Abstract
A synthetic peptide corresponding to amino acids (aa) 15–28 of the severe acute respiratory syndrome coronavirus (SARS‐CoV) 3a protein was used to raise polyclonal antibodies in rabbits. This anti‐3a N‐terminal antibody could detect 3a protein in infected cells, as did an anti‐3a C‐terminal antibody previously described. The latter targeted the C‐terminal cytoplasmic domain of 3a (aa 134–274). The anti‐3a N‐terminal antibody could detect intracellular 3a as well as 3a expressed on the cell surface. Interestingly, only the anti‐3a N‐terminal antibody can inhibit SARS‐CoV propagation in Vero E6 culture although the binding affinity of the anti‐3a N‐terminal antibody was lower than the anti‐3a C‐terminal antibody.
Keywords: Severe acute respiratory syndrome, Coronavirus, Group-specific viral protein, 3a Protein, Neutralizing activities
1. Introduction
The genome of the severe acute respiratory syndrome coronavirus (SARS‐CoV) encodes for eight group‐specific proteins with no significance sequence homology to viral proteins of other coronaviruses [1, 2, 3]. The largest of these group‐specific proteins is termed 3a and the 3a protein has also been shown to be expressed in SARS‐CoV infected cells [4, 5] and could be detected in tissues obtained from SARS patients [5, 6, 7]. Antibodies against 3a have also been detected in different cohorts of SARS patients [8, 9, 10, 11].
The 3a protein consists of 274 amino acids (aa) and contains three putative transmembrane domains and it is expressed on the cell surface [4, 12]. The topology of 3a on the cell surface was determined experimentally: its first 34 aa, i.e. before the first transmembrane domain, is facing the extracellular matrix and its C‐terminal after the third transmembrane domain (i.e. aa 134–274) is facing the cytoplasm [4]. As 3a is a novel coronavirus structural protein [12, 13], its N‐terminal ectodomain would be expected to protrude out of the virion. Interestingly, in two separate cohorts of SARS patients, one from Taiwan [14] and one from Hong Kong [15], B cells recognizing the N‐terminal region of 3a were isolated from patients. In addition, it was recently reported that the N terminal of 3a elicits strong and potentially protective humoral responses in infected patients [11]. In this study, rabbit polyclonal antibodies targeted against the N‐terminal ectodomain and the C‐terminal cytoplasmic domain of the 3a protein were tested for their abilities to inhibit SARS‐CoV propagation in Vero E6 culture.
2. Materials and methods
2.1. Cell‐line and virus
The Vero E6 cells and SARS‐CoV isolate used in this study have been previously described [16]. Culturing of 293T cells have been previously described [4].
2.2. Synthesis of peptide and production of rabbit polyclonal antibodies
A peptide ((C)AQPVKIDNASPAST), which corresponds to amino acids 15–28 of SARS‐CoV 3a protein, was synthesized by BioGenes GmbH (Berlin, Germany). The peptide was conjugated to a carrier, Limulus Polyphemus Hemocyanine (LPH) from horseshoe crab, and used to immunize two rabbits using standard protocols. All procedures were performed by BioGenes GmbH. The immunization schedule is showed in Table 1 . All the sera were tested by Western blot analysis.
Table Table 1.
Schedule for the immunization of rabbits (#2 and #3) with a synthetic peptide corresponding to 15–28 amino acids of SARS‐CoV 3a protein
| Day | Immunization no. | Bleed no. |
|---|---|---|
| 0 | 1 | Pre‐immune |
| 7 | 2 | – |
| 14 | 3 | – |
| 28 | 4 | – |
| 35 | – | 1 |
| 49 | 5 | – |
| 63 | – | 2 |
| 77 | 6 | – |
| 91 | – | 3 |
| 120 | 7 | – |
| 127 | – | 4 |
| 141 | 8 | – |
| 148 | – | 5 |
A rabbit polyclonal antibody raised against bacterially‐expressed GST‐3a (134‐274aa) has been previously described [4]. This antibody targets the C‐terminal cytoplasmic domain of 3a and the 6th bleed was used in this study. A neutralizing antibody (rabbit anti‐SΔ10) that targeted the SARS‐CoV spike (S) protein was also used in the neutralizing assays [17]. The last bleed obtained after 16 immunizations was used.
2.3. Western blot analysis and immunofluorescence experiments
In order to express recombinant 3a protein in mammalian cells, Vero E6 cells were transfected with a cDNA construct (pXJ‐3a) for expressing full‐length 3a protein, as previously described [4]. Transfected cells were then subjected to Western blot analysis and immunofluorescence experiments as previously described [4]. Briefly, cells were grown to 80% confluence in a 6 cm dish and transfected with 1 μg of the plasmid. The cells were harvested after ∼16 h and washed with PBS and then lysed in 1 ml of lysis buffer (50 mM Tris, pH 8, 150 mM NaCl, 0.5% NP40, 0.5% deoxycholic acid, 0.005% SDS, 1 mM PMSF). After 6 rounds of alternate freezing and thawing, the cells suspension was centrifuged at 13 000 rpm for 20 min at 4 °C. The lysates were used for Western blot analysis. Western blot analysis was also performed on Vero E6 cells infected with SARS‐CoV at a multiplicity of infection (MOI) of 1. At 24 h post‐infection, the cells were washed with PBS and lysed in 350 μl of lysis buffer and the lysate was subjected to Western blot analysis in a similar manner.
For immunofluorescence experiments, the cells were grown on coverslips and transfected as described above. About 16 h later, the cells were fixed with 4% paraformaldehyde and/or permeabilized with Triton X‐100, and incubated with the relevant antibodies as previously described [4].
2.4. Enzyme‐linked immunosorbent assay (ELISA)
For ELISA, 293T cells were used instead because of their higher transfection efficiency. Cell lysates obtained from untransfected 293T or 293T transiently transfected with pXJ‐3a (as described above) were diluted 1:12 with carbonate buffer (pH 9.6) prior to plate coating. 96‐well Nunc Maxisorp microtiter plates (Nalge Nunc International, Naperville, IL, USA) were coated with the protein mixtures at a volume of 50 μl per well by incubation overnight at 4 °C. All subsequent steps were performed at room temperature. The plates were washed three times with PBS containing 0.05% Tween‐20 (PBST) and non‐specific binding sites were blocked with 200 μl per well of blocking buffer (PBST containing 3% BSA) for 1 h. The plates were further washed another three times before the addition of serum samples diluted (1:2000 or 1:8000) in blocking buffer. Subsequently, the plates were incubated for 30 min followed by three washes with PBST. Horse‐radish peroxidase (HRP)‐conjugated goat anti‐rabbit IgG (Pierce, Rockford, USA; 1:2000 dilution) was added at 50 μl per well and incubated for 30 min. The plates were then washed three times in PBST, followed by three times with distilled water and allowed a colour development with the addition of 100 μl per well of TMB substrate solution (Pierce). After incubation for 15 min in the dark, the reaction was stopped by adding 100 μl per well of 1 M H2SO4. The optical densities (OD) were measured at 450 nm. All experiments were performed in duplicates and the average values with standard deviations are plotted.
2.5. Neutralization assay
Sera were diluted 1:10 using D‐MEM (Gibco) supplemented with 2% HEPES, 1% antibiotics and 2% inactivated fetal calf serum, and then inactivated at 56 °C for 30 min. The sera were diluted twofold in series using 100 μl of 2% medium supplemented as described above, in a 96 well plate. To the serum dilutions, virus was added; 25 μl virus stock (107 PFU ml−1) was added to 15 ml of medium and from that a 1/10 dilution was made and 100 μl/well of the last dilution was added to the serum dilutions. Thus, in all cases, a fixed concentration of the virus was used except for a virus control, which was diluted 10‐folds starting with the concentration that was added to the serum as described above. The plates were incubated at 37 °C for 60 min, then 100 μl from each well were transferred to 96 well plates containing Vero E6 cells. At each dilution, the antiserum and virus mixtures were added to 8 wells. The 96‐well plates were incubated in a 37 °C CO2 incubator for 48 h, and the neutralization titers were deduced by the 50% tissue culture infective dose (TCID50) calculated from the cytopathic effect induced in cell culture by the virus in the presence of different dilutions of antibodies as previously described [16, 18, 19]. Briefly, the ratios of infected wells to uninfected wells were determined by microscopy and the titers of the neutralizing antibodies were calculated using the Reed–Muench method [20]. All experiments were performed in duplicates.
3. Results and discussion
In this study, we compared the properties of sera obtained a rabbit that was immunized with bacterially expressed GST‐3a (134–274 aa) (i.e. C‐terminal, Rabbit #1, Ref. [4]), and two rabbits that were immunized with a synthetic peptide corresponding to 15–28 aa of 3a (i.e. N‐terminal, Rabbit #2 and Rabbit #3). This peptide corresponds to aa 15–28 of the SARS‐CoV 3a protein ectodomain which is predicted to face the extracellular matrix in infected cells [4]. Western blot analysis was performed to determine the specificity of the rabbit polyclonal antibodies. Total cell lysates obtained from Vero E6 cells transiently transfected with a DNA construct for expressing full‐length 3a or SARS‐CoV infected Vero E6 cells were used in Western blot analysis with sera collected from the immunized rabbits after different number of immunizations with the peptide. As shown in Fig. 1 A, the sera from 1st to 5th bleeds of one of the immunized rabbits (Rabbit #2) could react specifically with the full‐length 3a protein of ∼35 kDa. Similar results were obtained with the other immunized rabbit (Rabbit #3, Fig. 1B), but the relative reactivity to 3a is lower than for Rabbit #2. Hence, subsequent experiments were performed with the sera from Rabbit #2.
Figure 1.
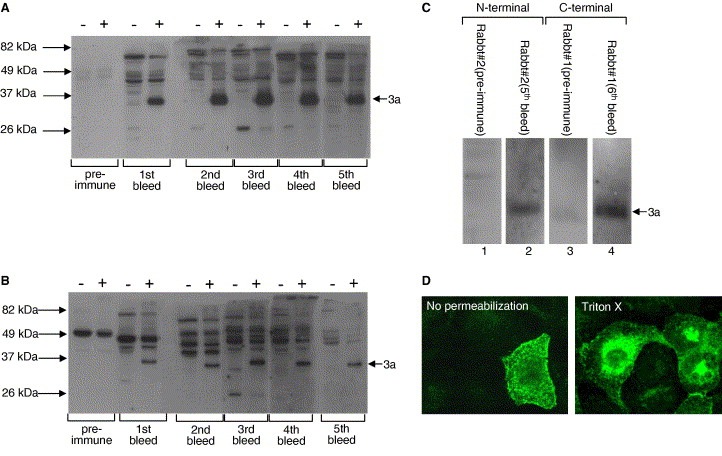
Detection of SARS‐CoV 3a protein expressed in Vero E6 cells with rabbit anti‐3a polyclonal antibodies. (A) Rabbit #2 and (B) Rabbit #3: Western blot analysis was performed using sera obtained from two rabbits that were immunized with a synthetic peptide which corresponds to amino acids 15–28 of the 3a protein (i.e. N‐terminal). All sera were diluted 1:2000 and the lysates were obtained from Vero E6 cells transiently transfected with a DNA construct for expressing full‐length 3a (+) or untransfected cells (−). (C) Vero E6 cells were infected with an MOI of 1 and then prepared for Western blot analysis. Pre‐immune serum from the rabbit (Rabbit #2) that was immunized with the 3a N‐terminal peptide showed no reactivity (lane 1), while the 5th bleed from the same rabbit after immunization detected the 3a protein in infected cells (lane 2). Similarly, pre‐immune serum from the rabbit (Rabbit #1) that was immunized with the 3a C‐terminal bacterially expressed protein showed no reactivity (lane 3), while the 6th bleed from the same rabbit after immunization detected the 3a protein in infected cells (lane 4). (D) Cellular localization of 3a in transfected Vero E6 cells as determined by indirect immunofluorescence. Serum from the 5th bleed of the rabbit (Rabbit #2) that was immunized with the 3a N‐terminal peptide was used at a dilution of 1:200 to detect for the 3a protein expressed on the cell surface (left panel, no permeabilization) and intracellularly (right panel, permeabilization with 0.2% Triton‐X 100).
Western blot analysis of SARS‐CoV infected cells also demonstrated that the anti‐3a N‐terminal antibody (Rabbit #2, 5th bleed) could react specifically with the 3a protein expressed in infected cells (Fig. 1C, lane 2). For comparison, the anti‐3a C‐terminal antibody (Rabbit #1, 6th bleed) also detected 3a in infected cells (Fig. 1C, lane 4). Consistent with previous studies [10, 11], the N‐terminal of 3a is sufficient to stimulate specificity antibody response in rabbits. The anti‐3a N‐terminal antibody (Rabbit #2, 5th bleed) was used in indirect immunofluorescence experiments and it could detect the 3a protein expressed on the cell surface (Fig. 1D, left panel) and intracellularly (Fig. 1D, right panel). It was previously showed that properly folded 3a protein is efficiently transported to the cell surface with its N‐terminal facing the extracellular matrix [4, 21]; hence, the results showed that the anti‐3a N‐terminal antibody could recognize the native form of 3a expressed on the cell surface.
Next, the rabbit polyclonal antibodies were tested for their abilities to inhibit SARS‐CoV propagation in Vero E6 culture. Microneutralization assays were performed by mixing a constant amount of virus with different dilutions of rabbit sera and then overlaying the mixture onto Vero E6 cells in 96‐well plates. Calculations were made from the cytopathic effect induced in cell culture by the virus as described previously [20]. As shown in Table 2 , the sera obtained from the rabbit immunized with the N‐terminal of 3a (Rabbit #2) showed a neutralization titer of 1:67 for the 1st bleed and 1:80 for the 2nd to 5th bleeds, while the pre‐immune serum did not have any neutralizing activity (titer <1:10). In contrast, the anti‐3a C‐terminal antibody did not show any neutralizing activity (Rabbit #1, 6th bleed, titer <1:10), neither did the pre‐immune serum from this rabbit. All experiments were performed three times and the average values are showed in Table 2. The neutralizing activities of the rabbit sera from Rabbit #2 were observed even though the components of the complement system have been inactivated by heating. In order to determine if this result is reproducible, the sera from another rabbit immunized with the same 3a N‐terminal peptide (Rabbit #3, Fig. 1B) were used for neutralizing assays. Indeed, the results showed that the sera from Rabbit #3 were also neutralizing, albeit with a lower titer of 1:40 (average value obtained for all the bleeds). For comparison, parallel experiments were also performed with a neutralizing antibody targeting 1055–1192 aa (SΔ10), which contains the heptad repeat 2 domain known to be important for mediating membrane fusion, of SARS‐CoV S protein [17]. In this experiment, the rabbit anti‐SΔ10 polyclonal antibody had a neutralizing titer of 1:160, which is consistent with our previous findings [17].
Table Table 2.
Neutralizing titers for the pre‐immune sera and the different bleeds obtained from rabbits that have been immunized with either the N‐ or C‐terminal of the SARS‐CoV 3a protein
| Bleed no. | N‐terminal (Rabbit #2) | C‐terminal (Rabbit #1) |
|---|---|---|
| Pre‐immune sera | <1:10 | <1:10 |
| 1 | 1:67 a | ND b |
| 2 | 1:80 a | ND b |
| 3 | 1:80 a | ND b |
| 4 | 1:80 a | ND b |
| 5 | 1:80 a | ND b |
| 6 | ND b | <1:10 |
Neutralizing titers were determined by the Reed–Muench method and computed as the average of three independent experiments.
ND means not determined.
To be certain that the inability of the anti‐3a C‐terminal antibody to neutralize the SARS‐CoV is not due to lower antibody concentrations, we determined the relative binding affinities of the anti‐3a N‐terminal and C‐terminal rabbit polyclonal antibodies to 3a protein expressed in 293T cells by ELISA. Diluted rabbit sera were added to wells coated with total cell lysates from untransfected cells or cells transiently transfected with a DNA construct for expressing the full‐length 3a protein. The OD difference (in arbitrary units) represented the specific binding of antibody to the 3a protein. As shown in Fig. 2 , the serum from Rabbit #1 (i.e. C‐terminal, 6th bleed) has relatively stronger binding affinity to the 3a protein than the sera from different bleeds of Rabbit #2 (i.e. N‐terminal). The pre‐immune sera from both rabbits showed little binding to 3a. Hence, it is clear that the anti‐3a C‐terminal antibody did not have any neutralizing activity, even though it contains a relatively higher level of 3a‐specific antibodies than the anti‐3a N‐terminal antibody.
Figure 2.
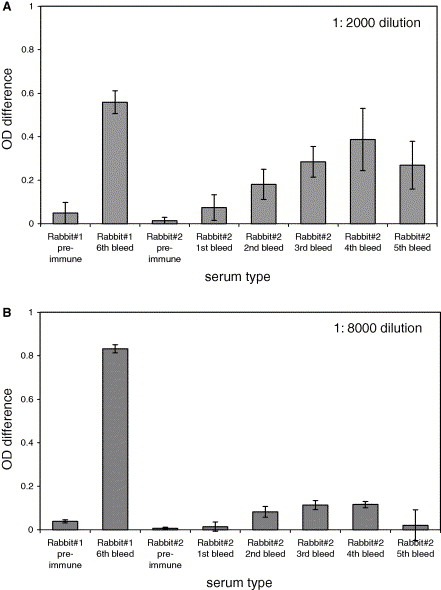
Binding affinity of rabbit polyclonal antibodies to 3a protein expressed in 293T cells as determined by ELISA. Rabbit sera were diluted 1:2000 (A) or 1:8000 (B) and added to wells coated with total cell lysates from untransfected cells or cells transiently transfected with a DNA construct for expressing the full‐length 3a protein. The OD difference (in arbitrary units) represented the specific binding of antibody to the 3a protein. Rabbit #1 was immunized with bacterially expressed GST‐3a (134–274 aa) (i.e. C‐terminal) and the pre‐immune serum and the serum from the 6th bleed were tested. Rabbit #2 was immunized with a synthetic peptide corresponding to amino acids 15–28 of 3a (i.e. N‐terminal). For Rabbit #2, the pre‐immune serum and the sera from the 1st to 5th bleeds were tested. All experiments were performed in duplicates and the average values with standard deviations are plotted.
Interestingly, B cells recognizing the N‐terminal ectodomain of the 3a protein were found in two separate cohorts of SARS patients [14, 15]. In another study, it was reported 48.8% of patients who recovered from SARS had antibodies against the N terminal of 3a while only 7.4% of the diseased patients has such antibodies [11]. It was further demonstrated that anti‐3a antibodies in the patient serum could bind cells expressing 3a and induce the elimination of these cells in the presence of the human complement system [11]. Several other studies also found that anti‐3a antibodies were presented only in a subset of SARS patients [4, 9, 10, 22]. In this study, we showed that rabbit polyclonal antibody targeting a 14 aa epitope (aa 15–28) in the N‐terminal ectodomain of 3a could neutralize the SARS‐CoV replication in Vero E6 cells in the absence of the complement system. On the other hand, serum obtained from a rabbit immunized with the C‐terminal cytoplasmic domain of 3a (aa 134–274) was not capable of neutralizing SARS‐CoV even though it contains a higher amount of 3a‐specific antibodies. Hence, it is clear that both the ectodomain and cytoplasmic domain of 3a are immunogenic but only the ectodomain stimulates neutralizing antibodies.
To our knowledge, this is the first report of a SARS‐CoV group‐specific protein that can induce neutralizing antibody. Our results showed that antibody targeting the N‐terminal of 3a can inhibit SARS‐CoV replication, suggesting that 3a can stimulate protective humoral responses during SARS infection. This finding is consistent with a recent report that a higher percentage of patients who recovered from SARS infection have antibodies against the N‐terminal of 3a, when compared to the patients who died from SARS infection [11]. Even though the 3a protein is not essential for SARS‐CoV replication in cell culture and the murine model [23], it is very likely that 3a contributes to viral replication or pathogenesis in the natural host(s). In future studies, it will be crucial to determine the role of 3a during SARS‐CoV infection in non‐human primate models, where the animals could develop a disease comparable to that in SARS patients [24]. The precise role of aa 15–28 in the N‐terminal ectodomain of the 3a protein during SARS‐CoV infection also remains to be elucidated.
Åkerström Sara,Tan Yee-Joo and Mirazimi Ali(2006), Amino acids 15–28 in the ectodomain of SARS coronavirus 3a protein induces neutralizing antibodies, FEBS Letters, 580, doi: 10.1016/j.febslet.2006.06.002
Contributor Information
Yee-Joo Tan, Email: mcbtanyj@imcb.a-star.edu.sg.
Ali Mirazimi, Email: Ali.Mirazimi@smi.ki.se.
References
- 1. Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L., The Genome sequence of the SARS-associated coronavirus. Science, 300, (2003), 1399– 1404. [DOI] [PubMed] [Google Scholar]
- 2. Snijder E.J., Bredenbeek P.J., Dobbe J.C., Thiel V., Ziebuhr J., Poon L.L., Guan Y., Rozanov M., Spaan W.J., Gorbalenya A.E., Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J. Mol. Biol., 331, (2003), 991– 1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tan Y.-J., Lim S.G., Hong W., Characterization of viral proteins encoded by the SARS-coronavirus genome. Antiviral Res., 65, (2005), 69– 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan Y.-J., Teng E., Shen S., Tan T.H.P., Goh P.-Y., Fielding B.C., Ooi E.-E., Tan H.-C., Lim S.G., Hong W., A novel SARS coronavirus protein, U274, is transported to the cell surface and undergoes endocytosis. J. Virol., 78, (2004), 6723– 6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu C.-J., Chen Y.-C., Hsiao C.-H., Kuo T.-C., Chang S.C., Lu C.-Y., Wei W.-C., Lee C.-H., Huang L.-M., Chang M.-F., Ho H.-N., Lee F.-J.S., Identification of a novel protein 3a from severe acute respiratory syndrome coronavirus. FEBS Lett., 565, (2004), 111– 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chan W.S., Wu C., Chow S.C., Cheung T., To K.F., Leung W.K., Chan P.K., Lee K.C., Ng H.K., Au D.M., Lo A.W., Coronaviral hypothetical and structural proteins were found in the intestinal surface enterocytes and pneumocytes of severe acute respiratory syndrome (SARS). Mod. Pathol., 18, (2005), 1432– 1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Law P.T., Wong C.H., Au T.C., Chuck C.P., Kong S.K., Chan P.K., To K.F., Lo A.W., Chan J.Y., Suen Y.K., Chan H.Y., Fung K.P., Waye M.M., Sung J.J., Lo Y.M., Tsui S.K., The 3a protein of severe acute respiratory syndrome-associated coronavirus induces apoptosis in Vero E6 cells. J. Gen. Virol., 86, (2005), 1921– 1930. [DOI] [PubMed] [Google Scholar]
- 8. Guo J.P., Petric M., Campbell W., McGeer P.L., SARS corona virus peptides recognized by antibodies in the sera of convalescent cases. Virology, 324, (2004), 251– 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tan Y.-J., Goh P.-Y., Fielding B.C., Shen S., Chou C.-F., Fu J.-L., Leong H.N., Leo Y.S., Ooi E.E., Ling A.E., Lim S.G., Hong W., Profile of antibody responses against SARS-coronavirus recombinant proteins and their potential use as diagnostic markers. Clin. Diag. Lab. Immunol., 11, (2004), 362– 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zeng R., Yang R.F., Shi M.D., Jiang M.R., Xie Y.H., Ruan H.Q., Jiang X.S., Shi L., Zhou H., Zhang L., Wu X.D., Lin Y., Ji Y.Y., Xiong L., Jin Y., Dai E.H., Wang X.Y., Si B.Y., Wang J., Wang H.X., Wang C.E., Gan Y.H., Li Y.C., Cao J.T., Zuo J.P., Shan S.F., Xie E., Chen S.H., Jiang Z.Q., Zhang X., Wang Y., Pei G., Sun B., Wu J.R., Characterization of the 3a protein of SARS-associated coronavirus in infected vero E6 cells and SARS patients. J. Mol. Biol., 341, (2004), 271– 279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhong X., Guo Z., Yang H., Peng L., Xie Y., Wong T.Y., Lai S.T., Guo Z., Amino terminal of the SARS coronavirus protein 3a elicits strong, potentially protective humoral responses in infected patients. J. Gen. Virol., 87, (2006), 369– 373. [DOI] [PubMed] [Google Scholar]
- 12. Ito N., Mossel E.C., Narayanan K., Popov V.L., Huang C., Inoue T., Peters C.J., Makino S., Severe acute respiratory syndrome coronavirus 3a protein is a viral structural protein. J. Virol., 79, (2005), 3182– 3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shen S., Lin P.-S., Chao Y.-C., Zhang A., Yang X., Lim S.G., Hong W., Tan Y.-J., The severe acute respiratory syndrome coronavirus 3a is a novel structural protein. Biochem. Biophys. Res. Commun., 330, (2005), 286– 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu I.J., Hsueh P.R., Lin C.T., Chiu C.Y., Kao C.L., Liao M.Y., Wu H.C., Disease-specific B cell epitopes for serum antibodies from patients with severe acute respiratory syndrome (SARS) and serologic detection of SARS antibodies by epitope-based peptide antigens. J. Infect. Dis., 190, (2004), 797– 809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhong X., Yang H., Guo Z.F., Sin W.Y., Chen W., Xu J., Fu L., Wu J., Mak C.K., Cheng C.S., Yang Y., Cao S., Wong T.Y., Lai S.T., Xie Y., Guo Z., B-cell responses in patients who have recovered from severe acute respiratory syndrome target a dominant site in the S2 domain of the surface spike glycoprotein. J.Virol., 79, (2005), 3401– 3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akerstrom S., Mousavi-Jazi M., Klingstrom J., Leijon M., Lundkvist A., Mirazimi A., Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J. Virol., 79, (2004), 1966– 1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Keng C.-T., Zhang A., Shen S., Lip K.M., Fielding B.C., Tan T.H.P., Chou C.-F., Loh C.B., Wang S., Fu J., Yang X., Lim S.G., Hong W., Tan Y.-J., Amino acids 1055 to 1192 in the S2 region of SARS coronavirus S protein induces neutralizing antibodies: implications for the development of vaccine and anti-viral agent. J. Virol., 79, (2005), 3289– 3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gillim-Ross L., Taylor J., Scholl D.R., Ridenour J., Masters P.S., Wentworth D.E., Discovery of novel human and animal cells infected by the severe acute respiratory syndrome coronavirus by replication-specific multiplex reverse transcription-PCR. J. Clin. Microbiol., 42, (2004), 3196– 3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li W., Greenough T.C., Moore M.J., Vasilieva N., Somasundaran M., Sullivan J.L., Farzan M., Choe H., Efficient replication of severe acute respiratory syndrome coronavirus in mouse cells is limited by murine angiotensin-converting enzyme 2. J. Virol., 78, (2004), 11429– 11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reed L., Muench H., A simple method of estimating fifty percent endpoints. Am. J. Hygiene, 27, (1938), 493– 497. [Google Scholar]
- 21. Huang C., Narayanan K., Ito N., Peters C.J., Makino S., Severe acute respiratory syndrome coronavirus 3a protein is released in membranous structures from 3a protein-expressing cells and infected cells. J. Virol., 80, (2006), 210– 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Guan M., Chan K.H., Peiris J.S., Kwan S.W., Lam S.Y., Pang C.M., Chu K.W., Chan K.M., Chen H.Y., Phuah E.B., Wong C.J., Evaluation and validation of an enzyme-linked immunosorbent assay and an immunochromatographic test for serological diagnosis of severe acute respiratory syndrome. Clin. Diag. Lab. Immunol., 11, (2004), 699– 703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yount B., Roberts R.S., Sims A.C., Deming D., Frieman M.B., Sparks J., Denison M.R., Davis N., Baric R.S., Severe acute respiratory syndrome coronavirus group-specific open reading frames encode nonessential functions for replication in cell cultures and mice. J. Virol., 79, (2005), 14909– 14922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Osterhaus A.D., Fouchier R.A., Kuiken T., The aetiology of SARS: Koch’s postulates fulfilled. Philos. Trans. R. Soc. Lond. B, 359, (2004), 1081– 1082. [DOI] [PMC free article] [PubMed] [Google Scholar]


