Flexible bronchoscopy (FB) is one of the most common and useful procedures performed by chest clinicians, with > 500,000 procedures performed annually in the United States.1 FB is performed frequently in immunocompromised patients and in health-care settings where exposures to increasingly virulent and drug-resistant microorganisms may occur. Recently reported bronchoscopy-associated infections (BAIs) and pseudoinfections1, 2, 3, 4 potentially affected > 800 patients and included instances in which clinically significant infections and fatalities might have been related to the procedure. Such outbreaks have generated major concerns regarding the possibility of iatrogenic infections due to FB and underscore the importance of reappraising this problem and optimizing preventive practices.5, 6 The potential sequelae of BAI and pseudoinfection are enormous. In addition to the possible morbidity and mortality associated with true infections, such events require expenditure of considerable time and resources for the careful assessment of the vast majority of instances in which no infections or harm to patients have occurred.
For many reasons it is remarkable that true infection due to FB appears to be an uncommon event. During performance of the procedure, host defenses are bypassed routinely as, most often, the bronchoscope is passed through the upper airways, which are invariably colonized by a myriad of potential pathogens. The patient’s cough and other protective reflexes are attenuated purposefully with a variety of medications, ensuring aspiration of microbes, and these and other solutions are instilled routinely into progressively more distal airways, potentially soiling peripheral lung parenchyma. Normal mucosal barriers to infection are disrupted during lung biopsies and an increasing array of interventional procedures. With the latter, lengthier procedure times may increase opportunities for hematogenous as well as local infections. Simultaneously, the progressive miniaturization of bronchoscopes and accessories introduces potential difficulties in effective cleaning and disinfection of these structurally complex instruments. In addition, the prevalence of HIV, multidrug-resistant tuberculosis, hepatitis B, and newly emerging pathogens such as the coronavirus agent of severe acute respiratory syndrome (SARS-CoV) heighten concerns about the relative risks posed by bronchoscopy.
Prakash7 has described several scenarios in which the bronchoscope may propagate infection. These include intrapulmonary or extrapulmonary spread of infection within the same patient, pathogen transmission from one patient to another, and the spread of infection from the patient to participating medical personnel.8, 9 Each of these possibilities poses unique challenges in implementing effective infection control practices. Other inherent difficulties also relate to meaningful definitions of infection or pseudoinfection, their accurate recognition in individual patients, appropriate monitoring of cleaning and disinfection of bronchoscopy instruments, laboratory maintenance, bronchoscopy staff education, and longitudinal monitoring and documentation of effective practices.
The exact incidence of infections caused by FB is unknown. Their apparent low frequency might reflect a truly uncommon occurrence. Alternatively, such events might be systematically underrecognized because of multiple factors. For example, new infection (patient-to-patient) induced by the procedure may be easily masked by the primary signs and symptoms for which FB was performed. In an individual patient, interpretation of a positive culture from a bronchoscopy specimen and the precise differentiation of colonization or infection from instrument contamination may be extraordinarily difficult. This dilemma may be especially problematic in the sickest patients. An individual bronchoscopist may not have access to the comprehensive experience of a bronchoscopy suite in order to discern whether telltale patterns of microbiologic isolates exist among patients undergoing bronchoscopy, and whether these are inappropriate for the patient’s clinical presentation. Other reasons for underrecognition of endemic infection may include inadequate surveillance of outpatient procedures, asymptomatic infection or prolonged incubation period prior to the development of the symptoms, and fear of medicolegal ramifications of reporting/publishing device-related transmission of infection.
At an institutional level, recognition of BAI requires regular systematic review of all aspects of the procedure, rigorous adherence, reinforcement and monitoring of well-established infection control practices, and established mechanisms for timely objective epidemiologic evaluation whenever a potential problem occurs. Such resources (and the funding needed to support such efforts) may not be consistently available at many centers.
While prevention of infection caused by FB is an obvious concern for all chest clinicians, several observations suggest that there is a widespread unfamiliarity with practice guidelines and that potentially consequential variations in primary and secondary prevention of BAI and pseudoinfections may occur. Although reprocessing guidelines exist, their focal point has been on GI endoscopes. Guidelines addressing bronchoscope reprocessing, for the most part, are derived from those dealing with the GI endoscope. Guidelines for reprocessing of flexible endoscopes, including bronchoscopes, have been promulgated by the Association for Professionals in Infection Control, Association of PeriOperative Registered Nurses, and the British Thoracic Society, among others.10, 11, 12, 13 Data suggest that these guidelines have not been effectively disseminated and/or followed by many clinicians. A comprehensive survey14 of bronchoscopists in the United Kingdom revealed that national guidelines for bronchoscope reprocessing were not followed consistently. Minimum disinfection times recommended before and after routine bronchoscopies were often (35%) not achieved, and no disinfection was carried out in 34% of medical centers before emergency bronchoscopies and in 19% of units after suspected cases of tuberculosis. Adequate rinsing of the bronchoscope with sterile or filtered water was not carried out by 43% of units. Staff rarely (7%) wore recommended protective clothing during bronchoscopy. In another survey involving US bronchoscopists, nearly two thirds of respondents, including medical directors of bronchoscopy suites, acknowledged that they were unfamiliar with national reprocessing recommendations. Interestingly, this survey was carried out just following a widely publicized Pseudomonas outbreak involving bronchoscopes.15 Moreover, many (39%) were not aware of the approaches to reprocessing at their own institutions! Although specific data are unavailable, anecdotal observations suggest that bronchoscope reprocessing techniques are inadequately emphasized during the training of many pulmonary fellows, and many bronchoscopists may take it for granted that the instruments they use are safe.
In order to help organize and disseminate information regarding validated practices for bronchoscope reprocessing and infection prevention and control, this committee was jointly convened by the American College of Chest Physicians and the American Association for Bronchology. In this document, we summarize data from the literature regarding the extent of the problem and specific risk factors (generally discovered during the comprehensive investigation of outbreaks). We also present principles related to the specific maintenance and disinfection of instruments together with provisional recommendations. Since many important factors extend beyond the purview of individual bronchoscopists and relate to the milieu in which the procedure is performed, we also address institutional aspects of the infrastructure and processes essential to bronchoscopy infection prevention and control.
The readers of this article should recognize that this is a consensus statement and does not represent evidence-based recommendations. Because of the relative absence of prospective investigations in this area, most of these recommendations are based on clinical experience and consensus opinion, rather than the higher grades of evidence generally required for true clinical practice guidelines. Accordingly, our recommendations represent an evolving perspective that provides numerous important opportunities for clinical outcomes research and that will require future critical refinement. In addition to general principles, the implementation of effective programs is institution specific and requires local modification through continued active dialogue among bronchoscopists, staff, and infection control teams.16 We would also like to highlight that these recommendations are not intended to ensure the inactivation or removal of causative agents of transmissible spongiform encephalopathies (prion proteins).
Literature Review
The true incidence of BAI is unknown, due in part to episodic reporting and lack of specific monitoring or institutional surveillance of such events. A review of the English-language literature from 1970 to 2003 revealed over three hundred references to endoscopy-related transmissions of infection. Sixty-two were specific to FB. Despite published guidelines for the reprocessing of endoscopes, reports of “true infections” and “pseudoinfections” or “pseudoepidemics” related to FB seem to be increasing. Most citations refer to pseudoinfections and pseudoepidemics as the isolation of organisms in bronchoscopy specimens due to colonization or contamination of the bronchoscope rather than true patient-to-patient transmission (“true infection”) [Table 1 ].1, 2, 3, 4, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72 The majority of reports are descriptive, with few case-controlled investigations. Typically, episodes can be traced to inadequate cleaning techniques or disinfection processes. Occasionally, the infection is due to contamination of water supplies, reprocessing equipment, or accessories such as stopcocks or cleaning brushes. Defects within the bronchoscope itself (suction valve port) have also been implicated in transmission of organisms from patient to patient (Table 2 ).17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 69, 70, 71, 73, 74
Table 1.
Bronchoscopy-Related Pseudoinfections*
| Organisms | Total Reports, No. | Affected Patients, No. | Reference(s) |
|---|---|---|---|
| Bacteria | |||
| Proteus sp | 2 | 8 | 3, 43 |
| Bacillus sp | 2 | 23 | 44, 45 |
| Serratia marcescens | 5 | 33†, ‡ | 40, 42, 46, 47, 48 |
| Pseudomonas aeruginosa | 8 | 220†, § | 1, 2, 48, 49, 66, 67, 68, 69, 70, 71 |
| Legionella pneumophilia | 1 | 5 | 38 |
| Klebsiella pneumonia | 2 | 19 | 3, 48 |
| Methylobacterium mesophilicum | 2 | 25 | 27, 34 |
| Morganella morganii | 1 | 1 | 3 |
| Fungi | |||
| Aureobasidium sp | 1 | 9 | 23 |
| Rhodotorula rubra | 3 | 56 | 21, 28, 41 |
| Blastomyces dermatitidis | 1 | 2 | 17 |
| Trichosporon cutaneum | 1 | 8 | 4, 5, 29 |
| Penicillium sp | 1 | 8 | 29 |
| Cladosporium sp | 1 | 1 | 29 |
| Phialospora sp | 1 | 1 | 29 |
| Mycobacteria | |||
| Mycobacterium tuberculosis | 9 | 24†, ‡ | 18, 20, 31, 32, 49, 50, 51, 52, 53 |
| Mycobacterium avium-intracellulare | 4 | 11†, ‡ | 20, 22, 39, 49 |
| Mycobacterium xenopi | 2 | 13†, ‱ | 25, 35 |
| Mycobacterium chelonae | 15 | 304†, ‡, ‱ | 19, 24, 25, 26, 27, 33, 54, 55, 56, 57, 58, 59, 60, 61, 62 |
| Mycobacterium fortuitum | 2 | 4†, ‱ | 25, 61 |
| Mycobacterium gordonae | 3 | 59†, ¶ | 24, 30, 36, 72 |
| Mycobacterium abscessus | 2 | 33 | 63, 64 |
| Various nontuberculous mycobacteria | 3 | 17† | 37, 60, 65 |
Modified from Culver et al.6
The precise number of pseudoinfections is not specified. The number of affected cases are estimated from the excess positive bronchoscopy culture results compared to control periods.2, 48, 60, 69
One report69 described 35 excess cases but did not differentiate the proportion of pseudoinfections and true infections. The same outbreak is described elsewhere.49, 70
One report25 described 15 patients with M xenopi, M chelonae, and/or M fortuitum pseudoinfections but did not specify the numbers of each.
Six patients with culture-positive M gordonae and two additional patients with smear-positive acid-fast bacilli only.36.
Table 2.
Major Sources of Contamination
| Source of Contamination | Reference(s) |
|---|---|
| Ineffective cleaning | |
| Inadequate cleaning | 17, 71 |
| Damaged internal channel | 18, 19 |
| Poorly mated internal components | 74 |
| Reusable suction valve | 20 |
| Suction channel | 20, 21 |
| Biopsy port | 71 |
| Accessories | |
| Sample collection tubing | 22 |
| Reused stopcocks for BAL fluid aspiration | 23 |
| Contaminated reprocessing equipment | |
| Automated washer | 69 |
| Rinsing tank | 24 |
| Tubing | 25 |
| Filter | 26 |
| Biofilm in reprocessor | 27, 69 |
| Cleaning brushes | 28 |
| Instilled solutions | |
| Topical anesthesia (cocaine) | 29 |
| Green dye (additive to anesthetic) | 30 |
| Atomizer | 31 |
| Disinfectant | |
| Inadequate activity | 32 |
| Incorrect disinfectant concentration dispensed by automated reprocessor | 73 |
| Contaminated glutaraldehyde | 23, 33 |
| Improper connector to reprocessor | 70 |
| Recontamination after disinfection | |
| Rinsing tap water (hospital supply) | 34, 35, 36, 37 |
| Contaminated water filters | 38, 39 |
| Reuse of “sterile water” for rinsing | 40 |
| Reassembly of valves prior to storage | 41 |
| Storage in coiled position/in cases: | 42 |
Only 18 publications have suggested “true” infection: the transmission of a specific pathogen associated with a clinically significant illness in a patient undergoing FB (Table 3 ).1, 2, 18, 19, 20, 31, 32, 42, 46, 49, 53, 66, 67, 68, 69, 70, 73, 75, 76 Two of the accounts were reported in 2003, involving 33 patients with three possible deaths.1, 2 The use of DNA probes was helpful in identifying patterns of transmission. In these reports, the FBs were cleaned using automated endoscope reprocessors (AERs) and the recommendations for disinfection of endoscopic equipment were strictly followed.
Table 3.
Bronchoscopy-Related True Infections*
| Organism | Mechanism | Outcome | Reference(s) | Year |
|---|---|---|---|---|
| S marcescens | Inadequate cleaning and disinfectant (alcohol) | Three true infections, 1 probable death, and 103 pseudoinfections | 46 | 1975 |
| Psudomonas sp | Suction attachment not detached prior to attempted disinfection | One true infection and five pseudoinfections | 66 | 1978 |
| Burkholderia pseudomallei† | Unknown (rigid bronchscope) | Causality tenuous | 67 | 1979 |
| P aeruginosa | Inadequate disinfectant (povidone-iodine) Insufficient disinfection time (5 min); reintroduction of cleaning brush after disinfection | One true infection and 10 pseudoinfections | 68 | 1982 |
| M tuberculosis | Inadequate disinfectant (povidone-iodine) | One patient each with true infection and pseudoinfection | 32 | 1983 |
| M chelonae | Damaged suction channel prevented adequate disinfection | Two true infections and 70 pseudoinfections | 19 | 1983 |
| M tuberculosis | Use of nondisposable suction valves | One patient acquired active TB, and two pseudoinfections each with M tuberculosis and MAI | 20 | 1989 |
| S marcescens | Regimen inadequate at multiple steps | Six cases (five possible true infections); causality tenuous | 42 | 1993 |
| M tuberculosis | Multiple deviations from APIC guidelines | One patient acquired active TB | 75 | 1997 |
| Multidrug-resistant M tuberculosis | Multiple deviations from APIC guidelines | One patient each with active TB (died due to TB) and skin test conversion, and one patient with pseudoinfection | 53 | 1997 |
| P aeruginosa | Contaminated AER | Number of true infections and pseudoinfections not specified | 69 | 1997 |
| P aeruginosa | Contaminated AER/not routinely serviced or cleaned | Two or more of eight total patients with true infection | 76 | 2000 |
| P aeruginosa | Inadequate disinfectant concentrations from automatic dispenser | Six ICU patients with colonization | 73 | 2001 |
| M tuberculosis | Reuse of lidocaine atomizers (?) | One patient each with lung and ocular TB | 31 | 2001 |
| P aeruginosa | Wrong connectors used for lumen disinfection by AER | Three true infections, and 14 pseudoinfections | 70 | 2001 |
| M tuberculosis | Punctured sheath/leak test not done | Two patients acquired active infection, and six patients had pseudoinfection | 18 | 2002 |
| P aeruginosa | Unclear (?) loose biopsy port cap prevented cleaning and disinfection | Twenty to 43 possible infections; pneumonias, sinusitis, bacteremias; and three possible deaths | 2 | 2003 |
| P aeruginosa | Same as Srinivasan (above) | Probable pneumonia; details scarce and/or causality tenuous in these reports. | 1 | 2003 |
APIC = Association for Professionals in Infection Control and Epidemiology; MAI = M avium-intracellulare. Used with permission from Culver et al.6
Formerly known as Pseudomonas pseudomallei.
The most common organisms implicated in bronchoscopy-related pseudoinfections include bacterial pathogens such as P aeruginosa or S marcescens, contagious and noncontagious mycobacteria, and environmental fungi4, (Table 1). Pseudomonas and Serratia species, and M tuberculosis are among the most common organisms reported in true infections (Table 3).
Viruses can be nominally classified into two groups based on the presence or absence of a lipid bilayer envelope. The latter provides a barrier to external digestive enzymes and therefore more resistance to disinfection. Patient-to-patient transmission of viral infections during bronchoscopy has not been reported, however. Interestingly, transmission of hepatitis B as well as hepatitis C has been previously reported in the setting of inadequately disinfected gastroendoscopes.77 While HIV is readily inactivated using standard disinfection techniques, HIV-RNA can be isolated from the bronchoscope after its use in patients with the virus. In addition, intact papilloma virus DNA can be isolated in the vapor plume from laser photoresection.78, 79, 80, 81 These observations further illustrate the potential for viral transmission during the FB procedure.
Reports of the transmission of airborne infections such as M tuberculosis or influenza to health-care providers suggest that there may be an additional occupational risk for bronchoscopy personnel. Recently, the outbreak of SARS-CoV afflicting health-care providers during the severe acute respiratory syndrome epidemic illustrated the risks of communicable airborne diseases. New evidence suggests that although transmission appears to occur from infectious droplets, there are occasional episodes in which airborne transmission cannot be excluded, including “aerosol”-generating procedures such as intubation, bilevel positive airway pressure ventilation, and bronchoscopy.82, 83, 84, 85 Cases of direct transmission of infectious diseases to staff during bronchoscopy are apparently rare. However, the increased incidence of latent tuberculosis among respiratory therapists and pulmonary fellows compared with other health-care providers suggests that there may be an increased risk to health-care providers who are involved in bronchoscopy procedures.86, 87
Recommendations
We reemphasize that our review of the literature reveals that the infrequent and sporadic recognition of bronchoscopy-related infection hinders development of evidence-based guidelines for this topic. However, review of the available literature suggests that all episodes are preventable. Many of the following recommendations are based on accumulated clinical experience and expert opinions involving several disciplines. The following recommendations have taken into account guidelines published by other societies and organizations.6, 10, 11, 12, 88, 89 Review of the literature also points out several flaws in current practices; we highlight measures to avoid such mistakes.
General Recommendations
All bronchoscopy personnel, including technical staff, physicians, and fellows should be educated in infection control practices including “sharp precautions,” all reprocessing steps, and material handling. All bronchoscopy personnel should be vaccinated against influenza as well as hepatitis B and should undergo a surveillance purified protein derivative test every 6 months as long as they are not tested positive at a prior testing interval.
All bronchoscopes should be properly maintained according to the recommendations of the manufacturer. The user manual should be easily accessible and provide information on the use of specific bronchoscope models. Use of bronchoscopes that are fully immersible and have disposable suction and biopsy valves is highly recommended. Nonimmersible bronchoscopes and those with a reuseable valve should be replaced as soon as possible.
There are no reports of subacute bacterial endocarditis or that of joint infections resulting from a bronchoscopy procedure. However, prophylactic antibiotic therapy should be considered for patients at high risk for these complications (Table 4, Table 5 ).7, 90 Patients with joint replacement within the past 2 years, history of previous prosthetic joint infection, inflammatory arthropathy, hemophilia, malnutrition, insulin-dependent diabetes mellitus, and immunocompromised status are susceptible to hematogenous total joint infection and, therefore, may benefit from prophylactic antibiotics.91 In view of the lack of data, application of such a practice could be recommended only on an individual basis.
Table 4.
Factors Associated With Bacterial Endocarditis*
| Patients susceptible to bacterial endocarditis |
| Individual factors† |
| History of bacterial endocarditis |
| Prosthetic heart valves including bioprosthetic and homograft valves |
| Cyanotic congenital heart diseases |
| Rheumatic valve disease |
| Hypertrophic cardiomyopathy |
| Mitral valve prolapse with regurgitation |
| Surgically corrected systemic-pulmonary shunts or conduits |
| Immunocompromised patients with lower respiratory tract infection |
| Procedural factors |
| Diagnostic tests that would induce mucosal trauma (brushing, endobronchial or bronchoscopic lung biopsy, transbronchial needle aspiration) |
| Therapeutic bronchoscopy causing mucosal trauma: laser photoresection, endobronchial electrosurgery, balloon bronchoplasty, stent placement |
| Patients susceptible to hematogenous total joint infection |
| Joint replacement within past 2 yr |
| Previous prosthetic joint infection |
| Inflammatory arthropathy |
| Immunocompromized patient |
| Hemophilia |
| Malnutrition |
| Insulin-dependent diabetes mellitus |
There are no reports of bacterial endocarditis or joint infections caused by the FB. Prophylaxis against these conditions should be considered in susceptible patients on individual basis.
Many center administer antibiotics during or immediately after bronchoscopic lung biopsy in lung transplant recipients to prevent BAI. To date, there are no data to support such practice.
Table 5.
FDA and CDC Public Health Advisory*
| 1. Follow the instructions for cleaning the endoscope provided by the manufacturer. |
| 2. Check with the manufacturers of the endoscope to determine whether the endoscope can be reprocessed in an AER. Also, check with the manufacturer to determine whether any specific steps are required prior to a specific type of endoscope being reprocessed in an AER. |
| 3. Compare the reprocessing instructions provided by the endoscope and AER manufacturers and resolve any conflicting recommendations. |
| 4. In the absence of specific technical instructions on automated reprocessing for each model of endoscope used in the facility, be sure to follow the manual reprocessing instructions of the endoscope manufacturer as well as the recommendations of the manufacturer of the chemical germicides at the facility. |
| 5. Regardless of manual reprocessing of the endoscope or use of an AER, consider incorporating a final drying step in the protocol. |
| 6. Ascertain that the instructions of the facility for preparing endoscopes for patient contact are appropriate and that the staff is adhering to these instructions. |
| 7. Provide comprehensive and intensive training for all staff assigned to reprocessing endoscopes to ensure that they understand the importance of proper reprocessing of all endoscopes used in the facility. |
| 8. Implement a comprehensive quality control program. |
Infections from endoscopes inadequately reprocessed by an AER system.
Infection control precautions (“standard precautions”) should be mandated for every procedure and should include full barrier clothing (gown, gloves, mask, and eye shields) and needle precautions for the bronchoscopist (Fig 1 ). Use of a fit-tested N95 particulate respirator by the bronchoscopist or a higher-grade respiratory precaution are recommended when mycobacterial infection is suspected. For highly contagious agents (eg, SARS-CoV), a power air-purifying respirator (PAPR) hood should be used (Fig 2 ).82, 83, 84, 85, 92 Prior to their use, medical clearance of proper fit-testing and familiarity with these masks is essential for all bronchoscopy personnel. There must be compelling indications for bronchoscopy in patients suspected of having highly contagious infections (see “Patient Selection”). When bronchoscopy is to be performed in such patients, it should be performed in a negative pressure-ventilated room, if one is available. These patients should also be required to wear a surgical mask so that the risk of dissemination of airborne infection can be minimized (Fig 3 ).
Figure 1.
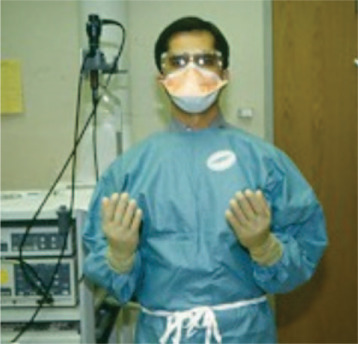
Standard precautions for bronchoscopy, showing bronchoscopist with gown, gloves, mask, and eye gear.
Figure 2.
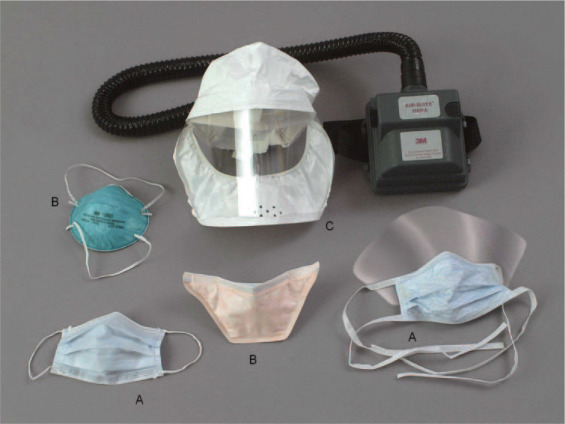
Facial masks used during bronchoscopy. Left, A, and right, A: The surgical mask helps prevent spread of droplet infection from the surgeon into the open surgical wounds. Left, B, and center, B: N95 particulate respirators help prevent spread of airborne infection from patient to the bronchoscopy personnel, and filter 95% of particles > 0.3 μm. Prefitting is required. Top, C: PAFR hood.
Figure 3.

Bronchoscopy personnel wearing a PAPR. The respirator circulates filter air through the hood.
Administration of adequate topical anesthetic agents or cough suppressants is recommended to minimize coughing resulting in dissemination of airborne pathogens. Sharp metal objects or needles should not be used to remove biopsy specimen from the forceps, because this may increase the risk of transmission of blood borne pathogens.77
A procedure log should be kept that includes the patient’s name and medical record number, the bronchoscope used, the name of the bronchoscopist(s), and the automated endoscope reprocessor (AER), if used, to assist in outbreak investigation. In addition, a maintenance record must be maintained for each bronchoscope and AER in use.
Patient Selection
To prevent contamination of the instrument and aerosolization of highly pathogenic organisms such as M tuberculosis or SARS-CoV,82, 83, 84, 85 every effort should be made to confirm the diagnosis by other techniques. Bronchoscopy for the purpose of diagnosis should be postponed until at least three sputum or gastric aspirate smear findings are negative for acid-fast bacilli in patients suspected of having pulmonary tuberculosis.93
Bronchoscopy Suite
The bronchoscopy suite as well as the postprocedure recovery areas should have engineering controls that will allow ≥ 12 air exchanges per hour for new construction as of year 2001 or 6 exchanges per hour for construction before 2001 and be under negative pressure.94 The air must be either discharged directly to the outside or to a monitored high-efficiency particulate air (HEPA) filtration system before recirculation. The adequacy of these air exchanges should be monitored on a regular basis with appropriate documentation by the engineering and/or infection control personnel of the institution. Designated “clean” and “dirty” (reprocessing) areas should be maintained in the bronchoscopy suite to separate used instruments from clean ones. Bronchoscopy supervisory staff should strictly implement and monitor such practices. No special preparation is required prior to the reuse of a well-ventilated facility after performing a procedure in a patient suspected of having infection with a virulent, airborne pathogen. All furniture and equipment used during the procedure should be wiped down following each procedure using hospital-approved cleaning solutions. The bronchoscopy suite floor should also be wiped with hospital approved cleaning solutions at the end of each working day, following the procedure appropriate for the soiling. If a procedure is being performed in a nondesignated area, a portable HEPA filter should be used when appropriate (high suspicion for airborne pathogens).
Reprocessing of the Bronchoscope
The sequence of reprocessing should be as follows.
Mechanical Cleaning
Mechanical cleaning begins immediately after the procedure to prevent drying or hardening of organic debris.10, 95 Personal protective equipment (gown, gloves, mask, and eye shields) must be worn while processing the contaminated bronchoscope. The outside of the bronchoscope should be wiped with a detergent-soaked gauze piece and detergent solution suctioned through the channel. All suction ports or biopsy attachments should be detached prior to leak testing, further cleaning, and inspecting the instrument for any damage. All disposable items must be discarded. The instrument should then be pressurized with a leak tester to detect any damage that could have occurred during the procedure. The bronchoscope is then fully immersed in water to confirm or to rule out any air leaks. Flexion and extension of the bending section should be performed under water to detect any minute leaks that may not be visualized otherwise. The presence of a leak indicates a breach in the integrity of the external or the luminal surface of the instrument. Such puncture sites and breaches will develop concretions of debris (blood, mucus) that cannot be disinfected. Any instrument with a positive leak test result should not be reused until fully repaired (Fig 4 ). Only after ensuring that there are no air leaks should an enzymatic cleaner be added to the water. Next, the bronchoscope should be soaked in cleaning solution for approximately 5 min. The external surface of the bronchoscope should be cleaned/wiped manually with an enzymatic detergent. Detergent solution or water is then flushed through all ports to loosen organic debris. The detergent preparation should not be reused. A cleaning brush of an appropriate size then should be passed multiple times through all ports of the instrument. Meticulous cleaning alone achieves a 3.5-to 4-log reduction in the organism load.96 After brushing, channels should be flushed again to remove all loosened material. Cleaning brushes should either be for single use or should receive mechanical cleaning followed by sterilization or high-level disinfection after each use.28 Finally the instrument and its channel should be rinsed with water to remove the enzymatic cleaner and prepare it for disinfection.
Figure 4.

Positive leak test result. Air bubbles emitting from the surface of the bronchoscope indicate a breach in its exterior.
Following a procedure performed in a nondesignated area (ie, in the ICU), the external surface of the bronchoscope should be wiped and its channel flushed with water. The instrument should then be placed in a water-tight polyethylene bag and returned to the bronchoscopy suite as soon as possible for a formal reprocessing. The bronchoscope reprocessing should not be performed in the procedure area.
The decision to process a bronchoscope with high-level disinfection vs sterilization is based on a classification system for medical devices that divides them by the risk of infection transmission. The Spaulding classification97 groups medical devices into three groups, allowing determination of the level of disinfection for each. The following is a description of each group.
Critical Devices
Devices that normally enter sterile tissue or the vascular system (eg, cardiac catheters, biopsy forceps). They should undergo sterilization, defined as the destruction of all microbial life.
Semicritical Devices
Devices that come in contact with intact mucous membranes and do not normally penetrate sterile tissue (eg, endoscopes). They should undergo high-level disinfection, defined as the destruction of all vegetative microorganisms, mycobacteria, viruses, fungal spores, and some, but not all, bacterial spores.
Noncritical Devices
Devices that do not ordinarily touch the patient or touch only intact skin (eg, stethoscopes or patient carts). These items may be cleaned by low-level disinfection.
Disinfection
According to the above scheme, the FB is a semicritical device, as it seldom comes in contact with breached mucosa or open surgical wounds.98 A minimum of high-level disinfection is required before its reuse. Sterilization with ethylene oxide gas, while highly effective, is likely to be associated with unacceptable delays between procedures due to the prolonged sterilization process and the need to aerate the instrument afterwards.
Disinfection is carried out either manually or by using an AER. Activated alkaline gluteraldehyde, peracetic acid, and orthophthaldehyde are the acceptable chemicals for disinfection.99, 100, 101 A complete list of approved disinfectant formulations and maximum reuse time can be found at www.fda.gov/cdrh/ode/germlab.html.89
All currently approved agents are effective high-level disinfectants in experimental conditions, by definition achieving a 6-log reduction in mycobacterial burden.95 The choice of specific disinfectant can vary by institution, depending on cost, volume of procedures, availability of AERs, and the number of bronchoscopes in use. Disinfection for 20 min in 2% alkaline gluteraldehyde at 20°C (“20–2-20”) provides adequate disinfection if cleaning with detergent precedes disinfection.7, 96, 98, 100 Meticulous cleaning and assiduous adherence to an appropriate protocol are much more important determinants of successful disinfection than the choice of a specific approved agent.100 It is important to note that dilution of disinfectants occurs over time.103 Depending on the formulation, the solutions may be reused for 14 to 28 days.89 Potency of the solution should be periodically tested with commercially available test kits and documented for proper record keeping.
Gluteraldehyde solution should be discarded after 14 days or 20 cycles and should not be used if the concentration is < 2%; the solution should be tested at the beginning of every day of use. Even if the concentration is adequate, the disinfectant should not be used longer than the recommended period, since the aldehyde moeity will polymerize over time attenuating its microbiocidal activity.102 If the AER use is the primary mode of disinfection, the advisory issued by the US Food and Drug Administration (FDA) and Centers for Disease Control and Prevention (CDC) should be followed closely (Table 4).89 It is essential to ensure compatibility between bronchoscopes and AERs.49, 65, 70 Chemical and biological indicators (sporicidal tests) should be strictly followed during the use of AERs. Automated reprocessors can be used for endoscopes other than FB, provided all AER users adhere to acceptable reprocessing protocol. All AERs should be properly maintained according to the recommendations of the manufacturer. User manuals should be easily accessible and provide information on which specific bronchoscope models have been tested for compatibility with the AER.
Postprocessing Procedure
Since many pathogens isolated from the bronchoscopes are from recontamination after disinfection,34, 35, 36, 37, 38, 39, 40 it is imperative to properly dry and store the instrument. Following disinfection or sterilization, thorough rinsing of the internal channel with sterile or filtered tap water is essential to prevent toxic effects of residual chemicals. Ideally, the instrument is dried by purging the channel with 70% alcohol and compressed air.97
Proper storage of the bronchoscope is an important step to prevent pathogen growth. Flexible bronchoscopes should be hung vertically without valves attached, in a roomy cabinet with adequate ventilation to prevent moisture accumulation. An additional step to decrease moisture is to place the bronchoscope in a drying cabinet that utilizes a desiccant to reduce relative humidity. The instrument should not be stored in its carrying case, which should be used only for long-distance transportation. Since the carrying case itself cannot be disinfected, it is unsuitable to maintain the bronchoscope in a “patient-ready” condition. All instruments transported in the case must be reprocessed before and after being placed in the case. For procedures performed at locations other than the bronchoscopy suite, a sterile sealed polyethylene bag should be used for transportation of the disinfected instrument.
Bronchoscopic Accessories
All nondisposable bronchoscopic accessories (such as forceps) that breach the bronchial mucosa require sterilization following a thorough mechanical cleaning.20, 23 Because they cannot be properly sterilized, needles used for aspiration biopsy are for single-patient-use only. All ancillary equipment (atomizers, filters, washers) must be cleaned and maintained according to the recommendation of the manufacturer. Reuse of atomizers between patients is unacceptable.31 Multiuse vials (eg, canisters of benzocaine) used for topical anesthesia should be wiped down with a hospital-approved disinfectant between use.
Rigid Bronchoscope
The rigid bronchoscope and ancillary equipment are made of durable steel or other metals. They can be steam-sterilized using standard autoclave methods of sterilization. Therefore, the potential for a rigid bronchoscope to be a vector of infection is minimal to nonexistent.
Secondary Measures of Infection Prevention
Secondary measures of infection prevention are often overlooked but become increasingly important for infection control during FB since total interdiction of pathogen transmission is impossible. Factors that increase the propensity for infection to be spread by bronchoscopy include increasing frequency of the procedure, duration and complexity of the procedure, the expanding population of immunosuppressed patients, economic pressures, and the hardiness of the pathogens. A review of prior outbreaks suggests that a large proportion of cases in each instance might have been prevented with earlier recognition of the problem. The mainstay of recognition, therefore, is effective pathogen surveillance. Each institution should develop bronchoscopy-specific protocols for surveillance for microbiological isolates. Input from bronchoscopists, infection control specialists, and microbiologists is ideal. Relying solely on individual practitioners to quickly identify trends in isolate patterns will prolong outbreaks, especially for users of shared facilities.
Because the diagnostic bronchoscopy is often performed to evaluate patients with fever, abnormal radiographic findings, and/or other signs of possible infection, it is not always possible to conclude with confidence that FB has caused an infection since these clinical indexes may wax and wane. If either a “true” or a “pseudo” infection is encountered, the bronchoscopy team must inform the institutional infection control officer, the bronchoscope manufacturer, the state health department, the FDA MedWatch program, and the CDC, as well as the patient(s) and referring physicians. If contamination is suspected, the instrument must be removed from service immediately and an investigation begun by culturing parts of the bronchoscope, hospital tap water, and reprocessing equipment. On the basis of this initial assessment, the infection control team and bronchoscopy personnel should proceed as needed to assess and ameliorate any breach in infection control practices. The use of routine environmental microbiological testing of bronchoscope for quality assurance has not yet been established.
Conclusions
Spread of infection during FB is underrecognized and underreported. Unless proper infection control practices are observed, the increasing numbers of procedures performed are likely to be associated with more frequent episodes of infections attributed to bronchoscopy. Even though the reported incidence is quite low, lethal outcomes can result. Prevention of BAI requires increased vigilance by physicians, assiduous implementation of reprocessing protocols, and closer collaboration between bronchoscopy personnel, infection control practitioners, microbiology laboratories, and instrument manufacturers. Formalized institutional monitoring of isolate patterns and epidemiologic analysis may aid in the early detection of potential epidemics. The use of molecular biology techniques such as DNA fingerprinting can help confirm such occurrences. Finally, future innovations in bronchoscope design will require attention to the principles of infection control. The bronchoscopist, health-care providers involved in bronchoscopy, and equipment manufacturers should actively continue to develop techniques and systems to prevent infections from FB. The following definitions and discussion are useful.104
Definitions
Critical Devices
Devices that normally enter sterile tissue or the vascular system (eg, cardiac catheters, biopsy forceps).
Semicritical Devices
Devices that come in contact with intact mucous membranes and do not normally penetrate sterile tissue (eg, endoscopes).
Noncritical Devices
Devices that do not ordinarily touch the patient or touch only intact skin (eg, stethoscopes or patient carts).
True Infection
Transmission of organisms during bronchoscopy causing new related illness in the patient under going the procedure.
Pseudoinfection and Pseudoepidemic
Isolation of transmitted organisms in the bronchoscopy specimens obtained from the patient or patients, without evidence of specific infection.
Low-Level Disinfection
Process that inactivates most bacteria, some viruses, and some fungi.
Intermediate-Level Disinfection
Process that inactivates M tuberculosis, vegetative bacteria, most viruses, and fungi
High-Level Disinfection
Process that achieves 6-log reduction in mycobacterial burden.
Sterilization
Complete elimination of all forms of microbial life, including bacterial and fungal spores.
Recommendations for Precleaning, Disinfection, and Postprocessing of the FB
The following prerequisites are required: (1) proper education and training, and (2) personal protective equipment must be worn.
Precleaning
Immediately After Use (at the Bedside)
(1) Flush water or saline solution through the channel for 20 s, making sure that the distal tip of the scope does not rest in the fluid without suction being applied; (2) wipe external surface of scope with wet gauze to remove any loose debris; and (3) place contaminated scope loosely in a sealed water-tight bag labeled biohazard, for transportation to the cleaning area, if necessary.
Cleaning, Disinfection, and Preparation
The following procedures should be followed: (1) Immediately after use (at beside), flush water or saline through the channel for 20 s making sure that the distal tip of the scope does not rest in the fluid without applying suction. (2) Transport the instrument to a processing area as soon as possible to avoid drying of debris over the instrument. (3) With proper personal protective equipment worn, remove the instrument from the bag and place it in the basin for precleaning after placing water-tight caps on to protect electrical components. (4) Remove all disposable parts (suction/biopsy valve). (5) Perform leak test before immersing the instrument in water. (6) Any instrument that fails the leak test must be removed from service until repaired. (7) Add enzymatic cleaner to the water and soak the instrument for 5 min. (8) Using the enzymatic solution, wipe external surfaces with wet gauze and flush the suction channel. (9) Insert an appropriate-size cleaning brush through the channel of the instrument and brush all ports until there is no more visible debris being removed from the instrument. Flush the channel again to remove all loosened material. (10) Drain the enzymatic solution from the basin. (11) Rinse all internal as well as external surfaces with water to prepare the instrument for disinfection.
High-Level Disinfection
For high-level disinfection, do the following: (1) Place the bronchoscope in either an AER or a basin used for manual disinfection. (2) Use only detergents and FDA-cleared disinfectants that are compatible with the bronchoscope as well as the reprocessor. Minimum effective concentration of the disinfectant solution must be checked with each cycle using the available test strips. (3) While using the AER, ascertain that proper connections are made with the internal channel of the instrument for the flushing of the disinfectant. (4) The bronchoscope is fully immersed in the disinfectant solution, exposing all surfaces to the solution for the proper time required. With the manual system, the channel is filled with the disinfectant using a syringe containing the solution.
Postprocessing Procedure
The following steps should be followed: (1) Once the proper time for the is met for high-level disinfection, the bronchoscope and its channel are rinsed with either sterile or filtered tap water according to the recommendations disinfectant supplier. (2) Proper drying of the channel is accomplished by purging 70% alcohol with forced air. (3) Remove the water-tight caps from the instrument and hang vertically in a storage cabinet devoid of any valves. (4) Proper documentation related to the use and the disinfection of the instrument (medical record number of the patient, date of the procedure, bronchoscopist performing the procedure, model and the serial number of the scope, and the date of reprocessing) is maintained for infection control purposes.
Footnotes
The following authors have disclosed financial relationships with a commercial party: Edward Haponik, MD, FCCP, who has previously provided continuing medical education/consultation supported at least in part by Olympus, Pentax, Bard Medical; and Gerard Silvestri, MD, FCCP, with Olympus Company and Bard Medical Company.
Support was provided by Olympus America, Inc, and Pentax Corporation.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (www.chestjournal.org/misc/reprints.shtml).
References
- 1.Kirschke DL, Jones TF, Craig AS. Outbreak ofPseudomonas aeruginosaandSeratia marcescensassociated with a manufacturing defect in bronchoscopes. N Engl J Med. 2003;348:214–220. doi: 10.1056/NEJMoa021791. [DOI] [PubMed] [Google Scholar]
- 2.Srinivasan A, Wolfenden LL, Song X. An outbreak ofPseudomonas aeruginosainfections associated with flexible bronchoscopes. N Engl J Med. 2003;348:221–227. doi: 10.1056/NEJMoa021808. [DOI] [PubMed] [Google Scholar]
- 3.Cetse JC, Vanhems P. Outbreak of infection associated with bronchoscopes. N Engl J Med. 2003;348:2039–2040. doi: 10.1056/NEJM200305153482021. [DOI] [PubMed] [Google Scholar]
- 4.Singh N, Belen O, Leger M. Cluster ofTrichosporon mucoidesin children associated with a faulty bronchoscope. Pediatr Infect Dis J. 2003;22:609–612. doi: 10.1097/01.inf.0000073301.27004.c1. [DOI] [PubMed] [Google Scholar]
- 5.Mehta AC, Minai OA. Infection control in the bronchoscopy suite: a review. Clin Chest Med. 1999;20:19–32. doi: 10.1016/s0272-5231(05)70123-4. [DOI] [PubMed] [Google Scholar]
- 6.Culver DA, Gordon SM, Mehta AC. Infection control in the bronchoscopy suite. Am J Respir Crit Care Med. 2003;167:1050–1056. doi: 10.1164/rccm.200208-797CC. [DOI] [PubMed] [Google Scholar]
- 7.Prakash UBS. Does the bronchoscope propagate infection? Chest. 1993;104:552–559. doi: 10.1378/chest.104.2.552. [DOI] [PubMed] [Google Scholar]
- 8.Rimmer J, Gibson B, Bryant DH. Extension of pulmonary TB after fiberoptic bronchoscopy. Tubercle. 1985;69:57–61. doi: 10.1016/0041-3879(88)90041-4. [DOI] [PubMed] [Google Scholar]
- 9.Pereira W, Kovnat DM, Khan MA. Fever and pneumonia after flexible fiberoptic bronchoscopy. Am Rev Respir Dis. 1975;112:19–38. doi: 10.1164/arrd.1975.112.1.59. [DOI] [PubMed] [Google Scholar]
- 10.Alvarado CJ, Reichelderfer M. APIC guideline for infection prevention and control in flexible endoscopy. Am J Infect Control. 2000;28:138–155. [PubMed] [Google Scholar]
- 11.Association of Operating Room Nurses. Recommended practices for use and care of endoscopes. AORN J. 1998;67:256–258. doi: 10.1016/s0001-2092(06)63206-7. 261–262. [DOI] [PubMed] [Google Scholar]
- 12.Honeybourne D, Babb J, Bowie P. British Thoracic Society guidelines on diagnostic flexible bronchoscopy. Thorax. 2001;56:I1–I21. doi: 10.1136/thorax.56.suppl_1.i1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.F1518-00 Standard practice for cleaning and disinfection of flexible fiberoptic and video endoscopes used in the examination of the hollow viscera. 2005 ASTM International. West Conshohocken, PA. [Google Scholar]
- 14.Honeybourne D, Neumann CS. An audit of bronchoscopy practice in the United Kingdom: a survey of adherence to national guidelines. Thorax. 1997;52:709–713. doi: 10.1136/thx.52.8.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Srinivasan A, Wolfenden LL, Song X. Bronchoscope reprocessing and infection prevention and control: bronchoscopy-specific guidelines are needed. Chest. 2004;125:307–314. doi: 10.1378/chest.125.1.307. [DOI] [PubMed] [Google Scholar]
- 16.Kaczmarek RG, Moore RM, Mc Crohan J. Multi-state investigation of the actual disinfection/sterilization of endoscopes in health care facilities. Am J Med. 1992;92:257–261. doi: 10.1016/0002-9343(92)90074-l. [DOI] [PubMed] [Google Scholar]
- 17.Nicolle LE, Mc Cleod J, Romance L. Pseudo-outbreak of blastomycosis associated with contaminated bronchoscope [letter] Infect Control Hosp Epidemiol. 1992;13:324. doi: 10.1086/646537. [DOI] [PubMed] [Google Scholar]
- 18.Ramsey AH, Oemig TV, Davis JP. An outbreak of Bronchoscopy-relatedMycobacterium tuberculosisinfections due to lack of bronchoscope leak testing. Chest. 2002;121:976–981. doi: 10.1378/chest.121.3.976. [DOI] [PubMed] [Google Scholar]
- 19.Pappas SA, Schaff DM, Di Costanzo MB. Contamination of flexible fiberoptic bronchoscopes [letter] Am Rev Respir Dis. 1983;127:391–392. doi: 10.1164/arrd.1983.127.3.391a. [DOI] [PubMed] [Google Scholar]
- 20.Wheeler PW, Lancaster D, Kaiser AB. Bronchopulmonary cross-colonization and infection related to mycobacterial contamination of suction valves of bronchoscopes. J Infect Dis. 1989;159:954–958. doi: 10.1093/infdis/159.5.954. [DOI] [PubMed] [Google Scholar]
- 21.Hagan ME, Klotz SA, Bartholomew W. A pseudoepidemic ofRhodotorula rubra: a marker for microbial contamination of the bronchoscope. Infect Control Hosp Epidemiol. 1995;16:727–728. doi: 10.1086/647048. [DOI] [PubMed] [Google Scholar]
- 22.Dawson DJ, Armstrong JG, Blacklock ZM. Mycobacterial cross-contamination of bronchoscopy specimens. Am Rev Respir Dis. 1982;126:1095–1097. doi: 10.1164/arrd.1982.126.6.1095. [DOI] [PubMed] [Google Scholar]
- 23.Wilson SJ, Everts RJ, Kirkland KB. A pseudo-outbreak of Aureobasidium species lower respiratory tract infections caused by reuse of single-use stopcocks during bronchoscopy. Infect Control Hosp Epidemiol. 2000;21:470–472. doi: 10.1086/501790. [DOI] [PubMed] [Google Scholar]
- 24.Gubler JG, Salfinger M, von Graevenitz A. Pseudoepidemic of nontuberculous mycobacteria due to a contaminated bronchoscope cleaning machine. Chest. 1992;101:1245–1249. doi: 10.1378/chest.101.5.1245. [DOI] [PubMed] [Google Scholar]
- 25.Brown NM, Hellyar EA, Harvey JE. Mycobacterial contamination of fibreoptic bronchoscopes. Thorax. 1993:1283–1285. doi: 10.1136/thx.48.12.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elston RA, Hay AJ. Acid-fast contamination of a bronchoscope washing machine [letter] J Hosp Infect. 1991;19:72–73. doi: 10.1016/0195-6701(91)90132-r. [DOI] [PubMed] [Google Scholar]
- 27.Kressel AB, Kidd F. Pseudo-outbreak ofMycobacterium chelonaeandMethylobacterium mesophilicumcaused by contamination of an automated endoscopy washer. Infect Control Hosp Epidemiol. 2001;22:414–418. doi: 10.1086/501926. [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann KK, Weber DJ, Rutala WA. Pseudoepidemic ofRhodotorula rubrain patients undergoing fiberoptic bronchoscopy. Infect Control Hosp Epidemiol. 1989;10:511–514. doi: 10.1086/645937. [DOI] [PubMed] [Google Scholar]
- 29.Schleupner CJ, Hamilton JR. A pseudoepidemic of pulmonary fungal infections related to fiberoptic bronchoscopy. Infect Control. 1980;1:38–42. doi: 10.1017/s0195941700052383. [DOI] [PubMed] [Google Scholar]
- 30.Steere AC, Corrales J, von Graevenitz A. A cluster ofMycobacterium gordonaeisolates from bronchoscopy specimens. Am Rev Respir Dis. 1979;120:214–216. doi: 10.1164/arrd.1979.120.1.214. [DOI] [PubMed] [Google Scholar]
- 31.Southwick KL, Hoffmann K, Ferree K. Cluster of tuberculosis cases in North Carolina: possible association with atomizer reuse. Am J Infect Control. 2001;29:1–6. doi: 10.1067/mic.2001.110213. [DOI] [PubMed] [Google Scholar]
- 32.Nelson KE, Larson PA, Schraufnagel DE. Transmission of tuberculosis by flexible fiberbronchoscopes. Am Rev Respir Dis. 1983;127:97–100. doi: 10.1164/arrd.1983.127.1.97. [DOI] [PubMed] [Google Scholar]
- 33.Takigawa K, Fujita J, Negayama K. Eradication of contaminatingMycobacterium chelonaefrom broncho fibrescopes and an automated bronchoscope disinfection machine. Respir Med. 1995;89:423–427. doi: 10.1016/0954-6111(95)90211-2. [DOI] [PubMed] [Google Scholar]
- 34.Flournoy DJ, Petrone RL, Voth DW. A pseudo-outbreak ofMethylobacterium mesophilicaisolated from patients undergoing bronchoscopy. Eur J Clin Microbiol Infect Dis. 1992;11:240–242. doi: 10.1007/BF02098087. [DOI] [PubMed] [Google Scholar]
- 35.Bennett SN, Peterson DE, Johnson DR. Bronchoscopy-associatedMycobacterium xenopipseudoinfections. Am J Respir Crit Care Med. 1994;150:245–250. doi: 10.1164/ajrccm.150.1.8025757. [DOI] [PubMed] [Google Scholar]
- 36.Stine TM, Harris AA, Levin S. A pseudoepidemic due to atypical mycobacteria in a hospital water supply. JAMA. 1987;258:809–811. [PubMed] [Google Scholar]
- 37.Duckworth G. Non-tuberculous mycobacteria in gastrointestinal biopsy specimens [letter] Lancet. 1988;2:284. doi: 10.1016/s0140-6736(88)92582-2. [DOI] [PubMed] [Google Scholar]
- 38.Mitchell DH, Hicks LJ, Chiew R. Pseudoepidemic ofLegionella pneumophiliaserogroup 6 associated with contaminated bronchoscopes. J Hosp Infect. 1997;37:19–23. doi: 10.1016/s0195-6701(97)90069-4. [DOI] [PubMed] [Google Scholar]
- 39.Pentony T, Haywood H, Smith G. Pseudo-outbreak ofMycobacterium aviumcomplex (MAC) in bronchoscopy specimens due to contaminated potable water supply [abstract] Infect Control Hosp Epidemiol. 1994;5:S33. [Google Scholar]
- 40.Siegman-Igra Y, Inbar G, Campus A. An “outbreak” of pulmonary pseudoinfection bySerratia marcescens. J Hosp Infect. 1985;6:218–220. doi: 10.1016/s0195-6701(85)80101-8. [DOI] [PubMed] [Google Scholar]
- 41.Whitlock WL, Dietrich RA, Steimke EH. Rhodotorula rubra contamination in fiberoptic bronchoscopy. Chest. 1992;102:1516–1519. doi: 10.1378/chest.102.5.1516. [DOI] [PubMed] [Google Scholar]
- 42.Vandenbroucke-Grauls CMJE, Baars ACM, Visser MR. An outbreak ofSerratia marcescenstraced to a contaminated bronchoscope. J Hosp Infect. 1993;23:263–270. doi: 10.1016/0195-6701(93)90143-n. [DOI] [PubMed] [Google Scholar]
- 43.Weinstein HJ, Bone RC, Ruth WE. Contamination of a fiberoptic bronchoscope with a Proteus species. Am Rev Respir Dis. 1977;116:541–543. doi: 10.1164/arrd.1977.116.3.541. [DOI] [PubMed] [Google Scholar]
- 44.Goldstein B, Abrutyn E. Pseudo-outbreak of Bacillus species related to fibreoptic bronchoscopy. J Hosp Infect. 1985;6:194–200. [PubMed] [Google Scholar]
- 45.Richardson AJ, Rothburn MM, Roberts C. Pseudo-outbreak of Bacillus species related to fibreoptic bronchoscopy [letter] J Hosp Infect. 1986;7:208–210. doi: 10.1016/0195-6701(86)90068-x. [DOI] [PubMed] [Google Scholar]
- 46.Webb SF, Vall-Spinosa A. Outbreak ofSerratia marcescensassociated with the flexible fiberbronchoscope. Chest. 1975;68:703–708. [Google Scholar]
- 47.Kellerhals S. A pseudo-outbreak ofSerratia marcescensfrom a contaminated fiberbronchoscope. APIC. 1978;6:5–7. [PubMed] [Google Scholar]
- 48.Suratt PM, Gruber B, Wellons HA. Absence of clinical pneumonia following bronchoscopy with contaminated and clean bronchofiberscopes. Chest. 1977;71:52–54. doi: 10.1378/chest.71.1.52. [DOI] [PubMed] [Google Scholar]
- 49.Centers for Disease Control and Prevention. Bronchoscopy-related infections and pseudoinfections-New York, 1996 and 1998. MMWR Morb Mortal Wkly Rep. 1999;48:557–560. [PubMed] [Google Scholar]
- 50.Leers WD. Disinfecting endoscopes: how not to transmitMycobacterium tuberculosisby bronchoscopy. Can Med Assoc J. 1980;123:275–280. [PMC free article] [PubMed] [Google Scholar]
- 51.Prigogine T, Glupczynski Y, Van Molle P. Mycobacterial cross-contamination of bronchoscopy specimens [letter] J Hosp Infect. 1988;11:93–95. doi: 10.1016/0195-6701(88)90047-3. [DOI] [PubMed] [Google Scholar]
- 52.Bryce EA, Walker M, Bevan C. Contamination of bronchoscopes withMycobacterium tuberculosis. Can J Infect Control. 1993;8:35–36. [PubMed] [Google Scholar]
- 53.Agerton T, Valway S, Gore B. Transmission of a highly drug-resistant strain (strain W1) ofMycobacterium tuberculosis. JAMA. 1997;278:1073–1077. [PubMed] [Google Scholar]
- 54.Nye K, Chadha DK, Hodgkin P. Mycobacterium chelonae isolation from broncho-alveolar lavage fluid and its practical implications. J Hosp Infect. 1990;16:257–261. doi: 10.1016/0195-6701(90)90114-4. [DOI] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention. Nosocomial infection and pseudoinfection from contaminated endoscopes and bronchoscopes-Wisconsin and Missouri. MMWR Morb Mortal Wkly Rev. 1991;40:675–678. [PubMed] [Google Scholar]
- 56.Fraser VJ, Jones M, Murray PR. Contamination of flexible fiberoptic bronchoscopes withMycobacterium chelonaelinked to an automated bronchoscope disinfection machine. Am Rev Respir Dis. 1992;145:853–855. doi: 10.1164/ajrccm/145.4_Pt_1.853. [DOI] [PubMed] [Google Scholar]
- 57.Campagnaro RL, Teichtahl H, Dwyer B. A pseudoepidemic ofMycobacterium chelonae: contamination of a bronchoscope and autocleaner. Aust N Z J Med. 1994;24:693–695. doi: 10.1111/j.1445-5994.1994.tb01785.x. [DOI] [PubMed] [Google Scholar]
- 58.Wang HC, Liaw YS, Yang PC. A pseudoepidemic ofMycobacterium chelonaeinfection caused by contamination of a fiberoptic bronchoscope suction channel. Eur Respir J. 1995;8:1259–1262. doi: 10.1183/09031936.95.08081259. [DOI] [PubMed] [Google Scholar]
- 59.Kiely JL, Sheehan S, Bredin CP. Isolation ofMycobacterium chelonaein a bronchoscopy unit and its subsequent eradication. Tuber Lung Dis. 1995;76:163–167. doi: 10.1016/0962-8479(95)90561-8. [DOI] [PubMed] [Google Scholar]
- 60.Cox R, de Borja K, Bach MC. A pseudo-outbreak ofMycobacterium chelonaeinfections related to bronchoscopy. Infect Control Hosp Epidemiol. 1997;18:136–137. doi: 10.1086/647570. [DOI] [PubMed] [Google Scholar]
- 61.Wallace RJ, Brown BA, Griffith DE. Nosocomial outbreaks/pseudo-outbreaks caused by nontuberculous mycobacteria. Annu Rev Microbiol. 1998;52:453–490. doi: 10.1146/annurev.micro.52.1.453. [DOI] [PubMed] [Google Scholar]
- 62.Belleguic C, Briens E, Lena H. Mycobacterium chelonaepseudoepidemic due to contamination of flexible bronchoscopes. J Bronchol. 1998;5:20–24. [Google Scholar]
- 63.Maloney S, Welbel S, Daves B. Mycobacterium abscessuspseudoinfection traced to an automated endoscope washer: utility of epidemiologic and laboratory investigation. J Infect Dis. 1994;169:1166–1169. doi: 10.1093/infdis/169.5.1166. [DOI] [PubMed] [Google Scholar]
- 64.Petersen K, Bus N, Walter V. Pseudoepidemic ofMycobacterium abscessusassociated with bronchoscopy [abstract] Infect Control Hosp Epidemiol. 1994;15:S32. [Google Scholar]
- 65.Strelczyk K. Pseudo-outbreak of acid-fast bacilli [abstract] Am J Infect Contro1. 1999;27:A198. [Google Scholar]
- 66.Hussain SA. Fiberoptic bronchoscope-related outbreak of infection with Pseudomonas [letter] Chest. 1978;74:483. doi: 10.1378/chest.74.4.483b. [DOI] [PubMed] [Google Scholar]
- 67.Markovitz A. Inoculation by bronchoscopy [letter] West J Med. 1979;131:6. [PMC free article] [PubMed] [Google Scholar]
- 68.Sammartino MT, Israel RH, Magnussen CR. Pseudomonas aeruginosacontamination of fibreoptic bronchoscopes. J Hosp Infect. 1982;3:65–71. doi: 10.1016/0195-6701(82)90032-9. [DOI] [PubMed] [Google Scholar]
- 69.Blanc DS, Parret T, Janin B. Nosocomial infections and pseudoinfections from contaminated bronchoscopes: two-year follow-up using molecular markers. Infect Control Hosp Epidemiol. 1997;18:134–136. doi: 10.1086/647569. [DOI] [PubMed] [Google Scholar]
- 70.Sorin M, Segal-Maurer S, Mariano N. Nosocomial transmission of imipenem-resistantPseudomonas aeruginosafollowing bronchoscopy associated with improper connection to the Steris System 1 processor. Infect Control Hosp Epidemiol. 2001;22:409–413. doi: 10.1086/501925. [DOI] [PubMed] [Google Scholar]
- 71.Kolmos HJ, Lerche A, Kristoffersen K. Pseudo-outbreak ofPseudomonas aeruginosain HIV infected patients undergoing fiberoptic bronchoscopy. Scand J Infect Dis. 1994;26:653–657. doi: 10.3109/00365549409008632. [DOI] [PubMed] [Google Scholar]
- 72.Jackson J, Leggett JE, Wilson D. Mycobacterium gordonaein fiberoptic bronchoscopes. Am J Infect Control. 1996;24:19–23. doi: 10.1016/s0196-6553(96)90049-8. [DOI] [PubMed] [Google Scholar]
- 73.Kramer MH, Krizek L, Gebel J, et al. Bronchoscopic transmission of Pseudomonas aeruginosa due to a contaminated disinfectant solution from an automated dispenser unit. In: Final Program of the 11th Annual Scientific Meeting of the Society of Healthcare Epidemiology of America; Toronto, ON, Canada; April 1–3, 2001; abstract 118
- 74.Centers for Disease Control and Prevention. Pseudomonas aeruginosainfections associated with defective bronchoscopes. MMWR Morb Mortal Wkly Rep. 2002;51:190. [Google Scholar]
- 75.Michele TM, Cronin WA, Graham NMH. Transmission ofMycobacterium tuberculosisby a fiberoptic bronchoscope. JAMA. 1997;278:1093–1095. [PubMed] [Google Scholar]
- 76.Schelenz S, French G. An outbreak of multidrug-resistantPseudomonas aeruginosainfection associated with contamination of bronchoscopes and an endoscope washer-disinfector. J Hosp Infect. 2000;46:23–30. doi: 10.1053/jhin.2000.0800. [DOI] [PubMed] [Google Scholar]
- 77.Birnie GG, Quigley EM, Clements GB. Endoscopic transmission of hepatitis B virus. Gut. 1983;24:171–174. doi: 10.1136/gut.24.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sawchuk WS, Weber PJ, Lowy DR. Infectious papillomavirus in the vapor of warts treated with carbon dioxide laser or electrocoagulation: detection and protection. J Am Acad Dermatol. 1989;21:41–49. doi: 10.1016/s0190-9622(89)70146-8. [DOI] [PubMed] [Google Scholar]
- 79.Baggish MS, Poiesz BJ, Joret D. Presence of human immunodeficiency virus DNA in laser smoke. Lasers Surg Med. 1991;11:197–203. doi: 10.1002/lsm.1900110302. [DOI] [PubMed] [Google Scholar]
- 80.Gloster HM, Roenigk RK. Risk of acquiring human papillomavirus from the plume produced by the carbon dioxide laser in the treatment of warts. J Am Acad Dermatol. 1995;32:436–441. doi: 10.1016/0190-9622(95)90065-9. [DOI] [PubMed] [Google Scholar]
- 81.Lobraico RV, Schifano MJ, Brader KR. A retrospective study on the hazards of the carbon dioxide laser plume. J Laser Appl. 1988;1:6–8. [Google Scholar]
- 82.Wenzel RP, Edmond M. Listening to SARS: lessons for infection control. Ann Intern Med. 2003;139:592–593. doi: 10.7326/0003-4819-139-7-200310070-00012. [DOI] [PubMed] [Google Scholar]
- 83.Ho AS, Sung JY, Chan-Yeung M. An outbreak of severe respiratory syndrome among hospital workers in a community hospital in Hong-Kong. Ann Intern Med. 2003;139:564–567. doi: 10.7326/0003-4819-139-7-200310070-00008. [DOI] [PubMed] [Google Scholar]
- 84.Booth CM, Matukas LM, Tomlinson GA. Clinical features and short-term outcomes of 144 patients with SARS in the Greater Toronto area. JAMA. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- 85.Ksiazek TG, Erdman D. A novel coronavirus associated with SARS [letter] N Engl J Med. 2003;348:1953. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 86.Malasky C, Jordan T, Potulski F. Occupational tuberculosis infections among pulmonary physicians in training. Am Rev Respir Dis. 1990;142:505–507. doi: 10.1164/ajrccm/142.3.505. [DOI] [PubMed] [Google Scholar]
- 87.Ball R, Van Wey M. Tuberculosis skin test conversion among health care workers at a military center. Mil Med. 1997;162:338–343. [PubMed] [Google Scholar]
- 88.Woodcock A, Campbell I, Collins JVC. Bronchoscopy and infection control. Lancet. 1989;2:270–271. doi: 10.1016/s0140-6736(89)90451-0. [DOI] [PubMed] [Google Scholar]
- 89.www.fda.gov/cdrh/ode/germlab.html Center for Devices and Radiologic Health, Food and Drug Administration. FDA-cleared sterilants and high level disinfectants with general claims for processing reusable medical and dental devices 1/30/02. Available at: Accessed September 22, 2004.
- 90.Prakash UBS. Prophylactic antibacterial therapy for bronchoscopy: indications. J Bronchol. 1997;4:281–285. [Google Scholar]
- 91.American Dental Association, American Academy of Orthopedic Surgeons. Antibiotic prophylaxis for dental patients with total joint replacements. J Am Dental Assoc. 1997;128:1004–1008. doi: 10.14219/jada.archive.1997.0307. [DOI] [PubMed] [Google Scholar]
- 92.Fennelly KP. Personal respiratory protection againstMycobacterium tuberculosis. Clin Chest Med. 1997;18:1–17. doi: 10.1016/s0272-5231(05)70352-x. [DOI] [PubMed] [Google Scholar]
- 93.Mc Williams T, Wells AU, Harrison AC. Induced sputum and bronchoscopy in the diagnosis of tuberculosis. Thorax. 2002;57:1010–1014. doi: 10.1136/thorax.57.12.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guidelines for environmental infection control in health care facilities. MMWR Morb Mortal Wkly Rep. 2003;52(RR10):1–42. [PubMed] [Google Scholar]
- 95.Alfa MJ, De Grange P, Olson N. Comparison of ion plasma, vaporized hydrogen peroxide and 100% ethylene oxide sterilizers to the 12/88 ethylene oxide gas sterilizer. Infect Control Hosp Epidemiol. 1996;17:92–100. doi: 10.1086/647252. [DOI] [PubMed] [Google Scholar]
- 96.Hanson PJV, Chadwick MV, Gaya H. A study of gluterladehyde disinfection of fiberoptic bronchoscope experimentally contaminated withMycobacterium tuberculosis. J Hosp Infect. 1992;22:137–142. doi: 10.1016/0195-6701(92)90097-6. [DOI] [PubMed] [Google Scholar]
- 97.Rutala WA, Weber DJ. Disinfection of endoscope: review of new chemical sterilents used for high-level disinfection. Infect Control Hosp Epidemiol. 1999;20:69–76. doi: 10.1086/501544. [DOI] [PubMed] [Google Scholar]
- 98.Kato H, Matsushima S, Smyth JP. Effects of disinfectants with and without the usage of detergent for the flexible fiberoptic bronchoscope. Jpn J Exp Med. 1979;49:337–342. [PubMed] [Google Scholar]
- 99.Rutala WA. APIC guideline for selection and use of disinfectants. Am J Infect Control. 1996;24:313–342. doi: 10.1016/s0196-6553(96)90066-8. [DOI] [PubMed] [Google Scholar]
- 100.Seballos RJ, Walsh AL, Mehta AC. Clinical evaluation of a liquid chemical sterilization system for the flexible bronchoscope. J Bronchol. 1995;2:192–199. [Google Scholar]
- 101.Mbithi JN, Springthorpe VS, Sattar SA. Bactericidal, virucidal, and mycobactericidal activities of reused alkaline glutaraldehyde in an endoscopy unit. J Clin Microbiol. 1993;31:2988–2995. doi: 10.1128/jcm.31.11.2988-2995.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Prakash UBS. Saunders; Philadelphia, PA: 2001. Prevention of bronchoscopy-induced infections; pp. 413–417. (Saunders infection control service). Adrutryn, E Goldmann, DA Scheckler, WE eds. [Google Scholar]
- 103.Multi-society guidelines for reprocessing flexible gastrointestinal endoscope. Gastrointest Endoscop. 2003;58:1–8. doi: 10.1016/s0016-5107(03)70109-6. [DOI] [PubMed] [Google Scholar]
- 104.Rultala W. Williams and Wilkins; Baltimore, MD: 1997. Disinfection, sterilization, and waste disposal. (Prevention and control of nosocomial infections). Wenzel, Reds 3rd ed. [Google Scholar]


