Abstract
Background & Aims: Celiac disease is a gluten-induced enteropathy characterized by the presence of gliadin-specific CD4+ T cells in the lamina propria and by a prominent intraepithelial T-cell infiltration of unknown mechanism. The aim of this study was to characterize the subset(s) of intraepithelial lymphocytes (IELs) expanding during active celiac disease to provide insights into the mechanisms involved in their expansion. Methods: Flow-cytometric analysis of isolated IELs and/or immunohistochemical staining of frozen sections were performed in 51 celiac patients and 50 controls with a panel of monoclonal antibodies against T-cell and natural killer (NK) receptors. In addition, in vitro studies were performed to identify candidate stimuli for NK receptor expression. Results: In normal intestine, different proportions of IELs, which were mainly T cells, expressed the NK receptors CD94/NKG2, NKR-P1A, KIR2D/3D, NKp46, Pen5, or CD56. During the active phase of celiac disease, the frequency of CD94+ IELs, which were mostly αβ T cells, was conspicuously increased over controls. In contrast, the expression of other NK markers was not modified. Furthermore, expression of CD94 could be selectively induced in vitro by T-cell receptor activation and/or interleukin 15, a cytokine produced by intestinal epithelial cells. Conclusions: The gut epithelium favors the development of T cells that express NK receptors. In active celiac disease, there is a specific and selective increase of IELs expressing CD94, the HLA-E–specific NK receptor that may be related to T-cell receptor activation and/or interleukin 15 secretion.
GASTROENTEROLOGY 2000;118:867-879
Abbreviations: EC , epithelial cell; FACS , fluorescence-activated cell sorter; FITC , fluorescein isothiocyanate; GFD , gluten-free diet; IEL , intraepithelial lymphocyte; IFN-γ , interferon gamma; MHC , major histocompatibility complex; NK , natural killer; PBL , peripheral blood lymphocyte; PE , phycoerythrin; TCR , T-cell receptor; T-IEL , IEL expressing T-cell receptor; TNF , tumor necrosis factor
Celiac disease is an enteropathy that is induced by gliadin in genetically susceptible individuals expressing HLA-DQ2 (DQA1*0501, B1*201) or HLA-DQ8 (DQA1*031, B1*302).1, 2 The nature of its pathogenesis remains unclear and may involve both direct toxic and immune-mediated effects of gliadin.1 Active celiac disease is associated with the presence of serum autoantibodies against a tissue transglutaminase,3 with activated CD4+CD25+ T cells in the lamina propria and with massive intraepithelial infiltration by proliferating T lymphocytes.4, 5 Interestingly, transglutaminase, the target of celiac disease autoantibodies, is an enzyme that enhances the binding of gliadin peptides to DQ2 and DQ8 molecules through deamidation of glutamine residues.6, 7 Furthermore, gliadin-specific DQ2- or DQ8-restricted T helper (Th) 1 CD4+ T cells have been derived from the lamina propria of celiac patients.6, 8 These findings suggest a model whereby enhanced presentation of gliadin peptides by DQ2 or DQ8 molecules to CD4+ cells in the lamina propria results in secretion of interferon gamma (IFN-γ) and other cytokines that may be deleterious to gut epithelial cells (ECs).9 In contrast, the role and mechanism of the intraepithelial T-cell infiltration, a hallmark of the disease, are debated. It does not appear, as previously suggested,4 that the expansion of the intraepithelial lymphocyte (IEL) population is a nonspecific reaction to the underlying inflammatory process, because IELs are not significantly increased in Crohn's disease10 and they are moderately expanded in autoimmune enteropathy of childhood, a disease with a strong inflammatory reaction in the lamina propria and a subtotal to total villous atrophy.11 In contrast, celiac disease–associated IEL infiltration seems to be of particular significance. It is detected as early as 12 hours after oral or rectal gluten challenge in patients receiving a long-term gluten-free diet (GFD) and precedes the onset of epithelial lesions.12, 13 Furthermore, IELs can undergo malignant transformation during the course of 2 rare but severe complications of celiac disease: enteropathy-associated T-cell lymphomas14 and refractory sprue.15 These complications, which are not observed in other inflammatory bowel diseases, support the idea that celiac disease IELs are permanently submitted to stimuli that promote their expansion and ultimately may favor their transformation. Altogether, these observations underline the importance of understanding the mechanism(s) that drives the expansion of IELs and their role in the pathogenesis of the epithelial lesions.
IELs represent a heterogeneous population of T lymphocytes with distinct ontogenic, phenotypic, and functional properties shown in mice.16 In humans, the main subset expresses αβ T-cell receptor (TCR), more often than CD4. The other subset expresses γδ TCR and is CD4−CD8− or CD8+. A variable albeit significant increase in the numbers of TCRγδ IELs, mainly using the Vδ1 chain, has been observed in treated as well as in untreated celiac disease.5, 17, 18 These γδ T cells, which recognize the MICA and MICB antigens, 2 closely related HLA molecules that are induced by stress on intestinal ECs, may therefore be part of a stress response to celiac disease–associated EC injury.19 In addition, a prominent increase in TCRαβ+CD8+ IELs has been observed in active celiac disease and reported to disappear after gluten avoidance and recovery of a normal villous architecture.5, 20 Because there is no evidence for gliadin-specific major histocompatibility complex (MHC)-restricted αβ T cells among CD8+ IELs, the mechanism driving their expansion remains to be elucidated.
Recent studies suggest that a subset of IELs express surface receptors and functional properties that are normally associated with the natural killer (NK) lineage.21, 22, 23, 24 These IELs may respond to modifications of ECs, as suggested by their natural cytotoxicity against corona virus–infected cells in mice25, 26 or against EC tumors in humans.27, 28 In mice, in which they have been extensively characterized, they constitute a fraction of both TCRαβ+ and TCRγδ+ IELs and express a CD8α+β+CD4− or CD8α−β−CD4− phenotype (reviewed by Rocha et al.29). Studies in humans have also identified the presence of IELs expressing NK receptors. CD56 and CD94 were found on both CD3-positive and -negative cells, whereas CD16 expression was mainly restricted to CD3-negative cells.21, 22, 24 The proportion of NK-like cells decreased in one study of active celiac disease,24 whereas another study suggested that the disappearance of CD16+ granular IELs was associated with a more severe and persistent disease.21
With the idea in mind that NK receptors may play an important role in the interactions between IELs and ECs in the normal or diseased human intestine, we used a panel of antibodies against NK receptors to characterize the surface phenotype of IELs in normal subjects and patients with celiac disease. We found that a significant proportion of normal human T-IELs (IELs expressing TCR) expressed one or several NK receptors. Remarkably, most of the TCRαβ+ IELs that expanded in active celiac disease consistently and selectively expressed CD94, the HLA-E–specific NK receptor.30, 31, 32 In contrast, there was no change in the proportion of IELs expressing NK receptors that bind classical MHC class I molecules or other ligands unrelated to MHC molecules. Furthermore, the increase in CD94+ IELs was specific of active celiac disease because it was not observed in various other inflammatory diseases and it subsided under GFD. Finally, in vitro studies with isolated IELs identified interleukin (IL)-15, a cytokine secreted by intestinal ECs,33 as a likely candidate for inducing CD94 expression. Altogether these findings suggest that the CD94/HLA-E and IL-15R/IL-15 receptor/ligand pairs may control the expansion and/or function of IELs in celiac disease.
Materials and methods
Celiac patients
Twenty-four children (age, 4 ± 3 years) had celiac disease according to EPSGAN criteria.34 At the time of the study, 10 had untreated celiac disease and total villous atrophy, 10 had been on a GFD for 1–3 years and had recovered a normal mucosa or showed a moderate villous atrophy, and 4 additional patients were studied before and after GFD.
Twenty-seven adults, showing symptoms of malabsorption and severe to subtotal villous atrophy, were diagnosed with celiac disease based on the presence of high titers of serum antiendomysium antibodies. All had the DQA1*0501/DQB1*0201 genotype. At the time of study, 16 patients (age, 43 ± 13 years) had active celiac disease with severe to subtotal villous atrophy and 11 (age, 57 ± 16 years) were on GFD-induced remission with normal mucosa or moderate villous atrophy.
Isolation of lymphocytes from biopsy specimens was performed in 15 of the adult celiac patients, 8 with active celiac disease and 7 on GFD with normal intestinal histology, and in 6 controls upon informed consent and approval by the Institutional Ethics Committee of Hôpital Necker-Enfants Malades.
Controls
Histologically normal intestinal biopsy specimens were obtained from 12 children (age, 6 ± 5 years), 2 with chronic colitis and 10 investigated for short stature syndrome without digestive symptoms, and from 11 adults undergoing duodenal biopsies for diagnostic purposes (age, 35 ± 20 years) but without small intestinal disease. For 3 of the adult patients, intestinal lymphocytes were isolated from the biopsy specimens.
Control ileal surgical samples with an important inflammatory infiltrate in the lamina propria were obtained from 2 children (aged 12 and 14 years) with active ileocolic Crohn's disease undergoing surgery for occlusive symptoms.
Control intestinal biopsy specimens from patients with villous atrophy distinct from celiac disease were obtained from 9 children and 3 adults. Four children with moderate villous atrophy had cow's milk intolerance (n = 1), intestinal giardiasis (n = 1), and intestinal graft rejection (n = 2).35 Five children (aged 1–8 years) with severe to total villous atrophy had autoimmune enteropathy (n = 2),11 epithelial dysplasia (n = 1),36 or graft-versus-host disease after bone marrow graft for severe combined immunodeficiency (n = 2). Three adult patients (aged 41–60 years) were included as controls for villous atrophy. In these patients, malabsorption and subtotal villous atrophy were not caused by celiac disease, as indicated by the absence of antiendomysium antibodies, lack of DQ2 or DQ8 haplotype, and lack of clinical or histological improvement after GFD for over 6 months. All 3 had severe infiltration of the lamina propria and the epithelium by CD3+CD4+ (n = 1), CD3+CD8+ (n = 1), or CD3−CD56+ (n = 1) small lymphocytes, suggesting low-grade lymphoproliferative disease without lymphoma.
Surgical samples of histologically normal proximal small intestine, 3–6 cm long, were obtained from 15 adult patients (aged 60 ± 12 years) undergoing intestinal surgery for gastric or pancreatic cancers or for chronic pancreatitis. Peripheral blood lymphocytes (PBLs) were obtained from 17 healthy adult volunteers.
Monoclonal antibodies and recombinant cytokines
Fluorescein isothiocyanate (FITC)- or phycoerythrin (PE)-conjugated antibodies against CD3, CD8, CD4, CD16, CD45RO, CD56, and CD57 were obtained from Becton Dickinson (Le Pont de Claix, France; and Mountain View, CA), and those against CD103, TCRαβ, and TCRγδ were from Coulter-Immunotech (Marseille, France). Purified HP-3B1 anti-CD94 was biotinylated according to standard procedures. This antibody recognizes CD94 alone or associated with members of the NKG2 family.30 The following anti-NK antibodies were used as supernatants (Table 1): Z199,37 5H10,38 Bab 281,39 NKVSF1,40 Z27,41 Q66,42 and 191B8,43 a gift of L. Moretta. The anti–IL-15 mouse monoclonal antibody was obtained from R&D Systems (Oxon, England). The anti–HLA-E antibody (3D12) was a gift from Dr. D. Geraghty.31 Recombinant IL-15, IL-12, IFN-γ, tumor necrosis factor (TNF)-α, and purified anti-CD3 were purchased from R&D Systems and Pharmingen (San Diego, CA).
Histology and immunohistochemistry
For histological studies, endoscopic duodenal biopsy specimens were fixed in 10% formalin. Villous atrophy was graded as moderate (crypt-to-villus ratio > 1) or severe (crypt-to-villus ratio ≤1) and subtotal or total.
For immunohistochemical staining with anti-CD94 antibody, endoscopic biopsy specimens were embedded in O.C.T. compound (Miles Inc., Elkhart, IN) and snap-frozen in liquid nitrogen. Serial cryostat sections, cut at 4 μm, were labeled with purified anti-CD94 antibody or with isotype-matched control immunoglobulin (Ig) G2a at 10 μg/mL (Coulter-Immunotech) and revealed using an indirect immunoperoxidase technique.44 The number of labeled IELs was estimated on serial sections by counting peroxidase-stained cells per 100 ECs. Five hundred to 1000 ECs were counted in each section. Comparable results were obtained by 2 different investigators (N.P., B.J.). Numbers of IELs in the different groups were compared using the Kruskal–Wallis nonparametric test. For immunohistochemical staining with anti–IL-15 antibody, samples were fixed in formalin and embedded in paraffin. Sections were dewaxed, microwave-heated (2 × 5 minutes at 750 W in citrate buffer, pH 6), rinsed in phosphate-buffered saline, and then incubated with anti–IL-15 antibody (2 μg/mL) overnight at 4°C. Staining was revealed using Cy3-labeled goat anti-mouse IgG (Amersham, Buckinghamshire, England). For control staining, a saturating concentration of recombinant IL-15 (R&D Systems) was added before staining with anti–IL-15 antibody.
Lymphocyte isolation
IELs were isolated from surgical intestinal samples, as described previously,45 or from biopsy specimens. Five to 6 endoscopic biopsy specimens were pooled and incubated under constant shaking for 30 minutes at 37°C in RPMI 1640 (GIBCO-BRL, Life Technology, Cergy-Pontoise, France) containing 1% dialyzed fetal calf serum (GIBCO-BRL), 1.5 mmol/L MgCl2, and 1 mmol/L ethylene glycol-bis(β-aminoethyl ether)-N,N,N',N'-tetraacetic acid. The supernatant containing the IELs was passed through a nylon filter (Falcon 2360; Becton Dickinson), and IELs were washed twice in phosphate-buffered saline supplemented with 5% human AB serum. PBLs were isolated on Ficoll-Hypaque (Lymphoprep; Nycomed Pharma, Oslo, Norway) according to standard procedures.
T-lymphocyte and cell cultures
IELs isolated from 6 normal surgical intestinal samples and PBLs from 3 normal individuals were labeled with anti-CD94 and anti-CD8 and sorted with a fluorescence-activated cell sorter (FACS) VANTAGE flow cytometer (Becton Dickinson). Next, 1 × 105 to 2 × 105 CD94-negative IELs (90%–95% TCRαβ+) and CD94-negative CD8-high PBLs (98% TCRαβ+) were cultured in RPMI plus 10% fetal calf serum for 18–48 hours in round-bottom microwells, alone or in the presence of 10 ng/mL IL-15. For TCR-mediated stimulation, cells were cultured in round-bottom microwells precoated overnight with 10 μg/mL anti-CD3 antibody. KATO, a human EC line of gastric origin, was cultured in RPMI with 10% fetal calf serum and antibiotics.
Flow-cytometric analysis
After isolation, lymphocytes were first incubated for 10 minutes in phosphate-buffered saline with 10% human AB serum to block Fc receptors. For each staining, 5 × 104 to 1 × 105 cells were used. For triple membrane staining with directly conjugated antibodies, cells were labeled with a combination of antibodies conjugated to red (PE) or green (FITC) fluorescent dyes or to biotin according to standard procedures. Biotinylated antibodies were revealed with streptavidin tricolor (Caltag Laboratories, San Francisco, CA). For single staining, supernatants containing antibodies were used at a 1:4 dilution and revealed using FITC-conjugated Fab'2 rat anti-mouse Igs at a 1:200 dilution (Jackson ImmunoResearch, West Grove, PA). For multicolor analysis with antibody-containing supernatants, cells were first incubated with a 1:4 dilution of antibody-containing supernatant, washed twice, and stained by an FITC-conjugated rat Fab'2 anti-mouse Ig. Free antibody sites were then blocked with a 50 μg/mL solution of purified mouse Igs (Sigma Chemical Co., St. Louis, MO) for 10 minutes before adding PE and biotin-conjugated antibodies for 15 additional minutes. Biotinylated antibodies were revealed by a final incubation in streptavidin tricolor.
Fluorescence was analyzed on a FACScan (Becton Dickinson). For analysis of CD94-positive lymphocytes, statistical quadrants were set so as to score as negative more than 99% of control-stained cells (cells stained with PE-conjugated IgG2a control isotype; Coulter Immunotech). CD94-positive cells include CD94-low and CD94-high cells, as previously reported for NK cells stained with either anti-CD94 or HLA-E tetramers.29, 30 The CD94-low cells, which include cells expressing CD94/NKG2C complexes, partially overlap with CD94-negative cells, and thus their frequency might have been underestimated. The CD94-high cells include cells expressing CD94/NKG2A complexes and are brightly stained.30, 31
Results
Human intraepithelial T lymphocytes express a distinct pattern of NK receptors
Expression of NK receptors was compared using flow cytometry on IELs isolated from 15 surgical samples of histologically normal small intestine and on PBLs obtained from 14 healthy volunteers. As shown in Table 1, a substantial fraction of IELs, mostly CD3+ T cells, expressed NK receptors.
Table 1.
Expression of NK receptors by IELs and PBLs
| Structural family | Molecule | Antibody | PBLs (n = 14) | CD3+ PBLs (n = 5) | IELs (n = 15) | CD3+ IELsa (n = 4) |
|---|---|---|---|---|---|---|
| KIRb | KIR2DL/S | NKVSF1 | 8 ± 4c | 1.5 ± 0.5 | 6 ± 4 | 3 ± 1 |
| KIR3DL1 | Z 27 | 2 ± 1 | 0.2 ± 0.2 | 1.5 ± 1.3 | ||
| KIR3DL2 | Q 66 | 0.5 ± 0.3 | 0.3 ± 0.3 | 2 ± 3 | ||
| Lectin-like | CD94/NKG2 | HP-3B1 | 23 ± 6 | 11 ± 6 | 27 ± 11 | 27 ± 7 |
| CD161 (NKR-P1A) | 191B8 | 21 ± 7 | 14 ± 6 | 64 ± 3 | 58 ± 4 | |
| Others | CD57 | Leu 7 | 14 ± 7 | 9 ± 8 | 0 | |
| CD56 | Leu 19 | 17 ± 6 | 6 ± 3 | 14 ± 5 | 8 ± 1 | |
| CD16 | Leu 11c | 16 ± 6 | 2 ± 2 | 1.5 ± 1.5 | ||
| NKp46 | Bab281 | 7 ± 6 | 0.3 ± 0.2 | 10 ± 5 | 7 ± 4 | |
| PEN5 | 5H10 | 8 ± 4 | 0.3 ± 0.1 | 13 ± 5 | 13 ± 7 | |
| aOf total IELs, 92% ± 6% are T cells. bMembers of the KIR family are designated according to the nomenclature proposed by Long et al.73cValues are expressed as arithmetic means ± SD of percentages. | ||||||
Two NK receptors of the C-lectin–like family were expressed by a large fraction of normal human IELs. NKR-P1A (CD161), a receptor with no defined ligand that is expressed by NK cells and a subset of peripheral T cells in humans,46 was present on 58% of intraepithelial T lymphocytes but only on 14% of blood T lymphocytes. This receptor can costimulate TCR-mediated signaling.47 Another C-lectin–like NK receptor is CD94, a glycoprotein that associates with molecules of the NKG2 family to form heterodimers that recognize the nonpolymorphic MHC class I molecule HLA-E and transduce either inhibitory or activating signals.31, 48 CD94 was expressed by 27% of T-IELs, a result comparable to that obtained by Eiras et al.,24 and by 11% of blood T lymphocytes.
Two NK receptors expressed by PBLs were virtually absent among IELs: CD57, a marker of unknown function, and CD16, an Fc receptor involved in antibody-dependent cytotoxicity. On the other hand, 2 NK receptors not found on blood T cells, Pen5, a receptor of unknown function,38 and NKp46, an activatory receptor of the Ig-like family,39, 49 were expressed by 13% and 7% T-IELs, respectively.
Other NK receptors were expressed at a similar frequency by both IELs and PBLs. A small subset of IELs (2%–10%) expressed NK receptors of the Ig-like KIR family, mainly KIR2DL/S receptors stained by the NKVSF1 antibody. These KIR receptors are biased toward recognition of HLA-Cw3 and HLA-Bw4 class I molecules and transduce either inhibitory (KIR2DL) or activating (KIR2DS) signals to NK cells.46 The frequency of KIR+ IELs was comparable to that of KIR+ PBLs, although the latter were mainly CD3-negative NK cells and the former included up to 50% CD3-positive T lymphocytes. In addition, CD56, an NK marker of unknown function, was expressed by a fraction of IELs, as reported previously.22, 24 The frequencies of CD56-positive cells among T-IELs and T-PBLs (PBLs expressing the TCR) were similar, approximately 7%.
Using multicolor analysis we determined the pattern of coexpression of several NK receptors with CD94 on IELs of 4 different individuals. The results indicated that, with the exception of CD161, which is present on most IELs, only a minor fraction of CD94+ IELs expressed the various other NK receptors (Figure 1).
Fig. 1.

Expression of NK receptors by CD94+ IELs. Flow-cytometric analysis using an anti-CD94 antibody associated with several anti-NK receptor antibodies was performed on normal IELs. The results are shown as percentage (+SD) of CD94+ IELs expressing individual NK receptors.
Conversely, CD94, which was only expressed by 27% of T-IELs, was present on most IELs expressing KIR2DL/S, CD161, CD56, Pen5, and NKp46+ IELs, indicating that these NK receptors tended to be coexpressed with CD94 (data not shown).
TCRαβ+ IELs expressing the CD94 NK receptor are selectively increased in active celiac disease
We first studied infiltration of the epithelium by CD3+ intraepithelial T cells in active celiac disease. As expected, the surface epithelium was massively infiltrated by CD3+ IELs in both children (91 ± 31 IELs/100 ECs vs. 20 ± 13 IELs/100 ECs in normal control biopsy specimens and 23 ± 15 IELs/100 ECs in control biopsy specimens with villous atrophy of other origin) and adults (100 ± 13 IELs/100 ECs vs. 34 ± 20 IELs/100 ECs in normal control biopsy specimens).
In preliminary immunohistochemical studies of intestinal frozen tissue sections, the pattern of staining of IELs with anti-CD56, -Pen5, -p46, -p58, and -CD94 antibodies was compared in 4 adult patients with active celiac disease and in 4 normal controls. CD56, p58, Pen5, and NKp46 were expressed by less than 5% of IELs in either group, and NKR-P1A was expressed by 70%–80% of IELs in both groups (not shown). Strikingly, CD94 was the only receptor whose frequency was markedly increased.
To confirm the increase in CD94+ IELs on a larger population of celiac patients and to study its specificity and its relationship with disease activity, we examined frozen sections from 24 children and 27 adults at various stages of celiac disease and from 32 controls (21 children, 12 with normal villi and 9 with villous atrophy not related to celiac disease, and 11 adults with normal villi). Counts of CD94+ IELs/100 ECs were determined. As shown in Figure 2, the number of CD94+ IELs/100 ECs was considerably increased over normal age-matched controls during active celiac disease in children (40 ± 11 vs. 5 ± 2; P < 0.001) as well as in adults (40 ± 16 vs. 10 ± 7; P < 0.001). In contrast, the numbers of CD94+ IELs/100 ECs in celiac patients on GFD (children, 13 ± 8; adults, 15 ± 7) were not significantly different from those in normal controls (children, 5 ± 2; adults, 10 ± 7) (Fig. 2, Fig. 2). Furthermore, a longitudinal follow-up of 4 children showed that high counts of CD94+ IELs before GFD returned to normal after GFD and recovery of a normal villous architecture (Figure 2B). Altogether, these results indicate that there is a selective increase in the number of CD94+ IELs that is dependent on the activity of the disease.
Fig. 2.

Expansion of CD94+ IELs is celiac disease specific and is associated with disease activity. Immunoperoxidase staining using anti-CD94 antibody was performed on frozen sections of duodenum in (A and B) 45 children and in (C) 38 adults. Results are expressed as numbers of CD94+ IELs per 100 ECs. In both (A) children and (C) adults, the number of CD94+ IELs was significantly increased in active celiac disease (P < 0.001) compared with controls with normal intestine, controls with villous atrophy unrelated to celiac disease, or patients receiving GFD. (B) A longitudinal study in 4 celiac children confirmed that counts of CD94+ IELs returned to normal after GFD.
We next asked whether the increase in the number of CD94+ IELs was specific to celiac disease by examining control patients with villous atrophy or patients with inflammatory bowel disease unrelated to celiac disease. No increase in the number of CD94+ IELs/100 ECs was observed in 4 children with moderate villous atrophy because of giardiasis (n = 1), cow's milk intolerance (n = 1), and intestinal graft rejection (n = 2), or in 5 children with total or subtotal villous atrophies because of epithelial dysplasia (n = 1), autoimmune enteropathy (n = 2), and graft-versus-host disease (n=2). Only one 2-year-old child with graft-versus-host disease and subtotal villous atrophy had a moderately increased number of CD94+ IELs (28 IELs/100 ECs) (Figure 2A). Finally, no increase in the number of CD94+ IELs/100 ECs was observed in 2 pediatric biopsy samples with severe Crohn's disease showing an important inflammatory infiltrate in the lamina propria but no villous atrophy (4 IELs/100 ECs and 8 IELs/100 ECs).
To determine whether the increase in CD94+ IELs simply reflected the overall increase of IELs or whether it was selective, flow-cytometric experiments were performed to precisely measure the proportion of CD94+ IELs among total IELs. IELs were isolated from biopsy specimens of 15 adult celiac patients and compared with those isolated from 6 adult controls (Figure 3). The percentage of CD94+ lymphocytes among total IELs was markedly increased in patients with active celiac disease (74% ± 13%; n = 8) over controls without villous atrophy (23% ± 9%; n = 3), controls with villous atrophy unrelated to celiac disease (adults 9% ± 12%; n = 3), or celiac patients receiving GFD (36% ± 13%; n = 7) (P < 0.001) (Fig. 3, Fig. 3). Additional controls included biopsy specimens from 2 children, 1 with Crohn's disease and 1 with autoimmune enteropathy, that also showed normal frequencies of CD94+ IELs (22% and 18%, respectively; data not shown). The frequencies of CD94+ IELs in control biopsy specimens without villous atrophy and in biopsy specimens from patients on GFD were similar to that observed in IELs isolated from surgical samples (23% ± 9% [n = 3] vs. 27% ± 10% [n=15]; Table 1 and Figure 3B). Despite a marked relative increase of CD94+ IELs in active celiac disease, the percentage of IELs expressing other NK receptors was within the normal range (Table 2).
Table 2.
Comparison between the expression of CD94 and other NK receptors by IELs in active celiac disease
| CD94 | CD161 | KIR 2DL/S | CD56 | NKp46 | PEN5 | |
|---|---|---|---|---|---|---|
| Normala (n = 15) | 27 ± 11b | 64 ± 3 | 6 ± 4 | 14 ± 5 | 10 ± 5 | 13 ± 5 |
| Active celiac disease (n = 3) | 71 ± 6 | 54 ± 11 | 3.6c | 13 ± 4 | 5 ± 1 | 5.5 ± 6 |
| aReference data as shown in Table 1. bValues are expressed as arithmetic means ± SD of percentages. cThe expression of KIR2DL/S on IELs has been studied in 2 patients; it was expressed by 3.7% and 3.5% of total IELs, respectively. | ||||||
These results show that untreated celiac disease is associated with a marked selective increase in CD94+ IELs.
Fig. 3.
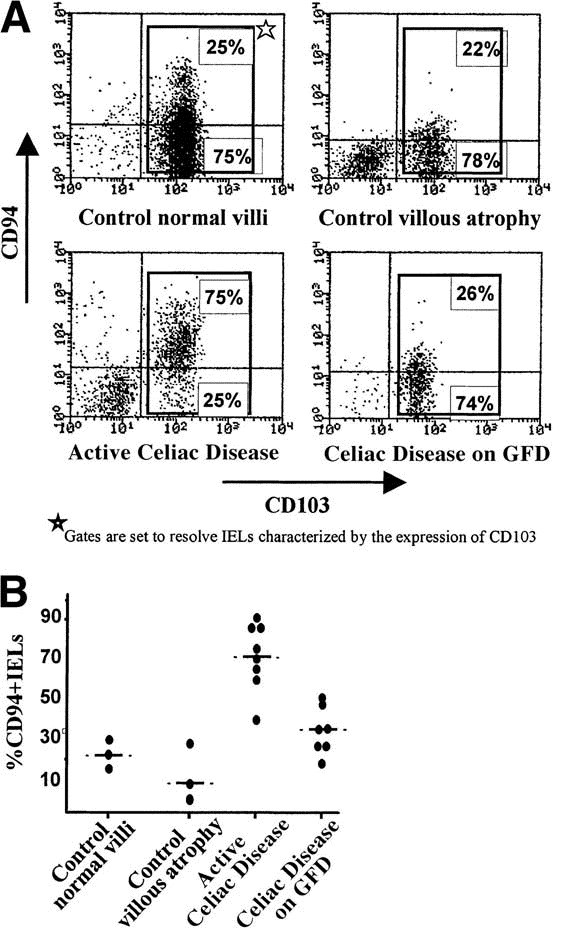
Flow-cytometric analysis of CD94+ IELs in celiac disease. Flow-cytometric analysis using conjugated anti-CD94 and anti-CD103 antibodies was performed on IELs. Expression of the CD103 integrin, characteristic of IELs, was used to distinguish IELs from ECs contaminating the lymphocyte preparation (lower-left quadrant). Results are expressed as percentages of CD94+ and CD94− cells among CD103+ IELs. (A) Representative examples of controls and patients with celiac disease (CD). (B) Summary of the results obtained in 6 controls and 15 celiac patients.
In addition, flow-cytometric analysis showed that the majority of CD94+ IELs in active celiac disease, as well as in controls, expressed the αβ TCR (celiac disease: 75% ± 15%, n = 6; control biopsy specimens: 83% ± 15%, n = 3; surgical controls: 81% ± 13%, n = 15) (Figure 4). Only a small and variable fraction of CD94+ IELs expressed the γδ TCR in active celiac disease (12% ± 10%, n = 6), a proportion comparable to that found in controls (control biopsy specimens: 19% ± 15%, n = 3; surgical controls: 19% ± 24%, n = 15) (Figure 4).
Fig. 4.

Most CD94+ IELs express the TCRαβ receptor. Flow-cytometric analysis of IELs using anti-CD94 vs. anti-TCRαβ or anti-TCRγδ antibodies in a representative patient with untreated celiac disease. Similar results were obtained in all 6 patients studied. ★Gates are set to resolve CD94+ cells.
CD94 is usually expressed as a heterodimer associated with various members of the NKG2 family, some of which inhibit and others enhance cell activation.30 Flow-cytometric analysis using an antibody directed against the inhibitory isoform NKG2A37 showed that, in active celiac disease, 5% ± 5% of CD94+ IELs expressed NKG2A (n = 4), whereas the frequency in normal controls was 29% ± 18% (n = 5). Thus, the increase in CD94+ IELs is not related to an expansion of cells expressing the CD94+NKG2A+ inhibitory receptor.
Finally, to investigate whether the CD94 receptor might participate to the interactions between IELs and ECs, we examined the expression of its ligand, the nonclassical MHC molecule HLA-E, on the surface of ECs. HLA-E was constitutively expressed on the surface of the KATO EC line and upon IFN-γ stimulation in KATO as well as in HT29 intestinal cell lines (Figure 5 and data not shown, respectively).
Induction of CD94 by IL-15 and/or TCR stimulation on normal IELs
Previous studies indicated that CD94 could be induced on PBLs, but that induction required the prolonged combination of TCR engagement and exposure to IL-15.50 This led us to examine the effect of TCR stimulation and IL-15, alone or in combination, on CD94 expression by IELs. IELs were isolated from histologically normal surgical samples, and CD94-negative cells were further purified by cell sorting. CD94-negative IELs, which included 90%–95% TCRαβ+CD8+ cells, were stimulated during a short period of time, 18–36 hours, with anti-CD3 antibodies and/or IL-15 before multicolor flow-cytometric analysis using anti-CD8 and CD94. Figure 6 shows that IL-15 alone, or TCR stimulation alone, rapidly and selectively induced CD94 on up to 40%–50% of IELs, whereas less than 10% of unstimulated cells induced CD94. Induction of CD94 was detected as early as 18 hours after culture and is shown at 24 hours. The prompt and important increase in the frequency of CD94 expression, in the absence of changes in cell recovery (data not shown), rules out the possibility of selective expansion or survival of a minor subset of CD94+ contaminants among the FACS-purified cells. Adding anti-CD3 antibody did not significantly enhance the effect of IL-15 in 6 separate experiments. In contrast, expression of CD56 and CD16 was consistently unaffected (data not shown). In addition, within a time frame ranging from 18 to 36 hours in 3 different experiments, IFN-γ had little effect on CD94 expression, and other cytokines that may be present in normal and/ or inflammatory intestine, IL-12, TNF-α, and transforming growth factor β, had no detectable effect alone or in combination with TCR stimulation. We confirmed in 3 separate experiments that, within this time frame, neither IL-15 alone nor the combination of IL-15 and TCR stimulation induced CD94 on CD8-high PBLs (which include 98% TCRαβ+), as previously reported.50 Furthermore, we showed that even the minor subset of CD45RO+CD8+ PBLs that may be functionally closer to IELs than CD45RO−CD8+ PBLs displayed little induction of CD94 (5%–7%) (Figure 6). Thus, in marked contrast with both RO+ and RO− CD8+ PBLs, a large fraction of IELs promptly expressed CD94 in response to IL-15 alone and, to a lesser degree, to TCR stimulation alone. Finally, to examine whether IL-15 might play a role in the expression of CD94 by IELs in celiac disease, we examined the presence of IL-15 in intestinal tissue sections. The IL-15 protein was mainly detected in ECs of the villi in the normal intestine and was strongly expressed by crypt and villi ECs as well as by numerous lamina propria cells in active celiac disease (Figure 7).
Fig. 7.
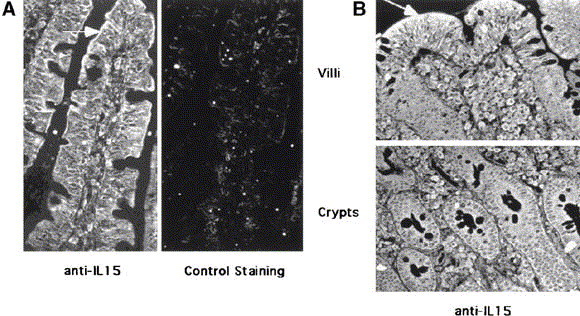
Expression of IL-15 protein in celiac intestine. Paraffin sections of duodenal biopsy specimens from (A) a control subject and (B) a patient with active celiac disease were stained with anti–IL-15 monoclonal antibody. For control staining (A), anti–IL-15 antibody was preincubated with recombinant IL-15 at neutralizing concentrations. In controls, IL-15 was mainly detected in the villous ECs. In celiac disease, IL-15 was strongly expressed by both crypt and villous enterocytes as well as by numerous lamina propria cells. Arrows indicate areas of strong positivity for IL-15.
Fig. 6.

IL-15 alone induces the expression of CD94 by IELs but not by PBLs. Altogether, 1 × 105 to 2 × 105 FACS-sorted CD94−CD8+ IELs and PBLs were cultured in RPMI plus 10% fetal calf serum alone or in the presence of saturating amounts of IL-15 in round-bottom microwells. For TCR-mediated stimulation, cells were cultured in round-bottom microwells precoated overnight with 10 μg/mL anti-CD3. Cells were analyzed by flow cytometry after 24 hours (IELs) or 48 hours (PBLs) in culture. PBLs are gated on CD8+CD45RO− (top row) and CD8+CD45RO+ (middle row), and IELs are gated on CD8+ (bottom row). Quadrants for statistical analysis were set so as to score as negative more than 99% of control-stained cells.
Discussion
We show in this study that most normal human T-IELs express at least 1 NK receptor and that the αβ TCR+ IELs that expand in untreated celiac disease specifically and selectively express the NK receptor CD94. We also provide functional evidence that increased expression of CD94 in active celiac disease may be the consequence of local release of IL-15, a potent cytokine produced in situ by intestinal ECs and activated macrophages (reviewed by Waldmann and Tagaya51) and/or direct TCR activation.
T cells that express various receptors of the NK lineage have been reported in mice and humans, in normal as well as in pathological states.23, 52, 53, 54, 55, 56, 57, 58, 59 NK receptor expression may be part of a genetic program of differentiation,52 or alternatively it may be imparted on activation of mature T cells.50, 60 In the present study we confirmed the expression of CD56 and CD94 by 7% and 27% T-IELs, respectively, and the paucity of CD16 expression, as previously reported on T-IELs.22, 24 We determined for the first time the pattern of expression of an extended set of NK receptors by T-IELs and compared it with that of T-PBLs. The results showed that IELs and PBLs markedly differed in the frequency of T cells expressing NK receptors as well as in the nature of the NK receptors expressed. Thus, more than 60% of T-IELs vs. only 20%–30% of T-PBLs expressed at least 1 NK receptor. NKR-P1A was expressed on a much larger subset of T-IELs than T-PBLs, and Pen5 and NKp46 were expressed by a subset of T-IELs but not by T-PBLs. Conversely, CD57 was expressed by a subset of T-PBLs but not by T-IELs. These NK receptor–expressing T-IELs, or a subset of them, might be the counterpart to the mouse NK receptor–expressing T-IELs, which are thought to differentiate in the gut wall and expand in response to self antigens.29, 61, 62 Alternatively, the expression of NK receptors on human T-IELs could be the consequence of particular activation events. In any case, our data suggest that the gut epithelium is a unique environment that favors the differentiation and/or expansion of particular subsets of NK receptor–expressing T cells in humans as well as in mice. Some NK receptors, such as CD16, endow T-IELs with NK-like properties23 that may contribute to the innate defense of the intestinal epithelium.25, 26, 27, 28 Other NK receptors, such as CD94,63, 64, 65 function as regulators of TCR-mediated signaling by modulating the activation threshold of the TCR. Accordingly, our recent studies of T-cell clones derived from CD8+TCRαβ+ IELs indicate that CD94 can modulate TCR-mediated cytotoxicity (unpublished data, Bana Jabri and Nadine Cerf-Bensussan, January 1999). These functional properties may profoundly influence the outcome of the local immune responses, and it is therefore of particular significance that distinct patterns of NK receptors may be expressed in particular types of inflammatory processes of the intestine.
A massive increase in the frequency of CD8+TCRαβ+ IELs was previously reported in active celiac disease and found to subside after gluten eviction.5, 20 This IEL expansion does not seem to be an obligatory consequence of the underlying Th1 response.17 In fact, IEL expansion in celiac disease could be dissociated from the Th1 response of the lamina propria because treatment with CTL4-Ig could block IFN-γ production without inhibiting the migration and/or expansion of IELs that occurred within 12 hours of gluten challenge.66 These observations suggested that gluten itself might, directly or indirectly, cause injury to the gut epithelial microenvironment, which in turn would induce the expansion and/or migration of IELs. In this context, it was important to further characterize the nature of the expanding cells, particularly with respect to their pattern of NK receptor expression, because NK receptors may regulate the interactions of IELs with the altered epithelium of celiac patients. Our results indicated that active celiac disease is associated with a striking increase in CD8+ TCRαβ+ IELs expressing the CD94 NK receptor. This increase was specific of active celiac disease and was not observed in various other types of villous atrophies, including autoimmune enteropathy, or in Crohn's disease. Furthermore, it was tightly associated with disease activity because it subsided after gluten eviction.
A remarkable finding of this study was that the induction of NK receptors seemed to be a selective process because CD94 was the only NK receptor tested whose expression was markedly increased in active celiac disease. This observation suggested that induction of CD94 could be dissociated from that of other NK receptors in vivo. Our in vitro studies further confirmed that CD94 induction was selective because CD94, but not the other NK receptors tested (such as CD16 and CD56, data not shown), could be induced on CD8+ T-IELs upon TCR and IL-15 stimulation. A selective induction of CD94 upon treatment with anti-TCR and IL-15 was also recently reported for PBLs.50 However, there seem to be some interesting differences between the response of PBLs and IELs to these stimuli. CD94 expression by PBLs required the combined stimulation by anti-TCR and IL-15 over a period of 4–6 days,50 but IELs expressed CD94 as early as 18 hours after stimulation with IL-15 alone, and, to a lesser degree, with TCR stimulation alone. This unusual property of IELs was not shared by the minor subset of CD45RO+CD8+ PBLs, which is functionally closer to IELs than the bulk of PBLs, suggesting that the ability to rapidly induce CD94 upon exposure to IL-15 is not a common feature of memory CD8+ T cells. A possible explanation for these differences is that, by prior exposure to antigens in the gut microenvironment, T-IELs were “primed” for CD94 induction. Our in vitro studies further indicated that cytokine-mediated induction of CD94 was the restricted property of IL-15 because other inflammatory cytokines such as IL-12, IFN-γ, and TNF-α consistently failed to induce high levels of CD94. IL-15 can be secreted by the intestinal epithelium33 and is a potent activator of human IELs, stimulating their proliferation, cytotoxicity, and IFN-γ secretion.67 In addition, IL-15 favors migration of lymphocytes68 and induces expression of several chemokines and chemokine receptors on lymphocytes.69 Altogether, these findings suggest a scenario whereby after TCR-mediated activation and exposure to the cytokines of the epithelial microenvironment, including IL-15, celiac disease IELs not only up-regulate CD94 expression, but also increase their cytotoxic properties through the induction of perforin and granzyme B.70 Our finding that IL-15 is abundant in the intestinal epithelium of celiac disease is consistent with this scenario. The local events leading to TCR engagement remain to be elucidated. One could speculate that celiac disease IELs recognize as yet undefined antigens induced on the intestinal epithelium in response to gliadin. Such a mode of response to stress has recently been suggested for TCRγδ+ IELs using the variable Vδ1 region, a subset of IELs known to be increased in celiac disease5, 17, 18 and that respond to the stress-induced MICA/MICB HLA molecules.19
The selective induction of CD94 expression by celiac disease IELs also points to a specific function of the CD94 receptor and its ligand, the nonpolymorphic MHC class I molecule HLA-E,31, 32, 48 in the immune processes associated with celiac disease. Although the surface expression of HLA-E on intestinal cells has not yet been shown with the available reagents, significant levels of messenger RNA have been reported.71 Furthermore, we found constitutive membrane expression of HLA-E and marked up-regulation by various cytokines, including IFN-γ, on intestinal EC lines such as HT29 colonic cells (Figure 5 and data not shown).
Fig. 5.
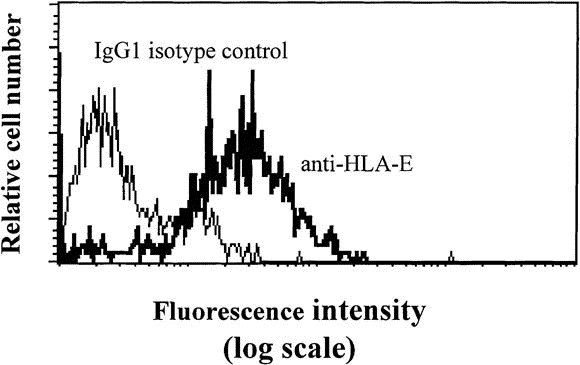
Expression of HLA-E on KATO EC line. The KATO EC line of gastric origin was stained with 3D12 anti–HLA-E monoclonal antibody or with IgG1 isotype control.
Depending on the associated NKG2 isotype, the engagement of CD94 induces a range of activating or inhibitory effects on proliferation, target killing, and cytokine secretion by NK cells.71 In CD94+ T cells, signaling via CD94 can modulate, positively or negatively, activation through the TCR, depending on the associated NKG2 molecule.54, 57, 59, 72 Because CD94+ IELs display cytotoxic activity and IFN-γ secretion after TCR stimulation (unpublished data, Bana Jabri and Nadine Cerf-Bensussan, January 1999), engagement of CD94 by HLA-E may modulate the consequences of TCR engagement. Interestingly, we found that the proportion of CD94+ T-IELs expressing the CD94/NKG2A inhibitory receptor was low in celiac disease. Further studies are in progress to define whether the NKG2 isotypes expressed by CD94+ IELs in celiac disease belong to the activating group and whether they modulate the cytotoxicity of IELs.
In conclusion, we report a selective and specific increase in the number of TCRαβ+ IELs that express the HLA-E–specific NK receptor CD94 during the active phase of celiac disease. We show that IL-15, which is expressed by intestinal ECs, or TCR engagement can selectively induce CD94 on IELs. These findings suggest a scenario whereby IELs primed in the celiac disease microenvironment may be locally regulated positively or negatively by IL-15 and HLA-E. Thus, EC injury and the resulting intestinal dysfunction in celiac disease may not only depend on the activation of gliadin-specific, DQ2-, or DQ8-restricted lamina propria CD4+ cells, but may also be regulated or mediated by CD94+TCRαβ+ IELs.
Acknowledgements
The authors thank A. Beavis for help with cell sorting, M. Leborgne for immunohistochemical staining, and Albert Bendelac for helpful comments on the manuscript.
Footnotes
Address requests for reprints to: Bana Jabri, M.D., Department of Molecular Biology, Schultz Laboratory, Princeton University, Washington Road, Princeton, New Jersey 08544. e-mail: bjabri@molbio.princeton.edu; or Nadine Cerf-Bensussan, M.D., INSERM E9925, Faculté Necker, 156, rue de Vangirard, 75730 Paris Cedex 15, France. e-mail: cerf@necker.fr.
Supported by grants from L'Association pour la Recherche contre le Cancer (contrats ARC 9216, 9962), Assistance Publique Hôpitaux de Paris (PHRC AOM96082), and la Société Nationale Française de Gastroentérologie.
References
- 1.Godkin A, Jewell D. The pathogenesis of celiac disease. Gastroenterology. 1998;115:206–210. doi: 10.1016/s0016-5085(98)70382-8. [DOI] [PubMed] [Google Scholar]
- 2.Sollid LM, Markussen G, Ek J, Gjerde H, Var tdal F, Thorsby E. Evidence for a primary association of celiac disease to a particular HLA-DQ α/β heterodimer. J Exp Med. 1989;169:345–350. doi: 10.1084/jem.169.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D. Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med. 1997;3:797–801. doi: 10.1038/nm0797-797. [DOI] [PubMed] [Google Scholar]
- 4.Halstensen TS, Brandtzaeg P. Activated T lymphocytes in the celiac lesion: non-proliferative activation (CD25) of CD4+ α/β cells in the lamina propria but proliferation (Ki-67) of α/β and γ/δ cells in the epithelium. Eur J Immunol. 1993;23:505–510. doi: 10.1002/eji.1830230231. [DOI] [PubMed] [Google Scholar]
- 5.Kutlu T, Brousse N, Rambaud C, Le Deist F, Schmitz J, Cerf-Bensussan N. Numbers of T cell receptor (TCR) αβ+ but not of TcR γδ+ intraepithelial lymphocytes correlate with the grade of villous atrophy in coeliac patients on a long term normal diet. Gut. 1993;34:208–214. doi: 10.1136/gut.34.2.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molberg O, McAdam SN, Korner R, Quarsten H, Kristiansen C, Madsen L, Fugger L, Scott H, Noren O, Roepstorff P, Lundin KE, Sjostrom H, Sollid LM. Tissue transglutaminase selectively modifies gliadin peptides that are recognized by gut-derived T cells in celiac disease. Nat Med. 1998;4:713–717. doi: 10.1038/nm0698-713. (published erratum appears in Nat Med 1998;4:974) [DOI] [PubMed] [Google Scholar]
- 7.van de Wal Y, Kooy Y, van Veelen P, Pena S, Mearin L, Papadopoulos G, Koning F. Selective deamidation by tissue transglutaminase strongly enhances gliadin-specific T cell reactivity. J Immunol. 1998;161:1585–1588. [PubMed] [Google Scholar]
- 8.Lundin KE, Sollid LM, Anthonsen D, Noren O, Molberg O, Thorsby E, Sjostrom H. Heterogeneous reactivity patterns of HLA-DQ–restricted, small intestinal T-cell clones from patients with celiac disease. Gastroenterology. 1997;112:752–759. doi: 10.1053/gast.1997.v112.pm9041236. [DOI] [PubMed] [Google Scholar]
- 9.Nilsen EM, Lundin KE, Krajci P, Scott H, Sollid LM, Brandtzaeg P. Gluten specific, HLA-DQ restricted T cells from coeliac mucosa produce cytokines with Th1 or Th0 profile dominated by interferon γ. Gut. 1995;37:766–776. doi: 10.1136/gut.37.6.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferguson A, Murray D. Quantitation of intraepithelial lymphocytes in human jejunum. Gut. 1971;12:988–994. doi: 10.1136/gut.12.12.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cuenod B, Brousse N, Goulet O, De Potter S, Mougenot JF, Ricour C, Guy-Grand D, Cerf-Bensussan N. Classification of intractable diarrhea in infancy using clinical and immunohistological criteria. Gastroenterology. 1990;99:1037–1043. doi: 10.1016/0016-5085(90)90624-a. [DOI] [PubMed] [Google Scholar]
- 12.Marsh MN, Loft DE, Garner VG, Gordon D. Time dose responses of celiac mucosae to graded oral challenges with Frazer fraction-III of gliadin. Eur J Gastroenterol Hepatol. 1992;4:667–673. [Google Scholar]
- 13.Marsh MN. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity (‘celiac sprue’) Gastroenterology. 1992;102:330–354. [PubMed] [Google Scholar]
- 14.Isaacson PG. Intestinal lymphoma and enteropathy. J Pathol. 1995;177:111–113. doi: 10.1002/path.1711770202. [DOI] [PubMed] [Google Scholar]
- 15.Cellier C, Patey N, Mauvieux L, Jabri B, Delabesse E, Cervoni JP, Burtin ML, Guy-Grand D, Bouhnik Y, Modigliani R, Barbier JP, Macintyre E, Brousse N, Cerf-Bensussan N. Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology. 1998;114:471–481. doi: 10.1016/s0016-5085(98)70530-x. [DOI] [PubMed] [Google Scholar]
- 16.Cerf-Bensussan N, Jabri B, Guy-Grand D. Subsets of intraepithelial lymphocytes in normal intestine and in coeliac disease. In: Mäki M, Collin P, Visakopi JK, editors. Vammalan Kirjapaino Oy; Vammala, Finland: 1997. pp. 291–309. (Proceedings of the seventh International Symposium on Coeliac Disease). [Google Scholar]
- 17.Halstensen TS, Scott H, Brandtzaeg P. Intraepithelial T cells of the TcR γ/δ+ CD8− and V δ 1/J δ 1+ phenotypes are increased in coeliac disease. Scand J Immunol. 1989;30:665–672. doi: 10.1111/j.1365-3083.1989.tb02474.x. [DOI] [PubMed] [Google Scholar]
- 18.Spencer J, Isaacson PG, Diss TC, MacDonald TT. Expression of disulfide-linked and non-disulfide-linked forms of the T cell receptor γ/δ heterodimer in human intestinal intraepithelial lymphocytes. Eur J Immunol. 1989;19:1335–1338. doi: 10.1002/eji.1830190728. [DOI] [PubMed] [Google Scholar]
- 19.Groh V, Steinle A, Bauer S, Spies T. Recognition of stress-induced MHC molecules by intestinal epithelial γδ T cells. Science. 1998;279:1737–1740. doi: 10.1126/science.279.5357.1737. [DOI] [PubMed] [Google Scholar]
- 20.Verkasalo MA, Arato A, Savilahti E, Tainio VM. Effect of diet and age on jejunal and circulating lymphocyte subsets in children with coeliac disease: persistence of CD4−8− intraepithelial T cells through treatment. Gut. 1990;31:422–425. doi: 10.1136/gut.31.4.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadziselimovic F, Emmons LR, Schaub U, Signer E, Burgin-Wolff A, Krstic R. Occurrence of large granular lymphocytes and natural killer cells in the epithelium of the gut distinguishes two different coeliac diseases. Gut. 1992;33:767–772. doi: 10.1136/gut.33.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundqvist C, Baranov V, Hammarstrom S, Athlin L, Hammarstrom ML. Intra-epithelial lymphocytes. Evidence for regional specialization and extrathymic T cell maturation in the human gut epithelium. Int Immunol. 1995;7:1473–1487. doi: 10.1093/intimm/7.9.1473. [DOI] [PubMed] [Google Scholar]
- 23.Guy-Grand D, Cuenod-Jabri B, Malassis-Seris M, Selz F, Vassalli P. Complexity of the mouse gut T cell immune system: identification of two distinct natural killer T cell intraepithelial lineages. Eur J Immunol. 1996;26:2248–2256. doi: 10.1002/eji.1830260942. [DOI] [PubMed] [Google Scholar]
- 24.Eiras P, Roldan E, Camarero C, Olivares F, Bootello A, Roy G. Flow cytometry description of a novel CD3− /CD7+ intraepithelial lymphocyte subset in human duodenal biopsies: potential diagnostic value in coeliac disease. Cytometry. 1998;34:95–102. doi: 10.1002/(sici)1097-0320(19980415)34:2<95::aid-cyto6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 25.Tagliabue A, Befus AD, Clark DA, Bienenstock J. Characteristics of natural killer cells in the murine intestinal epithelium and lamina propria. J Exp Med. 1982;155:1785–1796. doi: 10.1084/jem.155.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carman PS, Ernst PB, Rosenthal KL, Clark DA, Befus AD, Bienenstock J. Intraepithelial leukocytes contain a unique sub-population of NK-like cytotoxic cells active in the defense of gut epithelium to enteric murine coronavirus. J Immunol. 1986;136:1548–1553. [PubMed] [Google Scholar]
- 27.Taunk J, Roberts AI, Ebert EC. Spontaneous cytotoxicity of human intraepithelial lymphocytes against EC tumors. Gastroenterology. 1992;102:69–75. doi: 10.1016/0016-5085(92)91785-3. [DOI] [PubMed] [Google Scholar]
- 28.Roberts AI, O'Connell SM, Biancone L, Brolin RE, Ebert EC. Spontaneous cytotoxicity of intestinal intraepithelial lymphocytes: clues to the mechanism. Clin Exp Immunol. 1993;94:527–532. doi: 10.1111/j.1365-2249.1993.tb08229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rocha B, Guy-Grand D, Vassalli P. Extrathymic T cell differentiation. Curr Opin Immunol. 1995;7:235–242. doi: 10.1016/0952-7915(95)80008-5. [DOI] [PubMed] [Google Scholar]
- 30.Lopez-Botet M, Perez-Villar JJ, Carretero M, Rodriguez A, Melero I, Bellon T, Llano M, Navarro F. Structure and function of the CD94 C-type lectin receptor complex involved in recognition of HLA class I molecules. Immunol Rev. 1997;155:165–174. doi: 10.1111/j.1600-065x.1997.tb00949.x. [DOI] [PubMed] [Google Scholar]
- 31.Braud VM, Allan DS, O'Callaghan CA, Soderstrom K, D'Andrea A, Ogg GS, Lazetic S, Young NT, Bell JI, Phillips JH, Lanier LL, McMichael AJ. HLA-E binds to natural killer cell receptors CD94/NKG2A, B, and C. Nature. 1998;391:795–799. doi: 10.1038/35869. [DOI] [PubMed] [Google Scholar]
- 32.Lee N, Llano M, Carretero M, Ishitani A, Navarro F, Lopez-Botet M, Geraghty DE. HLA-E is a major ligand for the natural killer inhibitory receptor CD94/NKG2A. Proc Natl Acad Sci U S A. 1998;95:5199–5204. doi: 10.1073/pnas.95.9.5199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reinecker HC, MacDermott RP, Mirau S, Dignass A, Podolsky DK. Intestinal ECs both express and respond to interleukin 15. Gastroenterology. 1996;111:1706–1713. doi: 10.1016/s0016-5085(96)70036-7. [DOI] [PubMed] [Google Scholar]
- 34.Walker-Smith JA. Management of infantile gastroenteritis. Arch Dis Child. 1990;65:917–918. doi: 10.1136/adc.65.9.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fromont G, Cerf-Bensussan N, Patey N, Canioni D, Rambaud C, Goulet O, Jan D, Revillon Y, Ricour C, Brousse N. Small bowel transplantation in children: an immunohistochemical study of intestinal grafts. Gut. 1995;37:783–790. doi: 10.1136/gut.37.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goulet O, Kedinger M, Brousse N, Cuenod B, Colomb V, Patey N, de Potter S, Mougenot JF, Canioni D, Cerf-Bensussan N, Ricour C. Intractable diarrhea of infancy with epithelial and basement membrane abnormalities. J Pediatr. 1995;127:212–219. doi: 10.1016/s0022-3476(95)70297-0. [DOI] [PubMed] [Google Scholar]
- 37.Carretero M, Cantoni C, Bellon T, Bottino C, Biassoni R, Rodriguez A, Perez-Villar JJ, Moretta L, Moretta A, Lopez-Botet M. The CD94 and NKG2-A C-type lectins covalently assemble to form a natural killer cell inhibitory receptor for HLA class I molecules. Eur J Immunol. 1997;27:563–567. doi: 10.1002/eji.1830270230. [DOI] [PubMed] [Google Scholar]
- 38.Vivier E, Sorrell JM, Ackerly M, Robertson MJ, Rasmussen RA, Levine H, Anderson P. Developmental regulation of a mucin-like glycoprotein selectively expressed on natural killer cells. J Exp Med. 1993;178:2023–2033. doi: 10.1084/jem.178.6.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pessino A, Sivori S, Bottino C, Malaspina A, Morelli L, Moretta L, Biassoni R, Moretta A. Molecular cloning of NKp46: a novel member of the immunoglobulin superfamily involved in triggering of natural cytotoxicity. J Exp Med. 1998;188:953–960. doi: 10.1084/jem.188.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moretta A, Sivori S, Vitale M, Pende D, Morelli L, Augugliaro R, Bottino C, Moretta L. Existence of both inhibitory (p58) and activatory (p50) receptors for HLA-C molecules in human natural killer cells. J Exp Med. 1995;182:875–884. doi: 10.1084/jem.182.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vitale M, Sivori S, Pende D, Augugliaro R, Di Donato C, Amoroso A, Malnati M, Bottino C, Moretta L, Moretta A. Physical and functional independency of p70 and p58 natural killer (NK) cell receptors for HLA class I: their role in the definition of different groups of alloreactive NK cell clones. Proc Natl Acad Sci U S A. 1996;93:1453–1457. doi: 10.1073/pnas.93.4.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pende D, Biassoni R, Cantoni C, Verdiani S, Falco M, di Donato C, Accame L, Bottino C, Moretta A, Moretta L. The natural killer cell receptor specific for HLA-A allotypes: a novel member of the p58/p70 family of inhibitory receptors that is characterized by three immunoglobulin-like domains and is expressed as a 140-kD disulphide-linked dimer. J Exp Med. 1996;184:505–518. doi: 10.1084/jem.184.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Poggi A, Costa P, Tomasello E, Moretta L. IL-12-induced up-regulation of NKRP1A expression in human NK cells and consequent NKRP1A-mediated down-regulation of NK cell activation. Eur J Immunol. 1998;28:1611–1616. doi: 10.1002/(SICI)1521-4141(199805)28:05<1611::AID-IMMU1611>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 44.Jarr y A, Cerf-Bensussan N, Brousse N, Guy-Grand D, Muzeau F, Potet F. Same peculiar subset of HML1+ lymphocytes present within normal intestinal epithelium is associated with tumoral epithelium of gastrointestinal carcinomas. Gut. 1988;29:1632–1638. doi: 10.1136/gut.29.12.1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cerf-Bensussan N, Guy-Grand D, Griscelli C. Intraepithelial lymphocytes of human gut: isolation, characterisation and study of natural killer activity. Gut. 1985;26:81–88. doi: 10.1136/gut.26.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lanier LL. NK cell receptors. Annu Rev Immunol. 1998;16:359–393. doi: 10.1146/annurev.immunol.16.1.359. [DOI] [PubMed] [Google Scholar]
- 47.Exley M, Porcelli S, Furman M, Garcia J, Balk S. CD161 (NKR-P1A) costimulation of CD1d-dependent activation of human T cells expressing invariant Vα 24 J α Q T cell receptor α chains. J Exp Med. 1998;188:867–876. doi: 10.1084/jem.188.5.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Llano M, Lee N, Navarro F, Garcia P, Albar JP, Geraghty DE, Lopez-Botet M. HLA-E-bound peptides influence recognition by inhibitory and triggering CD94/NKG2 receptors: preferential response to an HLA-G-derived nonamer. Eur J Immunol. 1998;28:2854–2863. doi: 10.1002/(SICI)1521-4141(199809)28:09<2854::AID-IMMU2854>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 49.Sivori S, Vitale M, Morelli L, Sanseverino L, Augugliaro R, Bottino C, Moretta L, Moretta A. p46, a novel natural killer cell-specific surface molecule that mediates cell activation. J Exp Med. 1997;186:1129–1136. doi: 10.1084/jem.186.7.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mingari MC, Ponte M, Bertone S, Schiavetti F, Vitale C, Bellomo R, Moretta A, Moretta L. HLA class I-specific inhibitory receptors in human T lymphocytes: interleukin 15-induced expression of CD94/NKG2A in superantigen- or alloantigen-activated CD8+ T cells. Proc Natl Acad Sci U S A. 1998;95:1172–1177. doi: 10.1073/pnas.95.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Waldmann TA, Tagaya Y. The multifaceted regulation of interleukin-15 expression and the role of this cytokine in NK cell differentiation and host response to intracellular pathogens. Annu Rev Immunol. 1999;17:19–49. doi: 10.1146/annurev.immunol.17.1.19. [DOI] [PubMed] [Google Scholar]
- 52.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 53.Ferrini S, Cambiaggi A, Meazza R, Sforzini S, Marciano S, Mingari MC, Moretta L. T cell clones expressing the natural killer cell-related p58 receptor molecule display heterogeneity in phenotypic properties and p58 function. Eur J Immunol. 1994;24:2294–2298. doi: 10.1002/eji.1830241005. [DOI] [PubMed] [Google Scholar]
- 54.Mingari MC, Vitale C, Cambiaggi A, Schiavetti F, Melioli G, Ferrini S, Poggi A. Cytolytic T lymphocytes displaying natural killer (NK)-like activity: expression of NK-related functional receptors for HLA class I molecules (p58 and CD94) and inhibitory effect on the TCR-mediated target cell lysis or lymphokine production. Int Immunol. 1995;7:697–703. doi: 10.1093/intimm/7.4.697. [DOI] [PubMed] [Google Scholar]
- 55.Phillips JH, Gumperz JE, Parham P, Lanier LL. Superantigen-dependent, cell-mediated cytotoxicity inhibited by MHC class I receptors on T lymphocytes. Science. 1995;268:403–405. doi: 10.1126/science.7716542. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki H, Duncan GS, Takimoto H, Mak TW. Abnormal development of intestinal intraepithelial lymphocytes and peripheral natural killer cells in mice lacking the IL-2 receptor β chain. J Exp Med. 1997;185:499–505. doi: 10.1084/jem.185.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Carena I, Shamshiev A, Donda A, Colonna M, Libero GD. Major histocompatibility complex class I molecules modulate activation threshold and early signaling of T cell antigen receptor-γ/δ stimulated by nonpeptidic ligands. J Exp Med. 1997;186:1769–1774. doi: 10.1084/jem.186.10.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Maria A, Ferraris A, Guastella M, Pilia S, Cantoni C, Polero L, Mingari MC, Bassetti D, Fauci AS, Moretta L. Expression of HLA class I–specific inhibitory natural killer cell receptors in HIV-specific cytolytic T lymphocytes: impairment of specific cytolytic functions. Proc Natl Acad Sci U S A. 1997;94:10285–10288. doi: 10.1073/pnas.94.19.10285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Halary F, Peyrat MA, Champagne E, Lopez-Botet M, Moretta A, Moretta L, Vie H, Fournie JJ, Bonneville M. Control of self-reactive cytotoxic T lymphocytes expressing γ δ T cell receptors by natural killer inhibitory receptors. Eur J Immunol. 1997;27:2812–2821. doi: 10.1002/eji.1830271111. [DOI] [PubMed] [Google Scholar]
- 60.Lanier LL, Phillips JH. Inhibitory MHC class I receptors on NK cells and T cells. Immunol Today. 1996;17:86–91. doi: 10.1016/0167-5699(96)80585-8. [DOI] [PubMed] [Google Scholar]
- 61.Rocha B, von Boehmer H, Guy-Grand D. Selection of intraepithelial lymphocytes with CD8 α/α co-receptors by self-antigen in the murine gut. Proc Natl Acad Sci U S A. 1992;89:5336–5340. doi: 10.1073/pnas.89.12.5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Guehler SR, Bluestone JA, Barrett TA. Immune deviation of 2C transgenic intraepithelial lymphocytes in antigen-bearing hosts. J Exp Med. 1996;184:493–503. doi: 10.1084/jem.184.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poccia F, Cipriani B, Vendetti S, Colizzi V, Poquet Y, Battistini L, Lopez-Botet M, Fournie JJ, Gougeon ML. CD94/NKG2 inhibitory receptor complex modulates both anti-viral and antitumoral responses of polyclonal phosphoantigen-reactive Vγ9Vδ2 T lymphocytes. J Immunol. 1997;159:6009–6017. [PubMed] [Google Scholar]
- 64.Noppen C, Schaefer C, Zajac P, Schutz A, Kocher T, Kloth J, Heberer M, Colonna M, De Libero G, Spagnoli GC. C-type lectin-like receptors in peptide-specific HLA class I-restricted cytotoxic T lymphocytes: differential expression and modulation of effector functions in clones sharing identical TCR structure and epitope specificity. Eur J Immunol. 1998;28:1134–1142. doi: 10.1002/(SICI)1521-4141(199804)28:04<1134::AID-IMMU1134>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 65.Bellon T, Heredia AB, Llano M, Minguela A, Rodriguez A, Lopez-Botet M, Aparicio P. Triggering of effector functions on a CD8+ T cell clone upon the aggregation of an activatory CD94/kp39 heterodimer. J Immunol. 1999;162:3996–4002. [PubMed] [Google Scholar]
- 66.Maiuri L, Auricchio S, Coletta S, De Marco G, Picarelli A, Di Tola M, Quaratino S, Londei M. Blockage of T-cell costimulation inhibits T-cell action in celiac disease. Gastroenterology. 1998;115:564–572. doi: 10.1016/s0016-5085(98)70135-0. [DOI] [PubMed] [Google Scholar]
- 67.Ebert EC. Interleukin 15 is a potent stimulant of intraepithelial lymphocytes. Gastroenterology. 1998;115:1439–1445. doi: 10.1016/s0016-5085(98)70022-8. [DOI] [PubMed] [Google Scholar]
- 68.Sancho D, Yanez-Mo M, Tejedor R, Sanchez-Madrid F. Activation of peripheral blood T cells by interaction and migration through endothelium: role of lymphocyte function antigen-1/intercellular adhesion molecule-1 and interleukin-15. Blood. 1999;93:886–896. [PubMed] [Google Scholar]
- 69.Perera LP, Goldman CK, Waldmann TA. IL-15 induces the expression of chemokines and their receptors in T lymphocytes. J Immunol. 1999;162:2606–2612. [PubMed] [Google Scholar]
- 70.Oberhuber G, Vogelsang H, Stolte M, Muthenthaler S, Kummer AJ, Radaszkiewicz T. Evidence that intestinal intraepithelial lymphocytes are activated cytotoxic T cells in celiac disease but not in giardiasis. Am J Pathol. 1996;148:1351–1357. [PMC free article] [PubMed] [Google Scholar]
- 71.Wei XH, Orr HT. Differential expression of HLA-E, HLA-F, and HLA-G transcripts in human tissue. Hum Immunol. 1990;29:131–142. doi: 10.1016/0198-8859(90)90076-2. [DOI] [PubMed] [Google Scholar]
- 72.Lanier LL, Corliss B, Wu J, Phillips JH. Association of DAP12 with activating CD94/NKG2C NK cell receptors. Immunity. 1998;8:693–701. doi: 10.1016/s1074-7613(00)80574-9. [DOI] [PubMed] [Google Scholar]
- 73.Long EO, Colonna M, Lanier LL. Inhibitory MHC class I receptors on NK and T cells: a standard nomenclature (letter) Immunol Today. 1996;17:100. doi: 10.1016/0167-5699(96)80590-1. [DOI] [PubMed] [Google Scholar]


