Abstract
Background
Non-muscle invasive bladder cancer patients are at high risk for tumour recurrence and progression, hence an intensive follow-up procedure is recommended which is costly. Identification of factors that are associated with the risk of recurrence and progression may enable personalized follow-up schedules. Obesity and diabetes mellitus may be associated with a worse prognosis, but the evidence is limited and inconsistent. Our objective was to determine the associations of BMI and diabetes mellitus with risks of recurrence and progression among non-muscle invasive bladder cancer patients.
Methods
A population-based cohort of patients diagnosed with non-muscle invasive bladder cancer between 1995 and 2010 was retrospectively identified from the Netherlands Cancer Registry and invited to participate in the Nijmegen Bladder Cancer Study (n = 1,433). Average weight during adult life, height, and diabetes mellitus diagnosis were self-reported by use of a questionnaire. Clinical follow-up data were retrieved from medical files. Associations were quantified using proportional hazard analyses. For all analyses, minimal adjustment sets were selected using a Directed Acyclic Graph.
Results
Fourteen percent of the patients indicated to be diagnosed with diabetes mellitus, and more than half was overweight (45%) or obese (9%). Compared to healthy weight, overweight and obesity were not associated with risk of recurrence (adjusted hazard ratio (HR) = 1.02; 95% confidence interval (CI): 0.86–1.22, and HR = 1.02; 95% CI: 0.76–1.38, respectively) and overall progression (HR = 1.04; 95% CI: 0.74–1.44, and HR = 1.20; 95% CI: 0.69–2.09, respectively). Also, no clear associations of diabetes mellitus with risk of recurrence (HR = 1.22; 95% CI: 0.98–1.54) and overall progression (HR = 1.16; 95% CI: 0.76–1.76) were found.
Conclusion
Average BMI during adult life and diabetes mellitus were not clearly associated with risk of recurrence or progression in non-muscle invasive bladder cancer. Prospective cohort studies with detailed information on BMI and diabetes mellitus before and after diagnosis are needed to confirm these findings.
Introduction
With 550,000 new cases each year, urinary bladder cancer (UBC) is the tenth most frequently diagnosed cancer worldwide, particularly occurring in high income countries [1]. Patients with muscle invasive bladder cancer (MIBC) have a low five-year survival rate of up to approximately 35% [2, 3]. Patients with non-muscle invasive bladder cancer (NMIBC) have a high five-year disease-specific survival, ranging between 88.7% and 98.5%. However, the five-year risk of recurrence is 28.3% to 51.7% and the five-year risk of progression is 4.6% to 19.8% [4]. Therefore, regular check-ups after initial treatment are recommended and resection of recurrent tumours and subsequent treatment are frequently required [5]. As a consequence, UBC has the highest lifetime treatment costs per patient of all cancers, which significantly impacts the total healthcare costs of oncology [6]. To predict prognostic outcomes in NMIBC patients, the European Organization for Research and Treatment of Cancer (EORTC) and Spanish Urology Association for Oncological Treatment (CUETO) designed scoring tables [7, 8]. These tables focus mainly on primary tumour characteristics. However, lifestyle factors such as smoking and obesity, as well as diabetes mellitus (DM), may also be relevant in the prognosis of NMIBC [9], and may contribute to more personalized follow-up schedules.
Obesity and DM have an estimated global prevalence above 650 and 400 million cases, respectively [10, 11]. More than 90% of all DM diagnoses in high income countries concern type 2 diabetes mellitus (DM2) which is strongly related to overweight [11]. Both obesity and DM2 are associated with increased risks of a variety of cancers, including UBC [12–15]. A meta-analysis of Sun et al. (2015) reported a relative risk (RR) of 1.10 (95% confidence interval (CI): 1.06–1.14) for UBC risk in obese compared with healthy weight people [16]. For diabetics compared with non-diabetics, a meta-analysis of Zhu et al. (2013) showed a RR of 1.35 (95% CI: 1.17–1.56) for UBC risk [17].
Proposed mechanisms for the effect of obesity and DM2 on cancer include the occurrence of insulin resistance and consequently hyperinsulinemia [13, 18]. This has a direct effect by increased binding of insulin to the tumour cell’s insulin-receptor and an indirect effect by increasing the concentration of free insulin-like-growth-factor-1 (IGF-1), resulting in increased tumour growth. Moreover, adipose tissue is characterised by macrophage infiltration, which plays an important role in inflammation. Macrophages and adipocytes produce pro-inflammatory factors, resulting in elevated concentrations of circulating tumour necrosis factor-α (TNF-α), interleukin-6 (IL-6) and C-reactive protein (CRP), which are beneficial for tumour growth [13, 18]. Mechanisms may differ by cancer type, and the exact mechanisms for UBC risk, recurrence and progression remain to be elucidated.
Although obesity and DM seem to be associated with UBC risk, only few studies are published on their associations with risk of tumour recurrence or progression in NMIBC patients. In a recent meta-analysis based on three historical cohort studies, our group found a pooled hazard ratio (HR) of 1.82 (95% CI: 1.12–2.95) for recurrence and 1.90 (95% CI: 0.93–3.88) for progression in obese versus healthy weight NMIBC patients [9, 19–21]. Hwang et al. (2011) found increased risks of recurrence and progression for diabetic compared with non-diabetic NMIBC patients [22]. Two studies comparing diabetic NMIBC patients with or without metformin use, a first line glucose-lowering drug, with non-diabetic NMIBC patients showed contradictory results [23, 24].
Thus, evidence for body mass index (BMI) and DM in NMIBC prognosis is limited and inconsistent, based on relatively small cohort studies, and restricted to BMI at diagnosis which may have been affected by the disease. Since such evidence may contribute to personalized follow-up schedules, we aimed to determine the associations of average BMI during adult life and DM with risk of recurrence and progression in a large population-based cohort of NMIBC patients.
Methods
Study population and data collection
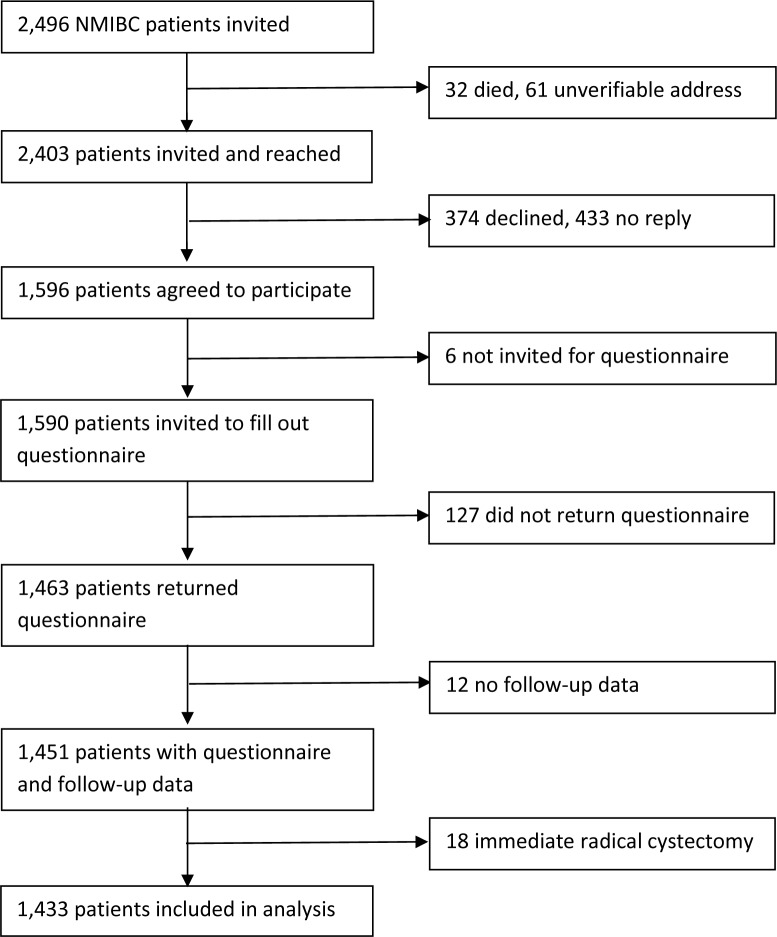
NMIBC patients were selected from the Nijmegen Bladder Cancer Study (NBCS) [25]. NMIBC patients who are registered in the Netherlands Cancer Registry (NCR), held by the Netherlands Comprehensive Cancer Organisation, were selected and invited to participate in the NBCS in four batches. Invitations for the first, second, third, and fourth batch were sent in May 2007, January 2009, November 2010, and February 2012, respectively. Only NMIBC patients under the age of 75 years and who were diagnosed between 1995 and 2010 in one of seven hospitals in the mid-eastern part of the Netherlands were invited. Also, a non-overlapping series of 280 NMIBC patients who were previously recruited for a separate study on gene-environment interactions was included [25]. Of all invited patients, 59% responded and filled out a questionnaire on general characteristics, medical history, lifestyle, and family history of cancer. The median time between diagnosis and filling out the questionnaire was 2.8 years (interquartile range: 1.8–6.7 years). Data on the primary tumour, treatment, and clinical follow-up were retrieved from medical files. Since clinical follow-up was assessed in the framework of a previous genome wide association study (GWAS), follow-up data were only available for NMIBC patients who donated a blood sample for DNA isolation [26]. Further, only patients who returned a questionnaire and did not undergo radical cystectomy directly after being diagnosed with the primary NMIBC were included (Fig 1).
Fig 1. Flow diagram.
The NBCS was approved by the Institutional Review Board of the Radboud university medical center. All participants gave written informed consent.
Exposure definitions
Patients were asked to report their average body weight during adult life and their height. Body weight was divided by the square of height to calculate average BMI during adult life, which will be further referred to as (adult) BMI. BMI was used as continuous and categorical variable. Obesity was defined as BMI ≥30.0, overweight as BMI ≥25.0 and <30.0, healthy weight as BMI ≥18.5 and <25.0, and underweight as BMI <18.5 kg/m2. No obesity was defined as a BMI <30.0 kg/m2 [27]. Only 2 patients had underweight, and were excluded from the analyses on BMI.
Patients were asked whether they were ever diagnosed with DM by a physician and at which age. Patients with missing values for DM diagnosis were assumed not having DM. Since DM type was not assessed, we classified type by using a proxy of age at DM diagnosis and BMI. Type 1 diabetes mellitus (DM1) was defined as a diagnosis at age ≤30 years, or at age 31–40 years in combination with having no obesity. Type 2 diabetes mellitus (DM2) was defined as a diagnosis at age >40 years, or at age 31–40 years in combination with being obese.
Lifestyle factors
Patients were asked for their smoking status at recruitment, age at smoking initiation and cessation, number of cigarettes smoked per day and duration of smoking in years. Smoking status at diagnosis and cumulative smoking exposure (in pack-years) was calculated using these variables, as previously described in more detail [28].
We also asked patients how many hours per week they spent walking, cycling, and sporting during adult life until 2 years before the NMIBC diagnosis, and with which frequency: <1 time/week, 1–2 times/week, 3–5 times/week or >5 times/week. For those with missing values for duration but known frequencies, missing values were imputed with calculated average durations per frequency stratum. The weekly duration of walking, cycling and sporting were summed to calculate the weekly duration of total physical activity.
UBC family history
Patients were asked to report whether a parent, sibling, or child ever had cancer, and if so, the type and year of diagnosis. Those who did not report a first-degree relative with UBC were included as having no family history.
Clinical data
Detailed clinical data concerning age at diagnosis, tumour stage, tumour grade, tumour number (single or multiple), tumour size (<3cm and ≥3cm), presence of concomitant carcinoma in situ (CIS), histological type, and initial treatment were collected through a medical file survey [28]. Tumour stage and grade were recorded according to the final conclusion in the pathology report. Tumours with World Health Organisation (WHO) 1973 differentiation grade 1 or 2, WHO/International Society of Urological Pathology (ISUP) 2004 low grade, or Malmström (Modified Bergkvist) grade 1 or 2a were considered low-grade tumours. We classified tumours with WHO 1973 differentiation grade 3, WHO/ISUP 2004 high grade, or Malmström (Modified Bergkvist) grade 2b or 3 as high-grade [29, 30]. Tumour aggressiveness was classified according to the risk of progression as follows: low-grade Ta tumours were classified as low-risk while all stage T1 tumours and CIS were classified as high-risk [31].
Outcome definitions
Outcomes were assessed by one of the authors (AJG) up to 5 years after diagnosis. Predefined instructions and a codebook were used to obtain consistency in retrieving the data. About 5% of medical records were seen twice to check consistency. A recurrence was defined as a new histologically confirmed tumour in the urinary bladder or prostatic urethra, after at least one tumour-negative follow-up cystoscopy or a radical re-transurethral resection of the primary tumour. For progression, the consensus definition of the International Bladder Cancer Group was used: the first occurrence of stage or grade progression, local or distant metastasis, and cystectomy for therapy-resistant disease, hereafter referred to as overall progression [32]. Additionally, a stricter definition was used where progression was defined as transition to MIBC (stage ≥T2).
Recurrence-free survival (RFS) and progression-free survival (PFS) were defined as the time between the date of diagnosis of the primary NMIBC and the date of first recurrence or recurrence with progression, respectively. In patients without recurrence or progression, follow-up was censored at the date of the last urological check-up, the date of death, or 5-year follow-up, whichever came first in time.
Statistical analyses
Standard descriptive statistics were calculated to describe patient and tumour characteristics for the total cohort, and by BMI and DM categories. Risks of recurrence and progression were calculated using the Kaplan-Meier method and presented in cumulative risk plots, stratified by exposure groups. Differences between the exposure groups were investigated using log rank tests. To calculate HRs and corresponding 95% CIs for the associations of BMI and DM with UBC outcomes, univariable and multivariable Cox proportional hazard analyses were performed for both RFS and PFS. The proportional hazards assumption was tested by modelling time-dependent covariables and using Schoenfeld residuals [33]. The assumption was fulfilled for all exposures of interest and potential covariables, except for BMI in relation to overall progression.
Sets of covariables for sufficient adjustment were initially selected using a Directed Acyclic Graph (DAG). This method has the advantage of keeping the included number of covariables to a minimum, resulting in more precise estimations of effects. The DAG was created using the software DAGitty [34]. First, the exposure, outcomes, covariables, and their causalities and associations known from literature were graphically depicted. The implications of independence between variables in the DAG were then tested in our data, using the software R, version 3.2.5 with the packages DAGitty [35], foreign 0.8–66 [36] and ggm 2.3 [37]. When this evaluation showed dependence between variables that were initially assumed independent, the DAG was improved by linking the variables. We refer to S1 Fig for the final DAG. Minimal sets of covariables for sufficient adjustment were subsequently selected from the final DAG, also using the software DAGitty. Covariables were selected for adjustment so that confounding pathways present in the DAG were blocked. It should be noted that adjusting for confounders deemed important (e.g. smoking or initial treatment) may not be required as a consequence of adjusting for other covariables in the same pathway. Details on the technical background of selecting covariables as applied by the software are described elsewhere [38].
In addition to the covariables that were selected using the DAG, most primary tumour characteristics of the EORTC and CUETO risk prediction tables were added to the adjustment sets [7, 8]. Prior recurrence rate was not included since all patients were newly diagnosed with NMIBC. Tumour size and number of tumours were not reported in the medical files of most patients, and therefore not included. The final adjustment set for the associations of BMI with recurrence and progression consisted of age at time of diagnosis, gender, highest completed level of education, weekly duration of physical activity, history of UBC among first degree relatives, tumour stage, tumour grade and presence of concomitant CIS. The final adjustment set for the associations of DM with recurrence and progression consisted of age at time of diagnosis, gender, BMI classes, tumour stage, tumour grade and presence of concomitant CIS. Patients with missing data on covariables (~3%) were excluded from the adjusted analyses.
Analyses were performed using the software IBM SPSS Statistics for Windows, release 22.0.0.1 (IBM Corp., Armonk, NY, USA), except for the analyses of testing the DAG. P-values ≤0.05 were considered statistically significant.
Results
Population characteristics
We included 1433 NMIBC patients. Patient characteristics on lifestyle and medical history, and characteristics regarding their primary tumour and the initially received treatment are presented in Table 1. The median age at time of NMIBC diagnosis was 64 years (interquartile range: 56–70 years). Most patients were male (83%) and current (37%) or former cigarette smokers (43%). The median adult BMI was 25.3 kg/m2 (interquartile range: 23.7–27.2 kg/m2), and 14% of the patients indicated to have been diagnosed with DM. Almost all patients had a urothelial cell carcinoma (99.4%) and received transurethral resection of the bladder tumour, with (50%) or without (47%) adjuvant intravesical instillations. The majority of patients had a Ta (70%) and low grade tumour (64%) and were at low risk of progression (59%). Most characteristics did not differ by BMI classes and DM status, except for educational level and physical activity which were lower among overweight and obese compared to healthy weight patients and patients with DM compared to no DM (S1 and S2 Tables).
Table 1. Patient characteristics on lifestyle and medical history, and characteristics regarding their primary bladder tumour and initial treatment.
| Patients (N = 1433) | |||
|---|---|---|---|
| n | (%) | ||
| Demographic characteristics | |||
| Age in years, median (P25, P75) a) | 64.0 | (56.0, 70.0) | |
| Male | 1187 | (82.8) | |
| Ethnicity b) | |||
| Dutch | 1294 | (90.3) | |
| Non-Dutch | 139 | (9.7) | |
| Highest completed level of education c) | |||
| Low | 794 | (55.4) | |
| Intermediate | 308 | (21.5) | |
| High | 329 | (23.0) | |
| Unknown | 2 | (0.1) | |
| Lifestyle factors | |||
| Adult BMI in kg/m2 | |||
| <18.5 | 2 | (0.1) | |
| ≥18.5 and <25.0 | 644 | (44.9) | |
| ≥25.0 and <30.0 | 642 | (44.8) | |
| ≥30.0 | 128 | (8.9) | |
| Unknown | 17 | (1.2) | |
| Median (P25, P75) | 25.3 | (23.7, 27.2) | |
| Cigarette smoking status a) | |||
| Current | 531 | (37.1) | |
| Former | 620 | (43.3) | |
| Never | 259 | (18.1) | |
| Unknown | 23 | (1.6) | |
| Cigarette pack-years, median (P25, P75) a) d) | 22.0 | (12.0, 36.0) | |
| Weekly duration of physical activity in hours, median (P25, P75) e) | 9.0 | (5.5, 15.0) | |
| Medical history | |||
| Diagnosed with diabetes mellitus f) | |||
| Yes | 198 | (13.8) | |
| No g) | 1235 | (86.2) | |
| Diabetes mellitus type f) h) | |||
| Type 1 | 13 | (6.6) | |
| Type 2 | 124 | (62.6) | |
| Unknown | 61 | (30.8) | |
| UBC history among first degree relatives f) | |||
| Yes | 98 | (6.8) | |
| No | 1335 | (93.2) | |
| Primary tumour characteristics | |||
| Stage, TNM 2002 classification | |||
| Ta | 1008 | (70.3) | |
| CIS | 54 | (3.8) | |
| T1 | 349 | (24.4) | |
| Unknown | 22 | (1.5) | |
| Grade i) | |||
| Low grade (G1 or G2) | 921 | (64.3) | |
| High grade (G3) | 496 | (34.6) | |
| Unknown | 16 | (1.1) | |
| Risk of progression j) | |||
| Low | 840 | (58.6) | |
| High | 573 | (40.0) | |
| Unknown | 20 | (1.4) | |
| Histology | |||
| Urothelial cell carcinoma | 1424 | (99.4) | |
| Other, including combinations of histology types | 2 | (0.1) | |
| Unknown | 7 | (0.5) | |
| Initial treatment | |||
| TURT with one intravesical chemotherapy instillation | 668 | (46.6) | |
| Adjuvant intravesical chemotherapy | 443 | (30.9) | |
| Adjuvant intravesical immunotherapy | 261 | (18.2) | |
| Adjuvant intravesical chemo- and immunotherapy | 18 | (1.3) | |
| Other | 1 | (0.1) | |
| Unknown | 42 | (2.9) | |
| Concomitant CIS | |||
| Yes | 105 | (7.3) | |
| No | 1310 | (91.4) | |
| Unknown | 18 | (1.3) | |
| Focality | |||
| Unifocal | 786 | (54.8) | |
| Multifocal | 564 | (39.4) | |
| Unknown | 83 | (5.8) | |
| Size in cm | |||
| <3 | 193 | (13.5) | |
| ≥3 | 111 | (7.8) | |
| Unknown | 1129 | (78.8) | |
P25: 25th percentile; P75: 75th percentile; BMI: body mass index; CIS: carcinoma in situ; TURT: transurethral resection of the bladder tumour.
[a] At the time of non-muscle invasive bladder cancer diagnosis.
[b] Based on the country of birth of the patients and their parents.
[c] Based on the International Standard Classification of Education: low level includes elementary, lower vocational and intermediate general education; intermediate level includes intermediate vocational and higher general education; high level includes higher vocational education and university.
[d] Presented for current and former cigarette smokers; unknown for 32 (former) smokers (2.8%).
[e] Based on the weekly duration of walking, cycling, and sporting during adult life until 2 years before diagnosis; unknown for 25 patients (1.7%).
[f] At the time of filling out the questionnaire.
[g] 66 patients (4.6%) had a missing value for diagnosis of diabetes mellitus and were included as not diagnosed.
[h] Presented for patients diagnosed with diabetes mellitus; type 1 was defined as diabetes mellitus diagnosis at age ≤30 years, or at age 31–40 years in combination with being not obese; type 2 was defined as diabetes mellitus diagnosis at age >40 years, or at age 31–40 years in combination with being obese.
[i] Tumours with WHO 1973 differentiation grade 1 or 2, WHO/ISUP 2004 low grade, or Malmström (Modified Bergkvist) grade 1 or 2a were considered low-grade tumours. Tumours with WHO 1973 differentiation grade 3, WHO/ISUP 2004 high grade, or Malmström (Modified Bergkvist) grade 2b or 3 as high-grade.
[j] Based on the European Association of Urology guidelines: low risk includes Ta-stage with low grade, high risk includes Ta-stage with high grade, T1-stage, T2-stage, T3-stage, T4-stage, and CIS.
Associations of adult BMI and DM with recurrence
In the first 5 years after diagnosis, 606 patients experienced at least one recurrence. The Kaplan-Meier method yielded a five-year risk of 48% (95% CI: 45%-50%) for recurrence.
BMI was not associated with risk of recurrence (S2 Fig and Table 2). The adjusted HRs for overweight and obesity, with healthy weight as reference, were 1.02 (95% CI: 0.86–1.22) and 1.02 (95% CI: 0.76–1.38), respectively. The analysis for continuous BMI yielded an adjusted HR of 1.00 (95% CI: 0.98–1.02) per 1 kg/m2 increase in BMI. Overall DM was associated with a non-statistically significant increased risk of recurrence (HR: 1.22, 95% CI: 0.98–1.54), compared with no DM. DM1 and DM2 were not statistically significantly associated with risk of recurrence compared with no DM (HR: 1.39, 95% CI: 0.66–2.95 and HR: 1.00, 95% CI: 0.75–1.33, respectively).
Table 2. Crude and adjusted hazard ratios (HR) with corresponding 95% confidence intervals (CI) for the associations of adult BMI and diabetes mellitus with risk of recurrence a) among non-muscle invasive bladder cancer patients.
| Crude analyses | Adjusted analyses b) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number at risk | Events c) | HR | (95% CI) | Number at risk | Events c) | HR | (95% CI) | ||
| BMI classes in kg/m2 d) | |||||||||
| ≥18.5 and <25.0 | 644 | 273 | Reference | 623 | 264 | Reference | |||
| ≥25.0 and <30.0 | 642 | 273 | 1.00 | (0.84–1.18) | 621 | 263 | 1.02 | (0.86–1.22) | |
| ≥30.0 | 128 | 53 | 0.96 | (0.72–1.29) | 125 | 53 | 1.02 | (0.76–1.38) | |
| Continuous BMI in kg/m2 d) | 1414 | 599 | 1.00 | (0.98–1.02) | 1369 | 580 | 1.00 | (0.98–1.02) | |
| Obesity d) | |||||||||
| No | 1286 | 546 | Reference | 1244 | 527 | Reference | |||
| Yes | 128 | 53 | 0.96 | (0.73–1.28) | 125 | 53 | 1.01 | (0.76–1.34) | |
| Diabetes mellitus status e) | |||||||||
| No | 1235 | 511 | Reference | 1201 | 496 | Reference | |||
| Yes | 198 | 95 | 1.18 | (0.95–1.46) | 191 | 94 | 1.22 | (0.98–1.54) | |
| Diabetes mellitus type e) | |||||||||
| No diabetes mellitus | 1235 | 511 | Reference | 1201 | 496 | Reference | |||
| Type 1 | 13 | 7 | 1.37 | (0.65–2.89) | 13 | 7 | 1.39 | (0.66–2.95) | |
| Type 2 | 124 | 55 | 1.02 | (0.77–1.34) | 123 | 54 | 1.00 | (0.75–1.33) | |
HR: hazard ratio; CI: confidence interval; BMI: body mass index.
[a] Defined as a new histologically confirmed tumour in the urinary bladder or prostatic urethra, after ≥1 tumour-negative follow-up cystoscopy result or a radical re-transurethral resection of the primary tumour.
[b] For the associations of BMI classes and obesity with recurrence, the adjustment set consists of age at time of UBC diagnosis, gender, highest completed level of education, weekly duration of physical activity, history of urinary bladder cancer among first degree relatives, tumour stage, tumour grade, and presence of concomitant CIS; for the associations of diabetes mellitus and type of diabetes mellitus with recurrence, the adjustment set consists of age at time of UBC diagnosis, gender, BMI classes, tumour stage, tumour grade, and presence of concomitant CIS.
[c] Number of incident events in the first 5 years after diagnosis of the primary non-muscle invasive urinary bladder cancer.
[d] Based on adult BMI; obesity was defined as a BMI ≥30.0 kg/m2, no obesity as a BMI <30.0 kg/m2.
[e] At the time of filling out the questionnaire; type 1 diabetes mellitus was defined as a diagnosis at age ≤30 years, or at age 31–40 years in combination without being obese, type 2 diabetes mellitus was defined as a diagnosis at age >40 years, or at age 31–40 years in combination with being obese.
Associations of adult BMI and DM with progression
Tumour progression was less frequent compared to tumour recurrence: 170 patients experienced progression in the first 5 years after diagnosis, 44 of whom experienced progression to MIBC. Five-year risks of 14% (95% CI: 12%-16%) and 4% (95% CI: 3%-4%) were calculated for overall progression and progression to MIBC, respectively.
BMI, DM, DM1, and DM2 had no statistically significant association with risk of overall progression or progression to MIBC (S3 and S4 Figs and Table 3). For BMI and risk of overall progression, the proportional hazards assumption was not fulfilled, i.e. the risk of overall progression for obesity compared to healthy weight seemed to be decreased up to about 3.5 years of follow-up and to be increased afterwards (S3 Fig). The average five-year adjusted HRs for overweight and obesity, with healthy weight as reference, were 1.04 (95% CI: 0.74–1.44) and 1.20 (95% CI: 0.69–2.09), respectively. The adjusted HR for DM compared with no DM was 1.16 (95% CI: 0.76–1.76). Regarding progression to MIBC, the analyses for overweight and obesity yielded adjusted HRs of 0.84 (95% CI: 0.43–1.63) and 0.93 (95% CI: 0.27–3.16), respectively. The adjusted HR for DM was 1.40 (95% CI: 0.64–3.07). BMI as continuous variable also showed no associations with overall progression or progression to MIBC, with adjusted HRs per 1 kg/m2 increase of 0.99 (95% CI: 0.95–1.04) and 0.94 (95% CI: 0.84–1.05), respectively.
Table 3. Crude and adjusted hazard ratios (HR) with corresponding 95% confidence intervals (CI) for the associations of adult BMI and diabetes mellitus with overall progression a) and progression to Muscle Invasive Bladder Cancer (MIBC) b) among non-muscle invasive bladder cancer patients.
| Crude analyses | Adjusted analyses c) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number at risk | Events d) | HR | (95% CI) | Number at risk | Events d) | HR | (95% CI) | ||
| Associations with overall progression | |||||||||
| BMI classes in kg/m2 e) | |||||||||
| ≥18.5 and <25.0 | 644 | 75 | Reference | 623 | 74 | Reference | |||
| ≥25.0 and <30.0 | 642 | 77 | 1.02 | (0.74–1.40) | 621 | 73 | 1.04 | (0.74–1.44) | |
| ≥30.0 | 128 | 16 | 1.08 | (0.63–1.86) | 125 | 16 | 1.20 | (0.69–2.09) | |
| Continuous BMI in kg/m2 e) | 1414 | 168 | 0.99 | (0.95–1.04) | 1369 | 163 | 0.99 | (0.95–1.04) | |
| Obesity e) | |||||||||
| No | 1288 | 152 | Reference | 1245 | 147 | Reference | |||
| Yes | 128 | 16 | 1.07 | (0.64–1.79) | 125 | 16 | 1.18 | (0.70–1.99) | |
| Diabetes mellitus status f) | |||||||||
| No | 1235 | 143 | Reference | 1201 | 139 | Reference | |||
| Yes | 198 | 27 | 1.16 | (0.77–1.75) | 191 | 27 | 1.16 | (0.76–1.76) | |
| Diabetes mellitus type f) | |||||||||
| No diabetes mellitus | 1235 | 143 | Reference | 1201 | 139 | Reference | |||
| Type 1 | 13 | 1 | 0.68 | (0.10–4.84) | 13 | 1 | 0.88 | (0.12–6.36) | |
| Type 2 | 124 | 16 | 1.09 | (0.65–1.83) | 123 | 16 | 1.02 | (0.60–1.72) | |
| Associations with progression to MIBC | |||||||||
| BMI classes in kg/m2 e) | |||||||||
| ≥18.5 and <25.0 | 644 | 22 | Reference | 623 | 21 | Reference | |||
| ≥25.0 and <30.0 | 642 | 18 | 0.81 | (0.44–1.51) | 621 | 16 | 0.84 | (0.43–1.63) | |
| ≥30.0 | 128 | 3 | 0.69 | (0.21–2.31) | 125 | 3 | 0.93 | (0.27–3.16) | |
| Continuous BMI in kg/m2 e) | 1414 | 43 | 0.94 | (0.85–1.05) | 1369 | 40 | 0.94 | (0.84–1.05) | |
| Obesity e) | |||||||||
| No | 1286 | 40 | Reference | 1244 | 37 | Reference | |||
| Yes | 128 | 3 | 0.76 | (0.24–2.47) | 125 | 3 | 1.01 | (0.31–3.31) | |
| Diabetes mellitus status f) | |||||||||
| No | 1235 | 36 | Reference | 1201 | 34 | Reference | |||
| Yes | 198 | 8 | 1.37 | (0.64–2.94) | 191 | 8 | 1.40 | (0.64–3.07) | |
| Diabetes mellitus type f) | |||||||||
| No diabetes mellitus | 1235 | 36 | Reference | 1201 | 34 | Reference | |||
| Type 1 | 13 | 1 | 2.82 | (0.39–20.55) | 13 | 1 | 5.65 | (0.73–43.44) | |
| Type 2 | 124 | 5 | 1.35 | (0.53–3.43) | 123 | 5 | 1.24 | (0.48–3.22) | |
HR: hazard ratio; CI: confidence interval; BMI: body mass index; MIBC: muscle invasive bladder cancer.
[a] Defined as the first occurrence of stage or grade progression, local or distant metastasis, and cystectomy for therapy-resistant disease.
[b] Defined as transition to MIBC (stage ≥T2).
[c] For the associations of BMI classes and obesity with progression, the adjustment set consists of age at time of UBC diagnosis, gender, highest completed level of education, weekly duration of physical activity, history of urinary bladder cancer among first degree relatives, tumour stage, tumour grade, and presence of concomitant CIS; for the associations of diabetes mellitus and type of diabetes mellitus with progression, the adjustment set consists of age at time of UBC diagnosis, gender, BMI classes, tumour stage, tumour grade, and presence of concomitant CIS.
[d] Number of incident events in the first 5 years after diagnosis of the primary non-muscle invasive urinary bladder cancer.
[e] Based on adult BMI; obesity was defined as a BMI ≥30.0 kg/m2, no obesity as a BMI <30.0 kg/m2.
[f] At the time of filling out the questionnaire; type 1 diabetes mellitus was defined as a diagnosis at age ≤30 years, or at age 31–40 years in combination without being obese, type 2 diabetes mellitus was defined as a diagnosis at age >40 years, or at age 31–40 years in combination with being obese.
Discussion
In our large population-based cohort of NMIBC patients, we showed that neither adult BMI nor DM showed clear associations with risk of recurrence, overall progression or progression to MIBC. Previous studies on BMI and DM in relation to NMIBC prognosis are limited and results are inconsistent.
Four historical cohort studies investigated the association of overweight and/or obesity with the risk of recurrence and progression in NMIBC patients [19–21, 39]. In a study of 469 NMIBC patients, Xu et al. (2015) compared overweight and obesity to healthy weight at the time of transurethral resection of the bladder tumour. For obesity, HRs of 1.71 (95% CI: 1.12–2.60) and 3.04 (95% CI: 1.24–7.42) were found for recurrence and progression, respectively [21]. In a study of 892 patients with high grade T1 tumours by Kluth et al. (2013), timing of obesity was not reported. Here, statistically significant associations with recurrence (HR; 2.66, 95% CI: 2.13–3.32) and progression (HR: 1.49, 95% CI: 1.00–2.21) were shown when comparing obesity with no obesity [19]. In a recent study by Ferro et al. in 1155 T1G3 NMIBC patients treated with maintenance Bacillus Calmette-Guerin (BCG), obesity versus normal weight at diagnosis was associated with an increased risk of recurrence (HR: 5.33; 95% CI: 4.16–6.83) and progression (HR:2.51, 95% CI: 1.76–3.57) as well, with similar but slightly lower risk estimates for overweight [39]. None of these three studies adjusted for smoking status. A study by Wyszynski et al. (2014) including 726 NMIBC patients did adjust for smoking status. Here, no statistically significant association with risk of recurrence (HR: 1.22, 95% CI: 0.80–1.87) was reported when comparing obese to healthy weight patients at time of diagnosis [20]. We conducted the largest population-based cohort on this topic to date and, in contrast to the previous studies, investigated average BMI during adult life. This reflects lifetime exposure to growth factors and pro-inflammatory factors and is not affected by possible weight changes due to cancer diagnosis. In our study, no statistically significant association of adult BMI with risk of recurrence and progression was found. Smoking status and cigarette pack years were included in our DAG but not in our minimal sets of sufficient adjustment, because tests on the DAG showed that there was sufficient adjustment for the effect of smoking by including the other covariables in our models. This was confirmed by the fact that a sensitivity analysis with additional adjustment for smoking status did not change our risk estimates (data not shown). Also, no associations were found in the subgroup of patients initially treated with adjuvant immunotherapy (S5 Table), in contrast to results of Kluth et al. [19] and Ferro et al. [39]. Our results are in line with those of Wyszynski et al. [20], who also found that associations for BMI did not differ by immunotherapy treatment status. However, power for our sensitivity analysis was limited.
In a historical cohort of 251 NMIBC patients, Hwang et al. (2011) classified diagnosis of DM based on treatment history and fasting glucose levels. When comparing DM with no DM, they found HRs of 2.11 (95% CI: 1.4–3.2) and 9.35 (95% CI: 3.1–28.6) for recurrence and progression, respectively [22]. Rieken et al. (2013) retrieved information on DM diagnosis from medical records in a historical cohort study of 1117 NMIBC patients, and stratified their analyses for use of metformin [23]. When comparing DM without use of metformin with no DM, they found HRs of 1.39 (95% CI: 1.04–1.86) and 2.21 (95% CI: 1.29–3.77) for recurrence and progression, respectively. For DM with use of metformin compared with no DM, HRs of 0.48 (95% CI: 0.26–0.89) and 0.34 (95% CI: 0.05–2.42) were reported for recurrence and progression, respectively [23]. In a historical cohort of 645 NMIBC patients, Ahn et al. (2016) assessed DM diagnosis based on medical records, treatment history, and fasting glucose levels [24]. No statistically significant association of DM with recurrence (HR = 1.22; 95% CI: 0.89–1.67) or progression (HR = 1.54; 95% CI: 0.95–2.50) were found. Univariable analyses comparing diabetics who ever used metformin with diabetics who never used metformin yielded non-significant HRs of 1.07 (95% CI: 0.64–1.80) and 1.52 (95% CI: 0.70–3.33) for recurrence and progression, respectively [24]. In our study, patients were asked whether they were ever diagnosed with DM by a physician and at which age. No association with risk of recurrence or progression was found. Since treatment for DM was not assessed in our study, effect modification by DM treatment could not be evaluated.
Our study had several limitations. First, patients were invited and completed questionnaires relatively long after diagnosis, and information about BMI and DM was self-reported. Also, the possibility of recall errors cannot be excluded although we do not expect these errors to be different between patients with or without an event. Another limitation was that we could not perform analyses stratified for the timing of DM diagnosis relative to the events of interest, because 31% of the DM diagnosed patients had missing values for age at DM diagnosis. Thus, also patients with a known age at DM diagnosis and who indicated to be diagnosed with DM between diagnosis and time of filling out the questionnaire were included as diabetics. Since 27% of patients with available age at DM diagnosis were diagnosed after NMIBC diagnosis, this may have influenced our results. Patients who had missing values for DM diagnosis were included as having no DM. To validate, we performed sensitivity analyses by comparing the study results with the results of two alternative methods to deal with missing DM status. In the first alternative, we assumed that patients only filled out questions on medical conditions with which they were diagnosed. Patients with missing DM status were only included as non-diabetics if they never answered negatively to a question regarding medical history and they answered positively to at least one medical history-question. In the second alternative, patients with missing values for DM diagnosis were excluded by default. Reassuringly, all methods resulted in similar findings (S3 and S4 Tables). Another limitation was the lack of information on DM type. We therefore used age at diagnosis and BMI to classify into DM1 and DM2. This may have caused some misclassification. Besides, diabetics who did not report age at diagnosis could not be classified and had to be excluded from the stratified analyses. Further, we could not draw conclusions from our study regarding changes in BMI up to or after diagnosis. Also, the possibility of residual confounding could not be excluded. Finally, we had limited power for the analyses of progression to MIBC due to a limited number of events, and as shown by the wide confidence intervals for these risk estimates.
Strengths of our study compared with previous studies are the inclusion of a large population-based series of patients. Since NMIBC patients included in the current analysis did not differ from invited NMIBC patients with respect to age, gender, tumour stage, and tumour grade (data not shown), our results are unlikely to have been affected by selection bias. Moreover, the five-year risks of recurrence and progression in our population are in line with estimates in the literature [4]. Also, we used a state-of-the-art approach in selecting the adjustment sets. By use of the DAG, minimal sets for sufficient adjustment were selected. Because over-adjustment was prevented, we achieved better precision in estimating the effect.
In conclusion, we did not find associations of adult BMI and DM with risk of recurrence and progression among NMIBC patients in the first five years after diagnosis. Considering the designs and results of our and previous studies, and their limitations, we cannot draw definitive conclusions. To obtain more insight in the associations of overweight and obesity, including weight change, and DM with risk of recurrence and progression in NMIBC patients, we recommend the conduction of prospective cohort studies assessing BMI, DM type and medication both before and after NMIBC diagnosis. Results of such studies may contribute to the development of personalized follow-up schedules and may also aid in advising NMIBC patients about weight and DM management to improve their prognosis.
Supporting information
(TIF)
Cumulative risk plots for recurrence, stratified by BMI class [A] and diabetes mellitus status [B].
(TIF)
Cumulative risk plots for overall progression [A] and progression to muscle invasive bladder cancer [B], stratified by BMI class.
(TIF)
Cumulative risk plots for overall progression [A] and progression to muscle invasive bladder cancer [B], stratified by diabetes mellitus (DM) status.
(TIF)
P25: 25th percentile; P75: 75th percentile; BMI: body mass index; CIS: carcinoma in situ; TURT: transurethral resection of the bladder tumour. [a] At the time of non-muscle invasive bladder cancer diagnosis. [b] Based on the country of birth of the patients and their parents. [c] Based on the International Standard Classification of Education: low level includes elementary, lower vocational and intermediate general education; intermediate level includes intermediate vocational and higher general education; high level includes higher vocational education and university. [d] Presented for current and former cigarette smokers. [e] Based on the weekly duration of walking, cycling, and sporting during adult life until 2 years before diagnosis; unknown for 25 patients (1.7%). [f] At the time of filling out the questionnaire. [g] 66 patients (4.6%) had a missing value for diagnosis of diabetes mellitus and were included as not diagnosed. [h] Presented for patients diagnosed with diabetes mellitus; type 1 was defined as diabetes mellitus diagnosis at age ≤30 years, or at age 31–40 years in combination with being not obese; type 2 was defined as diabetes mellitus diagnosis at age >40 years, or at age 31–40 years in combination with being obese. [i] Tumours with WHO 1973 differentiation grade 1 or 2, WHO/ISUP 2004 low grade, or Malmström (Modified Bergkvist) grade 1 or 2a were considered low-grade tumours. Tumours with WHO 1973 differentiation grade 3, WHO/ISUP 2004 high grade, or Malmström (Modified Bergkvist) grade 2b or 3 as high-grade. [j] Based on the European Association of Urology guidelines: low risk includes Ta-stage with low grade, high risk includes Ta-stage with high grade, T1-stage, T2-stage, T3-stage, T4-stage, and CIS.
(DOCX)
P25: 25th percentile; P75: 75th percentile; BMI: body mass index; CIS: carcinoma in situ; DM: diabetes mellitus; TURT: transurethral resection of the bladder tumour. [a] 66 patients (4.6%) had a missing value for diagnosis of diabetes mellitus and were included as not diagnosed. [b] Type 1 was defined as diabetes mellitus diagnosis at age ≤30 years, or at age 31–40 years in combination with being not obese; type 2 was defined as diabetes mellitus diagnosis at age >40 years, or at age 31–40 years in combination with being obese. [c] At the time of non-muscle invasive bladder cancer diagnosis. [d] Based on the country of birth of the patients and their parents. [e] Based on the International Standard Classification of Education: low level includes elementary, lower vocational and intermediate general education; intermediate level includes intermediate vocational and higher general education; high level includes higher vocational education and university. [f] Presented for current and former cigarette smokers. [g] Based on the weekly duration of walking, cycling, and sporting during adult life until 2 years before diagnosis; unknown for 25 patients (1.7%). [h] At the time of filling out the questionnaire. [i] Tumours with WHO 1973 differentiation grade 1 or 2, WHO/ISUP 2004 low grade, or Malmström (Modified Bergkvist) grade 1 or 2a were considered low-grade tumours. Tumours with WHO 1973 differentiation grade 3, WHO/ISUP 2004 high grade, or Malmström (Modified Bergkvist) grade 2b or 3 as high-grade. [j] Based on the European Association of Urology guidelines: low risk includes Ta-stage with low grade, high risk includes Ta-stage with high grade, T1-stage, T2-stage, T3-stage, T4-stage, and CIS.
(DOCX)
HR: hazard ratio; CI: confidence interval. [a] Defined as a new histologically confirmed tumour in the urinary bladder or prostatic urethra, after ≥1 tumour-negative follow-up cystoscopy result or a radical re-transurethral resection of the primary tumour. [b] The adjustment set consists of age at time of UBC diagnosis, gender, BMI classes, tumour stage, tumour grade, and presence of concomitant CIS. [c] Number of events in the first 5 years after diagnosis of the primary non-muscle invasive urinary bladder cancer. [d] At the time of filling out the questionnaire.
(DOCX)
HR: hazard ratio; CI: confidence interval; MIBC: muscle invasive bladder cancer. [a] The adjustment set consists of age at time of UBC diagnosis, gender, BMI classes, tumour stage, S5 Table. Crude and adjusted hazard ratios (HR) with corresponding 95% confidence intervals (CI) for the associations of BMI with recurrence, overall progression, and progression to MIBC, among non-muscle invasive bladder cancer patients who received transurethral resection of the bladder tumour (TURT) with one intravesical chemotherapy instillation and adjuvant intravesical immunotherapy. [b] Number of events in the first 5 years after diagnosis of the primary non-muscle invasive urinary bladder cancer. [c] Defined as the first occurrence of stage or grade progression, local or distant metastasis, and cystectomy for therapy-resistant disease. [d] At the time of filling out the questionnaire. [e] Defined as transition to MIBC (stage ≥T2).
(DOCX)
HR: hazard ratio; CI: confidence interval; BMI: body mass index. [a] Defined as a new histologically confirmed tumour in the urinary bladder or prostatic urethra, after ≥1 tumour-negative follow-up cystoscopy result or a radical re-transurethral resection of the primary tumour. [b] Defined as the first occurrence of stage or grade progression, local or distant metastasis, and cystectomy for therapy-resistant disease [c] Defined as transition to MIBC (stage ≥T2). [d] The adjustment set consists of age at time of UBC diagnosis, gender, highest completed level of education, weekly duration of physical activity, history of urinary bladder cancer among first degree relatives, tumour stage, tumour grade, and presence of concomitant CIS. [e] Number of incident events in the first 5 years after diagnosis of the primary non-muscle invasive urinary bladder cancer. [f] Based on average BMI during adult life.
(DOCX)
Acknowledgments
The authors thank all the participating patients and their treating physicians as well as the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry.
Data Availability
Data are available from the DANS database: https://doi.org/10.17026/dans-2zx-zqh2
Funding Statement
The authors received no specific funding for this work.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Ploeg M, Aben KK, Hulsbergen-van de Kaa CA, Schoenberg MP, Witjes JA, Kiemeney LA. Clinical epidemiology of nonurothelial bladder cancer: analysis of the Netherlands Cancer Registry. J Urol. 2010;183(3):915–20. 10.1016/j.juro.2009.11.018 [DOI] [PubMed] [Google Scholar]
- 3.Canadian Cancer Society. Survival statistics for bladder cancer [cited 13th June 2019] [Internet]. Available from: http://www.cancer.ca/en/cancer-information/cancer-type/bladder/prognosis-and-survival/survival-statistics/?region=bc
- 4.Cambier S, Sylvester RJ, Collette L, Gontero P, Brausi MA, van Andel G, et al. EORTC Nomograms and Risk Groups for Predicting Recurrence, Progression, and Disease-specific and Overall Survival in Non-Muscle-invasive Stage Ta-T1 Urothelial Bladder Cancer Patients Treated with 1–3 Years of Maintenance Bacillus Calmette-Guerin. Eur Urol. 2016;69(1):60–9. 10.1016/j.eururo.2015.06.045 [DOI] [PubMed] [Google Scholar]
- 5.Babjuk M, Bohle A, Burger M, Capoun O, Cohen D, Comperat EM, et al. EAU Guidelines on Non-Muscle-invasive Urothelial Carcinoma of the Bladder: Update 2016. Eur Urol. 2017;71(3):447–61. 10.1016/j.eururo.2016.05.041 [DOI] [PubMed] [Google Scholar]
- 6.Svatek RS, Hollenbeck BK, Holmang S, Lee R, Kim SP, Stenzl A, et al. The economics of bladder cancer: costs and considerations of caring for this disease. Eur Urol. 2014;66(2):253–62. 10.1016/j.eururo.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 7.Fernandez-Gomez J, Madero R, Solsona E, Unda M, Martinez-Pineiro L, Gonzalez M, et al. Predicting nonmuscle invasive bladder cancer recurrence and progression in patients treated with bacillus Calmette-Guerin: the CUETO scoring model. J Urol. 2009;182(5):2195–203. 10.1016/j.juro.2009.07.016 [DOI] [PubMed] [Google Scholar]
- 8.Sylvester RJ, van der Meijden AP, Oosterlinck W, Witjes JA, Bouffioux C, Denis L, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49(3):466–5; discussion 75–7. 10.1016/j.eururo.2005.12.031 [DOI] [PubMed] [Google Scholar]
- 9.Westhoff E, Witjes JA, Fleshner NE, Lerner SP, Shariat SF, Steineck G, et al. Body Mass Index, Diet-Related Factors, and Bladder Cancer Prognosis: A Systematic Review and Meta-Analysis. Bladder Cancer. 2018;4(1):91–112. 10.3233/BLC-170147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organisation. Obesity and overweight [cited 13th June 2019] [Internet]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 11.International Diabetes Federation. Diabetes Atlas 8th Edition 2017 [cited 13th June 2019] [Internet]. Available from: https://diabetesatlas.org/upload/resources/previous/files/8/IDF_DA_8e-EN-final.pdf
- 12.Gallagher EJ, LeRoith D. Obesity and Diabetes: The Increased Risk of Cancer and Cancer-Related Mortality. Physiol Rev. 2015;95(3):727–48. 10.1152/physrev.00030.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallagher EJ, LeRoith D. Diabetes, antihyperglycemic medications and cancer risk: smoke or fire? Curr Opin Endocrinol Diabetes Obes. 2013;20(5):485–94. 10.1097/01.med.0000433065.16918.83 [DOI] [PubMed] [Google Scholar]
- 14.Johnson JA, Carstensen B, Witte D, Bowker SL, Lipscombe L, Renehan AG, et al. Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia. 2012;55(6):1607–18. 10.1007/s00125-012-2525-1 [DOI] [PubMed] [Google Scholar]
- 15.Suh S, Kim KW. Diabetes and cancer: is diabetes causally related to cancer? Diabetes Metab J. 2011;35(3):193–8. 10.4093/dmj.2011.35.3.193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun JW, Zhao LG, Yang Y, Ma X, Wang YY, Xiang YB. Obesity and risk of bladder cancer: a dose-response meta-analysis of 15 cohort studies. PLoS One. 2015;10(3):e0119313 10.1371/journal.pone.0119313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu Z, Wang X, Shen Z, Lu Y, Zhong S, Xu C. Risk of bladder cancer in patients with diabetes mellitus: an updated meta-analysis of 36 observational studies. BMC Cancer. 2013;13:310 10.1186/1471-2407-13-310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gild P, Ehdaie B, Kluth LA. Effect of obesity on bladder cancer and renal cell carcinoma incidence and survival. Curr Opin Urol. 2017;27(5):409–14. 10.1097/MOU.0000000000000425 [DOI] [PubMed] [Google Scholar]
- 19.Kluth LA, Xylinas E, Crivelli JJ, Passoni N, Comploj E, Pycha A, et al. Obesity is associated with worse outcomes in patients with T1 high grade urothelial carcinoma of the bladder. J Urol. 2013;190(2):480–6. 10.1016/j.juro.2013.01.089 [DOI] [PubMed] [Google Scholar]
- 20.Wyszynski A, Tanyos SA, Rees JR, Marsit CJ, Kelsey KT, Schned AR, et al. Body mass and smoking are modifiable risk factors for recurrent bladder cancer. Cancer. 2014;120(3):408–14. 10.1002/cncr.28394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu T, Zhu Z, Wang X, Xia L, Zhang X, Zhong S, et al. Impact of body mass on recurrence and progression in Chinese patients with Ta, T1 urothelial bladder cancer. Int Urol Nephrol. 2015;47(7):1135–41. 10.1007/s11255-015-1013-1 [DOI] [PubMed] [Google Scholar]
- 22.Hwang EC, Kim YJ, Hwang IS, Hwang JE, Jung SI, Kwon DD, et al. Impact of diabetes mellitus on recurrence and progression in patients with non-muscle invasive bladder carcinoma: a retrospective cohort study. Int J Urol. 2011;18(11):769–76. 10.1111/j.1442-2042.2011.02845.x [DOI] [PubMed] [Google Scholar]
- 23.Rieken M, Xylinas E, Kluth L, Crivelli JJ, Chrystal J, Faison T, et al. Association of diabetes mellitus and metformin use with oncological outcomes of patients with non-muscle-invasive bladder cancer. BJU Int. 2013;112(8):1105–12. 10.1111/bju.12448 [DOI] [PubMed] [Google Scholar]
- 24.Ahn JH, Jung SI, Yim SU, Kim SW, Hwang EC, Kwon DD. Impact of Glycemic Control and Metformin Use on the Recurrence and Progression of Non-Muscle Invasive Bladder Cancer in Patients with Diabetes Mellitus. J Korean Med Sci. 2016;31(9):1464–71. 10.3346/jkms.2016.31.9.1464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rafnar T, Vermeulen SH, Sulem P, Thorleifsson G, Aben KK, Witjes JA, et al. European genome-wide association study identifies SLC14A1 as a new urinary bladder cancer susceptibility gene. Hum Mol Genet. 2011;20(21):4268–81. 10.1093/hmg/ddr303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grotenhuis AJ, Dudek AM, Verhaegh GW, Aben KK, Witjes JA, Kiemeney LA, et al. Independent Replication of Published Germline Polymorphisms Associated with Urinary Bladder Cancer Prognosis and Treatment Response. Bladder Cancer. 2016;2(1):77–89. 10.3233/BLC-150027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.World Health Organisation. Body mass index—BMI. [cited 13th June 2019] [Internet]. Available from: http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi
- 28.Barbosa ALA, Vermeulen S, Aben KK, Grotenhuis AJ, Vrieling A, Kiemeney LA. Smoking intensity and bladder cancer aggressiveness at diagnosis. PLoS One. 2018;13(3):e0194039 10.1371/journal.pone.0194039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mostofi FK, Sobin LH, Torloni H. Histological typing of urinary bladder tumours. World Health Organization. 1973. [cited 13th June 2019]. Available from: http://www.who.int/iris/handle/10665/41533 [Google Scholar]
- 30.Eble JN, Sauter G, Epstein J, Sesterhenn I. Pathology and genetics of tumours of the urinary system and male genital organs. World Health Organization. 2004. [cited 13th June 2019]. Available from: http://publications.iarc.fr/Book-And-Report-Series/Who-Iarc-Classification-Of-Tumours/Pathology-And-Genetics-Of-Tumours-Of-The-Urinary-System-And-Male-Genital-Organs-2004 [Google Scholar]
- 31.Kiemeney LA, Thorlacius S, Sulem P, Geller F, Aben KK, Stacey SN, et al. Sequence variant on 8q24 confers susceptibility to urinary bladder cancer. Nat Genet. 2008;40(11):1307–12. 10.1038/ng.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lamm D, Persad R, Brausi M, Buckley R, Witjes JA, Palou J, et al. Defining progression in nonmuscle invasive bladder cancer: it is time for a new, standard definition. J Urol. 2014;191(1):20–7. 10.1016/j.juro.2013.07.102 [DOI] [PubMed] [Google Scholar]
- 33.Schoenfeld D. Partial Residuals for the Proportional Hazards Regression-Model. Biometrika. 1982;69(1):239–41. [Google Scholar]
- 34.Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22(5):745. [DOI] [PubMed] [Google Scholar]
- 35.Textor J, Van der Zander B. dagitty: Graphical Analysis of Structural Causal Models. The Comprehensive R Archive Network. 2016. [cited 10th November 2019]. Available from: https://cran.r-project.org/web/packages/dagitty/index.html [Google Scholar]
- 36.R Core Team. foreign: Read Data Stored by 'Minitab', 'S', 'SAS', 'SPSS', 'Stata', 'Systat', 'Weka', 'dBase', … The Comprehensive R Archive Network. 2019 [cited 10th November 2019]. Available from: https://cran.r-project.org/web/packages/foreign/index.html
- 37.Marchetti GM, Drton M, Sadeghi K. Package ‘ggm’. The Comprehensive R Archive Network. 2015 [cited 10th November 2019]. Available from: https://cran.r-project.org/web/packages/ggm/ggm.pdf
- 38.Textor J. Drawing and Analyzing Causal DAGs with DAGitty. 2015. [cited 10th November 2019]. Available from:http://www.dagitty.net/manual-2.x.pdf
- 39.Ferro M, Vartolomei MD, Russo GI, Cantiello F, Farhan ARA, Terracciano D, et al. An increased body mass index is associated with a worse prognosis in patients administered BCG immunotherapy for T1 bladder cancer. World J Urol. 2019;37(3):507–14. 10.1007/s00345-018-2397-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIF)
Cumulative risk plots for recurrence, stratified by BMI class [A] and diabetes mellitus status [B].
(TIF)
Cumulative risk plots for overall progression [A] and progression to muscle invasive bladder cancer [B], stratified by BMI class.
(TIF)
Cumulative risk plots for overall progression [A] and progression to muscle invasive bladder cancer [B], stratified by diabetes mellitus (DM) status.
(TIF)
P25: 25th percentile; P75: 75th percentile; BMI: body mass index; CIS: carcinoma in situ; TURT: transurethral resection of the bladder tumour. [a] At the time of non-muscle invasive bladder cancer diagnosis. [b] Based on the country of birth of the patients and their parents. [c] Based on the International Standard Classification of Education: low level includes elementary, lower vocational and intermediate general education; intermediate level includes intermediate vocational and higher general education; high level includes higher vocational education and university. [d] Presented for current and former cigarette smokers. [e] Based on the weekly duration of walking, cycling, and sporting during adult life until 2 years before diagnosis; unknown for 25 patients (1.7%). [f] At the time of filling out the questionnaire. [g] 66 patients (4.6%) had a missing value for diagnosis of diabetes mellitus and were included as not diagnosed. [h] Presented for patients diagnosed with diabetes mellitus; type 1 was defined as diabetes mellitus diagnosis at age ≤30 years, or at age 31–40 years in combination with being not obese; type 2 was defined as diabetes mellitus diagnosis at age >40 years, or at age 31–40 years in combination with being obese. [i] Tumours with WHO 1973 differentiation grade 1 or 2, WHO/ISUP 2004 low grade, or Malmström (Modified Bergkvist) grade 1 or 2a were considered low-grade tumours. Tumours with WHO 1973 differentiation grade 3, WHO/ISUP 2004 high grade, or Malmström (Modified Bergkvist) grade 2b or 3 as high-grade. [j] Based on the European Association of Urology guidelines: low risk includes Ta-stage with low grade, high risk includes Ta-stage with high grade, T1-stage, T2-stage, T3-stage, T4-stage, and CIS.
(DOCX)
P25: 25th percentile; P75: 75th percentile; BMI: body mass index; CIS: carcinoma in situ; DM: diabetes mellitus; TURT: transurethral resection of the bladder tumour. [a] 66 patients (4.6%) had a missing value for diagnosis of diabetes mellitus and were included as not diagnosed. [b] Type 1 was defined as diabetes mellitus diagnosis at age ≤30 years, or at age 31–40 years in combination with being not obese; type 2 was defined as diabetes mellitus diagnosis at age >40 years, or at age 31–40 years in combination with being obese. [c] At the time of non-muscle invasive bladder cancer diagnosis. [d] Based on the country of birth of the patients and their parents. [e] Based on the International Standard Classification of Education: low level includes elementary, lower vocational and intermediate general education; intermediate level includes intermediate vocational and higher general education; high level includes higher vocational education and university. [f] Presented for current and former cigarette smokers. [g] Based on the weekly duration of walking, cycling, and sporting during adult life until 2 years before diagnosis; unknown for 25 patients (1.7%). [h] At the time of filling out the questionnaire. [i] Tumours with WHO 1973 differentiation grade 1 or 2, WHO/ISUP 2004 low grade, or Malmström (Modified Bergkvist) grade 1 or 2a were considered low-grade tumours. Tumours with WHO 1973 differentiation grade 3, WHO/ISUP 2004 high grade, or Malmström (Modified Bergkvist) grade 2b or 3 as high-grade. [j] Based on the European Association of Urology guidelines: low risk includes Ta-stage with low grade, high risk includes Ta-stage with high grade, T1-stage, T2-stage, T3-stage, T4-stage, and CIS.
(DOCX)
HR: hazard ratio; CI: confidence interval. [a] Defined as a new histologically confirmed tumour in the urinary bladder or prostatic urethra, after ≥1 tumour-negative follow-up cystoscopy result or a radical re-transurethral resection of the primary tumour. [b] The adjustment set consists of age at time of UBC diagnosis, gender, BMI classes, tumour stage, tumour grade, and presence of concomitant CIS. [c] Number of events in the first 5 years after diagnosis of the primary non-muscle invasive urinary bladder cancer. [d] At the time of filling out the questionnaire.
(DOCX)
HR: hazard ratio; CI: confidence interval; MIBC: muscle invasive bladder cancer. [a] The adjustment set consists of age at time of UBC diagnosis, gender, BMI classes, tumour stage, S5 Table. Crude and adjusted hazard ratios (HR) with corresponding 95% confidence intervals (CI) for the associations of BMI with recurrence, overall progression, and progression to MIBC, among non-muscle invasive bladder cancer patients who received transurethral resection of the bladder tumour (TURT) with one intravesical chemotherapy instillation and adjuvant intravesical immunotherapy. [b] Number of events in the first 5 years after diagnosis of the primary non-muscle invasive urinary bladder cancer. [c] Defined as the first occurrence of stage or grade progression, local or distant metastasis, and cystectomy for therapy-resistant disease. [d] At the time of filling out the questionnaire. [e] Defined as transition to MIBC (stage ≥T2).
(DOCX)
HR: hazard ratio; CI: confidence interval; BMI: body mass index. [a] Defined as a new histologically confirmed tumour in the urinary bladder or prostatic urethra, after ≥1 tumour-negative follow-up cystoscopy result or a radical re-transurethral resection of the primary tumour. [b] Defined as the first occurrence of stage or grade progression, local or distant metastasis, and cystectomy for therapy-resistant disease [c] Defined as transition to MIBC (stage ≥T2). [d] The adjustment set consists of age at time of UBC diagnosis, gender, highest completed level of education, weekly duration of physical activity, history of urinary bladder cancer among first degree relatives, tumour stage, tumour grade, and presence of concomitant CIS. [e] Number of incident events in the first 5 years after diagnosis of the primary non-muscle invasive urinary bladder cancer. [f] Based on average BMI during adult life.
(DOCX)
Data Availability Statement
Data are available from the DANS database: https://doi.org/10.17026/dans-2zx-zqh2