Graphical Abstract
Keywords: Extracellular vesicles, liver, liver disease, biomarkers, liquid biopsy
Introduction
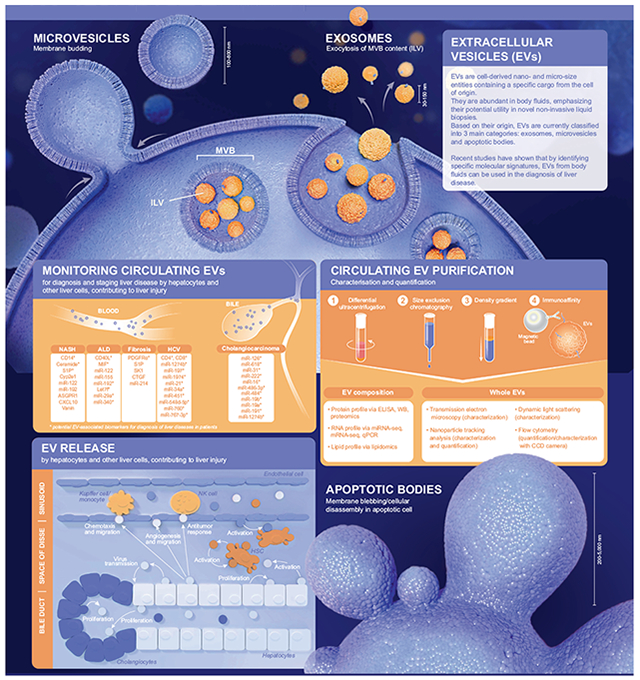
Extracellular vesicles (EVs) are cell-derived nano- and micro-size entities containing a specific cargo from the cell of origin. They are abundant in body fluids, emphasizing their potential utility in novel non-invasive liquid biopsies. Based on their specific molecular signature, EVs from body fluids can be used to diagnose liver diseases. This snapshot summarizes the current knowledge on EV classification, characterization, origin and cargo, while reviewing the potential of EVs as novel biomarkers in the context of liver diseases.
EV classification
Based on their origin, EVs are currently classified into 3 main categories: exosomes, microvesicles and apoptotic bodies.1 Exosomes originate from multivesicular bodies which fuse with the plasma membrane and release their intraluminal vesicles in the extracellular space. Their size ranges from 30 to 150 nm and they are enriched for markers such as CD81, CD63 and CD9. Microvesicles, which can be positive for CD81, CD63 and Annexin V, derive from plasma membrane budding and are between 100–800 nm in size. Apoptotic bodies originate from membrane blebbling and cellular disassembly from cells undergoing programmed cell death. They are heterogeneous in size with a diameter ranging from 200 to 5,000 nm and are enriched for Annexin V.1,2
Circulating EV purification, characterization and quantification
Many soluble factors are present in the non-EV fraction of the circulation. Thus, careful EV purification is an important first step towards their characterization and analysis. The most widely used method for EV purification is differential ultracentrifugation which eliminates several types of contaminants including large serum proteins. Other methods for EV purification include density gradient, size exclusion chromatography3 and magnetic bead immunoaffinity. Each technique varies with increased specificity traded for decreased sensitivity.4 The characterization of EVs is based on their size and concentration. The size of circulating EVs can be characterized using transmission electron microscopy, dynamic light scattering analysis, nanoparticle tracking analysis (NTA) and flow cytometry (FC). Moreover, NTA and FC can also measure EV concentration, which may be important in the context of disease.4 EV composition is another crucial aspect that can be explored in disease diagnosis. EVs are composed of proteins, RNAs and lipids. Protein cargo can be identified by ELISA, western blot and proteomics/mass spectrometry. RNAs can be measured using qPCR, mRNA-seq and miRNA-seq. Lipid content of EVs can be explored using lipidomics/mass spectrometry.
EV release by hepatocytes and other liver cells and contribution to liver injury
In the last decade, increasing interest has focused on EVs as important particles for cell-to-cell communication. In the liver, EVs are released from hepatocytes and nonparenchymal cells and may act as key mediators of various pathological responses contributing to the progression of liver diseases. EVs from injured hepatocytes (Hep-EVs) can target several cell types, in an autocrine or paracrine manner, and modulate their behavior. Hep-EVs induce hepatic stellate cell (HSC) activation,5 Kupffer cell/infiltrating macrophage recruitment and activation,6,7 antitumor responses by natural killer cells, and endothelial cell migration and angiogenesis.8 Hep-EVs can also act on the surrounding hepatocytes to induce cell proliferation and liver regeneration9 or transmit viral particles.10 From non-parenchymal cells, activated HSC-EVs promote neighboring HSC migration and regulate fibrogenesis.11,12 In the bile, Hep-EVs and cholangiocyte-derived EVs are mediators of cholangiocyte proliferation.13 These studies demonstrate that liver cell-derived EVs may be important contributors to hepatic regeneration, inflammation, angiogenesis and fibrosis. However, methods to specifically identify an EV’s cell of origin remain an unmet need.
Monitoring EVs from body fluids for diagnosis and staging liver disease
Several studies emphasize the potential of EV content as biomarkers of specific diseases. In the context of liver diseases, EVs have been collected from blood or bile. Blood EVs have been studied in non-alcoholic steatohepatitis, alcohol-related liver disease, cirrhosis and hepatitis C virus infection. They are reported to contain proteins and microRNAs important for disease progression.2,8,11,12,14–16 Bile EVs have been examined in the context of cholangiocarcinoma, with microRNAs being the center of attention of most of these investigations.2 Some of these EV-associated markers have been studied in patients with liver disease. Taken together, these studies suggest that a combination of several EV molecules could represent a signature of a specific liver disease in a liquid biopsy.
Acknowledgments
Financial support
This work was funded by NIH grants U01 AA022489, R01 DK113592, and U01 AA022489 for A.F. and AA21788 for V.H.S.
Footnotes
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2019.01.030.
References
Author names in bold designate shared co-first authorship
- [1].Lasser C, Jang SC, Lotvall J. Subpopulations of extracellular vesicles and their therapeutic potential. Mol Aspects Med 2018;60:1–14. [DOI] [PubMed] [Google Scholar]
- [2].Hirsova P, Ibrahim SH, Verma VK, Morton LA, Shah VH, LaRusso NF, et al. Extracellular vesicles in liver pathobiology: small particles with big impact. Hepatology 2016;64:2219–2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Boing AN, van der Pol E, Grootemaat AE, Coumans FA, Sturk A, Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles 2014:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sunkara V, Woo HK, Cho YK. Emerging techniques in the isolation and characterization of extracellular vesicles and their roles in cancer diagnostics and prognostics. Analyst 2016;141:371–381. [DOI] [PubMed] [Google Scholar]
- [5].Povero D, Panera N, Eguchi A, Johnson CD, Papouchado BG, de Araujo Horcel L, et al. Lipid-induced hepatocyte-derived extracellular vesicles regulate hepatic stellate cell via microRNAs targeting PPAR-gamma. Cell Mol Gastroenterol Hepatol 2015;1, 646–663 e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ibrahim SH, Hirsova P, Tomita K, Bronk SF, Werneburg NW, Harrison SA, et al. Mixed lineage kinase 3 mediates release of C-X-C motif ligand 10-bearing chemotactic extracellular vesicles from lipotoxic hepatocytes. Hepatology 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Verma VK, Li H, Wang R, Hirsova P, Mushref M, Liu Y, et al. Alcohol stimulates macrophage activation through caspase-dependent hepatocyte derived release of CD40L containing extracellular vesicles. J Hepatol 2016;64:651–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Povero D, Eguchi A, Niesman IR, Andronikou N, de Mollerat du Jeu X, Mulya A, et al. Lipid-induced toxicity stimulates hepatocytes to release angiogenic microparticles that require Vanin-1 for uptake by endothelial cells. Sci Signal 2013;6:ra88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Nojima H, Freeman CM, Schuster RM, Japtok L, Kleuser B, Edwards MJ, et al. Hepatocyte exosomes mediate liver repair and regeneration via sphingosine-1-phosphate. J Hepatol 2016;64:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ramakrishnaiah V, Thumann C, Fofana I, Habersetzer F, Pan Q, de Ruiter PE, et al. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci U S A 2013;110:13109–13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Chen L, Charrier A, Zhou Y, Chen R, Yu B, Agarwal K, et al. Epigenetic regulation of connective tissue growth factor by MicroRNA-214 delivery in exosomes from mouse or human hepatic stellate cells. Hepatology 2014;59:1118–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kostallari E, Hirsova P, Prasnicka A, Verma VK, Yaqoob U, Wongjarupong N, et al. Hepatic stellate cell-derived platelet-derived growth factor receptor-alpha-enriched extracellular vesicles promote liver fibrosis in mice through SHP2. Hepatology 2018;68:333–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Masyuk AI, Huang BQ, Ward CJ, Gradilone SA, Banales JM, Masyuk TV, et al. Biliary exosomes influence cholangiocyte regulatory mechanisms and proliferation through interaction with primary cilia. Am J Physiol Gastrointest Liver Physiol 2010;299:G990–G999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Charrier A, Chen R, Chen L, Kemper S, Hattori T, Takigawa M, et al. Exosomes mediate intercellular transfer of pro-fibrogenic connective tissue growth factor (CCN2) between hepatic stellate cells, the principal fibrotic cells in the liver. Surgery 2014;156:548–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Eguchi A, Lazaro RG, Wang J, Kim J, Povero D, Willliams B, et al. Extracellular vesicles released by hepatocytes from gastric infusion model of alcoholic liver disease contain a MicroRNA barcode that can be detected in blood. Hepatology 2017;65:475–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bala S, Petrasek J, Mundkur S, Catalano D, Levin I, Ward J, et al. Circulating microRNAs in exosomes indicate hepatocyte injury and inflammation in alcoholic, drug-induced, and inflammatory liver diseases. Hepatology 2012;56:1946–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]


