The new H7N9 influenza virus, recently emerged in China, can replicate in human airway cells and in the respiratory tract of ferrets to a higher level than can seasonal H3N2 virus and shows higher lethality in mice than genetically related H7N9 and H9N2 viruses, but shows limited transmission in ferrets by respiratory droplets.
Supplementary information
The online version of this article (doi:10.1038/nature12391) contains supplementary material, which is available to authorized users.
Subject terms: Viral transmission
Transmission of emerging H7N9 virus
By 20 July 2013, there had been 134 laboratory-confirmed human cases of infection with avian influenza A H7N9 virus infection, including 43 deaths.
Yoshihiro Kawaoka and colleagues characterize the biology of two recent isolates of the virus. They provide a wealth of data from infections in mice, pigs, macaques and ferrets. H7N9 virus is shown to be less sensitive to neuraminidase inhibitors than pandemic H1N1 virus, but equally susceptible to an experimental polymerase inhibitor. Terrence Tumpey and colleagues determine the capacity of two clinical H7N9 isolates to cause disease and transmit between mammals. They show that the virus can replicate in human airway cells and in the respiratory tract of ferrets to a higher level than can seasonal H3N2 virus, and show higher lethality in mice than genetically related H7N9 and H9N2 viruses. In transmission studies, the H7N9 virus showed limited transmission in ferrets by respiratory droplets. Ron Fouchier and colleagues investigate the transmissibility of H7N9 virus between ferrets. They show that airborne transmission can occur, but inefficiently. They also show that on passage in ferrets, virus variants that have higher avian receptor binding, higher pH of fusion and lower thermostability are selected, and they suggest that these characteristics may result in reduced transmissibility.
Supplementary information
The online version of this article (doi:10.1038/nature12391) contains supplementary material, which is available to authorized users.
Abstract
On 29 March 2013, the Chinese Center for Disease Control and Prevention confirmed the first reported case of human infection with an avian influenza A(H7N9) virus1. The recent human infections with H7N9 virus, totalling over 130 cases with 39 fatalities to date, have been characterized by severe pulmonary disease and acute respiratory distress syndrome (ARDS)2. This is concerning because H7 viruses have typically been associated with ocular disease in humans, rather than severe respiratory disease3. This recent outbreak underscores the need to better understand the pathogenesis and transmission of these viruses in mammals. Here we assess the ability of A/Anhui/1/2013 and A/Shanghai/1/2013 (H7N9) viruses, isolated from fatal human cases, to cause disease in mice and ferrets and to transmit to naive animals. Both H7N9 viruses replicated to higher titre in human airway epithelial cells and in the respiratory tract of ferrets compared to a seasonal H3N2 virus. Moreover, the H7N9 viruses showed greater infectivity and lethality in mice compared to genetically related H7N9 and H9N2 viruses. The H7N9 viruses were readily transmitted to naive ferrets through direct contact but, unlike the seasonal H3N2 virus, did not transmit readily by respiratory droplets. The lack of efficient respiratory droplet transmission was corroborated by low receptor-binding specificity for human-like α2,6-linked sialosides. Our results indicate that H7N9 viruses have the capacity for efficient replication in mammals and human airway cells and highlight the need for continued public health surveillance of this emerging virus.
Supplementary information
The online version of this article (doi:10.1038/nature12391) contains supplementary material, which is available to authorized users.
Main
Laboratory-confirmed H7N9 infections in humans have been reported in eight contiguous provinces in eastern China (Anhui, Fujian, Henan, Hunan, Jiangsu, Jiangxi, Shandong and Zhejiang), two municipalities (Beijing and Shanghai) and Taiwan to date4. Although historically this subtype resulted in mild illness among humans3, the majority of novel A(H7N9) cases have resulted in severe respiratory illness in adults, representing the largest occurrence of H7 virus infections in humans2. Unlike highly pathogenic avian influenza (HPAI) H5N1 viruses, which exhibit lethality in poultry and cause severe human disease, low pathogenic (LPAI) H7N9 viruses seem to be asymptomatic or cause mild disease in poultry and wild bird populations, possibly due to the presence of a single amino acid in the haemagglutinin (HA) cleavage site of this virus2,5. Similar to H5N1 viruses, human cases with H7N9 virus seem to result from direct contact with infected poultry, yet >20% of confirmed H7N9 cases have reported no exposure to live animals6. Although sustained human-to-human transmission has not been observed with this virus to date, reports of family clusters of A(H7N9) infection suggest the potential for virus spread between close contacts6. Understanding the properties of A(H7N9) viruses which contribute to human disease and their capacity for human-to-human transmission is a critical requirement for guidance of public health responses. The ferret (Mustela putorius furo) is recognized as the best small mammalian model for the concurrent study of influenza virus pathogenicity and transmissibility, and was used here to assess the virulence of A(H7N9) viruses.
A/Anhui/1/13 (Anhui/1) virus was isolated from a throat-swab specimen collected from an adult female patient with known poultry exposure and who died from ARDS 6 days after illness onset. A/Shanghai/1/13 (Shanghai/1) virus was isolated from an adult male patient with no known poultry exposure who died from refractory hypoxemia 13 days after illness onset2. These A(H7N9) viruses from China possess several molecular markers previously associated with human adaptation, including the presence of E627K in the PB2 protein and Q226L in the 210-loop in the HA gene2. Glycan microarray analysis of these phylogenetically distinct viruses revealed that Shanghai/1 (bearing Q226 in the HA (H3 numbering)) largely binds to avian α2,3 receptor analogues (glycans 4 to 40) (Fig. 1a and Supplementary Table 1), whereas Anhui/1 virus (bearing L226), revealed a mixed α2,3/α2,6 receptor preference (Fig. 1b and Supplementary Table 1); the single Q226L amino substitution has long been recognized to modulate receptor-binding specificity7. In particular, Anhui/1 virus bound to human biantennary structures (glycans 43 to 47), as well as the longer, linear α2,6 sialylated tri-N-acetyl lactosamine (glycans 58–60), some of which have been detected in N glycans of cultured human bronchial epithelial cells8. These findings are in accord with recent studies showing that Anhui/1 virus binds to human tracheal tissue sections, albeit at a lower intensity than other human-adapted viruses9,10.
Figure 1. Receptor specificity of A(H7N9) viruses.
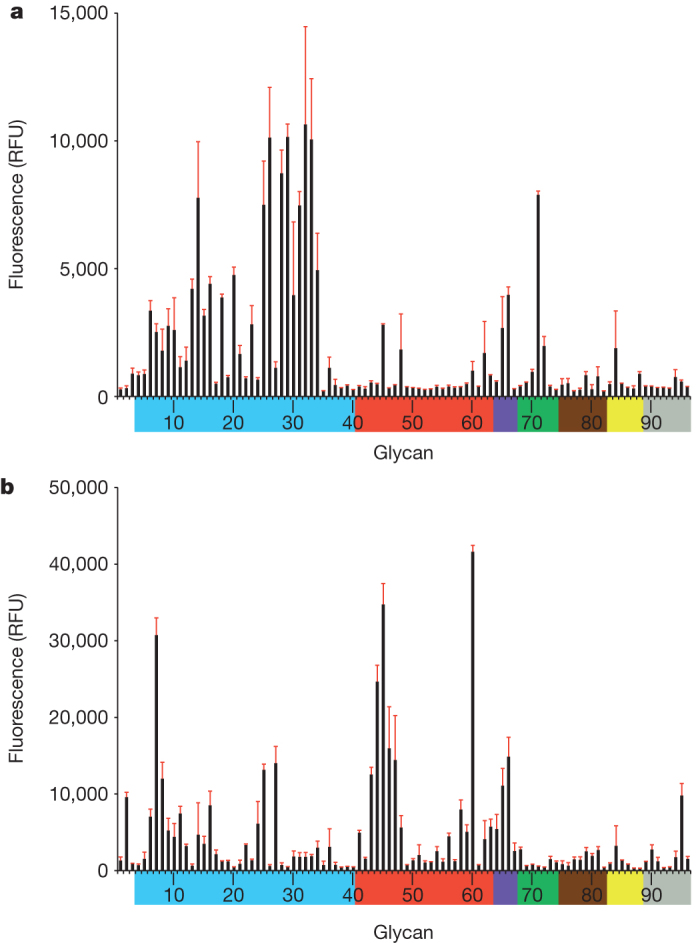
a, b, Glycan microarray analysis of Shanghai/1 (a) and Anhui/1 (b) viruses. Coloured bars highlight glycans that contain α2,3 sialosides (blue), α2,6 sialosides (red), α2,6/α2,3 mixed sialosides (purple), N-glycolyl sialosides (green), α2,8 sialosides (brown), β2,6 and 9-O-acetyl sialosides (yellow), and non-sialoside glycans (grey) and are expressed as relative fluorescence units (RFU). Error bars reflect the s.e.m. in the signal for six independent replicates on the array. Structures of each of the numbered glycans are found in Supplementary Table 1.
Select contemporary North American and Eurasian H7 viruses associated with human infection have previously demonstrated the capacity to transmit between ferrets in a direct-contact model, but not by respiratory droplets in the absence of direct contact11. To characterize the transmissibility of A(H7N9) isolates, we intranasally inoculated ferrets with 106 plaque-forming units (p.f.u.) of virus. Approximately 24 h post-inoculation (p.i.), inoculated–contact pairs were established by placing a naive ferret in the same cage as an inoculated ferret (direct contact transmission), or in an adjacent cage with perforated side-walls (respiratory droplet transmission)12. Nasal washes were collected on alternate days from all animals and titred for the presence of infectious virus12. The A(H7N9) viruses were compared to a seasonal H3N2 virus, A/Texas/50/2012 (Texas/50), representative of H3N2 viruses circulating in the 2012–2013 northern hemisphere season13.
Ferrets inoculated with Anhui/1 or Shanghai/1 virus showed modest weight loss and transient inactivity, but generally did not exhibit signs of severe disease (Table 1 and Supplementary Fig. 1). However, two of eight Shanghai/1 virus-inoculated ferrets were euthanized due to severe lethargy (laboured breathing, tremors, unresponsiveness day 4 p.i.) or excessive weight loss (26.6% drop from initial body weight day 12 p.i.) (Table 1 and Supplementary Fig. 1). A(H7N9) virus was detected at elevated titres in the nasal turbinates and trachea (Supplementary Fig. 2). However, unlike the seasonal H3N2 virus, A(H7N9) virus was detected in the lower respiratory tract (peak mean titre >105 p.f.u. per g lung tissue) (Supplementary Fig. 1)10,14. Infectious virus was detected in the brain and olfactory bulb of 1/3 and 2/3 ferrets inoculated with Anhui/1 and Shanghai/1 viruses, respectively (Supplementary Fig. 2); viraemia or systemic spread of virus to the spleen, kidney or liver was not detected. Similar to the 2009 H1N1 pandemic virus, gastrointestinal symptoms have been noted following A(H7N9) virus infection in humans5; rectal swabs were positive for virus detection in 7/11 and 5/8 Anhui/1 and Shanghai/1 virus-inoculated ferrets, respectively, whereas H3N2 virus was not detected in this sample (Supplementary Fig. 3). Furthermore, intestinal tract samples were positive in 3/3 Shanghai/1 inoculated ferrets day 3 p.i. (Supplementary Fig. 2). In agreement with reports of lymphopenia among A(H7N9) virus-infected patients5, ferrets showed transient lymphopenia that was sustained through day 7 p.i., with greater reductions in circulating lymphocytes in A(H7N9) virus-infected ferrets compared to ferrets infected with H3N2 virus (Supplementary Table 2).
Table 1.
Clinical signs, virus replication, and transmission of H7N9 and H3N2 viruses in ferrets
| Inoculated animals | Direct contact animals | Respiratory droplet contact animals | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Virus | Weight loss† (%) | Respiratory symptoms‡ | Lethargy§ | Lethality‖ | Nasal wash titre¶ | Virus detected | Seroconverted | Virus detected | Seroconverted |
| Anhui/1 | 8.8 | 3/11 | 1.1 (7/11) | 0/11 | 7.1 (1)* | 4/4 | 4/4 | 2/6 | 2/6 |
| Shanghai/1 | 9.2 | 5/8 | 1.3 (7/8) | 2/8 | 6.7 (1)* | 4/4 | 4/4 | 1/3 | 1/3 |
| Texas/50 | 10.1 | 4/6 | 1.0 (2/6) | 0/6 | 5.6 (1) | 3/3 | 3/3 | 3/3 | 3/3 |
†The percentage mean maximum weight loss observed during the first 10 days p.i.
‡Number of ferrets that exhibited sneezing or nasal discharge during the first 10 days p.i.
§Relative inactivity index of ferrets during the first 10 days p.i. (number of ferrets with observed lethargy in parentheses).
‖Number of animals euthanized before the end of the 14 day experimental period because of reaching a clinical end point.
¶Peak mean log10 (p.f.u. ml−1) nasal wash titre (day p.i. in parentheses). *P < 0.05 compared with Texas/50 by one-way ANOVA.
Ferrets inoculated with A(H7N9) virus shed high titres of virus in nasal washes as early as day 1 p.i., significantly higher than Texas/50 virus-infected ferrets at this time point (P < 0.05) (Table 1 and Supplementary Fig. 4). Mean titres in nasal washes were sustained at >105 p.f.u. in Anhui/1 and Shanghai/1 virus-infected ferrets through day 3 p.i. (Supplementary Fig. 4). Efficient direct contact transmission occurred by day 1 post-contact, with viral titres in nasal wash samples reaching comparable titres to inoculated ferrets by day 3 post-contact. Respiratory droplet transmission of Anhui/1 virus was observed in 2/6 ferrets, with detection of virus in contact ferret nasal washes delayed 2–4 days compared to seasonal H3N2 virus; Shanghai/1 virus was detected in 1/3 ferrets in this model (Supplementary Fig. 4). These results are in accord with a recently published study demonstrating only limited transmissibility of A/Shanghai/2/13 virus by the airborne route15. Sequence analysis of virus gene segments obtained from nasal wash samples of A(H7N9) respiratory droplet contact animals (total n = 3) revealed no substantial amino acid changes. No sequence changes were observed from nasal washes obtained from the Shanghai/1 respiratory droplet contact ferret compared to Shanghai/1 sequences submitted to GISAID. Sequencing of nasal wash samples obtained on days 5–7 p.i. from Anhui/1 respiratory droplet contact ferrets revealed only one amino acid substitution in the HA protein at R140M (H3 numbering), not found among Anhui/1 inoculated ferrets. This position is located in the HA 140 loop region of HA1 and is not known to be associated with changes in H7 HA receptor binding or influenza virus transmission. Although A(H7N9) viruses are not readily transmissible by respiratory droplets, the efficient virus transmission observed in the direct-contact model has not been observed with H5N1 viruses12 and may indicate the capacity of an A(H7N9)-like virus to acquire properties that would confer efficient airborne transmission.
Phylogenetic analysis showed that the H7N9 virus is a triple reassortant comprising an HA from an H7N3 virus, the neuraminidase (NA) of an H7N9 virus, and all six internal genes from an H9N2 virus2. To assess the relative contribution of these avian precursors to the pathogenicity of the 2013 H7N9 viruses, we inoculated BALB/c mice intranasally with A/shoveler/Egypt/00215-NAMRU3/07 (shv/Egypt/07)16, an H7N9 virus with high surface glycoprotein amino acid sequence identity to Anhui/1, A/chicken/Vietnam/NCVD-1156/2011 (ck/VN/11), an H9N2 virus possessing internal genes with high genetic similarity to Anhui/1 virus, and the 2013 A(H7N9) viruses to determine virus replication, morbidity (as measured by weight loss), the 50% mouse infectious dose (MID50), and the 50% mouse lethal dose (LD50). MID50 titres were markedly low for all viruses, demonstrating high infectivity of the avian viruses in this model; typically human viruses do not replicate efficiently in mice without prior host adaptation17 (Table 2). Mice inoculated with Anhui/1 or Shanghai/1 virus showed severe morbidity (>20% weight loss) and a LD50 of 103.3–103.4, a value comparable to the select group of HPAI H5N1 and H7N7 viruses which have a high-pathogenicity phenotype in this model18,19. In contrast, the avian precursor viruses shv/Egypt/07 and ck/VN/11 caused moderate morbidity in mice, but did not display a lethal phenotype (Table 2). Collectively, lung titres in Anhui/1 and Shanghai/1 virus-inoculated mice were significantly higher than LPAI precursor viruses examined day 3 p.i. (P < 0.05); by day 6 p.i., all H7N9 viruses were detected at comparable titre, >100-fold higher than the H9N2 virus at this time point (P < 0.05). All viruses examined replicated to low titres in the nose day 3 and 6 p.i. (Supplementary Fig. 5), and systemic spread of virus to the brain was not detected.
Table 2.
Pathogenicity of H7N9 and related viruses in BALB/c mice
| Virus | Subtype | Weight loss† | Lung titre (d 3)‡ | Lung titre (d 6)‡ | MID50§ | LD50§ |
|---|---|---|---|---|---|---|
| Anhui/1 | H7N9 | 22.2 (7) | 7.2 ± 0.6 | 5.5 ± 0.5 | 0.25 | 3.4 |
| Shanghai/1 | H7N9 | 25.6 (4) | 6.6 ± 0.2 | 5.6 ± 0.2 | 0.25 | 3.3 |
| shv/Egypt/07 | H7N9 | 16.5 (3) | 4.4 ± 0.2 | 5.6 ± 0.3 | 2.25 | >6 |
| ck/VN/11 | H9N2 | 18.1 (6) | 4.8 ± 0.2 | 3.1 ± 0.1 | 1.5 | >6 |
†Maximum per cent weight loss (5 mice per group) after infection with 106 p.f.u. of virus (day p.i. in parentheses).
‡Average lung titres of three mice on indicated days p.i., expressed as log10 (p.f.u. ml−1) ± SD.
§MID50 and LD50 are expressed as the log10 p.f.u. ml−1 required to give one MID50 or one LD50.
Unlike past human infections with H7 viruses, conjunctivitis has not been reported among A(H7N9)-infected individuals5. Viral titres in ferret eye or conjunctiva samples were not frequently detected in H7N9 or H3N2 virus-infected animals, although conjunctival washes from 50% of Anhui/1 virus-infected ferrets were positive for virus (Supplementary Figs 2 and 3). Ocular inoculation of mice with shv/Egypt, Anhui/1 or Shanghai/1 H7N9 viruses did not result in consistent virus replication in eye tissue (Supplementary Table 3). However, viral titres in the nose were detected days 3 and 6 p.i. following ocular inoculation with Anhui/1 and Shanghai/1 virus in mice with greater frequency compared to shv/Egypt/07 virus (Supplementary Table 3). These studies provide evidence that the 2013 A(H7N9) virus does not maintain the ocular tropism typical of H7 viruses, but is capable of using the eye as an entry portal to cause a productive respiratory infection.
The ability of Anhui/1 virus to form plaques in the presence or absence of the trypsin protease was assayed in MDCK cells by standard plaque methods20. Proteolytic cleavage of the HA molecule is a prerequisite for multi-cycle replication, and the ability of virus to replicate in the absence of trypsin is thought to be an important determinant of influenza virus pathogenicity in mammals21. Similar to the seasonal Texas/50 virus which requires an exogenous protease source for multi-cycle replication and plaque formation, the A(H7N9) virus failed to form visible plaques without the addition of trypsin (data not shown).
Because the airway epithelium is the primary site of influenza virus replication in humans, we investigated the A(H7N9) virus replication efficiency in Calu-3 cells, derived from human bronchial epithelium and grown on permeable cell culture membranes to resemble the in vivo airway epithelium20 (Fig. 2a). For all viruses tested, titres of progeny virus progressively increased during the first 48 h p.i. Anhui/1 virus showed a 20- to 400-fold increase in replication at 48 h p.i. when compared to the other virus subtypes tested in Calu-3 cells (P < 0.04). Notably, compared to the seasonal H3N2 virus, Anhui/1 virus showed an 80,000-fold increase in replication at 24 h p.i.
Figure 2. Replication kinetics of A(H7N9) influenza viruses in polarized human airway epithelial cells.
a, Replication at 37 °C; b, viral titre at 24 h post-infection. Calu-3 cells cultured for 1 week were inoculated with virus at an MOI of 0.01. Culture supernatants were collected at the times indicated and virus titres were determined by plaque assay. Titre values represent the average for three independent wells plus s.d. *indicates statistically significant difference between 37 °C and 33 °C for each virus, based on the Mann–Whitney test (P < 0.004).
As previous studies have suggested an association between the transmissibility of influenza viruses in ferrets and their ability to replicate efficiently at the lower temperature (33 °C), found in the environment of the mammalian upper airway22, we evaluated the replication kinetics of A(H7N9) virus in Calu-3 cells cultured at either 33 °C or 37 °C. Whereas the seasonal Texas/50 virus replicated equally well at both temperatures, A(H7N9) viruses showed significantly reduced replication at 33 °C compared with 37 °C at 24 h, characteristic of avian subtype viruses (Fig. 2b)21; comparable titres at either temperature were detected by 48 h for all viruses (data not shown). These data demonstrate that the A(H7N9) viruses replicate productively in polarized bronchial epithelial cells at 37 °C, but less efficiently at the lower temperature of the human proximal airways early after infection.
The recent cases of human infection with H7N9 viruses highlight the need to better understand the potential of these viruses to spread and cause disease in humans. Although the novel H7N9 influenza virus has caused severe illness and death among individuals in China, the initial epidemiologic findings suggest that there is no evidence of sustained human-to-human transmission of this virus. Our ferret transmission results are consistent with this observation and suggest that additional virus adaptation in mammals would be required to reach the high-transmissible phenotypes observed by the respiratory droplet route with pandemic and seasonal influenza A viruses10.
Methods Summary
Viruses and cells
H7N9 and H9N2 viruses were passaged in eggs; H1N1 virus was propagated in MDCK cells. Detailed culture conditions for Calu-3 cells are included in Methods. Virus titrations were performed in MDCK cells by standard plaque assay20.
Biosafety and biosecurity
Experiments were conducted in biosafety level 3 facilities with enhancements (BSL3+) in accordance with U.S. Federal and WHO guidelines. Features of the BSL3+ containment laboratories include a double door entrance, double door autoclave, HEPA filtration of intake and exhaust air, shower out room, and a liquid effluent sterilizer. Security is monitored through an automated access control system and entrance to the laboratory is restricted by guard stations, cameras, card-controlled doors and biometric readers. Use of powered air-purifying respirators in the laboratory and yearly training requirements are mandatory for all staff with access.
Glycan arrays
Microarray printing and influenza virus analyses were performed as previously described11; specific details on the glycans analysed are provided in Methods.
Animals
Six-to-eight week old female BALB/c mice (Jackson Laboratory) and six-to-seven month old influenza virus-seronegative male ferrets (Triple F Farms) were used in all experiments. All intranasally inoculated animals were administered 106 p.f.u. of virus except for determination of mouse infectivity and lethality, where mice were administered serial 10-fold dilutions of virus. Virus transmission in ferrets was measured by co-housing an inoculated and naive ferret (direct contact transmission) or by placing an inoculated and naive ferret in adjacent cages with a perforated side wall (respiratory droplet transmission)12. Transmission was determined by presence of virus in nasal washes and seroconversion of contact ferrets. Detailed procedures are included in Methods for pathotyping, transmission and tropism studies.
Online Methods
Viruses
All animal experiments were conducted under biosafety level 3 facilities with enhancements (BSL3+) in accordance with US Federal and World Health Organization guidelines. Biocontainment for the BSL3+ laboratories is based on the 5th Edition of Biosafety in Microbiological and Biomedical Laboratories. A/Anhui/1/13 (H7N9), A/Shanghai/1/13 (H7N9), A/shoveler/Egypt/00215-NAMRU3/07 (H7N9), A/chicken/Vietnam/NCVD-1156/2011 (H9N2) and A/Goose/Nebraska/17097-4/11 (H7N9) viruses were propagated in 10-day-old embryonated hens’ eggs for 42 h at 37 °C as previously described18,23. A/Texas/50/12 was propagated in Madin Darby canine kidney (MDCK) cells as previously described24.
Ferret experiments
All animal research was approved by the Centers for Disease Control and Prevention’s Institutional Animal Care and Use Committee and conducted in an Association for Assessment and Accreditation of Laboratory Animal Care International-accredited animal facility. Male Fitch ferrets (Triple F Farms), 6 to 7 months of age and serologically negative by haemagglutination inhibition (HI) assay for currently circulating influenza viruses, were used in all experiments. A minimum number of animals were used to achieve reproducible results in each experiment per ethical guidelines; investigators were not blinded to groups. Ferrets were housed in cages within a Duo-Flow Bioclean mobile clean room (Lab Products) and randomly assigned to experimental groups. Temperatures were measured using a subcutaneous implantable temperature transponder (BioMedic Data Systems). Ferrets were inoculated intranasally with 106 p.f.u. of virus in a 1 ml volume diluted in PBS, then monitored daily for changes in body temperature, weight and clinical signs of infection. Lethargy was measured as described previously14. Any ferret that lost >25% of its pre-inoculation body weight or showed neurological dysfunction was euthanized and submitted to post-mortem examination. Nasal washes, conjunctival washes and rectal swabs were collected on alternate days post-inoculation (p.i.) or post-contact, immediately frozen on dry ice and stored at −70 °C until titration as previously described25. Tissue specimens collected for virus titration were immediately frozen on dry ice and stored at −70 °C until titration as previously described18. Blood was collected from inoculated ferrets on indicated days p.i. in EDTA Vacutainer tubes (BD) and complete blood counts were quantified using a Hemavet HV950FS instrument per the manufacturer’s instructions (Drew Scientific).
For transmission experiments, ferrets were inoculated as described above. Approximately 24 h after inoculation, a naive ferret was placed in the same cage as an inoculated animal (for assessment of virus transmission in the presence of direct contact) or in an adjacent cage with perforated side-walls (holes 1–5 mm in diameter, with cages spaced ∼3 mm apart) to allow for air exchange between cages in the absence of direct or indirect contact (for assessment of virus transmission by respiratory droplets). The term ‘respiratory droplet transmission’ refers to virus transmission in the absence of direct or indirect contact and does not imply an understanding of the size of droplets or aerosols involved in the transmission events. To prevent inadvertent transmission in direct contact or respiratory droplet experiments, investigators always handled the contact animals first, and decontaminated all surfaces which came into contact with each ferret before handling the next animal.
Whole-genome sequencing of nasal washes
RNA was extracted from nasal wash samples from the peak day of virus shedding presenting in the inoculated and respiratory droplet contact (2/6 for Anhui/1 ferrets, and 1/3 for Shanghai/1 virus experiment) ferrets and subjected to RT–PCR (PCR with reverse transcription) followed by DNA sequencing analysis. Obtained sequences obtained from all virus gene segments were compared with relevant virus sequences available from The Global Initiative on Sharing All Influenza Data (GISAID) database.
Mouse experiments
Female BALB/c mice 6 to 9 weeks old were anaesthetized with an intraperitoneal injection of 0.15 ml of 2,2,2-tribromoethanol in tert-amyl alcohol (Avertin; Sigma-Aldrich) prior to virus inoculation. Intranasal inoculations were performed by instilling 50 μl of virus diluted in PBS onto the nares of the animals. The 50% mouse infectious dose (MID50, determined by virus detection in the lungs day 3 p.i) and 50% lethal dose (LD50) were determined by inoculating groups of eight mice with serial 10-fold dilutions of virus. Three mice per dilution were euthanized on day 3 p.i.; lungs were collected from each mouse, immediately frozen on dry ice, and stored at −70 °C. Tissues were thawed, homogenized in 1 ml cold PBS, and clarified by centrifugation prior to titration by standard plaque assay. Five mice per dilution were observed daily for 14 days for morbidity (as measured by weight loss) and mortality; any mouse which lost >25% of its pre-inoculation weight was euthanized. MID50 and LD50 values were determined following ref. 26. To investigate the ability of each virus to spread systemically in mice, an additional three mice were euthanized at days 3 and 6 p.i. from mice inoculated with 106 p.f.u. of each virus for collection of nose, lung and brain tissue, which were stored and processed as described above.
Ocular inoculations were performed by lightly scarifying the right eye of the mouse with a corneal trephine and instilling 5 μl of virus (106 p.f.u.) onto the surface of the eye and massaging the virus into the eye with the eyelid as previously described27. An additional three mice were inoculated and euthanized on days 3 and 6 p.i. for collection of eye, nose and lung tissue, which were stored and processed as described above.
Cell culture and viral replication
The human bronchial epithelial cell line Calu-3 (ATCC) was grown on membrane inserts as previously described20. Cells were grown to confluence in six-well plates for 1 week until the confluent monolayer reached a stable transepithelial resistance of >1,000 Ω cm2. Virus was added to cells apically in serum-free medium at a multiplicity of infection (MOI) of 0.01 for one hour before washing. Plates were incubated at either 37 °C or 33 °C for the duration of the experiment as indicated. Aliquots of culture supernatant taken p.i. were immediately frozen at −80 °C until titration. All samples collected for replication kinetics were titrated for the presence of infectious virus by standard plaque assay in MDCK cells.
Serology
HI assays were performed with ferret sera collected 16–20 days after inoculation or exposure to infected animals using 0.5% turkey erythrocytes against homologous virus28.
Cytokine and chemokine quantification
Clarified mouse lung tissue homogenates were analysed with the BioPlex protein array system (Bio-Rad), according to manufacturer’s instructions. A minimum of three independent samples were collected and tested in duplicate for each condition.
Glycan microarray analysis
Microarray printing and influenza virus analyses have been described previously11. A/Anhui/1/2013 virus was analysed at a HA titre of 512 while A/Shanghai/1/2013 was analysed at an HA titre of 256. Specific details on the glycans analysed in these studies are provided in Supplementary Table 1.
Statistics
Statistical significance (P < 0.05) for murine and ferret studies was determined by one-way analysis of variance (ANOVA) with a Bonferroni post-test. Statistical significance for in vitro studies was determined using the Mann–Whitney and Student’s t tests. All in vitro studies were conducted with sufficient sample size for statistical analyses performed; indicated tests were chosen as appropriate for each data set to be analysed and all data meet the assumptions of the tests.
Supplementary information
This file contains Supplementary Tables 1-3 and Supplementary Figures 1-6. (PDF 995 kb)
Acknowledgements
We thank the China CDC as part of the WHO Global Influenza Surveillance and Response System (GISRS) for facilitating access to viruses and A. Balish for preparation of viruses. The findings and conclusions in this report are those of the authors and do not necessarily reflect the views of the funding agency. Glycan microarray slides were produced under contract for the CDC using a glycan library generously provided by the Consortium for Functional Glycomics, funded by National Institute of General Medical Sciences Grant GM62116.
PowerPoint slides
Author Contributions
J.A.B., K.M.G., T.R.M., H.Z., X.S., P.J.C., J.S., J.M.K., and T.M.T. designed the experiments; J.A.B., K.M.G., M.B.P., T.R.M., H.Z., C.P., X.S., P.J.C., J.S., and T.M.T. performed the experiments; J.A.B., K.M.G., T.R.M., H.Z., C.P., X.S., P.J.C., J.M.V., J.S., J.M.K., and T.M.T. analysed data; J.A.B., T.R.M., H.Z., C.P., P.J.C., J.S., and J.M.K., T.M.T. wrote the manuscript.
Accession codes
Data deposits
GISAID (http://platform.gisaid.org/) accession numbers are as follows for ck/VN/11 (PB2, EPI457480; PB1, EPI457481; PA, EPI457479; HA, EPI457483; NP, EPI457476; NA, EPI457482; MP, EPI457478; NS, EPI457477) and shv/Egypt/07 (PB2, EPI372407; PB1, EPI372408; PA, EPI372406; HA, EPI372410; NP, EPI372403; NA, EPI372409; MP, EPI372405; NS, EPI372404).
Competing interests
The authors declare no competing financial interests.
References
- 1.WHO. Avian influenza A(H7N9) virushttp://www.who.int/influenza/human_animal_interface/influenza_h7n9/en/index.html (2013)
- 2.Gao R, et al. Human infection with a novel avian-origin influenza A (H7N9) virus. N. Engl. J. Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- 3.Belser JA, Bridges CB, Katz JM, Tumpey TM. Past, present, and possible future human infection with influenza virus A subtype H7. Emerg. Infect. Dis. 2009;15:859–865. doi: 10.3201/eid1506.090072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.CDC. Emergence of avian influenza A(H7N9) virus causing severe human illness - China, February-April 2013. MMWR Morb. Mortal. Wkly. Rep.62, 1–4 (2013) [PMC free article] [PubMed]
- 5.Chen Y, et al. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet. 2013;381:1916–1925. doi: 10.1016/S0140-6736(13)60903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li, Q. et al. Preliminary report: epidemiology of the avian influenza A (H7N9) outbreak in China. N. Engl. J. Med.10.1056/NEJMoa1304617 (2013)
- 7.Rogers GN, et al. Single amino acid substitutions in influenza haemagglutinin change receptor binding specificity. Nature. 1983;304:76–78. doi: 10.1038/304076a0. [DOI] [PubMed] [Google Scholar]
- 8.Chandrasekaran A, et al. Glycan topology determines human adaptation of avian H5N1 virus hemagglutinin. Nature Biotechnol. 2008;26:107–113. doi: 10.1038/nbt1375. [DOI] [PubMed] [Google Scholar]
- 9.Tharakaraman K, et al. Glycan receptor binding of the influenza A virus H7N9 hemagglutinin. Cell. 2013;153:1486–1493. doi: 10.1016/j.cell.2013.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maines TR, et al. Transmission and pathogenesis of swine-origin 2009 A(H1N1) influenza viruses in ferrets and mice. Science. 2009;325:484–487. doi: 10.1126/science.1177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belser JA, et al. Contemporary North American influenza H7 viruses possess human receptor specificity: Implications for virus transmissibility. Proc. Natl Acad. Sci. USA. 2008;105:7558–7563. doi: 10.1073/pnas.0801259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maines TR, et al. Lack of transmission of H5N1 avian-human reassortant influenza viruses in a ferret model. Proc. Natl Acad. Sci. USA. 2006;103:12121–12126. doi: 10.1073/pnas.0605134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Recommended composition of influenza virus vaccines for use in the 2013-2014 northern hemisphere influenza seasonhttp://www.who.int/influenza/vaccines/virus/recommendations/201302_recommendation.pdf (2013)
- 14.Zitzow LA, et al. Pathogenesis of avian influenza A (H5N1) viruses in ferrets. J. Virol. 2002;76:4420–4429. doi: 10.1128/JVI.76.9.4420-4429.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhu, H. et al. Infectivity, transmission, and pathology of human H7N9 influenza in ferrets and pigs. Science10.1126/science.1239844 (2013) [DOI] [PubMed]
- 16.Gerloff, N. A. et al. A high diversity of Eurasian lineage low pathogenicity avian influenza A viruses circulate among wild birds sampled in Egypt. PLoS ONE (in the press) [DOI] [PMC free article] [PubMed]
- 17.Hartley CA, Reading PC, Ward AC, Anders EM. Changes in the hemagglutinin molecule of influenza type A (H3N2) virus associated with increased virulence for mice. Arch. Virol. 1997;142:75–88. doi: 10.1007/s007050050060. [DOI] [PubMed] [Google Scholar]
- 18.Maines TR, et al. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J. Virol. 2005;79:11788–11800. doi: 10.1128/JVI.79.18.11788-11800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Wit E, et al. Protection of mice against lethal infection with highly pathogenic H7N7 influenza A virus by using a recombinant low-pathogenicity vaccine strain. J. Virol. 2005;79:12401–12407. doi: 10.1128/JVI.79.19.12401-12407.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zeng H, et al. Highly pathogenic avian influenza H5N1 viruses elicit an attenuated type I interferon response in polarized human bronchial epithelial cells. J. Virol. 2007;81:12439–12449. doi: 10.1128/JVI.01134-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeng H, et al. Tropism and infectivity of influenza virus, including highly pathogenic avian H5N1 virus, in ferret tracheal differentiated primary epithelial cell cultures. J. Virol. 2013;87:2597–2607. doi: 10.1128/JVI.02885-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Van Hoeven N, et al. Pathogenesis of 1918 pandemic and H5N1 influenza virus infections in a guinea pig model: antiviral potential of exogenous alpha interferon to reduce virus shedding. J. Virol. 2009;83:2851–2861. doi: 10.1128/JVI.02174-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belser JA, et al. Pathogenesis, transmissibility, and ocular tropism of a highly pathogenic avian influenza A (H7N3) virus associated with human conjunctivitis. J. Virol. 2013;87:5746–5754. doi: 10.1128/JVI.00154-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tumpey TM, et al. Characterization of the reconstructed 1918 Spanish influenza pandemic virus. Science. 2005;310:77–80. doi: 10.1126/science.1119392. [DOI] [PubMed] [Google Scholar]
- 25.Belser JA, et al. Influenza virus respiratory infection and transmission following ocular inoculation in ferrets. PLoS Pathog. 2012;8:e1002569. doi: 10.1371/journal.ppat.1002569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed LJ, Muench H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938;27:493–497. [Google Scholar]
- 27.Belser JA, Wadford DA, Xu J, Katz JM, Tumpey TM. Ocular infection of mice with influenza A (H7) viruses: a site of primary replication and spread to the respiratory tract. J. Virol. 2009;83:7075–7084. doi: 10.1128/JVI.00535-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stephenson I, Wood JM, Nicholson KG, Charlett A, Zambon MC. Detection of anti-H5 responses in human sera by HI using horse erythrocytes following MF59-adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res. 2004;103:91–95. doi: 10.1016/j.virusres.2004.02.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains Supplementary Tables 1-3 and Supplementary Figures 1-6. (PDF 995 kb)
Data Availability Statement
Data deposits
GISAID (http://platform.gisaid.org/) accession numbers are as follows for ck/VN/11 (PB2, EPI457480; PB1, EPI457481; PA, EPI457479; HA, EPI457483; NP, EPI457476; NA, EPI457482; MP, EPI457478; NS, EPI457477) and shv/Egypt/07 (PB2, EPI372407; PB1, EPI372408; PA, EPI372406; HA, EPI372410; NP, EPI372403; NA, EPI372409; MP, EPI372405; NS, EPI372404).


