Abstract
Bovine herpesvirus 5 (BHV5) infection of young cattle is frequently associated with fatal neurological disease and, as such, represents an attractive model for studying the pathogenesis of viral-induced meningoencephalitis. Following replication in the nasal mucosa, BHV5 invades the central nervous system (CNS) mainly through the olfactory pathway. The innate immune response triggered by the host face to virus replication through the olfactory route is poorly understood. Recently, an upregulation of conserved pathogen-associated molecular pattern, as Toll-like receptors (TLRs), has been demonstrated in the CNS of BHV5 experimentally infected cows. A new perspective to understand host-pathogen interactions has emerged elucidating microRNAs (miRNAs) network that interact with innate immune response during neurotropic viral infections. In this study, we demonstrated a link between the expression of TLRs 3, 7, and 9 and miR-155 transcription in the olfactory bulbs (OB) of 16 cows suffering from acute BHV5-induced neurological disease. The OBs were analyzed for viral antigens and genome, miR-155 and TLR 3, 7, and 9 expression considering three major regions: olfactory receptor neurons (ORNs), glomerular layer (GL), and mitral cell layer (ML). BHV5 antigens and viral genomes, corresponding to glycol-C gene, were detected in all OBs regions by fluorescent antibody assay (FA) and PCR, respectively. TLR 3, 7, and 9 transcripts were upregulated in ORNs and ML, yet only ORN layers revealed a positive correlation between TLR3 and miR-155 transcription. In ML, miR-155 correlated positively with all TLRs studied. Herein, our results evidence miR-155 transcription in BHV5 infected OB tissue associated to TLRs expression specifically ORNs which may be a new window for further studies.
Keywords: BHV5, miRNAs, Neuropathogenesis, Neuroinvasion, Innate immune response
Introduction
Bovine herpesvirus 5 (BHV5) belongs to the family Herpesviridae, subfamily Alphaherpesvirinae, and genus Varicellovirus (Davison et al. 2009; Davison 2010). BHV5 is a neurotropic virus that produces neurological disease mainly in young animals worldwide, especially in South American countries (Vogel et al. 2003). Following primary replication in the nasal mucosa, BHV5 is believed to invade the central nervous system (CNS) mainly through the olfactory route (Vogel et al. 2003; Mori et al. 2004; Perez et al. 2006). The olfactory mucosa is composed by a neuroepithelium divided into layers organized initially by nasal mucosa, olfactory receptor neurons (ORNs), glomerular layer (GL), cribriforme plate, mitral cell layer (ML), and limbic system (Mori et al. 2004). The olfactory receptor neurons (ORNs) are bipolar neuronal cells whose axons leave the epithelium, penetrate the cribriform plate, enter the olfactory bulb and, finally, make synapse with the dendrites of mitral cells in the glomerular layer (Mori et al. 2004; Shivkumar et al. 2013). Mitral cells project to the olfactory and limbic systems, mainly the olfactory cortex and hippocampus, respectively (Mori et al. 2004; van Riel et al. 2015). During acute infection, BHV5 gains access mostly to the CNS cranial areas, indicating that viral invasion occurs more directly along olfactory neurons (Chowdhury et al. 1997; Favier et al. 2014). Despite evidences indicating that BHV5 uses the olfactory pathway to invade the CNS, detailed information on the sequential progression of the virus—and the host-mediated immune events—through the OB layers is lacking.
Toll-like receptors (TLRs) are a broad family of conserved innate immune receptors that recognize pathogen-associated molecular patterns (PAMPs) (Xagorari and Chlichlia 2008). TLRs 3, 7, 8, and 9 are expressed in intracellular vesicles and recognize nucleic acids from viral origin (Cardoso et al. 2016a). Cattle experimentally infected with BHV5 showed an overexpression of TLRs 3, 7, and 9 in the frontal cerebral cortex, including the OBs, during acute infection and/or following virus reactivation (Marin et al. 2015). We have previously demonstrated that the host immune response and inflammation play a crucial role in the pathogenesis of acute encephalitis by BHV5 in cattle (Cardoso et al. 2016b). In many aspects, there is a consensus that BHV5 acute infection induces activation of pro-inflammatory cytokines and chemokines that eventually lead to brain injury, especially in frontal cortices (Marin et al. 2015). However, the portion/areas of the OBs more affected by inflammation process remain unclear.
MicroRNAs (miRNAs) represent small RNA species composed by ~ 20–14 nucleotides found in virtually all living organisms (Grey 2015; Sorel and Dewals 2016). Mature miRNAs are able to cause either mRNA degradation or translational repression regulating physiological process by target genes after their transcription (Glazov et al. 2009). Moreover, host miRNAs control herpesvirus infection by inhibiting viral or host transcripts (Piedade and Azevedo-Pereira 2016; Powdrill et al. 2016; Dickey et al. 2017). Recent data indicate that miR-155 plays a critical role in immunity, inflammation, and viral infections (Bhela et al. 2014; Dickey et al. 2016). Moreover, miR-155 is expressed in a variety of immune cells, including B cells, macrophages, types of T cells, NK cells, and dendritic cells Dickey et al. 2017). In addition, miR-155 modulation may act to control Japanese encephalitis virus infection (Pareek et al. 2004) and to enhance T cell trafficking in a model of coronavirus-induced neurological disease (Dickey et al. 2016). In fact, a number of studies have demonstrated that T cell responses are impaired in the absence of miR-155 during infection with some neurotropic viruses (Bhela et al. 2014; Bhela et al. 2015; Dickey et al. 2016; Dickey et al. 2017). Recently, induction of miR-155 was associated to HSV1 acute encephalitis in a mouse model (Majer et al. 2017). Considering the finding described for HSV1 encephalitis (Shivkumar et al. 2013; Bhela et al. 2014; Grey 2015; Sorel and Dewals 2016; Majer et al. 2017), it would be worth to investigate miRNA expression in the CNS during BHV5 infection.
In this study, we investigated TLRs 3, 7, and 9 transcription associated with the distribution of BHV5 antigens and genome in OB portions/layers of cattle naturally infected with BHV5. In addition, expression of host miR-155 was also examined. The results were compared in order to describe a possible participation of these components in the pathogenesis/immune response in natural BHV5 acute infections.
Materials and methods
Olfactory bulb samples
Brains of 16 cattle with positive diagnosis of acute BHV5 neurological disease (Cardoso et al. 2016b) and from eight healthy cattle were obtained from routine pathological diagnostics at the Laboratory of Animal Virology of University of São Paulo state, Brazil. The brain samples were selected from 38 cattle reported previously (Cardoso et al. 2016b) based on OB anatomical preservation. All applicable institutional guidelines for the care and use of animals were followed (CEEA 2015/09765). The whole brain was carefully removed, sectioned into two halves and the olfactory bulbs (OB) were gently removed to preserve the anatomical and histological structures. Two fragments of approximately 8.5 mm were collected from each OB (Fig. 1) and stored in RNA later®-ICE (Ambien®, Life Technologies™, Carlsbad, CA, USA) at − 86 °C for molecular analysis, and immersed in 10% formalin for immunofluorescence in histological sections (Marin et al. 2015; Vogel et al. 2003).
Fig. 1.
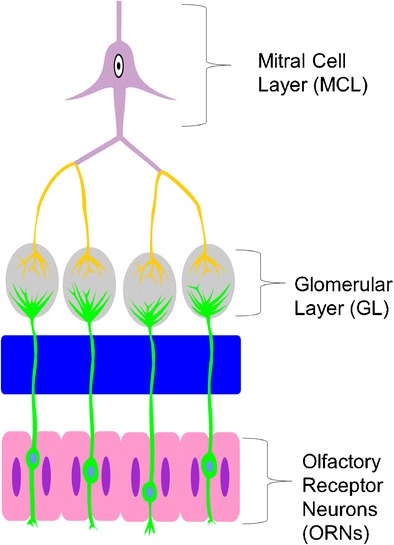
Schematic overview of the bovine olfactory bulb (OB) regions examined in this study: olfactory receptor neurons (ORNs), glomerular layer (GL), and mitral layer (ML)
BHV5 antigen detection in OBs
Briefly, OB histological slides (infected and controls) prepared as described previously (Cardoso et al. 2016b) were hydrated for 15 min followed by incubation overnight at 4 °C with 1:50 diluted monoclonal antibody to BHV5 (BHV52F9) (Goldoni et al. 2004) and 1:100 diluted N200 monoclonal antibody (Sigma-Aldrich®) produced against neurofilament. After three washes, slides were incubated with the respective goat secondary antibody (1:100) anti-mouse FITC (Sigma-Aldrich®) and nuclear staining was performed with 1 mg/ml of DAPI (4′-6-diamino-2-phenylindole; Sigma-Aldrich®) was diluted in Fluormount™ aqueous medium. The images were collected under an AxioImager® A.1 light and an ultraviolet (UV) microscope connected to an AxioCam®MRc (Carl Zeiss, Oberkochen, Germany). The images were processed using AxioVision® 4.8 software (Carl Zeiss) for viral antigen detection and neuron cells.
BHV5 DNA detection by quantitative real-time polymerase chain reaction
Briefly, total DNA from each OB region and respective negative control was extracted using DNAzol™ according to manufacturer’s instructions (Invitrogen®). An average of 100 ng of genomic DNA were used for quantitative real-time polymerase chain reaction (qPCR) and BHV5 primers and respective probes developed for glycol-C gene were used as described previously (Cardoso et al. 2016b). The data obtained from three replicates were carried out by qPCR reaction and analyzed by the software on a StepOnePlus™ real time instrument (Applied Biosystems®). Housekeeping Histone 2a gene (H2A) was used for normalization and comparative delta-delta Ct method applied to analyze the results (Livak and Schmittgen 2001).
RNA extraction, RNA integrity and cDNA generation
OB samples stored at − 80 °C in RNAlater™ (Ambien, Paisley, UK) were thawed on ice and RNA extracted using a Trizol™ protocol according to manufacturer’s instructions (Invitrogen®) with a DNase digestion step to remove contaminating genomic DNA using TurboDNase™ (Ambien, Paisley, UK). RNA concentration was determined using the Nanodrop™ ND-2000 spectrophotometer (Thermo-Scientific, Wilmington, DE, USA) and samples were diluted in nuclease-free water appropriately to give a final concentration of 40 ng/μL per sample. The RNA integrity was determined using an Agilent 2100 Bioanalyzer™ (Agilent Technologies, Waldbronn, Germany). All samples included in this study had an RNA Integrity Number (RIN) value in excess of six. cDNA was generated using an Enhanced Avian™ RT First Strand Synthesis Kit (Sigma-Aldrich®), aliquoted and stored at − 20 °C.
Molecular quantification of miR-155 and Toll-like receptors 3, 7, and 9
The qPCR was applied in order to quantify TLR 3, 7, and 9 genes using a single tube assay from 100 ng of cDNA prepared. Both TaqMan™ FAM-MGB primers and probes: TLR3 Bt03210267_m1 (cat # 4351372), TLR7 Bt03225261_s1 (cat # 4351373), and TLR9 Bt03224812_m1 (cat # 4331182). The expression of the housekeeping Histone 2a gene was also quantified in a similar way as viral DNA. The qPCR reaction was conducted as described previously (Cardoso et al. 2016b).The same amount of cDNA applied on TLR3, 7, and 9 transcription was used to detect sequence mature of bta-miR-155 (cat # 25576) and amplification was performed using Advanced miRNA Assay™ (Applied Biosystems®). All reactions were carried out and analyzed as described for BHV5 DNA detection.
Statistical analysis
All experiments were performed at least in duplicate. Descriptive statistics included the mean ± standard deviation (s.d.). A p value < 0.005 was considered significant. All statistical analyses were performed using Prism software (GraphPad® v.6, CA, USA). Gene expression values were transformed in logs and correlations were evaluated with Spearman correlation coefficient. The differences between various groups were examined for a significance using the non-parametric Mann-Whitney U test.
Results
BHV5 antigens and genome detection
The control samples, represented by OBs obtained from healthy animals, were included to elucidate the histology of each studied area, ORNs, GL, and ML (Fig. 2a–c, respectively). The distribution of BHV5 antigens in ONRs, GL, and ML regions is illustrated in Fig. 2d–f, respectively. The ONRs showed positive fluorescence in olfactory neurons and glomerular layer showed by boxed areas (Fig. 2d, e, respectively). The ML revealed positive BHV5 antigens not associated with mitral cells (Fig. 2f). After labeled with BHV52F9 monoclonal control, OBs did not show any positive signal for viral antigens in ONRs, GL, and ML regions (Fig. 2g–i). Detection of BHV5 in the OBs reinforces the role of the olfactory pathway as a route for BHV5 neuroinvasion. These findings correlated with the distribution of BHV5 genome, since all OBs regions were positive for glyco-C BHV5 expression (Fig. 3a).
Fig. 2.
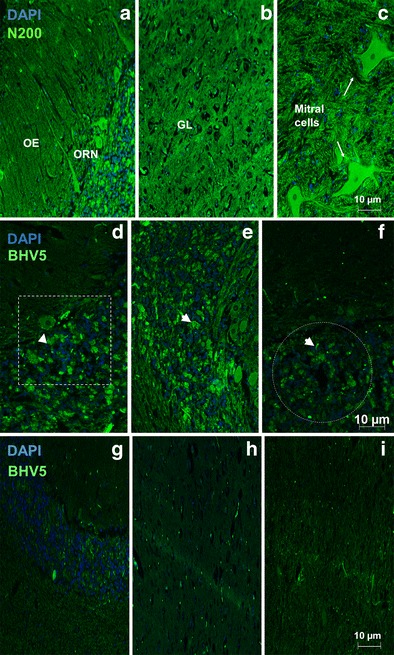
Analysis of negative samples showed OBs, divided into a ORNs, b GL, and c ML stained with N200 monoclonal antibody whereas OE-olfactory epithelium, olfactory receptor neurons, and GL-glomerular layer are represented, respectively. The photomicrography is a representative for all negative OBs analyzed. BHV5 antigen visualization by immunofluorescence performed using monoclonal antibody (BHV5 2F9) against BHV 5 (green) followed by DAPI (blue) counterstain. The photomicrography is a representative for all positive 16 OBs, divided into d ORNs, e GL, and f ML analyzed. The arrows indicate positive label for BHV5 and boxed areas represent viral antigens, ×100 magnification. The negative OB samples are illustrated g ORNs, h GL, and i ML, respectively
Fig. 3.
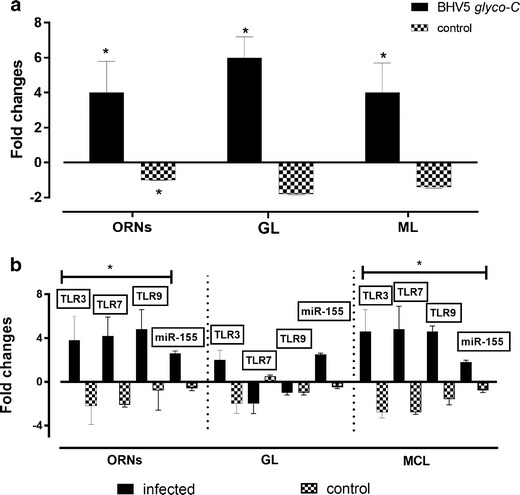
a Detection of viral DNA amplification in ORNs, GL, and ML from olfactory bulbs suspensions collected from positive and negative animals. b Transcription of TLR3, TLR7, TLR9, and miR-155 by qPCR in ORNs, GL, and ML from the same tissue samples. The data is a media (X) plus standard deviation (s.d) of positive and negative samples analyzed
TLR3, TLR7, and TLR9 expression associated to miR-155 transcription
Despite viral expression detected in all OBs regions, only ORNs and ML regions showed TLR3, 7, and 9 upregulation (Fig. 3b). Moreover, only TLR3 was upregulated in GL, while TLR7 and 9 were downregulated. No positive results were observed among control samples. In regard to miR-155 expression, only TLR3 correlated positively in ORNs, r = 0.92 (Fig. 4a). No correlation was observed between TLR and miR-155 expression in GL region (Fig. 4b). However, the highest correlation between the same parameter was found in ML, whereas r > 0.90 was observed in all OBs regions (Fig. 4c).
Fig. 4.
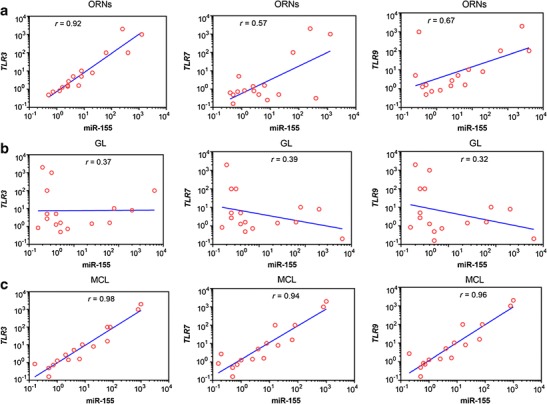
Correlation between TRL3, TLR7, TLR9, and miR-155 expression in a ORNs, b GL, and c ML from bovine olfactory bulbs naturally infected with BHV5. The correlation coefficient (r) was calculated according to the Spearman analysis and p < 0.005 was considered significant
Discussion
Toll-like receptors (TLRs) play an important role in host defense against pathogens, including viruses. Among these, TLR3, TLR7, and TLR9 are involved in antiviral responses through cytokine activation, such as the type-I IFNs (Xagorari and Chlichlia 2008). Studies focusing on the interaction between BHV5 and TLRs in the CNS are scarce (Marin et al. 2015). The results presented in the present study provide new information on the regulation of TLR3, TLR7, and TLR9 in ORNs, GL, and ML of OBs by association of their expression with miR-155 transcription. Taken together, these data indicate that BHV5 infection may result in a differential expression of TLRs in neural tissues. Basically, TLR3 showed correlation with miR-155 transcription in ORNs, and all studied TLRs were positively correlated with miR-155 transcription in ML.
The olfactory mucosa is an external barrier of the nasal cavity in human and animals (Mori et al. 2004; van Riel et al. 2015; Winkler et al. 2015). The olfactory neuroepithelium consists of a limited number of cell types, including ORN (Winkler et al. 2015). The course of neurotropic virus infection may include a latent phase in the OBs and neuroepithelium, representing potential for virus reactivation (Mori et al. 2004). For example, HSV1 established latent infection in the murine OBs after experimental infection (Shivkumar et al. 2013) and Equine herpesvirus type 1 (EHV1) remained latent in mitral cells after experimental infection of mice (Mori et al. 2004). Moreover, after experimental inoculation of rabbits, BHV5 is transported retrogradely from the nasal cavity and may be found in the OBs and limbic system (Ludlow et al. 2016). These findings are in agreement, to what has been described in the present study. Nonetheless, detection/localization of BHV5 antigens in ORNs and GL of OBs regions has not been described previously.
Despite the fact that TLR9 was the only receptor affected by viral infection, and only animals acutely infected showed high levels of TLR9 transcription, olfactory cortices were not the main brain region of TLR9 detection (Marin et al. 2015). These findings contrast our results, in which, in addition to OBs, ORNs, and ML regions also presented upregulation of TLRs. Moreover, BHV5 antigens and genomes were also detected in ORNs and GL regions.
In fact, it has been described that olfactory nerve acts as a shortcut for influenza and other viruses into the CNS (Van Riel et al. 2015) and the neuroepithelium for HSV 1 invasion (Shivkumar et al. 2013). Moreover, defects in TLR3-interferon (IFN) and IFN-responsive pathways were shown to facilitate HSV encephalitis (Verma and Bharti 2017). Therefore, TLR3-mediate immune response in CNS infection should be considered an important pathway to control neuroinvasion (Verma and Bharti 2017). In addition, capillaries in the OBs were shown to be susceptible to virus-induced vascular leak and to facilitate neuroinvasion (Winkler et al. 2015). Recently, a novel role for miR-155 in host defense in a model of viral-induced encephalomyelitis has been supported, by enhancing antiviral T cell responses including cytokine secretion, cytolytic activity, and homing to the CNS in response to coronavirus infection (Dickey et al. 2016). In conclusion, miR-155 may play either a host-protective or damaging role during neuroinflammation, depending on the disease trigger (Pareek et al. 2004). Interestingly, BHV5 has been shown to encode different microRNAs when compared with BHV1, which could help to explain the differences in cell/tissue tropism (Tang Qi et al. 2014). Taking our results together, we can speculate that ORNs could be the target cells for BHV5 neuroinvasion during natural infections. In, the HSV1 system, it was established a possible link between miR-155 overexpression and susceptibility of the CNS to virus infection (Bhela et al. 2014) in accordance to what has been proposed in the present study. In addition, induction of miR-155 has been recently associated with HSV 1 acute encephalitis in a mouse model (Majer et al. 2017).
The CNS changes observed during BHV5 infection are composed by an increase of lymphocyte migration during acute, late, or reactivation phases (Cardoso et al. 2016b; Perez et al. 2006). However, the neuropathogenesis of BHV5 neurological disease is not completely understood. Recently, a pro-inflammatory cytokines profile has been demonstrated and an association with CD3+ T cells was established mainly in the OBs (Cardoso et al. 2016b). This could help to understand the high levels of miR-155 transcription in this study, since an association of miR-155 with the immune response has been described for another alphaherpesviruses (Bhela et al. 2014). The importance of miR-155 immune properties has been described in mice infected with a recombinant influenza virus encoding miR-155 in the NS gene segment, resulting in expansion of CD8+ T cells and production of high levels of neutralizing antibodies (Izzard et al. 2017).
In summary, the overall results obtained in this work suggest that BHV5 is able to regulate the innate immunity by the TLR pathways associated to miR-155 expression in ORNs and ML. These preliminary results can be useful for further studies focusing on the innate immunity against BHV5 neurological infection under in vitro conditions.
Acknowledgments
This work was supported by the FAPESP (Grant 2012/16715-4) and CNPq (Grant 500063/2014-1) both Brazilian Council for Research. T.C. Cardoso and E.F. Flores are the recipients of CNPq fellowships.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Bhela S, Mulik S, Reddy PB, Richardson RL, Gimenez F, Rajasagi NK, Veiga-Parga T, Osmand AP, Rouse BT. Critical role of microRNA-155 in herpes simplex encephalitis. J Immunol. 2014;192:2734–2743. doi: 10.4049/jimmunol.1302326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhela S, Mulik S, Reedy PB, Richardson RL, Varanasi SK, Jaggi U, Xu J, Lu PY, Rouse BT. Role of miR-155 in the pathogenesis of herpetic stromal keratitis. Am J Pathol. 2015;185:1073–1084. doi: 10.1016/j.ajpath.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardoso AL, Guedes JR, Lima MCP. Role of microRNAs in the regulation of innate immune cells under neuroinflammatory conditions. Curr Op Pharmacol. 2016;26:1–9. doi: 10.1016/j.coph.2015.09.001. [DOI] [PubMed] [Google Scholar]
- Cardoso TC, Ferreira HL, Okamura LH, Giroto TP, Oliveira BRSM, Fabri CUF, Gameiro R, Flores EF. Cellular response markers and cytokine gene expression in the central nervous system of cattle naturally infected with bovine herpesvirus 5. Vet J. 2016;218:71–77. doi: 10.1016/j.tvjl.2016.11.014. [DOI] [PubMed] [Google Scholar]
- Chowdhury SI, Lee BJ, Moiser D, Sur JH, Osorio FA, Kennedy G, Weiss ML. Neuropathology of bovine herpesvirus type 5 (BHV-5) meningoencephalitis in a rabbit seizure model. J Comp Pathol. 1997;117:295–310. doi: 10.1016/S0021-9975(97)80078-3. [DOI] [PubMed] [Google Scholar]
- Davison AJ. Herpesvirus systematics. Vet Microbiol. 2010;143:52–69. doi: 10.1016/j.vetmic.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison AJ, Eberle R, Ehlers B, Hayward GS, McGeoch DJ, Minson AC, Pellet PE, Roizman B, Studdert MJ, Thiry E. The order Herpesvirales. Arch Virol. 2009;154:171–177. doi: 10.1007/s00705-008-0278-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickey LL, Hanley TM, Huffaker TB, Ramstead AG, O’Connel RM, Lane TE (2017) MicroRNA 155 and viral-induced neuroinflammation. J Neuroimmunol. doi:10.1016/j.jneuroim.2017.01.016 [DOI] [PMC free article] [PubMed]
- Dickey LL, Worne CL, Glover JL, Lane TE, O’Connel RM. MicroRNA-155 enhances T cell trafficking and antiviral effector function in a model of coronavirus-induced neurological disease. J Neuro-Oncol. 2016;13:240. doi: 10.1186/s12974-016-0699-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favier PA, Marin MS, Moran PE, Odeon AC, Verna AE, Perez SE. Latency of bovine herpesvirus type 5 (BoHV-5) in tonsils and peripheral blood leukocytes. Vet J. 2014;202:134–140. doi: 10.1016/j.tvjl.2014.06.017. [DOI] [PubMed] [Google Scholar]
- Glazov EA, Kogsuwan K, Assavalapsakul W, Horwood PF, Mitter N, Mahony TJ. Repertoire of bovine miRNA and miRNA-like small regulatory of bovine miRNA and miRNA-like small regulatory RNAs expressed upon viral infection. PLoS One. 2009;4:e6349. doi: 10.1371/journal.pone.0006349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grey F. Role of microRNAs in herpesvirus latency and persistence. J Gen Virol. 2015;96:739–751. doi: 10.1099/vir.0.070862-0. [DOI] [PubMed] [Google Scholar]
- Izzard L, Dlugoleski D, Xia Y, McMahon M, Middleton D, Stambas J (2017) Enhanced immunogenicity following miR-155 incorporation into influenza A virus genome. Virus Res doi. doi:10.1016/j.%20viruses.2017.04.002 [DOI] [PubMed]
- Livak K, Schmittgen T. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Ludlow M, Kortekaas J, Herden C, Hoffmann B, Tappe D, Trebst C, Griffin DE, Brindle HE, Solomon T, Brown AS, van Riel D, Wolthers KC, Pajkrt D, Wohlsein P, Martina KC, Baumgärtner VGM, Osterhaus ADME. Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol. 2016;131:159–184. doi: 10.1007/s00401-015-1511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majer A, Caligiuri KA, Gale KK, Niu Y, Phillipson CS, Booth TF, Booth SA. Induction of multiple miR-200/182 members in the brains of mice are associated with acute herpes simplex virus 1 encephalitis. PLoS. 2017;3:0169081. doi: 10.1371/journal.pone.0169081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin MS, Quintana S, Leunda MR, Odeon AC, Perez SE. Toll-like receptor expression in the nervous system of bovine alpha-herpesvirus-infected calves. Res Vet Sci. 2015;97:422–429. doi: 10.1016/j.rvsc.2014.06.014. [DOI] [PubMed] [Google Scholar]
- Mori I, Nishiyama Y, Yokochi T, Kimura Y. Olfactory transmission of neurotropic viruses. J Neuro-Oncol. 2004;11:129–137. doi: 10.1080/13550280590922793. [DOI] [PubMed] [Google Scholar]
- Oldoni I, Weiblen R, Inkelmann MA, Flores EF. Production and characterization of monoclonal antibodies to a Brazilian bovine herpesvirus type 5. Braz J Med Biol Res. 2004;37:213–221. doi: 10.1590/S0100-879X2004000200008. [DOI] [PubMed] [Google Scholar]
- Pareek S, Roy S, Kumari B, Jain P, Banerjee A, Vrati S. miR-155 induction in microglial cells suppress Japanese encephalitis virus replication and negatively modulates innate immune responses. J Neuroinflammation. 2004;11:97. doi: 10.1186/1742-2094-11-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez S, Lovato L, Zhou J, Doster A, Jones C. Comparison of inflammatory infiltrates in trigeminal ganglia of cattle infected with wild-type Bovine herpesvirus 1 versus a virus strain containing a mutation in the LR (latency-related) gene. J Neuro-Oncol. 2006;12:392–397. doi: 10.1080/13550280600936459. [DOI] [PubMed] [Google Scholar]
- Piedade D, Azevedo-Pereira M. The role of microRNAs in the pathogenesis of herpesvirus infection. Viruses. 2016;8:156. doi: 10.3390/v8060156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powdrill MH, Desrochers GF, Singaravelu R, Pezacki JP. The role of microRNAs in metabolic interactions between viruses and their hosts. Curr Op Virol. 2016;19:71–76. doi: 10.1016/j.coviro.2016.07.005. [DOI] [PubMed] [Google Scholar]
- Shivkumar M, Milho R, May JS, Nicoll MP, Efstahiou S, Stevenson PG. Herpes simplex virus 1 targets the murine olfactory neuroepithelium for host entry. J Virol. 2013;87:10477–10488. doi: 10.1128/JVI.01748-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorel O, Dewals BG. MicroRNAs in large herpesvirus DNA genomes: recent advances. BioMol Concepts. 2016;7:229–239. doi: 10.1515/bmc-2016-0017. [DOI] [PubMed] [Google Scholar]
- Tang Qi WY-Q, Chen D-S, Zhou Q, Chen H-C, Liu Z-F. Bovine herpesvirus 5 encodes a unique pattern of microRNAs compared with bovine herpesvirus 1. J Gen Virol. 2014;95:671–678. doi: 10.1099/vir.0.061093-0. [DOI] [PubMed] [Google Scholar]
- Van Riel D, Verdijk R, Kuiken T. The olfactory nerve: a shortcut for influenza and other viral diseases into the central nervous system. J Pathol. 2015;235:277–287. doi: 10.1002/path.4461. [DOI] [PubMed] [Google Scholar]
- Verma R, Bharti K. Toll like receptor 3 and viral infections of nervous system. J Neurol Sci. 2017;372:40–48. doi: 10.1016/j.jns.2016.11.034. [DOI] [PubMed] [Google Scholar]
- Vogel FS, Caron L, Flores EF, Weiblen R, Winkelmann ER, Mayer SV, Bastos RG. Distribution of bovine herpesvirus type 5 DNA in the central nervous systems of latently, experimentally infected calves. J Clin Microbiol. 2003;41:4512–4520. doi: 10.1128/JCM.41.10.4512-4520.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler CW, Race B, Philips K, Peterson KE. Capillaries in the olfactory bulb but not the cortex are highly susceptible to virus-induced vascular leak and promote viral neuroinvasion. Acta Neuropathol. 2015;130:233–245. doi: 10.1007/s00401-015-1433-0. [DOI] [PubMed] [Google Scholar]
- Xagorari A, Chlichlia K. Toll-like receptors and viruses: induction of innate antiviral immune responses. The Open Microbiol J. 2008;2:49–59. doi: 10.2174/1874285800802010049. [DOI] [PMC free article] [PubMed] [Google Scholar]


