Abstract
Travel and trade have grown immensely. Travelers interact with people and microbes during their journeys, and can introduce infectious agents to new areas and populations. Studying illnesses in travelers is a source of knowledge into diseases of resource-poor regions and the control of these diseases. Travel-associated illnesses also serve to detect emerging infections.
Keywords: Travelers, Travel volume, Emerging infections, Travel-associated illnesses, Disease surveillance
Global travel has evolved dramatically during the past 2 centuries, with ever escalating speed, distance, and volume. Because the geographic distribution of diseases is dynamic and influenced by ecologic, genetic, and human factors, travel allows humans to interact with microbes and introduce pathogens into new locations and populations. The increased numbers of travelers and their spatial mobility have reduced geographic barriers for microbes and heightened the potential for spread of infectious diseases.
Magnitude of travel and trade
Population Growth
Between 1950 and 2007, world population grew from 2.5 to more than 6.6 billion.1 The population growth favored centers of commerce, usually urban or periurban areas, which brought more humans into close contact with larger groups of people. Concurrently, progress in transportation led to speedier movement of humans and goods and microbial organisms.
Number of Travelers
The volume of travel has grown exponentially. International tourist arrivals increased from 25.3 million in 1950 to 898 million in 2007, an astounding 35-fold increase (Table 1 ).2 In recent years, the World Tourism Organization has estimated growth in travel at approximately 6% per year, and anticipates similar growths in upcoming decades.2
Table 1.
Growth in world population and international tourist arrivals
| Year | World Population (Millions) | International Tourist Arrivals (Millions) |
|---|---|---|
| 1950 | 2557 | 25.3 |
| 1985 | 4852 | 329 |
| 1995 | 5694 | 550 |
| 2007 | 6600 | 898 |
| Change from 1950 to 2007 | 2.6× | 35× |
Data from US Census Bureau and World Tourism Organization. Available at: http://www.census.gov/ipc/www/idb and http://www.world-tourism.org/facts/menu.html, respectively.
Human migration data provide another indicator of population mobility. Approximately 2% of the world's population (>200 million people), including immigrants, migrant workers, refugees, asylum seekers, and expatriates, now reside outside their country of birth.3 The United States Census Bureau4 estimated that in 2003, 33.5 million people residing in the United States were foreign-born, comprising 11.7% of the population. Most figures of foreign-born populations only reflect legal entrants, but provide some estimate of travel, because migration to foreign lands is associated with long-distance travel to visit families.
Reason for Travel
People travel voluntarily for numerous and varied reasons, including planned trips for pleasure, work, research, study, humanitarian aid, religious purposes, or missionary activities. They may travel to visit friends and families or for economic opportunities. However, people also migrate involuntarily because of catastrophic events, including environmental disasters and sociopolitical upheaval.
Between 1990 and 2006, the proportion of international tourist arrivals that traveled to visit friends and relatives, seek health care, or for religious reasons increased from 19.6% to 27%. Business and professional travel also increased (Table 2 ).5, 6 Although the total number of leisure travelers increased, the proportion traveling for leisure declined from 55.6% to 51%.5, 6
Table 2.
Comparison of arrivals by purpose of visit, 1990 and 2006
| Total International Arrivals (%) |
||
|---|---|---|
| Reason for Travel | 1990 (International Tourist Arrivals = 438 Million) | 2006 (International Tourist Arrivals = 846 Million) |
| Leisure, holiday | 55.6 | 51 |
| Business, professional | 13.8 | 16 |
| Visit friends and relatives, health, religion | 19.6 | 27 |
| Not specified | 11.0 | 6 |
Data from World Tourism Organization. Available at: http://www.unwto.org.
How populations are moving
Over the decades, the modes of transportation have also shifted from horses and sailing vessels to steamships, railways, automobiles, and aircrafts. In 1788, when long-distance travel primarily occurred on sailing vessels, a trip from England to Australia spanned 1 year.7 Clippers shortened travel time to 100 days by 1840; steamers reduced it to 50 days by 1910; and in the 21st century, aircraft can reach almost any major city on the globe in 24 hours.7 As a result of the augmented speed, sphere, and range of modern transportation, the spatial mobility of the average person grew 1000-fold over past 2 centuries.7
Air travel has accounted for the greatest gains in international travel. In 2006, air travel accounted for 46% of transport, followed by road at 43%, water at 7%, and rail at 4%.5 These figures indicate continued growth of long-haul travel, typically associated with the use of large aircraft, and connections between different ecosystems and their resident species.
Conveyances
Although conveyances have become faster, they have also become larger. Jumbo jets now carry several hundred passengers each. The risk for a traveler to acquire a communicable disease is estimated to increase fourfold when the aircraft size is doubled.7 The United States has 19,500 airports, of which 18 receive more than 500,000 international arrivals annually.8 Up to 5000 planes may be in United States airspace at one time. The global civilian aviation network connects most areas of the world, allowing rapid transit and mix of multiples species.
Ballast water from ships can transport pathogenic microbes (such as Vibrio cholerae) over long distances, and disperse to habitats where the species can persist.9 Cruises have become a popular leisure activity. Cruise ships can now carry more than 3000 passengers and crew. During 2003, 184 ships served the United States cruise industry, with an estimated 7.4 million passengers.8 Currently, 14 United States ports receive more than 150,000 maritime passengers annually.8 Worldwide, 11.5 million passengers traveled on cruise ships in 2005, each for an average of 7 days.10 Passengers converge from different countries; a cruise may involve multiple stops, where passengers may be dropped off or picked up. Passengers may also have brief visits at multiple ports. These patterns expand the potential pool of exposures.
Cruise ships have served as sites of outbreaks, with passengers then dispersing infections elsewhere. Many travelers on cruise ships are older and have chronic medical conditions, and therefore may be susceptible to more severe consequences of infections. The confined and crowded environs on cruise ships allow easy transmission of pathogens. The short durations of most cruises can allow an infected passenger to reach another location before onset of symptoms. The most commonly identified pathogens in cruise ship outbreaks have been norovirus and influenza, but Salmonella, Shigella, Staphylococcus, V cholerae and other vibrios, Legionella, Corynebacterium diphtheriae, and rubella have also been implicated.10, 11 Sources of gastrointestinal disease on cruise ships include water (eg, contaminated by sewage, inadequate disinfection, improper storage), food (poor handling, preparation, cooking), and use of sea water in the galley.10
During 2006, an unusually high number of norovirus outbreaks occurred on cruise ships. By July 5, 2006, 13 cruise ships traveling around Europe had reported 35 outbreaks of gastrointestinal infection.12 In all, investigators confirmed 43 outbreaks on 13 cruise ships.13 The norovirus from stool or environmental samples were of two distinct lineages of the GGII.4 genotype, which emerged separately in Europe and Pacific and caused concurrent outbreaks in the community.13 The cruise ship outbreaks were an early indicator of increased activity in the region and revealed strains that originated in distant locations.
Legionella is another pathogen associated with cruise ships, with more than 200 cases reported.10 A single cruise ship from New York to Bermuda was associated with 50 cases during nine separate voyages in 1994, with the whirlpool spa as the source.14 On another cruise ship, eight German passengers contracted legionellosis (attack rate, 4%), also linked to the spa pool.15
Transmission of pathogens also occurs on aircraft. Infections spread by the airborne or large droplet route are of greatest concern in aircraft transmission, and include influenza, meningococcal infections, measles, tuberculosis, and severe acute respiratory syndrome (SARS). However, the most commonly documented infections transmitted on aircraft have spread through contaminated food: Salmonella, Staphylococcus, norovirus, and cholera.16 Most foodborne transmissions on aircraft result from food contaminated before the flight. Only those with a short incubation manifest during the flight; most often toxin-related (eg, staphylococcal) or, rarely, infections such as V cholerae on a long flight.
Norovirus is exceptional in that it is a gastrointestinal pathogen that can be easily transmitted in a crowded environment. For example, probable transmission of norovirus occurred in 2002 among a flight crew, with limited transmission to passengers.17 Acute illness was reported on an 8-hour flight from London to Philadelphia, Pennsylvania. A survey found 8 of the 14 crew members had symptom onset during flight. Stool specimens from two hospitalized crew members had noroviruses with identical sequences using polymerase chain reaction. Among 93 passengers who returned the survey, 5 had probable norovirus gastroenteritis (5.4%).17
In-flight transmission of Mycobacterium tuberculosis is also possible. M tuberculosis is transmissible through large droplets and droplet nuclei with productive cough, and a single organism can cause infection.18 In 1994, a patient who had multidrug-resistant tuberculosis (MDR TB) traveled on commercial flights from Honolulu to Chicago, Chicago to Baltimore, and returned a month later.19 Contact tracing, questionnaire, and skin testing found up to 6% skin test conversions, with greatest risk in passengers seated within two rows of the case patient (31% conversion).19 Another traveler with MDR TB flew on a commercial airline from Delhi, India, to Chicago, Illinois, in December 2007.20 The incident required coordinated efforts among the Centers for Disease Control and Prevention (CDC) and multiple organizations (the airline, U.S. Customs and Border Protection, U.S. state and local health departments, and the Indian Ministry of Family Welfare) to notify and follow up on passengers and crew that may have been exposed.
Most common origins and destinations
The sphere of travel has enlarged over the years and travel patterns have become ever more complex. The trend of average daily distance traveled in France increased 10-fold with each generation, or more than 1000 times between 1800 and 2000.7 The bacillus causing plague, carried by rats, took 3 years to reach Britain from Italy during the 14th century.21 Today, aircraft can travel thousands of miles in less than a day, allowing infected passengers to carry their microbial baggage to distant destinations where susceptible populations may reside.
In recent years, the growth in travel to Africa, Asia and Pacific, and Middle East has exceeded that in other regions (Fig. 1 ). For example, the average annual growth from 1995 to 2004 was 3.9% for the world, but these three regions (Africa, Asia and Pacific, and Middle East) grew at higher rates: 5.7%, 6.5%, and 10.9%, respectively.6 These areas of rapid growth include many developing countries in tropical/subtropical regions, places characterized by greater species richness.22 Other attributes of these areas, including poor infrastructure, lack of clean water and sanitation, and poor vector control, may increase the risk that travelers will be exposed to local infections. Although Europe is expected to remain the most popular destination, its overall share in the market is projected to decline. Europe and America's combined share in world tourist arrivals was more than 95% in 1950, but declined to 82% in 1990 and 76% in 2000, and is predicted to fall to 64% by 2020.6 The shift of international tourist arrivals to less-developed regions predicts increased exposure to diseases endemic in those regions.
Fig. 1.
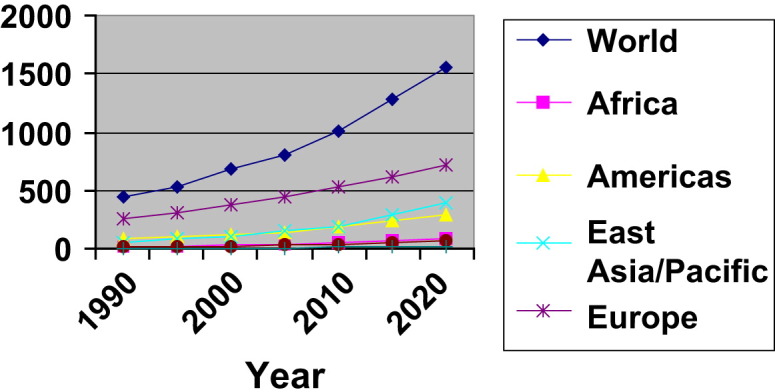
International tourist arrivals by region (millions) with forecast. (Data from WTO Tourism Highlights 2007 and World Tourism Barometer 2008;6(1). Available at http://www.world-tourism.org/facts/menu.html.)
Implication of Travel Pattern on Disease Outbreaks
Travel pattern influences disease outbreaks. Frequent travelers accelerate international spread if they are infected early and the outbreak does not otherwise expand rapidly.23 The travel routes, aviation network, number of flights departing from and arriving at airport, number of passengers carried, and size of aircraft are important considerations in estimating the spread of modern epidemics.24 For some types of infections, simulations illustrate that travel restrictions, particularly isolation of largest cities, will be a necessary component in epidemic control strategies.24
The present pattern of air travel could expedite the spread of an influenza pandemic compared with past pandemics. In 1968 to 1969, 160 million persons traveled internationally on commercial flights.25 The Hong Kong influenza strain of 1968 to 1969 spread globally through the network of cities by air travel: first to northern latitudes, then southern latitudes.25 Modeling of the epidemic with air transportation data in 2000 for 52 cities showed that influenza would spread concurrently to cities in both hemispheres, resulting in minimal seasonal swing and little time for public health intervention.26 Disease would reach nearby cities first, but also distant cities with high air travel volumes; a pandemic initiating in Hong Kong can now spread speedily to northern hemisphere cities 111 days earlier than in 1968.26 Understanding the local ecology and linkages through travel can provide projection of disease spread.
Interactions of travelers, microbes, and locations
Travelers have dynamic interactions with microbes and places. Travelers can carry microbes and their genetic material, and can play many roles with respect to microbes. Travelers can be victims, sentinels, couriers, processors, and transmitters of microbial pathogens.27 Conversely, arrival of travelers can affect host populations through contact with diverse groups of people and microbes throughout their trip and sharing environments sequentially. Travel should be considered a loop and not just an origin and destination.27
Travel can be associated with behavior that leads to transmission of pathogens through blood and body fluid exposure. Travelers may engage in sexual activities or pursue extreme sports or other injury-prone activities that they would not risk at home. A survey assessing possible exposures to hepatitis B among more than 9000 European travelers found that most had potential risk (60.8%–75.8%), including holiday romance (12.5% of all travelers), with 6.6%–11.2% at high risk.28 A Canadian study found that 15% of travelers had potential exposure to blood and body fluids through vehicles such as new sexual partner (9%); sharing instruments, such as razor or toothbrush (5%); receiving injection for medical treatment (3.2%); having acupuncture or other percutaneous nontraditional treatment (1%); tattooing or body piercing (0.5%); and abrasive injury (0.5%).29
Other investigators found that 5.6% of tourists departing from Cuzco engaged in sexual activity with a new partner during their stay.30 Although most reported having sex with other travelers (54.3%), some had sex with local partners (40.7%) or commercial sex workers (2.15%).30 Sexually transmitted infections (including hepatitis B, HIV, and HTLV-1) acquired during travel can further spread during the journey and after return home.
Recent Illustrations
Many examples from the past decade show the range of infections in travelers and the role that travelers can play in sparking outbreaks (Table 3 ). Some infections, such as legionellosis, can affect travelers but also have a wide geographic distribution. Diagnosis is important to allow appropriate treatment (and identification of a risky place, in some instances), but infected travelers do not pose a risk to others. Other infections, such as Lassa fever, can present a risk to close contacts but are not likely to lead to an outbreak in a new region where modern medical facilities are available.
Table 3.
Examples of recent infectious disease transmission associated with travel
| Pathogen | Location Where Illnesses Originated | Countries Where Illnesses Occurred | Comment | Reference |
|---|---|---|---|---|
| Infections with a wide geographic distribution that can affect travelers but pose no risk to others | ||||
| Legionella | Worldwide | Cruise ships | >200 cases have occurred in outbreaks associated with cruise ships | |
| Cruise ship New York to Bermuda | New York | One single cruise ship was implicated in 50 cases of legionellosis during nine separate voyages in 1994; the source was the whirlpool spa | Jernigan e al.14 | |
| Cruise ship to Nordic Sea | Germany | Eight German passengers developed infection after a cruise to the Nordic SeaLegionella pneumophila serogroup 1, subgroup “Knoxville” was isolatedThe attack rate was 4%, and disease was associated with prolonged exposure to the spa pool | Beyrer et al.15 | |
| Infections with risk to immediate contacts but unlikely to lead to an outbreak in a new region with good health care infrastructure | ||||
| Lassa fever | Liberia or Sierra Leone | New Jersey | A businessman born in Liberia but residing in United States returned from West Africa with a febrile illness; lassa fever was confirmed | CDC 200498 |
| Infections that can be introduced by a traveler and may lead to multiple generations of spread or establishment in a new region | ||||
| Chikungunya | Reunion, Comoros, Mauritius, Madagascar, Seychelles, India | Europe, United States, Australia, Hong Kong | Travelers acquired chikungunya in Indian Ocean Island countries and presented with illnesses when they returned home | |
| India | Italy | Traveler was infected in India, visited Italy, became index case in an outbreak that occurred in Italy | Rezza et al.46 | |
| Dengue | Tahiti | Hawaii | In 2001–2002, a returning traveler from Pacific Islands was the index case in the first autochthonous outbreak in Hawaii since 1944, with 122 laboratory-confirmed cases | Effler et al.48 |
| Meningococcal disease | Saudi Arabia | Worldwide | Hajj pilgrims and contacts have transmitted disease to many areas | |
| Norovirus | Worldwide | Cruise ships | The Vessel Sanitation Program at the CDC identified >12 outbreaks on cruise ships in 2002 | Widdowson et al.99 |
| Europe | Cruise ships | Increased outbreaks in Europe were associated with cruise ships | Lopman et al.100 | |
| Aircraft | An outbreak occurred among the crew of a flight with limited transmission to passengers | Widdowson et al.17 | ||
| Europe | Cruise ships | Outbreaks occurred in 2006 on cruise ships from the Netherlands, Scotland, England, most operating in the Baltic Sea | ||
| Severe acute respiratory syndrome | Hong Kong, Singapore | Worldwide | Between November 1, 2002, and July 31, 2003, SARS spread globally to >25 countries and caused 8096 reported infections and 774 deaths | |
| Tuberculosis | Worldwide, Saudi Arabia | Worldwide, air travel | The 2005 tuberculosis rate in foreign-born persons in the United States was 8.7 times that of United States–born persons; the incidence of multidrug-resistant tuberculosis is higher in low- and middle-income countries | |
| Saudi Arabia | Singapore | Comparison of tuberculosis tests using a whole-blood assay (QuantiFERON-TB assay) before and after return from the Hajj showed 10% conversion consistent with exposure during the pilgrimage | Wilder-Smith et al.87 | |
| Multidrug-resistant Mycobacterium tuberculosis | Honolulu, Chicago, and Baltimore | United States | Passengers on flights with an infectious patient had up to 6% skin test conversions; passengers seated within two rows of the case patient had the highest risk for skin test conversion at 31% | Kenyon et al.19 |
| Multidrug-resistant tuberculosis | Delhi, India | United States | A passenger with MDR TB traveled from Delhi to United States, and could potentially spread to others on flight | CDC20 |
| Infections that can be spread by a traveler, but vaccine-induced immunity of the population can limit spread | ||||
| Hepatitis A | Ethiopia, Russia, Philippines | United States | International adoptees have transmitted hepatitis A to their families and contacts | CDC 2007105 |
| Influenza | Cruise ships | Cruise ships, widespread | Multiple outbreaks occurred among cruise ship passengers between New York and Montreal, Tahiti and Hawaii, and Alaska and the Yukon Territory | |
| Measles | China | Many states in the United States, Denmark, Spain, and likely other countries | International adoptees, their family, and other contacts have acquired measles during travel and after arriving home | CDC 2000–200457, 58, 59, 60 |
| United Kingdom, Switzerland, Israel | Many states in the United States and Europe | Unvaccinated travelers have acquired measles during travel (to United Kingdom, Switzerland, Israel, and other countries), and led to outbreaks after return home | CDC 200865, 66 | |
| Israel, Switzerland, India, Japan | Many states in the United States | Visitors and travelers from other countries have presented in the United States with measles and led to outbreaks | CDC 200765, 66 | |
| Europe (Italy, Germany, Switzerland, Austria, France) | Australia | Traveler was diagnosed with measles in Australia after a 3-week holiday in Europe; molecular studies identified it to be a strain identified in United Kingdom | Riddell et al.64 | |
| Mumps | United Kingdom | Multiple states in the United States | Multistate outbreaks began in Iowa in December 2005, and 2597 cases were reported from 11 states between January 1and May 2, 2006; some cases were possibly infectious during air travel | CDC 2006107, 108 |
| United Kingdom | United States | Summer camp outbreak in New York involved 31 cases and was associated with a counselor from the United Kingdom; attack rate was 5.7% | CDC 2006109 | |
| Polio | Somalia | Kenya | Two children, aged 3 and 12 years and born in a camp in Kenya, developed paralytic polio with WPV1, which was consistent with isolates from Somalia | CDC 2008110 |
| Nigeria, India, Afghanistan, Pakistan | Angola, Burma, Chad, Democratic Republic of the Congo, Nepal, Niger, Somalia, Sudan | Wild poliovirus spread from endemic countries to numerous previously polio-free countries; introduction has led to sustained transmission in some countries | CDC 200875 | |
Most relevant to emerging infections are agents that can be introduced by a traveler that lead to multiple generations of spread or even establishment of a pathogen in a new region. Infections in the latter category include those spread from person to person, some with fecal–oral transmission, and some vector-borne infections, such as SARS, chikungunya, dengue, hepatitis A, influenza, measles, meningococcal disease, mumps, norovirus, pertussis, polio, and tuberculosis, including multidrug-resistant (MDR) and extensively drug-resistant (XDR). Populations may be partially or completely protected if vaccinated, as in the case of hepatitis A, influenza, measles, mumps, and polio.
The spread of some infections into new regions may lead to multiple generations in any population (tuberculosis). Other infections may spread only if the appropriate environmental conditions (eg, temperature, humidity) and vector or intermediate hosts are present (dengue, chikungunya). Yet others spread only if the community has susceptible/nonimmune individuals (measles, hepatitis A).
Severe Acute Respiratory Syndrome
The outbreak of SARS in 2003 exemplifies the impact of spatial mobility and the dynamic role of travelers. In 2002, a previously unrecognized coronavirus caused an outbreak of respiratory infections in the Guangdong Province of China. The virus apparently jumped species from civet cats to humans, although subsequent research suggests that the reservoir host is the fruit bat.31 The outbreak became visible to the world community when an infected physician from Guangdong, who stayed for a day in Hotel Metropole in Hong Kong, was the source of infection for multiple hotel guests, who then disseminated the virus to numerous other countries. By May, more than 8000 SARS infections had been reported.32 By July, 29 countries and territories across five continents reported outbreaks and attributed 774 fatalities to SARS.32 Transmission of SARS on aircraft occurred at rates of 0% to 18.3%, and occurred as far as seven rows from the source passenger.33
One particular SARS case showed the potential for rapid international dispersion of a pathogen that is spread from person to person.34 A businessman flew from Hong Kong to Frankfurt, Germany, on March 30, 2003. He traveled on seven flights throughout Europe during a 5-day period, including stops in Barcelona, London, Munich, and Hong Kong. He was hospitalized in Hong Kong on April 8 for suspected SARS, subsequently confirmed on April 10.34 Responding to SARS outbreak, the CDC issued advisories to avoid travel to the SARS-affected countries. Most countries in Asia instituted strict quarantine measures and restricted travel to reduce cross-border spread and as intracountry spread. The CDC temporarily suspended international adoption from China because of concern for dissemination.
SARS and the associated travel advisories led to a decline in international tourist arrivals in 2003; the World Trade Organization (WTO) reported that arrivals to some affected countries in Asia plunged to less than 50% of their usual levels.35 Although the region rebounded quickly, SARS was responsible for a 9% overall loss in travel volume for Asia in 2003 and had substantial economic impact.35
Chikungunya
Chikungunya virus, an alphavirus first isolated in Africa in 1952, is a mosquito-transmitted virus that was recently carried by travelers to geographically disparate regions on different continents. Recent outbreaks of chikungunya virus infection originated in Kenya in 2004, and major outbreaks followed in the Indian Ocean Island countries (Reunion, Mauritius, Comoros, Seychelles, Madagascar) in 2005 to 2006.36 Outbreaks ensued in India and Indonesia, and the virus was carried by travelers to Europe,37, 38, 39, 40, 41 the United States42, 43 Australia,44 and Hong Kong.45 A viremic traveler from India visiting the Ravenna province of Italy became the index case of an outbreak that infected 205 local residents, which was transmitted through local Aedes albopictus, a mosquito species introduced into Italy by ship in 1990.46
Dengue
Dengue virus, a flavivirus, is endemic in Southeast Asia, South Asia, the Pacific, Caribbean, and Central and South America, and its history illustrates the intricate interactions of travel, movement of goods, and translocation of infectious disease.47 Most cases of dengue virus infection diagnosed in the United States have been imported in travelers, although limited local transmission in Texas has also occurred recently. Less well-known is the fact that a competent vector, A albopictus, or Asian tiger mosquito, was introduced into the United States in 1980s by ships that carried used tires. Since then, the mosquito has established itself in many states, and could potentiate autochthonous dengue outbreaks.
In 2001 Hawaii experienced dengue outbreaks, the likely source being viremic travelers returning from French Polynesia. Dengue had been present in Hawaii until the 1940s (after World War II), when autochthonous transmission ceased. However, A albopictus became established in Hawaii, and in 2001was the primary vector in a local outbreak involving more than 100 cases.48
Influenza: Seasonal and Pandemic
Influenza remains an ongoing global challenge, given the large pool of influenza viruses in avian and other species and the capacity of the virus to recombine, reassort, and mutate. Spread through aerosol or direct contact, the aircraft provides an ideal enclosed space for transmission of influenza virus. In one well-characterized outbreak, a passenger who had influenza on an airplane with a nonfunctioning ventilation system for 3 hours probably transmitted the infection to 72% of 54 passengers.49 Movement of troops contributed to the spread of influenza in 1918 to 1919. Nowadays the expanded range and speed of travel can rapidly disseminate a pandemic strain of influenza.
Influenza has caused multiple outbreaks on cruise ships. A large outbreak of influenza A (estimated >33,000) cases during the summer of 1998 in Alaska and the Yukon Territory, Canada affected primarily tourists and workers in the tourism industry.50 Outbreaks also occurred on two cruise ships, affecting passengers between New York and Montreal, and Tahiti and Hawaii.51 A major outbreak on a cruise ship can affect thousands of individuals, and passengers can carry infection to their next destination.
In a study of Swiss travelers that included a questionnaire and paired serologic testing before and after travel (N = 1450), 2.8% of travelers tested positive for influenza and 1.2% had more than a fourfold increase in antibody titers. Investigators estimated the incidence for influenza-associated events to be 1.0 per 100 person-months abroad.52 These results indicate that influenza has become the most common vaccine-preventable disease in Swiss travelers to the tropics, and highlight the risk for spread through travel.
An analysis of the CDC's influenza and pneumonia mortality data from 1996 to 2005 found that international air travel influences the timing of influenza introduction, and that domestic airline travel volume in November correlates with the rate of spread in the United States.53 A study of the hemagglutinin of 13,000 human influenza A (H3N2) viruses during 2002 through 2007 indicated that most new strains emerge in East and Southeast Asia.54 The new strains circulate continuously in this region and cause epidemics, leading to epidemics in temperate regions. The new strains initially spread to Oceania, North America, and Europe, later reaching South America.54 The new influenza strains most likely reach other parts of the world through travelers.
Measles
Measles has exemplified the travel-related spread of an infectious pathogen for centuries. European explorers brought measles to the New World along with smallpox and other pathogens, decimating local populations and contributing to the collapse of civilizations in the New World. In the 1990s, as countries in the Americas attempted measles eradication and cases declined, numerous importations were clearly documented. The countries of origin included developed countries in Asia and Europe, and developing countries in these continents and Africa.55 The CDC reported 14 measles outbreaks in the United States between 2001 and 2004, with 7 originating from an American traveler.56
Measles outbreaks have recently resulted from travel for international adoption, including cases that were infectious during flights.57, 58, 59, 60 Clusters of internationally adopted children from China, their family members, and contacts contracted measles in 2000 and almost every year thereafter. Transmission was identified in the orphanages in China, causing the CDC to suspend adoption temporarily from the affected orphanages.
Measles outbreaks have also occurred in unvaccinated students returning from community service in developing countries, with subsequent spread to their communities.61, 62 During the Little League championships in 2007 in Pennsylvania, a player from Japan became infected and transmitted measles to other players and contacts.63 Measles was also acquired by a traveler on a 3-week holiday in Europe (Italy, Germany, Switzerland, Austria, France) and diagnosed after his return to Australia.64 Molecular analysis determined the genotype B3 strain to be one from the United Kingdom, where he had not visited, indicating unrecognized transmission of the strain in continental Europe.64
In February, 2008, an adult visitor from Switzerland was hospitalized in Arizona for measles and pneumonia.65 The individual acquired measles in Switzerland, where an outbreak was occurring. In the several weeks that followed, nine confirmed cases were linked, and additional cases were suspected to be associated.
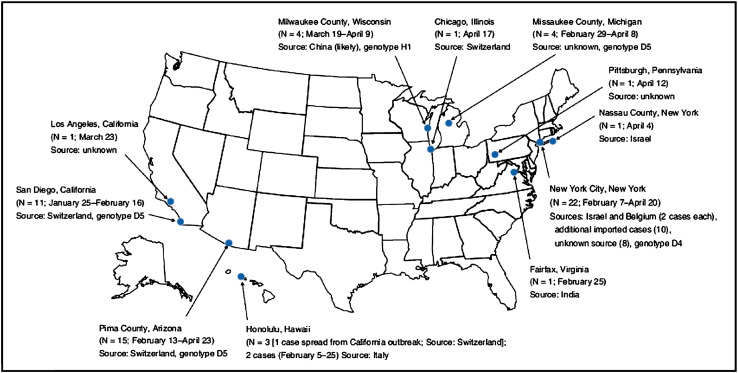
Similarly, an unvaccinated child became infected with measles during travel to Switzerland, which led to an outbreak of 11 cases in San Diego, and another patient who became ill in Hawaii.66 Both outbreaks involved genotype D5, which was circulating in Switzerland. Confirmed measles cases have also been reported in New York and Virginia, involving genotype D4, which has been causing large outbreaks in Israel (Fig. 2 ).65
Fig. 2.
Measles outbreaks in the United States from January 1 through April 25, 2008. (From CDC. Measles—United States, January 1–April 25, 2008. MMWR Morb Mortal Wkly Rep 2008;57(18):494–8. http://www.cdc.gov/mmwr/PDF/wk/mm5718.pdf; with permission.)
In Europe, 70% to 86% of measles cases have been associated with importation.67 Cases were noted in Spanish travelers returning from Thailand and Mozambique.67, 68 Because of its high reproductive rate, as long as the measles virus persists anywhere, it will remain a threat to the global population.
Meningococcal Disease
Meningococcal disease represents an infectious disease that impacts travel health requirements. After the meningococcal outbreaks associated with the Hajj pilgrimage in 1987, Saudi Arabia required meningococcal immunization for all pilgrims and local populations in pilgrimage sites.69 Despite this immunization requirement, outbreaks of serogroup W-135 associated with the Hajj occurred in 2000 and 2001.70, 71, 72 A study in the United States found that 1.3% of pilgrims returning from the Hajj were carrying serogroup W-135 N meningitidis,72 despite vaccination with a quadrivalent vaccine that included W-135.
Because of these meningococcal outbreaks associated with the Hajj, Saudi Arabia revised the meningococcal vaccination requirement to specifically use the quadrivalent vaccine for pilgrims and all local population at risk.73 The widely used meningococcal vaccines can reduce the risk for meningococcal disease but do not prevent nasopharyngeal carriage with Neisseria meningitidis.
Polio
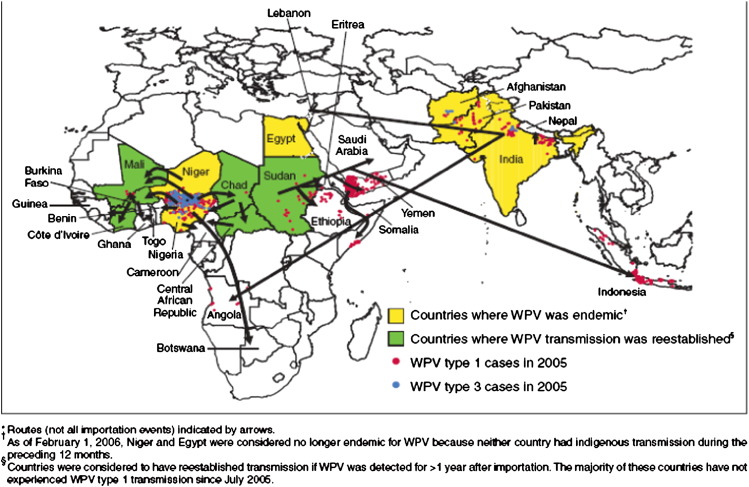
In 1988, the World Health Assembly resolved to eradicate polio globally by 2000. Although eradication has not been achieved, global incidence has declined.74 However, in the past several years, travel and migration have reintroduced poliovirus into many countries that had previously achieved polio-free status. From 2002 to 2005, wild poliovirus resurged and spread from 6 endemic countries to 21 countries (Fig. 3 ).74 Among 13 countries with sustained transmission for more than 6 months, polio vaccine coverage was 52%, whereas those without sustained transmission had coverage of 82%, clearly illustrating that higher immunization coverage is necessary to eradicate polio.74 As of April 2008, only 4 countries (Afghanistan, India, Nigeria, Pakistan) remain endemic for polio, but at least 13 have identified polio importation, including 4 that had been polio-free for at least 4 years (Bangladesh, Burma, the Democratic Republic of the Congo, Namibia) and 1 that had been polio-free for 10 years (Kenya).75
Fig. 3.
Wild poliovirus (WPV) cases in 2005 and importation routes during 2002–2005 worldwide. (From CDC. Resurgence of wild poliovirus type 1 transmission and consequences of importation—21 countries, 2002–2005. Morbidity Mortality Weekly Report 2006;55(6):145–50; with permission.)
In addition to wild poliovirus circulation, vaccine-derived poliovirus has reverted to virulent forms and has circulated in several countries (including Nepal, Myanmar, Philippines, Madagascar, Haiti, and Dominican Republic), associated with paralytic polio.75 A 22-year-old woman from the United States contracted paralytic polio from vaccine-strain poliovirus in 2005 while studying abroad in South and Central America.76 The source was probably an infant who had recently received the live polio vaccine.76
Also in 2005, an immunocompromised Amish infant (unvaccinated) in Minnesota was found to be infected with vaccine-derived poliovirus, although without paralysis.77 This finding led to the identification of four more children who had asymptomatic infection. Molecular analysis of the virus suggested that it probably had replicated for 2 years, and most likely originated in someone visiting the United States from a country that used the live oral polio vaccine.77
Animal and Vector Movement and Travel
Animals are a common source of human pathogens recently and remotely, with HIV/AIDS the most dramatic recent example of a pathogen of animal origin that entered and spread in the human population.78 Although humans travel widely, they also orchestrate the movement of many species of domestic and wild animals, legally and illegally.79 Wild-caught African animals imported into the United States were the source of a monkeypox outbreak in the Midwest. The African animals, which had unrecognized monkeypox infection, were housed with prairie dogs that were sold as pets.80 Dogs brought in from rabies-endemic areas have been an occasional source of rabies in Europe. Movement of avian species, including exotic birds sold to falconers, can be a potential route for introduction of H5N1 or other microbes potentially pathogenic for humans (Fig. 4 ).81 Some of these infections have the potential for persistence and dissemination in a new geographic region.
Fig. 4.
Crested Hawk-Eagles confiscated at Brussels International Airport in the hand luggage of a Thai passenger. The birds were wrapped in a cotton cloth, with the heads free, and each of them inserted in a wicker tube ∼60 cm in length, with one end open. (Courtesy of Paul Meuleneire, custom investigations officer, antidrug group. From Van Borm S, Thomas I, Hanquey G, et al. Highly pathogenic H5N1 influenza virus in smuggled Thai eagles, Belgium. Emerg Infect Dis 2005;11(5):702–5. Available from http://www.cdc.gov/ncidod/EID/vol11no05/05-0211.htm.)
Conveyances of travel and trade have also transported mosquito vectors and introduced them into new areas, where they have become established and have been important in disease outbreaks. With chikungunya virus infection, A albopictus mosquitoes were imported into Italy, probably by way of shipped used tires. Subsequently, a viremic travel carried the virus that was responsible for the outbreak in Italy in the summer of 2007.46 A albopictus was introduced through used tires into the United States in 1986 and spread broadly, and was the main vector identified in the dengue outbreak in Hawaii in 2001 to 2002. Regions that have a competent vector can be potentially vulnerable to outbreaks of new vector-borne infections, if the appropriate bioclimatic conditions exist.
Tatem and colleagues82 collected data on the volume of shipping and air traffic and the climate and used it to identify shipping routes with the highest risk for A albopictus invasion. Among 47 ports outside the original distribution of A albopictus, it invaded just more than half. Those invaded had similar climate and high sea traffic volumes. The authors concluded that “when climatic suitabilities are similar, shipping volume alone appears to determine invasion probability.”82 These studies may be useful in trying to identify areas at highest risk so that strict surveillance and control activities can be instituted. With increasing travel and trade, introductions of animals and insects are expected to continue.
Investigators also tried to quantify which airports have the greatest risk for local Plasmodium falciparum transmission through importation of infective mosquitoes from sub-Saharan Africa.83 They used global climate and air traffic data and analyzed risk according to season. They also estimated areas of greatest potential risk because of development of new routes. These quantitative risk assessments can be used to assess likely pathways of introductions into new regions.84
Mass Gatherings
When masses of people from different regions of the world congregate, great potential arises for the translocation of microorganisms. Religious pilgrimages are typical of these mass meetings of humans. Major sporting events where spectators and athletes from distant lands are also possible venues for microbial mixing.
Hajj pilgrimage
The Hajj is a gathering of approximately 2 million Muslim pilgrims, which takes place annually in Saudi Arabia. The Umra is a pilgrimage of a smaller scale, although pilgrims also gather in Saudi Arabia from all parts of the globe. The WHO has issued health recommendations for the Hajj, with specific directives for yellow fever, meningococcal disease, influenza, and poliomyelitis.85 Meningococcal disease in particular has demonstrated transmission during Hajj and its spread after pilgrims return to their home countries, despite vaccination.69, 70, 71, 72, 73, 86
Tuberculosis also poses a threat. A study of possible exposure to tuberculosis using whole-blood assay (QuantiFERON-TB) before travel and after return from the Hajj pilgrimage found that 10% of pilgrims had a rise in immune response to tuberculosis antigens.87 Influenza, measles, and pertussis also have potential for creating outbreaks associated with crowded events such as the Hajj pilgrimage.
Olympics
The Olympic Games are held every 4 years and attract approximately 10,500 athletes worldwide. For the Beijing Olympics in 2008, several hundred thousand spectators are expected at any one time, in addition to 20,000 media personnel.88 Although the events will be held in 37 venues and involve several cities, the athletes and visitors are expected to concentrate in densely populated cities of Beijing, Hong Kong, and Shanghai. Communicable diseases can potentially spread among athletes and spectators, and then into their home countries. Enterovirus 71 emerged in numerous Chinese provinces in the spring of 2008. With SARS as a reminder, health authorities are working to contain the enteroviral outbreaks before the Olympic Games.
During the Commonwealth Games in 2006, the case of measles in a returning traveler to Australia generated concern about the possibility of spread through the event.64 A measles outbreak occurring in Germany just preceding the Football World Cup in 2006 caused concern about transmission to spectators and further dispersal when they returned home. Fortunately, no major outbreaks occurred. However, the Little League Tournament in Pennsylvania in 2007 led to outbreak of measles, and strengthens the notion that mass gatherings facilitate dispersal of pathogens.63
Travel Medicine
Travel medicine is a specialty that coordinates various disciplines, including infectious diseases, tropical medicine, public health, migrant health, wilderness medicine, and psychiatry. In 1991, the International Society of Travel Medicine, was established with the goal of providing health promotion and disease prevention for travelers.89 This specialty integrates an understanding of global health issues into the health care of travelers,90 and many specialists in the field have led the research and teaching that have provided insight into the impact of travel on infectious diseases.91
Programs on Outbreak Reporting and Disease Surveillance
Several programs have been established to report on outbreaks or survey infectious diseases in travelers. ProMED, a program of the International Society of Infectious Diseases (www.isid.org), disseminates news on humans, animals, and plant diseases globally. GeoSentinel is a network of travel medicine clinics developed through a collaborative agreement between the International Society of Travel Medicine and the CDC. Participants collect information on travel-related illnesses and report on unusual illnesses in travelers.92 The GeoSentinel network now has 40 sites located in six continents. GeoSentinel analyses of illnesses in travelers help define geographic areas associated with risk for specific diseases, and thereby improve the health preparation of travelers.93
A recent GeoSentinel report on schistosomiasis in travelers returning from Tanzania alerted clinicians to exposure associated with swimming in an artificial pond. An analysis of dengue cases in the GeoSentinel database showed periodic increases, and the cyclic pattern corresponded to epidemics in Southeast Asia, illustrating usefulness of travelers as a sentinel population.94 GeoSentinel has notably reported on leptospirosis associated with Eco-Challenge in Borneo, SARS in Canadian travelers returning to from Asia, and malaria from resort holidays in Punta Cana, Dominican Republic. Additional cases were identified in travelers after the initial alerts, and public health responses followed.
TropNet Europe is another surveillance network of travel medicine providers that also focuses exclusively on travelers returning to Europe.95 These networks show useful information-sharing between clinicians and public health authorities.
Summary
Travel influences the emergence of infectious diseases. Travelers have contact and interactions with diverse microbes and people during their journeys, share environments with other people, and can have in-transit transmission. Travelers can carry microorganisms to new environments or allow mingling of organisms from different regions, resulting in mixing of genetic material or resistance characteristics. Travelers can become infected and then infect others. In some instances, this can lead to multiple generations of spread or sustained transmission in a new area. Finally, diagnoses of travelers in resource-rich regions can yield knowledge about infectious disease agents acquired in resource-poor areas. This knowledge can be used to alert the global community to the presence or susceptibility patterns of pathogens in different regions; inform strategies that can be used to control infections in developing countries; and prepare travelers to those areas and guide the care of those returning. Travelers should be considered an integral part of the global surveillance network for emerging infections.
References
- 1.United States Census Bureau. International database. Available at: http://www.census.gov/ipc/www/idb. Accessed March 1, 2008.
- 2.World Tourism Organization. UNWTO World Tourism Barometer January 2008;6(1). Available at: http://www.world-tourism.org/facts/menu.html. Accessed February 8, 2008.
- 3.Gushulak B.D., MacPherson D.W. Globalization of infectious diseases: the impact of migration. Clin Infect Dis. 2004;38:1742–1748. doi: 10.1086/421268. [DOI] [PubMed] [Google Scholar]
- 4.United States Census Bureau. The Foreign-Born Population in the United States—2003. Available at: http://www.census.gov/prod/2004pubs/p20-551.pdf. Accessed February 8, 2008.
- 5.World Tourism Organization. Tourism highlights, edition 2007. Available at: http://www.world-tourism.org/facts/menu.html. Accessed February 8, 2008.
- 6.World Tourism Organization. Tourism indicators. Available at: http://www.world-tourism.org/facts/menu.html. Accessed February 8, 2008.
- 7.Cliff A., Haggett P. Time, travel and infection. Br Med Bull. 2004;69:87–99. doi: 10.1093/bmb/ldh011. [DOI] [PubMed] [Google Scholar]
- 8.Sivitz L.B., Stratton K., Benjamin G.C., editors. Quarantine stations at ports of entry: protecting the public's health. Committee on measures to enhance the effectiveness of the CDC quarantine station expansion plan for U.S. ports of entry. Institute of Medicine of the National Academies. The National Academies Press; Washington, DC: 2006. [Google Scholar]
- 9.Ruiz G.M., Rawlings T.K., Dobbs F.C. Global spread of microorganisms by ships. Nature. 2000;408:49–50. doi: 10.1038/35040695. [DOI] [PubMed] [Google Scholar]
- 10.WHO Travel by sea: health considerations. Wkly Epidemiol Rec. 2007;82(34):305–308. [PubMed] [Google Scholar]
- 11.Minooee A., Rickman L.S. Infectious diseases on cruise ships. Clin Infect Dis. 1999;29:737–744. doi: 10.1086/520426. [DOI] [PubMed] [Google Scholar]
- 12.Koopmans M, Harris J, Verhoef L, et al. European investigation into recent norovirus outbreaks on cruise ships: update. Available at: http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=2997. Accessed March 1, 2008. [DOI] [PubMed]
- 13.Verhoef L., Depoortere E., Boxman I. Emergence of new norovirus variants on spring cruise ships and prediction of winter epidemics. Emerging Infect Dis. 2008;14(2):238–243. doi: 10.3201/eid1402.061567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jernigan D.B., Hofmann J., Cetron M.S. Outbreak of Legionnaires' disease among cruise ship passengers exposed to a contaminated whirlpool spa. Lancet. 1996;347(9000):494–499. doi: 10.1016/s0140-6736(96)91137-x. [DOI] [PubMed] [Google Scholar]
- 15.Beyrer K., Lai S., Dreesman J. Legionnaires' disease outbreak associated with a cruise liner, August 2003: epidemiological and microbiological findings. Epidemiol Infect. 2007;135(5):802–810. doi: 10.1017/S0950268806007473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mangili A., Gendreau M.A. Transmission of infectious diseases during commercial air travel. Lancet. 2005;365:989–996. doi: 10.1016/S0140-6736(05)71089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Widdowson M.-A., Glass R., Monroe S. Probable transmission of norovirus on an airplane. JAMA. 2005;293:1859–1860. doi: 10.1001/jama.293.15.1859. [DOI] [PubMed] [Google Scholar]
- 18.Musher D.M. How contagious are common respiratory tract infections? N Engl J Med. 2003;348(13):1256–1266. doi: 10.1056/NEJMra021771. [DOI] [PubMed] [Google Scholar]
- 19.Kenyon T.A., Valway S.E., Ihle W.W. Transmission of multidrug-resistant Mycobacterium tuberculosis during a long airplane flight. N Engl J Med. 1996;334(15):933–938. doi: 10.1056/NEJM199604113341501. [DOI] [PubMed] [Google Scholar]
- 20.CDC. CDC investigation of traveler with multidrug resistant tuberculosis (MDR TB). Available at: http://www.cdc.gov/tb/flightQA.htm. Accessed May 1, 2008.
- 21.Ozonoff D., Pepper L. Ticket to ride: spreading germs a mile high. Lancet. 2005;365(9463):917–919. doi: 10.1016/S0140-6736(05)71058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guernier V., Hockberg M.E., Guegan J.E. Ecology drives the worldwide distribution of human diseases. PLoS Biol. 2004;2(6):740–746. doi: 10.1371/journal.pbio.0020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollingsworth T.D., Ferguson N.M., Anderson R.M. Frequent travelers and rate of spread of epidemics. Emerging Infect Dis. 2007;13(9):1288–1294. doi: 10.3201/eid1309.070081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hufnagel L., Brockmann D., Geisel T. Forecast and control of epidemics in a globalized world. Proc Natl Acad Sci USA. 2004;101(42):15124–15129. doi: 10.1073/pnas.0308344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rvachev L., Longini I. A mathematical model for the global spread of influenza. Math Biosci. 1985;75:3–22. [Google Scholar]
- 26.Grais R.F., Ellis J.H., Glass G.E. Assessing the impact of airline travel on the geographic spread of pandemic influenza. Eur J Epidemiol. 2003;18:1065–1072. doi: 10.1023/a:1026140019146. [DOI] [PubMed] [Google Scholar]
- 27.Wilson M.E. The traveller and emerging infections: sentinel, courier, transmitter. J Appl Microbiol. 2003;94:1S–11S. doi: 10.1046/j.1365-2672.94.s1.1.x. [DOI] [PubMed] [Google Scholar]
- 28.Zuckerman J.N., Steffen R. Risks of hepatitis B in travelers as compared to immunization status. J Travel Med. 2000;7:170–174. doi: 10.2310/7060.2000.00054. [DOI] [PubMed] [Google Scholar]
- 29.Correia J.D., Shafer R.T., Patel V. Blood and body fluid exposure as a health risk for international travel. J Travel Med. 2001;8:263–266. doi: 10.2310/7060.2001.24033. [DOI] [PubMed] [Google Scholar]
- 30.Cabada M.M., Montoya M., Echevarria J.I. Sexual behavior in travelers visiting Cuzco. J Travel Med. 2003;10(4):214–218. doi: 10.2310/7060.2003.40508. [DOI] [PubMed] [Google Scholar]
- 31.Li W., Shi Z., Yu M. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Available at: http://www.who.int/csr/sars/country/table2004_04_21/en/. Accessed March 1, 2006.
- 33.Olsen S.J., Chang H.-L., Cheung T.Y.-Y. Transmission of the severe acute respiratory syndrome on aircraft. N Engl J Med. 2003;349:2416–2422. doi: 10.1056/NEJMoa031349. [DOI] [PubMed] [Google Scholar]
- 34.Breugelmans J.G., Zucs P., Porten K. SARS transmission and commercial aircraft. J Am Med Assoc. 2004;10(8):1502–1503. doi: 10.3201/eid1008.040093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Tourism Organization. Tourism highlights, edition 2004. Available at: http://www.unwto.org. Accessed October 27, 2005.
- 36.Charrel R.N., de Lamballerie X., Raoult D. Chikungunya outbreaks–the globalization of vectorborne diseases. N Engl J Med. 2007;356:769–771. doi: 10.1056/NEJMp078013. [DOI] [PubMed] [Google Scholar]
- 37.Panning M., Grywna K., van Esbroeck M. Chikungunya fever in travelers returning to Europe from the Indian Ocean region, 2006. Emerging Infect Dis. 2007;14(3):416–422. doi: 10.3201/eid1403.070906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Beltrame A., Angheben A., Bisoffi Z. Imported chikungunya infection, Italy. Emerging Infect Dis. 2007;13(8):1264–1266. doi: 10.3201/eid1308.070161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parola P., de Lamballerie X., Jourdan J. Novel chikungunya virus variant in travellers returning from Indian Ocean Islands. Emerging Infect Dis. 2006;12:1493–1499. doi: 10.3201/eid1210.060610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simon F, Parola P, Grandalam M, et al. Chikungunya infection: an emerging rheumatism among travellers returned from Indian Ocean Islands. A report of 47 patients. Medicine;86(3):123–37. [DOI] [PubMed]
- 41.Hochedez P., Jaureguiberry S., Debruyne M. Chikungunya infection in travelers. Emerging Infect Dis. 2006;12:1565–1567. doi: 10.3201/eid1210.060495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lanciotti R.S., Kosoy O.L., Laven J.J. Chikungunya virus in US travelers returning from India, 2006. Emerging Infect Dis. 2007;13(5):764–767. doi: 10.3201/eid1305.070015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.CDC Update: chikungunya fever diagnosed among international travellers, United States. MMWR Morb Mortal Wkly Rep. 2007;56(12):276–277. [PubMed] [Google Scholar]
- 44.Druce J.D., Johnson D.F., Tran T. Chikungunya virus infection in traveler to Australia. Emerging Infect Dis. 2007;13(3):509–510. doi: 10.3201/eid1303.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee N., Wong C.K., Lam W.Y. Chikungunya fever, Hong Kong. Emerging Infect Dis. 2006;12(11):1790–1792. doi: 10.3201/eid1211.060574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rezza G., Nicolleti L., Angelleti R. Infection with chikungunya virus in Italy: an outbreak in a temperate region. Lancet. 2007;370:1840–1846. doi: 10.1016/S0140-6736(07)61779-6. [DOI] [PubMed] [Google Scholar]
- 47.Gubler D.J. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10(2):100–102. doi: 10.1016/s0966-842x(01)02288-0. [DOI] [PubMed] [Google Scholar]
- 48.Effler P.V., Pang L., Kitsutani P. Dengue fever, Hawaii, 2001–2002. Emerging Infect Dis. 2005;11(5):742–749. doi: 10.3201/eid1105.041063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moser M.R., Bender T.R., Margolis H.S. An outbreak of influenza aboard a commercial airliner. Am J Epidemiol. 1979;110:1–6. doi: 10.1093/oxfordjournals.aje.a112781. [DOI] [PubMed] [Google Scholar]
- 50.Uyeki T.M., Zane S.B., Bodnar U.R. Large summertime influenza A outbreak among tourists in Alaska and the Yukon territory. Clin Infect Dis. 2003;36(9):1095–1102. doi: 10.1086/374053. [DOI] [PubMed] [Google Scholar]
- 51.CDC Update: influenza activity—United States, 1997–1998 season. MMWR Morb Mortal Wkly Rep. 1997;46:1094–1098. [PubMed] [Google Scholar]
- 52.Mutsch M., Tavernini M., Marx A. Influenza virus infection in travelers to tropical and subtropical countries. Clin Infect Dis. 2005;40(9):1282–1287. doi: 10.1086/429243. [DOI] [PubMed] [Google Scholar]
- 53.Brownstein J.S., Wolfe C.J., Mandl K.D. Empirical evidence for the effect of airline travel on inter-regional influenza spread in the United States. PLoS Med. 2006;3(10):e401. doi: 10.1371/journal.pmed.0030401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Russell C.A., Jones T.C., Barr I.G. The global circulation of influenza A (H3N2) viruses. Science. 2008;320(5874):340–346. doi: 10.1126/science.1154137. [DOI] [PubMed] [Google Scholar]
- 55.de Quadros C., Izurieta H., Venczel L. Measles eradication in the Americas: progress to date. J Infect Dis. 2004;189(Suppl 1):S227–S235. doi: 10.1086/377741. [DOI] [PubMed] [Google Scholar]
- 56.CDC Preventable measles among U.S. residents, 2001-2004. MMWR Morb Mortal Wkly Rep. 2005;54(33):817–820. [PubMed] [Google Scholar]
- 57.CDC Update: multistate investigation of measles among adoptees from China—April 16, 2004. MMWR Morb Mortal Wkly Rep. 2004;53:323–324. [PubMed] [Google Scholar]
- 58.Centers for Disease Control and Prevention (CDC) Measles outbreak associated with an imported case in an infant—Alabama, 2002. MMWR Morb Mortal Wkly Rep. 2004;53(2):30–33. [PubMed] [Google Scholar]
- 59.CDC Measles outbreak in adults associated with adoption of children in China—California, Missouri, Washington, July-August 2006. MMWR Morb Mortal Wkly Rep. 2007;56(7):144–146. [PubMed] [Google Scholar]
- 60.CDC Measles among adults associated with adoption of children in China—California, Missouri, Washington, July-August 2006. MMWR Morb Mortal Wkly Rep. 2007;56(07):144–146. [PubMed] [Google Scholar]
- 61.Centers for Disease Control and Prevention (CDC) Import-associated measles outbreak—Indiana, May-June 2005. MMWR Morb Mortal Wkly Rep. 2005;54:1073–1075. [PubMed] [Google Scholar]
- 62.Parker A.A., Staggs W., Dayan G.H. Implications of a 2005 measles outbreak in Indiana for sustained elimination of measles in the United States. N Engl J Med. 2006;355(5):447–455. doi: 10.1056/NEJMoa060775. [DOI] [PubMed] [Google Scholar]
- 63.CDC Multistate measles outbreak associated with an international youth sporting event—Pennsylvania, Texas, Michigan, August-September, 2007. MMWR Morb Mortal Wkly Rep. 2008;57(7):169–173. [PubMed] [Google Scholar]
- 64.Riddell M.A., Lynch P., Jin L. Measles case imported from Europe to Victoria, Australia, March 2006. Euro Surveill. 2006;11(20):2959. doi: 10.2807/esw.11.20.02959-en. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=2959 Available at: Accessed May 20, 2006. [DOI] [PubMed] [Google Scholar]
- 65.CDC Measles—United States, January 1-April 25, 2008. MMWR Morb Mortal Wkly Rep. 2008;57(18):494–498. [PubMed] [Google Scholar]
- 66.CDC Outbreak of measles—San Diego, California, January-February, 2008. MMWR Morb Mortal Wkly Rep. 2008;57(8):203–206. [PubMed] [Google Scholar]
- 67.Muscat M., Glismann S., Bang H. Measles in Europe in 2001–2002. Euro Surveill. 2003;8:123–129. doi: 10.2807/esm.08.06.00414-en. [DOI] [PubMed] [Google Scholar]
- 68.Munoz J., Alonso D., Vilella A. Measles in travelers: are we aware enough? J Travel Med. 2008;15:124–125. doi: 10.1111/j.1708-8305.2008.00190.x. [DOI] [PubMed] [Google Scholar]
- 69.Moore P.S., Harrison L.H., Telzak E.E. Group A meningococcal carriage in travelers returning from Saudi Arabia. JAMA. 1988;260(18):2686–2689. [PubMed] [Google Scholar]
- 70.CDC Serogroup meningococcal disease among travelers returning from Saudi Arabia: United States, 2000. MMWR Morb Mortal Wkly Rep. 2000;49:345–346. [PubMed] [Google Scholar]
- 71.CDC Public health dispatch: update: assessment of risk for meningococcal disease associated with the Hajj 2001. MMWR Morb Mortal Wkly Rep. 2001;50(12):221–222. [PubMed] [Google Scholar]
- 72.Dull P.M., Abdelwahab J., Sacchi C.T. Neisseria meningitidis serogroup W-135 carriage among US travelers to the 2001 Hajj. J Infect Dis. 2005;191:33–39. doi: 10.1086/425927. [DOI] [PubMed] [Google Scholar]
- 73.World Health Organization. 2001-Meningococcal disease, serogroup W135, disease outbreak news April 27, 2001. Available at: http://www.who.int/csr/don/2001_04_27/en/index.html. Accessed April 4, 2008.
- 74.Centers for Disease Control and Prevention (CDC) Resurgence of wild poliovirus type 1 transmission and consequences of importation—21 countries, 2002–2005. MMWR Morb Mortal Wkly Rep. 2006;55(6):145–150. [PubMed] [Google Scholar]
- 75.CDC Progress toward interruption of wild poliomyelitis transmission—Worldwide, January 2007–April 2008. MMWR Morb Mortal Wkly Rep. 2008;57(18):489–494. [PubMed] [Google Scholar]
- 76.CDC Imported vaccine-associated paralytic poliomyelitis—United States, 2005. MMWR Morb Mortal Wkly Rep. 2006;55(4):97–99. [PubMed] [Google Scholar]
- 77.CDC Poliovirus infections in four unvaccinated children—Minnesota, August-October 2005. MMWR Morb Mortal Wkly Rep. 2005;54:1053–1055. [PubMed] [Google Scholar]
- 78.Hahn B., Shaw G.M., De Cock K.M. AIDS as a zoonosis: scientific and public health implications. Science. 2000;287:607–614. doi: 10.1126/science.287.5453.607. [DOI] [PubMed] [Google Scholar]
- 79.Karesh W.B., Cook R.A., Gilbert M. Implications of wildlife trade on the movement of avian influenza and other infectious diseases. J Wildl Dis. 2007;43(3):S55–S59. [Google Scholar]
- 80.Reed K.D., Melski J.W., Braham M.B. The detection of monkeypox in humans in the Western Hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 81.Van Borm S., Thomas I., Hanquet G. Highly pathogenic H1N1 influenza virus in smuggled Thai eagles, Belgium. Emerging Infect Dis. 2005;11(5):702–705. doi: 10.3201/eid1105.050211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tatem A.J., Hay S.I., Rogers D.J. Global traffic and disease vector dispersal. Proc Natl Acad Sci USA. 2006;103:6242–6247. doi: 10.1073/pnas.0508391103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tatem A.J., Rogers D.J., Hay S.I. Estimating the malaria risk of African mosquito movement by air travel. Malar J. 2006;5:57. doi: 10.1186/1475-2875-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kilpatrick A.M., Gluzberg Y., Burgett J. Quantitative risk assessment of the pathways by which West Nile virus could reach Hawaii. Ecohealth. 2004;1:205–209. [Google Scholar]
- 85.WHO Health conditions for travellers to Saudi Arabia for the pilgrimage to Mecca (Hajj) Wkly Epidemiol Rec. 2007;82(44):385–388. [PubMed] [Google Scholar]
- 86.Wilder-Smith A., Barkham T.M., Earnest A. Acquisition of W135 meningococcal carriage in Hajj pilgrims and transmission to household contacts: prospective study. Br Med J. 2002;325:365–366. doi: 10.1136/bmj.325.7360.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wilder-Smith A., Foo W., Earnest A. High risk of Mycobacterium tuberculosis infection during the Hajj pilgrimage. Trop Med Int Health. 2005;10(4):336–339. doi: 10.1111/j.1365-3156.2005.01395.x. [DOI] [PubMed] [Google Scholar]
- 88.Shaw M.T., Leggat P.A., Borwein S. Travelling to China for the Beijing 2008 Olympic and Paralympic games. Travel Med Infect Dis. 2007;5:365–373. doi: 10.1016/j.tmaid.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 89.Kozarsky P.E., Keystone J.S. Introduction to travel medicine. In: Keystone J.E., Kozarsky P.E., Freedman D.O., editors. Travel medicine. Mosby; Edinburgh (UK): 2004. pp. 1–3. [Google Scholar]
- 90.Steffen R. Travel medicine—prevention based on epidemiological data. Trans R Soc Trop Med Hyg. 1991;85:156–162. doi: 10.1016/0035-9203(91)90005-j. [DOI] [PubMed] [Google Scholar]
- 91.Wilson M.E. Travel and the emergence of infectious diseases. Emerging Infect Dis. 1995;1:39–46. doi: 10.3201/eid0102.950201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Freedman D.O., Kozarsky P.E., Weld L.H. GeoSentinel: the global emerging infections sentinel network of the International Society of Travel Medicine. J Travel Med. 1999;6(2):94–98. doi: 10.1111/j.1708-8305.1999.tb00839.x. [DOI] [PubMed] [Google Scholar]
- 93.Freedman D.O., Weld L.H., Kozarsky P.E. Spectrum of disease and relation to place of exposure among ill returned travelers. N Engl J Med. 2006;354(2):119–130. doi: 10.1056/NEJMoa051331. [DOI] [PubMed] [Google Scholar]
- 94.Schwartz E., Weld L.H., Wilder-Smith A. Seasonality, annual trends, and characteristics of dengue in ill returned travelers, 1997–2006. Emerg Infect Dis. 2008;14(7):1081–1088. doi: 10.3201/eid1407.071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ross K. Tracking the spread of infectious diseases. EMBO Rep. 2006;7(9):855–858. doi: 10.1038/sj.embor.7400797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Centers for Disease Control and Prevention (CDC) Cruise-ship associated Legionnaires disease, November 2003-May 2004. MMWR Morb Mortal Wkly Rep. 2005;54:1153–1155. [PubMed] [Google Scholar]
- 97.Kura F., Amemura-Maekawa J., Yagita K. Outbreak of Legionnaires' disease on a cruise ship linked to spa-bath filter stones contaminated with Legionella pneumophila serogroup 5. Epidemiol Infect. 2006;134:385–391. doi: 10.1017/S095026880500508X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.CDC Imported Lassa fever—New Jersey, 2004. MMWR Morb Mortal Wkly Rep. 2004;53(38):894–897. [PubMed] [Google Scholar]
- 99.Widdowson M.-A., Cramer E.H., Hadley L. Outbreaks of acute gastroenteritis on cruise ships and on land: identification of a predominant circulating strain of norovirus—United States 2002. J Infect Dis. 2004;90:27–36. doi: 10.1086/420888. [DOI] [PubMed] [Google Scholar]
- 100.Lopman B., Vennema H., Kohli E. Increase in viral gastroenteritis outbreaks in Europe and epidemic spread of new norovirus variant. Lancet. 2004;363:682–688. doi: 10.1016/S0140-6736(04)15641-9. [DOI] [PubMed] [Google Scholar]
- 101.Bull R.A., Tu E.T., McIver C.J. Emergence of a new norovirus genotype II.4 variant associated with global outbreaks of gastroenteritis. J Clin Microbiol. 2006;44(2):327–333. doi: 10.1128/JCM.44.2.327-333.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.CDC Severe acute respiratory syndrome—Singapore, 2003. MMWR Morb Mortal Wkly Rep. 2003;52(18):405–411. [PubMed] [Google Scholar]
- 103.CDC Trends in tuberculosis—United States, 2005. MMWR Morb Mortal Wkly Rep. 2006;55(11):305–308. [PubMed] [Google Scholar]
- 104.CDC Emergence of mycobacterium tuberculosis with extensive resistance to second-line drugs—worldwide, 2000–2004. MMWR Morb Mortal Wkly Rep. 2006;55(11):301–305. [PubMed] [Google Scholar]
- 105.CDC. Health advisory—Hepatitis A infections linked to children adopted from Ethiopia and their family contacts. July 18, 2007. Available at: http://www.cdc.gov/ncidod/diseases/hepatitis/a/HAHealthAdvisory.pdf. Accessed March 1, 2008.
- 106.Brotherton J.M., Delpech V.C., Gilbert G.L. A large outbreak of influenza A and B on a cruise ship causing widespread morbidity. Epidemiol Infect. 2003;130:263–271. doi: 10.1017/s0950268802008166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.CDC Exposure to mumps during air travel—United States, April 2006. MMWR Morb Mortal Wkly Rep. 2006;55(14):401–402. [PubMed] [Google Scholar]
- 108.CDC Update: multistate outbreak of mumps—United States, January 1–May 2, 2006. MMWR Morb Mortal Wkly Rep. 2006;55(20):559–563. [PubMed] [Google Scholar]
- 109.Centers for Disease Control and Prevention (CDC) Mumps outbreak at a summer camp in New York, 2005. MMWR Morb Mortal Wkly Rep. 2006;55(7):175–177. [PubMed] [Google Scholar]
- 110.CDC US-incurred costs of wild poliovirus infections in a camp with US-bound refugees—Kenya, 2006. MMWR Morb Mortal Wkly Rep. 2008;57(9):232–235. [PubMed] [Google Scholar]