Abstract
Purpose
Uncertainty about the severity of the A(H1N1) pandemia persists. Information about disease severity can be obtained by investigating intensive care unit (ICU) admissions, especially when historical comparisons can be made with cases of community-acquired pneumonia (CAP).
Methods
This prospective observational study was conducted in 155 ICUs contributing to the GiViTI national database. To assess the impact on ICU workload, the occupancy rate during the epidemic phase was compared with influenza periods in previous years. A logistic regression model was developed to assess the prognostic importance of A(H1N1) influenza.
Results
The characteristics of the 319 A(H1N1) cases were similar to those reported in other studies, confirming the young age of patients (mean 43 years) and the higher prevalence among pregnant women and obese people. At the epidemic’s peak (October–December 2009) the occupancy rate did not significantly differ from the same period of the previous year, and was significantly lower than the 2009 seasonal influenza outbreak (January–March 2009). Compared with CAP of other origin (3,678 patients), A(H1N1) pneumonia was associated with a lower risk of death. However, after adjusting for confounding this was no longer the case (OR 0.88; 95% CI 0.59–1.31; p = 0.52).
Conclusion
This study confirmed the specific features of critically ill A(H1N1) patients (i.e., young age, pregnancy, obesity). The pandemic did not increase ICU workload compared with other periods. A(H1N1) pneumonia did not have a higher risk of death than CAP of different origin among patients admitted to the ICU.
Electronic supplementary material
The online version of this article (doi:10.1007/s00134-011-2339-5) contains supplementary material, which is available to authorized users.
Keywords: Intensive care units, Influenza A(H1N1), Mortality, Bed occupancy
Introduction
In June 2009, over 40 years after the 1968 A(H3N2) Hong Kong pandemic influenza, the World Health Organization confirmed a new pandemic outbreak caused by an A(H1N1) virus [1]. The first confirmed cases were reported in the USA in April [2]; then, from Mexico, the virus spread all over the world [3]. Particular concern centered on its contagiousness, the propensity to affect young people, including pregnant women, and the potential risk of progression toward severe pneumonia [3].
The severity of an influenza epidemic is usually determined by the case fatality ratio (CFR, i.e., the proportion of disease-related deaths out of the number of diagnosed cases over a certain period of time). Unfortunately, preliminary CFR estimates for A(H1N1) influenza varied widely both between and within countries, ranging from 0.0008 to 4.5% [4–6]. Studies published during and after the emergency indicate there is still a substantial degree of uncertainty about the virulence and mortality risk of the A(H1N1) virus [7–12]. Ascertaining the total number of cases and related deaths, on which the estimates are based, is in fact influenced by many undependable factors (e.g., the level of population alarm and of spontaneous medical referral).
Because intensive care unit (ICU) admission criteria are not subject to significant changes over time and the number of cases and deaths are clearly identifiable, the question of A(H1N1) severity can be approached from the ICU perspective [6]. Several studies on A(H1N1) patients admitted to ICUs have not, however, provided the historical comparisons needed to interpret ICU-based indicators [13–16].
In Italy a large network of ICUs (GiViTI—Italian Group for the Evaluation of Interventions in Intensive Care Medicine), established to evaluate and improve the quality of care in this field, has been operative since 1991 [17]. In 2002 the GiViTI launched Project Margherita, a national research campaign currently involving 230 ICUs, which collects and analyzes clinical data on all patients admitted to the participating ICUs [17]. A prognostic model is developed yearly as the basis for quality assessment. In October 2009, GiViTI set up the A(H1N1) registry with the following aims: to describe the epidemiology of the phenomenon in Italy and compare it with other international surveys; to assess the impact of the pandemic on the work of Italian ICUs; to assess whether a patient with A(H1N1) pneumonia had a different risk of death from a patient with severe community-acquired pneumonia (CAP) of different origin.
Methods
Data collection and patients
The Margherita Project is based on an electronic form which was extended to meet the requirements of the A(H1N1) registry. We also developed an online case report form to allow participation in the survey by ICUs not contributing to the project. Patients with a suspected or proven A(H1N1) infection were eligible for inclusion in the registry. The core data of the Margherita Project included demographics, admission diagnoses, severity of infection on admission, comorbidities, where the patient had been before admission, surgical status, reasons for admission, Simplified Acute Physiology Score II (SAPS II) variables [18], failures and diseases while in the ICU, maximum severity of infection, major procedures and interventions, ICU and hospital outcomes. Additional variables were collected in the A(H1N1) registry on onset of influenza symptoms, vaccination, risk factors for severe infection, polymerase chain reaction-confirmed diagnosis, worst PaO2/FiO2 ratio, and specific treatments during the ICU stay. We were careful to spot patients transferred from one ICU to another, so as to count them only once. We only considered the outcome at the final ICU destination.
During the epidemic, weekly electronic reports were published on the website. Informed consent was waived because the study was fully observational and no information was collected that could identify patients.
Quality control
In each ICU a senior intensivist (see “Appendix”) was responsible for data integrity. A detailed online operating manual, which was easily accessible during data input, explained all the definitions employed. As many as 140 different validity checks were performed concurrently with data entry. The system allowed inconsistent or implausible data to be saved, but marked the record as problematic. Data were further reviewed by the coordinating center, and any queries solved with the individual ICUs. A call center was fully operative. All units were contacted by phone to ensure that all eligible patients were included in the registry.
Statistical analysis
Proportion was used as a descriptive statistic for categorical and ordinal variables, median and interquartile range (IQR) for ordinal variables, mean and standard deviation (SD), or median and IQR for continuous variables; 95% test-based confidence intervals were computed for each estimate of interest. The number of patients admitted with A(H1N1) infection was standardized to 1,000 ICU beds. Because the curve of the cumulative frequency over time was sigmoid, we fitted a logistic function to calculate its cumulative estimated frequencies. This function was derived to obtain the disease density function, expressed as the number of patients with A(H1N1) influenza per 1,000 ICU beds.
Two important series of critically ill A(H1N1) cases have been published from Australia/New Zealand and Canada [13, 15]. To allow direct comparison, we reconstructed the same variables as in these series.
To investigate the impact of the influenza pandemic on Italian ICU workload, we computed the average weekly occupancy rate, from October 2008 to December 2010, for the ICUs taking part in the Margherita Project at that time. The same analysis was carried out on the reference center patients.
To assess the relative impact of the A(H1N1) pandemic on the incidence of severe pneumonia, we calculated the density function of patients with CAP per 1,000 ICU beds and compared the curves for the last 6 years, obtained from Margherita Project data.
To determine the prognostic value of A(H1N1) influenza, we developed a multivariate logistic regression model of all patients with CAP admitted to the ICUs from October 2008 to December 2009, distinguishing between those with and without A(H1N1) infection. The dependent variable was hospital mortality. In the case of patients transferred to an ICU in another hospital, we considered mortality at discharge from the last hospital, because their acute illness could not be considered resolved at transfer from the first hospital.
Because the literature on influenza pandemics has indicated age, body mass index (BMI), important comorbidities, and pregnancy as risk factors for the disease, all these variables (except pregnancy, which was not available in the Margherita Project database) were tested in the model, along with the other variables identified as prognostically relevant in the 2009 GiViTI mortality prediction model (see electronic supplementary material, ESM). We also included a variable identifying the seasonal influenza period of each year, as reported by the permanent influenza surveillance program of the Italian Ministry of Health (http://www.iss.it/iflu/, see ESM). There were 47 potential prognostic factors tested in the model. We tested the assumption that the logit was linear in the quantitative variables by analyzing the estimated coefficients of designed variables representing the quartiles of the original variable distribution [19]. Whenever suggested by this analysis, we tested a second-order model or log transformation of the variable. If these approaches failed to fit the data, the variable was divided into classes, and dummy variables were used [19]. After forcing the inclusion of both A(H1N1) infection and influenza period in the model, step by step we added the covariate that maximized the increment in likelihood, in a forward approach. The model was selected using an information criterion with a penalizing parameter of 1.
All tests were two-tailed, with 0.05 as the level of significance. Data were analyzed using SAS software (9.02, SAS system, NC) and R (2.12.1, The R Foundation for Statistical Computing).
Results
In 2009, 180 out of the about 450 general ICUs in Italy took part in the GiViTI Margherita Project; 155 of these contributed to the A(H1N1) registry. The median number of beds per ICU was 6 (IQR 5–8); 55 units (35.5%) were university-affiliated. The Italian Ministry of Health designated 14 reference ICUs for the centralization of more complex cases and those requiring extracorporeal membrane oxygenation (ECMO); 9 (64%) took part in the survey.
A total of 319 A(H1N1) cases were admitted to the ICUs between October 2009 and April 2010. All of them, as verified by phone, were registered in the survey. Figure 1 illustrates the density of A(H1N1) cases; the peak in the second week of November 2009 reached 7 patients per 1,000 ICU beds. Table 1 summarizes the characteristics of these patients, and those of the Australian and Canadian series.
Fig. 1.
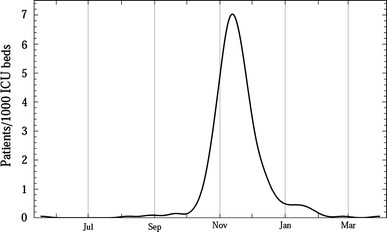
Density function of A(H1N1) cases in Italian ICUs
Table 1.
Baseline characteristics of the patients, and comparison with the Australian and Canadian series
| Italy (GiViTI) | Australia/New Zealand (ANZICS) | Canada | |
|---|---|---|---|
| No. ICUs | 155 | 187 | 38 |
| No. ICU beds | 1,086 | 1,879 | 608 |
| No. patients | 319 | 856 | 168 |
| Study period | October 2009–April 2010 | June–August 2009 | April–August 2009 |
| Type of ICU | Adult (151) pediatric (4) | Adult and pediatric | Adult and pediatric |
| PCR confirmed diagnosis | 94.8% (23 missing) | 84.3% | 96.4% |
| Age (years) | Mean 43.0; SD 19.6; median 44 | Median 40 | Mean 32.3; SD 21.4 |
| <1 | 1.6% | 4%a | |
| 1–4 | 2.8% | 3%a | |
| 5–24 | 13.5% | 2%a | |
| 25–49 | 43.3% | 52%a | |
| 50–64 | 24.5% | 31%a | |
| >64 | 14.4% | 8%a | |
| Female | 42.8% (1 missing) | 52.1% | 67.3% |
| Pregnancy (% on females) | 9.6% (38 missing) | 18.0% | 11.5% |
| Obesity | |||
| BMI > 35 | 11.6% | 28.6% | – |
| BMI > 30 | 22.9% | – | 33.3% |
| Diabetes | 15.4% | 16.0% | 20.8% |
| Asthma/COPD | 32.6% (9 missing) | 32.7% | – |
| Immune suppression | 8.8% (2 missing) | 19.6% | |
| Hematologic malignancy | 8.5% (2 missing) | – | 3.0% |
| Metastatic cancer | 0.7% | – | 0.6% |
| Autoimmune disease | 2.8% (2 missing) | – | 4.8% |
| Cardiac failure | 9.0% | 10.5% | 7.1% |
| Chronic renal failure | 4.3% | – | 7.1% |
| ARDS | 53.0% (2 missing) | 48.8% | – |
ICU intensive care unit, PCR polymerase chain reaction, BMI body mass index, COPD chronic obstructive pulmonary disease, ARDS acute respiratory distress syndrome, SD standard deviation, ANZICS Australian and New Zealand Intensive Care Society
aEstimated from Fig. 2a of Ref. [13]
In Italy, the percentages of women, pregnant women, and obese people were lower than in the other surveys. The remaining characteristics were fairly similar.
Table 2 describes the clinical management of the patients and the outcome. Only 64.6% of the patients were ventilated in Australia–New Zealand, but more in Canadian and Italian ICUs. ECMO was used more frequently in Italy and less in Australia–New Zealand and Canada. ICU mortality was similar in the three series.
Table 2.
Management of the patients and outcomes in Italy, Australia, and Canada
| Italy (GiViTI) | Australia/New Zealand (ANZICS) | Canada | |
|---|---|---|---|
| Days from first symptoms to hospital admission | Median 4 | Median 4 | Median 4 |
| IQR 2–6 (22 missing) | IQR 2–7 | IQR 2–7 | |
| Mechanical ventilation | 93.4% (2 missing) | 64.6% | 81.0% |
| Invasive ventilation | 69.2% | – | 76.2% |
| NIV | 45.5% | – | 32.7% |
| ECMO (% of ventilated) | 14.3% (11 missing) | 11.6% | 5.1% |
| iNO (% of ventilated) | 8.6% (11 missing) | – | 16.9% |
| HFO (% of ventilated) | 2.5% (11 missing) | – | 14.7% |
| Pronation (% of ventilated) | 22.5% (11 missing) | – | 3.7% |
| Antivirals | 88.5% (12 missing) | – | 90.5% |
| Antibiotics | 86.6% | – | 98.8% |
| Steroids | 54.5% (18 missing) | 18.4% | 50.6% |
| High dose | 9.4% (18 missing) | – | |
| Low dose | 47.5% (18 missing) | – | |
| ICU stay (days) | Mean 13.2; SD 14.4 | Median 7.4 | Median 12 |
| Median 9; IQR 3–17 (4 missing) | IQR 3.0–16.0 | IQR 5–20 | |
| Hospital stay (days) | Mean 23.5; SD 20.3 | Median 12.3; IQR 6.4–22.1 | – |
| Median 18; IQR 10–30 (22 missing) | |||
| ICU mortality | 17.1% (4 missing) | – | 16.7% |
| Hospital mortality | 20.2% (17 missing) | 16.9% | 17.3% |
NIV noninvasive ventilation, ECMO extracorporeal membrane oxygenation, iNO inhaled nitric oxide, HFO high frequency oscillator, ICU intensive care unit, IQR interquartile range, SD standard deviation, ANZICS Australian and New Zealand Intensive Care Society
Table 3 compares patients affected by A(H1N1)-related pneumonia with those affected by a non-A(H1N1) CAP, admitted between March 2008 and April 2010 to the 136 ICUs taking part in the Margherita Project in March 2008. The latter served as the control group in the logistic regression model to assess the prognostic value of A(H1N1) influenza.
Table 3.
Characteristics of patients with A(H1N1) pneumonia, and comparison with those admitted from March 2008 to April 2010 with a non-A(H1N1) CAP, in the subset of 136 ICUs taking part in the Margherita Project since March 2008
| A(H1N1) pneumonia (N = 213) | Non-A(H1N1) CAP (N = 3,678) | p value | |
|---|---|---|---|
| Age | Mean 48.9; SD 15.5; median 48 | Mean 66.2; SD 16.1; median 70 | <0.001 |
| Female | 43.2% | 36.8% | 0.059 |
| None of the following (excluding asthma) | 35.7% | 26.7% (21 missing) | 0.004 |
| Obesity | |||
| BMI > 35 | 10.8% | 6.6% | 0.020 |
| BMI > 30 | 23.5% | 16.0% (21 missing) | 0.004 |
| Diabetes | 17.4% | 22.4% | 0.087 |
| COPD | 31.9% | 45.4% | <0.001 |
| Asthma | 11.1% | – | – |
| Immune suppression | 9.4% | 4.5% | 0.001 |
| Hematologic malignancy | 9.4% | 4.8% | 0.003 |
| Metastatic cancer | 0.9% | 2.3% | 0.188 |
| Autoimmune disease | 3.3% | 4.5% | 0.417 |
| Cardiac failure | 9.9% | 22.0% | <0.001 |
| Chronic renal failure | 4.2% | 13.9% | <0.001 |
| SAPS II | Mean 37.2; SD 17.3; median 34 | Mean 48.5; SD 18.6; median 46 (4 missing) | <0.001 |
| Infection severity | |||
| Infection/sepsis | 57.4% | 62.6% | |
| Severe sepsis | 30.8% | 20.6% | 0.001 |
| Septic shock | 11.9% (2 missing) | 16.8% | |
| PaO2/FiO2 | |||
| ≥200 | 17.4% | 22.7% | |
| 100–199 | 42.7% | 48.0% | <0.001 |
| <100 | 36.6% | 23.3% (5 missing) | |
| Mechanical ventilation | 98.1% | 95.2% (1 missing) | 0.048 |
| Invasive ventilation | 70.4% | 78.7% (1 missing) | 0.004 |
| NIV | 48.4% | 31.8% (1 missing) | <0.001 |
| Vasoactive drugs | 46.5% | 48.6% (1 missing) | 0.542 |
| Dialysis or CVVH | 2.4% | 2.3% | 0.973 |
| ICU stay (days) | Mean 13.7; SD 14.0; median 10; IQR 4–18 | Mean 11.0; SD 14.7; Median 6; IQR 3–14 (1 missing) | 0.009 |
| ICU mortality | 20.2% | 29.5% (1 missing) | 0.004 |
| Hospital mortality | 22.1% | 38.2% (1 missing) | <0.001 |
SD standard deviation, BMI body mass index, COPD chronic obstructive pulmonary disease, SAPS simplified acute physiology scores, NIV noninvasive ventilation, CVVH continuous venovenous hemodialysis, ICU intensive care unit, IQR interquartile range
The occupancy rate in the ICUs during the pandemic was much the same as in the corresponding period of the previous year (October–December 2008; p = 0.072), but significantly lower than during the 2009 seasonal influenza period (January–March 2009; p = 0.001, see Fig. 2). The results were similar when the analysis was restricted to the reference centers.
Fig. 2.
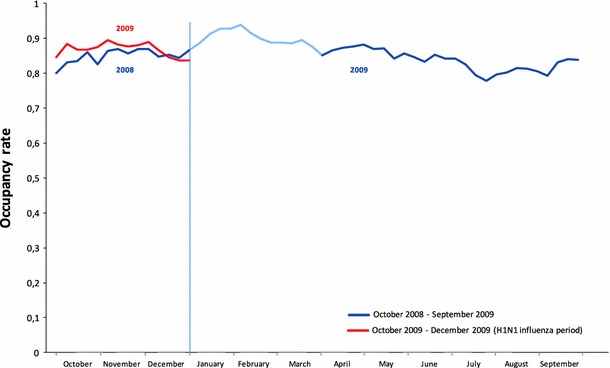
ICU occupancy rate over time The light blue line indicates the 2009 seasonal influenza period (January–March). The average weekly occupancy rate was obtained by dividing the number of occupied beds each hour by the total number of beds available in the ICUs, and averaging the hourly rates over the week. This provided a very precise estimate that takes account of patients staying just a few hours in the ICU
Figure 3 shows the density of cases of CAP per 1,000 ICU beds from 2005 to 2009, in the 85 ICUs in the A(H1N1) registry. In 2009 the peak was earlier, higher, and narrower than in the previous years.
Fig. 3.
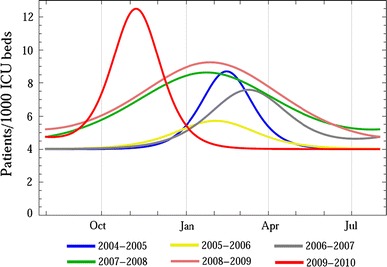
Density functions of CAP during the seasonal influenza periods in different years
The logistic regression model was used to assess the prognostic value of influenza A(H1N1) on 3,891 CAP admissions to the ICUs between March 2008 and April 2010. Rough mortality was lower among patients with influenza A(H1N1) than the controls (22.1 vs. 38.2%; p < 0.0001). A total of 21 variables entered the final model (see ESM). Figure 4 shows the odds ratio (OR) for A(H1N1) influenza, unadjusted and after adjustment for each variable individually (left-hand column) and cumulatively in the forward models (right-hand column). Interestingly, age and SAPS II were able, even alone, to adjust the rough OR of A(H1N1) influenza to a nonsignificant value (Fig. 4, left-hand column). The two confounders partly overlapped, so their combined effect was lower than the sum of their single components (Fig. 4, right-hand column). Conversely, ARDS lowered the A(H1N1) OR from 0.47 to 0.38. In the full model, A(H1N1) had no significant influence on hospital mortality (OR 0.88; 95% CI 0.59–1.31; p = 0.52), as shown in the last row of Fig. 4, right-hand column. The variable identifying the seasonal influenza period was not significant (OR 1.02; 95% CI 0.85–1.22; p = 0.83).
Fig. 4.
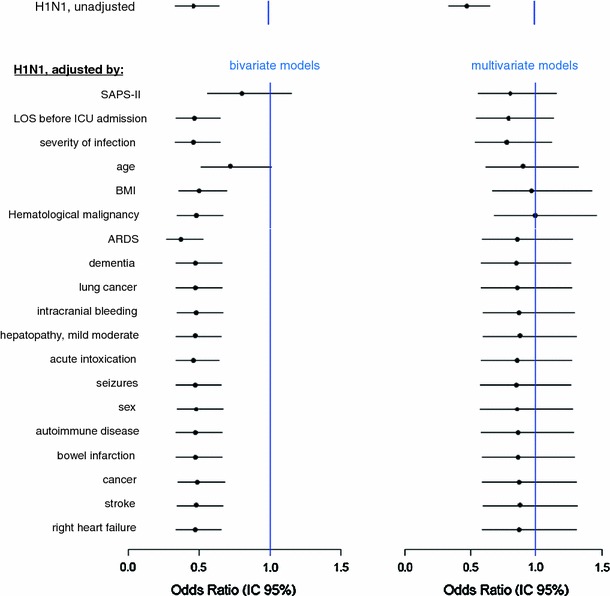
OR of hospital mortality due to A(H1N1) pneumonia compared with non-A(H1N1) CAP, from different multivariate models, and OR of H1N1 after different adjustments. In the left-hand column the H1N1 OR is adjusted by the only variable specified in the corresponding row, in a bivariate model. In the right-hand column, the H1N1 OR is adjusted by the variable specified in the corresponding row plus all the variables listed before, in a multivariate model. Hence, for example, the third row of the left column gives the odds ratio of H1N1 after adjustment for severity of infection; the same row on the right gives the odds ratio of H1N1 after adjustment for severity of infection, length of stay (LOS) before ICU admission, and SAPS II. In the full model, the OR of H1N1 was not statistically significant (0.88; 95% CI 0.59–1.31; p = 0.52), as shown in the last row of the right column
Discussion
Our study has achieved two main results. First, it describes A(H1N1) infections in Italian ICUs and compares them with other studies. Second, it provides new information on the severity of the disease. During the epidemic phase in Italy, the vaccination campaign was not yet underway or was in its early stages, and only about 1.4% of the population had been vaccinated at the end of the campaign. Accordingly, our results should not have been substantially influenced by the effects of vaccination.
Epidemiological description
There were more young people among the patients with A(H1N1)-related CAP than in the control group with CAP of different origin. Compared with the Australian–New Zealand and Canadian series, fewer women were admitted to the ICU in Italy. Fewer were pregnant, particularly compared with Australia (9.6 vs. 17.6%). However, if we directly standardize the percentage of Italian pregnant women to the Australian population structure by sex and birthrate [20], we obtain the figure of 14.2%, which is much closer to the Australian one.
Looking at the raw data, obesity seemed to be a less important risk factor for ICU admission in Italy than in Australia and Canada. However, adjusting for the lower prevalence of obesity in Italy [21], these differences disappear. Obesity appeared to be a risk factor for ICU admission, compared with the control group, but it did not prove to be a mortality predictor in the logistic regression model. Similar results were found in Australian–New Zealand [13].
Another important finding is that the ICU occupancy rate was only slightly and not significantly higher during the A(H1N1) Italian epidemic phase than in the same period in 2008, but significantly lower than in the 2009 seasonal influenza period. In the absence of detailed information, we cannot ascribe the increase in ICU admissions at the beginning of 2009 to seasonal influenza. In other words, we cannot state that patients with 2009 seasonal influenza were more prevalent in the ICUs than those with A(H1N1) influenza, at their corresponding peaks. Nevertheless, on comparing the density of CAP per 1,000 ICU beds in the last 7 years (Fig. 3) with the incidence of seasonal influenza provided by the Ministry of Heath (see ESM) the two phenomena are seen to clearly overlap. This suggests that at least the peak in ICU CAP may be related to seasonal influenza. At any rate, the observation that the Italian ICUs were not overcrowded during the A(H1N1) influenza outbreak, proves that the ICU system, which already has very high occupancy rates, was able to absorb the H1N1 wave without additional workload. However, we do not know whether this was achieved by transferring critically ill patients, delaying elective surgery, or by other strategies. Only the Australian–New Zealand study provided the percentages of ICU beds occupied by A(H1N1) patients (on average 5.2% over the 3-month period), but did not make any comparison with previous periods [13].
Severity of A(H1N1) influenza from the ICU perspective
The severity of influenza epidemics is usually expressed by the CFR, but it is hard to give a precise indication. Usually, only people who spontaneously consult a physician enter the count. Thus patients with mild forms are rarely included, whereas those who die are more likely to enter the analysis [6], causing bias. Moreover, different ways of calculating the CFR lead to very different results [8, 22]. In addition, widespread alarm about the pandemic probably made more people consult their physician, consequently increasing the number of confirmed cases, hampering any comparison with previous epidemics [23]. Then too, CFR is not adjusted for prognostic factors, further undermining the comparison.
Our alternative was to investigate the severity of A(H1N1) influenza through the ICU, where most severe cases are admitted. Although we have historical data, direct comparison with other viral CAP was impracticable, as virological tests were seldom done before 2009 so few such cases have been reported in the Margherita database. We were, however, able to compare A(H1N1) patients with pneumonia and those with non-A(H1N1)-related CAP, providing adjusted mortality comparisons. This assesses whether patients with A(H1N1)-related CAP were at higher risk of death than those with CAP of different etiology. In Italy, on account of the limited numbers of ICU beds, admission criteria are quite strict, selecting CAP patients who mostly need ventilatory support, often have severe sepsis or septic shock, and are thus a high risk of mortality (Table 3).
The logistic regression model was restricted to the ICUs taking part in the Margherita Project in 2008. This way we included the 2008–2009 seasonal influenza wave, which was accounted for in the logistic regression analysis but it did not prove to be prognostically relevant, indicating that CAP was not more serious during the influenza period than during the rest of the year.
Our main finding is that A(H1N1) was not a predictor of mortality. Although overall crude hospital mortality was lower for A(H1N1) CAPs than for the control group (22.1 vs. 38.2%), age and severity of illness were strong confounders. The relatively low crude mortality rate among ICU patients with A(H1N1) CAP was mainly attributable to younger age and a lower SAPS II. After adjusting for these important confounders, patients with A(H1N1) infection were found to have the same probability of dying as those with severe non-A(H1N1) CAP.
In a sensitivity analysis without SAPS II, to avoid the chance that it might have masked the influence of important risk factors, the result about A(H1N1) pneumonia did not change. The following risk factors entered the model: deep coma on admission, chronic renal failure, coming from a medical ward, cardiogenic shock, hypovolemic shock, and acute liver failure. These risk factors were shared by A(H1N1) CAP and non-A(H1N1) CAP as no interaction with A(H1N1) infection was found.
Our data do not show whether the widespread use of ECMO (14.3%, Table 2), hardly ever employed to treat CAP outside the H1N1 pandemic, significantly lowered the expected mortality, limiting the validity of our control group.
In summary, these findings cast fresh light on the importance of the pandemic, considering its low crude mortality rate [24], and the alarm raised after reports of a high CFR among young people [4, 25].
The influenza pandemic had certain peculiar features, mainly affecting young people, many without serious comorbidities, and directly causing atypical pneumonia and deep hypoxia. However, the probability of dying from an A(H1N1) CAP was comparable to that of extra-pandemic periods. Although this cools the alarm about the severity of the disease, it is still true that the pandemic affected many young people, and the burden in terms of young lives lost cannot be overlooked [12].
The creation of registries and dedicated case report forms for national and international surveillance of worldwide epidemics has been recommended and an international research group, the International Forum of Acute Care Trialists (InFACT), set up a global registry for A(H1N1) patients admitted to ICUs [26, 27]. Permanent databases are important to rapidly establish case report forms for new, specific problems, in the context of an existing data-collection network. ICUs are largely accustomed to data collection and this allows timely responses using high quality data. Registries that have been running for many years also provide data for historical controls, which serve to measure what is happening in the present.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors substantially contributed to the conception and design (all authors), analysis (GB, SF, MM, and CR), and interpretation (all authors) of data, drafting the article (GB and DP), or critically revising it (all authors). All authors approved the final version of the manuscript. None of the authors has any conflict of interest in relation to this work. GB and CR had full access to all of the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. The study was wholly funded by GiViTI-Istituto di Ricerche Farmacologiche Mario Negri, Bergamo (Italy). GiViTI is the recipient of unconditioned grants from Bellco, Brahms, and Astellas, which did not, however, have any role in this study. The authors would like to thank Abramo Anghileri and Michele Giardino (Istituto di Ricerche Farmacologiche Mario Negri) for their help to developing and maintaining the software for data collection. Special thanks go to Judith Baggott and Joanne Fleming for their invaluable help in editing the manuscript.
Appendix
List of participating clinicians (with their location in brackets):
Cristina Caracciolo (Abano Terme, PD), Adalgisa Caracciolo (Acquaviva delle Fonti, BA), Cristina Chiani (Adria, RO), Silvia Scarrone (Alessandria, AL), Massimo Gianni (Aosta, AO), Rita Gabini (Arezzo, AR), Maurizio Greco (Ariano Irpino, AV), Ernesto Della Mora (Arzignano, VI), Claudia Acciarri (Ascoli Piceno, AP), Eleonora Costanzo (Asti, AT), Angelo Blasetti (Avezzano, AQ), Daniele Poole (Belluno, BL), Vittorio Zanni (Bentivoglio, BO), Gianmariano Marchesi (Bergamo, BG), Daniela Codazzi (Bergamo, BG), Ermanno Spagarino (Biella, BI), Elena Chinelli (Bologna, BO), Vieri Parrini (Borgo San Lorenzo, FI), Arcangelo Bartoccini (Borgomanero, NO), Annamaria Acquarolo (Brescia, BS), Ricardo Martinez Escobar (Brescia, BS), Corinna Boniotti (Brescia, BS), Nicola Bronzini (Brescia, BS), Alessandra Besozzi (Busto Arsizio, VA), Stefano Mancosu (Cagliari, CA), Simonetta Pastorini (Camposampiero, PD), Francesco Bona (Candiolo, TO), Giovanni Bassi (Carrara, MS), Maria Angela Scolari (Casalmaggiore, CR), Giancarlo Negro (Casarano, LE), Angelo Benedetti (Castel San Giovanni, PC), Maurizio Pegoraro (Castelfranco Veneto, TV), Pierfrancesco Di Masi (Castellana Grotte, BA), Pasqualino Quattrocchi (Catania, CT), Giacomo Castiglione (Catania, CT), Giuseppe Garofalo (Catania, CT), Francesco Ferla (Cefalù, PA), Martino Gregorio Legnani (Cento, FE), Andreina Grioni (Cernusco sul Naviglio, MI), Emiliano Gamberini (Cesena, FC), Alessandro Mastroianni (Chieri, TO), Paolo Perino (Ciriè, TO), Livio Todesco (Cittadella, PD), Maria Federica Magatti (Como, CO), Paolo Dal Cero (Conegliano, TV), Licia Lucchetta (Cosenza, CS), Luciano Crema (Cremona, CR), Giandomenico Zonta (Desenzano, BS), Eduardo Beck (Desio, MI), Lorella Altafini (Dolo, VE), Paola Rosa Solda’ (Domodossola, VB), Lamberto Padovan (Este, PD), Pierpaolo Casalini (Faenza, RA), Giorgio Mantovani (Ferrara, FE), Lorenzo Doni (Firenze, FI), Rossana Fiaschi (Firenze, FI), Valerio Mangani (Firenze, FI), Lea Fabbri (Firenze, FI), Massimo Barattini (Firenze, FI), Manuela Bonizzoli (Firenze, FI), Emilio Fabbri (Forlì, FC), Enrico Arditi (Genova, GE), Michele Isetta (Genova, GE), Marcus Ferretti (Genova, GE), Antonio Lapolla (Genova, GE), Roberto Madonna (Grosseto, GR), Rosa Salcuni (Ivrea, TO), Roberto Buonanno (Lacco Ameno, NA), Erminio Righini (Lagosanto, FE), Mario Tavola (Lecco, LC), Leonardo Bossi (Legnano, MI), Massimiliano Nardini (Lido di Camaiore, LU), Daniela Boccalatte (Lucca, LU), Maria Babini (Lugo di Romagna, RA), Emanuela Brunori (Macerata, MC), Giovanni Negri (Magenta, MI), Benvenuto Antonini (Manerbio, BS), Alberto Baratta (Massa, MS), Elisa Barberi (Massa, MS), Maria Grazia Schievenin (Matera, MT), Maurizio Rossi (Menaggio, CO), Antonio David (Messina, ME), Domenica Claudia Risitano (Messina, ME), Alberto Sicignano (Milan, MI), Marco Pulici (Milan, MI), Massimo Raffaeli (Milan, MI), Angelo Pezzi (Milan, MI), Sergio Colombo (Milan, MI), Martin Langer (Milan, MI), Edi Prandi (Milan, MI), Paola Bruzzone (Milan, MI), Marco Guido Alberto Cigada (Milan, MI), Marco Rambaldi (Modena, MO), Ilaria Cavazzuti (Modena, MO), Gilberto Fiore (Moncalieri, TO), Paola Bignone (Mondovì, CN), Andrea Bianchin (Montebelluna, TV), Valeria Roticiani (Montevarchi, AR), Roberto Rona (Monza, MI), Maria Giovanna De Cristofaro (Napoli, NA), Pio Zannetti (Napoli, NA), Maurizio Prato (Novi Ligure, AL), Franco Pala (Olbia, OT), Lorenzo Odetto (Orbassano, TO), Giovanni Pasetti (Orbetello Scalo, GR), Giuseppina Bonaccorso (Padova, PD), Paola Cogo (Padova, PD), Paolo Persona (Padova, PD), Andrea Cracchiolo (Palermo, PA), Maria Barbagallo (Parma, PR), Teresa Sabina Mediani (Pavia, PV), Mirko Belliato (Pavia, PV), Adonella Gorietti (Perugia, PG), Cesare Breschi (Pesaro, PU), Claudia Cipollone (Pescara, PE), Cesare Benanti (Pescia, PT), Piero Aurelio Segalini (Piacenza, PC), Giulio Briano (Pietra Ligure, SV), Elisa Bellocchio (Pieve di Coriano, MN), Luca Doroni (Pisa, PI), Manuela Carli (Pistoia, PT), Daniela Laudano (Poggibonsi, SI), Armando Alborghetti (Ponte San Pietro, BG), Eugenia Roberto (Pontedera, PI), Alberto Garelli (Ravenna, RA), Valter Bottari (Reggio Emilia, RE), Simona Rossi (Rho, MI), Francesca Facondini (Rimini, RN), Pasquale De Negri (Rionero in Vulture, PZ), Massimiliano Parlanti Garbero (Rivoli, TO), Roberto Alberto De Blasi (Rome, RM), Giuseppe Nardi (Rome, RM), Giuseppe Angelo Vulcano (Rossano, CS), Mara Olga Bernasconi (Rovigo, RO), Valentina Bellato (Rozzano, MI), Graziano Cortis (Rozzano, MI), Giuseppe Calicchio (Salerno, SA), Silvano Papiri (San Benedetto del Tronto, AP), Flavio Badii (San Donà di Piave, VE), Giuseppe Piredda (Sassari, SS), Maddalena Coaloa (Savigliano, CN), Maurizio Rizzi (Seriate, BG), Edith Casadei (Siena, SI), Riccardo Pannacci (Spoleto, PG), Daniela Silengo (Turin, TO), Vincenzo Segala (Turin, TO), Sally Calva (Turin, TO), Marcella Converso (Turin, TO), Giorgio Pettazzi (Tortona, AL), Mario Morbelli (Treviglio, BG), Lorenzo Menato (Treviso, TV), Alessandro Rech (Varese, VA), Cristina Blando (Vercelli, VC), Silvio Marafon (Vicenza, VI), Pietro Vecchiarelli (Viterbo, VT), Marialuisa Pizzaballa (Zingonia, BG).
GiViTI Steering Committee (in alphabetic order, with their location in brackets):
Guido Bertolini (Ranica, BG), Daniela Boccalatte (Lucca), Arturo Chieregato (Cesena), Daniela Codazzi (Bari), Roberto Fumagalli (Monza), Giorgio Gambale (Forlì), Martin Langer (Milan), Sergio Livigni (Turin), Giuseppe Nardi (Rome), Paolo Malacarne (Pisa), Daniele Poole (Belluno), Danilo Radrizzani (Legnano, MI), Mario Tavola (Lecco).
Footnotes
Gruppo Italiano per la Valutazione degli Interventi in Terapia Intensiva (Italian Group for the Evaluation of Interventions in Intensive Care Medicine) is an independent collaboration network of Italian ICUs.
The complete list of study participants appears in the appendix.
References
- 1.Chan M (2009) World now at the start of 2009 influenza pandemic. Available via http://www.who.int/mediacentre/news/statements/2009/h1n1_pandemic_phase6_20090611/en/index.html. Accessed 8 Aug 2011
- 2.Centers for Disease Control and Prevention (2009) Swine influenza A(H1N1) infection in two children—Southern California, March–April 2009. Morb Mortal Wkly Rep 58(15):400–402. Available via http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5815a5.htm. Accessed 8 Aug 2011 [PubMed]
- 3.Chan M (2009) Influenza A(H1N1): lessons learned and preparedness. Available via http://www.who.int/dg/speeches/2009/influenza_h1n1_lessons_20090702/en/index.html. Accessed 8 Aug 2011
- 4.Vaillant L, La Ruche G, Tarantola A, Barboza P, for the epidemic intelligence team at InVS (2009) Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill 14(33):pii=19309. Available via http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19309. Accessed 8 Aug 2011 [DOI] [PubMed]
- 5.Fraser C, Donnelly CA, Cauchemez S, Hanage WP, Van Kerkhove MD, Hollingsworth TD, Griffin J, Baggaley RF, Jenkins HE, Lyons EJ, Jombart T, Hinsley WR, Grassly NC, Balloux F, Ghani AC, Ferguson NM, Rambaut A, Pybus OG, Lopez-Gatell H, Alpuche-Aranda CM, Chapela IB, Zavala EP, Guevara DM, Checchi F, Garcia E, Hugonnet S, Roth C. Pandemic potential of a strain of influenza A (H1N1): early findings. Science. 2009;324:1557–1561. doi: 10.1126/science.1176062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garske T, Legrand J, Donnelly CA, Ward H, Cauchemez S, Fraser C, Ferguson NM, Ghani AC. Assessing the severity of the novel influenza A/H1N1 pandemic. BMJ. 2009;339:b2840. doi: 10.1136/bmj.b2840. [DOI] [PubMed] [Google Scholar]
- 7.Dawood FS, Hope KG, Durrheim DN, Givney R, Fry AM, Dalton CB. Estimating the disease burden of pandemic (H1N1) 2009 virus infection in Hunter New England, Northern New South Wales, Australia, 2009. PLoS One. 2010;5:e9880. doi: 10.1371/journal.pone.0009880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Presanis AM, De Angelis D, Hagy A, Reed C, Riley S, Cooper BS, Finelli L, Biedrzycki P, Lipsitch M. The severity of pandemic H1N1 influenza in the United States, from April to July 2009: a Bayesian analysis. PLoS Med. 2009;6:e1000207. doi: 10.1371/journal.pmed.1000207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donaldson LJ, Rutter PD, Ellis BM, Greaves FE, Mytton OT, Pebody RG, Yardley IE. Mortality from pandemic A/H1N1 2009 influenza in England: public health surveillance study. BMJ. 2009;339:b5213. doi: 10.1136/bmj.b5213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuite AR, Greer AL, Whelan M, Winter AL, Lee B, Yan P, Wu J, Moghadas S, Buckeridge D, Pourbohloul B, Fisman DN. Estimated epidemiologic parameters and morbidity associated with pandemic H1N1 influenza. CMAJ. 2010;182:131–136. doi: 10.1503/cmaj.091807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishiura H. The relationship between the cumulative numbers of cases and deaths reveals the confirmed case fatality ratio of a novel influenza A (H1N1) virus. Jpn J Infect Dis. 2010;63:154–156. [PubMed] [Google Scholar]
- 12.Viboud C, Miller M, Olson D, Osterholm M, Simonsen L (2010) Preliminary estimates of mortality and years of life lost associated with the 2009 A/H1N1 pandemic in the US and comparison with past influenza seasons. PLoS Curr:RRN1153 [DOI] [PMC free article] [PubMed]
- 13.Webb SA, Pettila V, Seppelt I, Bellomo R, Bailey M, Cooper DJ, Cretikos M, Davies AR, Finfer S, Harrigan PW, Hart GK, Howe B, Iredell JR, McArthur C, Mitchell I, Morrison S, Nichol AD, Paterson DL, Peake S, Richards B, Stephens D, Turner A, Yung M. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361:1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 14.Jain S, Kamimoto L, Bramley AM, Schmitz AM, Benoit SR, Louie J, Sugerman DE, Druckenmiller JK, Ritger KA, Chugh R, Jasuja S, Deutscher M, Chen S, Walker JD, Duchin JS, Lett S, Soliva S, Wells EV, Swerdlow D, Uyeki TM, Fiore AE, Olsen SJ, Fry AM, Bridges CB, Finelli L. Hospitalized patients with 2009 H1N1 influenza in the United States, April–June 2009. N Engl J Med. 2009;361:1935–1944. doi: 10.1056/NEJMoa0906695. [DOI] [PubMed] [Google Scholar]
- 15.Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, Turgeon AF, Lapinsky S, Ahern SP, Smith O, Siddiqui F, Jouvet P, Khwaja K, McIntyre L, Menon K, Hutchison J, Hornstein D, Joffe A, Lauzier F, Singh J, Karachi T, Wiebe K, Olafson K, Ramsey C, Sharma S, Dodek P, Meade M, Hall R, Fowler RA. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. Jama. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 16.Dominguez-Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa-Perez L, de la Torre A, Poblano-Morales M, Baltazar-Torres JA, Bautista E, Martinez A, Martinez MA, Rivero E, Valdez R, Ruiz-Palacios G, Hernandez M, Stewart TE, Fowler RA. Critically Ill patients with 2009 influenza A(H1N1) in Mexico. Jama. 2009;302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 17.Boffelli S. Continuous quality improvement in intensive care medicine. The GiViTI Margherita Project. Report 2005. Minerva Anestesiol. 2005;72:1935–1940. [PubMed] [Google Scholar]
- 18.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. Jama. 1993;270:2957–2963. doi: 10.1001/jama.270.24.2957. [DOI] [PubMed] [Google Scholar]
- 19.Hosmer D, Lemeshow S. Applied logistic regression. New York: Wiley; 1989. [Google Scholar]
- 20.Schaible T, Hermle D, Loersch F, Demirakca S, Reinshagen K, Varnholt V. A 20-year experience on neonatal extracorporeal membrane oxygenation in a referral center. Intensive Care Med. 2010;36:1229–1234. doi: 10.1007/s00134-010-1886-5. [DOI] [PubMed] [Google Scholar]
- 21.National Obesity Observatory of the National Health Service (2009) International comparisons of obesity prevalence. Available via http://www.noo.org.uk/uploads/doc799_2_International_Comparisons_Obesity_Prevalence2.pdf. Accessed 8 Aug 2011
- 22.Wilson N, Baker MG (2009) The emerging influenza pandemic: estimating the case fatality ratio. Euro Surveill 14(26):pii=19255. Available via http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=19255. Accessed 8 Aug 2011 [PubMed]
- 23.Nishiura H. Case fatality ratio of pandemic influenza. Lancet Infect Dis. 2010;10:443–444. doi: 10.1016/S1473-3099(10)70120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chowell G, Bertozzi SM, Colchero MA, Lopez-Gatell H, Alpuche-Aranda C, Hernandez M, Miller MA. Severe respiratory disease concurrent with the circulation of H1N1 influenza. N Engl J Med. 2009;361:674–679. doi: 10.1056/NEJMoa0904023. [DOI] [PubMed] [Google Scholar]
- 25.Doshi P. How should we plan for pandemics? BMJ. 2009;339:b3471. doi: 10.1136/bmj.b3471. [DOI] [PubMed] [Google Scholar]
- 26.Bouadma L, Mourvillier B, Deiler V, Derennes N, Le Corre B, Lolom I, Regnier B, Wolff M, Lucet JC. Changes in knowledge, beliefs, and perceptions throughout a multifaceted behavioral program aimed at preventing ventilator-associated pneumonia. Intensive Care Med. 2010;36:1341–1347. doi: 10.1007/s00134-010-1890-9. [DOI] [PubMed] [Google Scholar]
- 27.Fowler RA, Webb SA, Rowan KM, Sprung CL, Thompson BT, Randolph AG, Jouvet P, Lapinsky S, Rubinson L, Rello J, Cobb JP, Rice TW, Uyeki T, Marshall JC. Early observational research and registries during the 2009–2010 influenza A pandemic. Crit Care Med. 2010;38:e120–e132. doi: 10.1097/CCM.0b013e3181d20c77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


