Abstract
Purpose
To determine whether macrolide-based treatment is associated with mortality in critically ill H1N1 patients with primary viral pneumonia.
Methods
Secondary analysis of a prospective, observational, multicenter study conducted across 148 Intensive Care Units (ICU) in Spain.
Results
Primary viral pneumonia was present in 733 ICU patients with pandemic influenza A (H1N1) virus infection with severe respiratory failure. Macrolide-based treatment was administered to 190 (25.9 %) patients. Patients who received macrolides had chronic obstructive pulmonary disease more often, lower severity on admission (APACHE II score on ICU admission (13.1 ± 6.8 vs. 14.4 ± 7.4 points, p < 0.05), and multiple organ dysfunction syndrome less often (23.4 vs. 30.1 %, p < 0.05). Length of ICU stay in survivors was not significantly different in patients who received macrolides compared to patients who did not (10 (IQR 4–20) vs. 10 (IQR 5–20), p = 0.9). ICU mortality was 24.1 % (n = 177). Patients with macrolide-based treatment had lower ICU mortality in the univariate analysis (19.2 vs. 28.1 %, p = 0.02); however, a propensity score analysis showed no effect of macrolide-based treatment on ICU mortality (OR = 0.87; 95 % CI 0.55–1.37, p = 0.5). Moreover, the sensitivity analysis revealed very similar results (OR = 0.91; 95 % CI 0.58–1.44, p = 0.7). A separate analysis of patients under mechanical ventilation yielded similar results (OR = 0.77; 95 % CI 0.44–1.35, p = 0.4).
Conclusion
Our results suggest that macrolide-based treatment was not associated with improved survival in critically ill H1N1 patients with primary viral pneumonia.
Keywords: Community-acquired infection, Antimicrobial agents, Viral infections, Mechanical ventilation: clinical studies
Introduction
Pandemic (H1N1)v influenza A infection often presents with severe acute respiratory symptoms in hospitalized patients [1]. What made this infection different from the normal seasonal varieties of influenza was the high fatality rates despite aggressive therapy. The principal clinical syndrome leading to hospitalization and intensive care was diffuse viral pneumonitis associated with severe hypoxemia, acute respiratory distress syndrome (ARDS), and sometimes shock and renal failure [2–5].
As early as June 2009, Perez-Padilla et al. [11] reported 38 % mortality in Mexico, and high rates were also reported in Spain (25 %), Canada (17.3 %), and Australia and New Zealand (14.3 %) [6–8]. Several recommendations have been based on the literature published during this time, mainly related to the identification of risk factors such as community-acquired respiratory co-infection (CARC) [9] and/or obesity [10].
There is often an abnormal inflammatory pathway that can cause irreversible damage to the lung tissue. No survival benefit has been found for systemic corticosteroids [11, 12], and early oseltamivir administration [13, 14] seems the only feasible option. Immune modulation concomitant to antibiotic therapy improves outcome in bacterial community-acquired pneumonia (CAP) [15]. The impact of potential adjuncts to antimicrobials on inflammation involves modulation of leukocyte biology, altered antigen-presenting cell function, and changes in epithelial and endothelial cells [16].
The main objective of this study was to determine whether a macrolide-based regimen was associated with mortality in critically ill H1N1 patients with primary viral pneumonia.
Materials and methods
We conducted a secondary analysis of a prospective, observational cohort study of patients in 148 intensive care units (ICU) in Spain. Data were obtained from a voluntary registry created by the Spanish Society of Intensive Care Medicine (SEMICYUC), the Spanish Network for Research on Infectious Disease (REIPI), and the Spanish Biomedical Research Network on Respiratory Diseases (CIBERES). The Joan XXIII University Hospital Ethics Committee approved the study (IRB NEUMAGRIP/11809) and waived the requirement for informed consent because patient anonymity was guaranteed and the study was observational and formed part of an emergency public health response [6].
Data were reported by the attending physician after reviewing medical charts and radiological and laboratory records. We analyzed data from all consecutive patients within the cohort diagnosed with pandemic (H1N1)v influenza A infection in two periods: the 2009 pandemic (H1N1)v infection period between epidemiological weeks 23–52 of 2009 and the post-pandemic influenza (H1N1)v infection period between epidemiological weeks 50–52 of 2010 and weeks 1–9 of 2011. Children under 15 years old were not included in the registry. A/H1N1 infection was confirmed by real-time reverse-transcription-polymerase chain reaction (RT-PCR) on either nasopharyngeal swab samples or tracheal secretions ordered by the attending physicians on admission to the ICU. RT-PCR was performed in each institution or in a centralized reference laboratory when local resources were not available. RT-PCR methods and further details are described elsewhere [6]. A confirmed case was defined as acute respiratory illness with laboratory-confirmed A/H1N1. Only confirmed cases were included in the current report.
The ICU admission criteria and treatment decisions for all patients, including determination of the need for intubation and type of antibiotic or antiviral therapy administered, were made at the discretion of the attending physician and not standardized. Septic shock and multiple organ dysfunction score (MODS) were defined following the criteria of the American College of Chest Physicians and the Society of Critical Care Medicine [17].
Systemic corticosteroids were administered when patients developed shock (hydrocortisone) or as adjuvant treatment for pneumonia (methylprednisolone). Attending physicians decided whether to administer oral oseltamivir (150 or 300 mg/24 h) or intravenous zanamivir (600 mg/12 h).
Primary viral pneumonia was defined as unequivocal alveolar opacities involving two or more lobes in patients presenting acute respiratory distress and negative respiratory and blood bacterial cultures during the acute phase of influenza virus infection. Immunosuppression was defined as any primary immunodeficiency or immunodeficiency secondary to HIV infection (the CD4 count was not recorded in HIV+ patients), active malignancies, radiation treatment, cytotoxic drugs, steroid drugs (daily doses > 40 mg of prednisolone or the equivalent for >2 weeks), immunological disease, or solid organ transplant. Hematological disease was defined as acute lymphoblastic leukemia, acute myeloblastic leukemia, chronic lymphocytic leukemia, chronic myelogenous leukemia, Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, myeloma, graft-versus-host disease, or post-bone marrow transplantation. Obese patients were defined as those with a body mass index (BMI) > 30 kg/m2 [10]. Smoking history was not recorded. The diagnosis and management of all patients were based on standardized guidelines [18, 19] and specific protocols published by the Spanish Ministry of Health [20]. Blood samples for cultures and serologic studies were routinely collected at ICU admission. Bronchoalveolar lavage was not performed because of the high risk of generating aerosols. Pleural effusion cultures were obtained in patients with documented pleural effusion. Bacterial identification and susceptibility testing were performed by standard methods based on local guidelines. An organism was considered the definitive etiologic agent [21] only if it could be isolated from blood or pleural fluid. Other microorganisms isolated from quantitative endotracheal aspirate (ETA) were considered “probable” pathogens [21]. Acute kidney injury and its stages were diagnosed according to the glomerular filtration rate (GFR) criteria of the current Acute Kidney Injury Network definitions [22]. Continuous renal replacement therapy (CRRT) in the course of acute kidney injury was initiated when indicated for pulmonary edema, oliguria (defined as urine output <0.5 mL/kg body weight per hour for >6 h), metabolic acidosis or hyperkalemia not responding to conventional treatment, or uremia (defined as urea nitrogen >100 mg/dL). CRRT was available 24 h a day, and no patient requiring CRRT was denied treatment due to likely futility.
Statistical analysis
Discrete variables were expressed as counts (percentage) and continuous variables as mean ± standard deviation (SD) or medians with 25th to 75th interquartile range (IQR). For the demographic and clinical characteristics of the patients, differences between groups were assessed using the chi-square test or Fisher’s exact test for categorical variables and Student’s t test or the Mann–Whitney U test for continuous variables when appropriate. Multivariable stepwise logistic regression analysis was used to assess the impact of explanatory variables on outcome (ICU mortality). To avoid spurious associations, only variables with a relationship in univariate analysis (p ≤ 0.1) or a potential plausible relationship with outcome were entered in the logistic regression models. In addition, the effectiveness of the macrolides on ICU mortality was further estimated using propensity scores. Propensity score analysis aims to balance observed covariates between treated and untreated patients from the study to mimic what happens in a randomized study [23], thus creating a quasi-randomized experiment from a non-randomized observational study. In our study, propensity scores were estimated by fitting a logistic regression. The covariates included in the propensity score model were those with a potential impact on outcome measured previous to macrolide treatment: age, APACHE II score, gender, chronic obstructive pulmonary disease (COPD), asthma, cardiac heart failure, chronic renal failure, hematological disease, pregnancy, obesity, diabetes, immunosuppression, and empiric oseltamivir treatment. Propensity score quintiles were derived, and boxplots of the estimated propensity scores for treated and untreated patients within each quintile of the propensity scores were plotted to assess the validity of the analysis. Finally, we fitted a logistic model for outcome including as covariates the propensity score quintiles and treatment. Results are presented as odds ratios (OR) and 95 % confidence intervals (CI). Additionally, a sensitivity analysis was conducted including the post-treatment variables in the propensity score analysis. These variables were invasive mechanical ventilation (MV), septic shock, multiorgan dysfunction/failure, corticosteroid use, and CRRT. For all analyses, p values <0.05 were considered significant. We used SPSS for Windows 20.0 (IBM SPSS Statistics, Chicago, IL, US) for all analyses.
Results
Primary viral pneumonia was present in 733 patients (440 (60.3 %) male) with RT-PCR-confirmed pandemic influenza A (H1N1) virus infection and severe respiratory failure in ICUs in 148 hospitals in Spain. Patients’ median age was 46 (IQR 35–55) years, and 669 (91.3 %) patients were aged below 65 years. The mean APACHE II score was 14.3 ± 7.3. A total of 509 (69.9 %) patients had comorbidities, including obesity [n = 279 (38.3 %)], COPD [n = 95 (13.0 %)], and diabetes mellitus [n = 88 (12.1 %)].
Macrolides were administered in 190 (25.9 %) patients; in 188 (98.9 %) of these, macrolides were administered in combination therapy. Clarithromycin was administered in 99 patients (52.1 %), azithromycin in 90 (47.4 %), and erythromycin in 1 patient (0.5 %). In the 543 patients who did not receive macrolide-based regimens, 451 patients (83.1 %) received double combination therapy and 57 (10.5 %) received triple combination therapy. In the patients for whom data was available (n = 697), the duration of antibiotic therapy did not differ significantly between patients administered macrolide-based regimens and those administered antibiotics without macrolides (9.4 ± 4.1 vs. 9.9 ± 4.1 days, p = 0.1). Patients who received macrolides had lower APACHE II scores on ICU admission (13.1 ± 6.8 vs. 14.4 ± 7.4 points, p < 0.05). Macrolides were administered more often in pregnant women (p = 0.0001) and COPD patients (p = 0.03). The mean interval between symptom onset and antibiotic administration was similar between patients who received macrolides and those who did not (5.1 ± 2.3 vs. 5.3 ± 3.3 days, p = 0.5). Table 1 shows the demographic data and clinical characteristics of patients with pandemic (H1N1)v influenza A infection treated with macrolide-based regimens versus those treated without macrolide-based regimens. MODS developed less frequently in patients who received macrolide-based treatment than in those who did not (23.4 vs. 30.1 %, p < 0.05) (Table 2). Length of ICU stay in survivors did not differ between patients who received macrolide-based regimens and those who did not (10 (IQR 4–20) vs. 10 (IQR 5–20), p = 0.9).
Table 1.
Comparison of demographic and clinical characteristics between patients with pandemic 2009 influenza A (H1N1) virus infection who received macrolides and those who received no macrolides
| Variables | No macrolide-based treatment N = 543 |
Macrolide-based treatment N = 190 |
p |
|---|---|---|---|
|
Age, years Mean (SD) |
46 ± 13.9 | 44 ± 14.0 | 0.2 |
|
Male sex n (%) |
331 (61.3) | 109 (57.4) | 0.3 |
|
APACHE II score Mean (SD) |
14.4 ± 7.4 | 13.1 ± 6.8 | 0.04 |
|
COPD n (%) |
62 (11.5) | 33 (17.5) | 0.03 |
|
Asthma n (%) |
41 (7.6) | 12 (6.3) | 0.5 |
|
Chronic heart failure n (%) |
31 (5.8) | 8 (4.2) | 0.4 |
|
Chronic renal disease n (%) |
30 (5.6) | 6 (3.2) | 0.1 |
|
Hematological disease n (%) |
58 (10.8) | 8 (4.2) | 0.007 |
|
Pregnancy n (%) |
20 (3.7) | 20 (10.6) | 0.0001 |
|
Obesity n (%) |
205 (38.0) | 74 (39.2) | 0.7 |
|
Diabetes n (%) |
64 (11.9) | 24 (12.7) | 0.7 |
|
Autoimmune disease n (%) |
24 (4.5) | 6 (3.2) | 0.5 |
|
Immunosuppression n (%) |
61 (11.1) | 58 (33) | 0.0001 |
APACHE acute physiology and chronic health evaluation, COPD chronic obstructive pulmonary disease
Table 2.
Comparison of clinical characteristics between patients with primary viral pneumonia caused by pandemic 2009 influenza A (H1N1) virus infection who received macrolides and those who received no macrolides
| Variables | No macrolide-based treatment N = 543 |
Macrolide-based treatment N = 190 |
p |
|---|---|---|---|
|
Invasive mechanical ventilation n (%) |
357 (66.7) | 118 (62.1) | 0.2 |
|
Septic shock n (%) |
268 (50.5) | 82 (43.4) | 0.09 |
|
MODS n (%) |
341 (63.4) | 104 (55.0) | 0.04 |
|
CRRT n (%) |
58 (10.8) | 14 (7.4) | 0.2 |
|
Empirical oseltamivir n (%) |
555 (99.6) | 175 (97.7) | 0.01 |
|
Corticosteroids n (%) |
216 (39.2) | 91 (53.2) | 0.001 |
MODS multiple organ dysfunction syndrome, CRRT continuous renal replacement therapy
The overall ICU mortality was 24.1 % (n = 177). The use of macrolides was associated with lower ICU mortality in the univariate analysis (19.2 vs. 28.1 %, p = 0.02). Table 3 shows details of risk factors for ICU mortality. Briefly, chronic renal disease, hematological disease, and immunosuppression were comorbid conditions significantly associated with higher ICU mortality; in addition, MV, septic shock, MODS, corticosteroids, and CRRT were more frequent in patients who died. A logistic regression analysis adjusted for severity (APACHE) and potential confounding factors (COPD, congestive heart failure, pregnancy, chronic renal failure, morbid obesity, immunosuppression and hematological disease) found the use of macrolides was not significantly associated with lower ICU mortality rate (OR = 0.89; 95 % CI 0.53–1.49, p = 0.6).When the analysis was done on the subgroup of patients receiving MV, similar results were obtained (OR = 0.77; 95 % CI 0.44–1.35, p = 0.4). The propensity score analysis found no effect of macrolide treatment on outcome (OR = 0.87; 95 % CI 0.55–1.37, p = 0.5), and the sensitivity analysis revealed a very similar result (OR = 0.91; 95 % CI 0.58–1.44, p = 0.7). Moreover, the overlapping of the propensity scores for treated and untreated patients within each propensity score quintile reinforced the validity of the propensity score analysis (Fig. 1). Finally, the length of the ICU stay in survivors was similar in patients who received macrolides and in those who did not (10 (IQR, 4–20) vs. 10(IQR 5–20), p = 0.6).
Table 3.
Risk factors for ICU mortality among patients with primary viral pneumonia caused by pandemic 2009 influenza A (H1N1) virus infection
| Variables | Alive N = 564 |
Dead N = 181 |
p |
|---|---|---|---|
|
Age, years Mean (SD) |
46.6 ± 14.2 | 48.9 ± 14.9 | 0.01 |
|
Male sex n (%) |
323 (58.4) | 117 (66.1) | 0.04 |
|
APACHE II score Mean (SD) |
12.4 ± 6.0 | 19.5 ± 8.6 | 0.0001 |
|
COPD n (%) |
66 (12.0) | 29 (16.5) | 0.1 |
|
Asthma n (%) |
42 (7.6) | 11 (6.3) | 0.4 |
|
Chronic heart failure n (%) |
25 (4.5) | 14 (8.0) | 0.08 |
|
Chronic renal disease n (%) |
19 (3.4) | 17 (9.7) | 0.002 |
|
Hematological disease n (%) |
29 (5.3) | 37 (21.0) | 0.0001 |
|
Immunosuppression n (%) |
29 (5.3) | 37 (21.0) | 0.0001 |
|
Pregnancy n (%) |
32 (5.8) | 8 (4.5) | 0.5 |
|
Obesity n (%) |
212 (38.4) | 67 (37.1) | 0.9 |
|
Diabetes n (%) |
67 (12.1) | 21 (11.9) | 0.9 |
|
Invasive mechanical ventilation n (%) |
309 (56.4) | 166 (93.8) | 0.0001 |
|
Shock n (%) |
220 (40.4) | 130 (74.3) | 0.0001 |
|
MODS n (%) |
296 (53.6) | 149 (85.1) | 0.0001 |
|
Corticosteroids n (%) |
216 (39.8) | 91 (53.8) | 0.001 |
|
CRRT n (%) |
21 (3.8) | 51 (29.1) | 0.0001 |
|
Oseltamivir n (%) |
547 (99.6) | 173 (98.3) | 0.09 |
APACHE acute physiology and chronic health evaluation, COPD chronic obstructive pulmonary disease, HIV human immunodeficiency virus, MODS multiple organ dysfunction syndrome, CRRT continuous renal replacement therapy
Fig. 1.
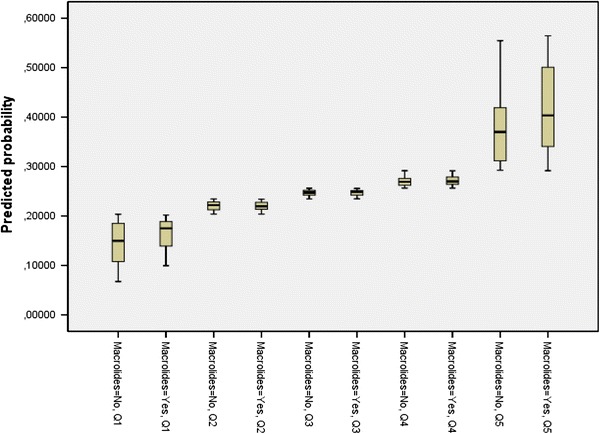
Propensity score distributions for patients who received macrolide-based regimens and those who did not within each propensity score quintile
Discussion
This secondary analysis of a multicenter prospective observational cohort study in ICU patients with pandemic (H1N1)v influenza A infection suggests that the use of macrolides does not result in a reduction in mortality in patients with primary viral pneumonia.
Several studies have found better outcomes in patients with CAP administered combinations of antibiotics [24–26] and also, more specifically, in patients with CAP administered combinations of macrolides with other antibiotics [27–30]. Restrepo et al. [31] found that the use of macrolides in combination therapy improved outcomes in patients with CAP and severe sepsis. Tessmer et al. [32] analyzed a large cohort of patients with CAP and reported better 14-day outcome for patients with high CRB65 (confusion, respiratory rate, blood pressure and age over 65 years) risk classes receiving beta-lactam/macrolide compared to those receiving beta-lactam alone. More recently, our group analyzed a cohort of critically ill patients requiring MV for severe CAP in a multicenter study and found that treatment with macrolides in combination therapy according with 2007 IDSA/ATS guidelines improves survival compared to fluoroquinolones [15]. Interestingly, this protective effect of macrolide therapy was more pronounced in the more severe patients. Thus, combination therapy and especially combination therapy including macrolides seem recommended in patients with CAP caused by bacteria. The rationale for combining antibiotics in patients with CAP is based on their different mechanisms of action, resulting in synergistic killing and a broader antimicrobial spectrum; however, macrolides are linked to an anti-inflammatory effects more than anti-infective properties [33–35]. Although there is a strong body of evidence for considering combinations including a macrolide in patients with bacterial CAP, evidence for this approach in patients with viral pneumonia without bacterial co-infection is lacking. The Spanish Society of Intensive Care Medicine currently recommends patients admitted to an ICU with pandemic (H1N1)v influenza A infection should be administered combination antibiotic therapy including a third generation cephalosporin and either a macrolide or a fluoroquinolone. This recommendation is based on the current guidelines for CAP management from the Spanish Thoracic Society (SEPAR), Spanish Society of Clinical Microbiology and Infectious Diseases (SEIMC), and Spanish Society of Intensive Care Medicine (SEMICYUC) [18]. Antibiotic therapy in patients with primary viral pneumonia had to be maintained until cultures rule out bacterial infection; however, the final decision should be individualized to reduce resistance and complications related to antibiotic overuse.
Whether pandemic (H1N1)v influenza A infection proves fatal depends on the degree to which the influenza virus depresses local and general pulmonary defense mechanisms. The most common pulmonary presentation of patients affected by pandemic (H1N1)v influenza A infection is a rapidly progressive viral pneumonia with bilateral alveolar infiltrates on chest radiographs and ARDS. The presentation of ARDS with severe refractory hypoxemia has been particularly common in patients with this disease and might be linked to an abnormal immune response [36]. Investigating this hypothesis, To et al. [37] demonstrated that, due to excessive cytokine activation, patients with ARDS and those who died had slower control of viral load than patients with mild disease.
Several studies have shown promising results with the use of macrolides in viral infections. Macrolides have remarkable anti-inflammatory properties that exceed their antibacterial activity [33]. Animal models of H2N2 influenza virus have shown that macrolides bring about beneficial effects by decreasing interferon gamma (IFNg) and reducing the level of nitrite/nitrate (metabolites of nitric oxide) [38]. In addition, a double-blind randomized placebo-controlled trial in the treatment of respiratory syncytial virus bronchiolitis found that treatment with clarithromycin was associated with significant reductions in the length of hospital stay, the duration of need for supplemental oxygen, and the need for beta2-agonist treatment [39]. However, scant information about the impact of macrolides on the outcome of patients with H1N1 is available [40]. To our knowledge, the only study to consider the role of macrolides was reported by Viasus et al. [41]. These authors analyzed a cohort of 197 patients with pandemic influenza A (H1N1) 2009 complicated by pneumonia, only 31 of whom were treated with macrolides. They found immunomodulatory therapies (corticosteroids, macrolides, and statins) as a group were not associated with a lower risk for developing severe disease, defined as ICU admission or death; however, the overall mortality was low (n = 3.5 %).
The present study has several potential limitations. First, given the epidemiological nature of this study, no data about the number of patients in whom respiratory samples were obtained before antibiotic administration or the number of patients that received antibiotics before ICU admission, and this is important because the causative agent of CAP remains unidentified in 30–50 % of cases (18). In addition, because, we included all patients with negative bacterial respiratory or blood cultures, one-third of whom were not receiving mechanical ventilation, the analysis was also restricted to the subgroup of ventilated patients and similar results were obtained. Furthermore, this was an observational, non-interventional study rather than a randomized, double-blind, controlled trial in which interventions could be tightly managed and the control and treatment groups matched. However, the epidemiological characteristics and emergency nature of this disease preclude clinical trials, so we did a propensity score analysis, which replicates some of the characteristics of a randomized controlled trial, to verify and validate our results. Second, the ICUs from 148 hospitals that participated in this observational, non-interventional study were self-selected, and the decision to prescribe macrolides was made in accordance with local protocols. Although our study includes more than 70 % of all patients admitted to ICUs in Spain during the present pandemic influenza A (H1N1) virus infection [13], the present analysis enrolled patients prospectively and represents a homogeneous population from critical care settings. Likewise, we had no data on viral load secreted by the respiratory tract in our cohort of patients; however, early administration of neuraminidase inhibitors has proven a protective factor in severe cases of 2009 pandemic influenza, and the results of our study reinforce the importance of therapeutic strategies based on the early administration of antiviral drugs to control viral replication.
Our results suggest that macrolide-based treatment do not confer a survival benefit in critically ill H1N1 patients with primary viral pneumonia. One possible explanation for the lack of survival benefit is that once the exacerbated inflammatory reaction reaches a certain point, it is too late for the immunomodulatory effects of macrolides to influence the course of the disease. The potential benefit of early administration of macrolides in these patients before the instauration of respiratory failure remains to be elucidated.
Acknowledgments
SEMICYUC and Programa de investigación comisionada en gripe, ISCIII, GR09/0021.
Appendix: H1N1 SEMICYUC/REIPI/CIBERES Working Group investigators
Andalucía: Pedro Cobo (Hospital Punta de Europa, Algeciras); Javier Martins (Hospital Santa Ana Motril, Granada); Cecilia Carbayo (Hospital Torrecardenas, Almería); Emilio Robles-Musso, Antonio Cárdenas, JavierFierro(Hospital del Poniente, Almería); Dolores Ocaña Fernández (Hospital Huercal–Overa, Almería); Rafael Sierra (Hospital Puerta del Mar, Cádiz); Mª JesúsHuertos(Hospital Puerto Real, Cádiz); Juan Carlos Pozo, R. Guerrero (Hospital Reina Sofía, Córdoba); Enrique Márquez (Hospital Infanta Elena, Huelva); Manuel Rodríguez-Carvajal (Hospital Juan Ramón Jiménez, Huelva); Ángel Estella (Hospital del SAS de Jerez, Jerez de la Frontera); José Pomares, José Luis Ballesteros (Hospital Universitario San Cecilio, Granada); Yolanda Fernández, Francisco Lobato, José F. Prieto, José Albofedo-Sánchez (Hospital Costa del Sol, Marbella); Pilar Martínez; María Victoria de la Torre; María Nieto (Hospital Vírgen de la Victoria, Málaga); Miguel Angel DíazCastellanos, (Hospital Santa Ana de Motril, Granada); Guillermo Sevilla, (Clínica Sagrado Corazón, Sevilla); José Garnacho-Montero, Rafael Hinojosa, Esteban Fernández, (Hospital Virgen del Rocío, Sevilla); Ana Loza, Cristóbal León (Hospital Universitario NuestraSeñora de Valme, Sevilla); Angel Arenzana, (Hospital Virgen de la Macarena, Sevilla), Dolores Ocaña (Hospital de la Inmaculada, Sevilla), Inés Navarrete (Hospital Virgen de las Nieves, Granada), Medhi ZaheriBeryanaki (Hospital de Antequera); Ignacio Sánchez (Hospital NISA Sevilla ALJARAFE, Sevilla).
Aragón: Manuel Luis Avellanas, Arantxa Lander, S Garrido Ramírez de Arellano, MIMarquina Lacueva (Hospital San Jorge, Huesca); Pilar Luque; Elena Plumed Serrano; Juan Francisco Martín Lázaro (Hospital Lozano Blesa, Zaragoza); Ignacio González (Hospital MiquelServet, Zaragoza); Jose Mª Montón (Hospital Obispo Polanco, Teruel); Paloma Dorado Regil(Hospital Royo Villanova, Zaragoza)
Asturias: Lisardo Iglesias, Carmen Pascual González (Hospital Universitario Central de Asturias – HUCA, Oviedo); Quiroga (Hospital De Cabueñes, Gijón); Águeda García-Rodríguez (Hospital Valle del Nalón, Langreo).
Baleares: Lorenzo Socias, Pedro Ibánez, Marcío Borges-Sa; A. Socias, Del Castillo A (Hospital Son LLatzer,Palma de Mallorca); RicardJordà Marcos (Clínica Rotger, Palma de Mallorca); José M Bonell (USP. Clínica Palmaplanas, Palma de Mallorca); Ignacio Amestarán (Hospital Son Dureta, Palma de Mallorca).
Canarias: Sergio Ruiz- Santana, Juan José Díaz,(Hospital DrNegrín,Las Palmas de Gran Canaria); Sisón (Hospital Doctor José Molina, Lanzarote); David Hernández, Ana Trujillo, Luis Regalado, (Hospital General la Palma, La Palma); Leonardo Lorente (Hospital Universitario de Canarias, Tenerife); Mar Martín (Hospital de la Candelaria, Tenerife), Sergio Martínez, J.J.Cáceres (Hospital Insular de Gran Canaria).
Cantabria: Borja Suberviola, P. Ugarte, (Hospital Universitario Marqués de Valdecilla, Santander);
Castilla La Mancha: Fernando García-López, (Hospital General, Albacete); Angel Álvaro Alonso, Antonio Pasilla (Hospital General La Mancha Centro, Alcázar de San Juan); Mª Luisa Gómez Grande (Hospital General de Ciudad Real, Ciudad Real); Antonio Albaya, (Hospital Universitario de Guadalajara, Guadalajara); Alfonso Canabal, Luis Marina, (Hospital Virgen de la Salud, Toledo); Almudena Simón (Hospital Nuestra Señora del Prado, Toledo); José María Añón(Hospital Virgen de la Luz, Cuenca)
CastillayLeón: Juan B López Messa, (Complejo Asistencial de Palencia, Palencia), Mª Jesús López Pueyo, Ortíz María del valle (Hospital General Yagüe, Burgos); Zulema Ferreras, (Hospital Universitario de Salamanca, Salamanca); Santiago Macias, (Hospital General de Segovia, Segovia); José Ángel Berezo, Jesús Blanco Varela, (Hospital Universitario Río Hortega, Valladolid), Andaluz Ojeda A (Hospital Universitario, Valladolid); Antonio Álvarez Terrero (Hospital Virgen de la Concha, Zamora), Fabiola Tena Ezpeleta (Hospital Santa Bárbara, Soria); Zulema Paez; Álvaro García (Hospital Virgen Vega, Salamanca)
Cataluña: Rosa Mª Catalán (Hospital General de Vic, Vic); Miquel Ferrer, Antoni Torres, Catia Cilloniz (Hospital Clínic, Barcelona); Sandra Barbadillo (Hospital General de Catalunya–CAPIO, Barcelona); Lluís Cabré, Ignacio Baeza (Hospital de Barcelona, Barcelona); Assumpta Rovira (Hospital General de l’Hospitalet, L’Hospitalet); Francisco Álvarez-Lerma, Antonia Vázquez, Joan Nolla (Hospital Del Mar, Barcelona); Francisco Fernández, Joaquim Ramón Cervelló; Raquel Iglesia (Centro Médico Delfos, Barcelona); Rafael Mañéz, J. Ballús, Rosa Mª Granada (Hospital de Bellvitge, Barcelona); Jordi Vallés, Marta Ortíz, C. Guía (Hospital de Sabadell, Sabadell); Fernando Arméstar, Joaquim Páez (Hospital Dos De Mayo, Barcelona); Jordi Almirall,Xavier Balanzo (Hospital de Mataró, Mataró); Jordi Rello, Elena Arnau,Marcos Pérez; César Laborda; Jesica Souto, Mercedes Palomar (Hospital Valld’Hebron, Barcelona); Iñaki Catalán (Hospital Sant Joan de Déu, Manresa); Josep Mª Sirvent, Cristina Ferri, Nerea López de Arbina (Hospital Josep Trueta, Girona); Mariona Badía, Montserrat Valverdú- Vidal, Fernando Barcenilla (Hospital Arnau de Vilanova, Lleida); MònicaMagret, (Hospital Sant Joan de Reus, Reus); MF Esteban, José Luna, (Hospital Verge de la Cinta, Tortosa); Juan Mª Nava, J González de Molina, (Hospital Universitario Mutua de Terrassa, Terrassa);Zoran Josic (Hospital de Igualada, Igualada); Francisco Gurri; Paula Rodríguez (Hospital Quirón, Barcelona, Alejandro Rodríguez, Thiago Lisboa, Ángel Pobo, Sandra Trefler (Hospital Universitario Joan XXIII, Tarragona), Rosa María Díaz (Hospital San Camil. Sant Pere de Ribes, Barcelona); Eduard Mesalles(Hospital GermansTrias i Pujol, Badalona); Diego de Mendoza (Hospital M. Broggi, Sant Joan Despí).
Extremadura: Juliá-Narváez José (Hospital Infanta Cristina, Badajóz), Alberto Fernández-Zapata, Teresa Recio, Abilio Arrascaeta, Mª José García-Ramos, Elena Gallego (Hospital San Pedro de Alcántara, Cáceres); Fernándo Bueno (Hospital Virgen del Puerto, Plasencia); Mercedes Díaz (Hospital de Mérida, Mérida).
Galicia: Mª Lourdes Cordero, José A. Pastor, Luis Álvarez–Rocha (CHUAC, A Coruña); Dolores Vila, (Hospital Do Meixoeiro, Vigo); Ana Díaz Lamas (Hospital Arquitecto Marcide, Ferrol); Javier Blanco Pérez, M Ortiz Piquer, (Hospital Xeral–Calde, Lugo); Eleuterio Merayo, VictorJose López-Ciudad, Juan Cortes Cañones, Eva Vilaboy, José Villar Chao (Complejo Hospitalario de Ourense, Ourense); Eva MariaSaborido, (Hospital Montecelo, Pontevedra); Raul José González, (H. Miguel Domínguez, Pontevedra); Santiago Freita, Enrique Alemparte; Ana Ortega (Complejo Hospitalario de Pontevedra, Pontevedra); Ana María López; Julio Canabal, Enrique Ferres (Clinica Universitaria Santiago de Compostela, Santiago).
La Rioja: José Luis Monzón, Félix Goñi (Hospital San Pedro, Logroño).
Madrid: Frutos Del Nogal Sáez, M Blasco Navalpotro (Hospital Severo Ochoa, Madrid); Mª Carmen García-Torrejón, (Hospital Infanta Elena, Madrid);César Pérez –Calvo, Diego López(Fundación Jiménez Díaz, Madrid); Luis Arnaiz, S.Sánchez- Alonso, Carlos Velayos, (Hospital Fuenlabrada, Madrid); Francisco del Río, Miguel Ángel González (Hospital Clínico San Carlos, Madrid); María Cruz Martín, José Mª Molina (Hospital Nuestra Señora de América, Madrid); Juan Carlos Montejo, Mercedes Catalán (Hospital Universitario 12 de Octubre, Madrid); Patricia Albert, Ana de Pablo (Hospital del Sureste, Arganda del rey); José Eugenio Guerrero, María Zurita; Jaime BenitezPeyrat (Hospital Gregorio Marañón, Madrid); Enrique Cerdá, Manuel Alvarez, Carlos Pey, (Hospital Infanta Cristina, Madrid); Montse Rodríguez, Eduardo Palencia (Hospital Infanta Leonor, Madrid); Rafael Caballero,(Hospital de San Rafael, Madrid); Concepción Vaquero, Francisco Mariscal, S. García, (Hospital Infanta Sofía, Madrid); Nieves Carrasco, (Hospital Universitario La Princesa, Madrid); Isidro Prieto, A Liétor, R. Ramos (Hospital Ramón y Cajal, Madrid); Beatríz Galván, Juan C. Figueira, M. Cruz Soriano (Hospital La Paz, Madrid); P Galdós; Bárbara Balandin Moreno (Hospital Puerta de Hierro, Madrid); Fernández del Cabo (Hospital Monte Príncipe, Madrid); Cecilia Hermosa, Federico Gordo (Hospital de Henares, Madrid); Alejandro Algora (Hospital Universitario Fundación Alcorcón, Madrid); Amparo Paredes(Hospital Sur de Alcorcón, Madrid); JA Cambronero (Hospital Universitario Príncipe de Asturias, Madrid); Sonia Gómez-Rosado, (Hospital de Móstoles, Madrid); Luis Miguel Prado López (Hospital Sanitas La Zarzuela, Madrid); Esteban A, Lorente JA, Nin N(Hospital de Getafe, Madrid).
Murcia: Sofía Martínez (Hospital Santa María del Rosell, Murcia); F. Felices Abad, (Hospital Universitario Reina Sofía, Murcia); Mariano Martínez, (Hospital Universitario Virgen de la Arrixaca, Murcia); Sergio Manuel Butí, Bernardo Gil Rueda, Francisco García (Hospital Morales Messeguer, Murcia).
Navarra: Laura Macaya, Enrique Maraví-Poma, I Jimenez Urra, L Macaya Redin, A Tellería (Hospital Virgen del Camino, Pamplona); Josu Insansti, (Hospital de Navarra, Pamplona).
País Vasco: Nagore González, Pilar Marco, Loreto Vidaur (Hospital de Donostia, San Sebastián); B. Santamaría, Tomás Rodríguez (Hospital de Basurto, Bilbao); Juan Carlos Vergara, JoseRamonIruretagoyenaAmiano, (Hospital de Cruces, Bilbao); Alberto Manzano, (Hospital Santiago Apóstol, Vitoria); Carlos Castillo Arenal (Hospital Txagorritxu, Vitoria); Pedro María Olaechea, Higinio Martín (Hospital Galdakao-Usansolo, Vizcaya) .
Andorra: Antoni Ribas (Hospital Nuestra Señora de Meritxell, Andorra).
Valencia: José Blanquer (Hospital ClinicUniversitari, Valencia); Roberto Reig Valero, A. Belenger, Susana Altaba (Hospital General de Castellón, Castellón); Bernabé Álvarez -Sánchez, (Hospital General de Alicante, Alicante); Santiago Alberto Picos, (Hospital Torrevieja Salud, Alicante); Ángel Sánchez-Miralles, (Hospital San Juan, Alicante); Juan Bonastre, M. Palamo, Javier Cebrian, José Cuñat(Hospital La Fe, Valencia); Belén Romero (Hospital de Manises, Valencia); Rafael Zaragoza, Constantino Tormo (Hospital DrPeset, Valencia); Virgilio Paricio, (Hospital de Requena, Valencia); Asunción Marques, S. Sánchez-Morcillo, S. Tormo (Hospital de la Ribera, Valencia). J. Latour (H.G Universitario de Elche, Valencia), M Ángel García (Hospital de Sagunto, Castellón).
Footnotes
Members of the SEMICYUC/REIPI/CIBERES H1N1 Working Group are listed in the Appendix.
References
- 1.Pérez-Padilla R, de la Rosa-Zamboni D, Ponce de León S, et al. Pneumonia and respiratory failure from swine-origin influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 2.Louie JK, Acosta M, Winter K, et al. Factors associated with death or hospitalization due to pandemic 2009 influenza A(H1N1) infection in California. JAMA. 2009;302:1896–1902. doi: 10.1001/jama.2009.1583. [DOI] [PubMed] [Google Scholar]
- 3.The ANZIC Influenza Investigators Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361:1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 4.Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 5.WHO Clinical aspects of pandemic 2009 influenza A (H1N1) virus infection. N Engl J Med. 2010;362:1708–1719. doi: 10.1056/NEJMra1000449. [DOI] [PubMed] [Google Scholar]
- 6.Rello J, Rodríguez A, Ibañez P, Socias L, Cebrian J, Marques A, Guerrero J, Ruiz-Santana S, Marquez E, Del Nogal-Saez F, Alvarez-Lerma F, Martínez S, Ferrer M, Avellanas M, Granada R, Maraví-Poma E, Albert P, Sierra R, Vidaur L, Ortiz P, Prieto del Portillo I, Galván B, León-Gil C, H1N1 SEMICYUC Working Group Intensive care adult patients with severe respiratory failure caused by influenza A (H1N1)v in Spain. Crit Care. 2009;13(5):R148. doi: 10.1186/cc8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar A, Zarychanski R, Pinto R, et al. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 8.ANZIC Influenza Investigators. Webb SA, Pettilä V, Seppelt I, et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361(20):1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 9.Martín-Loeches I, Sanchez-Corral A, Diaz E, Granada RM, Zaragoza R, Villavicencio C, Albaya A, Cerdá E, Catalán RM, Luque P, Paredes A, Navarrete I, Rello J, Rodríguez A, H1N1 SEMICYUC Working Group Community-acquired respiratory co-infection (CARC) in critically ill patients infected with pandemic 2009 influenza A (H1N1) virus infection. Chest. 2011;139:555–562. doi: 10.1378/chest.10-1396. [DOI] [PubMed] [Google Scholar]
- 10.Díaz E, Rodríguez A, Martin-Loeches I, Lorente L, del Mar Martín M, Pozo JC, Montejo JC, Estella A, Arenzana A, Rello J, H1N1 SEMICYUC Working Group Impact of obesity in patients infected with new influenza A (H1N1)v. Chest. 2011;139:382–386. doi: 10.1378/chest.10-1160. [DOI] [PubMed] [Google Scholar]
- 11.Martin-Loeches I, Lisboa T, Rhodes A, Moreno RP, Silva E, Sprung C, Chiche JD, Barahona D, Villabon M, Balasini C, Pearse RM, Matos R, Rello J, ESICM H1N1 Registry Contributors Use of early corticosteroid therapy on ICU admission in patients affected by severe pandemic (H1N1)v influenza A infection. Intensive Care Med. 2011;37:272–283. doi: 10.1007/s00134-010-2078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim SH, Hong SB, Yun SC, Choi WI, Ahn JJ, Lee YJ, Lee HB, Lim CM, Koh Y, Korean Society of Critical Care Medicine H1N1 Collaborative Corticosteroid treatment in critically ill patients with pandemic influenza A/H1N1 2009 infection: analytic strategy using propensity scores. Am J Respir Crit Care Med. 2011;183:1207–1214. doi: 10.1164/rccm.201101-0110OC. [DOI] [PubMed] [Google Scholar]
- 13.Rodríguez A, Díaz E, Martín-Loeches I, Sandiumenge A, Canadell L, Díaz JJ, Figueira JC, Marques A, Alvarez-Lerma F, Vallés J, Baladín B, García-López F, Suberviola B, Zaragoza R, Trefler S, Bonastre J, Blanquer J, Rello J, H1N1 SEMICYUC Working Group Impact of early oseltamivir treatment on outcome in critically ill patients with 2009 pandemic influenza A. J Antimicrob Chemother. 2011;66:1140–1149. doi: 10.1093/jac/dkq511. [DOI] [PubMed] [Google Scholar]
- 14.Hiba V, Chowers M, Levi-Vinograd I, Rubinovitch B, Leibovici L, Paul M. Benefit of early treatment with oseltamivir in hospitalized patients with documented 2009 influenza A (H1N1): retrospective cohort study. J Antimicrob Chemother. 2011;66:1150–1155. doi: 10.1093/jac/dkr089. [DOI] [PubMed] [Google Scholar]
- 15.Martin-Loeches I, Lisboa T, Rodriguez A, Putensen C, Annane D, Garnacho-Montero J, Restrepo MI, Rello J. Combination antibiotic therapy with macrolides improves survival in intubated patients with community acquired pneumonia. Intensive Care Med. 2010;36:612–620. doi: 10.1007/s00134-009-1730-y. [DOI] [PubMed] [Google Scholar]
- 16.Wunderink RG, Mandell L. Adjunctive therapy in community-acquired pneumonia. Semin Respir Crit Care Med. 2012;33:311–318. doi: 10.1055/s-0032-1315643. [DOI] [PubMed] [Google Scholar]
- 17.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, SCCM/ESICM/ACCP/ATS/SIS 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med. 2003;31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 18.Alfageme I, Aspa J, Bello S, et al. Guidelines for the diagnosis and management of community-acquired pneumonia. Spanish Society of Pulmonology and Thoracic Surgery (SEPAR) Arch Bronconeumol. 2005;41:272–289. doi: 10.1157/13074594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious diseases society of America/American thoracic society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.http://www.msc.es/profesionales/saludPublica/gripeA/guiasProtocolosInf/pdf/ProtocoloGripeAenUCI.pdf. Accessed 07 August 2009
- 21.Woodhead MA, Arrowsmith J, Chamverlain-Webber R, et al. The value of routine microbial investigation in community-acquired pneumonia. Respir Med. 1991;85:313–317. doi: 10.1016/S0954-6111(06)80103-4. [DOI] [PubMed] [Google Scholar]
- 22.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A, Acute Kidney Injury Network Acute kidney injury network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suarez D, Faries DE, Propensity score regression and stratification (2010) In: Faries DE, Leon AC, Haro JM, Obenchain RL (eds) SAS Institute Inc Analysis of Observational Health Care Data Using SAS, Cary, NC, pp 23–50
- 24.Waterer GW, Somes GW, Wunderink RG. Monotherapy may be suboptimal for severe bacteremic pneumococcal pneumonia. Arch Intern Med. 2001;161:1837–1842. doi: 10.1001/archinte.161.15.1837. [DOI] [PubMed] [Google Scholar]
- 25.Baddour LM, Yu VL, Klugman KP, Feldman C, Ortkvist A, Rello J, Morris AJ, Luna CM, Snydman DR, Ko WC, Chedid BF, Hui DS, Andremont A, Chiou CCC, and the International Pneumococcal Study Group Combination antibiotic therapy may lower mortality in severely ill patients with Streptococcus pneumoniae bacteremia. Am J Respir Crit Care Med. 2004;170:400–404. doi: 10.1164/rccm.200305-648OC. [DOI] [PubMed] [Google Scholar]
- 26.Rodríguez A, Mendia A, Sirvent JM, Barcenilla F, de la Torre-Prados MV, Solé-Violán J, Rello J; CAPUCI Study Group Combination antibiotic therapy improves survival in patients with community-acquired pneumonia and shock. Crit Care Med. 2007;35:1493–1498. doi: 10.1097/01.CCM.0000266755.75844.05. [DOI] [PubMed] [Google Scholar]
- 27.Brown RB, Iannini P, Gross P, Kunkel M. Impact of initial antibiotic choice on clinical outcomes in community-acquired pneumonia: analysis of a hospital claims-made database. Chest. 2003;123:1503–1511. doi: 10.1378/chest.123.5.1503. [DOI] [PubMed] [Google Scholar]
- 28.Gleason PP, Meehan TP, Fine JM, Galusha DH, Fine MJ. Associations between initial antimicrobial therapy and medical outcomes for hospitalized elderly patients with pneumonia. Arch Intern Med. 1999;159:2562–2572. doi: 10.1001/archinte.159.21.2562. [DOI] [PubMed] [Google Scholar]
- 29.Metersky ML, Ma A, Houck PM, Bratzler DW. Antibiotics for bacteremic pneumonia: improved outcomes with macrolides but not fluoroquinolones. Chest. 2007;131:466–473. doi: 10.1378/chest.06-1426. [DOI] [PubMed] [Google Scholar]
- 30.Mortensen EM, Restrepo MI, Anzueto A, Pugh J. The impact of empiric antimicrobial therapy with a β-lactam and fluoroquinolone on mortality for patients hospitalized with severe pneumonia. Crit Care. 2006;10:R8. doi: 10.1186/cc3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Restrepo MI, Mortensen EM, Waterer GW, Wunderink RG, Coalson JJ, Anzueto A. Impact of macrolide therapy on mortality for patients with severe sepsis due to pneumonia. Eur Respir J. 2009;33:153–159. doi: 10.1183/09031936.00054108. [DOI] [PubMed] [Google Scholar]
- 32.Tessmer A, Welte T, Martus P, Schnoor M, Marre R, Suttorp N. Impact of intravenous β-lactam/macrolide versus β-lactam monotherapy on mortality in hospitalized patients with community-acquired pneumonia. J Antimicrob Chemother. 2009;63:1025–1033. doi: 10.1093/jac/dkp088. [DOI] [PubMed] [Google Scholar]
- 33.Tamaoki J, Kadota J, Takizawa H. Clinical implications of the immunomodulatory effects of macrolides. Am J Med. 2004;117(suppl 9A):5S–11S. doi: 10.1016/j.amjmed.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 34.Healy DP. Macrolide immunomodulation of chronic respiratory diseases. Curr Infect Dis Rep. 2007;9(1):7–13. doi: 10.1007/s11908-007-0016-1. [DOI] [PubMed] [Google Scholar]
- 35.Vanaudenaerde BM, Wuyts WA, Geudens N, Dupont LJ, Schoofs K, Smeets S, Van Raemdonck DE, Verleden GM. Macrolides inhibit IL17-induced IL8 and 8-isoprostane release from human airway smooth muscle cells. Am J Transpl. 2007;7:76–82. doi: 10.1111/j.1600-6143.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- 36.Bermejo-Martin JF, Ortiz de Lejarazu R, Pumarola T, Rello J, Almansa R, Ramírez P, Martin-Loeches I, Varillas D, Gallegos MC, Serón C, Micheloud D, Gomez JM, Tenorio-Abreu A, Ramos MJ, Molina ML, Huidobro S, Sanchez E, Gordón M, Fernández V, Del Castillo A, Marcos MA, Villanueva B, López CJ, Rodríguez-Domínguez M, Galan JC, Cantón R, Lietor A, Rojo S, Eiros JM, Hinojosa C, Gonzalez I, Torner N, Banner D, Leon A, Cuesta P, Rowe T, Kelvin DJ. Th1 and Th17 hypercytokinemia as early host response signature in severe pandemic influenza. Crit Care. 2009;13(6):R201. doi: 10.1186/cc8208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.To KK, Hung IF, Li IW, et al. Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin Infect Dis. 2010;50:850–859. doi: 10.1086/650581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato K, Suga M, Akaike T, Fujii S, Muranaka H, Doi T, Maeda H, Ando M. Therapeutic effect of erythromycin on influenza virus-induced lung injury in mice. Am J Respir Crit Care Med. 1998;157:853–857. doi: 10.1164/ajrccm.157.3.9703098. [DOI] [PubMed] [Google Scholar]
- 39.Tahan F, Ozcan A, Koc N. Clarithromycin in the treatment of RSV bronchiolitis: a double-blind, randomised, placebo-controlled trial. Eur Respir J. 2007;29:91–97. doi: 10.1183/09031936.00029206. [DOI] [PubMed] [Google Scholar]
- 40.Bermejo-Martin JF, Kelvin DJ, Eiros JM, Castrodeza J, Ortiz de Lejarazu R. Macrolides for the treatment of severe respiratory illness caused by novel H1N1 swine influenza viral strains. J Infect Dev Ctries. 2009;30:159–161. doi: 10.3855/jidc.18. [DOI] [PubMed] [Google Scholar]
- 41.Viasus D, Paño-Pardo JR, Cordero E, Campins A, López-Medrano F, Villoslada A, Fariñas MC, Moreno A, Rodríguez-Baño J, Oteo JA, Martínez-Montauti J, Torre-Cisneros J, Segura F, Carratalà J, Novel Influenza A (H1N1) Study Group, Spanish Network for Research in Infectious Diseases Effect of immunomodulatory therapies in patients with pandemic influenza A (H1N1) 2009 complicated by pneumonia. J Infect. 2011;62:193–199. doi: 10.1016/j.jinf.2011.01.014. [DOI] [PubMed] [Google Scholar]


