Abstract
Seasonal, pandemic, and avian influenza virus infections may be associated with central nervous system pathology, albeit with varying frequency and different mechanisms. Here, we demonstrate that differentiated human astrocytic (T98G) and neuronal (SH-SY5Y) cells can be infected by avian H7N9 and pandemic H1N1 viruses. However, infectious progeny viruses can only be detected in H7N9 virus infected human neuronal cells. Neither of these viral strains can generate infectious progeny virus in human astrocytes despite replication of viral genome was observed. Furthermore, H7N9 virus triggered high pro-inflammatory cytokine expression, while pandemic H1N1 virus induced only low cytokine expression in either brain cell type. The experimental finding here is the first data to demonstrate that avian H7N9 virus can infect, transcribe, and replicate its viral genome; induce cytokine upregulation; and cause cytopathic effects in human brain cells, which may potentially lead to profound central nervous system injury. Observation for neurological problems due to H7N9 virus infection deserves further attention when managing these patients.
Keywords: Cytokines, Neuroinflammation, Encephalitis, Encephalopathy, Neurodegenerative diseases, Neurological complications
Introduction
The recent avian H7N9 outbreak has caused concern owing to its transmissibility to humans and apparent severity. Since early 2013 to October 2017, there have been 1622 laboratory-confirmed human cases with 619 deaths, although the overall number of human infected cases is believed to be at least 100-fold greater (Yu et al. 2013). An avian virus repeatedly infecting humans on such a scale poses a significant pandemic threat.
The main symptoms of H7N9 infection include fever, cough, and dyspnea; however, the disease sometimes progresses to acute respiratory distress syndrome (ARDS) (Gao et al. 2013a; Shi et al. 2013). Hospitalized patients present with clinical features of primary viral pneumonia, including bilateral ground-glass opacity and consolidation of the lungs; other symptoms include chills, shivering, nausea, vomiting, and diarrhea (Chen et al. 2013; Gao et al. 2013a; Shi et al. 2013).
Neurologic complications associated with influenza infection are rare, but often severe. Seizures and encephalopathy are the most common neurological complications associated with influenza, most frequently observed in young children (Gao et al. 2013b; Togashi et al. 2004). Influenza has been reported to be associated with 2–11% of cases in childhood encephalitis study cohorts (Fowler et al. 2008; Ishikawa et al. 1993). In vivo study demonstrated using ferret model showed that H7N9 virus caused a more severe disease than did the 2009 pandemic H1N1 (pdmH1N1) virus (Yum et al. 2015) and viral H7N9 RNA was detected in the brain of experimentally infected ferrets, suggesting the potential of avian H7N9 virus to spread to the mammalian brain (Kalthoff et al. 2014).
In this study, we investigated the neuropathogenicity of H7N9 virus in vitro using differentiated human brain cells.
Material and methods
Viruses
The influenza viruses used in this study were avian H7N9 virus, A/Shanghai/2/2013 (H7/SH2/13), avian H5N1 virus, A/Vietnam/3212/2004 (H5/3212/04), and two pdmH1N1 strains including A/Hong Kong/415742/2009 (H1/415742/09), isolated from infected patient in Hong Kong 2009 (pdmH1N1) and A/Hong Kong/1750/2011 (H1/1750/11), isolated from a pdmH1N1 case with neurological complications (pdmH1N1-complicated). From their initial isolation, the viruses were propagated in Madin-Darby canine kidney (MDCK) cells. Virus infectivity was determined on MDCK cells and quantified as 50% tissue culture infectious dose (TCID50). All experiments involving H7N9 and H5N1 viruses were conducted in a Biosafety Level 3 facility at the Centre of Influenza Research, The University of Hong Kong.
Cell cultures
MDCK cells were maintained in Minimal Essential Medium (MEM, Invitrogen) supplemented with 10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml of streptomycin and cultured at 37 °C with 5% CO2. Human glioblastoma cells, T98G were maintained in Minimum Essential Medium alpha supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin, at 37 °C in 5% CO2. For astrocyte differentiation, the cells were cultured in medium supplemented with 0.5% serum and 1 μM all-trans retinoic acid (Sigma-Aldrich) for 7 days. Culture medium was changed every 3 days with 1 μM of all-trans retinoic acid. Human neuroblastoma cell line, SH-SY5Y, was maintained in MEM supplemented with 10% FBS, 100 U/ml penicillin and 100 μg/ml streptomycin, at 37 °C in 5% CO2. To induce neuronal differentiation, cells were treated in medium supplemented with 0.5% serum and 10 μM all-trans retinoic acid for 7 days. Culture medium was changed every 3 days with 10 μM of all-trans retinoic acid. Primary human monocyte-derived macrophages were cultured and derived according to the approved protocol by the ethics committee of the University of Hong Kong. Briefly, mononuclear cells were separated from whole blood by gradient centrifugation (Ficoll-paque Plus, GE Healthcare) and purified by plastic adherence. Cells were let differentiate in vitro in RPMI 1640 medium (Invitrogen) supplemented with 5% autologous human serum for 14 days before the infectious experiments.
Virus infection of differentiated human astrocytic and neuronal cells
Differentiated cells were infected at a multiplicity of infection (MOI) of 2 or 0.001 as indicated. The cells were incubated with the virus inoculum for 45 min, then washed with warm PBS and replenished with the appropriate growth medium. Mock-infected cells were incubated with corresponding growth medium throughout the experiment. At the indicated time, culture supernatants were collected for TCID50 study. Cell lysates were collected for RNA extraction using the RNeasy Mini kit (Qiagen), and the cDNA was synthesized for gene expression analysis. Cytopathic effect (CPE) of virus-infected cells was examined under light microscopy (TS100, Nikon).
Real-time quantitative RT-PCR assays
The cDNA was synthesized from mRNA with poly(dT) primers and SuperScript III reverse transcriptase (Invitrogen). For the measurement of viral RNA (vRNA), cDNA was synthesized from vRNA with Uni12 primer (Hoffmann et al. 2001) and ProtoScript II reverse transcriptase (New England Biolabs). Transcript expression was monitored using SYBR Fast qPCR master mix kit (KAPA Biosystems, Wilmington, MA) with corresponding primers. The fluorescence signals were measured using the 7500 real-time PCR system (Applied Biosystems). The specificity of the SYBR® Green PCR signal was confirmed by melting curve analysis. The threshold cycle (CT) was defined as the fractional cycle number at which the fluorescence reached 10 times the standard deviation of the base-line (from cycle 2 to 10). The ratio change in target gene relative to the β-actin control gene was determined by the 2-ΔΔCT method. Specific primers sequences for influenza virus matrix (M) gene and β-actin gene have been described previously (Lee et al. 2008; Lee et al. 2011); pro-inflammatory cytokines: tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), IL-8 and chemokine (C-C motif) ligand 2 (CCL2), and interferon-beta (IFN-β) were described in Table 1.
Table 1.
Primers used in the study
| Gene | Forward primers (5′ — 3′) | Reverse primers (5′ — 3′) |
|---|---|---|
| β-actin | CATGTACGTTGCTATCCAGGC | CTCCTTAATGTCACGCACGAT |
| TNF-α | ATGAGCACTGAAAGCATGATCC | GAGGGCTGATTAGAGAGAGGTC |
| IL-6 | AAATTCGGTACATCCTCGACGG | GGAAGGTTCAGGTTGTTTTCTGC |
| IL-8 | ACTGAGAGTGATTGAGAGTGGAC | AACCCTCTGCACCCAGTTTTC |
| CCL2 | CTGCTCATAGCAGCCACCTT | CAGATCTCCTTGGCCACAAT |
| IFN-β | ATGACCAACAAGTGTCTCCTCC | GCTCATGGAAAGAGCTGTAGTG |
| M gene | CTTCTAACCGAGGTCGAAACG | AGGGCATTTTGGACAAAGCGTCTA |
Statistical analysis
Results from the real-time RT-PCR were analyzed by two-tailed Student’s t test. A p value ≤ 0.05 was considered to be statistically significant.
Results
Cytopathic effect of avian H7N9 virus-infected human astrocytic and neuronal cells
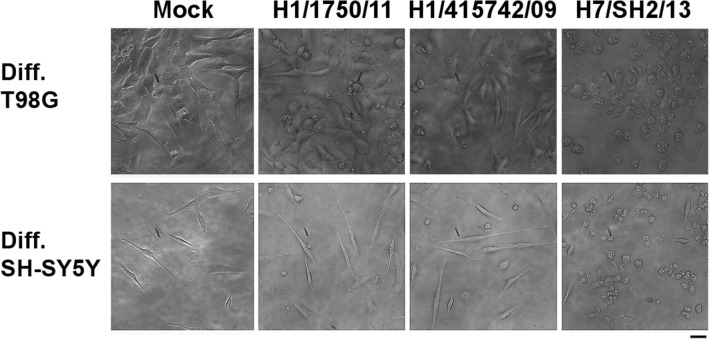
Differentiation of T98G glioblastoma cells and SH-SY5Y neuroblastoma cells are shown in Fig. 1. Treatment of T98G and SH-SY5Y cells with all-trans retinoic acid for 7 days induced differentiation phenotypes. Differentiation of T98G cells into astrocyte lineage (Fig. 1a) and SH-SY5Y cells with retinoic acid induced neurite outgrowth (Fig. 1b) which is consistent to the previously reported studies (Das et al. 2009; Haque et al. 2007; Lovat et al. 1993). Differentiated human T98G glioblastoma cells and SH-SY5Y neuroblastoma cells (Ng et al. 2010) were infected with influenza A H7N9 virus, A/Shanghai/2/2013 (isolated from a fatal human case in Shanghai, China 2013) and pdmH1N1 strains including A/Hong Kong/415742/2009, isolated from pdmH1N1 virus-infected patient and A/Hong Kong/1750/2011, isolated from a patient with neurological complications at a multiplicity of infection (MOI) of 2. CPE (such as cell rounding, vacuolation, and cell detachment) of influenza A virus-infected cells were examined. After 24 h of infection, distinct CPE was observed in astrocytes and neuronal cells infected by H7N9 virus. In contrast, there was only minor cytopathic effect observed in both cell types infected by the two pdmH1N1 virus strains (Fig. 2).
Fig. 1.
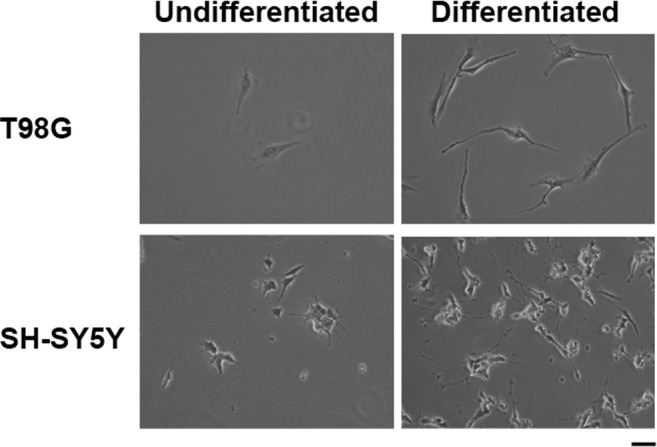
Differentiation of T98G glioblastoma cells and SH-SY5Y neuroblastoma cells with all-trans retinoic acid. a Treatment of T98G cells with all-trans retinoic acid for 7 days differentiated into astrocyte lineage. b Treatment of SH-SY5Y cells with all-trans retinoic acid for 7 days induced differentiation phenotypes with neurite outgrowth. Scale bar, 20 μm
Fig. 2.
Cytopathic effects of avian influenza A H7N9 virus-infected human brain cells. Morphological examination of differentiated human T98G and SH-SY5Y cells after influenza A virus infection. Cells were infected with two strains of influenza A pdmH1N1 viruses and avian H7N9 virus at a MOI of 2. Cytopathic effects such as cell rounding, vacuolation and cell detachment were determined at 24 h after infection. Scale bar, 20 μm
Viral replication kinetics of avian H7N9 in astrocytic and neuronal cells
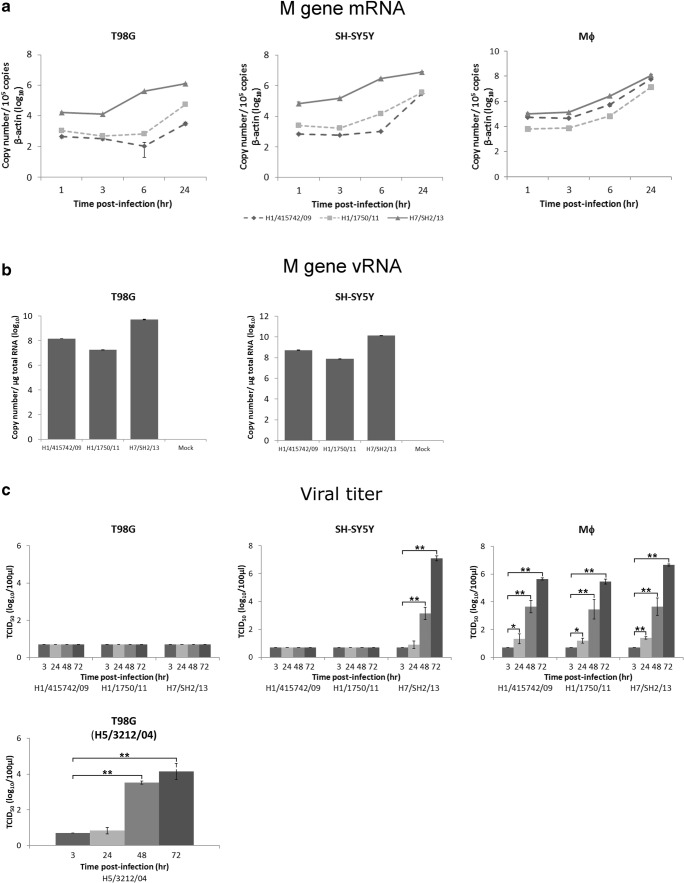
To examine the viral gene transcription and replication kinetics in H7N9 virus and pdmH1N1 virus-infected human brain cells, cell lysates were collected for RNA extraction using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA), cDNA were synthesized by reverse transcription and PCR was performed using real-time PCR with specific primers (Table 1). Detection of the influenza A viral matrix (M) gene has been described previously (Lee et al. 2011). Human primary monocytes-derived macrophages, reported as a permissive host for H7N9 virus (Zhao et al. 2016) were included for comparison.
The detection and increase of viral M gene mRNA expression in astrocytic and neuronal cells infected by virus at MOI of 2 to investigate the viral gene expression in one round of virus replication indicates that H7N9 virus is able to infect and transcribe its viral genome in human brain cells. Induction of viral M gene can also be detected in both cell types after infection by pdmH1N1 viruses, but to a lesser extent compared to that of H7N9 virus at all post-infection time under investigation (Fig. 3a). Higher amount of viral M gene mRNA for H7N9 virus were detected in both brain cell types compared to that of pdmH1N1 virus, while there is no differential difference between H7N9 virus and pdmH1N1 virus in infected primary human monocyte-derived macrophages (Fig. 3a), suggesting an intrinsic difference in viral gene transcription efficiency between H7N9 virus and pdmH1N1 virus in human brain cells. In addition to M gene mRNA, we also detected the replication of vRNA through cRNA synthesis. Similarly, both H7N9 virus and pdmH1N1 virus can efficiently replicate vRNA, with H7N9 virus replicates to a higher magnitude compared with the two pdmH1N1 viruses (Fig. 3b).
Fig. 3.
Replication kinetics of avian influenza A H7N9 virus in human astrocytes, neuronal cells, and primary monocyte-derived macrophages. a Differentiated human astrocytes (T98G), neuronal cells (SH-SY5Y) cells, and human primary monocyte-derived macrophages (Mϕ) were infected by avian H7N9 virus and pdmH1N1 viruses at MOI of 2. Mock-infected cells served as controls. Total RNA was extracted from the infected cells at the indicated times. Influenza A viral matrix mRNA expression was measured by real-time PCR and normalized to that of β-actin. b DifferentiatedT98G and SH-SY5Y cells were infected with influenza A viruses at MOI of 2 for 24 h. Copy number of influenza M gene vRNA was measured by real-time PCR and normalized per microgram of total RNA. c Kinetics of influenza progeny virus production. Differentiated T98G, SH-SY5Y cells or human Mϕ were infected with different influenza A viruses at MOI of 0.001. The culture supernatants were collected at the indicated times, and the viral titers were determined by TCID50 assay. Viral titers of different strains at different time points were compared to that at 3 h post-infection. Data are mean ± standard deviation of representative results from at least three independent experiments. *p ≤ 0.05, **p ≤ 0.005
To further determine the kinetics of infectious progeny virus production in multiple rounds of virus replication, supernatants of cells infected by H7N9 virus and both pdmH1N1 viruses at MOI of 0.001 were collected at different time points and determined the viral titers using a TCID50 assay (Fig. 3c). In human astrocytes, the viral titer of H7N9 virus was undetectable. Interestingly, in infected neuronal cells, H7N9 virus replicated effectively and produced infectious progeny viruses with viral titer significantly increasing from 48 h onwards after infection. No detectable progeny of the two pdmH1N1 viruses was detected from the infected astrocytic and neuronal cells. For comparison, primary human monocyte-derived macrophages produced infectious progeny virus efficiently for all three viral strains, with significant increase in viral titer from 24 to 72 h after infection. As astrocytes seemed not to support the progeny viral production for all the three viral strains examined, we have included an avian H5N1 influenza virus (A/Vietnam/3212/2004) which reported previously to produce infectious progeny virus in astrocytes (Ng et al. 2010) for comparison (Fig. 3c). Significant increase in H5N1 progeny viral titer was detectable from 48 h post-infection onwards, suggesting T98G cells are in fact permissive to influenza virus infection.
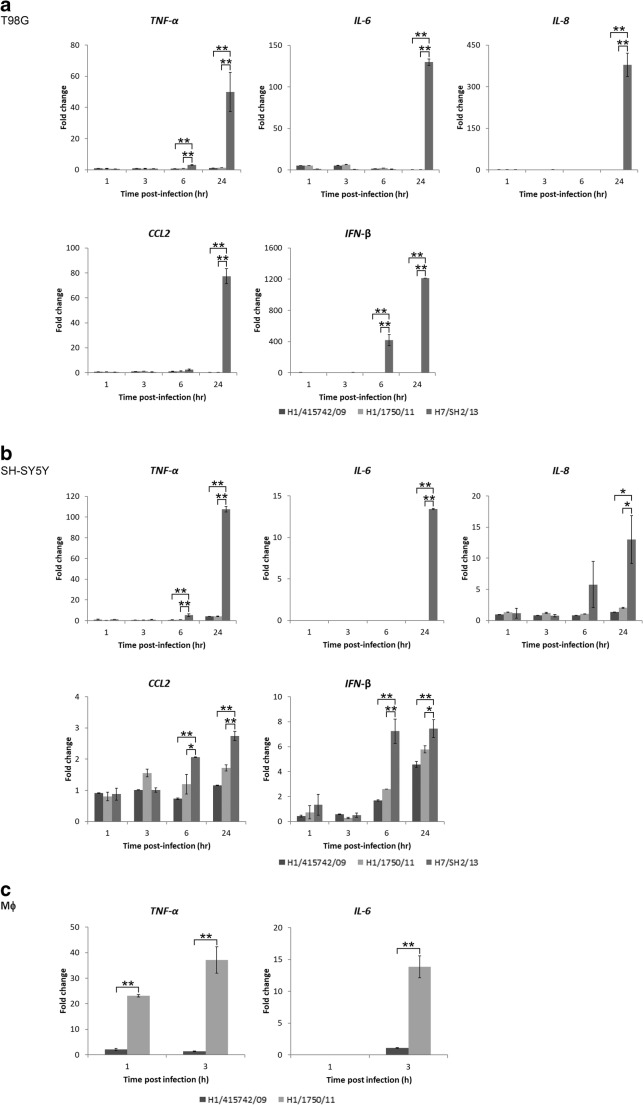
Pro-inflammatory cytokine expression in avian H7N9 virus-infected astrocytic and neuronal cells
During an influenza viral infection, signs of inflammation can be observed in respiratory tissues (Chan et al., 2005). Here, we determined the cytokine expression profiles in human brain cells infected by H7N9 virus compared to that of pdmH1N1 virus infection. In both human astrocytes and neuronal cells, pro-inflammatory cytokines including TNF-α, IL-6, IL-8, and CCL2 as well as type I IFN, IFN-β were significantly upregulated at 24 h after infection by H7N9 virus, while both pdmH1N1 virus strains induced only little to no cytokine expression (Fig. 4a, b). Of note, H7N9 virus induced IL-6, IL-8, CCL2, and IFN-β expression was found to be more potent in infected astrocytes compared to that in neuronal cells. TNF-α and IL-6 have been reported to be associated with influenza virus-related acute encephalitis/encephalopathy (Aiba et al. 2001; Ichiyama et al. 1998), and are commonly expressed upon influenza virus infection (Cheung et al. 2002; Paquette et al. 2012). Absence of induction of TNF-α and IL-6 in pdmH1N1 virus-infected human astrocytic and neuronal cells prompted us to investigate if this effect is cell-type specific. Notwithstanding with the outcome observed in human astrocytes and neuronal cells, expression of TNF-α in primary human monocyte-derived macrophages (Fig. 4c) could be detected as early as at 1 h post-infection and further increased at 3 h after infection by pdmH1N1-complicated virus (H1/1750/11) and was significantly higher compared to that by pdmH1N1 (H1/415742/09) virus. Similarly, expression of IL-6 was significantly higher in pdmH1N1-complicated (H1/1750/11) virus compared to pdmH1N1 (H1/415742/09) virus-infected human macrophages. This data suggested that pdmH1N1 can in fact hyper-induced pro-inflammatory cytokine expression in a cell-type and viral strain-specific manner.
Fig. 4.
Expression of pro-inflammatory cytokines and IFN in avian influenza A H7N9 virus-infected human astrocytes and neuronal cells. a Differentiated human astrocytes (T98G) and b neuronal cells (SH-SY5Y) were infected with different influenza A viruses at MOI of 2. Mock-infected cells served as controls. Total RNA was collected at the indicated times. TNF-α, IL-6, IL-8, CCL2, and IFN-β mRNA expression was examined by real-time PCR. c Human primary monocyte-derived-macrophages (Mϕ) were infected with influenza A viruses at MOI of 2 and total RNA were isolated for quantification of TNF-α and IL-6 expression at 1 and 3 h post-infection time by real-time PCR. The results are expressed as the mean fold change ± standard deviation from at least three independent experiments versus that of corresponding mock controls normalized to β-actin expression. *p ≤ 0.05, **p ≤ 0.005
Discussion
The neurological and cognitive effects of influenza have been recognized for a long time (Jelliffe 1918; Menninger 1994). During the 1918 Spanish flu pandemic, which was the deadliest influenza outbreak, several patients who recovered from the disease subsequently developed psychosis and encephalitic Parkinsonism (Dickman 2001). Accordingly, encephalopathy is important complication associated with influenza infection, described most frequently in young children, particularly in Japan and East Asia (Ekstrand 2012). There were human pdmH1N1 cases reported to have neurological complications including acute encephalopathy, altered mental status, and seizures. Cerebral edema and brainstem lesions can be detected by computed tomography scanning in some patients. Santini et al. report the cases of three pdmH1N1 virus-infected adult patients with viral RNA in cerebrospinal fluid (Santini et al. 2012). In our study, pdmH1N1 viruses could infect and transcribe their viral genome in astrocytic and neuronal cells. However, these viruses failed to produce infectious progeny viruses and did not cause or just exhibited minor cytopathic effects in the two brain resident cells. Although, there were no remarkable difference between the two pdmH1N1 strains with respect to viral replication kinetics and cytokine induction in human brain cells, our data (Fig. 4c) here demonstrated that pdmH1N1 viruses isolated from neurologically complicated case (H1/1750/11) induced much higher pro-inflammatory cytokines, TNF-α and IL-6 in primary human macrophages at early post-infection time. This suggests that pdmH1N1-associated neurological complications are more likely to be a consequence of peripheral viral infection rather than caused by direct invasion of the brain, while the detailed mechanisms underlying the neurological symptoms deserves further investigation.
We previously demonstrated that the highly pathogenic avian H5N1 influenza can infect human astrocytic and neuronal cells, resulting in the induction of severe CPE and pro-inflammatory cytokine cascades (Ng et al. 2010). In the present study, we extend our study to investigate the tropism and replication competence of lately emerging avian H7N9 virus and found that H7N9 virus could also infect, replicate, produce infectious progeny viruses, and cause CPE in human neuronal cells. More remarkably, H7N9 virus induced potent cytokine expression in human brain cells, suggesting that H7N9 virus infection may potentially cause profound CNS injury. Of note, our present data suggested that neurons could be a major source for H7N9 virus production in human brain (Fig. 3c), whereas astrocytes being the most abundant glial cells in human brain (Guillamon-Vivancos et al. 2015) could be an important producer in the brain infected with H7N9 virus. There was a more differential upregulation of pro-inflammatory cytokine expression (IL-6, IL-8, CCL2, and IFN-β) in infected astrocytes (Fig. 4a) compared to that in neuronal cells (Fig. 4b). In particular, the induction level of IL-6 was remarkably elevated in astrocytes, which normally secrete neurotrophic factors and cytokines to regulate immune responses in the brain (Jensen et al. 2013; van Heteren et al. 2008). As a result, the dysregulated cytokine profile of astrocytes could lead to CNS complications and further trigger inflammatory cascade and cause damage in the brain in addition to the direct CPE due to influenza infection. Indeed, some studies report a significant association between increased secretion of these pro-inflammatory cytokines and the incidence of influenza virus-related acute encephalitis/encephalopathy (Ichiyama et al. 1998; Ito et al. 1999). In addition, astrocytes and neuronal cells could cross talk and interact in an autocrine/paracrine manner. The treatment of differentiated astrocytes with TNF-α gradually increases IL-6 expression in astrocytes for up to 3 days and causes a substantial loss of cell adhesion (Ng et al. 2010; van Kralingen et al. 2013). Astrocytes produce IL-8 in respond to TNF-α stimulation (Aloisi et al. 1992). IL-8 secreted into the cerebrospinal fluid has been suggested to correlate with the dysfunction in the blood-brain barrier (BBB) (Kossmann et al. 1997). As the BBB is important in controlling viral invasion into the central nervous system, hyper-induction of IL-8 by astrocytes may lead to a detrimental disease outcome during H7N9 infection via increasing the BBB permeability to virus infection. Another cytokine, CCL2 has also been reported to be induced upon TNF-α stimulation (He et al. 2016). CCL2 produced from astrocytes and neurons activates microglials to M1 phenotype (He et al. 2016) and recruits inflammatory monocytes to the brain (Howe et al. 2017) which could lead to neurodegeneration (He et al. 2016; Howe et al. 2017). In the present study, most cytokine induced by H7N9 infection in astrocytes was detected at a later infection stage, implying that cytokine induction may not be a direct effect of virus infection but is more likely to be a result of cross talk with other infected cells by autocrine/paracrine feedback in a pro-inflammatory cascade. Furthermore, it has been demonstrated that upon viral infection, astrocytes are one of the major resident IFN-producing cells in the CNS (Kallfass et al. 2012); this is corroborated by the findings of the present study as H7N9-infected astrocytes exhibited over a 1000-fold increase in IFN-β induction.
Intrinsic difference in the magnitude of viral mRNA transcription was observed between H7N9 and pdmH1N1 virus in astrocytes and neuronal cells but not in macrophages. H7N9 virus has a more pronounced viral mRNA transcription in differentiated human brain cells compared to that of H1N1 virus. In fact, both viral mRNA and vRNA can be detected suggesting a productive transcription of viral mRNA from vRNA and replication of vRNA through cRNA synthesis in H7N9 and pdmH1N1 virus-infected astrocytes and neuronal cells. Failure in infectious progeny virus production in astrocytes may be due to many factors such as inhibition in viral mRNA translation, aberrant post-translation modifications, attenuation in viral assembly, or budding during the replication cycle. For example, aberrant post-translation modifications of viral proteins such as viral HA N-glycosylation (Rossignol et al. 2009) has been reported to affect influenza virus progeny production. Failure in nuclear export of nascent viral ribonucleoprotein complex as a result of expression of host restriction factors (Qiao et al. 2018), perturbation in intracellular trafficking of influenza viral protein due to disruption in intracellular trafficking machineries (Ramos-Nascimento et al. 2017), or attenuation in viral assembly or budding during the replication cycle. Of interest, astrocytes are believed to be a major cholesterol producer in the brain (Ferris et al. 2017) and cholesterol homeostasis has been suggested to play important role in the production of progeny virus (Musiol et al. 2013), whether this could be part of an explanation for the lack of progeny virus generated from H7N9 and pdmH1N1 virus-infected astrocytes remained to be investigated.
To our best knowledge, this is the first study to investigate the tropism, replication competence, and innate immune response of avian H7N9 virus in differentiated human astrocytes and neurons. The findings here provide important insights into the neuropathogenesis of this virus. In summary, we demonstrated that avian H7N9 virus effectively produce infectious progeny and cause severe CPE in human neuronal cells. The pdmH1N1 viruses cannot produce progeny viruses in either cell type. In comparison to pdmH1N1 viruses, the H7N9 virus exhibits a higher capacity to elicit pro-inflammatory innate immune responses in human brain cells. These phenotypic differences among viruses may be relevant to the pathogenesis of neurological complications occurring after H7N9 virus infection. Observation for neurological problems due to H7N9 virus infection deserves further attention when managing these patients.
Acknowledgements
The authors thank Mr. Li Ping-hung, Ms. Aisha Selim, and Ms. Li Shu-ting for their technical support, Dr. Fanny Ip for the helpful discussion, and Ms. Capucine Jacob-Chavagnac for revising the manuscript.
Funding information
This research was supported in part by Hong Kong Research Grants Council (HKUST 1/06C), Area of Excellence Scheme (AoE/B-15/01 and AoE/M-12/06), Theme-based Research Scheme (T11-705/14-N), Health and Medical Research Fund (12111822 and 14130662), and Hong Kong Jockey Club, and Roche R&D Center (China) Ltd. (RRDCCL12SC01).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Aiba H, Mochizuki M, Kimura M, Hojo H. Predictive value of serum interleukin-6 level in influenza virus-associated encephalopathy. Neurology. 2001;57:295–299. doi: 10.1212/WNL.57.2.295. [DOI] [PubMed] [Google Scholar]
- Aloisi F, Care A, Borsellino G, Gallo P, Rosa S, Bassani A, Cabibbo A, Testa U, Levi G, Peschle C. Production of hemolymphopoietic cytokines (IL-6, IL-8, colony-stimulating factors) by normal human astrocytes in response to IL-1 beta and tumor necrosis factor-alpha. J Immunol. 1992;149:2358–2366. [PubMed] [Google Scholar]
- Chan MC, Cheung CY, Chui WH, Tsao SW, Nicholls JM, Chan YO, Chan RW, Long HT, Poon LL, Guan Y, Peiris JS. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir Res. 2005;6:135. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Liang W, Yang S, Wu N, Gao H, Sheng J, Yao H, Wo J, Fang Q, Cui D, Li Y, Yao X, Zhang Y, Wu H, Zheng S, Diao H, Xia S, Zhang Y, Chan KH, Tsoi HW, Teng JL, Song W, Wang P, Lau SY, Zheng M, Chan JF, To KK. Chen H, Li L, Yuen KY. Human infections with the emerging avian influenza A H7N9 virus from wet market poultry: clinical analysis and characterisation of viral genome. Lancet. 2013;381:1916–1925. doi: 10.1016/S0140-6736(13)60903-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CY, Poon LL, Lau AS, Luk W, Lau YL, Shortridge KF, Gordon S, Guan Y, Peiris JS. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831–1837. doi: 10.1016/S0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- Das A, Banik NL, Ray SK. Molecular mechanisms of the combination of retinoid and interferon-gamma for inducing differentiation and increasing apoptosis in human glioblastoma T98G and U87MG cells. Neurochem Res. 2009;34:87–101. doi: 10.1007/s11064-008-9669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman MS. von Economo encephalitis. Arch Neurol. 2001;58:1696–1698. doi: 10.1001/archneur.58.10.1696. [DOI] [PubMed] [Google Scholar]
- Ekstrand JJ. Neurologic complications of influenza. Semin Pediatr Neurol. 2012;19:96–100. doi: 10.1016/j.spen.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Ferris HA, Perry RJ, Moreira GV, Shulman GI, Horton JD, Kahn CR. Loss of astrocyte cholesterol synthesis disrupts neuronal function and alters whole-body metabolism. Proc Natl Acad Sci U S A. 2017;114:1189–1194. doi: 10.1073/pnas.1620506114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler A, Stodberg T, Eriksson M, Wickstrom R. Childhood encephalitis in Sweden: etiology, clinical presentation and outcome. Eur J Paediatr Neurol. 2008;12:484–490. doi: 10.1016/j.ejpn.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Gao HN, Lu HZ, Cao B, Du B, Shang H, Gan JH, Lu SH, Yang YD, Fang Q, Shen YZ, Xi XM, Gu Q, Zhou XM, Qu HP, Yan Z, Li FM, Zhao W, Gao ZC, Wang GF, Ruan LX, Wang WH, Ye J, Cao HF, Li XW, Zhang WH, Fang XC, He J, Liang WF, Xie J, Zeng M, Wu XZ, Li J, Xia Q, Jin ZC, Chen Q, Tang C, Zhang ZY, Hou BM, Feng ZX, Sheng JF, Zhong NS, Li LJ. Clinical findings in 111 cases of influenza A (H7N9) virus infection. N Engl J Med. 2013;368:2277–2285. doi: 10.1056/NEJMoa1305584. [DOI] [PubMed] [Google Scholar]
- Gao R, Cao B, Hu Y, Feng Z, Wang D, Hu W, Chen J, Jie Z, Qiu H, Xu K, Xu X, Lu H, Zhu W, Gao Z, Xiang N, Shen Y, He Z, Gu Y, Zhang Z, Yang Y, Zhao X, Zhou L, Li X, Zou S, Zhang Y, Li X, Yang L, Guo J, Dong J, Li Q, Dong L, Zhu Y, Bai T, Wang S, Hao P, Yang W, Zhang Y, Han J, Yu H, Li D, Gao GF, Wu G, Wang Y, Yuan Z, Shu Y. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–1897. doi: 10.1056/NEJMoa1304459. [DOI] [PubMed] [Google Scholar]
- Guillamon-Vivancos T, Gomez-Pinedo U, Matias-Guiu J. Astrocytes in neurodegenerative diseases (I): function and molecular description. Neurologia. 2015;30:119–129. doi: 10.1016/j.nrl.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Haque A, Das A, Hajiaghamohseni LM, Younger A, Banik NL, Ray SK. Induction of apoptosis and immune response by all-trans retinoic acid plus interferon-gamma in human malignant glioblastoma T98G and U87MG cells. Cancer Immunol Immunother. 2007;56:615–625. doi: 10.1007/s00262-006-0219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M, Dong H, Huang Y, Lu S, Zhang S, Qian Y, Jin W. Astrocyte-derived CCL2 is associated with M1 activation and recruitment of cultured microglial cells. Cell Physiol Biochem. 2016;38:859–870. doi: 10.1159/000443040. [DOI] [PubMed] [Google Scholar]
- Hoffmann E, Stech J, Guan Y, Webster RG, Perez DR. Universal primer set for the full-length amplification of all influenza A viruses. Arch Virol. 2001;146:2275–2289. doi: 10.1007/s007050170002. [DOI] [PubMed] [Google Scholar]
- Howe CL, LaFrance-Corey RG, Goddery EN, Johnson RK, Mirchia K. Neuronal CCL2 expression drives inflammatory monocyte infiltration into the brain during acute virus infection. J Neuroinflammation. 2017;14:238. doi: 10.1186/s12974-017-1015-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama T, Nishikawa M, Yoshitomi T, Hayashi T, Furukawa S. Tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-6 in cerebrospinal fluid from children with prolonged febrile seizures. Comparison with acute encephalitis/encephalopathy. Neurology. 1998;50:407–411. doi: 10.1212/WNL.50.2.407. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Asano Y, Morishima T, Nagashima M, Sobue G, Watanabe K, Yamaguchi H. Epidemiology of acute childhood encephalitis. Aichi Prefecture, Japan, 1984-90. Brain Dev. 1993;15:192–197. doi: 10.1016/0387-7604(93)90064-F. [DOI] [PubMed] [Google Scholar]
- Ito Y, Ichiyama T, Kimura H, Shibata M, Ishiwada N, Kuroki H, Furukawa S, Morishima T. Detection of influenza virus RNA by reverse transcription-PCR and proinflammatory cytokines in influenza-virus-associated encephalopathy. J Med Virol. 1999;58:420–425. doi: 10.1002/(SICI)1096-9071(199908)58:4<420::AID-JMV16>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Jelliffe SE. Nervous and mental disturbances of influenza. New York: A.R. Elliott Publishing Company; 1918. [Google Scholar]
- Jensen CJ, Massie A, De Keyser J. Immune players in the CNS: the astrocyte. J NeuroImmune Pharmacol. 2013;8:824–839. doi: 10.1007/s11481-013-9480-6. [DOI] [PubMed] [Google Scholar]
- Kallfass C, Ackerman A, Lienenklaus S, Weiss S, Heimrich B, Staeheli P. Visualizing production of beta interferon by astrocytes and microglia in brain of La Crosse virus-infected mice. J Virol. 2012;86:11223–11230. doi: 10.1128/JVI.01093-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalthoff D, Bogs J, Grund C, Tauscher K, Teifke JP, Starick E, Harder T, Beer M. Avian influenza H7N9/13 and H7N7/13: a comparative virulence study in chickens, pigeons, and ferrets. J Virol. 2014;88:9153–9165. doi: 10.1128/JVI.01241-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kossmann T, Stahel PF, Lenzlinger PM, Redl H, Dubs RW, Trentz O, Schlag G, Morganti-Kossmann MC. Interleukin-8 released into the cerebrospinal fluid after brain injury is associated with blood-brain barrier dysfunction and nerve growth factor production. J Cereb Blood Flow Metab. 1997;17:280–289. doi: 10.1097/00004647-199703000-00005. [DOI] [PubMed] [Google Scholar]
- Lee SM, Cheung CY, Nicholls JM, Hui KP, Leung CY, Uiprasertkul M, Tipoe GL, Lau YL, Poon LL, Ip NY, Guan Y, Peiris JS. Hyperinduction of cyclooxygenase-2-mediated proinflammatory cascade: a mechanism for the pathogenesis of avian influenza H5N1 infection. J Infect Dis. 2008;198:525–535. doi: 10.1086/590499. [DOI] [PubMed] [Google Scholar]
- Lee SM, Gai WW, Cheung TK, Peiris JS. Antiviral effect of a selective COX-2 inhibitor on H5N1 infection in vitro. Antivir Res. 2011;91:330–334. doi: 10.1016/j.antiviral.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Lovat PE, Pearson AD, Malcolm A, Redfern CP. Retinoic acid receptor expression during the in vitro differentiation of human neuroblastoma. Neurosci Lett. 1993;162:109–113. doi: 10.1016/0304-3940(93)90572-3. [DOI] [PubMed] [Google Scholar]
- Menninger KA. Influenza and schizophrenia. An analysis of post-influenzal “dementia precox,” as of 1918, and five years later further studies of the psychiatric aspects of influenza. 1926. Am J Psychiatry. 1994;151:182–187. doi: 10.1176/ajp.151.6.182. [DOI] [PubMed] [Google Scholar]
- Musiol A, Gran S, Ehrhardt C, Ludwig S, Grewal T, Gerke V, Rescher U. Annexin A6-balanced late endosomal cholesterol controls influenza A replication and propagation. MBio. 2013;4:e00608–e00613. doi: 10.1128/mBio.00608-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng YP, Lee SM, Cheung TK, Nicholls JM, Peiris JS, Ip NY. Avian influenza H5N1 virus induces cytopathy and proinflammatory cytokine responses in human astrocytic and neuronal cell lines. Neuroscience. 2010;168:613–623. doi: 10.1016/j.neuroscience.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Paquette SG, Banner D, Zhao Z, Fang Y, Huang SS, Leomicronn AJ, Ng DC, Almansa R, Martin-Loeches I, Ramirez P, Socias L, Loza A, Blanco J, Sansonetti P, Rello J, Andaluz D, Shum B, Rubino S, de Lejarazu RO, Tran D, Delogu G, Fadda G, Krajden S, Rubin BB, Bermejo-Martin JF, Kelvin AA, Kelvin DJ. Interleukin-6 is a potential biomarker for severe pandemic H1N1 influenza A infection. PLoS One. 2012;7:e38214. doi: 10.1371/journal.pone.0038214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y, Yan Y, Tan KS, Tan SSL, Seet JE, Arumugam TV, Chow VT, Wang Y, Tran T. CD151, a novel host factor of nuclear export signaling in influenza virus infection. J Allergy Clin Immunol. 2018;141:1799–1817. doi: 10.1016/j.jaci.2017.11.032. [DOI] [PubMed] [Google Scholar]
- Ramos-Nascimento A, Kellen B, Ferreira F, Alenquer M, Vale-Costa S, Raposo G, Delevoye C, Amorim MJ. KIF13A mediates trafficking of influenza A virus ribonucleoproteins. J Cell Sci. 2017;130:4038–4050. doi: 10.1242/jcs.210807. [DOI] [PubMed] [Google Scholar]
- Rossignol JF, La Frazia S, Chiappa L, Ciucci A, Santoro MG. Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at the post-translational level. J Biol Chem. 2009;284:29798–29808. doi: 10.1074/jbc.M109.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini M, Kutlesa M, Zarkovic K, Drazenovic V, Barsic B. Influenza A 2009 H1N1 encephalitis in adults with viral RNA in cerebrospinal fluid. Scand J Infect Dis. 2012;44:992–996. doi: 10.3109/00365548.2012.689849. [DOI] [PubMed] [Google Scholar]
- Shi J, Xie J, He Z, Hu Y, He Y, Huang Q, Leng B, He W, Sheng Y, Li F, Song Y, Bai C, Gu Y, Jie Z. A detailed epidemiological and clinical description of 6 human cases of avian-origin influenza A (H7N9) virus infection in Shanghai. PLoS One. 2013;8:e77651. doi: 10.1371/journal.pone.0077651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togashi T, Matsuzono Y, Narita M, Morishima T. Influenza-associated acute encephalopathy in Japanese children in 1994-2002. Virus Res. 2004;103:75–78. doi: 10.1016/j.virusres.2004.02.016. [DOI] [PubMed] [Google Scholar]
- van Heteren JT, Rozenberg F, Aronica E, Troost D, Lebon P, Kuijpers TW. Astrocytes produce interferon-alpha and CXCL10, but not IL-6 or CXCL8, in Aicardi-Goutieres syndrome. Glia. 2008;56:568–578. doi: 10.1002/glia.20639. [DOI] [PubMed] [Google Scholar]
- van Kralingen C, Kho DT, Costa J, Angel CE, Graham ES. Exposure to inflammatory cytokines IL-1beta and TNFalpha induces compromise and death of astrocytes; implications for chronic neuroinflammation. PLoS One. 2013;8:e84269. doi: 10.1371/journal.pone.0084269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Cowling BJ, Feng L, Lau EH, Liao Q, Tsang TK, Peng Z, Wu P, Liu F, Fang VJ, Zhang H, Li M, Zeng L, Xu Z, Li Z, Luo H, Li Q, Feng Z, Cao B, Yang W, Wu JT, Wang Y, Leung GM. Human infection with avian influenza a H7N9 virus: an assessment of clinical severity. Lancet. 2013;382:138–145. doi: 10.1016/S0140-6736(13)61207-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yum J, Ku KB, Kim HS, Seo SH. H7N9 influenza virus is more virulent in ferrets than 2009 pandemic H1N1 influenza virus. Viral Immunol. 2015;28:590–599. doi: 10.1089/vim.2015.0052. [DOI] [PubMed] [Google Scholar]
- Zhao C, Qi X, Ding M, Sun X, Zhou Z, Zhang S, Zen K, Li X. Pro-inflammatory cytokine dysregulation is associated with novel avian influenza A (H7N9) virus in primary human macrophages. J Gen Virol. 2016;97:299–305. doi: 10.1099/jgv.0.000357. [DOI] [PubMed] [Google Scholar]