INTRODUCTION
Early screening of first-degree relatives (FDRs) of patients with colorectal cancer (CRC) has always been a clinical focus. However, the significant risk to FDRs of those with advanced colorectal polyps (ACPs) and the need for earlier initiation of screening may be overlooked. For the purposes of this manuscript, we use the term ACP to describe advanced adenomas (AAs) (the term traditionally used in the literature) and advanced serrated polyps.
Both CRC and ACPs diagnosed in a proband require FDRs (parents, siblings, and children) to be screened at 40 years of age or 10 years before the proband's diagnosis, whichever is earlier (1). In addition, ACPs are high-risk lesions that warrant shorter surveillance intervals in the proband. Given the increasing incidence of early-onset CRC, it is imperative to increase awareness of ACPs among gastroenterologists, primary care physicians, and other providers to assure adherence to earlier screening among FDRs.
Herein, we provide a guide to (i) appreciate recommended surveillance intervals for patients with ACP and early screening for FDRs and (ii) communicate risk to patients with ACP and their FDRs. The impetus for this guide was the development of the Advanced Colorectal Polyp GI brief (2) (Figure 1) developed by the American Cancer Society and the National Colorectal Cancer Roundtable (NCCRT) Advanced Adenoma Working Group (https://nccrt.org/resource/advanced-colorectal-polyp-brief/).
Figure 1.
National Colorectal Cancer Roundtable Advanced Colorectal Polyp GI brief. Reprinted with permission from the National Colorectal Cancer Roundtable, American Cancer Society. GI, gastrointestinal.
DEFINITION AND EPIDEMIOLOGY OF ACPS
ACPs are defined as any one of the following: (i) tubular adenoma ≥1 cm or any adenoma with villous features or high-grade dysplasia regardless of the size, (ii) sessile serrated polyp (SSP) ≥1 cm or SSP with cytologic dysplasia, or (iii) traditional serrated adenoma of any size. ACPs are the immediate precursors of CRC (3) and critical target lesions for screening.
During screening colonoscopy, approximately 10% of average-risk individuals are diagnosed with an AA (4). AA prevalence is higher among men (5) but appears similar among blacks and whites (6). The prevalence of any SSP ranges from 2 to 9% among average-risk adults undergoing screening, with approximately half ≥1 cm (7) and <1% showing cytologic dysplasia (8). Traditional serrated adenomas are more rare (prevalence 0.1–2.3%) (9).
PRACTICE ADVICE FOR THE ENDOSCOPIST
Step 1. Define the patient at risk
Knowing a patient's risk is essential to providing recommendations that can be lifesaving. Individuals with AAs have a 15.9%–19.3% risk of metachronous AA and 0.8%–1.3% risk of metachronous CRC (10). The recommended surveillance interval for ACPs is 3 years, with earlier follow-up for piecemeal or incompletely resected lesions (11).
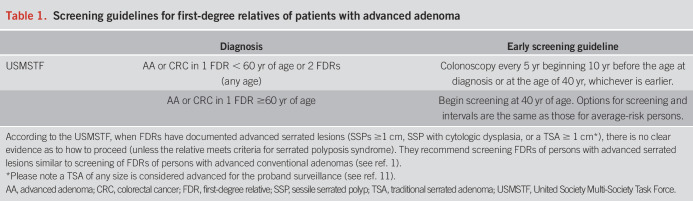
FDRs of patients with AA carry a 1.68–3.90-fold increased risk of developing CRC and 6.05-fold increased odds of developing AAs compared with those without a family history (12,13). FDRs of patients with advanced serrated lesions may be at a similarly increased risk, but additional data are needed (1). The United Society Multi-Society Task Force recommends that FDRs of patients with AAs or advanced serrated lesions initiate screening at age 40 years or 10 years before the patient's diagnosis, whichever is earlier (1) (Table 1). Early screening among FDRs of patients with ACP is underutilized and represents an area where gastroenterologists could have a larger impact on CRC prevention.
Table 1.
Screening guidelines for first-degree relatives of patients with advanced adenoma
Common clinical scenarios.
Scenario #1. An asymptomatic 39-year-old man is referred to gastroenterology because his father had a 1.2-cm tubular adenoma at the age of 67 years. Recommendation: Because of a FDR with an AA, screening should commence at 40 years of age.
Scenario #2. A 64-year-old woman has a 1.1-cm tubular adenoma on screening colonoscopy. Recommendation: Surveillance colonoscopy in 3 years, and counsel patient that FDRs are at increased risk and should undergo screening at 40 years of age. In this scenario, the endoscopist needs to not only think about surveillance colonoscopy intervals in the proband but also be mindful of the increased risk to FDRs. Because gastroenterologists routinely make decisions about surveillance intervals (because of high polyp prevalence), surveillance guidelines are at the forefront of the physician's approach, but communicating familial risk may potentially be overlooked. The patient should notify their children and siblings to talk to their physician about earlier screening.
Step 2. Take a thorough family history to exclude hereditary syndromes
Documenting the family history of CRC, colorectal polyps, and other malignancies in all patients is essential to identifying those with underlying hereditary cancer syndromes, including Lynch syndrome and others.
Furthermore, in the setting of multiple adenomas (lifetime cumulative adenomas and on a single colonoscopy), polyposis syndromes need to be considered. Patients with hereditary syndromes fall outside the average-risk screening guidelines. This also has implications for family members. The following strategies for collecting family history can be used, even in busy endoscopy units: (i) in advance of the visit, provide patients with family history worksheets, (ii) use a clinical prediction algorithm (i.e., PREMM 5 Model) to quantify the likelihood of a Lynch syndrome gene mutation (https://premm.dfci.harvard.edu), and (iii) refer to the NCCRT Risk Assessment and Screening Toolkit to Detect Familial, Hereditary, and Early Onset Colorectal Cancer (https://nccrt.org/resource/risk-assessment-and-screening-toolkit-to-detect-familial-hereditary-and-early-onset-colorectal-cancer/)
Step 3. Communicate risk to your patient and their FDRs
Postpolypectomy risk communication is critical for CRC prevention. Preliminary data show 80% of patients with adenoma are unaware that they may be at higher risk than the general population, 21% do not know follow-up is needed, and 68% have inaccurate knowledge of their results (Molmenti, unpublished data). Furthermore, multiple communication channels exist by which patients receive colonoscopy results and risk information with no standard of care established. A more streamlined approach to risk communication that begins with the patient and reaches FDRs may improve the quality of care we provide (Figure 2). It is recommended that all such communications be documented clearly in the medical record.
Figure 2.
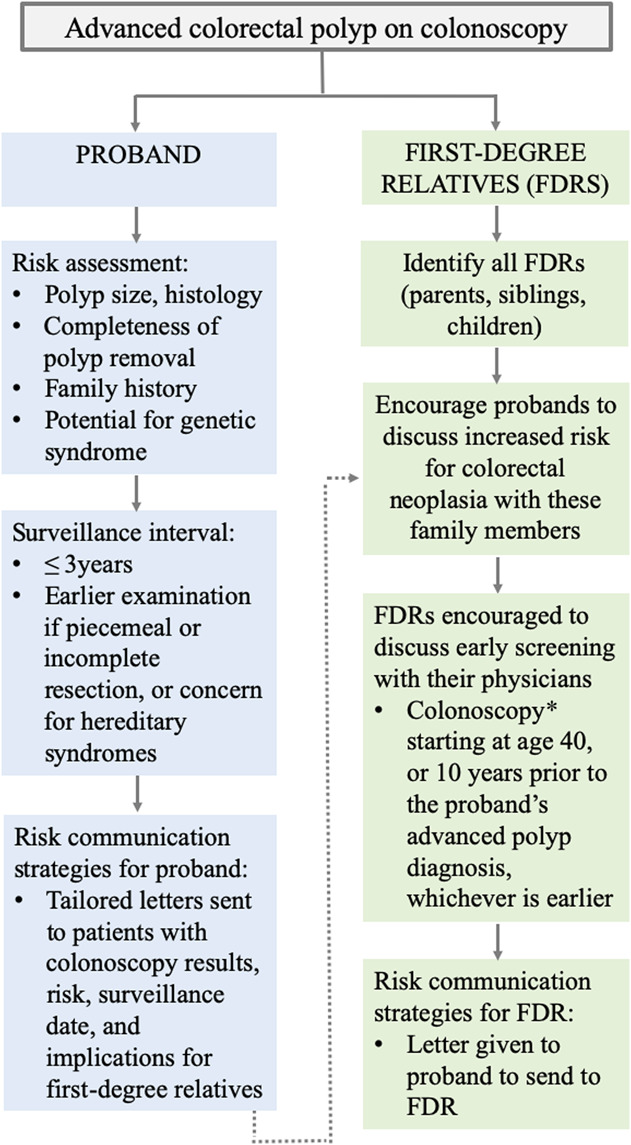
Risk communication flow. *Persons with a single first-degree relative diagnosed at ≥60 years with an advanced colorectal polyp can be offered average-risk screening options at age 40 years.
The use of a computer-based bedside educational tool, administered before discharge from endoscopy units in combination with personalized letters sent through mail, is effective at improving the patient's knowledge of results and risk perception (for themselves and their relatives) and increases the likelihood that patients contact their relatives, compared with standard of care (14). Template letters developed by the NCCRT (2) can be downloaded online, tailored to your patient, and embedded as macros into electronic health record systems (https://nccrt.org/wp-content/uploads/GI-Brief_ADVANCED-POLYPS-Colonoscopy-Report-Letter_final.pdf) (Figure 3).
Figure 3.
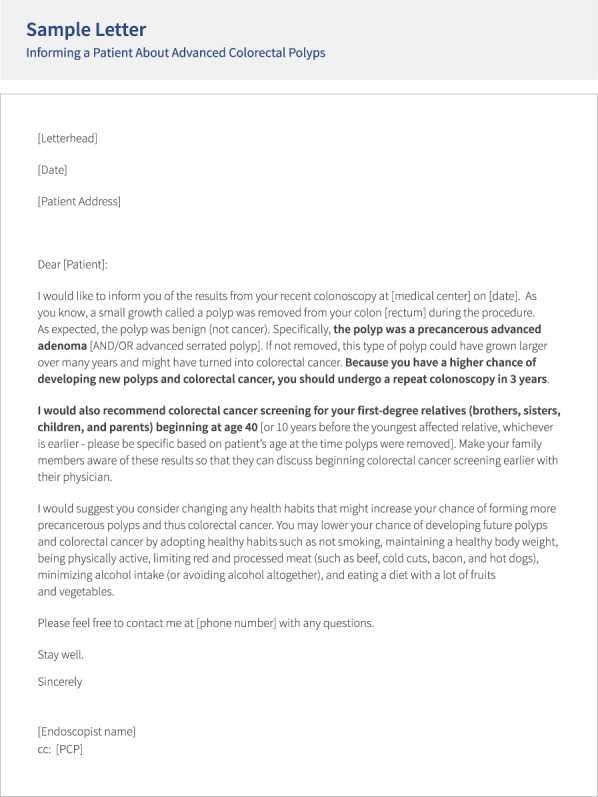
Template letter included in the National Colorectal Cancer Roundtable Advanced Colorectal Polyp GI brief. GI, gastrointestinal. Reprinted with permission from the National Colorectal Cancer Roundtable, American Cancer Society.
Another approach can be used at the time of endoscopy. If an adenomatous or sessile serrated appearing polyp ≥1 cm is found, a preliminary discussion regarding potential earlier screening of FDRs before discharge can be carried out (and documented in the colonoscopy report and discharge materials as there are often recall issues because of sedation or issues related to patient loss to follow up). A caveat is that occasionally, histology will reveal a nonprecancerous polyp (i.e., inflammatory polyp). Although this method can be useful as an adjunct, it should not serve as a replacement for direct confirmatory communication with patients once pathology results return.
FUTURE DIRECTIONS/SUMMARY
There has always been a focus on the risk to relatives after a proband's CRC diagnosis, yet the United Society Multi-Society Task Force early screening guidelines for FDRs of patients with ACPs may be underappreciated. Improved strategies to communicate risk for colorectal neoplasia among probands and FDRs are imperative. Furthermore, there is a need to create a culture of awareness of ACPs among gastroenterologists, primary care physicians, and others, whereby patients are routinely asked not only about their family history of CRC but also about their family history of ACPs. By increasing the dialogue regarding these advanced lesions, we can continue to make meaningful progress toward reducing the overall burden of CRC, including early-onset disease.
CONFLICTS OF INTEREST
Guarantor of the article: Christine L. Molmenti, PhD, MPH, and Jordan J. Karlitz, MD.
Specific author contributions: C.L.M. and J.J.K. were responsible for the idea. All authors were involved in drafting and critically revising the manuscript. All authors approved the final draft submitted.
Financial support: J.M.K. is supported in part by the NIH Gastrointestinal Diseases Training Grant (T32-DK007038).
Potential competing interests: J.J.K. at the time of publication: Advisor Exact Sciences, Consultant and Speakers Bureau Myriad Genetics, and equity position in Gastro Girl. C.M. at the time of publication: Consultant Pfizer. The contents in this manuscript do not necessarily reflect the views of the Department of Veterans Affairs or the U.S. Government.
ACKNOWLEDGMENTS
We would like to extend our sincere gratitude to Drs. Dennis Ahnen and Paul Schroy for their invaluable contributions to the development of this manuscript.
REFERENCES
- 1.Rex DK, Boland CR, Dominitz JA, et al. Colorectal cancer screening: Recommendations for physicians and patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2017;153(1):307–23. [DOI] [PubMed] [Google Scholar]
- 2.National Colorectal Cancer Roundtable. Resource Center. https://nccrt.org/resource/advanced-colorectal-polyp-brief/. Accessed December 4, 2019. [Google Scholar]
- 3.Burnett-Hartman AN, Newcomb PA, Phipps AI, et al. Colorectal endoscopy, advanced adenomas, and sessile serrated polyps: Implications for proximal colon cancer. Am J Gastroenterol 2012;107:1213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lieberman DA, Weiss DG, Bond JH, et al. Use of colonoscopy to screen asymptomatic adults for colorectal cancer. Veterans Affairs Cooperative Study Group 380. N Engl J Med 2000;343(3):162–8. [DOI] [PubMed] [Google Scholar]
- 5.Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal-cancer screening for detection of advanced neoplasia. N Engl J Med 2006;355(18):1863–72. [DOI] [PubMed] [Google Scholar]
- 6.Imperiale TF, Abhyankar PR, Stump TE, et al. Prevalence of advanced, precancerous colorectal neoplasms in black and white populations: A systematic review and meta-analysis. Gastroenterology 2018;155(6):1776–86 e1771. [DOI] [PubMed] [Google Scholar]
- 7.Makkar R, Pai RK, Burke CA. Sessile serrated polyps: Cancer risk and appropriate surveillance. Cleve Clin J Med 2012;79(12):865–71. [DOI] [PubMed] [Google Scholar]
- 8.Abdeljawad K, Vemulapalli KC, Kahi CJ, et al. Sessile serrated polyp prevalence determined by a colonoscopist with a high lesion detection rate and an experienced pathologist. Gastrointest Endosc 2015;81(3):517–24. [DOI] [PubMed] [Google Scholar]
- 9.East JE, Atkin WS, Bateman AC, et al. British Society of Gastroenterology position statement on serrated polyps in the colon and rectum. Gut 2017;66(7):1181–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Martinez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology 2009;136(3):832–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: A consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143(3):844–57. [DOI] [PubMed] [Google Scholar]
- 12.Lowery JT, Ahnen DJ, Schroy PC, III, et al. Understanding the contribution of family history to colorectal cancer risk and its clinical implications: A state-of-the-science review. Cancer 2016;122(17):2633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng SC, Lau JY, Chan FK, et al. Risk of advanced adenomas in siblings of individuals with advanced adenomas: A cross-sectional study. Gastroenterology 2016;150(3):608–16, quiz e616-607. [DOI] [PubMed] [Google Scholar]
- 14.Schroy PC, III, Glick JT, Wilson S, et al. An effective educational strategy for improving knowledge, risk perception, and risk communication among colorectal adenoma patients. J Clin Gastroenterol 2008;42(6):708–14. [DOI] [PubMed] [Google Scholar]