Abstract
Objectives
To report epidemiological features, clinical characteristics, and outcomes of human rhinovirus (HRV) infections in comparison with other community acquired respiratory virus (CRV) infections in patients hospitalized for two consecutive years.
Methods
This was a cross-sectional study. Clinical, epidemiological, and laboratory data of patients hospitalized with acute respiratory syndrome in a tertiary care hospital from 2012 to 2013 were reviewed.
Results
HRV was the most common CRV observed (36%, 162/444) and was present in the majority of viral co-detections (69%, 88/128), mainly in association with human enterovirus (45%). Most HRV-infected patients were younger than 2 years (57%). Overall, patients infected with HRV had a lower frequency of severe acute respiratory infection than those infected with other CRVs (60% and 84%, respectively, p = 0.006), but had more comorbidities (40% and 27%, respectively; p = 0.043). However, in the adjusted analysis this association was not significant. The mortality rate within the HRV group was 3%. Detection of HRV was more prevalent during autumn and winter, with a moderately negative correlation between viral infection frequency and temperature (r = −0.636, p < 0.001) but no correlation with rainfall (r = −0.036, p = 0.866).
Conclusion
HRV is usually detected in hospitalized children with respiratory infections and is often present in viral co-detections. Comorbidities are closely associated with HRV infections. These infections show seasonal variation, with predominance during colder seasons.
Keywords: Human rhinovirus, Acute respiratory infections, Respiratory virus
Resumo
Objetivos
Relatar as características epidemiológicas, as características clínicas e os resultados das infecções por rinovírus humano (RVH) em comparação a outras infecções por vírus respiratórios adquiridos na comunidade (VRCs) em pacientes internados por dois anos consecutivos.
Métodos
Este foi um estudo transversal. Foram revisados os dados clínicos, epidemiológicos e laboratoriais de pacientes internados com síndrome respiratória aguda em um hospital terciário de 2012 a 2013.
Resultados
O RVH foi o VRC mais comum observado (36%, 162/444) e esteve presente na maior parte das codetecções virais (69%, 88/128), principalmente em associação ao enterovírus humano (45%). A maioria dos pacientes infectados por RVH possuía menos de 2 anos (57%). De modo geral, os pacientes com RVH apresentaram uma menor frequência de infecção respiratória aguda grave que os pacientes infectados por outros VRCs (60% e 84%, respectivamente, p = 0,006), porém mais comorbidades (40% e 27%, respectivamente; p = 0,043). Contudo, em uma análise ajustada, essa associação não foi significativa. A taxa de mortalidade no grupo RVH foi 3%. A detecção de RVH foi mais prevalente durante o outono e inverno, com uma correlação negativa moderada entre a frequência de infecção viral e a temperatura (r = -0,636, p < 0,001), porém nenhuma correlação com a precipitação (r = -0,036, p = 0,866).
Conclusão
O RVH é normalmente detectado em crianças internadas com infecções respiratórias e normalmente está presente em codetecções virais. As comorbidades estão estreitamente associadas a infecções por RVH. Essas infecções mostram variação sazonal, com predominância durante as estações mais frias.
Palavras-chave: Rinovírus humano, Infecções respiratórias agudas, Vírus respiratório
Introduction
Human rhinoviruses (HRVs) belong to Picornaviridae family, genus Enterovirus, and are divided in three species (HRV-A, HRV-B, and HRV-C) with about 100 serotypes within these species.1, 2 The development of highly-sensitive molecular techniques for characterization of the HRV genome has recently allowed recognition of the HRV-C species.3 There is already evidence that this new species may be more virulent and more strongly associated with lower respiratory tract infections than HRV-A and HRV-B.4
HRV is the most common cause of upper respiratory tract infections, being responsible for at least 50% of cases of the common cold. This leads to considerable economic burden in terms of medical visits and both school and work absenteeism.2, 4 HRVs have also been linked to lower airway effects that result in significant morbidity and mortality,1 such as exacerbations of chronic pulmonary disease, severe bronchiolitis in infants and children, as well as fatal pneumonia in elderly and immunocompromised adults.4, 5 In general, HRV infections occur during spring and autumn,6 and manifest differently depending on whether the lower or upper respiratory tract is infected. Infections of the upper respiratory tract ordinarily include symptoms of the common cold, but can present as acute otitis media or rhinosinusitis. On the other hand, infections of the lower respiratory tract can cause severe symptoms and result in bronchiolitis and pneumonia.4
In Brazil, since the influenza A pandemic of 2009, referral hospitals have been conducting active surveillance to detect respiratory viruses. Such surveillance includes notification and laboratory investigation of cases meeting the diagnostic criteria of severe acute respiratory infection (SARI). This viral respiratory infection monitoring has resulted in important information about the circulation of other community-acquired respiratory viruses (CRV). The present study reports the epidemiological features, clinical characteristics, and outcomes of HRV infections in comparison with other CRV infections in patients hospitalized in a referral hospital in Southern Brazil, for two consecutive years.
Methods
Patient selection and data acquisition
Patients hospitalized at an academic tertiary care center in Southern Brazil from whom respiratory samples were collected and sent for investigation, or who were diagnosed with SARI in 2012 or 2013, were included in the study. Respiratory samples (nasal swab or nasopharyngeal aspirate, or bronchoalveolar lavage) were collected in different periods of hospitalization according to medical recommendation. Individuals with more than one sample collected during the same symptomatic period were considered as a single case and only the first result was evaluated, so that the number of respiratory viral detections was not overestimated. The medical records and influenza notification forms of patients with detectable respiratory virus were reviewed, focusing on epidemiology, clinical manifestation, outcome, laboratory findings, and diagnosis of SARI. SARI was defined as a flu-like syndrome with signs of severity (dyspnea or oxygen saturation below 95%).
During the study, a total of 1002 cases were identified in both databases. Of these, five cases were excluded because the researchers could not access the medical records and a further 242 were excluded as no samples had been sent for virus detection. Thus, 755 patients with respiratory samples investigated for respiratory virus detection were included. The Institutional Ethics Review Board approved the study (No. #18714013.4.0000.0096).
Respiratory virus (RV) detection
RVs were detected using a multiplex reverse transcription polymerase chain reaction (RT-PCR) technique. The viral genome was extracted using a High Pure Viral RNA Kit (Roche Inc., Mannheim, Germany) in accordance with the manufacturer's instructions. First strand cDNA synthesis was achieved using random primers and an ImProm-II reverse transcription system (Promega Inc., WI, USA). The resulting cDNA was then subject to PCR by using a Seeplex® RV15 ACE Detection Kit (Seegene Inc., Korea), in accordance with the manufacturer's protocol. This multiplex PCR technology enables a simultaneous detection of 15 respiratory viruses: human adenovirus (AdV), human metapneumovirus (MPV), parainfluenza virus type 1, 2, 3, and 4 (PIV-1, PIV-2, PIV-3, and PIV-4), influenza A (FLUA), influenza B (FLUB), respiratory syncytial virus type A and B (RSV-A, RSV-B), human rhinovirus types A/B/C (HRV), human enterovirus (EV), human bocavirus (BoV), and human coronavirus (CoV) types 229E/NL63 (alpha coronaviruses) and OC43/HKU1 (beta coronaviruses).
Statistical analysis
Data were compiled using JMP version 5.2.1 (SAS Institute Inc., Cary, NC, USA) and analyzed using GraphPad Prism version 5.03 (GraphPad Software Inc., CA, USA). Parametric and non-parametric tests were used as appropriate. The nonparametric Spearman correlation coefficient was used to analyze meteorological data. Variables with an associated p-value < 0.05 in the univariate analysis and those considered as confounding factors (age and length of hospitalization) were subjected to multivariate logistic regression to identify independent predictors for severe disease. All p-values were two-tailed and a value of <0.05 was considered significant.
Meteorological data
Curitiba is located in Southern Brazil and has a temperate climate. Data on monthly measures of temperature and rainfall were supplied by the Meteorological System of Paraná (Sistema Meteorológico do Paraná [SIMEPAR]).
Results
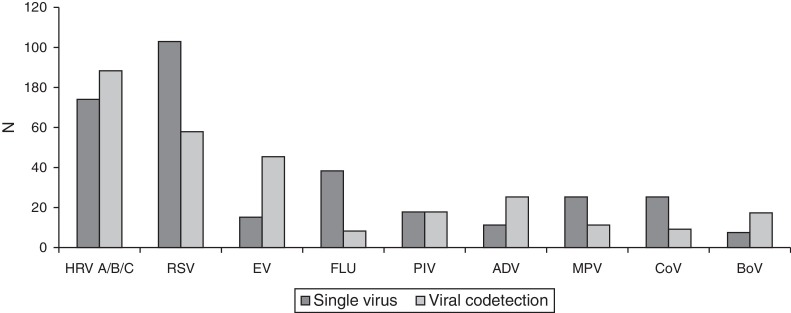
A total of 444 (444/755; 59%) samples were positive for CRV infection, including 201 (201/444; 45%) cases in 2012 and 243 (55%) in 2013. The most frequently detected viruses were HRV (37%) and RSV (36%; Fig. 1 ). There were 162 cases positive for HRV over the study period: 83 (51%) in 2012 and 79 (49%) in 2013, with 282 cases of other respiratory viruses infections during the same period.
Figure 1.
Respiratory virus detected in hospitalized patients with acute respiratory infection in a tertiary hospital, Southern Brazil, 2012–2013 (n = 755). HRV A/B/C, human rhinovirus types A/B/C; RSV, respiratory syncytial virus; EV, enterovirus; FLU, influenza A and B viruses; PIV, parainfluenza viruses; ADV, adenovirus; MPV, human metapneumovirus; CoV, human coronaviruses; BoV, human bocavirus.
To evaluate the epidemiological profile and clinical impact HRV infections, data from HRV infected patients were compared with those with other CRV infections, groups 1 and 2, respectively) (Table 1 ). For this analysis, only cases with single infections were included, excluding nosocomial infections, since given that patients with nosocomial infections were already in the hospital, it would be expected that they would have some underlying illness, which could overestimate the severity of infections. Most HRV infected patients were younger than 2 years (57%), 53% were younger than 1 year, and 37% were younger than 6 months of age. The groups showed no statistically significant difference in median age. Gender was unequally distributed between the groups.
Table 1.
Comparison of the clinical and epidemiological profile of patients hospitalized with HRV- vs. other CRV-monoinfections, 2012–2013.
| HRV (group 1) | CRV (group 2) | Unadjusted analyses | Adjusted analyses | |
|---|---|---|---|---|
| n = 60 (%) | n = 196 (%) | p-value | p-value | |
| Gender | ||||
| Male | 35 (58) | 92 (47) | 0.14 | – |
| Age | ||||
| <2 years | 34 (57) | 138 (70) | ||
| 2–5 years | 9 (15) | 15 (8) | ||
| 5–14 years | 4 (7) | 10 (6) | ||
| 14–50 years | 7 (12) | 11 (7) | ||
| >50 years | 6 (9) | 17 (9) | ||
| Median, years | 1.3 | 0.6 | 0.16 | NS |
| (IQR) | (0.2–8) | (0.2–2.8) | ||
| Length of hospitalization | ||||
| <5 days | 30 (50) | 49 (25) | ||
| 5–15 days | 24 (40) | 109 (55) | ||
| 15–30 days | 4 (7) | 19 (10) | ||
| >30 days | 2 (3) | 19 (10) | ||
| Median, days | 4.5 | 7 | 0.0032 | NS |
| (IQR) | (2–9) | (4.2–12) | ||
| Symptoms | ||||
| Fever | 37 (62) | 168 (85) | 0.001 | NS |
| Cough | 55 (92) | 189 (96) | 0.15 | |
| Dyspnea | 56 (93) | 183 (93) | 0.95 | |
| Radiological findings | ||||
| Missed | 34 (57) | 76 (39) | ||
| Normal | 6 (10) | 18(9) | 0.98 | |
| Interstitial infiltrate | 8 (13) | 38 (19) | ||
| Pulmonary consolidation | 9 (15) | 35 (15) | ||
| Mixed patterns | 2 (3) | 13 (9) | ||
| Other findingsa | 1 (2) | 16 (9) | ||
| Comorbidities | ||||
| Yes | 24 (40) | 52 (27) | 0.043 | 0.033 |
| Immunosuppression | 10 (17) | 23 (12) | 0.37 | |
| Chronic lung disease | 10 (17) | 24 (12) | 0.37 | |
| Chronic heart disease | 5 (8) | 9 (5) | 0.067 | |
| Mechanical ventilation | 12 (20) | 36 (18) | 0.72 | |
| ICU | 18 (30) | 65 (33) | 0.60 | |
| Death | 2 (3) | 6 (3) | 0.53 | |
| SARI diagnosis | 36 (60) | 165 (84) | 0.006 | NS |
HRV, human rhinovirus; CRV, community-acquired respiratory virus; IQR, interquartile range, ICU, intensive care unit; SARI, severe acute respiratory infections; NS, not significant, OR, odds ratio.
Bold values are in statistically significant.
Pleural effusion, atelectasis.
The main clinical manifestations observed in both groups included fever, cough, and dyspnea. However, the HRV positive group had a significantly lower proportion of cases with fever (62%, p = 0.001), and more comorbidities than the other group (40% vs. 27%, p = 0.043). Although immunosuppression, chronic lung disease, and heart disease were the major medical conditions present in both groups, chronic lung disease was more frequent in the HRV group (17%), but without significant difference. Concerning the diagnosis of SARI, there were fewer patients diagnosed with this syndrome in the HRV group (60%, p = 0.006) than in the other CRV group. In the multivariate analysis of the relationship between clinical characteristics significantly different between the HRV- and other CRV-groups, only the higher prevalence of previous comorbidities in the HRV group was significant. Patients with no comorbidities were 1.6 (95% CI: 1.04–2.34) times more likely to be infected CRVs other than HRV, in comparison to patients with comorbidities.
Despite the fact that most of chest X-rays of HRV infected cases were missed (53%), radiologic alterations were described in 83% of the performed exams. Most of the findings observed were interstitial infiltrate (33%) and pulmonary consolidation (28%), with no significant difference between both groups.
Severe disease was defined as that requiring mechanical ventilatory support, or intensive care unit (ICU) admission, or death during hospitalization; no significant difference was found between the groups. Two HRV infected patients died during hospitalization, both from respiratory infection complications.
HRV had the highest rates of coinfection (69%) of the 128 samples with more than one CRV detected. The viruses most co-detected with HRV were EV (40/88; 45%) and RSV (29/88; 33%), as shown in Fig. 1. In 52% of cases, the co-detection involved HRV plus another virus, and in 16% of cases, HRV was associated with two or more viruses. In 65 cases, HRV was the only virus detected.
A comparison of the clinical characteristics of mono-infected HRV patients with co-infected HRV patients was performed, and no relation between viral co-detection and disease severity (p = 0.717) was found. The clinical features significantly associated with HRV co-detection were as follows: age 6–48 months (OR = 3.2; 95% CI: 1.3–8.1), length of hospitalization more than 30 days (OR = 9.7; 95% CI: 2.5–36.8), and no SARI diagnosis (OR = 4.0; 95% CI: 1.7–9.3). As co-infection with HRV alone was not related with severe disease, a second analysis was performed comparing disease severity between HRV co-infected patients and patients infected with other CRVs. Neither the presence nor absence of HRV co-detection was associated with disease severity (p = 0.196).
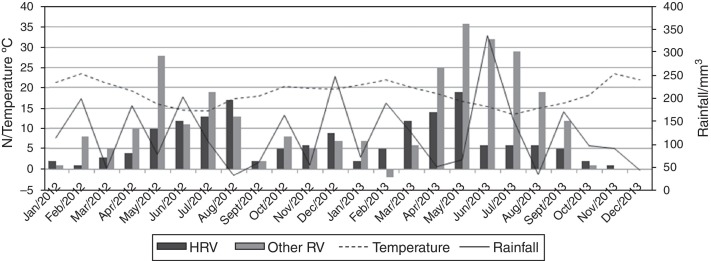
Cases of HRV infection occurred throughout the study period (Fig. 2 ). The months of May to August demonstrated the highest number of HRV infections during 2012, peaking in August (17 cases; 10%). In 2013, the seasonality of HRV infection was from March to May, peaking in May (19 cases; 12%). Comparing the monthly distribution of HRV cases with average temperature (°C) and rainfall (mm), a negative correlation between the number of HRV cases and the average temperature (rs = −0.636, p < 0.001) was demonstrated, but there was no significant correlation with rainfall (rs = −0.036, p = 0.866).
Figure 2.
Human rhinovirus infections: seasonality, monthly median temperature, and rainfall data, 2012–2013, Curitiba, Brazil. HRV, human rhinovirus; RV, respiratory viruses.
Discussion
Since the implementation of systematic investigation into respiratory viruses in hospitalized patients with SARI, HRV has been found with high frequency, either alone or co-detected with other respiratory viruses. Since some of these infections may have a poor prognosis, including death, it is critical to know and better characterize this infection to be prepared for its early diagnosis, appropriate clinical management, and prevention of nosocomial spread.
The high frequency of HRV found in clinical samples from patients with SARI was similar to that reported by Kim et al.7 in a tertiary hospital in Korea. He et al.8 analyzed a hospitalized pediatric population and found a prevalence of 48%, while Walker and Ison9 found a prevalence of 14% in a hospitalized adult population. This difference between age groups was observed in this study, emphasizing the greater vulnerability of children to this infection, probably as consequence of a more intense inflammatory process triggered by primary infection or by favorable anatomical conditions of the respiratory tract in younger children.
RSV was the first pathogen identified to be associated with the severe respiratory disease in children. However, since the development of molecular techniques to detect HRV, this virus has been the focus of the most recent studies in this field. Nowadays, HRV infections have been linked with exacerbations of chronic lung diseases and fatal pneumonia in patients at the extremes of age or with pre-existing comorbidities.4, 5 The importance of one study that evaluated the characteristics of HRV infections in a tertiary hospital is based on these new concerns. Marcone et al.10 reported a higher number of HRV infections in hospitalized children in Argentina when compared with pediatric outpatients with acute respiratory infection, demonstrating a scenario that contributed to the increased concern about HRV infection in the hospital setting.
Previous studies have reported that the HRV infection rate in hospitals varies from 21% to 41%.8, 11, 12 However, this variation is probably accounted for by the differences in age and clinical characteristics of patients analyzed in each study. Although this study included all age groups, a predominance of pediatric patients infected with HRV was observed, mostly younger than 1 year old. In addition, the HRV-positive group had a greater proportion of patients with pre-existing comorbidities than the group with other identified CRVs.6, 7, 11, 12, 13, 14 Among the comorbidities observed in this study, chronic lung diseases were frequent in the HRV group, a correlation that has already been demonstrated in another similar study,14 which corroborates the close association of HRV infection and exacerbations of chronic lung diseases, such as chronic obstructive pulmonary disease (COPD), asthma, and cystic fibrosis.4
Of 444 samples positive for CRVs, 128 samples (29%) had at least two co-detected viruses, in keeping with the expected 10–31% encountered in hospitalized individuals.7, 8, 15 The viruses most frequently co-detected with HRV were EV (45%), RSV (33%), and AdV (18%); consistent with previously reported patterns.14, 15 However, the high frequency of EV and HRV co-detection may be as a result of using the 5′NRT region of the viral genome to identify both pathogens, which may have lowered the specificity of the RT-PCR test.16 A more appropriate means to discern between these species would be to carry out the RT-PCR followed by nucleotide sequencing of amplicons.17 The high level of dual infection involving HRV and RSV is often explained both by the coexistence of these viruses throughout the year and their similar seasonal variation,15 but this hypothesis is not unanimous.12
In contrast to findings reported by Goka et al.,15 this study did not show an association between disease severity and viral co-detections. Furthermore, HRV was not found to have a protective influence in cases where it was involved in co-detection, as there was no significant difference in disease severity when comparing the groups with or without HRV co-detection. Likewise, in contradiction to the present findings, Asner et al.18 observed an increased disease severity in children infected with HRV alone. Among the probable factors associated with these contrasting findings, the following may be cited: (i) analysis in different groups of patients (outpatient and hospitalized), (ii) assessing a small number of patients, and (iii) adoption of different severity criteria.
Fever was less frequent in patients infected with HRV compared to other CRVs.19, 20 Chest X-ray findings were normal in 17%, showed interstitial infiltrate in 33%, and demonstrated pulmonary consolidation in 28% of cases, with no significant difference between the HRV and other CRV groups. These patterns are similar to those reported by Fica et al.11 in hospitalized adults in Chile. There were significantly fewer cases of SARI diagnosed in the patients infected with HRV than other CRVs. Overall, 14% (110/770) of patients with SARI were infected with HRV. This concurs with a previous study.20
Reports about the seasonality of HRV infection, including a study from Argentina,10 which is in close geographical proximity of Southern Brazil, show that it circulates mainly in autumn and spring.7, 8, 19 Although HRV occurred in almost all months of the study period, different peaks were observed in 2012 and 2013, and neither peak included spring. In 2013, the highest prevalence of HRV infection was in autumn, followed by winter, and vice versa in 2012. The analysis of meteorological data found a negative correlation between the number of HRV cases and the average temperature, but no significant correlation with rainfall. However, in order to more accurately establish the seasonality of HRV infections, the analysis should include additional years.
This research had some limitations: (i) it was a retrospective study and some medical records were incomplete; (ii) HRV species were not identified, which would have yielded important data since the genotypes reportedly have different virulence; (iii) this analysis was carried out only with hospitalized patients, which may have overestimated the impact of HRV infection; and (iv) it was not possible to evaluate the frequency of nosocomial HRV infection, data critical to guide preventive measures in the most affected settings. However, this is the first report about HRV infections in the region and a critical analysis of the data is important to obtain greater awareness of the dynamics of dispersion and impact of these respiratory viruses in the community.
In conclusion, HRV has a high prevalence in the hospitalized children and was present in cases of severe disease, including death. However, a dependent relationship between the presence of HRV in viral co-detections and severity of disease was not observed in this study. Conflicts in the literature demand a closer analysis of cases of co-detection involving HRV with review of the data to determine the impact of this, since the lack of standardization between studies probably contributes to the divergent data. HRV infection is closely associated with comorbidities, mainly chronic lung diseases, and is an important factor in exacerbations of these underlying lung diseases. The colder seasons were the period with higher frequency of HRV infections in Southern Brazil, and therefore must be a period to warn clinicians about young age children affected by respiratory infections, especially those with comorbidities such as chronic lung disease. Patients with this profile should have respiratory samples collected to identify possible viral infection, and if HRV is detected, the medical staff should be ready for adequate management, taking into consideration the possible poor outcomes.
Funding
SMR is sponsored by a CNPq fellowship.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Please cite this article as: Leotte J, Trombetta H, Faggion HZ, Almeida BM, Nogueira MB, Vidal LR, et al. Impact and seasonality of human rhinovirus infection in hospitalized patients for two consecutive years. J Pediatr (Rio J). 2017;93:294–300.
References
- 1.Palmenberg A.C., Rathe J.A., Liggett S.B. Analysis of the complete genome sequences of human rhinovirus. J Allergy Clin Immunol. 2010;125:1190–1201. doi: 10.1016/j.jaci.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gern J.E. The ABCs of rhinoviruses, wheezing, and asthma. J Virol. 2010;84:7418–7426. doi: 10.1128/JVI.02290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bochkov Y.A., Gern J.E. Clinical and molecular features of human rhinovirus C. Microbes Infect. 2012;14:485–494. doi: 10.1016/j.micinf.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobs S.E., Lamson D.M., St. George K., Walsh T.J. Human rhinoviruses. Clin Microbiol Rev. 2013;26:135–162. doi: 10.1128/CMR.00077-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson D.J., Gangnon R.E., Evans M.D., Roberg K.A., Anderson E.L., Pappas T.E. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178:667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Litwin C.M., Bosley J.G. Seasonality and prevalence of respiratory pathogens detected by multiplex PCR at a tertiary care medical center. Arch Virol. 2014;159:65–72. doi: 10.1007/s00705-013-1794-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J.K., Jeon J.S., Kim J.W., Rheem I. Epidemiology of respiratory viral infection using multiplex rt-PCR in Cheonan, Korea (2006–2010) J Microbiol Biotechnol. 2013;23:267–273. doi: 10.4014/jmb.1212.12050. [DOI] [PubMed] [Google Scholar]
- 8.He Y., Lin G.Y., Wang Q., Cai X.Y., Zhang Y.H., Lin C.X. A 3-year prospective study of the epidemiology of acute respiratory viral infections in hospitalized children in Shenzhen, China. Influenza Other Respir Viruses. 2014;8:443–451. doi: 10.1111/irv.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker E., Ison M.G. Respiratory viral infections among hospitalized adults: experience of a single tertiary healthcare hospital. Influenza Other Respir Viruses. 2014;8:282–292. doi: 10.1111/irv.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marcone D.N., Culasso A., Carballal G., Campos R., Echavarría M. Genetic diversity and clinical impact of human rhinoviruses in hospitalized and outpatient children with acute respiratory infection, Argentina. J Clin Virol. 2014;61:558–564. doi: 10.1016/j.jcv.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fica A., Dabanch J., Andrade W., Bustos P., Carvajal I., Ceroni C. Clinical relevance of rhinovirus infections among adult hospitalized patients. Braz J Infect Dis. 2015;19:118–124. doi: 10.1016/j.bjid.2014.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greer R.M., McErlean P., Arden K.E., Faux C.E., Nitsche A., Lambert S.B. Do rhinoviruses reduce the probability of viral co-detection during acute respiratory tract infections? J Clin Virol. 2009;45:10–15. doi: 10.1016/j.jcv.2009.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pretorius M.A., Madhi S.A., Cohen C., Naidoo D., Groome M., Moyes J. Respiratory viral coinfections identified by a 10-plex real-time reverse-transcription polymerase chain reaction assay in patients hospitalized with severe acute respiratory illness – South Africa, 2009–2010. J Infect Dis. 2012;206:S159–S165. doi: 10.1093/infdis/jis538. [DOI] [PubMed] [Google Scholar]
- 14.Rahamat-Langendoen J.C., Riezebos-Brilman A., Hak E., Schölvinck E.H., Niesters H.G. The significance of rhinovirus detection in hospitalized children: clinical, epidemiological and virological features. Clin Microbiol Infect. 2013;19:E435–E442. doi: 10.1111/1469-0691.12242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goka E.A., Vallely P.J., Mutton K.J., Klapper P.E. Single, dual and multiple respiratory virus infections and risk of hospitalization and mortality. Epidemiol Infect. 2015;143:37–47. doi: 10.1017/S0950268814000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gharabaghi F., Hawan A., Drews S.J., Richardson S.E. Evaluation of multiple commercial molecular and conventional diagnostic assays for the detection of respiratory viruses in children. Clin Microbiol Infect. 2011;17:1900–1906. doi: 10.1111/j.1469-0691.2011.03529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva E.R., Watanabe A.S., Carraro E., Perosa A.H., Granato C.F., Bellei N.C. Rhinovirus detection using different PCR-based strategies. Braz J Microbiol. 2012;43:739–743. doi: 10.1590/S1517-83822012000200038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Asner S.A., Rose W., Petrich A., Richardson S., Tran D.J. Is virus coinfection a predictor of severity in children with viral respiratory infections? Clin Microbiol Infect. 2015;21:e1–e6. doi: 10.1016/j.cmi.2014.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aponte F.E., Taboada B., Espinoza M.A., Arias-Ortiz M.A., Monge-Martínez J., Rodríguez-Vázquez R. Rhinovirus is an important pathogen in upper and lower respiratory tract infections in Mexican children. Virol J. 2015;12:31. doi: 10.1186/s12985-015-0262-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radin J.M., Hawksworth A.W., Kammerer P.E., Balansay M., Raman R., Lindsay S.P. Epidemiology of pathogen-specific respiratory infections among three US populations. PLOS ONE. 2014;9:e114871. doi: 10.1371/journal.pone.0114871. [DOI] [PMC free article] [PubMed] [Google Scholar]