Abstract
Objective:
Lateralized dysfunction has been suggested in Obsessive-Compulsive Disorder (OCD). However, it is currently unclear whether OCD is characterized by abnormal patterns of structural brain asymmetry. Here we carried out by far the largest study of brain structural asymmetry in OCD.
Method:
We studied a collection of 16 pediatric datasets (501 OCD patients and 439 healthy controls), as well as 30 adult datasets (1777 patients and 1654 controls) from the OCD Working Group within the ENIGMA (Enhancing Neuro-Imaging Genetics through Meta-Analysis) consortium. Asymmetries of the volumes of subcortical structures, and of regional cortical thickness and surface area measures, were assessed based on T1-weighted MRI scans, using harmonized image analysis and quality control protocols. We investigated possible alterations of brain asymmetry in OCD patients. We also explored potential associations of asymmetry with specific aspects of the disorder and medication status.
Results:
In the pediatric datasets, the largest case-control differences were observed for volume asymmetry of the thalamus (more leftward; Cohen’s d = 0.19) and the pallidum (less leftward; d = −0.21). Additional analyses suggested putative links between these asymmetry patterns and medication status, OCD severity, and/or anxiety and depression comorbidities. No significant case-control differences were found in the adult datasets.
Conclusions:
The results suggest subtle changes of the average asymmetry of subcortical structures in pediatric OCD, which are not detectable in adults with the disorder. These findings may reflect altered neurodevelopmental processes in OCD.
Keywords: laterality, brain asymmetry, obsessive-compulsive disorder, thalamus, pallidum, mega-analysis
Introduction
Obsessive-Compulsive Disorder (OCD) is a psychiatric disorder with a lifetime prevalence of approximately 2% (1–4). OCD involves persistent, intrusive and unwanted thoughts (obsessions) as well as repetitive behaviors which might be accompanied by mental acts (compulsions) (4). As a heterogeneous neuropsychiatric condition with considerable heritability of roughly 40% (5), OCD has significant genetic and non-genetic determinants (4), but the pathophysiology of this complex disorder remains unclear.
Left-right asymmetry is an important aspect of human brain organization for multiple functions (6). For example visual-spatial processing and emotions that elicit withdrawal behaviors are usually right-lateralized in healthy people (7–10), whereas language-related processes, hand motor dominance, and emotions that elicit approach behaviors tend to be left-lateralized in the brain (11, 12). Alterations of asymmetry have been reported in various psychiatric and neurocognitive conditions, including schizophrenia (13, 14), autism (15) and dyslexia (16). Altered functional laterality has also been investigated in OCD (17, 18), partly due to observations of psychometric deficits within the visual-spatial domain (19–21), as well as altered emotional processing (22–25). For example, a behavioral study found reduced functional asymmetry for spatial attention in OCD patients, and also that less typical asymmetry was correlated with more serious obsessions (20). Several studies found greater impairment in visual-spatial memory compared with verbal memory in OCD, suggestive of right-sided dysfunction (17, 18, 26). Increased left-right asymmetry of electroencephalographic (EEG) activity at rest, or reduced activity in the right hemisphere linked to approach/avoidance motivation, has also been reported in OCD compared to healthy controls (19, 22). However, left-sided dysfunction has also been suggested in OCD, on the basis of neuropsychological data (23) as well as neuroimaging studies (27–29). Reduced right-ear advantage, which can indicate left-hemisphere dysfunction, was reported in OCD for certain tasks (23). In addition, hyper-responsiveness was observed in the left hemisphere based on event-related potentials (27, 30). More recently, left lateralized differences in functional connectivity of the amygdala were reported in OCD versus controls, using task fMRI (31). Studies with animal models of OCD (32), and transcranial magnetic stimulation (TMS) in treatment-resistant OCD patients (33) have suggested that left-lateralized stimulation is more effective compared to right. Therefore, overall, the literature suggests altered hemispheric functional balance in OCD, but does not point consistently to one of the hemispheres as being the primary site of disruption.
Importantly, any structural basis linked to altered functional laterality in OCD is still unclear. Two previous studies explored brain structural asymmetry in OCD as a specific outcome of interest, but with low sample sizes. In one of these studies, with 16 OCD patients, leftward asymmetry (i.e., left > right) of cortical thickness in the anterior cingulate region was found in OCD patients and their siblings but not in matched controls, and this was claimed to present a potential endophenotype linked to increased hereditary risk for OCD (34). In the other study, with 32 patients, significant differences of frontal white matter volume asymmetry were found in both medicated (N = 19) and non-medicated (N = 13) patients, as compared with healthy controls (35). Unfortunately, small sample sizes tend to limit the reliability of findings in human neuroscience (36), and the extent of any association between OCD and structural brain asymmetry remains uncertain.
The OCD working group within the Enhancing Neuro-Imaging Genetics through Meta-Analysis (ENIGMA) consortium (37) recently achieved more highly powered analyses of brain changes in OCD, based on a sample size of over 1500 OCD individuals and a similar number of controls (38). They reported several regional case-control differences in cerebral cortical measures which involved only one hemisphere (38). However, these analyses did not examine whether effect sizes were significantly different on the left and right sides, and asymmetry was not quantitatively characterized. Unilateral patterns in this and other studies may arise from small but uniform bilateral effect sizes; the fact that statistical significance was achieved on one side, but not on the other, does not necessarily indicate a significant change in asymmetry. Furthermore, a post-hoc statistical comparison of the left and right-sided effect sizes as reported by the previous ENIGMA study (38) would not yield the same level of statistical power as can be provided by utilizing the individual-level, paired left and right data, to analyze asymmetry alterations in OCD. In addition, a previous ENIGMA study of subcortical volumes in OCD only reported combined left and right volumes (39).
Here, we used the latest data for both subcortical and cortical structures from the ENIGMA OCD Working Group, and targeted hemispheric structural asymmetry across subcortical and cortical measures, as assessed by subject-specific asymmetry indexes, AI = (Left-Right)/((Left+Right)/2) (40). The AI is a widely used approach in studies of brain asymmetry (e.g., (41, 42)). Our primary interest was to compare structural asymmetries between patients and healthy controls, but we also performed post-hoc analyses to investigate possible associations of brain asymmetries with medication status, age at disease onset, disease duration, OCD severity, and presence of anxiety and depression comorbidities. As the recent studies from the ENIGMA OCD working group had indicated distinct alterations in pediatric and adult patients (38, 39), and because asymmetries of both cortical and subcortical structures are also known to change subtly with age in the healthy population (40, 43), we carried out all analyses for the pediatric (<18 year old) and adult (>=18 year old) data separately (see also (44)).
Materials and Methods
See Supplementary Materials for detailed methods.
Datasets.
The datasets used in this study were provided by members of the OCD Working Group within the ENIGMA Consortium (37). There were 46 independent datasets from 16 countries: 16 pediatric datasets comprising 501 OCD patients and 439 healthy controls, and 30 adult datasets comprising 1777 OCD patients and 1654 healthy controls (Table 1, Figure S1–2 and Table S1). All local institutional reviews boards permitted the use of extracted measures from their anonymized data. In addition, we leveraged publicly available summary statistics which describe the average form of brain regional asymmetries, based on our previous larger studies of healthy individuals (40, 43).
Table 1.
Summary information on the case-control datasets included in the present study.
| Group | Site | Field Strength | Age in Years | Male (%) | N Controls | N OCD | Total N | ||
|---|---|---|---|---|---|---|---|---|---|
| Controls | OCD | Controls | OCD | ||||||
| Pediatric | James | 1.5 T | 16.63 (1.23) | 16.3 (1.42) | 58 | 54 | 12 | 13 | 25 |
| Lazaro | 1.5 T | 14.63 (2.3) | 14.61 (2.04) | 47 | 58 | 32 | 31 | 63 | |
| Buitelaar | 1.5 T | 10.93 (1.04) | 10.57 (1.41) | 72 | 64 | 61 | 22 | 83 | |
| Fitzgerald | 3 T | 12.96 (2.73) | 14.17 (2.59) | 51 | 48 | 59 | 62 | 121 | |
| Gruner | 3 T | 14.19 (2.21) | 14.33 (2.09) | 52 | 57 | 23 | 23 | 46 | |
| Arnold | 3 T | 12.3 (2.19) | 12.86 (2.35) | 54 | 61 | 13 | 36 | 49 | |
| Hoexter | 3 T | 12 (2.42) | 12.61 (2.45) | 57 | 61 | 28 | 28 | 56 | |
| Huyser | 3 T | 13.32 (2.55) | 13.59 (2.47) | 36 | 37 | 25 | 27 | 52 | |
| Stewart | 3 T | 14.02 (3.48) | 15.04 (2.68) | 40 | 39 | 30 | 28 | 58 | |
| Lazaro | 3 T | 14.57 (2.1) | 14.57 (2.04) | 55 | 60 | 44 | 58 | 102 | |
| Nurmi | 3 T | 13.3 (2.49) | 12.53 (2.84) | 50 | 54 | 36 | 59 | 95 | |
| Walitza | 3 T | 14.64 (1.34) | 15.68 (1.45) | 50 | 81 | 20 | 16 | 36 | |
| Reddy | 3 T | 13.07 (2.06) | 14.56 (1.98) | 50 | 56 | 14 | 18 | 32 | |
| Marsh | 3 T | 9.14 (2.48) | 12.12 (3.4) | 57 | 52 | 14 | 25 | 39 | |
| Hirano | 3 T | 15.33 (1.03) | 14 (2.18) | 67 | 65 | 6 | 20 | 26 | |
| Soreni | 3 T | 11.09 (3.02) | 13.09 (2.47) | 50 | 37 | 22 | 35 | 57 | |
| Pediatric Samples Combined | 13.06 (2.77) | 13.67 (2.65) | 53 | 54 | 439 | 501 | 940 | ||
| Adult | Menchon | 1.5 T | 33.06 (10.19) | 34.83 (9.17) | 45 | 50 | 66 | 117 | 183 |
| Cheng | 1.5 T | 31.43 (7.96) | 30.63 (10.21) | 33 | 38 | 40 | 24 | 64 | |
| KwonNMC | 1.5 T | 24.05 (3.63) | 24.76 (5.36) | 56 | 76 | 104 | 45 | 149 | |
| KwonSNU | 1.5 T | 24.89 (5.35) | 28.1 (6.71) | 64 | 63 | 45 | 41 | 86 | |
| Nakamae | 1.5 T | 30.44 (7.9) | 31.61 (9.15) | 46 | 48 | 48 | 82 | 130 | |
| Morgado | 1.5 T | 27.58 (6.23) | 27.69 (7.4) | 38 | 47 | 53 | 59 | 112 | |
| Mataix_Cols | 1.5 T | 36.12 (11.26) | 38.68 (10.9) | 36 | 43 | 33 | 44 | 77 | |
| Reddy | 1.5 T | 27.22 (6.45) | 27.45 (6.31) | 74 | 59 | 46 | 44 | 90 | |
| Hoexter | 1.5 T | 27.62 (7.75) | 31.46 (10.06) | 35 | 44 | 37 | 50 | 87 | |
| van den Heuvel | 1.5 T | 31.57 (7.67) | 33.54 (9.19) | 39 | 30 | 49 | 54 | 103 | |
| Beucke | 1.5 T | 31.92 (9.5) | 32.41 (9.74) | 49 | 50 | 104 | 92 | 196 | |
| Cheng | 3 T | 26.19 (4.18) | 32.89 (10.57) | 28 | 55 | 95 | 56 | 151 | |
| Nakamae | 3 T | 29.57 (7.27) | 32.82 (9.74) | 45 | 35 | 42 | 34 | 76 | |
| Brennan | 3 T | 32.38 (12.14) | 28.84 (9.99) | 45 | 56 | 29 | 98 | 127 | |
| van den Heuvel | 3 T | 39.61 (11.37) | 38.32 (10.07) | 47 | 48 | 38 | 42 | 80 | |
| Denys | 3 T | 39.64 (10.32) | 35.26 (9.17) | 44 | 26 | 25 | 31 | 56 | |
| Kwon | 3 T | 26.26 (6.9) | 26.7 (7.28) | 61 | 62 | 89 | 90 | 179 | |
| Benedetti | 3 T | 33.98 (12.35) | 35.02 (10.39) | 73 | 71 | 62 | 66 | 128 | |
| Hirano | 3 T | 30.95 (8.36) | 33.11 (7.82) | 45 | 36 | 44 | 47 | 91 | |
| Koch | 3 T | 30.27 (9.04) | 30.91 (9.55) | 39 | 37 | 74 | 76 | 150 | |
| Stein | 3 T | 30.59 (10.76) | 30.48 (10.63) | 38 | 48 | 29 | 23 | 52 | |
| Tolin | 3 T | 48 (11.87) | 32.11 (12.04) | 22 | 67 | 32 | 27 | 59 | |
| Simpson | 3 T | 28.27 (8.04) | 29.62 (7.98) | 52 | 52 | 33 | 33 | 66 | |
| Nakao | 3 T | 39.34 (12.99) | 36.6 (10.02) | 39 | 42 | 41 | 81 | 122 | |
| Spalletta | 3 T | 36.52 (10.55) | 36.67 (11.56) | 59 | 67 | 128 | 84 | 212 | |
| Stern | 3 T | 28.17 (7.15) | 27.87 (6.9) | 44 | 33 | 18 | 15 | 33 | |
| Wang | 3 T | 26.24 (7.55) | 29.47 (9.33) | 54 | 55 | 37 | 53 | 90 | |
| Nurmi | 3 T | 30.76 (11.77) | 33.31 (11.04) | 56 | 51 | 25 | 49 | 74 | |
| Walitza | 3 T | 32.89 (9.21) | 30.72 (7.76) | 28 | 47 | 18 | 17 | 35 | |
| Reddy | 3 T | 26.59 (4.88) | 29.5 (6.74) | 64 | 53 | 170 | 203 | 373 | |
| Adult Samples Combined | 30.55 (9.73) | 31.74 (9.66) | 50 | 51 | 1654 | 1777 | 3431 | ||
Site indicate the representative author of each dataset; Numbers in parenthesis indicate the standard deviation of age.
Image Acquisition and Processing.
Structural T1-weighted MRI scans were acquired and processed locally at each collection site. Images were acquired at different field strengths (1.5 T and 3T). All images were analyzed using one automated and validated pipeline, i.e. “recon-all” as implemented in FreeSurfer. For each subject, surface area and mean thickness was extracted for each of the 68 cortical regions (34 per hemisphere) in the Desikan-Killiany parcellation scheme (45), as well as total hemispheric surface area, and the average mean thickness over each hemisphere. In addition, volumes of eight subcortical regions of interest, including seven subcortical structures (nucleus accumbens, amygdala, caudate, hippocampus, pallidum, putamen, and thalamus), and the lateral ventricle volume, were calculated.
Asymmetry indexes.
The aim of this study was to investigate differences in subcortical and cortical asymmetry related to OCD. To this end, for each participant, and each subcortical or cortical measure, an Asymmetry Index (AI) was defined as (L-R)/((L+R)/2), where L and R represent the corresponding left and right volume measures (from subcortical regions), or thickness and surface area measures (from cortical regions). This AI formula has been widely used in previous brain asymmetry studies (41, 42, 46), including our own (8, 40, 43).
Case-control analyses.
Separately for the pediatric and adult data, and for each AI, we pooled data from all available individuals from each dataset, and used a mega-analytical framework to investigate the case-control effects. Specifically, for each AI, we used a linear mixed-effect model (using lme4 R package), with AI as the outcome variable, and a binary indicator of diagnosis (0=controls, 1=OCD patients) as the predictor of interest. In each model, a binary variable for sex, and a continuous measure for age (in years at time of scan) were included as confounding factors, and the categorical variable ‘dataset’ as a random-effect term.
Separately for thickness and surface area, we additionally calculated an overall ‘typicality score’ per subject, which indexed how much a given subject deviated from the population mean asymmetry profile, when considered simultaneously across all 34 cortical regions. A lower typicality score indicates more deviation from the mean asymmetry profile in the population.
OCD case-only analyses of clinical characteristics.
For AIs which were potentially associated with OCD in the main analysis (see Results), we further investigated, within cases only, whether the AIs were associated with specific aspects of the disorder and medication status.
Results
An overview of the datasets is provided in Table 1, Figure S1–2, and Table S1.
Pediatric data.
The results for both subcortical and cortical AIs in the pediatric data, including the effect size estimates for diagnosis on each AI, are presented in Figure 1 and Tables S2–S4.
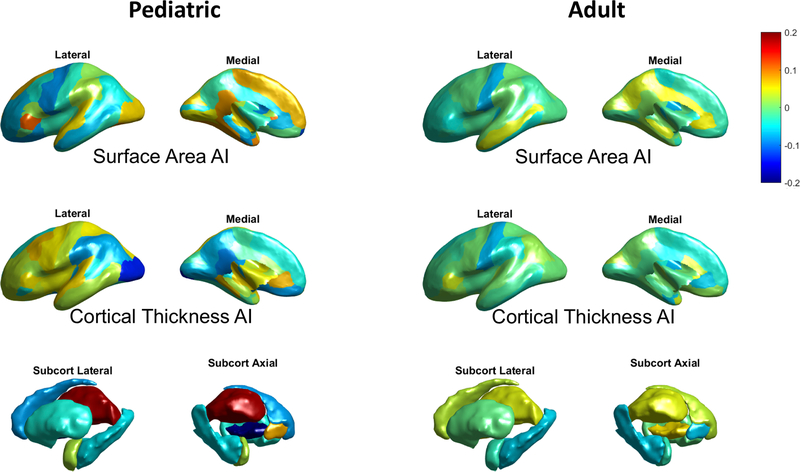
Figure 1. Effect size (Cohen’s d) distributions for diagnosis on regional AIs in the pediatric (left) and adult (right) data.
In terms of cortical asymmetries in the pediatric data, no significant case-control differences in the global hemispheric AI for either cortical thickness or surface area were found (ps >0.40). Regionally, only one AI showed a nominally significant effect (i.e. prior to multiple testing correction) of diagnosis, which was for thickness asymmetry of the lateral occipital cortex (greater rightward asymmetry in OCD patients; t = −2.08, p = 0.038, d = −0.14; Figure 2). This did not survive multiple testing correction. No other AIs in case-control comparisons within the pediatric data showed significant effects (uncorrected ps >0.05).
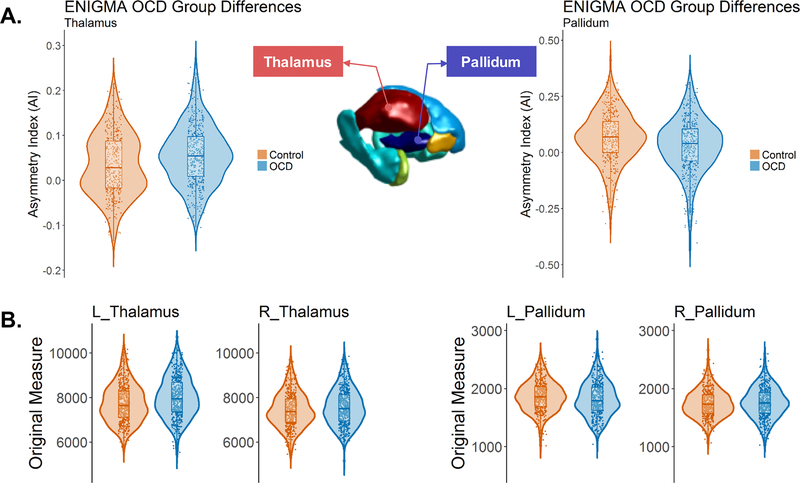
The largest effects of diagnosis in pediatric cases were more leftward asymmetry of the thalamus (t = 2.84, p = 0.0047, d = 0.19; Figure 1–2), and less leftward asymmetry of the pallidum volume (t = −3.17, p = 0.0016, d = −0.21; Figure 1–2). These two findings were significant when controlling the FDR at 0.05 (see Materials and Methods). Post hoc analyses showed that these case-control differences were mainly due to a left thalamus which was relatively larger in OCD patients than controls (Left: t = 4.08, p = 4.89e-05, d = 0.27; Right: t = 2.12, p = 0.034, d = 0.14), and a left pallidum which was relatively smaller in OCD patients than controls (Left: t = −1.98, p = 0.048, d = −0.13; Right: t <1.0, p = 0.35, d = 0.062) (see also Figure 2B for distribution and group differences of each unilateral volume measure). In addition, we confirmed that the effects remained when excluding possible outliers in each AI per dataset (see Methods) (pediatric thalamus volume asymmetry: t = 2.90, p = 0.0038, d = 0.19; pediatric pallidum volume asymmetry: t = −3.16, p = 0.0016, d = −0.21).
Figure 2. Subcortical structures showing altered volumetric asymmetry in pediatric OCD patients: the thalamus and the pallidum.
The violin plots show the distributions and group differences of the volume asymmetry (A) and the lateral volume measures (in mm3) in each hemisphere (B) for the thalamus and the pallidum. Note that the main analyses were based on linear mixed-effect modelling with ‘dataset’ as a random-effect term, whereas data are plotted here without correction for the ‘dataset’ variable, for display purposes only.
Within pediatric patients only, there were no differences of the thalamus or pallidum AIs between medicated and unmedicated subjects (uncorrected ps >0.20), nor with respect to current anxiety or depression comorbidity (ps >0.20), or age at disease onset or disease duration (ps >0.05). In terms of OCD symptom, the pallidum AI showed significant association with two of the 5 major Y-BOCS symptom components: hoarding (t = −2.37, p = 0.0065) and cleaning/contamination (t = −2.29, p = 0.014), such that cases with these symptoms had reduced leftward asymmetry of the pallidum compared to cases without these symptoms. No significant associations of symptom severity were observed with the thalamus AI, within the pediatric cases (ps >0.10).
When repeating the main analysis including age2 in the model, in case of substantial non-linear effects of age on AIs, all of the Cohen’s d for the effects of diagnosis remained within 0.005 of their values before having included age2, and the same two AIs (thalamus volume AI, pallidum volume AI) remained significant after FDR correction. None of the AIs showed significant scanner effects in the pediatric data (ps >0.05), and the significant effects of diagnosis remained when adding scanner field strength as a predictor variable to the main analysis models (pediatric thalamus volume asymmetry: t = 2.81, p = 0.0050, d = 0.19; pediatric pallidum volume asymmetry: t = −3.02, p = 0.0025, d = −0.20).
We calculated per-subject ‘typicality scores’ (see Methods), and compared the typicality scores between patients and controls. However, no significant differences were found in the pediatric data for either thickness or surface area asymmetries (ps >0.15). This analysis might have been sensitive to multi-regional disruptions of laterality that are not consistent in direction, as could conceivably arise from generally increased developmental instability.
Adult data.
The results for both subcortical and cortical AIs in the adult data, including the effect size estimates for diagnosis on each AI, are presented in Figure 1 and Tables S5–S7. All effects were subtle (Cohen’s d between −0.086 and 0.066), and not as strong as found in the pediatric data.
The largest effect in adults was a case-control difference in the AI of global hemispheric surface area (t = −2.48, p = 0.013, d = −0.086), indicating that adult OCD was associated with slightly more rightward overall asymmetry in surface area, compared with controls. However, this did not survive multiple testing correction when accounting for all regional surface area AI comparisons. Post hoc analyses showed that this difference was mainly due to relatively smaller surface area in the left hemisphere (Left: t = −2.80, p = 0.0051, d = −0.098; Right: t = −2.18, p = 0.029, d = −0.076) in adult OCD patients than controls. The effect on this AI remained after excluding potential outliers (see Methods) (t = −3.03, p = 0.0025, d = −0.10). No significant case-control difference in the total average asymmetry of cortical thickness was found (p =0.35). No significant differences were found in regional asymmetries after multiple testing correction (Supplementary Materials).
Although the observed effect of diagnosis on the AI of global hemispheric surface area did not survive multiple testing correction, we were interested to explore associations of this AI with case-only variables, as it is a global rather than regional measure. Within the adult OCD patients, there was a trend towards unmedicated cases showing a mean AI difference compared to medicated cases (t = −1.77, p = 0.077, d =−0.086; i.e., more rightward asymmetry in medicated cases). Adult cases with current depression showed a mean AI difference compared to those without (t = −2.15, p = 0.032, d = −0.17; i.e., more rightward asymmetry in cases with current depression), while no effect of current anxiety comorbidity was observed (p =0.48). There was no correlation of this AI with the age at disease onset (t <1.0, p = 0.53) or the disease duration (t = −1.03, p =0.30). In terms of OCD severity measures, no significant associations were found with either the severity in total score or the subcomponent variables (ps >0.10).
Including age2 or scanner field strength did not change the main results (Supplementary Materials). Typicality scores (see Methods) showed no case-control differences in the adult data, for either thickness or surface area asymmetry (ps >0.15).
The effect sizes of the AI case-control differences in the pediatric and adult data were found to be uncorrelated across the 34 cortical regions, for either thickness AIs or surface area AIs (ps >0.40).
Discussion
In this study we aimed to map differences in brain asymmetry between OCD patients and healthy controls, by leveraging a collection of 16 pediatric datasets and 30 adult datasets, via the ENIGMA Consortium. Using by far the largest sample size to address this issue to date, the results revealed a small number of asymmetry differences in OCD patients. The largest effects were in the pediatric patients for the volume asymmetry of the thalamus and the pallidum. These effects both had Cohen’s d values of around 0.2, which indicates their subtlety and suggests that altered structural brain asymmetry alone is unlikely to be a clinically useful predictor of OCD. Nonetheless, these effect sizes were comparable to those reported by previous large-scale studies of disorder-related changes in brain structure, in which asymmetry was not studied, including studies of OCD as well as major depression, schizophrenia, and autism (e.g., (38, 39, 47–51)). Given that the effect sizes in the present study were estimated based on large sample sizes, relatively accurate estimations of the true effects were possible, whether they were statistically significant or not. As such, the effects are informative to share with the field.
Our finding of subtle changes in thalamus asymmetry in pediatric patients is broadly in accordance with previous disease models for OCD as regards the cortico-striato-thalamo-cortical (CSTC) circuitry, which is involved in a wide range of cognitive, motivational and emotional processes (44). Boedhoe et al. (39) observed a mean increase in bilateral thalamus volume (left plus right) in pediatric OCD patients versus controls, while in the present study, with a larger collection of 16 datasets (including 10 datasets used by Boedhoe et al.), we found that this OCD-related volume alteration was largely left-lateralized and resulted in altered thalamus asymmetry. It is not clear what pathophysiological mechanisms might link altered thalamus asymmetry to OCD. Within OCD individuals, we found no associations of thalamus asymmetry with medication status, age at a disease onset, disease duration, current anxiety and depression comorbidity, or disease symptoms, which might have given some insights into the observed differences. The thalamus is involved in diverse interactions among cortical, subcortical, and brainstem nuclei, and many of its functions are asymmetrical in normal subjects (52). In addition, the thalamus is subdivided into cytoarchitectonically distinct nuclei with different functions (53). Future studies using higher resolution mapping of internal thalamus subsegments’ structure and function may therefore be informative in pediatric OCD.
For the pallidum, no total volume change (left plus right) was reported by Boedhoe et al. in pediatric OCD patients, while here, with a larger collection of 16 pediatric datasets (including 10 used by Boedhoe et al.), we found an asymmetry difference of the pallidum which was largely driven by a significantly reduced left-sided volume in pediatric OCD patients. Boedhoe et al. also reported that adult OCD patients showed a larger pallidum (again left plus right) than controls, driven by patients with a childhood-onset of disease (39). We saw no significant effect on pallidum asymmetry in adult patients, in either the subgroups of early- or late-onset of disease (Supplemental Materials). This overall pattern of results suggests that disease chronicity, cumulative treatment effects and/or late adolescent volumetric changes in patients are linked to a bilateral increase in pallidum volume, but that reduced left sided volume in pediatric patients reflects a different, earlier developmental process. Moreover, pallidum asymmetry in the pediatric patients showed associations with symptom components “hoarding” and “cleaning/contamination”. Although recently “hoarding disorder” was suggested as a separate diagnostic entity (54), in the present data there was only 1 case with hoarding behavior in the absence of other symptoms. Thus, we do not consider this tentative effect on asymmetry to relate to hoarding disorder specifically.
The pallidum, linking with the striatum and the thalamus within the CSTC circuitry (44), has roles in reward and motivation, as well as broader cognitive, affective and sensorimotor processes (44, 55). Further studies on specific functions of the (left) pallidum in compulsive symptoms, cleaning/contamination behaviors specifically, are needed. While it is not clear why lateralized changes in particular should be involved, in general terms our findings in pediatric cases help to characterize the brain structural changes in this disorder, and suggest altered subcortical neurodevelopment affecting the cortico-striato-thalamo-cortical circuitry. Further research will be needed to clarify any potential functional relevance of asymmetrical alterations in particular.
In terms of cortical measures in the pediatric data, we found no significant case-control differences in the asymmetry of regional or global measures of cortical thickness or surface area. This indicates that none of the cortical case-control differences reported by the previous large-scale ENIGMA study (38) are significantly lateralized, even when they might have been reported with respect to only one side. We also used a multivariable measure to describe the ‘typicality’ of each subject’s asymmetry pattern over all cortical regions with respect to a healthy and general population database (40). However, no case-control differences in this measure were found. Together these analyses indicate that alterations of cerebral cortical anatomical asymmetry are not notable features of pediatric OCD.
In the adult data, there was no evidence for case-control differences of regional asymmetries, for either subcortical or cortical measures. The strongest cortical effect in adults was at the total hemispheric level, whereby cases showed slightly more rightward asymmetry of total surface area, mainly due to having a relatively smaller surface area in the left hemisphere than controls. However, this very small effect, with Cohen’s d of 0.086, was not significant in the context of multiple testing, so that further studies with even larger sample sizes will be needed to confirm or refute this result. The effect was more pronounced in cases with comorbid depression, although this observation also remains tentative in the context of multiple testing.
Consistently with the previous findings of distinct alterations between pediatric and adult patients by the ENIGMA OCD Working Group (38, 39), the present study of structural asymmetry also showed different OCD-related effects between pediatric and adult data. There was also no correlation of case-control asymmetry differences between pediatric and adult data across the 34 cortical regions, which further supported the distinct OCD-related effects between pediatric and adult patients. Nonetheless, it is intriguing that the most notable effects in the pediatric and adult data all involved predominantly left-hemisphere alterations, which might support previous models of left-hemisphere dysfunction in OCD, as have been suggested by some functional imaging and neuropsychological findings (see Introduction) (23, 27–29). However, it will be important for future functional imaging studies to avoid reporting lateralized dysfunction on the basis that only one of the two hemispheres shows significant case-control differences. This is because, as noted in the Introduction, a hemispheric difference of significance does not necessarily indicate a significant difference of effects between hemispheres.
OCD is a heterogeneous neuropsychiatric condition with a heritability of roughly 40%, as has been observed using both twin/family based estimation and SNP-based estimation (5, 56). A recent study showed that genetic variation across the genome, which impacts risk for OCD, also includes variation which affects the volumes of the nucleus accumbens and putamen (57). The structural brain asymmetries which showed the strongest associations with OCD in the present study have been shown to have significant heritability: 23% for the volume asymmetry of the thalamus, 15% for the volume asymmetry of the pallidum (43), and 17% for the total hemispheric asymmetry of cerebral cortical surface area (40). It may therefore be useful in future studies to assess the genetic correlation between these aspects of brain asymmetry and OCD, which might lead towards genome-wide association studies (58) to identify individual genetic loci that are involved in OCD-related asymmetry abnormalities.
This study has several limitations. First, the cross-sectional study design limits the interpretation of the results particularly with respect to age-related changes. Further work using longitudinal studies, and incorporating genetic and environmental variables, may be useful to understand the mechanisms underlying the potential associations reported here. Second, while the region-based approach used in this study is feasible for large-scale, collaborative projects, it is necessarily limited in terms of spatial resolution, and this might have contributed to some of the null results for regional cortical or subcortical regions. Investigation with more fined definition of regions (e.g., sub-regions of the thalamus (59)) or a vertex-wise approach combined with cross-hemispheric registration methods will be likely to be useful for future cortical asymmetry studies (60, 61). Third, the symptoms of OCD are heterogeneous (4). Identifying potential subtypes of OCD could therefore provide further insights into the pathophysiology.
In summary, we mapped structural brain asymmetry in pediatric and adult OCD as compared to controls, using by far the largest sample size to date. Effects were small overall, and most pronounced in the thalamus and the pallidum in pediatric patients, which also showed potential links with medication status, disorder severity, and/or anxiety and depression comorbidities. Our study adds to literature implicating the thalamus in the pathophysiology of pediatric OCD, and additionally implicates the pallidum in pediatric cases. The full set of results from this study is available in the SI Tables and online for easy access (https://conxz.github.io/AsymOCD/).
Supplementary Material
Highlights:
Brain structural asymmetry alterations in patients with OCD were investigated.
This study was performed with a large sample size via the ENIGMA Consortium.
The largest case-control mean differences were found in the thalamus and pallidum in pediatric OCD patients.
Alterations of structural asymmetry in OCD were subtle and restricted to pediatric cases.
Acknowledgments
The ENIGMA-OCD Working Group. Xiangzhen Kong (1), Premika S.W. Boedhoe (2,3), Yoshinari Abe (4), Pino Alonso (5,6,7), Stephanie H. Ameis (8,9), Alan Anticevic (10), Paul D. Arnold (11,12), Francesca Assogna (13), Justin T. Baker (14), Nerisa Banaj (13), Nuria Bargalló (15,16), Marcelo C. Batistuzzo (17), Francesco Benedetti (18), Jan C. Beucke (19), Irene Bollettini (18), Anushree Bose (20), Daniel Brandeis (21,22), Silvia Brem (23,24), Brian P. Brennan (25), Jan Buitelaar (26), Geraldo F. Busatto (17), Anna Calvo (15), Rosa Calvo (27,28,29), Yuqi Cheng (30), Kang Ik K. Cho (31), Valentina Ciullo (13,32), Sara Dallaspezia (18), Damiaan Denys (33,34), Froukje E. de Vries (2), Stella J. de Wit (2), Erin Dickie (35), Renate Drechsler (21), Benjamin A. Ely (36), Madalena Esteves (37,38,39), Andrea Falini (40), Yu Fang (41), Jamie Feusner (42), Martijn Figee (43,33), Kate D. Fitzgerald (41), Martine Fontaine (44), Jean-Paul Fouche (45,46), Egill A. Fridgeirsson (33), Patricia Gruner (10), Deniz A. Gürsel (47,48), Geoff Hall (49), Sayo Hamatani (50), Gregory L. Hanna (41), Bjarne Hansen (51,52), Tobias U. Hauser (23,53,54), Yoshiyuki Hirano (50), Marcelo Q. Hoexter (17), Hao Hu (55), Chaim Huyser (56,57), Keisuke Ikari (58), Neda Jahanshad (59), Anthony James (60), Fern Jaspers-Fayer (61), Norbert Kathmann (19), Christian Kaufmann (19), Kathrin Koch (47,48), Masaru Kuno (50), Gerd Kvale (51,52), Jun Soo Kwon (62,63), Luisa Lazaro (27,64,28,29), Yanni Liu (41), Christine Lochner (65), Ricardo Magalhães (37,38,39), Paulo Marques (37,38,39), Rachel Marsh (44,66), Ignacio Martínez-Zalacaín (5,7), Yasutaka Masuda (67), David Mataix-Cols (68), Koji Matsumoto (67), James T. McCracken (42), José M. Menchón (5,6,7), Euripedes C. Miguel (17), Luciano Minuzzi (69), Pedro S Moreira (37,38,39), Astrid Morer (27,64,28,29), Pedro Morgado (37,38,39), Akiko Nakagawa (50), Takashi Nakamae (4), Tomohiro Nakao (58), Janardhanan. C. Narayanaswamy (20), Jin Narumoto (4), Seiji Nishida (4), Erika L. Nurmi (42), Joseph O’Neill (42), Jose C. Pariente (15), Chris Perriello (25,70), John Piacentini (42), Fabrizio Piras (13), Federica Piras (13), Christopher Pittenger (71), Sara Poletti (18), Y.C. Janardhan Reddy (20), Tim Reess (47,48), Oana Georgiana Rus-Oswald (72), Yuki Sakai (73,4), Joao R. Sato (74), Lianne Schmaal (75,76), Eiji Shimizu (50,77), H. Blair Simpson (44,78), Noam Soreni (79), Carles Soriano-Mas (5,6,80), Nuno Sousa (37,38,39), Gianfranco Spalletta (13,81), Emily R. Stern (82,83), Michael C. Stevens (84,85), S. Evelyn Stewart (86,87), Philip R. Szeszko (88,89), Jumpei Takahashi (50), Jinsong Tang (42), Anders Lillevik Thorsen (51,52,90), David F. Tolin (91,92), Aki Tsuchiyagaito (50,93), Daan van Rooij (26), Guido A. van Wingen (33), Ysbrand D. van der Werf (3), Dick J. Veltman (2), Daniela Vecchio (13), Ganesan Venkatasubramanian (20), Susanne Walitza (21), Zhen Wang (55,94), Anri Watanabe (4), Jian Xu (95), Xiufeng Xu (30), Kei Yamada (96), Tokiko Yoshida (50), Je-Yeon Yun (97,98), Mojtaba Zarei (99), Qing Zhao (55), Cong Zhou (30), Paul M. Thompson (100), Dan J. Stein (101), Odile A. van den Heuvel (2,3), Clyde Francks (1,102)
1. Language and Genetics Department, Max Planck Institute for Psycholinguistics, Nijmegen, The Netherlands; 2. Amsterdam UMC, Vrije Universteit Amsterdam, Department of Psychiatry, Amsterdam Neuroscience, Amsterdam, The Netherlands; 3. Amsterdam UMC, Vrije Universiteit Amsterdam, Department of Anatomy & Neurosciences, Amsterdam Neuroscience, Amsterdam, The Netherlands; 4. Department of Psychiatry, Graduate School of Medical Science, Kyoto Prefectural University of Medicine, Kyoto, Japan; 5. Department of Psychiatry, Bellvitge University Hospital, Bellvitge Biomedical Research Institute-IDIBELL, L’Hospitalet de Llobregat, Barcelona, Spain; 6. Centro de Investigación Biomèdica en Red de Salud Mental-CIBERSAM, Barcelona, Spain; 7. Department of Clinical Sciences, University of Barcelona, Spain; 8. The Margaret and Wallace McCain Centre for Child, Youth & Family Mental Health, Campbell Family Mental Health Research Institute, The Centre for Addiction and Mental Health, Department of Psychiatry, Faculty of Medicine, University of Toronto, Toronto, Canada; 9. Centre for Brain and Mental Health, The Hospital for Sick Children, Toronto, Canada; 10. Department of Psychiatry, Yale University School of Medicine, New Haven, Connecticut, U.S.A.; 11. Mathison Centre for Mental Health Research & Education, Hotchkiss Brain Institute, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada; 12. Department of Psychiatry, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada; 13. Laboratory of Neuropsychiatry, Department of Clinical and Behavioral Neurology, IRCCS Santa Lucia Foundation, Rome, Italy; 14. McLean Hopsital, Harvard Medical School, Belmont, MA, U.S.A.; 15. Magnetic Resonance Image Core Facility, IDIBAPS (Institut d’Investigacions Biomèdiques August Pi i Sunyer), Barcelona, Spain; 16. Image Diagnostic Center, Hospital Clínic, Barcelona, Spain; 17. Departamento e Instituto de Psiquiatria do Hospital das Clinicas, IPQ HCFMUSP, Faculdade de Medicina, Universidade de Sao Paulo, SP, Brasil.; 18. Psychiatry and Clinical Psychobiology, Division of Neuroscience, Scientific Institute Ospedale San Raffaele, Milano, Italy; 19. Department of Psychology, Humboldt-Universität zu Berlin, Berlin, Germany; 20. Obsessive-Compulsive Disorder (OCD) Clinic Department of Psychiatry National Institute of Mental Health & Neurosciences, Bangalore, India; 21. Department of Child and Adolescent Psychiatry and Psychotherapy, Psychiatric Hospital, University of Zurich, Zurich Switzerland; 22. Department of Child and Adolescent Psychiatry and Psychotherapy, Central Institute of Mental Health, Medical Faculty, Mannheim, Heidelberg University, Mannheim, Germany; 23. Department of Child and Adolescent Psychiatry and Psychotherapy, Psychiatric Hospital, University of Zurich, Zurich, Switzerland; 24. Neuroscience Center Zurich, University of Zurich and ETH Zurich, Zurich, Switzerland; 25. McLean Hospital, Harvard Medical School, Belmont, MA, U.S.A.; 26. Department of Cognitive Neurosicence, Donders Institute for Brain, Cognition and Behavior, Radboudumc, Nijmegen, The Netherlands; 27. Department of Child and Adolescent Psychiatry and Psychology, Institute of Neurosciences, Hospital Clínic Universitari, Barcelona, Spain; 28. Department of Medicine, University of Barcelona, Barcelona, Spain; 29. Centro de Investigación Biomédica en red de Salud Mental (CIBERSAM), Spain; 30. Department of Psychiatry, First Affiliated Hospital of Kunming Medical University, Kunming, China; 31. Institute of Human Behavioral Medicine, SNU-MRC, Seoul, Republic of Korea; 32. Department of Neurosciences, Psychology, Drug Research and Child Health (NEUROFARBA), University of Florence, Italy; 33. Amsterdam UMC, University of Amsterdam, Department of Psychiatry, Amsterdam Neuroscience, Amsterdam, Netherlands; 34. Netherlands Institute for Neuroscience, Royal Netherlands Academy of Arts and Sciences, Amsterdam, The Netherlands; 35. Campbell Family Mental Health Research Institute, The Centre for Addiction and Mental Health, Department of Psychiatry, Faculty of Medicine, University of Toronto, Toronto, Canada; 36. Department of Neuroscience, Graduate School of Biomedical Sciences, Icahn School of Medicine at Mount Sinai, New York, NY, U.S.A; 37. Life and Health Sciences Research Institute (ICVS), School of Medicine, University of Minho, Braga, Portugal.; 38. ICVS/3B’s, PT Government Associate Laboratory, Braga/Guimarães, Portugal.; 39. Clinical Academic Center-Braga, Braga, Portugal.; 40. Neuroradiology, Division of Neuroscience, Scientific Institute Ospedale San Raffaele, Milano, Italy; 41. Department of Psychiatry, University of Michigan, Ann Arbor, Michigan, U.S.A.; 42. Department of Psychiatry and Biobehavioral Sciences, University of California, Los Angeles, CA, U.S.A.; 43. Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, USA.; 44. Columbia University Irving Medical Center, Columbia University, New York, NY, U.S.A.; 45. Department of Psychiatry, University of Cape Town, Cape Town, South Africa; 46. Department of Psychiatry, University of Stellenbosch, Cape Town, South Africa; 47. Department of Neuroradiology, Klinikum rechts der Isar, Technische Universität München, Germany; 48. TUM-Neuroimaging Center (TUM-NIC) of Klinikum rechts der Isar, Technische Universität München, Germany; 49. Department of Psychology, McMster University, Hamilton, Ontario, Canada; 50. Research Center for Child Mental Development, Chiba University, Chiba, Japan; 51. OCD-team, Haukeland University Hospital, Bergen, Norway; 52. Department of Clinical Psychology, University of Bergen, Bergen, Norway; 53. Max Planck UCL Centre for Computational Psychiatry and Ageing Research, London, UK; 54. Wellcome Centre for Human Neuroimaging, University College London, London, UK; 55. Shanghai Mental Health Center Shanghai Jiao Tong University School of Medicine, PR China; 56. De Bascule, Academic Center for Child and Adolescent Psychiatry, Amsterdam, the Netherlands; 57. Department of child and adolescent psychiatry Amsterdam UMC, Amsterdam, The Netherlands; 58. Department of Neuropsychiatry, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan; 59. Imaging Genetics Center, Mark and Mary Stevens Neuroimaging and Informatics Institute, Keck School of Medicine of USC, Marina del Rey, CA 90292 USA; 60. Department of Psychiatry, Oxford University, Oxford, U.K.; 61. University of British Columbia, Vancouver, BC, Canada; 62. Department of Psychiatry, Seoul National University College of Medicine, Seoul, Republic of Korea; 63. Department of Brain & Cognitive Sciences, Seoul National University College of Natural Sciences, Seoul, Korea; 64. Institut d’Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain; 65. SU/UCT MRC Unit on Anxiety & Stress Disorders, Department of Psychiatry, University of Stellenbosch, South Africa; 66. The Division of Child and Adolescent Psychiatry, New York State Psychiatric Institute, Columbia University, New York, NY, U.S.A.; 67. Department of Radiology, Chiba University Hospital, Chiba, Japan; 68. Department of Clinical Neuroscience, Centre for Psychiatry Research, Karolinska Institutet, Stockholm, Sweden; 69. Mood Disorders Clinic, St. Joseph’s HealthCare, Hamilton, Ontario, Canada; 70. University of Illinois at Urbana-Champaign, Champaign, IL, U.S.A.; 71. Department of Psychiatry, Yale University School of Medicine, New Haven, Connecticut, U.S.A; 72. University of Zürich, University Hospital Zürich, Dept. Neuroradiology, Zürich, Germany; 73. ATR Brain Information Communication Research Laboratory Group, Kyoto, Japan; 74. Center of Mathematics, Computing and Cognition, Universidade Federal do ABC, Santo Andre, Brazil; 75. Orygen, The National Centre of Excellence in Youth Mental Health, Parkville, VIC, Australia; 76. Centre for Youth Mental Health, The University of Melbourne, Melbourne, VIC, Australia; 77. Department of Cognitive Behavioral Physiology, Graduate School of Medicine, Chiba University, Chiba, Japan; 78. Center for OCD and Related Disorders, New York State Psychiatric Institute, New York, NY, U.S.A.; 79. Pediatric OCD Consultation service, Anxiety Treatment and Research Center, St. Joseph’s HealthCare, Hamilton, Ontario, Canada; 80. Department of Psychobiology and Methodology of Health Sciences, Universitat Autònoma de Barcelona, Spain; 81. Beth K. and Stuart C. Yudofsky Division of Neuropsychiatry, Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, Texas, USA; 82. Department of Psychiatry, New York University School of Medicine, New York, NY, U.S.A.; 83. Nathan Kline Institute for Psychiatric Research, Orangeburg, NY, U.S.A.; 84. Yale University School of Medicine, New Haven, Conneticut, U.S.A; 85. Clinical Neuroscience and Development Laboratory, Olin Neuropsychiatry Research Center, Hartford, Connecticut, U.S.A.; 86. Department of Psychiatry, University of British Columbia, Vancouver, BC, Canada; 87. Provincial Obsessive-Compulsive Disorder Program, British Columbia Children’s Hospital, Vancouver, BC, Canada; 88. Icahn School of Medicine at Mount Sinai, New York, U.S.A.; 89. James J. Peters VA Medical Center, Bronx, New York, U.S.A; 90. Department of Anatomy and Neurosciences, Amsterdam UMC, Amsterdam, The Netherlands; 91. Institute of Living/Hartford Hospital, Hartford, Connecticut, USA; 92. Yale University School of Medicine, New Haven, Connecticut, U.S.A; 93. Laureate Institute for Brain Research, Tulsa, Oklahoma, U.S.A; 94. Shanghai Key Laboratory of Psychotic Disorders, PR China; 95. Department of Internal Medicine, First Affiliated Hospital of Kunming Medical University, Kunming, China; 96. Department of Radiology, Graduate School of Medical Science Kyoto Prefectural University of Medicine, Kyoto, Japan; 97. Seoul National University Hospital, Seoul, Republic of Korea; 98. Yeongeon Student Support Center, Seoul national University College of Medicine, Seoul, Republic of Korea; 99. Institute of Medical Science and Technology, Shahid Beheshti University, Tehran, Iran; 100. Imaging Genetics Center, Mark and Mary Stevens Neuroimaging & Informatics Institute, Keck School of Medicine of the University of Southern California, Marina del Rey, U.S.A.; 101. SU/UCT MRC Unit on Risk & Resilience in Mental Disorders, Department of Psychiatry and Mental Health, University of Cape Town, South Africa; 102. Donders Institute for Brain, Cognition and Behavior, Radboud University, Nijmegen, The Netherlands
Yoshinari Abe was supported by JSPS KAKENHI Grant Numbeer 18K15523; Pino Alonso was supported by Project grant PI14/00419 from the Carlos III Health Institute.; Stephanie H. Ameis was supported by funding for this project from an Ontario Mental Health Foundation Research Training Fellowship; Paul D. Arnold was supported by Funding for this project from Alberta Innovates Translational Health Chair in Child and Youth Mental Health and the Ontario Brain Institute; Justin T. Baker was supported by NIMH K23MH104515; Marcelo C. Batistuzzo was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo Research Foundation; Grant no. 201½1357–9); Silvia Brem was supported by the Swiss National Science Foundation Grant (no. 320030_130237, principal investigator: Susanne Walitza) and the Hartmann Müller Foundation (no. 1460); Brian P. Brennan was supported by Grant K23-MH092397 from the National Institute of Mental Health (BPB) and the David Judah Fund at the Massachusetts General Hospital (BPB). Grant K23-MH104515 from the National Institute of Mental Health (JTB); Jan Buitelaar was supported by EU FP7 project TACTICS (grant nr 278948); Geraldo F. Busatto was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo Research Foundation; Grant no. 201½1357–9); Yuqi Cheng was supported by the National Natural Science Foundation of China (81560233); Kate D. Fitzgerald was supported by NIMH K23MH082176; Patricia Gruner was supported by IOCDF Award and K23 MH115206; Tobias U. Hauser was supported by a Wellcome Sir Henry Dale Fellowship (211155/Z/18/Z), a grant from the Jacobs Foundation, and a 2018 NARSAD Young Investigator grant (27023) from the Brain & Behavior Research Foundation.; Yoshiyuki Hirano was supported by AMED under Grant Number JP18dm0307002, JSPS KAKENHI Grant Number 16K04344; Marcelo Q. Hoexter was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo Research Foundation; Grant no. 201½1357–9); Fern Jaspers-Fayer was supported by Michael Smith Foundation for Health Research; Norbert Kathmann was supported by BMBF-01GW0724 from the Federal Ministry of Education and Research of Germany; Kathrin Koch was supported by Deutsche Forschungsgemeinschaft (DFG) grant (KO 3744/7–1); Gerd Kvale was supported by Funding by Helse Vest Health Authority (911754, 911880); Norwegian Research Council, HELSEFORSK 243675; Luisa Lazaro was supported by The Marató TV3 Foundation grants 0½010 and 091710; The Carlos III Health Institute (PI040829) co-funded by FEDER funds/European Regional Development Fund (ERDF), a way to build Europe; AGAUR (2017 SGR 881); Ricardo Magalhães was supported by the FCT fellowship grant with the number PDE/BDE/113604/2015 from the PhD-iHES program; Rachel Marsh was supported by NIMH R21MH101441; Ignacio Martínez-Zalacaín was supported by Grant FI17/00294 from the Carlos III Health Institute.; José M. Menchón was supported by Project grant PI16/00950 from the Carlos III Health Institute and AGAUR 2017 SGR 1247 from the Generalitat de Catalunya.; Euripedes C. Miguel was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, São Paulo Research Foundation; Grant no. 201½1357–9); Pedro S Moreira was supported by the FCT fellowship grant with the number PDE/BDE/11360½015 from the PhD-iHES program; Takashi Nakamae was supported by JSPS KAKENHI Grant Numbeer 16K19778 and 18K07608; Tomohiro Nakao was supported by Grant-in-Aid for Scientific Research (C) (22591262) (25461732) (16K10253) from the Japanese Ministry of Education, Culture, Sports, Science and Technology; Janardhanan. C. Narayanaswamy was supported by Government of India grants to Dr. Narayanaswamy from Department of Science and Technology (DST INSPIRE faculty grant -IFA12-LSBM-26) & Department of Biotechnology (BT/06/IYBA/2012); Joseph O’Neill was supported by NIMH grants R01MH081864 (to Drs. O’Neill and Piacentini) and R01MH085900 (to Drs. O’Neill and Feusner); John Piacentini was supported by NIMH grants R01MH081864 (to Drs. O’Neill and Piacentini) and R01MH085900 (to Drs. O’Neill and Feusner); Y.C. Janardhan Reddy was supported by Government of India grants to Prof. Reddy from Department of Science and Technology (SR/S0/HS/0016/2011) & Department of Biotechnology(No.BT/PR13334/Med/30/259/2009); H. Blair Simpson was supported by NIMH R21MH093889 and by the New York State Office of Mental Health; Carles Soriano-Mas was supported by Grant CPII16/00048 and Project grants PI13/01958 and PI16/00889 from the Carlos III Health Institute, co-funded by FEDER funds/European Regional Development Fund (ERDF), a way to build Europe.; Gianfranco Spalletta was supported by Italian Ministry of Health RC13–14-15–16A; Emily R. Stern was supported by UL1TR000067/KL2TR00069 from the National Center for Advancing Translational Sciences.; S. Evelyn Stewart was supported by Canadian Institutes of Health Research, Michael Smith Foundation for Health Research, British Columbia Provincial Health Services Authority; Guido A. van Wingen was supported by Netherlands Organization for Scientific Research (NWO/ZonMW Vidi 917.15.318); Ganesan Venkatasubramanian was supported by Wellcome-DBT India Alliance grant to Dr. Venkatasubramanian (500236/Z/11/Z); Zhen Wang was supported by National Natural Science Foundation of China (81371340) and the Shanghai Key Laboratory of Psychotic Disorders (No.13dz2260500). Akiko Nakagawa was JSPS KAKENHI Grant Number 26461762.
Justin T. Baker: Dr. Baker has received consulting income from Pear Therapeutics and Niraax Therapeutics; Brian P. Brennan: Dr. Brennan has received consulting fees from Rugen Therapeutics and Nobilis Therapeutics and research grant support from Eli Lilly, Transcept Pharmaceuticals, and Biohaven Pharmaceuticals.; David Mataix-Cols: Prof. Mataix-Cols receives royalties for contributing articles to UpToDate, Wolters Kluwer Health, and fees from Elsevier in his role as associate editor; H. Blair Simpson: Dr. Simpson has received royalties from UpToDate, Inc and Cambridge University Press and is currently receiving research support from Biohaven for a multi-site industry-sponsored clinical trial.
Footnotes
Disclosures
The authors declare no conflicts of interest except for the authors below:
References
- 1.Ruscio AM, Stein DJ, Chiu WT, Kessler RC (2010): The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry. 15:53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wittchen HU, Jacobi F (2005): Size and burden of mental disorders in Europe--a critical review and appraisal of 27 studies. Eur Neuropsychopharmacol. 15:357–376. [DOI] [PubMed] [Google Scholar]
- 3.Nestadt G, Samuels J, Riddle M, Bienvenu OJ 3rd, Liang KY, LaBuda M, et al. (2000): A family study of obsessive-compulsive disorder. Arch Gen Psychiatry. 57:358–363. [DOI] [PubMed] [Google Scholar]
- 4.Pauls DL, Abramovitch A, Rauch SL, Geller DA (2014): Obsessive-compulsive disorder: an integrative genetic and neurobiological perspective. Nat Rev Neurosci. 15:410–424. [DOI] [PubMed] [Google Scholar]
- 5.Geschwind DH, Flint J (2015): Genetics and genomics of psychiatric disease. Science. 349:1489–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hugdahl K, Davidson RJ (2004): The asymmetrical brain. MIT press. [Google Scholar]
- 7.Zago L, Petit L, Jobard G, Hay J, Mazoyer B, Tzourio-Mazoyer N, et al. (2017): Pseudoneglect in line bisection judgement is associated with a modulation of right hemispheric spatial attention dominance in right-handers. Neuropsychologia. 94:75–83. [DOI] [PubMed] [Google Scholar]
- 8.Zhen Z, Kong XZ, Huang L, Yang Z, Wang X, Hao X, et al. (2017): Quantifying the variability of scene-selective regions: Interindividual, interhemispheric, and sex differences. Hum Brain Mapp. 38:2260–2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coan JA, Allen JJB (2004): Frontal EEG asymmetry as a moderator and mediator of emotion. Biological Psychology. 67:7–49. [DOI] [PubMed] [Google Scholar]
- 10.Wheeler RE, Davidson RJ, Tomarken AJ (1993): Frontal Brain Asymmetry and Emotional Reactivity - a Biological Substrate of Affective Style. Psychophysiology. 30:82–89. [DOI] [PubMed] [Google Scholar]
- 11.Vigneau M, Beaucousin V, Herve PY, Duffau H, Crivello F, Houde O, et al. (2006): Meta-analyzing left hemisphere language areas: Phonology, semantics, and sentence processing. Neuroimage. 30:1414–1432. [DOI] [PubMed] [Google Scholar]
- 12.Corballis MC (2003): From mouth to hand: gesture, speech, and the evolution of right-handedness. Behav Brain Sci. 26:199–208; discussion 208–160. [DOI] [PubMed] [Google Scholar]
- 13.Crow TJ (1990): Temporal-Lobe Asymmetries as the Key to the Etiology of Schizophrenia. Schizophrenia Bulletin. 16:433-&. [DOI] [PubMed] [Google Scholar]
- 14.Yucel M, Stuart GW, Maruff P, Wood SJ, Savage GR, Smith DJ, et al. (2002): Paracingulate morphologic differences in males with established schizophrenia: a magnetic resonance imaging morphometric study. Biol Psychiatry. 52:15–23. [DOI] [PubMed] [Google Scholar]
- 15.Eyler LT, Pierce K, Courchesne E (2012): A failure of left temporal cortex to specialize for language is an early emerging and fundamental property of autism. Brain. 135:949–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Altarelli I, Leroy F, Monzalvo K, Fluss J, Billard C, Dehaene-Lambertz G, et al. (2014): Planum temporale asymmetry in developmental dyslexia: Revisiting an old question. Hum Brain Mapp. 35:5717–5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuelz AK, Hohagen F, Voderholzer U (2004): Neuropsychological performance in obsessive-compulsive disorder: a critical review. Biol Psychol. 65:185–236. [DOI] [PubMed] [Google Scholar]
- 18.Abramovitch A, Abramowitz JS, Mittelman A (2013): The neuropsychology of adult obsessive-compulsive disorder: a meta-analysis. Clinical psychology review. 33:1163–1171. [DOI] [PubMed] [Google Scholar]
- 19.Kuskowski MA, Malone SM, Kim SW, Dysken MW, Okaya AJ, Christensen KJ (1993): Quantitative EEG in obsessive-compulsive disorder. Biol Psychiatry. 33:423–430. [DOI] [PubMed] [Google Scholar]
- 20.Maril S, Hermesh H, Gross-Isseroff R, Tomer R (2007): Spatial attention and neural asymmetry in obsessive-compulsive disorder. Psychiatry Res. 153:189–193. [DOI] [PubMed] [Google Scholar]
- 21.Rao NP, Arasappa R, Reddy NN, Venkatasubramanian G, Reddy Y.C. J (2015): Lateralisation abnormalities in obsessive-compulsive disorder: a line bisection study. Acta Neuropsychiatrica. 27:242–247. [DOI] [PubMed] [Google Scholar]
- 22.Ischebeck M, Endrass T, Simon D, Kathmann N (2014): Altered frontal EEG asymmetry in obsessive-compulsive disorder. Psychophysiology. 51:596–601. [DOI] [PubMed] [Google Scholar]
- 23.Wexler BE, Goodman WK (1991): Cerebral laterality, perception of emotion, and treatment response in obsessive-compulsive disorder. Biol Psychiatry. 29:900–908. [DOI] [PubMed] [Google Scholar]
- 24.Schienle A, Schafer A, Stark R, Walter B, Vaitl D (2005): Neural responses of OCD patients towards disorder-relevant, generally disgust-inducing and fear-inducing pictures. Int J Psychophysiol. 57:69–77. [DOI] [PubMed] [Google Scholar]
- 25.Simon D, Kaufmann C, Musch K, Kischkel E, Kathmann N (2010): Fronto-striato-limbic hyperactivation in obsessive-compulsive disorder during individually tailored symptom provocation. Psychophysiology. 47:728–738. [DOI] [PubMed] [Google Scholar]
- 26.Rao NP, Reddy YC, Kumar KJ, Kandavel T, Chandrashekar CR (2008): Are neuropsychological deficits trait markers in OCD? Prog Neuropsychopharmacol Biol Psychiatry. 32:1574–1579. [DOI] [PubMed] [Google Scholar]
- 27.Shagass C, Roemer RA, Straumanis JJ, Josiassen RC (1984): Distinctive Somatosensory Evoked-Potential Features in Obsessive-Compulsive Disorder. Biological Psychiatry. 19:1507–1524. [PubMed] [Google Scholar]
- 28.Tot S, Ozge A, Comelekoglu U, Yazici K, Bal N (2002): Association of QEEG findings with clinical characteristics of OCD: evidence of left frontotemporal dysfunction. Can J Psychiatry. 47:538–545. [DOI] [PubMed] [Google Scholar]
- 29.Shin YW, Ha TH, Kim SY, Kwon JS (2004): Association between EEG alpha power and visuospatial function in obsessive-compulsive disorder. Psychiatry Clin Neurosci. 58:16–20. [DOI] [PubMed] [Google Scholar]
- 30.Towey J, Bruder G, Tenke C, Leite P, Decaria C, Friedman D, et al. (1993): Event-Related Potential and Clinical Correlates of Neurodysfunction in Obsessive-Compulsive Disorder. Psychiatry Research. 49:167–181. [DOI] [PubMed] [Google Scholar]
- 31.Rus OG, Reess TJ, Wagner G, Zimmer C, Zaudig M, Koch K (2017): Functional and structural connectivity of the amygdala in obsessive-compulsive disorder. Neuroimage Clin. 13:246–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ahmari SE, Spellman T, Douglass NL, Kheirbek MA, Simpson HB, Deisseroth K, et al. (2013): Repeated cortico-striatal stimulation generates persistent OCD-like behavior. Science. 340:1234–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mondino M, Haesebaert F, Poulet E, Saoud M, Brunelin J (2015): Efficacy of Cathodal Transcranial Direct Current Stimulation Over the Left Orbitofrontal Cortex in a Patient With Treatment-Resistant Obsessive-Compulsive Disorder. J ECT. 31:271–272. [DOI] [PubMed] [Google Scholar]
- 34.Peng Z, Li G, Shi F, Shi C, Yang Q, Chan RC, et al. (2015): Cortical asymmetries in unaffected siblings of patients with obsessive-compulsive disorder. Psychiatry Res. 234:346–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garber HJ, Ananth JV, Chiu LC, Griswold VJ, Oldendorf WH (1989): Nuclear magnetic resonance study of obsessive-compulsive disorder. Am J Psychiatry. 146:1001–1005. [DOI] [PubMed] [Google Scholar]
- 36.Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, et al. (2013): Power failure: why small sample size undermines the reliability of neuroscience. Nature Reviews Neuroscience. 14:365–376. [DOI] [PubMed] [Google Scholar]
- 37.Thompson PM, Stein JL, Medland SE, Hibar DP, Vasquez AA, Renteria ME, et al. (2014): The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain imaging and behavior. 8:153–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boedhoe P, Schmaal L, Abe Y, Alonso P, Ameis SH, Anticevic A, et al. (2018): Cortical Abnormalities Associated With Pediatric and Adult Obsessive-Compulsive Disorder: Findings From the ENIGMA Obsessive-Compulsive Disorder Working Group. Am J Psychiatry. 175:453–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boedhoe P, Schmaal L, Abe Y, Ameis SH, Arnold PD, Batistuzzo MC, et al. (2017): Distinct Subcortical Volume Alterations in Pediatric and Adult OCD: A Worldwide Meta- and Mega-Analysis. Am J Psychiatry. 174:60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong X-Z, Mathias SR, Guadalupe T, Group ELW, Glahn DC, Franke B, et al. (2018): Mapping cortical brain asymmetry in 17,141 healthy individuals worldwide via the ENIGMA Consortium. PNAS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kurth F, Gaser C, Luders E (2015): A 12-step user guide for analyzing voxel-wise gray matter asymmetries in statistical parametric mapping (SPM). Nature Protocols. 10:293–304. [DOI] [PubMed] [Google Scholar]
- 42.Leroy F, Cai Q, Bogart SL, Dubois J, Coulon O, Monzalvo K, et al. (2015): New human-specific brain landmark: the depth asymmetry of superior temporal sulcus. Proc Natl Acad Sci U S A. 112:1208–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guadalupe T, Mathias SR, vanErp TG, Whelan CD, Zwiers MP, Abe Y, et al. (2016): Human subcortical brain asymmetries in 15,847 people worldwide reveal effects of age and sex. Brain Imaging Behav. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van den Heuvel OA, van Wingen G, Soriano-Mas C, Alonso P, Chamberlain SR, Nakamae T, et al. (2016): Brain circuitry of compulsivity. Eur Neuropsychopharmacol. 26:810–827. [DOI] [PubMed] [Google Scholar]
- 45.Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 31:968–980. [DOI] [PubMed] [Google Scholar]
- 46.Zhong S, He Y, Shu H, Gong G (2017): Developmental Changes in Topological Asymmetry Between Hemispheric Brain White Matter Networks from Adolescence to Young Adulthood. Cereb Cortex. 27:2560–2570. [DOI] [PubMed] [Google Scholar]
- 47.Schmaal L, Hibar DP, Samann PG, Hall GB, Baune BT, Jahanshad N, et al. (2017): Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 22:900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmaal L, Veltman DJ, van Erp TG, Samann PG, Frodl T, Jahanshad N, et al. (2016): Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. 21:806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Erp TGM, Walton E, Hibar DP, Schmaal L, Jiang W, Glahn DC, et al. (2018): Cortical Brain Abnormalities in 4474 Individuals With Schizophrenia and 5098 Control Subjects via the Enhancing Neuro Imaging Genetics Through Meta Analysis (ENIGMA) Consortium. Biol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Logue MW, van Rooij SJH, Dennis EL, Davis SL, Hayes JP, Stevens JS, et al. (2018): Smaller Hippocampal Volume in Posttraumatic Stress Disorder: A Multisite ENIGMA-PGC Study: Subcortical Volumetry Results From Posttraumatic Stress Disorder Consortia. Biol Psychiatry. 83:244–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Rooij D, Anagnostou E, Arango C, Auzias G, Behrmann M, Busatto GF, et al. (2018): Cortical and Subcortical Brain Morphometry Differences Between Patients With Autism Spectrum Disorder and Healthy Individuals Across the Lifespan: Results From the ENIGMA ASD Working Group. Am J Psychiatry. 175:359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ojemann GA (1977): Asymmetric function of the thalamus in man. Ann N Y Acad Sci. 299:380–396. [DOI] [PubMed] [Google Scholar]
- 53.Behrens TEJ, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CAM, Boulby PA, et al. (2003): Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nature Neuroscience. 6:750–757. [DOI] [PubMed] [Google Scholar]
- 54.Mataix-Cols D, Frost RO, Pertusa A, Clark LA, Saxena S, Leckman JF, et al. (2010): Hoarding disorder: a new diagnosis for DSM-V? Depress Anxiety. 27:556–572. [DOI] [PubMed] [Google Scholar]
- 55.Smith KS, Tindell AJ, Aldridge JW, Berridge KC (2009): Ventral pallidum roles in reward and motivation. Behav Brain Res. 196:155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mataix-Cols D, Boman M, Monzani B, Ruck C, Serlachius E, Langstrom N, et al. (2013): Population-based, multigenerational family clustering study of obsessive-compulsive disorder. JAMA psychiatry. 70:709–717. [DOI] [PubMed] [Google Scholar]
- 57.Hibar DP, Cheung JW, Medland SE, Mufford MS, Jahanshad N, Dalvie S, et al. (2018): Significant concordance of genetic variation that increases both the risk for obsessive-compulsive disorder and the volumes of the nucleus accumbens and putamen. Br J Psychiatry. 213:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.International Obsessive Compulsive Disorder Foundation Genetics C, Studies OCDCGA (2018): Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatry. 23:1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Johansen-Berg H, Behrens TEJ, Sillery E, Ciccarelli O, Thompson AJ, Smith SM, et al. (2005): Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cerebral Cortex. 15:31–39. [DOI] [PubMed] [Google Scholar]
- 60.Maingault S, Tzourio-Mazoyer N, Mazoyer B, Crivello F (2016): Regional correlations between cortical thickness and surface area asymmetries: A surface-based morphometry study of 250 adults. Neuropsychologia. 93:350–364. [DOI] [PubMed] [Google Scholar]
- 61.Van Essen DC, Glasser MF, Dierker DL, Harwell J, Coalson T (2012): Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cereb Cortex. 22:2241–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.