Abstract
The 90 kDa ribosomal S6 kinases (p90RSKs) are a family of broadly expressed serine/threonine kinases with two kinase domains activated by extracellular signal‐regulated protein kinase in response to many growth factors. Our recent study demonstrated that severe acute respiratory syndrome (SARS)‐coronavirus (CoV) infection of monkey kidney Vero E6 cells induces phosphorylation and dephosphorylation of signaling pathways, resulting in apoptosis. In the present study, we investigated the phosphorylation status of p90RSK, which is a well‐known substrate of these signaling pathways, in SARS‐CoV‐infected cells. Vero E6 mainly expressed p90RSK1 and showed weak expression of p90RSK2. In the absence of viral infection, Ser221 in the N‐terminal kinase domain was phosphorylated constitutively, whereas both Thr573 in the C‐terminal kinase domain and Ser380 between the two kinase domains were not phosphorylated in confluent cells. Ser380, which has been reported to be involved in autophosphorylation by activation of the C‐terminal kinase domain, was phosphorylated in confluent SARS‐CoV‐infected cells, and this phosphorylation was inhibited by http://SB203580, which is an inhibitor of p38 mitogen‐activated protein kinases (MAPK). Phosphorylation of Thr573 was not upregulated in SARS‐CoV‐infected cells. Thus, in virus‐infected cells, phosphorylation of Thr573 was not necessary to induce phosphorylation of Ser380. On the other hand, Both Thr573 and Ser380 were phosphorylated by treatment with epidermal growth factor (EGF) in the absence of p38 MAPK activation. Ser220 was constitutively phosphorylated despite infection. These results indicated that phosphorylation status of p90RSK by SARS‐CoV infection is different from that by stimulation of EGF. This is the first detailed report regarding regulation of p90RSK phosphorylation by virus infection.
Keywords: 90 kDa ribosomal S6 kinases, Phosphorylation, Severe acute respiratory syndrome
1. Introduction
The signaling pathway of extracellular signal‐regulated kinase (ERK) regulates cellular processes, including growth, cell proliferation, survival, and motility [1]. The p90 ribosomal S6 kinases (RSK), a family of serine/threonine kinases, are important substrates of ERK [2]. The RSK family consists of four isoforms (RSK1, 2, 3, and 4) and two structurally related RSK‐like protein kinases (RLPK/MSK1) and RSK‐B (MSK2) in humans [2, 3, 4, 5]. Members of the RSK family contain two distinct kinase domains. The C‐terminal kinase domain is thought to be involved in autophosphorylation at the critical step in 90 kDa ribosomal S6 kinase (p90RSK) activation [6, 7, 8]. On the other hand, the N‐terminal kinase domain is capable of phosphorylation of substrates. Recent studies have clarified the mechanisms of activation of p90RSK. p90RSK1 is phosphorylated at Thr573 in the activation loop of the C‐terminal kinase domain by ERK because the C‐terminal of p90RSK has an ERK docking site [9, 10]. Autophosphorylation at Ser380 in the linker region is thought to be induced by activation of the C‐terminal kinase domain [11], and then PDK1 phosphorylates at Ser221 in the activation loop of the N‐terminal kinase domain [12, 13, 14].
p90RSK is thought to have multiple functions. In quiescent cells, p90RSK is present in the cytoplasm, and p90RSK activated via the ERK signaling pathway by growth factors is imported into the nucleus. p90RSK activates nuclear factor‐κB by phosphorylation of Iκ‐B and phosphorylates the transcription factors, c‐Fos and cAMP‐response element‐binding protein (CREB) [2]. It has been shown that p90RSK plays important roles in apoptosis and the cell cycle. p90RSK phosphorylates Bad [15, 16] and C/EBPβ [17], which protects cells against apoptosis. Furthermore, p90RSK phosphorylates and inhibits Myt1, which is a p34cdc2 inhibitory kinase, resulting in G2 arrest in Xenopus extracts [18, 19]. In mouse oocytes, Emi1 and p90RSK2 cooperate to induce metaphase arrest [20]. p90RSK also functions as a serum‐stimulated Na+/H+ exchanger‐1 kinase and regulates its activity [21]. Recently, it has been shown that p90RSK activation induces H2O2‐mediated cardiac troponin I phosphorylation, which depresses the acto‐myosin interaction and is important during the progression of heart failure [21]. Thus, p90RSK has been demonstrated to play key roles in regulating cellular functions in the ERK signaling pathway in vitro and in vivo.
Severe acute respiratory syndrome (SARS) is a newly discovered infectious disease caused by a novel coronavirus, SARS coronavirus (SARS‐CoV) [22, 23], which spread to more than 30 countries in late 2002, causing severe outbreaks of atypical pneumonia. Our recent studies using the monkey kidney cell line, Vero E6, demonstrated that a variety of signaling pathways are activated upon infection with SARS‐CoV. Especially, p38 mitogen‐activated protein kinase (MAPK) is throught to be involved in induction of apoptosis because a p38 inhibitor was able to partially prevent cytopathic effects induced by SARS‐CoV infection [24]. Signal transducer and activator of transcription (STAT)‐3, which is ordinarily phosphorylated at a tyrosine residue, is dephosphorylated by SARS‐CoV‐induced activation of p38 [25]. c‐Jun N‐terminal protein kinase (JNK) and Akt are important for establishing persistent SARS‐CoV infection [26]. In confluent virus‐infected cells, Akt is first phosphorylated at a single serine residue shortly after SARS‐CoV infection, and subsequently dephosphorylated during the course of viral infection [27], whereas Akt, which is ordinary phosphorylated at a serine residue, was dephosphorylated by SARS‐CoV infection without any upregulation of its phosphorylation in subconfluent cells [28]. This downregulation of Akt phosphorylation induces inhibition of cell proliferation by SARS‐CoV infection and weak activation of Akt cannot induce escape from SARS‐CoV‐induced apoptosis. Nucleocapsid protein, X1 and spike proteins of SARS‐CoV are able to induce apoptosis in their expressing cells [29, 30, 31, 32]. Especially, N protein is able to upregulation of phosphorylation of JNK and p38 MAPK, but not ERK and Akt [29]. Although ERK was shown to be phosphorylated in SARS‐CoV‐infected cells [25], the function is not clear. p90RSK is a well‐known substrate for ERK as described above.
In the present study, we showed that Ser380 of p90RSK, which is thought to be auto‐phosphorylated after activation of the C‐terminal kinase domain, is phosphorylated without upregulation of Thr573 phosphorylation in the C‐terminal kinase domain, in SARS‐CoV‐infected Vero E6 cells. Furthermore, we demonstrated that activation of p38 MAPK was responsible for phosphorylation of Ser380 in virus‐infected cells. These results indicated signaling pathways, which are different from those induced by growth factor, contribute to phosphorylation of p90RSK in SARS‐CoV‐infected cells.
2. Materials and methods
2.1. Cells and virus
Vero E6 cells were subcultured routinely in 75‐cm3 flasks in Dulbecco's modified Eagle's medium (DMEM; Sigma, St. Louis, MO, USA) supplemented with 0.2 mM l‐glutamine, 100 units/ml penicillin, 100 μg/ml streptomycin, and 5% (v/v) fetal bovine serum (FBS), and maintained at 37 °C in an atmosphere of 5% CO2. For use in the experiments, the cells were split once onto 6‐ and 24‐well tissue culture plate inserts and cultured under subconfluent and confluent conditions. SARS‐CoV, which was isolated as Frankfurt 1 and kindly provided by Dr. J. Ziebuhr, was used in the present study. Infection was usually performed at a multiplicity of infection (m.o.i.) of 10. The number of cells was counted using the WST‐1 cell proliferation assay system (Takara, Shiga, Japan).
2.2. Inhibitor
The p38 MAPK inhibitor, http://SB203580, which was purchased from Calbiochem (La Jolla, CA, USA), was dissolved in dimethyl sulfoxide (DMSO) at a concentration of 10 mM. The same volume of DMSO alone was used as a control. As shown in previous reports [24, 27], http://SB203580 and http://PD98059 had no effect on viral replication including viral protein synthesis.
2.3. Western blotting
The whole‐cell extracts were electrophoresed on 5–20% gradient polyacrylamide gels, and transferred electrophoretically onto PVDF membranes (Immobilon‐P; Millipore, Bedford, MA, USA). In the present study, we applied two sets of samples to polyacrylamide gels, and the membranes were divided into two halves after blotting using a LumiGLO Elite chemiluminescent system (Kirkegaard and Perry Laboratories, Gaithersburg, ML, USA). When it was necessary to strip the membranes, Restore Western blot stripping buffer (Pierce, Rockford, IL, USA) was used. The following antibodies, obtained from Cell Signaling Technology Inc. (Beverly, MA, USA), were used in the present study at a dilution of 1:1000: rabbit anti‐phospho Akt (Ser473) antibody, rabbit anti‐Akt antibody, rabbit anti‐phospho‐PDK1 (Ser241) antibody, rabbit anti‐phospho STAT3 (Tyr‐705) antibody, rabbit anti‐p38 MAPK (Thr180/Tyr182) antibody, rabbit anti‐p38 MAPK antibody, rabbit anti‐phospho‐ERK1/2 (Thr202/Tyr204) antibody, rabbit anti‐ERK antibody, rabbit anti‐phospho‐MEK1/2 (Ser217/221) antibody, rabbit anti‐MEK1/2 antibody, rabbit anti‐p90RSK1/2/3 antibody, rabbit anti‐cleaved Caspase‐3 (Asp175) antibody, rabbit anti‐cleaved Caspase‐7 (Asp198) antibody. Rabbit Mouse anti‐STAT3 antibody (diluted 1:2500) was obtained from BD Biosciences (Franklin Lakes, NJ, USA). Rabbit anti‐p90RSK1 monoclonal antibody, which was purchased from Epitomics, Inc. (Burlingame, CA, USA), was diluted at 1:1000. Rabbit anti‐p90RSK2 (C‐term), p90RSK3 (Mid) and p90RSK4 (N‐term) were purchased from Zymed Laboratory, Inc. (South San Francisco, CA, USA). Anti‐p90RSK2 and 4 antibodies were diluted 1:250, and anti‐p90RSK3 was diluted 1:500. Rabbit anti‐PARP p85 fragment antibody was purchased from Promega (Madison, WI, USA) and diluted 1:100. Mouse anti‐β‐actin antibody was purchased from Sigma (St. Louis, MO, USA) and used at a dilution of 1:5000. Rabbit anti‐SARS N and M antibodies were described previously [24]. K562 and Jurkat cell lysates were purchased from Clontech Laboratories Inc. (Mountain View, CA, USA).
3. Results
3.1. Signaling pathways in SARS‐CoV‐sensitive cell lines
As shown in Fig. 1 , SARS‐CoV‐infected confluent Vero E6 cells induced phosphorylation or dephosphorylation of signaling pathways as also described in previous studies [24, 27, 28]. The cytopathic effects (CPEs) were observed in Vero E6 cells at 24‐h post‐infection (h.p.i.). DNA fragmentation as an indicator of apoptosis was detected at 24 h.p.i. [24]. Among the signaling pathways activated by SARS‐CoV infection, p38 MAPK is thought to act as a pro‐apoptotic signaling pathway, whereas Akt has an anti‐apoptotic effect. Vero cells, the parental cells of Vero E6, are also sensitive to SARS‐CoV infection. However, the time point of the appearance of CPE on Vero cells by SARS‐CoV infection is later than that of Vero E6 cells at 24 h.p.i. (data not shown). As shown in Fig. 1A, nucleocapsid (N) protein of SARS‐CoV was detected at 17 h.p.i. in both cell lines. The level of N protein in Vero cells was only slightly lower than that in Vero E6 cells, indicating that replication of SARS‐CoV is not markedly different between the two cell lines. Anti‐apoptotic Akt was phosphorylated at 17 h.p.i. in both cell lines, and was dephosphorylated at 27 h.p.i. (Fig. 1B). Our previous study indicated that phosphorylation level of Akt around 17 h.p.i. is only 20% of phosphorylated Akt in growing cells [28]. Therefore, this low level of activation cannot prevent apoptosis by SARS‐CoV infection. Fig. 1B also shows that ERK was phosphorylated at 17 h.p.i. in both cell lines, similar to the observations regarding Akt. The apoptotic markers, PARP (p85) and cleaved caspase‐3 and ‐7, were detected in Vero E6 and Vero cells at 27 and 44 h.p.i., respectively (Fig. 1A). The p38 MAPK in virus‐infected Vero E6 and Vero cells was phosphorylated at 17 and 27 h.p.i., respectively (Fig. 1A). Tyrosine of STAT3 was also dephosphorylated via phosphorylation of p38 MAPK as reported previously [25]. Thus, SARS‐CoV‐induced apoptosis related to time‐dependent activation of the pro‐apoptotic signaling pathway, p38 MAPK. Taken together, these results suggested that substrates of PI3K/Akt, ERK1/2, and p38 MAPK are important for understanding the cytopathic effects of SARS‐CoV infection.
Figure 1.
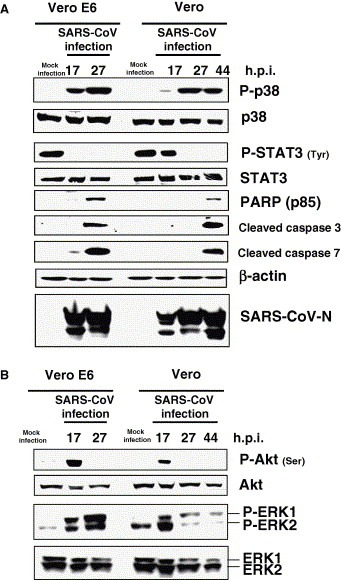
Phosphorylation of signaling pathways in SARS‐CoV‐infected cells. Vero and Vero E6 cells were prepared at confluence in 24‐well plates and the cells were infected with SARS‐CoV at 10 m.o.i. Protein samples were obtained at 17, 24, and 44 h.p.i. The protein of Vero E6 cells at 44 h.p.i. could not be obtained due to strong morphological changes caused by apoptosis. Western blotting analyses were performed to examine signaling pathways (A) and apoptotic marker proteins (B).
3.2. Phosphorylation of p90RSK Ser221 in Vero E6 cells
p90RSK is phosphorylated by both PDK‐1 and ERK [2, 13, 14]. Ser221 of p90RSK1 is phosphorylated by PDK‐1 and Thr359, Ser363, and Thr573 of p90RSK‐1 are phosphorylated by ERK. p90RSK is thought to play important roles in apoptosis and the cell cycle. Both PI3K/Akt and ERK signaling pathways are activated early post‐infection with SARS‐CoV in both Vero and Vero E6 cells (Fig. 1B). Therefore, p90RSK may be a target of both signaling pathways in SARS‐CoV‐infected cells. At least four species of p90RSK (p90RSK1, 2, 3, and 4) has been reported to date [2, 3, 4, 5]. We used anti‐p90RSK antibodies, which do not cross‐react with other p90RSK family members, as described in the legend of Fig. 2 . We measured densities of RSK1 and 2 bands in Vero E6 cells using LAS‐3000 mini system (Fuji Photo Film Co. Ltd, Tokyo, Japan). The amount of p90RSK1 in subconfluent cells, p90RSK2 in confluent cells and p90RSK2 in subconfluent cells were 73.05%, 39.94% and 14.70% of RSK1 in confluent cells, respectively. Although affinity of each antibody is different, this result suggested that p90RSK1 was expressed stronger than p90RSK2 in Vero E6 cells. p90RSK1 is expressed mainly in the human kidney, lung, and pancreas, whereas p90RSK2 is expressed in skeletal muscle, heart, and pancreas [33]. p90RSK3 and 4 were not detected in Vero E6 and HeLa cells in the present study. The p90RSK4 was weakly detected in K562 and Jurkat cells.
Figure 2.
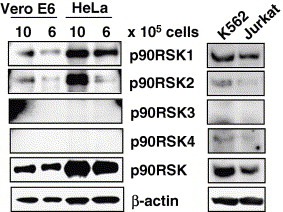
p90RSK family expressed in Vero E6 cells. Vero E6 cells were prepared at densities of 1 × 106 and 0.6 × 105 in 6‐well plates. HeLa cells, a clonal cell line for another study, were used as controls. Both K562 and Jurkat cell lysates were obtained from Clontech Laboratories Inc. Western blotting analysis was performed using the same amounts of protein. According to the antibody product data sheets, anti‐human p90RSK1 monoclonal antibody (Epitomics) does not cross‐react with other RSK family members. Anti‐human and mouse p90RSK2 antibody (Zymed) does not react with overexpressed p90RSK1, 3, or 4. Anti‐human p90RSK3 antibody (Zymed) does not react with overexpressed p90RSK1, 2, or 4. Anti‐human p90RSK antibody (Zymed) does not react with p90RSK1, 2, or 3. Anti‐p90RSK1/2/3 antibody (Cell Signaling) detects endogenous levels of RSK1, RSK2, and RSK3 proteins. In Vero E6 cells, the band of RSK1 was stronger than that of RSK2 as described in the text. The weak bands were enhanced using Adobe Photoshop.
We examined the phosphorylation status of p90RSK Ser221 in Vero E6 cells. Our previous study indicated that very low levels of Akt in confluent Vero E6 cells are phosphorylated at serine, whereas the level is high in subconfluent cells [28]. However, the phosphorylated threonine of Akt is difficult to detect in subconfluent Vero E6 cells. We only detected it weakly when the cells were treated with epidermal growth factor (EGF) at 1 min [28]. As threonine of Akt was transiently phosphorylated by EGF treatment, it was never detected after 5 min. On the other hand, serine of Akt was easily phosphorylated by treatment with EGF after 3 min. The threonine residue of Akt is phosphorylated by PDK‐1, and the serine residue of Akt is thought to be phosphorylated by the putative kinase, PDK‐2. To compare the phosphorylation level of PDK‐1 at different cell densities, Vero E6 cells were prepared at 10, 5, 2.5, 1.25, 0.6, and 0.3 × 105 cells in 5% FBS containing DMEM per well in 6‐well plates. At a density of 10 × 105 cells, Vero E6 cells showed 100% confluence in this experiment. As shown in Fig. 3 A, the level of PDK‐1 phosphorylation was similar at all cell densities. Ser221 of p90RSK was also phosphorylated at a similar level. The confluency of Vero E6 cells in the present study is shown in Fig. 3B. To confirm that the phosphorylation levels of PDK‐1 and p90RSK Ser221 were unaffected by cell proliferation, Vero E6 cells were cultured in medium containing low and high concentrations of FBS. Cell proliferation of Vero E6 cells was partially suppressed in medium containing 0.2% FBS as compared with 5% FBS (Fig. 3C). Confluent and subconfluent cells in medium containing 5–0.2% FBS showed similar phosphorylation levels of PDK‐1 and p90RSK at Ser221 (Fig. 3D). Thus, the phosphorylation level of Ser221 of p90RSK is not influenced by the status of cell proliferation.
Figure 3.
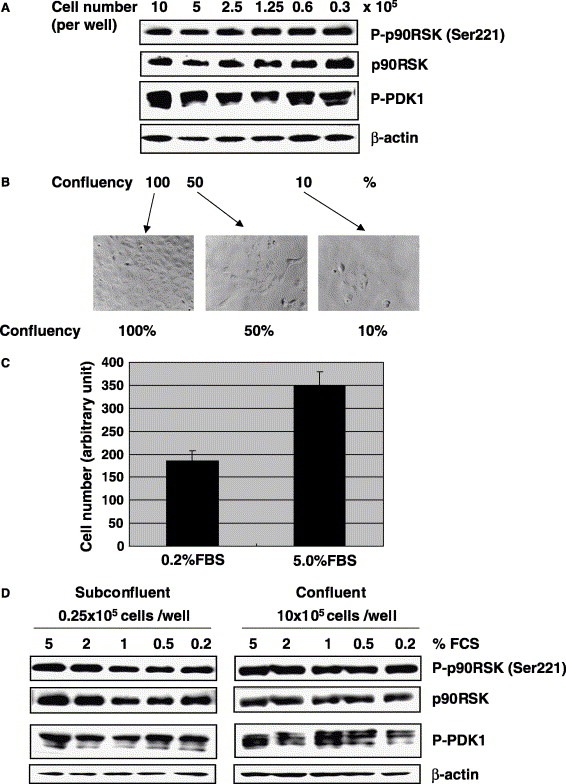
Phosphorylation of p90RSK Ser221 in Vero E6 cells. (A) Vero E6 cells were prepared at densities of 10, 5, 2.5, 1.25, 0.6, and 0.3 × 105 cells in DMEM containing 5% FBS per well in 6‐well plates. Proteins were obtained from these cells after 24 h, and Western blotting was performed using anti‐phospho p90RSK (Ser221). (B) The confluency of Vero E6 cells used in this study is shown. (C) 2 × 103 cells in DMEM containing 0.2% and 5% FBS were prepared in 96‐well plates. After 4 days, cell number was counted using a WST‐1 cell proliferation assay kit. (D) 0.25 and 10 × 105 cells in DMEM containing various concentrations of FBS were prepared in 6‐well plates. Western blotting was performed using proteins obtained after 24 h.
3.3. Phosphorylation of p90RSK Ser380 and Thr573 in Vero E6 cells
p90RSK1 is phosphorylated at Thr573 in the activation loop of the C‐terminal kinase domain [9, 10], and this activation of the C‐terminal kinase domain is thought to lead to autophosphorylation at Ser380 [11]. Activation of the C‐terminal domain by the ERK signaling pathway is thought to be necessary for phosphorylation at Ser380 [34]. Fig. 4 A indicates that EGF treatment induces phosphorylation of ERK in Vero E6 cells. Both Thr573 and Ser380 of p90RSK were phosphorylated early after EGF treatment. Interestingly, the phosphorylation level of p90RSK Ser221 was not altered by EGF treatment. To investigate whether cell density affects phosphorylation level of p90RSK Thr573 and Ser380, Western blotting analysis was performed using proteins obtained from 10, 5, 2.5, 1.25, 0.6, and 0.3 × 105 cells in DMEM containing 5% FBS per well in 6‐well plates. Fig. 4B shows that Ser380 of p90RSK phosphorylation was increased by decreasing cell density. Although Thr573 was also increased phosphorylation by decreasing cell density, the amount was very low. The amount of Thr573 phosphorylated p90RSK in 0.3 × 105 cells is only 11.53% of Ser380 using LAS‐3000 mini system. Therefore, the band of Thr573 phosphorylated p90RSK was difficult to see in Fig. 4B.
Figure 4.
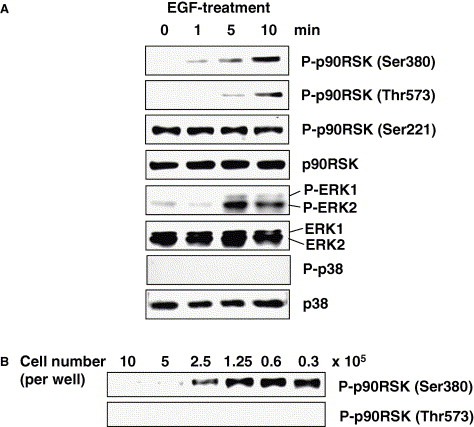
Phosphorylation of p90RSK Thr573 and Ser380 in Vero E6 cells. Confluent Vero E6 cells in 24‐well plates were treated with EGF. Western blotting analysis was performed using proteins obtained at 0, 1, 5, and 10 min (A). (B) Vero E6 cells were prepared at 10, 5, 2.5, 1.25, 0.6, and 0.3 × 105 cells in 6‐well plates. Proteins were obtained from these cells after 24 h, and Western blotting was performed using anti‐phospho p90RSK (Thr573 and Ser380). The proteins used in (B) were the same as those in Fig. 3A, and equal amount of proteins were blotted.
3.4. Phosphorylation of p90RSK in SARS‐CoV‐infected cells
To investigate regulation of p90RSK phosphorylation in SARS‐CoV‐infected cells, confluent Vero E6 cells were infected with SARS‐CoV at approximately 50 m.o.i., and Western blotting analysis was performed using proteins at 26 and 24 h.p.i. As shown in Fig. 5 A, no significant differences in phosphorylation levels of PDK‐1 or p90RSK at Ser221 were observed between confluent virus‐infected cells at 16 and 24 h.p.i. Phosphorylation of Thr573 was not upregulated by viral infection. Ser380 of p90RSK is phosphorylated in virus‐infected confluent cells. Previous reports indicated autophosphorylation of Ser380 after activation of C‐terminal kinase domain [11]. Thus, phosphorylation of p90RSK Ser380 is upregulated without upregulation of Thr573 in SARS‐CoV‐infected cells.
Figure 5.
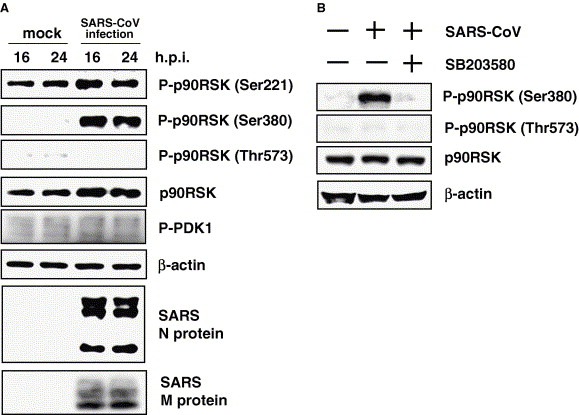
Phosphorylation of p90RSK in SARS‐CoV‐infected Vero E6 cells. (A) 1 × 106 cells in 6‐well plates were prepared (100% confluency). The cells were infected with SARS‐CoV at 50 m.o.i. Western blotting analysis was performed using proteins obtained at 16 and 24 h.p.i. (B) One hour after viral inoculation, cells were treated with http://SB203580 (20 μM). Proteins were obtained at 24 h.p.i. for Western blotting analysis. Mock‐infected cells were treated with DMSO as a control.
3.5. Phosphorylation of p90RSK Ser380 under ERK and p38 MAPK signaling pathways
These observations raise a question regarding which signaling pathway regulates phosphorylation of Ser380 of p90RSK in SARS‐CoV‐infected cells. p90RSK is thought to act in response to stimulation and p38 MAPK plays key roles in cytopathic effects in SARS‐CoV‐infected cells, as shown in Fig. 1 and in our previous study [24]. As shown in Fig. 5A, Ser380 was phosphorylated without phosphorylation of Thr573 in virus‐infected cells, suggesting that the ERK signaling pathway is not important for phosphorylation of Ser380. We next investigated whether p38 MAPK inhibitor can inhibit phosphorylation of Ser380. Confluent cells were prepared in 24‐well plates. Cells were infected with SARS‐CoV for 1 h, and then http://SB203580 was added as a p38 MAPK inhibitor. Western blotting analysis was performed using proteins at 24 h.p.i. As shown in Fig. 5B, phosphorylation of Ser380 was decreased in http://SB203580‐treated cells.
4. Discussion
In the present study, we showed that p90RSK, the best‐known substrate of ERK and PDK‐1, was regulated phosphorylation in SARS‐CoV‐infected Vero E6 cells. There has been one previous report regarding phosphorylation of p90RSK by viral infection. Rous sarcoma virus has the ability to phosphorylate p90RSK [35], but there have been no detailed analyses of p90RSK phosphorylation. Investigation of the phosphorylation status of p90RSK by viral infection is important as activation of p90RSK is involved in control of apoptosis.
Thr573 of p90RSK in mock infected cells was phosphorylated by EGF stimulation (Fig. 4A). The Thr573 was slightly phosphorylated in subconfluent mock infected cells compared with confluent mock infected cells (Fig. 4B). However, the phosphorylation was decreased by SARS‐CoV‐infection and was abolished by the MEK1/2‐specific inhibitor, http://PD98059 (data not shown). Therefore, the ERK signaling pathway is involved in phosphorylation of Thr573 in Vero E6 cells. These observations raise a question regarding the role of ERK in SARS‐CoV‐infected cells. PD98059‐treated SARS‐CoV‐infected Vero E6 cells showed no significant changes in activated caspase‐3 or ‐7 at 18 h.p.i. (data not shown). This result suggested that phosphorylation of ERK was not sufficient to prevent apoptosis by SARS‐CoV infection, as discussed previously regarding the lack of an inhibitory effect on apoptosis due to low activation of Akt in virus‐infected cells [27]. Furthermore, we found different phosphorylation kinetics between ERK1 and ERK2 in EGF‐treated and SARS‐CoV‐infected cells. Interestingly, the phosphorylation level of ERK1 is similar to that of ERK2 in SARS‐CoV‐infected Vero E6 cells (Fig. 1B). Among several experiments, the phosphorylation level of ERK1 was sometimes higher than that of ERK2, as in the case of virus‐infected Vero cells at 27 and 44 h.p.i. (Fig. 1B). The total amounts of ERK1 were lower than those of total ERK2 in both mock‐ and SARS‐CoV‐infected cells. To confirm that factors contained in seed virus do not upregulate phosphorylation of ERK1, SARS‐CoV in seed virus was completely neutralized by anti‐SARS‐CoV antibody, and then added to cells, resulting in no upregulation of the phosphorylation of ERK1/2 (data not shown). Thus, the strong phosphorylation of ERK1 occurred specifically in SARS‐CoV‐infected cells. In the case of EGF stimulation, the phosphorylation level of ERK1 was lower than that of ERK2 (Fig. 4A). Eblen et al. showed that ERK2 phosphorylates p90RSK [34]. Angenstein et al. identified p90RSK, ERK2, and GSK‐3β as poly‐associated proteins, suggesting that polyribosome‐bound ERK2 activates p90RSK, and then inhibits GSK‐3β [36]. Thus, ERK2 activation is important for phosphorylation of p90RSK in the absence of viral infection. On the other hand, the strong phoshorylation of ERK1 in SARS‐CoV‐infected cells may affect on phosphorylation status of p90RSK as discussed below.
p90RSK is phosphorylated at Thr573 in the activation loop of the C‐terminal kinase domain, and then autophosphorylation at Ser380 in the linker region is thought to be led by this C‐terminal kinase domain [11, 40]. The phosphorylation level of Ser380 in confluent Vero E6 cells was very low, and SARS‐CoV infection induced phosphorylation of Ser380 (Fig. 5). However, as described above, upregulation of Thr573 was not observed in virus‐infected cells. There may be differences in regulation of Ser380 in SARS‐CoV‐infected cells from other stimuli. In the present study, we showed that p38 MAPK can induce phosphorylation of Ser380. Several reports have suggested that p90RSK activation results in phosphorylation of CREB [2, 41]. Our previous study showed that SARS‐CoV infection of Vero E6 cells induces phosphorylation of CREB, and treatment with http://SB203580 can inhibit this phosphorylation [24]. Thus, phosphorylation of CREB is regulated by p38 MAPK in SARS‐CoV‐infected cells. In addition, phosphorylation of ERKs was partially downregulated by treatment with http://SB203580 in virus‐infected cells in viral infected cells (data not shown). Although there is a possibility of nonspecific reaction by http://SB203580, cross‐talk between ERK and p38 has been reported [37, 38, 39]. On the other hand, EGF stimulation induces phosphorylation of ERK without phosphorylation of p38 MAPK (Fig. 4A). Several signaling pathways of p38 MAPK and ERKs including cross‐talk may exist in Vero E6 cells. These results may indicate a signaling cascade, p38 MAPK > (ERK >) p90RSK > CREB, in virus‐infected cells. Further investigations are necessary to clarify the roles of p90RSK in virus‐infected cells.
Based on these results, we conclude that phosphorylation of p90RSK Ser380 is regulated by p38 MAPK, in the absence of upregulation of Thr573 phosphorylation in SARS‐CoV‐infected cells. These new observations provide valuable insights into the biological effects of p90RSK in SARS‐CoV infection.
Acknowledgements
We thank Dr. Funaba (Azabu University, Japan) for helpful suggestions. We also thank Ms. M. Ogata (National Institute of Infectious Diseases, Japan) for her assistance. This work was supported in part by the Japan Health Science Foundation and Grants‐in‐Aid for Scientific Research, Tokyo, Japan.
Mizutani Tetsuya,Fukushi Shuetsu,Saijo Masayuki,Kurane Ichiro and Morikawa Shigeru(2006), Regulation of p90RSK phosphorylation by SARS-CoV infection in Vero E6 cells, FEBS Letters, 580, doi: 10.1016/j.febslet.2006.01.066
References
- 1. Pearson G., Robinson F., Beers Gibson T., Xu B.E., Karandikar M., Berman K., Cobb M.H., Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev., 22, (2001), 153– 183. [DOI] [PubMed] [Google Scholar]
- 2. Frodin M., Gammeltoft S., Role and regulation of 90 kDa ribosomal S6 kinase (RSK) in signal transduction. Mol. Cell. Endocrinol., 151, (1999), 65– 77. [DOI] [PubMed] [Google Scholar]
- 3. Moller D.E., Xia C.H., Tang W., Zhu A.X., Jakubowski M., Human rsk isoforms: cloning and characterization of tissue-specific expression. Am. J. Physiol., 266, (1994), C351– C359. [DOI] [PubMed] [Google Scholar]
- 4. Zhao Y., Bjorbaek C., Weremowicz S., Morton C.C., Moller D.E., RSK3 encodes a novel pp90rsk isoform with a unique N-terminal sequence: growth factor-stimulated kinase function and nuclear translocation. Mol. Cell. Biol., 15, (1995), 4353– 4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yntema H.G., van den Helm B., Kissing J., van Duijnhoven G., Poppelaars F., Chelly J., Moraine C., Fryns J.P., Hamel B.C., Heilbronner H., Pander H.J., Brunner H.G., Ropers H.H., Cremers F.P., van Bokhoven H., A novel ribosomal S6-kinase (RSK4; RPS6KA6) is commonly deleted in patients with complex X-linked mental retardation. Genomics, 62, (1999), 332– 343. [DOI] [PubMed] [Google Scholar]
- 6. Bjorbeak C., Zhao Y., Moller D.E., Divergent functional roles for p90RSK kinase domains. J. Biol. Chem., 270, (1995), 18848– 18852. [DOI] [PubMed] [Google Scholar]
- 7. Fisher T.L., Blenis J., Evidence for two catalytically active kinase domains in pp90rsk. Mol. Cell. Biol., 16, (1996), 1212– 1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vik T.A., Ryder J.W., Identification of serine 380 as the major site of autophosphorylation of xenopus pp90rsk. Biochem. Biophys. Res. Commun., 235, (1997), 398– 402. [DOI] [PubMed] [Google Scholar]
- 9. Gavin A.C., Nebreda A.R., A MAP kinase docking site is required for phosphorylation and activation of p90rsk/MAPKAP kinase-1. Curr. Biol., 9, (1999), 281– 284. [DOI] [PubMed] [Google Scholar]
- 10. Smith J.A., Poteet-Smith C.E., Malarkey K., Sturgill T.W., Identification of an extracellular signal-regulated kinase (ERK) docking site in ribosomal S6 kinase, a sequence critical for activation by ERK in vivo. J. Biol. Chem., 274, (1999), 2893– 2898. [DOI] [PubMed] [Google Scholar]
- 11. Vik T.A., Sweet L.J., Erikson R.L., Coinfection of insect cells with recombinant baculovirus expressing pp60v-src results in the activation of a serine-specific protein kinase pp90rsk. Proc. Natl. Acad. Sci. USA, 87, (1990), 2685– 2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frödin M., Jensen C.J., Merienne K., Gammeltoft S., A phosphoserine-regulated docking site in the protein kinase RSK2 that recruits and activates PDK1. EMBO J., 19, (2000), 2924– 2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jensen C.J., Buch M.B., Krag T.O., Hemmings B.A., Gammeltoft S., Frodin M., 90-kDa ribosomal S6 kinase is phosphorylated and activated by 3-phosphoinositide-dependent protein kinase-1. J. Biol. Chem., 274, (1999), 27168– 27176. [DOI] [PubMed] [Google Scholar]
- 14. Richards S.A., Fu J., Romanelli A., Shimamura A., Blenis J., Ribosomal S6 kinase 1 (RSK1) activation requires signals dependent on and independent of the MAP kinase ERK. Curr. Biol., 9, (1999), 810– 820. [DOI] [PubMed] [Google Scholar]
- 15. Bonni A., Brunet A., West A.E., Datta S.R., Takasu M.A., Greenberg M.E., Cell survival promoted by the Ras-MAPK signaling pathway by transcription-dependent and -independent mechanisms. Science, 286, (1999), 1358– 1362. [DOI] [PubMed] [Google Scholar]
- 16. Dalby K.N., Morrice N., Caudwell F.B., Avruch J., Cohen P., Identification of regulatory phosphorylation sites in mitogenactivated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J Biol. Chem., 273, (1988), 1496– 1505. [DOI] [PubMed] [Google Scholar]
- 17. Buck M., Poli V., Hunter T., Chojkier M., C/EBPβ phosphorylation by RSK creates a functional XEXD caspase inhibitory box critical for cell survival. Mol. Cell, 8, (2001), 807– 816. [DOI] [PubMed] [Google Scholar]
- 18. Palmer A., Gavin A.C., Nebreda A.R., A link between MAP kinase and p34cdc2/cyclin B during oocyte maturation: p90rsk phosphorylates and inactivates the p34cdc2 inhibitory kinase Myt1. EMBO J., 17, (1998), 5037– 5047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chun J., Chau A.S., Maingat F.G., Edmonds S.D., Ostergaard H.L., Shibuya E.K., Phosphorylation of Cdc25C by pp90Rsk contributes to a G2 cell cycle arrest in Xenopus cycling egg extracts. Cell Cycle, 4, (2005), 148– 154. [DOI] [PubMed] [Google Scholar]
- 20. Paronetto M.P., Giorda E., Carsetti R., Rossi P., Geremia R., Sette C., Functional interaction between p90Rsk2 and Emi1contributes to the metaphase arrest of mouse oocytes. EMBO J., 23, (2004), 4649– 4659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Itoh S., Ding B., Bains C.P., Wang N., Takeishi Y., Jalili T., King G.L., Walsh R.A., Yan C., Abe J., Role of p90 ribosomal S6 kinase (p90RSK) in reactive oxygen species and protein kinase Cβ (PKC-β)-mediated cardiac troponin I phosphorylation. J. Biol. Chem., 280, (2005), 24135– 24142. [DOI] [PubMed] [Google Scholar]
- 22. Marra M.A., Jones S.J., Astell C.R., Holt R.A., Brooks-Wilson A., Butterfield Y.S., Khattra J., Asano J.K., Barber S.A., Chan S.Y., Cloutier A., Coughlin S.M., Freeman D., Girn N., Griffith O.L., Leach S.R., Mayo M., McDonald H., Montgomery S.B., Pandoh P.K., Petrescu A.S., Robertson A.G., Schein J.E., Siddiqui A., Smailus D.E., Stott J.M., Yang G.S., Plummer F., Andonov A., Artsob H., Bastien N., Bernard K., Booth T.F., Bowness D., Czub M., Drebot M., Fernando L., Flick R., Garbutt M., Gray M., Grolla A., Jones S., Feldmann H., Meyers A., Kabani A., Li Y., Normand S., Stroher U., Tipples G.A., Tyler S., Vogrig R., Ward D., Watson B., Brunham R.C., Krajden M., Petric M., Skowronski D.M., Upton C., Roper R.L., The genome sequence of the SARS-associated coronavirus. Science, 300, (2003), 1399– 1404. [DOI] [PubMed] [Google Scholar]
- 23. Rota P.A., Oberste M.S., Monroe S.S., Nix W.A., Campagnoli R., Icenogle J.P., Penaranda S., Bankamp B., Maher K., Chen M.H., Tong S., Tamin A., Lowe L., Frace M., DeRisi J.L., Chen Q., Wang D., Erdman D.D., Peret T.C., Burns C., Ksiazek T.G., Rollin P.E., Sanchez A., Liffick S., Holloway B., Limor J., McCaustland K., Olsen- Rasmussen M., Fouchier R., Gunther S., Osterhaus A.D., Drosten C., Pallansch M.A., Anderson L.J., Bellini W.J., Characterization of a novel coronavirus associated with severe acute respiratory syndrome. Science, 300, (2003), 1394– 1399. [DOI] [PubMed] [Google Scholar]
- 24. Mizutani T., Fukushi S., Saijo M., Kurane I., Morikawa S., Phosphorylation of p38 MAPK and its downstream targets in SARS coronavirus-infected cells. Biochem. Biophys. Res. Commun., 319, (2004), 1228– 1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mizutani T., Fukushi S., Murakami M., Hirano T., Saijo M., Kurane I., Morikawa S., Tyrosine dephosphorylation of STAT3 in SARS coronavirus-infected Vero E6 cells. FEBS Lett., 577, (2004), 187– 192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mizutani T., Fukushi S., Saijo M., Kurane I., Morikawa S., JNK and PI3k/Akt signaling pathways are required for establishing persistent SARS-CoV infection in Vero E6 cells. Biochem. Biophys. Acta, 1741, (2005), 4– 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mizutani T., Fukushi S., Saijo M., Kurane I., Morikawa S., Importance of Akt signaling pathway for apoptosis in SARS-CoV-infected Vero E6 cells. Virology, 327, (2004), 169– 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Watanabe H., de Caestecker M.P., Yamada Y., Transcriptional cross-talk between Smad, ERK1/2, and p38 mitogen-activated protein kinase pathways regulates transforming growth factor-beta-induced aggrecan gene expression in chondrogenic ATDC5 cells. J. Biol. Chem., 276, (2001), 14466– 14473. [DOI] [PubMed] [Google Scholar]
- 29. Surjit M., Liu B., Jameel S., Chow V.T., Lal S.K., The SARS coronavirus nucleocapsid protein induces actin reorganization and apoptosis in COS-1 cells in the absence of growth factors. Biochem. J., 383, (2004), 13– 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. He R., Leeson A., Andonov A., Li Y., Bastien N., Cao J., Osiowy C., Dobie F., Cutts T., Ballantine M., Li X., Activation of AP-1 signal transduction pathway by SARS coronavirus nucleocapsid protein. Biochem. Biophys. Res. Commun., 311, (2003), 870– 876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tan Y.J., Fielding B.C., Goh P.Y., Shen S., Tan T.H., Lim S.G., Hong W., Overexpression of 7a, a protein specifically encoded by the severe acute respiratory syndrome coronavirus, induces apoptosis via a caspase-dependent pathway. J. Virol., 78, (2004), 14043– 14047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chang Y.J., Liu C.Y., Chiang B.L., Chao Y.C., Chen C.C., Induction of IL-8 release in lung cells via activator protein-1 by recombinant baculovirus displaying severe acute respiratory syndrome-coronavirus spike proteins: identification of two functional regions. J. Immunol., 173, (2004), 7602– 7614. [DOI] [PubMed] [Google Scholar]
- 33. Zeniou M., Ding T., Trivier E., Hanauer A., Expression analysis of RSK gene family members: the RSK2 gene, mutated in Coffin–Lowry syndrome, is prominently expressed in brain structures essential for cognitive function and learning. Hum. Mol. Genet., 11, (2002), 2929– 2940. [DOI] [PubMed] [Google Scholar]
- 34. Eblen S.T., Catling A.D., Assanah M.C., Weber M.J., Biochemical and biological functions of the N-terminal, noncatalytic domain of extracellular signal-regulated kinase 2. Mol. Cell. Biol., 21, (2001), 249– 259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang H.C., Erikson R.L., Activation of protein serine/threonine kinases p42, p63, and p87 in Rous sarcoma virus-transformed cells: signal transduction/transformation-dependent MBP kinases. Mol. Biol. Cell., 3, (1992), 1329– 1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Angenstein F., Greenough W.T., Weiler I.J., Metabotropic glutamate receptor-initiated translocation of protein kinase p90rsk to polyribosomes: a possible factor regulating synaptic protein synthesis. Proc. Natl. Acad. Sci. USA, 95, (1998), 15078– 15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xiao Y.Q., Malcolm K., Worthen G.S., Gardai S., Schiemann W.P., Fadok V.A., Bratton D.L., Henson P.M., Cross-talk between ERK and p38 MAPK mediates selective suppression of pro-inflammatory cytokines by transforming growth factor-β. J. Biol. Chem., 277, (2002), 14884– 14893. [DOI] [PubMed] [Google Scholar]
- 38. Mizutani, T., Fukushi, S., Iizuka, D., Inanami, O., Kuwabara, M., Takashima, H., Yanagawa, H., Saijo, M., Kurane, I. and Morikawa, S. Inhibition of cell proliferation by SARS-CoV infection in Vero E6 cells. FEMS Immunol. Med. Microbiol. (in press). [DOI] [PMC free article] [PubMed]
- 39. Houliston R.A., Pearson J.D., Wheeler-Jones C.P.D., Agonist-specific cross talk between ERKs and p38mapk regulates PGI2 synthesis in endothelium. Am. J. Physiol. Cell Physiol., 281, (2001), C1266– C1276. [DOI] [PubMed] [Google Scholar]
- 40. Grove J.R., Price D.J., Banerjee P., Balasubramanyam A., Ahmad M.F., Avruch J., Regulation of an epitope-tagged recombinant Rsk-1 S6 kinase by phorbol ester and erk/MAP kinase. Biochemistry, 32, (1993), 7727– 7738. [DOI] [PubMed] [Google Scholar]
- 41. Böhm M., Moellmann G., Cheng E., Alvarez-Franco M.S.W., Sassone-Corsi P.R.H., Identification of p90RSK as the probable CREB-Ser133 kinase in human melanocytes. Cell Growth Differ., 6, (1955), 291– 302. [PubMed] [Google Scholar]


