Abstract
Phthalates are associated with several adverse health outcomes, but few studies have evaluated phthalate exposures in Mexican populations, particularly pregnant women. Between 2007 and 2011, 948 pregnant women from Mexico City were recruited as part of the PROGRESS cohort. We quantified 17 metabolites of phthalates and phthalate alternatives in urine samples collected during the second and third trimesters and examined temporal trends of metabolites concentrations, within-person reproducibility, and relations of individual metabolites with sociodemographic, lifestyle, and occupational factors. Concentrations of mono-2-ethyl-5-carboxypentyl terephthalate, a metabolite of the alternative phthalate di-2-ethylhexyl terephthalate, increased monotonically from 2007 to 2010 (31% per year, 95% confidence interval: 23%, 39%). We observed moderate to high correlations among metabolites collected at the same visit, but there was high variability between second and third trimester phthalate metabolite concentrations (intraclass correlation coefficients: 0.17–0.35). In general, higher SES was associated with higher phthalate concentrations. Some metabolites were associated with maternal age and education, but no consistent patterns were observed. Women working in the home and those who worked in administration had higher concentrations of several phthalate metabolites relative to students, professionals, and those in customer service. Biomonitoring efforts are warranted to investigate present and future exposure trends and patterns.
Keywords: Mexico, phthalates, pregnancy, gestation, DINCH
1. Introduction
Phthalates are a class of synthetic organic chemicals commonly used in industrial and commercial products. High molecular weight (HMW) phthalates are used in plastic tubing, food packaging, toys, containers and building materials1,2, and low molecular weight (LMW) phthalates are typically used in some personal care products, solvents, fixatives, medications, or alcohol denaturants2–5. Because phthalates are not covalently bound to the commercial products, they are released in the environment, resulting in widespread human exposures globally6.
Epidemiological data suggest associations between phthalate exposure and a variety of adverse health outcomes, including reproductive7–10, perinatal11–19, and offspring health outcomes20–25. Di(2-ethylhexyl) phthalate (DEHP) is a known reproductive toxicant26 and its use, along with several other phthalates, have been restricted by the European Union27, United States28, and other legislative bodies. As data on the health effects of phthalates and their metabolites accumulate, phthalates with suspected harmful effects have been replaced with alternative phthalates or phthalate substitutes. For example, DEHP is being replaced with alternative phthalates such as di(2-ethylhexyl) terephthalate (DEHTP), a structural isomer of DEHP29, and the non-phthalate plasticizer di(isononyl)cyclohexane-1,2-dicarboxylate (DINCH)30. U.S. biomonitoring data from 2001–2016 have shown corresponding temporal changes in the profiles of urinary phthalate metabolites, with declining concentrations of some phthalates as the use of alternative phthalates or phthalate substitutes has increased31,32.
To date, most biomonitoring studies of phthalates have been conducted on North American, European and Asian populations, relatively little data are available for Central and South American populations33. Despite the recognized impact of phthalates on human health and near ubiquitous human exposure, Mexico has not yet adopted any regulations on phthalates use in commercial products. Only two previous studies have reported data on phthalate exposures in Mexican adults34,35, including a small cohort pregnant women35, and neither reported on temporal trends and sociodemographic correlates. Such knowledge is vital to our understanding of the global trends and exposure patterns and can help guide public health and research priorities. In light of potential health impacts of phthalate exposure, particularly for sensitive populations such as pregnant women19,35, we described the temporal trends and sociodemographic determinants of urinary phthalate concentrations in a cohort of pregnant women from Mexico City.
2. Methods
2.1. Cohort Recruitment and Follow Up:
From July 2007 to February 2011, the Programming Research in Obesity, Growth, Environment and Social Stressors (PROGRESS) study recruited 1054 women with singleton pregnancies from Mexico City who were receiving prenatal care from Mexican Social Security System (IMSS) and 948 women remained until delivery. Women were eligible if they were 18 years or older, less than 20 weeks gestation at the time of recruitment, planning to stay in Mexico City for the next 3 years, free of heart or kidney disease, did not use steroids or anti-epilepsy drugs, not daily consumers of alcohol, and had access to a telephone. Written informed consent was obtained from all participants. The study protocols were approved by institutional review boards at the Harvard School of Public Health, Icahn School of Medicine at Mount Sinai, and the Research, Ethics in Research and Biosafety Committees in the Mexican National Institute of Public Health. The analysis of blinded specimens at the Centers for Disease Control and Prevention (CDC) laboratory was determined not to constitute engagement in human subjects research.
2.2. Sociodemographic, Anthropometric, and Lifestyle Factors:
We assessed maternal age (years), education (<high school, high school, >high school), socioeconomic status (SES) based on the Mexican Association of Research and Public Opinion Agencies (AMAI) guidelines36), parity (0, 1, 2, >2), alcohol use (binary), ever smoking (binary), and secondhand smoking (binary) via questionnaire during the second trimester. Height and weight were measured by trained personnel using Health-O-Meter combined scale and stadiometer (Scaleomatics inc, Cleveland, OH). We calculated gestation age at the time of each study visit based on self-reported last menstrual period and the Capurro method37. We additionally asked the women regarding their occupation and subsequently combined responses to form five mutually exclusive groups: administrative tasks and services (includes cashier, secretary, and supervisory roles), customer service (e.g. chef, tourist guide, shop assistant, etc.), student, professional services (engineer, doctor, teach, etc.), and those working in the home.
2.3. Urine Collection and Phthalate and DINCH Metabolites Quantification:
Maternal urine samples were collected during the second and third trimester study visits in phthalate-free specimen collection cups and 2 mL aliquots were stored at −800C. Phthalate and DINCH metabolites quantification was conducted at the Centers for Disease Control and Prevention (CDC) using isotope dilution high-performance liquid chromatography coupled with tandem mass spectrometry as previously described38.
Samples were analyzed for 15 phthalate metabolites and two metabolites of DINCH: mono-n-butyl phthalate (MBP), mono-isobutyl phthalate (MiBP), mono-hydroxybutyl phthalate (MHBP), mono-hydroxyisobutyl phthalate (MHiBP), mono-3-carboxypropyl phthalate (MCPP), monoethyl phthalate (MEP), mono-2-ethyl-5-carboxypentyl phthalate (MECPP), mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), monobenzyl phthalate (MBzP), mono(carboxy-isononyl) phthalate (MCNP) mono(carboxy-isooctyl) phthalate (MCOP), monooxononyl phthalate (MONP), mono-2-ethyl-5-carboxypentyl terephthalate (MECPTP), cyclohexane-1,2-dicarboxylic acid, monocarboxy isooctyl ester (MCOCH), and cyclohexane-1,2-dicarboxylic acid, monohydroxy isononyl ester (MHiNCH).
Limits of detection (LOD) ranged from 0.2 to 1.2 ng/mL, depending on the metabolite. For phthalate metabolites, concentrations below the LOD were replaced by the instrumental reported value and zero values were replaced by the lowest instrumental reported value for that metabolite. Distributions and associations observed in this study did not meaningfully change when we corrected for LOD by imputing all values below LOD with LOD/sqrt(2)39. To summarize multiple metabolites of the same parent compound for presentation, we calculated molar sums of DEHP (ΣDEHP = MEHP + MEHHP + MEOHP +MECPP), DiNP (ΣDiNP = MONP + MCOP), DiBP (ΣDiBP = MHiBP + MiBP), and DBP (ΣDBP = MBP + MHBP) and then multiplied these molar sums by the molecular weight of one metabolite (MECPP for ΣDEHP, MCOP for ΣDiNP, MHiBP for ΣDiBP, MHBP for ΣDBP). For DINCH metabolites MCOCH and MHiNCH, a dichotomous variable was created (above vs. below LOD) due to low detection frequencies.
In addition to the standard analytical quality control protocols of the CDC laboratory, a pool of anonymous human adult urine (BioIV, NY, USA) was included as a blinded replicate 92 times randomly inserted throughout the study samples. As shown in Supplemental Figure 1, the variations observed in the QC samples were small compared to the population distribution.
Urine specific gravity (SG) was measured using a digital handheld refractometer (AR200, Reichert Technologies, Buffalo, NY). The formula40 for dilution standardization of phthalate measurement by specific gravity is Pc = P[(SGm-1)/(SG-1)] where Pc is the SG-corrected metabolite concentration (ng/mL), P is the measured phthalate metabolite concentration, SGm is the median SG value of all samples, and SG is the specific gravity value for that individual urine sample. Imputation of the median value of the study samples (1.016) was used for 101 samples from the second trimester visit with missing specific gravity measures. All presented results and trends do not meaningfully change when restricted only to those with measured SG.
2.4. Statistical Analysis:
We calculated geometric means, quantiles, and detection frequencies to describe the distributions of urinary metabolites for both visits. We used spearman correlation test to examine pairwise correlations between metabolites at each visit and the Kolmogorov-Smirnov test to examine differences in distribution of metabolites between trimesters. To estimate the within- and between-person variability between the second and third trimester samples, we calculated the intraclass correlation coefficient (ICC) and estimated the 95% confidence interval (CI) via 10,000 bootstraps.
We used linear regression with general estimating equation (GEE) to compare second and third trimester urinary metabolite concentrations where we modeled trimester of visit as the exposure and log metabolite concentrations as outcomes. We used a similar model to assess temporal trends in metabolite concentrations from 2007 to 2010. We decided to exclude year 2011 from the temporal trends analysis due to the low number of participants recruited that year. Second and third trimester visits were treated as repeated observations where year of visit was modeled as the exposure and log metabolite concentrations as outcomes. For all GEE models where log2 metabolite concentrations were modeled as outcomes, we calculated concentration ratios (CR) by taking the exponential of the beta coefficient, which represents the relative difference in urinary metabolite concentrations, and their corresponding 95% confidence intervals (CI). For presentation of the temporal trends, we additionally calculated geometric means and 95% CIs by year. We used unadjusted linear regression models to assess bivariate relations between second trimester urinary metabolites with sociodemographic and lifestyle factors. The resulting effect estimates are presented as CRs and 95% CIs. In all statistical models, log2 transformed SG-corrected metabolite concentrations were used.
3. Results
At second trimester (16–22 weeks gestation), 948 PROGRESS participants had available urinary phthalate and DINCH metabolite data while 792 participants additionally had urinary metabolite data in the third trimester (27–34 weeks gestation). A total of 183, 308, 245, 188, and 24 participants were recruited in years 2007, 2008, 2009, 2010, and 2011. The mean age and BMI of the participants at the time of recruitment were 27.3 years (SD=5.5) and 26.9 kg/m2 (SD=4.2), respectively. The majority of participants did not complete high school (76%) and were not current smokers (99%) or alcohol consumers (97%). The study participants were generally of low SES, with 74% of individuals in the bottom three categories of the AMAI index. Additional demographic data can be found in Supplemental Table 1.
3.1. Distribution of Phthalates and DINCH among Pregnant Women from Mexico City:
In both the second and third trimesters, 14 of the 15 measured phthalate metabolites were detected in ≥92% of all samples (Table 1). In contrast, the DINCH metabolites MCOCH and MHiNCH were detected in ≤4% second trimester samples and in ≤8% of all samples in the third trimester. Due to the low detection frequencies, the DINCH metabolites were not examined in subsequent analyses. Compared to a similar cohort of pregnant women recruited in Mexico City from 1997–2005 35, PROGRESS participants had higher concentrations of MBP, MiBP, and MBzP (Figure 2). In contrast, in comparison to a group of older women recruited as controls for a case-control study of breast cancer in Northern Mexico from 2007 to 2008 34, PROGRESS participants had lower concentrations of DEHP metabolites and MCPP.
Table 1.
Distribution of Uncorrected Urinary Phthalate Metabolite Concentrations (ng/mL) in the PROGRESS cohort by Trimester
| Second Trimester (N=948) | Third Trimester (N=792) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parent Class | Parent | Metabolite | 25% | 50% | 75% | 90% | Detection Rate | 25% | 50% | 75% | 90% | Detection Rate |
| High Molecular Weight | DEHP | MEHP | 2.7 | 5.7 | 10.7 | 17.1 | 96% | 2.8 | 5.4 | 9.6 | 18.3 | 96% |
| MEOHP | 9.4 | 17.3 | 34.4 | 55.6 | 100% | 12.3 | 21.3 | 37.1 | 59.4 | 100% | ||
| MEHHP | 10.4 | 18.5 | 37.7 | 61.2 | 100% | 12.3 | 21.2 | 38.8 | 64.9 | 100% | ||
| MECPP | 22.0 | 40.2 | 73.2 | 115.0 | 100% | 26.8 | 44.3 | 76.1 | 127.0 | 100% | ||
| DEHTP | MECPTP | 0.9 | 1.7 | 3.2 | 6.2 | 98% | 1.1 | 2.1 | 3.7 | 7.6 | 98% | |
| DiNP | MONP | 0.6 | 1.2 | 2.4 | 4.6 | 85% | 0.8 | 1.5 | 2.6 | 4.9 | 93% | |
| MCOP | 2.3 | 4.4 | 7.7 | 13.3 | 100% | 2.5 | 4.1 | 7.0 | 12.5 | 100% | ||
| DiDP | MCNP | 0.6 | 0.9 | 1.5 | 2.2 | 97% | 0.6 | 0.9 | 1.5 | 2.2 | 98% | |
| DOP | MCPP* | 0.8 | 1.4 | 2.4 | 3.9 | 92% | 0.9 | 1.5 | 2.4 | 3.7 | 94% | |
| BBzP | MBzP | 2.5 | 5.0 | 10.4 | 21.6 | 99% | 2.6 | 5.6 | 10.9 | 19.1 | 99% | |
| Low Molecular Weight | DiBP | MHiBP | 1.8 | 3.4 | 6.2 | 10.2 | 99% | 1.9 | 3.5 | 6.1 | 10.7 | 99% |
| MiBP | 4.6 | 8.9 | 16.1 | 25.4 | 99% | 5.1 | 9.5 | 16.8 | 29.5 | 99% | ||
| DBP | MBP** | 38.0 | 79.4 | 150.3 | 255.0 | 100% | 42.9 | 80.4 | 148.3 | 267.7 | 100% | |
| MHBP | 3.4 | 6.9 | 13.3 | 24.5 | 99% | 3.8 | 7.5 | 14.0 | 25.4 | 99% | ||
| DEP | MEP | 52.2 | 119.0 | 296.3 | 771.2 | 100% | 53.9 | 123.5 | 354.5 | 997.0 | 100% | |
| Phthalate Alternatives | DINCH | MCOCH | <LOD | <LOD | <LOD | <LOD | 2% | <LOD | <LOD | <LOD | <LOD | 3% |
| MHiNCH | <LOD | <LOD | <LOD | <LOD | 4% | <LOD | <LOD | <LOD | <LOD | 8% | ||
Also a minor metabolite of several high molecular weight phthalates
Also a minor metabolite of BBzP
<LOD: Below the limit of detection. LODs were 0.5 ng/mL (MCOCH) and 0.4 ng/mL (MHiNCH)
Figure 2.
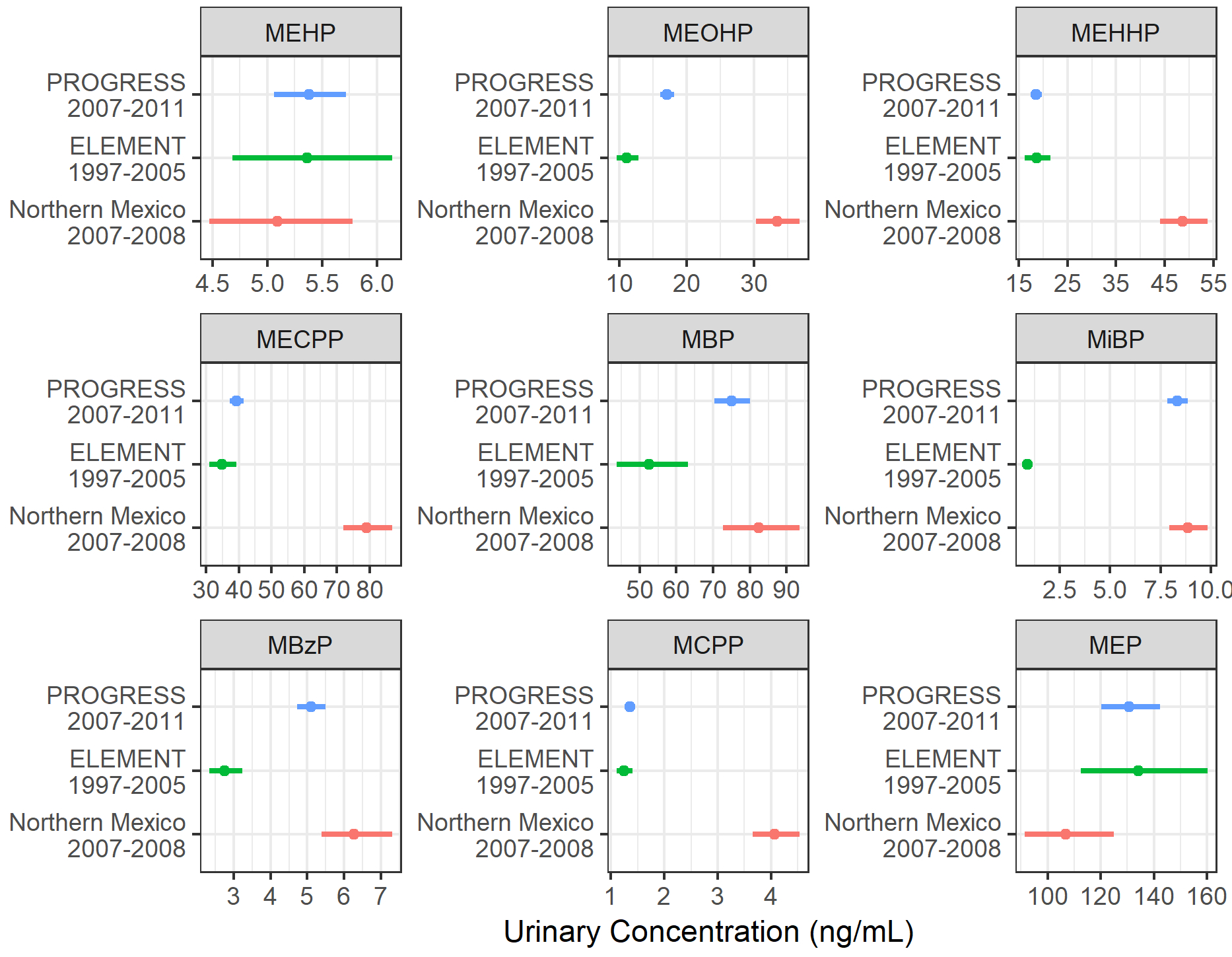
A comparison of urinary phthalate metabolite concentrations between PROGRESS and other reports of urinary phthalate concentrations in Mexican adults. Geometric means and 95% confidence intervals for urinary phthalate metabolite concentrations are depicted for the PROGRESS cohort (pregnant women from Mexico City, recruited 2008–2011, N=941), ELEMENT cohort (pregnant women from Mexico City, recruited 1997–2005, N=153)35,50, and a group of women from Northern Mexico (controls of a breast cancer case-control study, 2007–2008, N=221)34.
Phthalate metabolites from different parent compounds showed moderate to strong pairwise correlations with one another at each visit (Spearman rho=0.33–0.74), with the exception of MEP, which was weakly correlated with all other metabolites (Spearman rho=0.15–0.41) (Supplemental Figure 2). As expected, metabolites from the same parent compounds exhibited very strong pairwise correlations with each other (0.75–0.98).
3.2. Variability Between Second and Third Trimesters:
For all metabolites, we observed low ICCs between second and third trimester concentrations within individuals (ICC: 0.18–0.35, Table 2). Among participants with both second and third trimester samples, third trimester urinary concentrations was higher in the third trimester compared to the second trimester for metabolites MEOHP (CR=1.28, 95% CI: 1.16, 1.39), MEHHP (CR=1.20, 95% CI: 1.08, 1.32), MECPP (CR=1.20, 95% CI: 1.09, 1.31), MECPTP (CR=1.30, 95% CI: 1.18, 1.42), MONP (CR=1.28, 95% CI: 1.15, 1.41), MHiBP (CR=1.11, 95% CI:1.00, 1.23), MiBP (CR=1.18, 95% CI: 1.07, 1.29), MBP (CR=1.11, 95% CI: 0.99, 1.24), MHBP (CR=1.12, 95% CI: 0.99, 1.25), and MEP (CR=1.10, 95% CI: 0.94, 1.26). Kolmogorov-Smirnov test using scaled metabolite concentrations showed no statistically significant differences in the distributions of metabolites between visits (data not shown).
Table 2.
Comparison of second and third trimester specific gravity corrected urinary phthalate metabolite concentrations among those with urine samples at both timepoints.
| Parent | Metabolite | Intraclass Correlation Coefficient (95% CI) | Concentration Ratio (95% CI)* |
|---|---|---|---|
| DEHP | MEHP | 0.28 (0.21, 0.35) | 1.03 (0.90, 1.15) |
| MEOHP | 0.25 (0.18, 0.32) | 1.28 (1.16, 1.39) | |
| MEHHP | 0.25 (0.18, 0.32) | 1.20 (1.08, 1.32) | |
| MECPP | 0.23 (0.16, 0.30) | 1.20 (1.09, 1.31) | |
| DEHTP | MECPTP | 0.35 (0.29, 0.42) | 1.30 (1.18, 1.42) |
| DiNP | MONP | 0.18 (0.11, 0.25) | 1.28 (1.15, 1.41) |
| MCOP | 0.19 (0.12, 0.26) | 1.03 (0.91, 1.14) | |
| DiDP | MCNP | 0.18 (0.11, 0.25) | 1.07 (0.97, 1.17) |
| DOP | MCPP | 0.23 (0.16, 0.30) | 1.08 (0.97, 1.19) |
| BBzP | MBzP | 0.33 (0.27, 0.39) | 1.10 (0.97, 1.24) |
| DiBP | MHiBP | 0.34 (0.27, 0.40) | 1.11 (1.00, 1.23) |
| MiBP | 0.33 (0.26, 0.39) | 1.18 (1.07, 1.29) | |
| DBP | MBP | 0.31 (0.23, 0.37) | 1.11 (0.99, 1.24) |
| MHBP | 0.32 (0.25, 0.39) | 1.12 (0.99, 1.25) | |
| DEP | MEP | 0.35 (0.28, 0.41) | 1.10 (0.94, 1.26) |
Third trimester compared to second trimester
3.3. Temporal Trends:
Figure 1 shows the temporal trends of measured phthalate metabolites from 2007 to 2010 among PROGRESS participants. There was a clear monotonic increase in urinary MECPTP concentrations from 2007 to 2010 (112% increase in 2010 compared to 2007, 95% CI: 87%, 137%, Supplemental Table 2). ΣDiBP and MBzP were higher in 2008–2010 compared to 2007, but these were primarily driven by lower concentrations in 2007 as no increases were observed from 2008 to 2010 (Supplemental Table 2). MEP was the only metabolite to show decreased urinary concentrations over time, most notably from 2008 to 2010.
Figure 1.
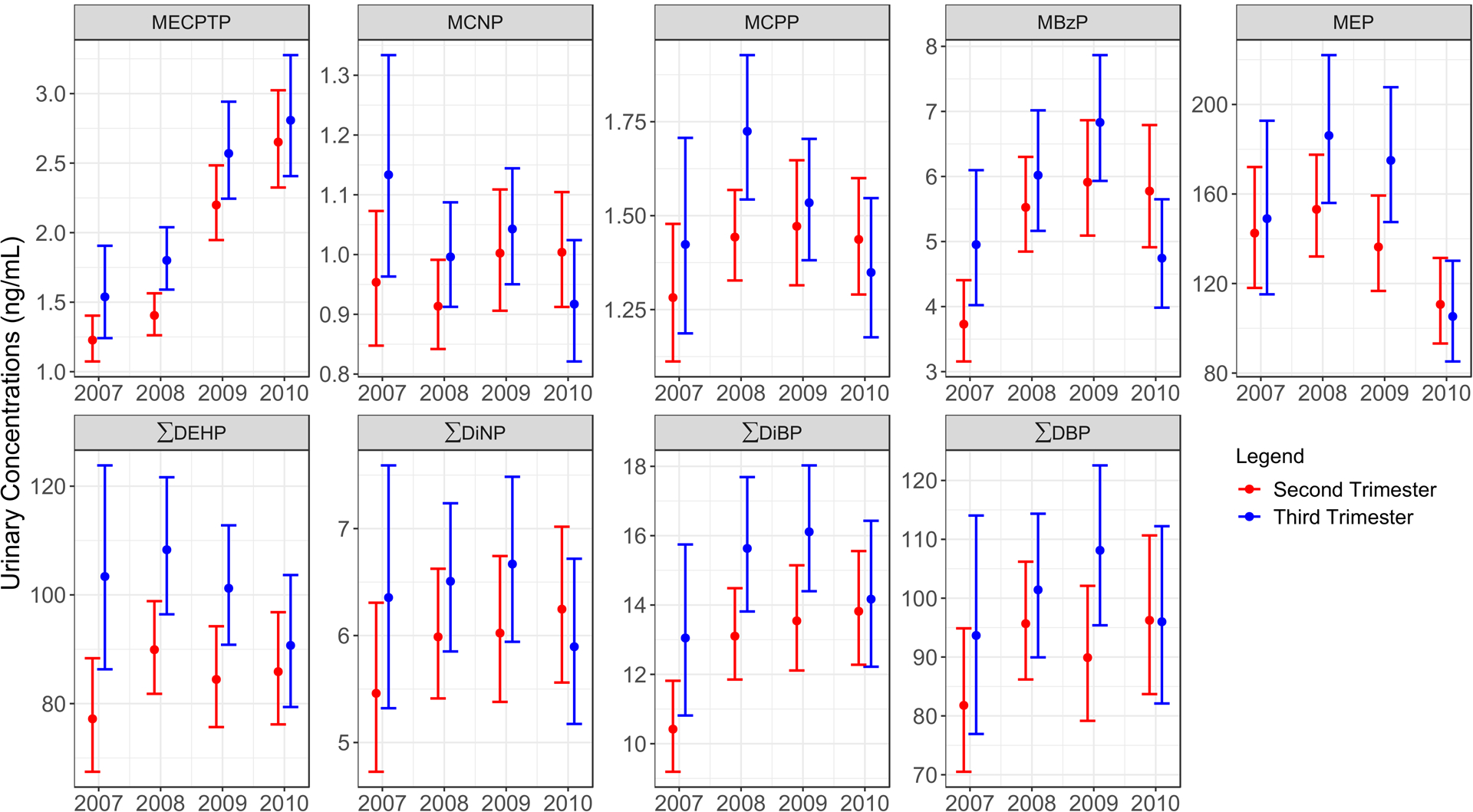
Temporal trends in geometric means (95% confidence intervals) of urinary phthalate metabolite concentrations among PROGRESS participants from 2007–2010. A monotonic increase was observed for MECPTP, a major metabolite of DEHTP, across all four years. MEP was the only metabolite to show decreased urinary concentrations during the observed timeframe. Similar trends were observed for both second (N=942) and third trimester samples (n=791).
3.4. Sociodemographic and Lifestyle Factors Related to Phthalates Exposure:
There were several nominal associations between measured sociodemographic factors and second trimester urinary phthalate metabolite concentrations. Older age was associated with decreased concentrations of MBzP (CR: 0.66, 95% CI: 0.51–0.85, >35 years compared to <25 years, Table 3). SES was associated with increased concentrations of DBP and DEP metabolites (Table 3). Compared to those in the lowest category of SES, those in the highest two categories of SES had 43% (95% CI: 7%, 90%), 46% (95% CI: 7%, 99%), and 57% (95% CI: 8%, 129%) higher concentrations of MBP, MHBP, and MEP, respectively. There were suggestions that SES was also associated with higher concentrations of DiNP metabolites MONP and MCOP. Attainment of a post high school degree was associated with lower concentrations of MECPP (CR: 0.86, 95% CI: 0.75, 0.99, vs. those who did not complete high school) and MECPTP (CR: 0.74, 95% CI: 0.63, 0.88, vs. those who did not complete high school) (Table 3).
Table 3.
Concentration Ratios (CRs) and Associated 95% Confidence Intervals (95% CI) of Specific Gravity Corrected Second Trimester Phthalate Metabolite Urinary Concentrations with Select Demographic and Anthropometric Factors.
| Metabolites of High Molecular Weight Phthalates | Metabolites of Low Molecular Weight Phthalates | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DEHP | DEHTP | DiNP | DiDP | DOP | BBzP | DiBP | DBP | DEP | |||||||
| MEHP | MEOHP | MEHHP | MECPP | MECPTP | MONP | MCOP | MCNP | MCPP | MBzP | MHiBP | MiBP | MBP | MHBP | MEP | |
| CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | |
| Age (Second Trimester) | |||||||||||||||
| <25 | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| 25–35 | 1.08 (0.94, 1.25) | 0.98 (0.86, 1.12) | 1.02 (0.89, 1.16) | 1.00 (0.88, 1.12) | 0.90 (0.78, 1.04) | 0.97 (0.84, 1.12) | 0.99 (0.87, 1.13) | 0.96 (0.86, 1.07) | 0.98 (0.87, 1.10) | 0.90 (0.76, 1.06) | 1.00 (0.88, 1.14) | 1.02 (0.90, 1.16) | 1.06 (0.92, 1.22) | 1.05 (0.90, 1.22) | 1.13 (0.94, 1.36) |
| >35 | 1.00 (0.81, 1.25) | 0.91 (0.74, 1.11) | 0.97 (0.79, 1.19) | 0.95 (0.79, 1.14) | 0.83 (0.66, 1.03) | 0.98 (0.78, 1.22) | 1.01 (0.83, 1.23) | 0.95 (0.80, 1.12) | 1.03 (0.86, 1.25) | 0.66 (0.51, 0.85) | 0.99 (0.81, 1.21) | 1.05 (0.86, 1.27) | 1.19 (0.96, 1.48) | 1.14 (0.90, 1.44) | 1.06 (0.79, 1.40) |
| BMI (second trimester) | |||||||||||||||
| <25 | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| 25–30 | 0.92 (0.79, 1.07) | 0.95 (0.82, 1.08) | 0.97 (0.84, 1.12) | 0.97 (0.85, 1.09) | 0.90 (0.78, 1.05) | 0.98 (0.84, 1.14) | 0.95 (0.83, 1.09) | 0.92 (0.82, 1.03) | 0.93 (0.82, 1.05) | 0.86 (0.72, 1.02) | 0.98 (0.85, 1.12) | 0.99 (0.86, 1.13) | 0.93 (0.81, 1.08) | 0.91 (0.78, 1.07) | 1.19 (0.98, 1.44) |
| >30 | 0.94 (0.79, 1.12) | 1.00 (0.85, 1.18) | 1.05 (0.89, 1.24) | 1.07 (0.93, 1.25) | 0.96 (0.81, 1.15) | 1.11 (0.93, 1.32) | 1.14 (0.97, 1.33) | 1.06 (0.93, 1.21) | 0.99 (0.85, 1.15) | 0.90 (0.73, 1.1) | 1.04 (0.89, 1.22) | 1.16 (0.99, 1.36) | 1.05 (0.88, 1.24) | 0.94 (0.78, 1.13) | 1.15 (0.92, 1.44) |
| Socioeconomic Status | |||||||||||||||
| 1 (lowest) | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| 2 | 1.23 (0.97, 1.56) | 1.15 (0.92, 1.43) | 1.16 (0.93, 1.46) | 1.16 (0.95, 1.42) | 1.08 (0.85, 1.37) | 1.28 (1.01, 1.63) | 1.26 (1.02, 1.56) | 1.13 (0.94, 1.35) | 1.12 (0.91, 1.37) | 1.31 (0.99, 1.73) | 1.14 (0.92, 1.42) | 1.12 (0.9, 1.38) | 1.31 (1.03, 1.66) | 1.31 (1.01, 1.68) | 1.34 (0.99, 1.83) |
| 3 | 1.24 (0.96, 1.60) | 1.02 (0.80, 1.29) | 1.05 (0.82, 1.33) | 1.01 (0.82, 1.26) | 0.98 (0.75, 1.27) | 1.12 (0.86, 1.45) | 1.10 (0.87, 1.38) | 0.99 (0.82, 1.21) | 1.10 (0.88, 1.36) | 1.22 (0.91, 1.65) | 1.09 (0.86, 1.37) | 1.04 (0.82, 1.30) | 1.28 (1.00, 1.65) | 1.33 (1.01, 1.74) | 1.42 (1.02, 1.97) |
| 4 | 1.16 (0.88, 1.53) | 1.02 (0.79, 1.32) | 1.07 (0.82, 1.39) | 0.99 (0.78, 1.25) | 0.87 (0.66, 1.15) | 1.27 (0.96, 1.68) | 1.20 (0.94, 1.54) | 1.04 (0.85, 1.29) | 1.08 (0.86, 1.37) | 1.18 (0.86, 1.64) | 1.12 (0.87, 1.44) | 1.06 (0.83, 1.36) | 1.32 (1.01, 1.73) | 1.35 (1.01, 1.81) | 1.53 (1.07, 2.18) |
| 5–6 (highest) | 1.37 (1.02, 1.83) | 1.10 (0.84, 1.44) | 1.10 (0.84, 1.45) | 1.03 (0.80, 1.32) | 0.86 (0.64, 1.16) | 1.22 (0.91, 1.65) | 1.04 (0.80, 1.36) | 1.03 (0.82, 1.28) | 1.13 (0.88, 1.44) | 1.24 (0.88, 1.75) | 1.25 (0.96, 1.62) | 1.22 (0.94, 1.59) | 1.43 (1.07, 1.90) | 1.46 (1.07, 1.99) | 1.57 (1.08, 2.29) |
| Education | |||||||||||||||
| < High School | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| High School | 1.08 (0.93, 1.25) | 1.07 (0.93, 1.23) | 1.06 (0.92, 1.22) | 1.04 (0.92, 1.18) | 1.01 (0.87, 1.18) | 1.02 (0.87, 1.18) | 1.01 (0.88, 1.15) | 1.03 (0.92, 1.16) | 1.08 (0.95, 1.23) | 1.20 (1.01, 1.43) | 1.08 (0.94, 1.23) | 1.05 (0.91, 1.20) | 1.08 (0.93, 1.25) | 1.16 (0.99, 1.35) | 1.16 (0.95, 1.41) |
| >High School | 1.01 (0.85, 1.19) | 0.90 (0.77, 1.05) | 0.91 (0.78, 1.07) | 0.86 (0.75, 0.99) | 0.74 (0.63, 0.88) | 0.92 (0.78, 1.09) | 0.91 (0.78, 1.06) | 1.00 (0.88, 1.13) | 0.97 (0.84, 1.12) | 0.83 (0.68, 1.01) | 0.96 (0.83, 1.12) | 0.95 (0.82, 1.11) | 1.02 (0.87, 1.21) | 1.07 (0.89, 1.28) | 1.13 (0.91, 1.40) |
| First Pregnancy | |||||||||||||||
| Yes | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| No | 1.09 (0.95, 1.25) | 1.02 (0.90, 1.15) | 1.05 (0.92, 1.19) | 1.03 (0.92, 1.16) | 1.22 (1.06, 1.39) | 1.02 (0.89, 1.17) | 1.05 (0.93, 1.19) | 0.96 (0.86, 1.06) | 0.97 (0.86, 1.09) | 1.04 (0.88, 1.21) | 1.08 (0.96, 1.22) | 1.15 (1.02, 1.30) | 1.06 (0.92, 1.21) | 0.97 (0.84, 1.11) | 0.89 (0.75, 1.06) |
Exposure to environmental smoking at home was not associated with any urinary phthalate metabolite concentrations. Ever use of alcohol was nominally associated with 21% decrease in MECPTP concentrations (CR: 0.79, 95% CI: 0.64, 0.99, Table 4). Notably, almost all metabolites showed identical patterns where mothers who self-reported as working in the home or working in administrative tasks and services during pregnancy had higher urinary phthalate metabolite concentrations compared to those who self-reported as students, professionals (doctors, lawyers, etc.), or those in customer service.
Table 4.
Concentration Ratios (CR) and 95% Confidence Intervals (95% CI) of Specific Gravity Corrected Second Trimester Phthalate Metabolite Urinary Concentrations with Select Demographic and Anthropometric Factors.
| Metabolites of High Molecular Weight Phthalates | Metabolites of Low Molecular Weight Phthalates | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DEHP | DEHTP | DiNP | DiDP | DOP | BBzP | DiBP | DBP | DEP | |||||||
| MEHP | MEOHP | MEHHP | MECPP | MECPTP | MONP | MCOP | MCNP | MCPP | MBzP | MHiBP | MiBP | MBP | MHBP | MEP | |
| CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | CR (95% CI) | |
| Environmental Smoking at Home | |||||||||||||||
| No | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| Yes | 1.05 (0.91, 1.21) | 1.00 (0.88, 1.14) | 1.00 (0.87, 1.14) | 0.99 (0.88, 1.11) | 0.95 (0.83, 1.10) | 0.98 (0.85, 1.13) | 0.98 (0.86, 1.11) | 1.01 (0.91, 1.12) | 0.97 (0.86, 1.10) | 1.11 (0.94, 1.31) | 1.04 (0.92, 1.19) | 1.03 (0.91, 1.17) | 1.01 (0.88, 1.16) | 0.98 (0.85, 1.14) | 1.13 (0.94, 1.35) |
| Ever Alcohol | |||||||||||||||
| No | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| Yes | 0.99 (0.80, 1.22) | 0.91 (0.74, 1.11) | 0.92 (0.75, 1.13) | 0.91 (0.76, 1.09) | 0.79 (0.64, 0.99) | 0.91 (0.73, 1.13) | 0.96 (0.79, 1.16) | 0.98 (0.83, 1.15) | 0.95 (0.79, 1.14) | 1.03 (0.80, 1.32) | 1.04 (0.86, 1.27) | 1.09 (0.90, 1.32) | 1.08 (0.87, 1.33) | 1.01 (0.80, 1.27) | 1.28 (0.97, 1.68) |
| Occupation During Pregnancy | |||||||||||||||
| Administrative Tasks and Services | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref | ref |
| Women working in the home | 0.94 (0.76, 1.16) | 0.98 (0.81, 1.20) | 0.96 (0.79, 1.18) | 1.02 (0.86, 1.22) | 1.02 (0.83, 1.26) | 0.99 (0.80, 1.23) | 1.06 (0.87, 1.28) | 1.03 (0.87, 1.21) | 0.97 (0.81, 1.17) | 0.97 (0.76, 1.24) | 0.92 (0.76, 1.11) | 0.91 (0.76, 1.10) | 0.91 (0.74, 1.12) | 0.88 (0.70, 1.10) | 0.76 (0.58, 0.99) |
| Customer Service | 0.81 (0.63, 1.03) | 0.81 (0.65, 1.02) | 0.77 (0.61, 0.98) | 0.86 (0.70, 1.06) | 0.88 (0.69, 1.12) | 0.87 (0.68, 1.11) | 0.94 (0.75, 1.17) | 0.93 (0.77, 1.12) | 0.91 (0.74, 1.12) | 0.83 (0.63, 1.09) | 0.85 (0.68, 1.05) | 0.85 (0.69, 1.05) | 0.86 (0.68, 1.10) | 0.84 (0.65, 1.09) | 0.67 (0.49, 0.91) |
| Professional | 0.80 (0.56, 1.14) | 0.72 (0.52, 1.00) | 0.70 (0.50, 0.98) | 0.76 (0.56, 1.02) | 0.75 (0.53, 1.07) | 0.64 (0.44, 0.91) | 0.69 (0.50, 0.95) | 0.86 (0.66, 1.12) | 1.00 (0.74, 1.36) | 0.57 (0.38, 0.85) | 0.75 (0.55, 1.02) | 0.75 (0.55, 1.02) | 1.12 (0.79, 1.60) | 1.09 (0.75, 1.59) | 0.77 (0.49, 1.20) |
| Student | 0.77 (0.54, 1.09) | 0.77 (0.56, 1.07) | 0.73 (0.52, 1.01) | 0.79 (0.59, 1.06) | 0.74 (0.52, 1.05) | 0.77 (0.54, 1.10) | 0.81 (0.59, 1.11) | 0.82 (0.63, 1.07) | 0.82 (0.60, 1.10) | 0.93 (0.62, 1.38) | 0.75 (0.55, 1.02) | 0.76 (0.56, 1.03) | 0.66 (0.47, 0.94) | 0.69 (0.47, 1.00) | 0.52 (0.34, 0.81) |
4. Discussion
Despite evidence that pregnant women and their offspring are sensitive to gestational environmental exposure to phthalates19,25,35, Mexico has not yet enacted any regulations to restrict phthalates use in any commercial products. In this report, we described phthalate biomarkers profiles among pregnant women in Mexico and provided novel exposure data in this particularly vulnerable population. We observed that urinary concentrations of most metabolites did not increase from 2007 to 2010 within the PROGRESS cohort, except a clear increase from 2007 to 2010 for MECPTP, a metabolite of DEHTP, which likely reflects the broader rise of DEHTP as a substitute for DEHP. We observed that age, SES, and education were associated with select urinary phthalate metabolite concentrations. Also, women working in administrative tasks and services and those working in the home had higher urinary concentrations of phthalate metabolites compared to other occupational groups. Together, our study highlights a clear need to examine current phthalate exposure profiles in the Mexican population.
It is well established that because of short biological half-lives and in the episodic nature of phthalate exposures, urinary phthalate metabolites should be measured using multiple samples to reduce measurement error and increase statistical power in analyses41. In addition, there may also be critical windows of exposure or other differences in offspring health outcomes that can be explored and identified using multiple samples from different gestation periods. For example, previous studies have identified gestation time dependent associations between prenatal phthalate exposures and preterm birth42 and developmental outcomes in male infants43,44. In our study, we observed weak ICCs (0.18–0.35) between second and third trimester samples for each individual metabolite, which is consistent with this known variability, albeit slightly weaker compared to previously publications42,45–48. Our study also observed that in general, third trimester phthalate metabolite concentrations were higher compared to the second trimester, but this relationship is not consistent across studies45,49 and it is unclear whether this was driven by greater exposure to phthalate as a result of changes in dietary and other factors such as personal product use, or intrinsic metabolic differences.
To our knowledge, there has been only one other pregnancy cohort from Mexico, recruited from 1997 to 2005, which reported urinary phthalate metabolite concentrations35,50. Compared to this cohort, the participants in our study, recruited from 2007 to 2011, had higher concentrations for three (MBP, MiBP, MBzP) of nine metabolites measured in both cohorts. Demographic differences between cohorts and use of different laboratories equipment, reagents, and personnel for urinary phthalate metabolite concentration quantification may explain some of these observed differences. However, the methods for phthalates metabolite quantification used within each laboratory for analyzing the samples were identical and it seems likely that there was an overall increase in DBP, DiBP, and BBzP exposure from 1997–2005 to 2007–2011 for women. This is consistent with our temporal analysis where we observed higher concentrations of DiBP and BBzP metabolites in 2008–2010 compared to 2007. There was also a clear and monotonic increase in urinary MECPTP concentration in the PROGRESS cohort from 2007 to 2010. This particular trend is mirrored by a recent study that reported a threefold increase in geometric mean of DEHTP metabolites in pregnant women in Puerto Rico between 2014 to 201751. This particular trend is likely explained by the restriction of DEHP use in certain products starting in the mid-2000s by several countries and legislative, which led to increases in DEHP alternatives such as DEHTP and DINCH29,32,52. To date, no studies have reported on the potential health impact of DEHTP. Thus, moving forward, in addition to phthalates metabolites with previously documented health effects, MECPTP and other metabolites of replacement compounds (e.g., DINCH) such as MCOCH and MHiNCH should be monitored. Finally, our study observed a decline in MEP concentrations from 2008 to 2010, which is consistent with the observed trends in the U.S. biomonitoring data31. DEP, most commonly found in fragrances and other personal care products, has not been subjected to the same regulatory scrutiny as other phthalates. Thus, our data suggest that there has been a change in either the formulation of the personal care products or the types of products used by pregnant women in Mexico during this time.
We observed that mothers who reported working in the home or working in administrative tasks and services had the highest phthalates burden while students and those in professional services (engineer, doctor, teachers, etc.) had the lowest. The source for this disparity appeared to be unrelated to SES as our analysis showed generally increased phthalates burden with increasing SES. Because of the ubiquitous nature of phthalates in the environment and commercial products1–5 and the heterogeneity of the occupational categories in our study, it is difficult to speculate on the potential sources that may explain the disparity in exposures. For example, administrative tasks and services comprise a range of potential occupations that differ in their exposures53 and our categorization does not allow us to identify the most highly burdened occupations. In addition, we had relatively few individuals who self-identified as students (n=45, 5% of the population) or those in professional services (n=46, 5% of the population). Thus, additional studies should further investigate occupational differences in phthalates exposure and determine potential methods for exposure reduction.
Our study has some notable strengths and limitations. We were able to characterize and describe 15 metabolites of eight phthalates and two DEHP replacements in a Mexican population. We have a relatively large sample size and excellent QC data that demonstrates reliability in the urinary biomarkers detection method. Furthermore, we were able to examine temporal trends both within the cohort and relative to other Mexican cohorts in the past. One major limitation of our study is that our participants were recruited from 2007 to 2011, and only a few enrolled in 2011 so we could not examine exposure profiles past 2010. The relatively short three year period may also have prevented us from identifying more subtle trends in phthalate metabolite concentrations. Future biomonitoring studies should examine current phthalate exposure trends. Another notable limitation is the lack of detailed dietary and personal care product use data during pregnancy, both major sources of phthalates exposure. Such information could be helpful in explaining the apparent differences in metabolite concentrations between the second and third trimesters and be used to inform pregnant women and the general Mexican population on how to reduce phthalate exposures.
Overall, we observed relatively high burdens of phthalates exposure in this cohort of pregnant women from Mexico City. These burdens appear to be correlated with several sociodemographic and occupational factors with no indication of decline over time. Future efforts are warranted to both continuously monitor the population’s exposure to traditional and replacement compounds and to investigate the potential public health impact of such exposures.
Supplementary Material
Supplemental Figure 1. Boxplot of 15 phthalate metabolites comparing the distribution of urinary concentrations across the PROGRESS population to the quality control data using 92 blinded replicates randomly distributed across batches.
Supplemental Figure 2. Correlation matrices of specific gravity corrected urinary phthalate metabolites concentrations measured within the second and third trimester urine samples from PROGRESS participants (N=941 and 792, respectively).
Supplemental Table 1. Demographics of PROGRESS Participants (n=948).
Supplemental Table 2. Estimated Relative Change and 95% Confidence Interval in Specific Gravity Corrected Urinary Phthalate Metabolite Concentrations from 2007 to 2010 by Year.
Acknowledgements:
We gratefully acknowledge all members of the PROGRESS team for their tireless efforts in maintain the cohort and the American British Cowdray Hospital for providing research facilities for the PROGRESS study. We also acknowledge Manori Silva, Ella Samandar, Jim Preau, and Tao Jia (CDC) for the urinary biomarkers measurements. Lastly, we would like to thank the study participants, without whom none of this would have been possible.
Funding:
This work was supported by grants from the National Institute of Environmental Health Sciences (R00 ES023474 to ALD, R01 ES021357 and P30 ES009089 to AAB, R01 ES024381 to JMB, R01 ES013744 and R24 ES028522 to ROW). The funding source did not have any role in the interpretation of the study results, writing of the manuscript, or decision to submit for publication.
Footnotes
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services.
References
- (1).Jurewicz J; Hanke W Exposure to Phthalates: Reproductive Outcome and Children Health. A Review of Epidemiological Studies. Int J Occup Med Environ Health 2011, 24 (2), 115–141. 10.2478/s13382-011-0022-2. [DOI] [PubMed] [Google Scholar]
- (2).Phthalates, N. R. C. (US) C. on the H. R. of Phthalate Exposure Assessment in Humans; National Academies Press; (US: ), 2008. [Google Scholar]
- (3).Wormuth M; Scheringer M; Vollenweider M; Hungerbühler K What Are the Sources of Exposure to Eight Frequently Used Phthalic Acid Esters in Europeans? Risk Analysis 2006, 26 (3), 803–824. 10.1111/j.1539-6924.2006.00770.x. [DOI] [PubMed] [Google Scholar]
- (4).Lyche JL; Gutleb AC; Bergman A; Eriksen GS; Murk AJ; Ropstad E; Saunders M; Skaare JU Reproductive and Developmental Toxicity of Phthalates. J Toxicol Environ Health B Crit Rev 2009, 12 (4), 225–249. 10.1080/10937400903094091. [DOI] [PubMed] [Google Scholar]
- (5).Hauser R; Duty S; Godfrey-Bailey L; Calafat AM Medications as a Source of Human Exposure to Phthalates. Environmental Health Perspectives 2004, 112 (6), 751–753. 10.1289/ehp.6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Katsikantami I; Sifakis S; Tzatzarakis MN; Vakonaki E; Kalantzi O-I; Tsatsakis AM; Rizos AK A Global Assessment of Phthalates Burden and Related Links to Health Effects. Environ Int 2016, 97, 212–236. 10.1016/j.envint.2016.09.013. [DOI] [PubMed] [Google Scholar]
- (7).Buck Louis GM; Sundaram R; Sweeney AM; Schisterman EF; Maisog J; Kannan K Urinary Bisphenol A, Phthalates, and Couple Fecundity: The Longitudinal Investigation of Fertility and the Environment (LIFE) Study . Fertil. Steril 2014, 101 (5), 1359–1366. 10.1016/j.fertnstert.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Dodge LE; Williams PL; Williams MA; Missmer SA; Souter I; Calafat AM; Hauser R; EARTH Study Team. Associations between Paternal Urinary Phthalate Metabolite Concentrations and Reproductive Outcomes among Couples Seeking Fertility Treatment. Reprod. Toxicol 2015, 58, 184–193. 10.1016/j.reprotox.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Mínguez-Alarcón L; Messerlian C; Bellavia A; Gaskins AJ; Chiu Y-H; Ford JB; Azevedo AR; Petrozza JC; Calafat AM; Hauser R; Williams PL; EARTH Study Team. Urinary Concentrations of Bisphenol A, Parabens and Phthalate Metabolite Mixtures in Relation to Reproductive Success among Women Undergoing in Vitro Fertilization. Environ Int 2019, 126, 355–362. 10.1016/j.envint.2019.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Wu H; Ashcraft L; Whitcomb BW; Rahil T; Tougias E; Sites CK; Pilsner JR Parental Contributions to Early Embryo Development: Influences of Urinary Phthalate and Phthalate Alternatives among Couples Undergoing IVF Treatment. Hum. Reprod 2017, 32 (1), 65–75. 10.1093/humrep/dew301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Bedrosian LD; Ferguson KK; Cantonwine DE; McElrath TF; Meeker JD Urinary Phthalate Metabolite Concentrations in Relation to Levels of Circulating Matrix Metalloproteinases in Pregnant Women. Sci. Total Environ 2018, 613–614, 1349–1352. 10.1016/j.scitotenv.2017.09.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Bellavia A; Hauser R; Seely EW; Meeker JD; Ferguson KK; McElrath TF; James-Todd T Urinary Phthalate Metabolite Concentrations and Maternal Weight during Early Pregnancy. Int J Hyg Environ Health 2017, 220 (8), 1347–1355. 10.1016/j.ijheh.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Boss J; Zhai J; Aung MT; Ferguson KK; Johns LE; McElrath TF; Meeker JD; Mukherjee B Associations between Mixtures of Urinary Phthalate Metabolites with Gestational Age at Delivery: A Time to Event Analysis Using Summative Phthalate Risk Scores. Environ Health 2018, 17 (1), 56 10.1186/s12940-018-0400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Chin HB; Jukic AM; Wilcox AJ; Weinberg CR; Ferguson KK; Calafat AM; McConnaughey DR; Baird DD Association of Urinary Concentrations of Phthalate Metabolites and Bisphenol A with Early Pregnancy Endpoints. Environ. Res 2019, 168, 254–260. 10.1016/j.envres.2018.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Johns LE; Ferguson KK; Cantonwine DE; McElrath TF; Mukherjee B; Meeker JD Urinary BPA and Phthalate Metabolite Concentrations and Plasma Vitamin D Levels in Pregnant Women: A Repeated Measures Analysis. Environ. Health Perspect 2017, 125 (8), 087026 10.1289/EHP1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Johns LE; Ferguson KK; McElrath TF; Mukherjee B; Meeker JD Associations between Repeated Measures of Maternal Urinary Phthalate Metabolites and Thyroid Hormone Parameters during Pregnancy. Environ. Health Perspect. 2016, 124 (11), 1808–1815. 10.1289/EHP170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Shaffer RM; Ferguson KK; Sheppard L; James-Todd T; Butts S; Chandrasekaran S; Swan SH; Barrett ES; Nguyen R; Bush N; McElrath TF; Sathyanarayana S Maternal Urinary Phthalate Metabolites in Relation to Gestational Diabetes and Glucose Intolerance during Pregnancy. Environ Int 2019, 123, 588–596. 10.1016/j.envint.2018.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).van T Erve TJ; Rosen EM; Barrett ES; Nguyen RHN; Sathyanarayana S; Milne GL; Calafat AM; Swan SH; Ferguson KK Phthalates and Phthalate Alternatives Have Diverse Associations with Oxidative Stress and Inflammation in Pregnant Women. Environ. Sci. Technol 2019, 53 (6), 3258–3267. 10.1021/acs.est.8b05729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Warembourg C; Basagaña X; Seminati C; de Bont J; Granum B; Lyon-Caen S; Manzano-Salgado CB; Pin I; Sakhi AK; Siroux V; Slama R; Urquiza J; Vrijheid M; Thomsen C; Casas M Exposure to Phthalate Metabolites, Phenols and Organophosphate Pesticide Metabolites and Blood Pressure during Pregnancy. Int J Hyg Environ Health 2019, 222 (3), 446–454. 10.1016/j.ijheh.2018.12.011. [DOI] [PubMed] [Google Scholar]
- (20).Botton J; Philippat C; Calafat AM; Carles S; Charles M-A; Slama R; The Eden Mother-Child Cohort Study Group, null. Phthalate Pregnancy Exposure and Male Offspring Growth from the Intra-Uterine Period to Five Years of Age. Environ. Res 2016, 151, 601–609. 10.1016/j.envres.2016.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Dorman DC; Chiu W; Hales BF; Hauser R; Johnson KJ; Mantus E; Martel S; Robinson KA; Rooney AA; Rudel R; Sathyanarayana S; Schantz SL; Waters KM Systematic Reviews and Meta-Analyses of Human and Animal Evidence of Prenatal Diethylhexyl Phthalate Exposure and Changes in Male Anogenital Distance. Journal of Toxicology and Environmental Health, Part B 2018, 21 (4), 207–226. 10.1080/10937404.2018.1505354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Li M-C; Chen C-H; Guo YL Phthalate Esters and Childhood Asthma: A Systematic Review and Congener-Specific Meta-Analysis. Environ. Pollut 2017, 229, 655–660. 10.1016/j.envpol.2017.06.083. [DOI] [PubMed] [Google Scholar]
- (23).Vafeiadi M; Myridakis A; Roumeliotaki T; Margetaki K; Chalkiadaki G; Dermitzaki E; Venihaki M; Sarri K; Vassilaki M; Leventakou V; Stephanou EG; Kogevinas M; Chatz L; Association of Early Life Exposure to Phthalates With Obesity and Cardiometabolic Traits in Childhood: Sex Specific Associations. Front Public Health 2018, 6, 327 10.3389/fpubh.2018.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Zhang Q; Chen X-Z; Huang X; Wang M; Wu J The Association between Prenatal Exposure to Phthalates and Cognition and Neurobehavior of Children-Evidence from Birth Cohorts. Neurotoxicology 2019, 73, 199–212. 10.1016/j.neuro.2019.04.007. [DOI] [PubMed] [Google Scholar]
- (25).Braun JM Early-Life Exposure to EDCs: Role in Childhood Obesity and Neurodevelopment. Nat Rev Endocrinol 2017, 13 (3), 161–173. 10.1038/nrendo.2016.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).European Chemicals Agency. Classifications - CL Inventory https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/10536 (accessed Jun 3, 2019).
- (27).EU Phthalates Directive 2005/84/EC; 2006.
- (28).CONSUMER PRODUCT SAFETY IMPROVEMENT ACT OF 2008; 2008.
- (29).Silva MJ; Wong L-Y; Samandar E; Preau JL; Jia LT; Calafat AM Exposure to Di-2-Ethylhexyl Terephthalate in the U.S. General Population from the 2015–2016 National Health and Nutrition Examination Survey. Environment International 2019, 123, 141–147. 10.1016/j.envint.2018.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Kasper-Sonnenberg M; Koch HM; Apel P; Rüther M; Pälmke C; Brüning T; Kolossa-Gehring M Time Trend of Exposure to the Phthalate Plasticizer Substitute DINCH in Germany from 1999 to 2017: Biomonitoring Data on Young Adults from the Environmental Specimen Bank (ESB). Int J Hyg Environ Health 2019, 222 (8), 1084–1092. 10.1016/j.ijheh.2019.07.011. [DOI] [PubMed] [Google Scholar]
- (31).Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals, Updated Tables; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, 2019. [Google Scholar]
- (32).Zota AR; Calafat AM; Woodruff TJ Temporal Trends in Phthalate Exposures: Findings from the National Health and Nutrition Examination Survey, 2001–2010. Environ. Health Perspect 2014, 122 (3), 235–241. 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Wang Y; Zhu H; Kannan K A Review of Biomonitoring of Phthalate Exposures. Toxics 2019, 7 (2). 10.3390/toxics7020021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).López-Carrillo L; Hernández-Ramírez RU; Calafat AM; Torres-Sánchez L; Galván-Portillo; Needham LL; Ruiz-Ramos R; Cebrián ME Exposure to Phthalates and Breast Cancer Risk in Northern Mexico. Environmental Health Perspectives 2010, 118 (4), 539–544. 10.1289/ehp.0901091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Rodríguez-Carmona Y; Cantoral A; Trejo-Valdivia B; Téllez-Rojo MM; Svensson K; Peterson KE; Meeker JD; Schnaas L; Solano M; Watkins DJ Phthalate Exposure during Pregnancy and Long-Term Weight Gain in Women. Environ. Res 2019, 169, 26–32. 10.1016/j.envres.2018.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Adrian Villegas Carrasco. The AMAI System of Classifying Households by Socio-Economic Level. ESOMAR; 2002. [Google Scholar]
- (37).Capurro H; Konichezky S; Fonseca D; Caldeyro-Barcia R A Simplified Method for Diagnosis of Gestational Age in the Newborn Infant. J. Pediatr 1978, 93 (1), 120–122. 10.1016/s0022-3476(78)80621-0. [DOI] [PubMed] [Google Scholar]
- (38).Silva MJ; Samandar E; Preau JL; Reidy JA; Needham LL; Calafat AM Quantification of 22 Phthalate Metabolites in Human Urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 2007, 860 (1), 106–112. 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- (39).Hornung RW; Reed LD Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene 1990, 5 (1), 46–51. 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- (40).Duty SM; Silva MJ; Barr DB; Brock JW; Ryan L; Chen Z; Herrick RF; Christiani DC; Hauser R Phthalate Exposure and Human Semen Parameters. Epidemiology 2003, 14 (3), 269–277. [PubMed] [Google Scholar]
- (41).Johns LE; Cooper GS; Galizia A; Meeker JD Exposure Assessment Issues in Epidemiology Studies of Phthalates. Environ Int 2015, 85, 27–39. 10.1016/j.envint.2015.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Ferguson KK; McElrath TF; Ko Y-A; Mukherjee B; Meeker JD Variability in Urinary Phthalate Metabolite Levels across Pregnancy and Sensitive Windows of Exposure for the Risk of Preterm Birth. Environ Int 2014, 70, 118–124. 10.1016/j.envint.2014.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Watkins DJ; Sánchez BN; Téllez-Rojo MM; Lee JM; Mercado-García A; Blank-Goldenberg C; Peterson KE; Meeker JD Impact of Phthalate and BPA Exposure during in Utero Windows of Susceptibility on Reproductive Hormones and Sexual Maturation in Peripubertal Males. Environ Health 2017, 16 (1), 69 10.1186/s12940-017-0278-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Martino-Andrade AJ; Liu F; Sathyanarayana S; Barrett ES; Redmon JB; Nguyen RHN; Levine H; Swan SH; TIDES Study Team. Timing of Prenatal Phthalate Exposure in Relation to Genital Endpoints in Male Newborns. Andrology 2016, 4 (4), 585–593. 10.1111/andr.12180. [DOI] [PubMed] [Google Scholar]
- (45).Cantonwine DE; Cordero JF; Rivera-González LO; Del Toro LVA; Ferguson KK; Mukherjee B; Calafat AM; Crespo N; Jiménez-Vélez B; Padilla IY; Alshawabkeh AN; Meeker JD Urinary Phthalate Metabolite Concentrations among Pregnant Women in Northern Puerto Rico: Distribution, Temporal Variability, and Predictors. Environ Int 2014, 62 10.1016/j.envint.2013.09.014.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Adibi JJ; Whyatt RM; Williams PL; Calafat AM; Camann D; Herrick R; Nelson H; Bhat HK; Perera FP; Silva MJ; Hauser R Characterization of Phthalate Exposure among Pregnant Women Assessed by Repeat Air and Urine Samples. Environ. Health Perspect 2008, 116 (4), 467–473. 10.1289/ehp.10749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Peck JD; Sweeney AM; Symanski E; Gardiner J; Silva MJ; Calafat AM; Schantz SL Intra- and Inter-Individual Variability of Urinary Phthalate Metabolite Concentrations in Hmong Women of Reproductive Age. J Expo Sci Environ Epidemiol 2010, 20 (1), 90–100. 10.1038/jes.2009.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Braun JM; Smith KW; Williams PL; Calafat AM; Berry K; Ehrlich S; Hauser R Variability of Urinary Phthalate Metabolite and Bisphenol A Concentrations before and during Pregnancy. Environ. Health Perspect 2012, 120 (5), 739–745. 10.1289/ehp.1104139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Fisher M; Arbuckle TE; Mallick R; LeBlanc A; Hauser R; Feeley M; Koniecki D; Ramsay T; Provencher G; Bérubé R; Walker M Bisphenol A and Phthalate Metabolite Urinary Concentrations: Daily and across Pregnancy Variability. J Expo Sci Environ Epidemiol 2015, 25 (3), 231–239. 10.1038/jes.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Yang TC; Peterson KE; Meeker JD; Sánchez BN; Zhang Z; Cantoral A; Solano M; Tellez-Rojo MM Exposure to Bisphenol A and Phthalates Metabolites in the Third Trimester of Pregnancy and BMI Trajectories. Pediatr Obes 2018, 13 (9), 550–557. 10.1111/ijpo.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Rodríguez-Carmona Y; Ashrap P; Calafat AM; Ye X; Rosario Z; Bedrosian LD; Huerta-Montanez G; Vélez-Vega CM; Alshawabkeh A; Cordero JF; Meeker JD; Watkins D Determinants and Characterization of Exposure to Phthalates, DEHTP and DINCH among Pregnant Women in the PROTECT Birth Cohort in Puerto Rico. J Expo Sci Environ Epidemiol 2020, 30 (1), 56–69. 10.1038/s41370-019-0168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Lessmann F; Kolossa-Gehring M; Apel P; Rüther M; Pälmke C; Harth V; Brüning T; Koch HM German Environmental Specimen Bank: 24-Hour Urine Samples from 1999 to 2017 Reveal Rapid Increase in Exposure to the Para-Phthalate Plasticizer Di(2-Ethylhexyl) Terephthalate (DEHTP). Environ Int 2019, 132, 105102 10.1016/j.envint.2019.105102. [DOI] [PubMed] [Google Scholar]
- (53).Brouwers MM; van Tongeren M; Hirst AA; Bretveld RW; Roeleveld N Occupational Exposure to Potential Endocrine Disruptors: Further Development of a Job Exposure Matrix. Occup Environ Med 2009, 66 (9), 607–614. 10.1136/oem.2008.042184. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Boxplot of 15 phthalate metabolites comparing the distribution of urinary concentrations across the PROGRESS population to the quality control data using 92 blinded replicates randomly distributed across batches.
Supplemental Figure 2. Correlation matrices of specific gravity corrected urinary phthalate metabolites concentrations measured within the second and third trimester urine samples from PROGRESS participants (N=941 and 792, respectively).
Supplemental Table 1. Demographics of PROGRESS Participants (n=948).
Supplemental Table 2. Estimated Relative Change and 95% Confidence Interval in Specific Gravity Corrected Urinary Phthalate Metabolite Concentrations from 2007 to 2010 by Year.


