.Mudumbai S.C., Cronkite R., Hu K.U. Association of admission hematocrit with 6-month and 1-year mortality in intensive care unit patients. Transfusion. 2011;51:2148–2159. doi: 10.1111/j.1537-2995.2011.03134.x. [DOI] [PubMed] [Google Scholar]
- 52.Hajjar L.A., Vincent J.L., Galas F.R. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559–1567. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 53.Carson J.L., Terrin M.L., Noveck H. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453–2462. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Villanueva C., Colomo A., Bosch A. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368:11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 55.Carson J.L., Brooks M.M., Abbott J.D. Liberal versus restrictive transfusion thresholds for patients with symptomatic coronary artery disease. Am Heart J. 2013;165:964–971.e1. doi: 10.1016/j.ahj.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murphy G.J., Pike K., Rogers C.A. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. 2015;372:997–1008. doi: 10.1056/NEJMoa1403612. [DOI] [PubMed] [Google Scholar]
- 57.Hovaguimian F., Myles P.S. Restrictive versus liberal transfusion strategy in the perioperative and acute care settings: A context-specific systematic review and meta-analysis of randomized controlled trials. Anesthesiology. 2016;125(1):46–61. doi: 10.1097/ALN.0000000000001162. [DOI] [PubMed] [Google Scholar]
- 58.Consensus Conference. Perioperative red blood cell transfusion. JAMA. 1988;260:2700–2703. [PubMed] [Google Scholar]
- 59.Welch H.G., Meehan K.R., Goodnough L.T. Prudent strategies for elective red blood cell transfusion. Ann Intern Med. 1992;116:393–402. doi: 10.7326/0003-4819-116-5-393. [DOI] [PubMed] [Google Scholar]
- 60.Practice Guidelines for blood component therapy: a report by the American Society of Anesthesiologists Task Force on Blood Component Therapy. Anesthesiology. 1996;84:732–747. [PubMed] [Google Scholar]
- 61.Practice guidelines for perioperative blood transfusion and adjuvant therapies: an updated report by the American Society of Anesthesiologists Task Force on Perioperative Blood Transfusion and Adjuvant Therapies. Anesthesiology. 2006;105:198–208. doi: 10.1097/00000542-200607000-00030. [DOI] [PubMed] [Google Scholar]
- 62.Innes G. Guidelines for red blood cells and plasma transfusion for adults and children: an emergency physician's overview of the 1997 Canadian blood transfusion guidelines. Part 1: red blood cell transfusion. Canadian Medical Association Expert Working Group. J Emerg Med. 1998;16:129–131. doi: 10.1016/s0736-4679(97)00253-9. [DOI] [PubMed] [Google Scholar]
- 63.Simon T.L., Alverson D.C., AuBuchon J. Practice parameter for the use of red blood cell transfusions: developed by the Red Blood Cell Administration Practice Guideline Development Task Force of the College of American Pathologists. Arch Pathol Lab Med. 1998;122:130–138. [PubMed] [Google Scholar]
- 64.Murphy M.F., Wallington T.B., Kelsey P. Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113:24–31. doi: 10.1046/j.1365-2141.2001.02701.x. [DOI] [PubMed] [Google Scholar]
- 65.British Committee for Standards in Haematology (BCSH) Guideline on the Administration of blood components. Available at: http://www.bcshguidelines.com/documents/BCSH_Blood_Admin_-_addendum_August_2012.pdf. Accessed June 9, 2016.
- 66.Australasian Society of Blood Transfusion. Clinical Practice Guidelines: Appropriate use of red blood cells. Available at: http://www.nhmrc.gov.au/_files_nhmrc/publications/attachments/cp78.pdf. Accessed June 9, 2016.
- 67.Society of Thoracic Surgeons Blood Conservation Guideline Task Force, Ferraris V.A., Ferraris S.P. Perioperative blood transfusion and blood conservation in cardiac surgery: the Society of Thoracic Surgeons and The Society of Cardiovascular Anesthesiologists clinical practice guideline. Ann Thorac Surg. 2007;83:S27–S86. doi: 10.1016/j.athoracsur.2007.02.099. [DOI] [PubMed] [Google Scholar]
- 68.Society of Thoracic Surgeons Blood Conservation Guideline Task Force, Ferraris V.A., Brown J.R. 2011 Update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91:944–982. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 69.Napolitano L.M., Kurek S., Luchette F.A. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009;37:3124–3157. doi: 10.1097/CCM.0b013e3181b39f1b. [DOI] [PubMed] [Google Scholar]
- 70.Napolitano L.M., Kurek S., Luchette F.A. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. J Trauma. 2009;67:1439–1442. doi: 10.1097/TA.0b013e3181ba7074. [DOI] [PubMed] [Google Scholar]
- 71.Shander A., Fink A., Javidroozi M. Appropriateness of allogeneic red blood cell transfusion: the international consensus conference on transfusion outcomes. Transfus Med Rev. 2011;25:232–246.e53. doi: 10.1016/j.tmrv.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 72.Carson J.L., Grossman B.J., Kleinman S. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157:49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 73.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group KDIGO Clinical Practice Guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2:279–335. [Google Scholar]
- 74.Rodgers G.M., 3rd, Becker P.S., Blinder M. Cancer- and chemotherapy-induced anemia. J Natl Compr Canc Netw. 2012;10:628–653. doi: 10.6004/jnccn.2012.0064. [DOI] [PubMed] [Google Scholar]
- 75.National Blood Authority, Australia. Patient blood management guidelines. Available at: https://www.blood.gov.au/pbm-guidelines. Accessed June 9, 2016.
- 76.Goodnough L.T., Shah N. Is there a “magic” hemoglobin number? Clinical decision support promoting restrictive blood transfusion practices. Am J Hematol. 2015;90:927–933. doi: 10.1002/ajh.24101. [DOI] [PubMed] [Google Scholar]
- 77.Carson J.L., Hebert P.C. Should we universally adopt a restrictive approach to blood transfusion? It's all about the number. Am J Med. 2014;127:103–104. doi: 10.1016/j.amjmed.2013.10.026. [DOI] [PubMed] [Google Scholar]
- 78.Callum J.L., Waters J.H., Shaz B.H. The AABB recommendations for the Choosing Wisely campaign of the American Board of Internal Medicine. Transfusion. 2014;54:2344–2352. doi: 10.1111/trf.12802. [DOI] [PubMed] [Google Scholar]
- 79.Hicks L.K., Bering H., Carson K.R. The ASH choosing wisely campaign: five hematologic tests and treatments to question. Blood. 2013;122:3879–3883. doi: 10.1182/blood-2013-07-518423. [DOI] [PubMed] [Google Scholar]
- 80.Audet A.M., Goodnough L.T., Parvin C.A. Evaluating the appropriateness of red blood cell transfusions: the limitations of retrospective medical record reviews. Int J Qual Health Care. 1996;8:41–49. doi: 10.1093/intqhc/8.1.41. [DOI] [PubMed] [Google Scholar]
- 81.Adams E.S., Longhurst C.A., Pageler N. Computerized physician order entry with decision support decreases blood transfusions in children. Pediatrics. 2011;127:e1112–e1119. doi: 10.1542/peds.2010-3252. [DOI] [PubMed] [Google Scholar]
- 82.Goodnough L.T., Shieh L., Hadhazy E. Improved blood utilization using real-time clinical decision support. Transfusion. 2014;54:1358–1365. doi: 10.1111/trf.12445. [DOI] [PubMed] [Google Scholar]
- 83.Goodnough L.T., Maggio P., Hadhazy E. Restrictive blood transfusion practices are associated with improved patient outcomes. Transfusion. 2014;54:2753–2759. doi: 10.1111/trf.12723. [DOI] [PubMed] [Google Scholar]
- 84.Goodnough L.T., Shah N. The next chapter in patient blood management: real-time clinical decision support. Am J Clin Pathol. 2014;142:741–747. doi: 10.1309/AJCP4W5CCFOZUJFU. [DOI] [PubMed] [Google Scholar]
- 85.Tim Goodnough L., Andrew Baker S., Shah N. How I use clinical decision support to improve blood cell utilization. Transfusion. 2016;56(10):2406–2411. doi: 10.1111/trf.13767. [DOI] [PubMed] [Google Scholar]
- 86.McKinney Z.J., Peters J.M., Gorlin J.B. Improving red blood cell orders, utilization, and management with point-of-care clinical decision support. Transfusion. 2015;55:2086–2094. doi: 10.1111/trf.13103. [DOI] [PubMed] [Google Scholar]
- 87.Roubinian N.H., Escobar G.J., Liu V. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion. 2014;54:2678–2686. doi: 10.1111/trf.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hibbs S., Miles D., Staves J. Is undertransfusion a problem in modern clinical practice? Transfusion. 2015;55:906–910. doi: 10.1111/trf.12893. [DOI] [PubMed] [Google Scholar]
- 89.Yeh D.D. A clinician's perspective on laboratory utilization management. Clin Chim Acta. 2014;427:145–150. doi: 10.1016/j.cca.2013.09.023. [DOI] [PubMed] [Google Scholar]
- 90.Yerrabothala S., Desrosiers K.P., Szczepiorkowski Z.M. Significant reduction in red blood cell transfusions in a general hospital after successful implementation of a restrictive transfusion policy supported by prospective computerized order auditing. Transfusion. 2014;54:2640–2645. doi: 10.1111/trf.12627. [DOI] [PubMed] [Google Scholar]
- 91.Oliver J.C., Griffin R.L., Hannon T. The success of our patient blood management program depended on an institution-wide change in transfusion practices. Transfusion. 2014;54:2617–2624. doi: 10.1111/trf.12536. [DOI] [PubMed] [Google Scholar]
- 92.Hibbs S.P., Nielsen N.D., Brunskill S. The impact of electronic decision support on transfusion practice: A systematic review. Transfus Med Rev. 2015;29:14–23. doi: 10.1016/j.tmrv.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 93.Shah N.K., Shepard J., Hadhazy E. Decreasing Inappropriate Plasma (FFP) Transfusion with Real-time Clinical Decision Support (CDS) Transfusion. 2015;55:107A. [Google Scholar]
- 94.Notable & Quotable: Atul Gawande. The Wall Street Journal; 12/12/2014. Available at: http://www.wsj.com/articles/notable-quotable-atul-gawande-1418425543?tesla=y&mod=djemITP_h&mg=reno64-wsj. Accessed June 9, 2016.
- 95.McWilliams B., Triulzi D.J., Waters J.H. Trends in RBC ordering and use after implementing adaptive alerts in the electronic computerized physician order entry system. Am J Clin Pathol. 2014;141:534–541. doi: 10.1309/AJCPEN6VHT0ECAFI. [DOI] [PubMed] [Google Scholar]
- 96.Berwick D.M. Continuous improvement as an ideal in health care. N Engl J Med. 1989;320:53–56. doi: 10.1056/NEJM198901053200110. [DOI] [PubMed] [Google Scholar]