Abstract
Aims: Vinegar-baked Radix Bupleuri (VBRB) potentiates the activity of anticancer drugs in the liver by increasing their hepatic distribution. However, this phenomenon may be associated with drug transporters. We investigated the effect of saikosaponin b2 (SSb2; the main component of VBRB) on the activity and expression of different drug transporters in both normal cells and those that overexpress the transporter.
Main methods: The activities of transporters were analyzed by concentration of their cellular substrates. Concentrations of colchicine (substrate of Pgp and MRP1) and cisplatin (substrate of OCT2 and MRP2) were determined by high-performance liquid chromatography (HPLC). The concentration of rhodamine B was determined by flow cytometry. The expression of transporter gene and protein were determined by qRT-PCR and Western blotting analysis.
Key findings: SSb2 increased colchicine efflux in HEK293 cells by primarily increasing Mrp1 activity, independent of gene and protein expression. SSb2 enhanced Mrp2 function and increased cisplatin efflux in BRL3A cells by upregulating Mrp2 gene expression, with a marginal effect on Pgp in normal cells. SSb2 increased OCT2 activity in OCT2-HEK293 cells by increasing the expression of OCT2 protein and mRNA; however, SSb2 inhibited MRP2 activity in MRP2-HEK293 cells by decreasing MRP2 protein expression, and decreased Pgp and MRP1 activity in Pgp- and MRP1-HEK293 cells.
Significance: SSb2 might potentially be the key active component of VBRB that enhances the hepatotargeting of anticancer drugs through the inhibition of multidrug resistance-associated drug transporters (Pgp, MRP1, and MRP2) in an environment-dependent manner.
Keywords: Drug transporters, MRP, OCT2, Pgp, Saikosaponin b2
1. Introduction
Conventional cancer chemotherapeutics induce systemic side effects and have poor local selectivity. Tumor-targeting drugs with high efficacy and selectivity have found widespread application in the treatment of some tumors. However, the high cost of treatment and poor biocompatibility are some limitations of tumor-targeted therapies. The development of safe and effective anticancer drugs is, therefore, a key clinical imperative.
In traditional Chinese medicine, medicinal guide herbs are used to enhance the effects of chemotherapeutic agents. Compared to targeted drug delivery, this approach is simple, safe, and economical. However, a lack of understanding of the mechanisms of action underlying the effects of this approach limits its wider application and development.
VBRB, the vinegar-baked product of Radix Bupleuri (the dry radix of Bupleurum Chinese DC and Bupleurum scozonerifolium), is the medicinal hepato-targeting herb. In a study by Cheung et al., VBRB was shown to increase the efficacy of anticancer drugs in patients with unresectable hepatocellular carcinoma [1]. In our previous study, VBRB showed good hepato-targeting efficiency by increasing the distribution of rhein, oxymatrine, and resveratrol in the liver of rats and mice [[2], [3], [4]]. These findings suggest that VBRB potentiates hepatic drug concentration, although the specific VBRB component responsible for the hepato-targeting effect and its mechanism of action are not known.
There are different types of compounds, including saikosaponin a, b2, c, and d; polysaccharides; lignin; and volatile oils, in VBRB. Several recent studies have identified saikosaponins as the principal active component of VBRB, and have explored the activity of the native saponins (saikosaponin a, c, and d) [[5], [6], [7], [8], [9]]. However, other research suggests that the secondary saponins (saikosaponin b1 and b2), and not the native saponins (saikosaponin a, c, d), are the main components of VBRB in Radix Bupleuri [10,11]. VBRB is mainly used in the treatment of hepatic diseases. As indicated by previous studies, the effect of VBRB may be associated with the drug transporters in hepatic cells. In our previous study, we found that the hepatoprotective effect of VBRB was enhanced and the content of saikosaponin b2 increased when higher concentrations of vinegar were used to process it [12]. Overall, these studies indicate that saikosaponin b2 may play a key role in the enhanced hepato-targeting effect of VBRB.
Changes in tissue drug distribution are modulated by the complex interplay of factors in the cell signal transduction pathways, with drug transporters being one of the key players [13]. In this study, we explored the effect of saikosaponin b2 on the activity and expressions of P-glycoprotein (Pgp), multiresistance-related protein 1 and 2 (Mrp1 and Mrp2), and organic cation transporter 2 (Oct2), both in cells with normal expression and over-expression of transporters..
2. Materials and methods
2.1. Cell culture and chemicals
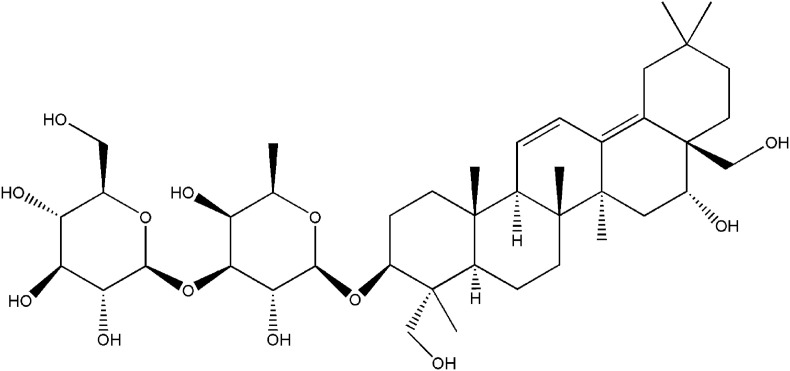
HEK293 and BRL3A cells were obtained from the American Type Culture Collection (ATCC; Manassas, VA, USA). Cells from both cell lines were maintained in Dulbecco's Modified Eagle's Medium (DMEM; Gibco, Grand Island, USA), which was supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin mixture (Gibco, Grand Island, USA). A glutathione (GSH)-stimulated HEK293 cell model was established through the addition of GSH (2 mM) as a stimulant. The stable overexpression of Pgp, OCT2, and MRP2 in HEK293 cells were established by lentiviral vectors according to previously described protocols [14]. Saikosaponin b2 (purity > 98%) was purchased from Winherb Medical Technology (Fig. 1 ; Shanghai, China). The OCT2, Pgp, Mrp1, and Mrp2 mouse monoclonal antibodies and anti-mouse immunoglobulin G (IgG) horseradish peroxidase (HRP)-conjugated antibodies were purchased from Abcam (Cambridge, UK). The GAPDH antibody was purchased from Cell Signaling Technology (MA, USA). The Pierce® BCA protein assay kit and TRIzol® reagent were purchased from Invitrogen (Carlsbad, US); the Revert Aid First Strand cDNA Synthesis and DyNAmo™ Color Flash SYBR® Green qPCR kits were obtained from ThermoFisher Scientific (Rockford, US). Colchicine, cisplatin, rhodamine B, verapamil, MK571, and 3-[4, 5-dimethylthiazol-2-yl]-2, 5-diphenyl-tetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, US). All other chemicals were commercially available and of reagent grade.
Fig. 1.
The structure of saikosaponin b2.
2.2. Determination of cell viability by MTT assay
The BRL3A and HEK293 cells were seeded into 96-well plates at a cell density of 2 × 103 and 4 × 103 cells per well, respectively, and incubated for 24 h. Thereafter, these cells were treated with different concentrations of saikosaponin b2 (128.0, 64.0, 25.6, 12.8, 6.4, 1.28, and 0.128 μM) for 48 h. Subsequently, 20 μL MTT (50 g/L) was added to the cells, and incubated for 4 h. When formazan was formed, the culture medium was removed and 150 μL DMSO was added to dissolve the formazan. The optical density was measured at 570 nm.
2.3. Substrate-uptake assays
For cisplatin and colchicine uptake assays, cells were seeded into six-well plates, and saikosaponin b2 and/or transporter inhibitors (50 μM MK571 or 50 μM verapamil) were added and incubated for 24 h. Subsequently, the cells were treated with 50 μg/mL cisplatin for 4 h or 50 μM colchicine for 1 h. Then, the cells were harvested and lysed by the freeze–thaw method (−80 °C to 37 °C, three times). The cell lysate was centrifuged, and the supernatant was collected and subjected to high-performance liquid chromatography (HPLC) analysis. Thereafter, separation was conducted using a Diamonsil C18 column (250 × 4.6 mm, 5 μm). The mobile phase was water:methanol:acetonitrile (23:46:31; flow rate 1.5 mL·min−1 for cisplatin) and water:methanol (45,55; flow rate 1 mL·min−1 for colchicine) and detection wavelengths were 254 and 353 nm, respectively. The total protein concentration was determined by using the BCA Protein Assay Kit according to the manufacturer's instructions. The substrate uptake was expressed as cisplatin/mg protein or colchicine/mg protein.
For rhodamine B uptake assays, cells were treated with saikosaponin b2 for 24 h; thereafter, the culture medium was discarded and 200 nM rhodamine B was added for 30 min and incubated in the dark. Then, the cells were harvested for flow cytometry (10,000 cells/time), and we detected the relative fluorescent intensity of intracellular rhodamine B.
2.4. Western blotting analysis
After treatment with saikosaponin b2 for 24 h, the cells were harvested and lysed in RIPA buffer containing protease inhibitors. Protein concentrations were determined by the BCA Protein Assay kit. Equal amounts of protein were separated by SDS-PAGE. After membrane transfer and blocking, the membrane was treated with primary antibodies [OCT2 (1:500), Mrp1 (1:1,000), Mrp2 (1:500), and Pgp (1:1,000)] overnight at 4 °C and incubated with HRP-linked anti-rabbit IgG for 1 h at room temperature. We used ECL to develop immunoreactive bands that were exposed on Kodak Medical X-Ray film. The intensity was analyzed by Gel Doc™ XR Quantity One® 1-D analysis software (Bio-Rad Laboratories, Richmond, CA, US) and normalized by GAPDH expression in each sample.
2.5. Quantitative real-time PCR
After 24-h treatment with saikosaponin b2, the cells were harvested and total RNA was extracted with the TRIzol® reagent. We used 1 μg total RNA as a template for cDNA synthesis. We synthesized first strand cDNA by using the Revert Aid First Strand cDNA synthesis kit according to the manufacturer's protocol. This cDNA was used for q RT-PCR, together with the primers listed in Table 1 . The amplification reactions were undertaken using the ABI 7500 real-time PCR machine (Applied Biosystems, USA) under the following conditions: denaturation for 10 min at 95 °C, followed by 40 cycles at 95 °C for 15 s and 60 °C for 1 min. The 2−ΔΔCt method was used for data analysis.
Table 1.
PCR primers of transporters.
| Transporters | Forward primer | Reverse primer |
|---|---|---|
| Rat Oct2 | 5′-CCGAGAATATGCAGAGGCCAA-3′ | 5′-AAGTCAGCTCCAGCAGCAAT-3′ |
| Rat Mrp2 | 5′-CCCGCCAGCTGAGACGGTTG-3′ | 5′-GCTGGTGCTCAAAGGCACGGA-3′ |
| Rat GADPH | 5′-ATGATTCTACCCACGGCAAG-3′ | 5′-CTGGAAGATGGTGATGGGTT-3′ |
| Human Pgp | 5′-ACTTGTCACAATGCAGACAGCAGG-3′ | 5′-TGTGATCCACGGACACTCCTACG-3′ |
| Human MRP1 | 5′-GGGGGAGAAAAGGTCGGCATCG-3′ | 5′-GTGCAGGCCGATCTTGGCGA-3′ |
| Human MRP2 | 5′-ACAGTCCGAGATGTGAACCTG-3′ | 5′-TGAATCCAGGACTGCTGTGG-3′ |
| Human OCT2 | 5′-TGCAGCTGGAGTTCTCATGG-3′ | 5′-CTCCGATATCTCCGCCCAAC-3′ |
| Human GAPDH | 5′-TGTGATCCACGGACACTCCTACG-3′ | 5′-GATCATCAGCAATGCCTCCTGCACC-3′ |
2.6. Statistical analysis
All experiments were conducted in triplicate, and the results are expressed as mean ± standard deviation (SD). Data processing and analysis were undertaken with the SPSS 17.0 software. Independent samples t-test was used for pair-wise comparisons, whereas Fisher's Least Significant Difference (LSD) test was used for multiple comparisons. P < 0.05 or P < 0.01 was considered indicative of statistical significance.
3. Results
3.1. Cell viability assays after treatment with saikosaponin b2
With the MTT assay, we investigated the effect of saikosaponin b2 (0.128–128.0 μM) on the viability of BRL3A and HEK293 cells. More than 80% of BRL3A and HEK293 cells survived at concentrations of up to 128.0 and 64.0 μM, respectively. Therefore, we used these two concentrations in subsequent experiments.
3.2. Effect of saikosaponin b2 on the activity and expressions of Pgp and MRP1 in HEK293 cells
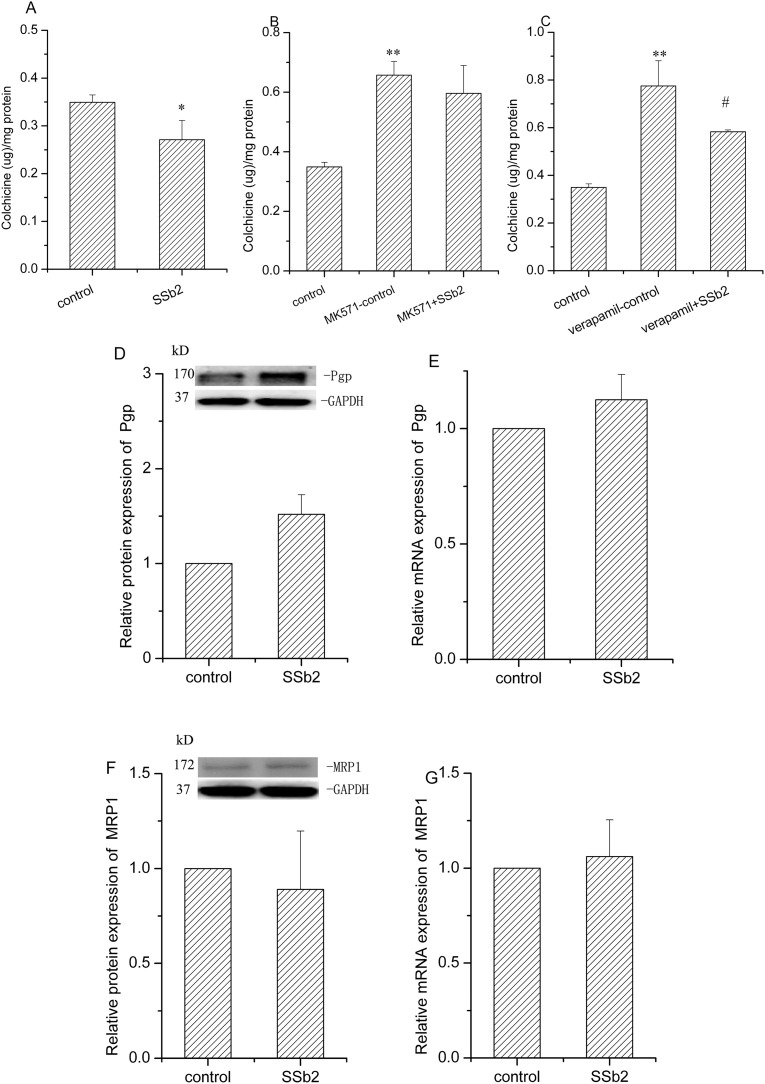
Colchicine was used to study the activity of Pgp and MRP1 because it is a substrate for both of these transporters. To exclude their interference with each other, we added the MRP1 inhibitor MK571 and the Pgp inhibitor verapamil. After 48-h treatment of HEK293 cells with saikosaponin b2 (64.0 μM), the colchicine uptake was decreased by 25.2% (P < 0.05) as compared to that in the control group (Fig. 2A). However, 48 h after the addition of the inhibitors MK571 or verapamil, both inhibitors significantly increased colchicine uptake (P < 0.01). These results indicated that both Pgp and MRP1 contributed to colchicine efflux. Compared to the verapamil-control group, treatment with saikosaponin b2 decreased colchicine uptake by 28.5% (P < 0.05; Fig. 2C). However, saikosaponin b2 showed no significant effect when compared to the MK571-control group (Fig. 2B). Furthermore, we studied the effect of saikosaponin b2 on the Pgp and MRP1 protein and mRNA levels. Saikosaponin b2 had a marginal effect on both Pgp (Fig. 2D, E), and Mrp1 (Fig. 2F, G) protein and mRNA, respectively. These findings indicated that saikosaponin b2 decreased the accumulation of colchicine in HEK293 cells, mainly by increasing Mrp1 activity.
Fig. 2.
Effect of saikosaponin b2 on Mrp1 and Pgp in HEK293 cells. Colchicine uptake was determined by high performance liquid chromatography after 48-h treatment of cells with saikosaponin b2 (A), MK571 or MK571 plus saikosaponin b2 (B), verapamil and verapamil plus saikosaponin b2 (C), followed by the addition of 50 μM colchicine for an additional 1 h. Protein expressions of Pgp (D) and Mrp1 (F) in HEK293 cells treated with saikosaponin b2 for 48 h were determined by Western blotting analysis. The mRNA expressions of Pgp (E) and Mrp1 (G) were measured by qRT-PCR analysis after 48-h treatment of cells with saikosaponin b2. GAPDH was used as the loading control. Relative protein and mRNA expressions were calculated by sample/control. Mean + SD values from three independent experiments are presented. *P < 0.05, **P < 0.01 versus control, #P < 0.05 versus verapamil-control.
3.3. Effect of saikosaponin b2 on the activity and expressions of Mrp2 and OCT2 in BRL3A cells
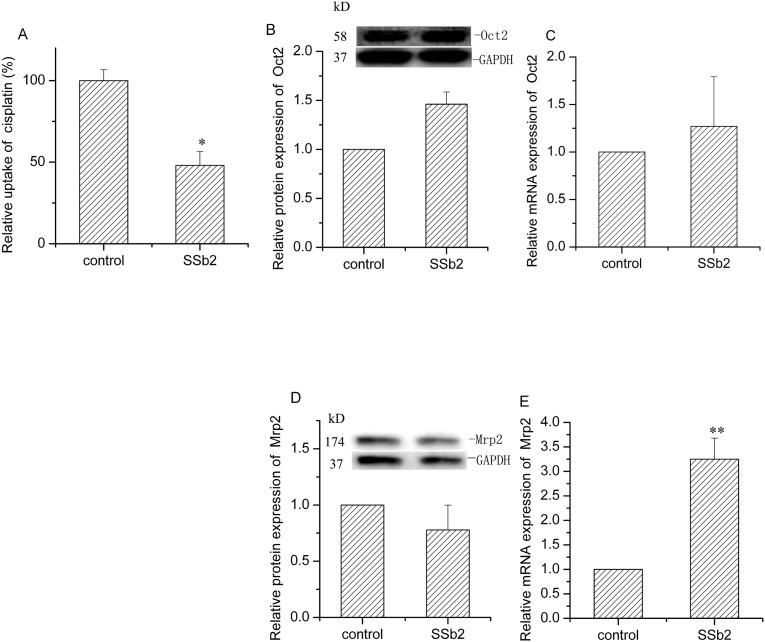
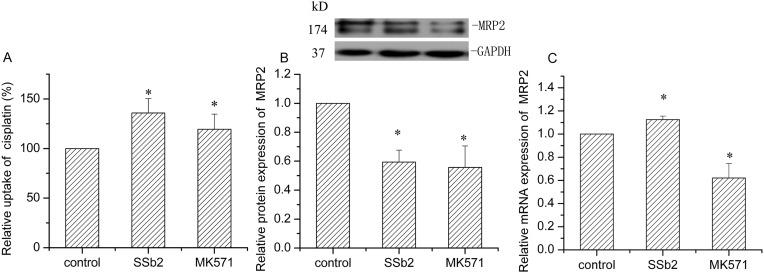
Cisplatin is a platinum-based drug that is a substrate of both OCT2 and Mrp2. Therefore, we investigated the effect of saikosaponin b2 on OCT2 and Mrp2 activities by detecting the accumulation of cisplatin in BRL3A cells. After 24-h treatment with 128.0 μM saikosaponin b2, cisplatin accumulation decreased by 53.0% (P < 0.05, Fig. 3A). Subsequently, we determined the protein and mRNA expressions of OCT2 and Mrp2 to study the underlying mechanisms. Saikosaponin b2 had no remarkable effect on OCT2 protein and OCT2 mRNA expression (Fig. 3C). Furthermore, saikosaponin b2 did not have a notable effect on Mrp2 protein; however, it increased the Mrp2 mRNA expression by 223.5% (Fig. 3D, E). These results indicated that saikosaponin b2 hampered cisplatin uptake in normal cells mainly by increasing the Mrp2 gene expression.
Fig. 3.
Effect of saikosaponin b2 on Mrp2 and Oct2 in BRL cells. Cisplatin uptake was determined by high performance liquid chromatography after 24-h treatment of cells with saikosaponin b2, t, followed by the addition of 50 μg/mL cisplatin for another 4 h (A). Protein expressions of Oct2 (B) and Mrp2 in cells treated with saikosaponin b2 were analyzed by Western blotting analysis. The mRNA expressions of Oct2 (C) and Mrp2 (E) were measured by qRT-PCR analysis after 24-h treatment of cells with saikosaponin b2. The relative protein and mRNA expressions were calculated by sample/control. Mean + SD values from three independent experiments are presented. *P < 0.05, **P < 0.01 versus control.
3.4. Saikosaponin b2 increased OCT2 activity in OCT2-HEK293 cells but decreased MRP2 activity in MRP2-HEK293 cells
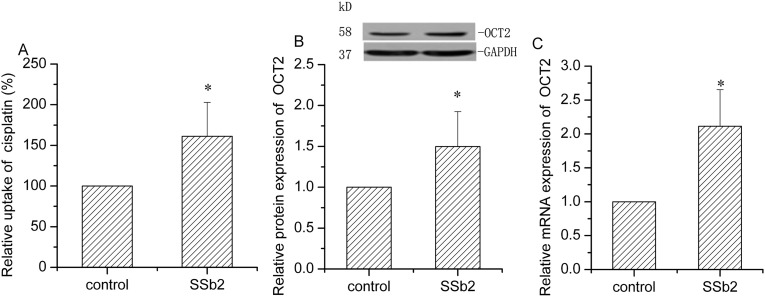
After 24-h treatment of OCT2-HEK293 cells, saikosaponin b2 increased cisplatin uptake by 61.3% (P < 0.05, Fig. 4 ). Both protein and mRNA expressions of OCT2 were increased by 49.7% (P < 0.05) and 111.3% (P < 0.05), respectively. These results indicate that saikosaponin b2 increases OCT2 activity in a protein- and gene-dependent manner.
Fig. 4.
Effect of Saikosaponin b2 on OCT2 activity and expression in OCT2-HEK293 cells. (A) Cisplatin uptake was analyzed by high performance liquid chromatography after 24-h treatment of cells with saikosaponin b2, followed by incubation with 50 μg/mL cisplatin for another 4 h. (B) OCT2 protein expression was determined by Western blotting analysis after 24-h treatment of cells saikosaponin b2. GAPDH was used as the loading control. (C) OCT2 mRNA expression was measured by qRT-PCR analysis after 24 h-treatment of cells with saikosaponin b2. The relative protein and mRNA expressions were calculated by sample/control. Mean + SD values from three independent experiments are presented. *P < 0.05, **P < 0.01 versus control.
In MRP2-HEK293 cells, saikosaponin b2 increased the uptake of cisplatin by 35.8% (P < 0.05), whereas MK571 (the inhibitor of MRP2) increased the uptake of cisplatin by 19.5% (P < 0.05, Fig. 5A). The MRP2 protein expression was decreased by 40.6% and 51.3% after treatment with saikosaponin b2 and MK571, respectively (P < 0.05, Fig. 5B, C).
Fig. 5.
Effect of Saikosaponin b2 on MRP2 activity and expression in MRP2-HEK293 cells. (A) Cisplatin uptake was analyzed by high performance liquid chromatography after 24-h treatment of cells with saikosaponin b2 or MK571, followed by incubation with 50 μg/mL cisplatin for another 4 h. (B) MRP2 protein expression was determined by Western blotting analysis after 24-h treatment of cells with saikosaponin b2 or MK571. GAPDH was used as the loading control. (C) MRP2 mRNA expression was measured by qRT-PCR analysis after 24-h treatment of cells with saikosaponin b2 or MK571. The relative protein and mRNA expressions were calculated by sample/control. Mean + SD values from three independent experiments are presented. *P < 0.05 versus control.
3.5. Saikosaponin b2 decreased Pgp activity in Pgp-HEK293 cells and decreased MRP1 activity in GSH-stimulated HEK293 cells
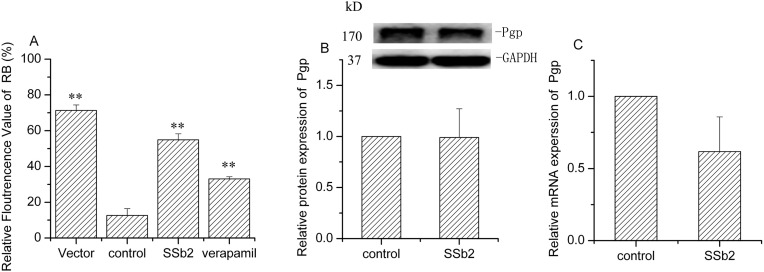
Rhodamine B is a substrate of Pgp. As shown in Fig. 6 , the relative fluorescence of rhodamine B decreased significantly (P < 0.01) in Pgp-HEK293 cells, compared to that with the vectors. Following the addition of saikosaponin b2 or verapamil (a Pgp inhibitor) to Pgp-HEK293 cells, the relative fluorescence of rhodamine B was significantly increased by 337.4% and 163.1% (P < 0.01), respectively. These results indicate that saikosaponin b2 inhibits Pgp activity when Pgp is overexpressed, and the effect was stronger than that of verapamil. However, both Pgp gene and protein expressions showed no remarkable changes after treatment of Pgp-HEK293 cells with saikosaponin b2. This result suggests that saikosaponin b2 may affect Pgp activity through post-translational regulation.
Fig. 6.
Effect of Saikosaponin b2 on Pgp activity and expression in Pgp-HEK293 cells. (A) Rhodamine B uptake was determined by flow cytometry after 24-h treatment of cells with saikosaponin b2 or verapamil, followed by treatment with rhodamine B for another 30 min. (B) Pgp protein expression was determined by Western blotting analysis after 24-h treatment of cells with saikosaponin b2. GAPDH was used as the loading control. (C) Pgp mRNA expression was measured by qRT-PCR analysis after 24-h treatment of cells with saikosaponin b2. Relative protein and mRNA expressions were calculated by sample/control. Mean + SD values from three independent experiments are presented. *P < 0.05, **P < 0.01 versus control.
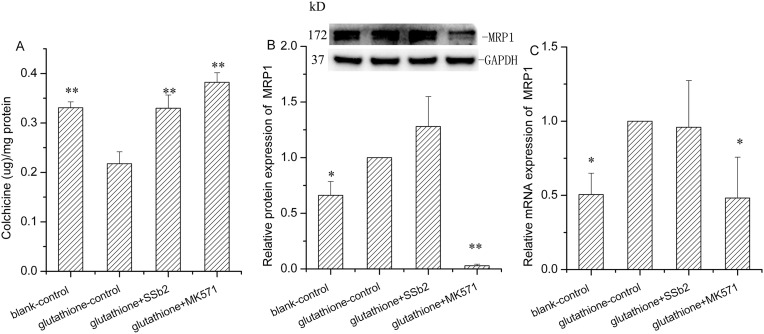
To examine the effect of saikosaponin b2 on the overexpression of MRP1, we constructed a GSH-stimulated HEK293 cell model because MRP1 is a GSH-dependent protein. When stimulated by GSH, the uptake of colchicine in HEK293 cells significantly declined, and the MRP1 gene and protein expressions notably increased (P < 0.01, P < 0.05). After the addition of MK571 (the MRP1 inhibitor), colchicine uptake increased by 75.6% (P < 0.01), whereas MRP1 gene and protein expressions decreased by 48.5% (P < 0.05) and 97.1% (P < 0.01, Fig. 7 ), respectively. These data indicate the successful establishment of the cell model. Treatment with saikosaponin b2 increased colchicine uptake by 51.4% (P < 0.05), but did not affect MRP1 protein and mRNA expressions.
Fig. 7.
Effect of Saikosaponin b2 on MRP1 activity and expression in glutathione (GSH)-stimulated HEK293 cells. (A) Colchicine uptake was determined by high performance liquid chromatography after 24-h treatment of cells with GSH, GSH plus saikosaponin b2, and GSH plus MK571, followed by incubation with colchicine for an another 1 h. (B) MRP1 protein expression was determined by Western blotting analysis after 24-h treatment of cells with GSH, GSH plus saikosaponin b2 and GSH plus MK571. GAPDH was used as the loading control. (C) MRP1 mRNA expression was measured by qRT-PCR analysis after 24-h treatment of cells with GSH, GSH plus saikosaponin b2 and GSH plus MK571. Relative protein and mRNA expressions were calculated by samples/glutathione-control. Mean + SD values from three independent experiments are presented. *P < 0.05, **P < 0.01 versus glutathione-control.
4. Discussion
For many years, medicinal targeting herbs have been used in combination with antitumor drugs in the treatment of tumors in Chinese medicine clinics. The rationale for the use of medicinal targeting herbs during tumor treatment is that they increase the distribution of antitumor compounds in the target tissue. VBRB is commonly used as a medicinal hepato-targeting herb. However, the active ingredient of VBRB which targets hepatic cells and its underlying mechanism of action are not well characterized.
Drug transporters are determinants of tissue distribution and are amenable to modulation by foreign substances. The active ingredients of medicinal targeting drugs may serve as inducers or inhibitors of transporters, which may then modify the tissue distribution of other drugs. Although saikosaponin a and d are the index components of Radix Bupleuri, both of these native saponins can be transformed to secondary saponins (saikosaponin b1 and b2) through processing as decoction and with vinegar. Because saikosaponin a is relatively stable, saikosaponin b2 is considered the index component of VBRB. However, there are limited reports with regard to its bioactivity. Studies have shown that VBRB has a good inhibitory effect on the human corona virus 229 E [15]. In addition, VBRB was shown to inhibit the entry of the hepatitis C virus [16]. Furthermore, saikosaponin b2 was shown to inhibit etoposide-induced NF-κB activation in B16F10 melanoma cells, which exposed a new approach to overcome cancer chemoresistance [17]. Moreover, it was shown to cause IgE-induced mast cell degranulation, which was related to the inflammatory response [18]. To the best of our knowledge, this is the first study to document the effect of saikosaponin b2 on drug transporters. Indeed, saikosaponin b2 may be the active component which is responsible for the enhanced hepato-targeting effects of VBRB.
Transporters are transmembrane proteins that facilitate the movement of a wide variety of substrates across membrane bilayers. Not only are these transporters responsible for the delivery of nutrients and endogenous substances, but they may also help protect against endogenous and exogenous toxicity. The functions of these transporters are influenced by the body state as well as by the types and concentrations of exogenous substances. Because anticancer therapy typically requires a long duration of administration and given that safety is a key concern during anticancer therapy, we studied the effect of saikosaponin b2 on drug transporters in both normal cells and those that overexpress transporters.
The Pgp transporter is ubiquitously expressed and abundantly expressed in the liver, especially in hepatocellular carcinoma cells. Saikosaponin b2 had marginal effect on Pgp activity in the normal state; however, it significantly inhibited Pgp activity in Pgp-HEK293 cells. This finding suggested that the coadministration of antitumor drugs with VBRB may lead to hepatic accumulation of the drug and-, thereby, exhibit a synergistic effect toward achieving a clinical effect with less generalized systemic toxicity [1]. Furthermore, in normal HEK293 or in Pgp-overexpressing HEK293 cells, the protein and gene expressions were inconsistent with their activities. Pgp is an ATP-binding cassette transporter, whose function is not only dependent on the protein and mRNA levels, but is also related to the energy supply and other variables such as hepatic nuclear factors [19]. Other studies have shown that certain drugs modulate the expression of Pgp through certain cell signaling pathways. Kim et al. [20] found that metformin activated AMPK and suppressed MDR1 expression in MCF-7/adr cells by inhibiting the activation of NF-κB and CREB. Seo et al. [21] showed that downregulation of Pgp through inhibition of the DNA-PKcs/Akt/GSK-3β pathway may ameliorate multidrug resistance. Further studies are required to determine whether saikosaponin b2 modulates the function of Pgp via these signaling pathways.
Furthermore, we studied the other two efflux transporters (MRP1/Mrp1 and MRP2/Mrp2) that are related to tumor multidrug resistance. The effect of saikosaponin b2 on both transporters was similar. Saikosaponin b2 enhanced MRP1/Mrp1 and MRP2/Mrp2 function in normal cells, but significantly inhibited MRP1 and MRP2 activity in MRP1-HEK293 cells and MRP2-HEK293 cells, respectively. Both MRP1/Mrp1 and MRP2/Mrp2 normally participate in the detoxification process [22]. Thus, enhancing their function in the normal state helps to avoid the harmful effects of exogenous compounds in the body. However, high expression levels of MRP1 and MRP2 in lung, breast, ovarian, and hepatocellular carcinoma cells were associated with unfavorable outcomes and poor prognosis [[23], [24], [25], [26], [27], [28]]. Our results indicate that saikosaponin b2 may improve the therapeutic efficacy of antitumor drugs against multidrug-resistant cancers that cause overexpression of MRP1/Mrp1 and MRP2/Mrp2. Unlike the former three transporters, OCT2/Oct2 is an influx transporter that mainly transports cationic compounds. The frequently used antitumor drugs cisplatin and oxaliplatin are substrates of OCT2. Moreover, compensatory renal overexpression of OCT2/Oct2 has been demonstrated in hepatic diseases [29]; therefore, we studied the effect of saikosaponin b2 on OCT2/Oct2 under normal and overexpressed conditions. Saikosaponin b2 enhanced OCT2 activity in the overexpressed condition by upregulating both mRNA and protein expressions. Our study indicates saikosaponin b2 may aggravate the renal toxicity of platinum-containing anticancer drugs when used for the treatment of hepatocellular carcinoma.
5. Conclusions
Saikosaponin b2 might potentially be the key active ingredient of VBRB that enhances the effect of hepatotargeted anticancer drugs through the inhibition of multidrug resistance-associated drug transporters (Pgp, MRP1/Mrp1, and MRP2/Mrp2). Thus, saikosaponin b2 enhanced the efficiency of chemotherapy drugs and prevented the development of multidrug resistance in hepatocellular carcinoma. However, the use of platinum-containing anticancer drugs should be avoided in combination with saikosaponin b2 treatment.
Declaration of Competing Interest
The authors declare they have no conflict of interests.
Acknowledgements
This work was generously supported by the National Natural Science Foundation of China (grant no. 81073063 and 81573612), the Natural Science Foundation of Guangdong Province of China (grant no. 2015A030313357), and the Science and Technology Planning Project of Guangdong Province of China (grant no. 2017A020211013).
References
- 1.Cheung F., Wang X., Wang N., Yuen M.F., Ziea T.C., Tong Y., Wong V.T., Feng Y. Chinese medicines as an adjuvant therapy for unresectable hepatocellular carcinoma during transarterial chemoembolization: a meta-analysis of randomized controlled trials. Evid. Based Complement. Alternat. Med. 2013;2013 doi: 10.1155/2013/487919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao R.Z., Chen Y.J., Cai J.X. Bioinformatics and Biomedicine Workshops (BIBMW), 2011 IEEE International Conference on. 2011. Liver targeting effect of vinegar-baked Radix Bupleuri on oxymatrine in mice; pp. 740–745. [Google Scholar]
- 3.Zhao R.Z., Yuan D., Liu S.J., Chen Y.J., Liu L.J., Zhao Y. Liver targeting effect of vinegar-baked Radix Bupleuri on rhein in rats. J. Ethnopharmacol. 2010;132:421–428. doi: 10.1016/j.jep.2010.08.021. [DOI] [PubMed] [Google Scholar]
- 4.Zhao R.Z., Liu S.J., Mao S.R., Wang Y.J. Study on liver targeting effect of vinegar-baked Radix Bupleuri on resveratrol in mice. J. Ethnopharmacol. 2009;126:415–420. doi: 10.1016/j.jep.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Ye R.P., Chen Z.D. Saikosaponin A, an active glycoside from Radix bupleuri, reverses P-glycoprotein-mediated multidrug resistance in MCF-7/ADR cells and HepG2/ADM cells. Xenobiotica. 2017;47:176–184. doi: 10.3109/00498254.2016.1171932. [DOI] [PubMed] [Google Scholar]
- 6.Lorrai I., Maccioni P., Carai M.A., Capra A., Castelli M.P., Riva A., Morazzoni P., Gessa G.L., Colombo G. Suppressing effect of saikosaponin A, an active ingredient of Bupleurum falcatum, on chocolate self-administration and reinstatement of chocolate seeking in rats. Neurosci. Lett. 2017;638:211–217. doi: 10.1016/j.neulet.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 7.Li Y., Cai T., Zhang W., Zhu W., Lv S. Effects of Saikosaponin D on apoptosis in human U87 glioblastoma cells. Mol. Med. Rep. 2017;16:1459–1464. doi: 10.3892/mmr.2017.6765. [DOI] [PubMed] [Google Scholar]
- 8.Li C., Guan X., Xue H., Wang P., Wang M., Gai X. Reversal of P-glycoprotein-mediated multidrug resistance is induced by saikosaponin D in breast cancer MCF-7/adriamycin cells. Pathol. Res. Pract. 2017;213:848–853. doi: 10.1016/j.prp.2017.01.022. [DOI] [PubMed] [Google Scholar]
- 9.Lee T.H., Chang J., Kim B.M. Saikosaponin C inhibits lipopolysaccharide-induced apoptosis by suppressing caspase-3 activation and subsequent degradation of focal adhesion kinase in human umbilical vein endothelial cells. Biochem. Biophys. Res. Commun. 2014;445:615–621. doi: 10.1016/j.bbrc.2014.02.046. [DOI] [PubMed] [Google Scholar]
- 10.Li Z.Y., Sun H.M., Xing J., Qin X.M., Du G.H. Chemical and biological comparison of raw and vinegar-baked Radix Bupleuri. J. Ethnopharmacol. 2015;165:20–28. doi: 10.1016/j.jep.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 11.Jiang H., Li J., Shi R.B., Yin W.P. Influence of processing on four saikosaponins in Radix Bupleuri. Chin. Pharm. J. 2009;44:1618–1621. [Google Scholar]
- 12.Zhao Y., Wang Y.-J., Zhao R.-Z., Xiang F.-J. Vinegar amount in the process affected the components of vinegar-baked Radix Bupleuri and its hepatoprotective effect. BMC Complement. Altern. Med. 2016;16:346. doi: 10.1186/s12906-016-1333-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shitara Y., Horie T., Sugiyama Y. Transporters as a determinant of drug clearance and tissue distribution. Eur. J. Pharm. Sci. 2006;27:425–446. doi: 10.1016/j.ejps.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 14.Zhao Y., Feng L.M., Liu L.J., Zhang X., Zhao R.Z. Clerosterol from vinegar-baked Radix Bupleuri modifies drug transport. Oncotarget. 2017;8:21351–21361. doi: 10.18632/oncotarget.15212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng P.W., Ng L.T., Chiang L.C., Lin C.C. Antiviral effects of saikosaponins on human coronacirus 299E in vitro. Clin. Exp. Pharmacol. Physiol. 2006;33:612–616. doi: 10.1111/j.1440-1681.2006.04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin L.T., Chung C.Y., Hsu W.C., Chang S.P., Hung T.C., Shields J., Russell R.S., Lin C.C., Li C.F., Yen M.H., Tyrrell D.L., Lin C.C., Richardson C.D. Saikosaponin b2 is a naturally occurring terpenoid that efficiently inhibits hepatitis C virus entry. J. Hepatol. 2015;62:541–548. doi: 10.1016/j.jhep.2014.10.040. [DOI] [PubMed] [Google Scholar]
- 17.Ma H., Yokoyama S., Saiki I., Hayakawa Y. Chemosensitizing effect of saikosaponin B on B16F10 melanoma cells. Nutr. Cancer. 2017;69:505–511. doi: 10.1080/01635581.2017.1285407. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y.X., Wang X., Li X., Peng S., Wang S.F., Huang C.Z., Huang C.Z., Zhang Q., Li D., Jiang J., Ouyang Q. Identification of a specific agonist of human TAS2R14 from Radix Bupleuri through virtual screening, functional evaluation and binding studies. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-11720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Priyamvada S., Anwar M., Kumar A., Anbazhagan A.N., Gill R.K., Alrefai W.A., Dudeja P.K., Saksena S. Hepatocyte nuclear factor 1 beta upregulates P-glycoprotein expression in human intestinal epithelial cells. Gastroenterology. 2017;152:S66–S67. [Google Scholar]
- 20.Kim H.G., Hien T.T., Han E.H., Hwang Y.P., Choi J.H., Kang K.W., Kwon K.I., Kim B.H., Kim S.K., Song G.Y., Jeong T.C., Jeong H.G. Metformin inhibits P-glycoprotein expression via the NF-kappaB pathway and CRE transcriptional activity through AMPK activation. Br. J. Pharmacol. 2011;162:1096–1108. doi: 10.1111/j.1476-5381.2010.01101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seo S.B., Hur J.G., Kim M.J., Lee J.W., Kim H.B., Bae J.H., Kim D.W., Kang C.D., Kim S.H. TRAIL sensitize MDR cells to MDR-related drugs by down-regulation of P-glycoprotein through inhibition of DNA-PKcs/Akt/GSK-3beta pathway and activation of caspases. Mol. Cancer. 2010;9:199. doi: 10.1186/1476-4598-9-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hipfner D.R., Deeley R.G., Cole S.P. Structural, mechanistic and clinical aspects of MRP1. Biochim. Biophys. Acta. 1999;1461:359–376. doi: 10.1016/s0005-2736(99)00168-6. [DOI] [PubMed] [Google Scholar]
- 23.Li J., Li Z.N., Du Y.J., Li X.Q., Bao Q.L., Chen P. Expression of MRP1, BCRP, LRP, and ERCC1 in advanced non-small-cell lung cancer: correlation with response to chemotherapy and survival. Clin. Lung Cancer. 2009;10:414–421. doi: 10.3816/CLC.2009.n.078. [DOI] [PubMed] [Google Scholar]
- 24.Faggad A., Darb-Esfahani S., Wirtz R., Sinn B., Sehouli J., Konsgen D., Lage H., Noske A., Weichert W., Buckendahl A.C., Budczies J., Muller B.M., Elwali N.E., Dietel M., Denkert C. Expression of multidrug resistance-associated protein 1 in invasive ovarian carcinoma: implication for prognosis. Histopathology. 2009;54:657–666. doi: 10.1111/j.1365-2559.2009.03297.x. [DOI] [PubMed] [Google Scholar]
- 25.Atalay C., Demirkazik A., Gunduz U. Role of ABCB1 and ABCC1 gene induction on survival in locally advanced breast cancer. J. Chemother. 2008;20:734–739. doi: 10.1179/joc.2008.20.6.734. [DOI] [PubMed] [Google Scholar]
- 26.Haber M., Smith J., Bordow S.B., Flemming C., Cohn S.L., London W.B., Marshall G.M., Norris M.D. Association of high-level MRP1 expression with poor clinical outcome in a large prospective study of primary neuroblastoma. J. Clin. Oncol. 2006;24:1546–1553. doi: 10.1200/JCO.2005.01.6196. [DOI] [PubMed] [Google Scholar]
- 27.Consoli U., Santonocito A., Stagno F., Fiumara P., Privitera A., Parisi G., Giustolisi G.M., Pavone B., Palumbo G.A., Di Raimondo F., Milone G., Guglielmo P., Giustolisi R. Multidrug resistance mechanisms in chronic lymphocytic leukaemia. Br. J. Haematol. 2002;116:774–780. doi: 10.1046/j.0007-1048.2002.03344.x. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan G.F., Amenta P.S., Villanueva J.D., Alvarez C.J., Yang J.M., Hait W.N. The expression of drug resistance gene products during the progression of human prostate cancer. Clin. Cancer Res. 1998;4:1393–1403. [PubMed] [Google Scholar]
- 29.Kurata T., Muraki Y., Mizutani H., Iwamoto T., Okuda M. Elevated systemic elimination of cimetidine in rats with acute biliary obstruction: the role of renal organic cation transporter OCT2. Drug Metab. Pharmacokinet. 2010;25:328–334. doi: 10.2133/dmpk.dmpk-10-rg-004. [DOI] [PubMed] [Google Scholar]