Abstract
Ventilator-associated pneumonia (VAP) is one of the most frequent ICU-acquired infections. Reported incidences vary widely from 5 to 40% depending on the setting and diagnostic criteria. VAP is associated with prolonged duration of mechanical ventilation and ICU stay. The estimated attributable mortality of VAP is around 10%, with higher mortality rates in surgical ICU patients and in patients with mid-range severity scores at admission. Microbiological confirmation of infection is strongly encouraged. Which sampling method to use is still a matter of controversy. Emerging microbiological tools will likely modify our routine approach to diagnosing and treating VAP in the next future. Prevention of VAP is based on minimizing the exposure to mechanical ventilation and encouraging early liberation. Bundles that combine multiple prevention strategies may improve outcomes, but large randomized trials are needed to confirm this. Treatment should be limited to 7 days in the vast majority of the cases. Patients should be reassessed daily to confirm ongoing suspicion of disease, antibiotics should be narrowed as soon as antibiotic susceptibility results are available, and clinicians should consider stopping antibiotics if cultures are negative.
Keywords: Ventilator-associated pneumonia, Bronchoscopy, Mechanical ventilation, Bronchoalveolar lavage, Endotracheal aspirate, Antibiotics, Multiple-drug resistance, Treatment, Prevention, epidemiology, Incidence, Mortality
Take-home message
| Microbiological confirmation is strongly recommended when considering a diagnosis of ventilator-associated pneumonia (VAP). Combination therapy is recommended for the initial treatment of most patients with VAP except for those with early-onset disease without risk factors for multidrug-resistant pathogens being treated in settings with low rates of resistance. De-escalation to a monotherapy once culture results are available and treating for a total of 7 days is recommended for most patients. |
Introduction
Ventilator-associated pneumonia (VAP) is defined by infection of the pulmonary parenchyma in patients exposed to invasive mechanical ventilation for at least 48 h and is part of ICU-acquired pneumonia. VAP remains one of the most common infections in patients requiring invasive mechanical ventilation. Despite recent advances in microbiological tools, the epidemiology and diagnostic criteria for VAP are still controversial, complicating the interpretation of treatment, prevention, and outcomes studies. VAP imposes a significant economic burden. A recent cost evaluation from the USA estimated that the attributable cost of VAP to be $40,144 (95% CI $36,286–$44,220) [1]. We will focus this review on current understanding of the epidemiology, diagnosis, prevention, and treatment of ventilator-associated pneumonia. Other conditions such as ventilator-associated tracheobronchitis are not detailed.
Epidemiology
Incidence
VAP is reported to affect 5–40% of patients receiving invasive mechanical ventilation for more than 2 days, with large variations depending upon the country, ICU type, and criteria used to identify VAP [2–4]. VAP rates in North American hospitals have been reported to be as low as 1–2.5 cases per 1000 ventilator-days [5]. European centers, however, report much higher rates. The EU-VAP/CAP study, for example, reported an incidence density of 18.3 VAP episodes per 1000 ventilator-days [6]. Lower–middle-income countries also report higher rates compared to US hospitals and high-income countries in particular (18.5 vs 9.0 per 1000 ventilator-days; P = .035) [7]. These large discrepancies are at least in part explained by differences in definitions, differences in how definitions are applied, diagnostic limitations of all definitions, and differences in microbiological sampling methods [8]. The daily risk of VAP peaks between days 5–9 of mechanical ventilation, whereas the cumulative incidence is closely related to total duration of mechanical ventilation [9, 10].
The Centers for Disease Control and Prevention’s National Healthcare Safety Network (NHSN) has reported large decreases in the incidence of VAP for both medical and surgical ICUs over the past 15 years [11]. These results were not confirmed, however, by an analysis using a stable definition for VAP conducted by the Medicare Patient Safety Monitoring System (MPSMS) from 2005 through 2013 [12]. The incidence of VAP was roughly 10% throughout the study period in a selected population of patients of at least 65 years with principal diagnoses of acute myocardial infarction, heart failure, pneumonia, and selected major surgical procedures [12]. These discrepancies suggest the possibility of variations in how surveillance criteria are applied and support the use of more objective surveillance parameters [13]. Comprehensive research to identify novel diagnostic biomarkers could be of interest in this context.
Incidence rates greatly vary based on the studied population. For example, VAP rates as high as 24.5/1000 ventilator-days have been reported in cancer patients [14]. A high incidence is also reported in trauma patients (17.8% in one series of 511 patients) [15], explained at least in part by the alteration of immune function after major traumatic injury, aspiration resulting from brain injury and lung contusion [6]. The increased incidence observed in chronic obstructive pulmonary disease (COPD) patients might be explained by prolonged duration of invasive mechanical ventilation (muscular weakness), high incidence of microaspiration and bacterial colonization (defective mucociliary clearance), and altered local and general host defense mechanisms [16]. Acute respiratory distress syndrome (ARDS) is also associated with a high risk of VAP. Even with the use of lung-protective strategies, incidence as high as 29% has been reported [10] among ARDS patients in general and 35% in patients receiving extra-corporeal membrane oxygenation (ECMO) [17].
Age does not appear to be particularly associated with risk of pneumonia in ventilated patients. A secondary analysis of a European cohort study [18] reported 13.7 VAPs per 1000 ventilation days in middle-aged (45–64 years) patients, 16.6 in old patients (65–74 years), and 13.0 in very old patients (≥ 75 years). Logistic regression analysis was unable to identify a higher risk of VAP among elderly patients [18]. In contrast, male gender is generally recognized as an independent risk factor for VAP [19]. The most important risk factor for VAP, however, is likely the underlying medical conditions of mechanically ventilated patients including their comorbidities and severity of illness. Accounting for difference in patient populations and VAP definitions is crucial for the implementation of suitable surveillance programs, analyzing differences in VAP rates between different ICUs, and evaluating potential therapeutic approaches, and prevention strategies. The systematic use of incidence density as a parameter to evaluate VAP epidemiology would also be helpful to reach these latter objectives.
Outcomes
Although all-cause mortality associated with VAP has been reported to be as high as 50%, there is still considerable controversy regarding the extent to which VAP contributes to death in ICU patients. In contrast, VAP has been consistently associated with prolonging duration of both mechanical ventilation and ICU stay.
Different methods have been used to evaluate the attributable mortality of VAP. Observational cohort studies done in the nineties reported conflicting results [20, 21]. However, these studies included heterogenous populations and were not prospective [22]. As the risk of acquiring VAP is not constant throughout the duration of mechanical ventilation (the risk is higher during the first 10 days), there is a risk of bias due to ICU mortality and discharge acting as competing endpoints (the sickest patients may have very short lengths of stay because of early death). More sophisticated statistical approaches have therefore been used, such as multistate and competing risks models, to estimate the attributable mortality of VAP. A competing risk survival analysis, treating ICU discharge as a competing risk of ICU mortality among 4479 patients treated in French ICUs, reported that the ICU mortality attributable to VAP was very low, about 1% on day 30 and 1.5% on day 60 [23]. In ARDS patients, crude mortality rates of up to 41.8% have been reported in patients with VAP versus 30.7% in patients without VAP (P = 0.05) [10]. However, after adjusting for confounding factors, VAP was no longer associated with ICU death [10]. Even after using a multistate approach controlling for the same risk factors, the occurrence of a bacterial VAP was not associated with the risk of ICU death [10]. This is consistent with recent reports in cancer patients [14] and in traumatic brain injury patients [24], in which VAP was not associated with death.
Another approach to limit the risk of biases related to the presence of confounding factors is to use randomized-controlled trials evaluating the preventive effects on VAP and mortality. Based on aggregate data from 58 randomized studies on VAP prevention, the estimated attributable mortality rate of VAP was 9% [25]. A similar approach using individual patient data for meta-analysis, including 6284 patients from 24 VAP prevention trials, estimated an attributable mortality of 13%, with higher mortality rates in surgical ICU patients and in patients with mid-range severity scores at admission (i.e., Acute Physiology and Chronic Health Evaluation scores [APACHE 2] 20–29 and Simplified Acute Physiology Score [SAPS 2] scores of 35–58 [26] In contrast, attributable mortality was close to zero in trauma patients, medical patients, and patients with low or high severity of illness scores [26]. Antimicrobial resistant pathogens may increase the mortality rates associated with VAP although this is controversial [18, 27]}. In summary, VAP is associated with prolonged duration of mechanical ventilation and prolonged ICU stay, whereas mortality is mainly driven by patients’ underlying conditions and illness severity. Future studies should focus on more homogeneous groups of patients in order to better elucidate the differential contributions of underlying disease, type, and number of organ failures and pathogen identity and resistance profile to the risk of death associated with VAP.
Microorganisms responsible for VAP
The organisms associated with VAP vary according to many factors including duration of mechanical ventilation, length of hospital and ICU stays before VAPs, timing and cumulative exposure to antimicrobials, the local ecology, and the occurrence of any potential epidemic phenomena in a given ICU. Usual Gram-negative microorganisms involved in VAP are Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, and Acinetobacter species; Staphylococcus aureus is the major Gram-positive microorganism [28–33]. It is generally recognized that early-onset VAP (within the first 4 days of hospitalization) in previously healthy patients not receiving antibiotics usually involves normal oropharyngeal flora, whereas late-onset VAP (occurring after at least 5 days of hospitalization) and VAP in patients with risk factors for multidrug resistant (MDR) pathogens are more likely to be due to MDR pathogens [34]. However, MDR pathogens may be isolated in early-onset VAP, mainly in the presence of certain risk factors such as antimicrobial exposure within the preceding 90 days [34–36]. Some reports have found comparable rates of MDR pathogens in patients with early- versus late-onset VAP [27, 36, 37]. Other risk factors for MDR pathogens generally recognized include prior colonization or infection with MDR pathogens, ARDS preceding VAP, acute renal replacement therapy prior to VAP, and the presence of septic shock at time of VAP [34]. The recent International Guidelines of the European Respiratory Society, European Society of Intensive Care Medicine, European Society of Clinical Microbiology and Infectious Diseases and Asociación Latinoamericana del Tórax suggested that additional risk factors should be taken into account such as high local rates of MDR pathogens, recent prolonged hospital stay (> 5 days of hospitalization) and previous colonization with MDR pathogens [38]. Resistance to third- and fourth-generation cephalosporins in Enterobacteriaceae strains due to the expression of acquired extended-spectrum β-lactamases (ESBLs) and/or AmpC β-lactamases is a major worry [39]. The spread of carbapenemase-producing strains is also a growing concern. MDR isolates of Pseudomonas aeruginosa are increasingly prevalent [40]; one-half to two-thirds of Acinetobacter baumannii strains causing VAP are currently carbapenem-resistant [41]. Colistin resistance has increased following rising rates of colistin consumption to treat extensively drug-resistant (XDR) organisms [42]. VAP may be caused by multiple pathogens which can complicate the therapeutic approach [32, 43, 44]. Fungi rarely cause VAP [45]. Candida spp. is the most common yeast isolated in respiratory samples [46]. Colonization of the lower respiratory tract by Candida spp. affects up to 27% of mechanically ventilated patients and could be associated with an increased risk of bacterial VAP, most notably caused by Pseudomonas aeruginosa [47]. However, available data do not support a direct role of Candida spp. as a VAP-causative pathogen [45]. In a recent report, the relationship between Candida spp. colonization and bacterial VAP was prospectively evaluated in 213 patients presenting with multiple organ failure [48]. Whereas 146 patients (68.5%) had tracheal colonization with Candida spp., no association with bacterial VAP was found [48]. Aspergillus spp. (mainly Aspergillus fumigatus) may be involved in some late-onset VAP, particularly in patients with a recent history of influenza [49]. A recently proposed clinical algorithm assessed the relevance of positive cultures and might be helpful for clinicians to decide whether to treat or not [50]. Finally, respiratory viruses including influenza, respiratory syncytial virus, and others may be responsible for VAP [51–54]. The Herpesviridae Herpes simplex virus (HSV) and Cytomegalovirus (CMV) can cause viral reactivation pneumonia in immunocompromised and non-immunocompromised mechanically ventilated patients. Histopathological evidence of HSV bronchopneumonitis has been reported in up to 21% of mechanically ventilated patients with worsening respiratory status [55]. CMV reactivation is observed in 20–30% of critically ill patients, especially in those with multi-organ failure and prolonged ICU stays [56, 57]. Histologically proven CMV pneumonia has been reported in ARDS patients with persistent clinical deterioration and negative bronchoalveolar lavage bacterial culture [58–61]. Other viruses have been identified in mechanically ventilated patients, but their pathogenicity needs to be confirmed [62, 63].
Diagnosis of VAP
VAP diagnosis is traditionally defined by the concomitant presence of the three following criteria: clinical suspicion, new or progressive and persistent radiographic infiltrates, and positive microbiological cultures from lower respiratory tract specimens [34, 38, 64, 65].
Clinical diagnosis
The first step to diagnose VAP is clinical suspicion. Many criteria for suspecting VAP exist (fever, leukocytosis, decline in oxygenation…), but their usefulness, alone or in combination, is not sufficient to diagnose VAP [66]. Scores have been proposed to help improve diagnostic accuracy, the most used being the Clinical Pulmonary Infection Score (CPIS) developed by Pugin et al. [67]: the original description of this score is based on 6 variables (temperature, blood leukocytes, tracheal secretions aspect, oxygenation, radiographic infiltrates, and semiquantitative cultures of tracheal aspirates with Gram stain), and patients with a score above 6 are at risk of having VAP. One randomized study found that using CPIS to determine when to stop antibiotics led to less antibiotic consumption compared to a clinical strategy of fixed durations of antibiotics [68]. However, using CPIS to determine when to start antibiotics may be associated with undue antibiotic use due to its low specificity, particularly as compared to obtaining lower respiratory tract specimens for culture [69]. Therefore, recent guidelines do not recommend CPIS to diagnose VAP [34, 65]. VAP should rather be suspected in patients with clinical signs of infection, such as at least two of the following criteria: new onset of fever, purulent endotracheal secretions, leukocytosis or leucopenia, increase in minute ventilation, decline in oxygenation, and/or increased need for vasopressors to maintain blood pressure. These signs are not specific for VAP, however, and can often be observed in the many conditions that mimic VAP (e.g., pulmonary edema, pulmonary contusion, pulmonary hemorrhage, mucous plugging, atelectasis, thromboembolic disease, etc.).
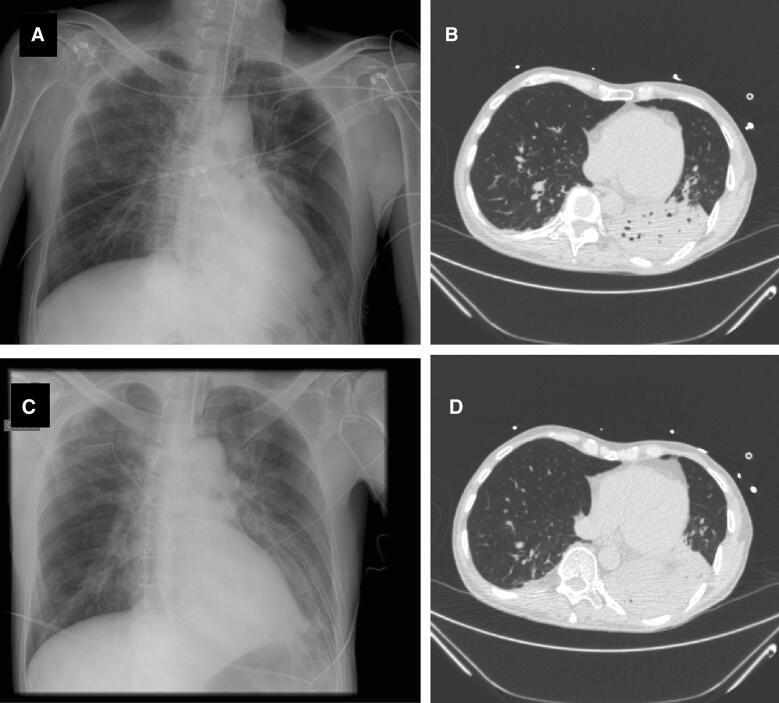
Although almost all definitions for suspecting (and diagnosing) VAP include radiographic criteria (new or progressive and persistent infiltrates), it is well known that chest X-rays are neither sensitive nor specific for VAP [64, 66, 70]. Figures 1 and 2 display two patients for whom radiological criteria were falsely negative (Fig. 1) or not contributive (Fig. 2). Computed tomography (CT) scan may be a good alternative since it is more sensitive; however, a strategy based on systematic lung CT-scan has obvious drawbacks, the main issues being feasibility, maintaining patient safety during transport, and availability. Lung ultrasound has recently been proposed as a diagnostic aid for VAP; however, data on its sensitivity and specificity are lacking [71].
Fig. 1.
Chest X-rays and CT-scan of a 65-year-old man who developed ventilator-associated pneumonia. Chest X-ray performed the day VAP was suspected seems normal (a), whereas the CT-scan performed the same day showed consolidation of the left inferior lobe (b, d). Bronchoalveolar lavage yielded 105Enterobacter aerogenes. The next day, chest X-ray showed progression of pulmonary infiltrates (c). VAP diagnosis based on chest X-ray would have been delayed
Fig. 2.
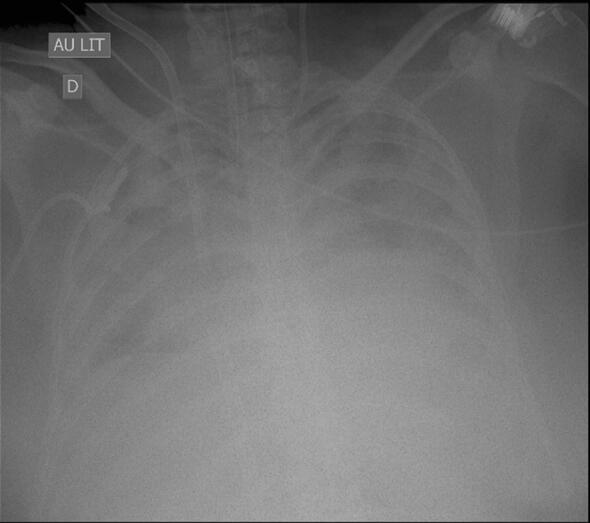
Chest X-ray of a 35-year-old woman with H1N1 influenza-associated acute respiratory distress syndrome (“white lungs”). She developed fever, leukocytosis, purulent tracheal secretions and bronchoalveolar lavage (obtained during fiber optic bronchoscopy) yielded 105Pseudomonas aeruginosa. Chest X-ray was unchanged (same chest X-ray since 1 week) and obviously not useful for suspecting/diagnosing ventilator-associated pneumonia
Biomarkers such as C-reactive protein, procalcitonin or soluble triggering receptor expressed on myeloid cells (sTREM-1) have been proposed as diagnostic markers for VAP; however, they lack accuracy and their use is, to date, not recommended for VAP diagnosis [34, 65, 72–75].
More research is needed to identify sensitive and specific biomarkers that could help the clinician to diagnose VAP, identify the causative pathogen, and guide antibiotic therapy. A translational approach, with application of genomic, proteomic, and metabolomic methodologies, may be helpful in improving our understanding of the pathophysiology of VAP and helping identify judicious biomarkers or profiles that could help clinicians [76].
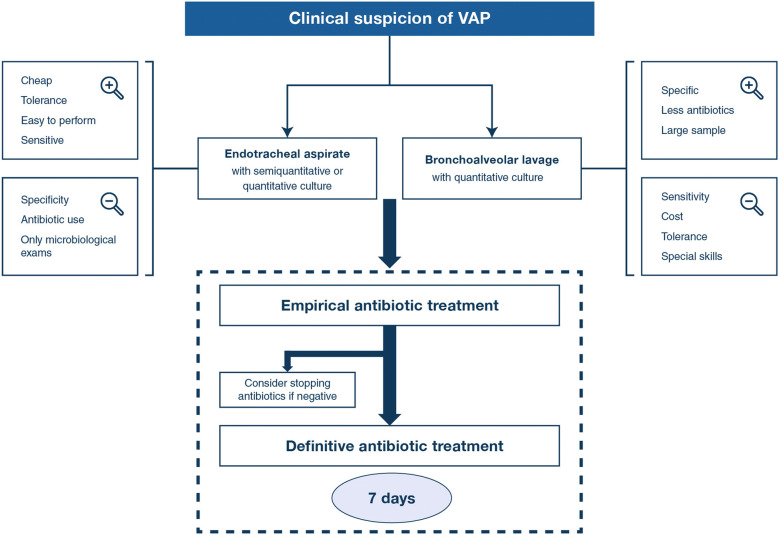
In summary, there is no single clinical criterion, biomarker or score that is accurate enough to diagnose VAP. Therefore, VAP should be considered whenever there are new signs of respiratory deterioration potentially attributable to infection (e.g., fever, purulent sputum, leukocytosis, worsening oxygenation, unexplained hypotension, or increasing vasopressor requirements), with or without new or progressive pulmonary infiltrates. Once VAP is suspected, the second step of the diagnostic workup is to perform microbiological sampling (Fig. 3).
Fig. 3.
Schematic representation of VAP diagnosis and treatment. Clinical suspicion of VAP refers to the association of some of the following criteria: fever, purulent sputum, leukocytosis, impaired oxygenation, unexplained hypotension or shock, new (or progression of) pulmonary infiltrates on chest X-ray (not always observed). Empirical treatment takes into account the underlying disease and its severity, the presence of risk factors for multiple-drug-resistant pathogens (antibiotic therapy in the previous 90 days, hospital stay > 5 days, septic shock at VAP onset, ARDS prior to VAP onset, acute renal replacement therapy prior to VAP onset, previous colonization with MDR pathogen) and local pattern of antimicrobial susceptibility. Immunocompromised patients, patients with empyema, lung abscess or necrotising pneumonia should receive prolonged antimicrobial course [38]
Microbiological diagnosis
Recently, scientific societies from North America and Europe proposed recommendations to diagnose VAP [34, 38] (Table 1). The European guidelines [38] suggested obtaining distal quantitative samples before antibiotic treatment, since it is known that, if samples are obtained after starting antibiotic treatment, the results may be altered or emerge as negative. The use of distal quantitative cultures, which may be more specific than blind (non-directed) sampling techniques, may help to reduce overutilization of antibiotics particularly if clinicians only start antibiotics in patients with positive gram stains, positive cultures, or suspected septic shock [77].
Table 1.
| 2016 Clinical Practice Guidelines by the Infectious Diseases Society of America and the American Thoracic Society [34] | 2017 International ERS/ESICM/ESCMID/ALAT guidelines [38] |
|---|---|
|
Should patients with suspected VAP be treated based on the results of invasive sampling (BAL, PSB, blind mini-BAL) with quantitative culture results, noninvasive sampling (endotracheal aspiration) with quantitative culture results, or noninvasive sampling with semiquantitative culture results? Recommendation We suggest noninvasive sampling with semiquantitative cultures to diagnose VAP, rather than invasive sampling with quantitative cultures and rather than noninvasive sampling with quantitative cultures (weak recommendation, low-quality evidence) If invasive quantitative cultures are performed, should patients with suspected VAP whose culture results are below the diagnostic threshold for VAP (PSB with < 103 CFU/mL, BAL with < 104 CFU/mL) have their antibiotics withheld rather than continued? Recommendation Noninvasive sampling with semiquantitative cultures is the preferred methodology to diagnose VAP; however, the panel recognizes that invasive quantitative cultures will occasionally be performed by some clinicians. For patients with suspected VAP whose invasive quantitative culture results are below the diagnostic threshold for VAP, we suggest that antibiotics be withheld rather than continued (weak recommendation, very low-quality evidence) |
In intubated patients suspected of having VAP, should distal quantitative samples be obtained instead of proximal quantitative samples? Recommendation We suggest obtaining distal quantitative samples (prior to any antibiotic treatment) in order to reduce antibiotic exposure in stable patients with suspected VAP and to improve the accuracy of the results. (weak recommendation, low quality of evidence) We recommend obtaining a lower respiratory tract sample (distal quantitative or proximal quantitative or qualitative culture) to focus and narrow the initial empiric antibiotic therapy. (strong recommendation, low quality of evidence) |
BAL Bronchoalveolar lavage, PSB protected specimen brush, CFU colony-forming units
Direct examination and Gram staining use are controversial. The American guidelines [34] suggest that a high-quality Gram stain from a respiratory specimen with numerous and predominant organisms provides further support for the diagnosis of VAP. The absence of microorganisms on Gram stain, however, does not reliably exclude VAP, and so it is important to also review culture results. The limited sensitivity and specificity of Gram stain are another reason why we need to identify additional rapid diagnostic strategies including biomarkers and rapid microorganism identification and susceptibility assays.
Presentations of diagnostic sampling techniques for VAPs are sometimes confusing. Invasive techniques are those which are distal and directed by bronchoscopy, such as bronchoalveolar lavage (BAL), protected specimen brush (PSB) or lung biopsies (an uncommon sampling method). Blind mini-BAL using a plugged telescopic catheter (or blind protected specimen brush) is a non-directed sampling technique which is not always distal (because there is no confirmation of the correct placement of the tip of the catheter) and which is considered as “non-invasive” even though bleeding and pneumothoraces are possible complications.
These “invasive techniques” also present several disadvantages: the need for qualified personnel to perform these procedures (even though it is now a conditional skill to become an intensivist in many countries), potential risks for the patient (hypoxemia, barotrauma, bleeding), and the associated costs especially when using disposable bronchoscopes. However, the use of bronchoscopic BAL combined with quantitative cultures may achieve more reliable identification of causative agents with a higher specificity than qualitative sampling methods and allows sufficient fluid return to perform complementary analyses (i.e., cytology, albumin levels, viruses identification, galactomannan determination, procollagen III in ARDS patients). Qualitative cultures obtaining using proximal sampling methods such as endotracheal aspirates may overestimate the presence of bacteria potentially lead to unnecessary antibiotics use, and promotion of antibiotic resistance. However, they can be performed more quickly and simply compared to bronchoscopy, with fewer complications and resources (Fig. 3). A meta-analysis of 5 randomized trials comparing invasive microbiological sampling techniques with quantitative cultures versus noninvasive sampling methods with either quantitative or semiquantitative cultures did not find any differences in patients’ outcomes [78, 79].
A common dilemma is the question of whether to start antibiotics when invasive quantitative culture results are negative or below the diagnostic threshold for patients with suspected VAP. The Infectious Diseases Society of America (IDSA)/American Thoracic Society (ATS) guidelines suggest withholding antibiotics in such cases so long as they are clinically stable [34]. This strategy might be associated with less unnecessary antibiotic use, which should reduce antibiotic-related adverse events (such as Clostridium difficile emergence and rising antibiotic resistance) and costs. However, intensivists should always use their clinical judgment to temper this decision if there is other compelling evidence of pulmonary infection, if the patient received antimicrobial therapy prior to microbiological sampling, if there is associated septic shock, if the patient is immunocompromised, and/or if the patient fails to improve despite managing potential non-infectious causes of clinical deterioration.
Although not recommend by the recent guidelines, some patients may receive antibiotics before microbiological sampling, results of this latter being therefore negative many times. In such cases, giving a full course (7 days, see below) of antibiotics may expose the patient to prolonged undue antibiotics and may promote antibiotic resistance. Therefore, our recommendation is to re-evaluate the patient at 48–72 h; if the clinical course is favorable, and the likelihood of infection is low, antibiotics could be stopped. Another solution is to use procalcitonin to stop antibiotics at 48–72 h if the procalcitonin level is < 0.5 ng/mL or has decrease of more than 80% as compared to the peak value [34, 80, 81]. Another common situation is a patient who receives antibiotics for more than 48 h at the time of microbiological sampling (whatever the indication, for an extrapulmonary infection for example). If microbiological results are negative, that suggests that the patient does not have VAP. Therefore, no new antibiotics should be started, and the decision on what to do with the current antimicrobial treatment should be based on the initial indication.
Microbiological diagnosis in the near future
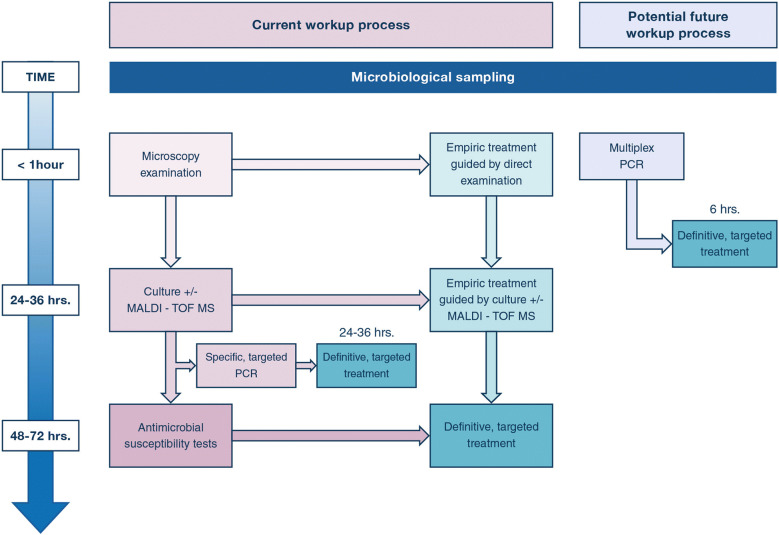
One of the challenges in diagnosing VAP, whatever the technique used (endotracheal aspirates or bronchoscopic-guided BAL), is to decrease the time from sampling to pathogen identification. Indeed, it currently takes at least 24–48 h using conventional microbiological methods to identify the pathogen(s) responsible for infection and its (their) sensitivity to antimicrobial treatment (Fig. 4). During that time, empiric broad-spectrum antibiotics are often given [34, 38, 82]. One of the key issues in antimicrobial stewardship is to decrease the consumption of broad-spectrum antibiotics [82] both by limiting their prescription and shortening their duration. Over the past few years, molecular methods have been developed to decrease the time between sampling organism identification, and determination of antibiotic susceptibilities. For example, the use of polymerase chain reaction (PCR) to detect bacterial DNA can shorten the time of organism identification and susceptibilities, but it is restricted to specific pathogens and resistance mechanisms, for example mecA to detect methicillin resistance in Staphyloccocus aureus strains [83]. Although this technique is not available to determine resistance patterns for pathogens commonly responsible for VAP such as Pseudomonas aeruginosa [84] or it requires a positive culture to detect resistance mechanisms [83], it is routinely used in many places to allow for very early de-escalation and narrowing of antimicrobial treatment in specific situations, for example to withdraw or withhold anti-MRSA antibiotics (Fig. 4).
Fig. 4.
Current and potential future workup processes for identification of pathogens responsible for VAP. To date, it takes 48–72 h. to identify pathogen responsible for ventilator-associated pneumonia (VAP) and its susceptibility to antibiotics (purple boxes), delaying the definitive, targeted treatment at that time (green boxes). Awaiting these results, physicians prescribe empiric broad-spectrum antimicrobial treatment. The use of specific, targeted polymerase chain reaction (PCR) may allow shortening this time to 24–36 h., but for specific pathogens and specific resistance mechanisms. A potential future workup process will be to use multiplex PCR (blue box) to identify within less than 6 h pathogens responsible for VAP and their resistance to antimicrobials
Recently, new tools using multiplex PCR directly applied to fresh (bronchoscopic) samples have been developed to identify pathogens. Some tests screen just for the main pathogens responsible for VAP, and some of them also screen for selected resistance mechanisms. The pneumonia application of the Unyvero system (Curetis AG, Holzgerlingen, Germany) allows testing for 20 different bacteria and one fungus, including those most frequently responsible for VAP, as well as 19 resistance markers, directly in clinical specimens, with a turnaround time of 4 to 5 h [85]. Recent studies have evaluated this new technique as compared to conventional microbiological methods and found a concordance rate between the two techniques of 50–60% for pathogen identification, and of 70–75% for identifying resistance [85–88]. However, this kind of technique is limited by the risk of over-detection, i.e., detection of DNA of non-viable organisms, detection of pathogens at non-pathogenic thresholds, and the detection of non-pathogenic organisms (i.e., colonizers rather than invaders). This kind of technique will probably facilitate major advances in the management of VAP in the near future, allowing clinicians to tailor antibiotics within a few hours (Fig. 4). However, improvements in the breadth and sensitivity of the technique as well as studies evaluating the safety and efficacy of rapid diagnostics to improve the suitability and duration of antimicrobial treatment as well as impacts on patients’ outcomes are needed before it can be routinely recommended.
Surveillance
The clinical signs used to diagnose VAP are neither sensitive nor specific either alone or in combination. Even lung biopsies are not definitive because of the uneven distribution of lung lesions and variability in pathologists’ interpretations. As such, it is highly unlikely that there will be a worldwide consensus on how best to define and conduct surveillance. This bespeaks the critical need for further research to develop and validate new diagnostic tools to support surveillance, prevention, and treatment studies as well as quality improvement initiatives. This need is particularly acute in the USA where regulators and legislators have considered including hospitals’ VAP rates in benchmarking and reimbursement policies. In this context, the US Centers for Disease Control and Prevention developed the concept of ventilator-associated events (VAE), a surveillance strategy designed to broaden the focus of surveillance to encompass multiple causes of respiratory deterioration in ventilated patients, not just pneumonia, to make surveillance more objective, and to allow for the possibility of automated surveillance using electronic clinical data. The definition includes subcriteria to try to identify the subset of VAEs that might be infection-related and which might be due to pneumonia in particular, but there are no data to suggest that VAE definitions are any more (or less) accurate than traditional surveillance definitions [13].
While lower respiratory tract surveillance cultures may help to predict the involvement of MDR microorganisms in patients that develop VAP and thus decrease unnecessary broad-spectrum antibiotics use, there are no clear data that this strategy improves clinical outcomes or lowers costs [89, 90].
Consensus diagnostic criteria that can be objectively applied are needed to compare incidence rates between hospitals and countries for the purposes of public health planning and reimbursement.
Prevention of VAP
Many of our presumptions about how best to prevent VAP have recently been challenged. Oral care with chlorhexidine and stress ulcer prophylaxis may be harmful, new data affirm the long-held fear that selective oral and digestive decontamination may not be effective in ICUs with high baseline rates of antibiotic resistance, and subglottic secretion drainage may not shorten duration of mechanical ventilation or ICU length-of-stay as was once thought [91–95]. The practices most consistently associated with earlier extubation and/or lower mortality rates are those focused on limiting exposure to invasive mechanical ventilation by avoiding intubation and speeding extubation [96].
Interpreting the VAP prevention literature is challenging because many initiatives have been reported to lower VAP rates, but the limitations of VAP diagnostic tools and criteria make it difficult to discern the true effect of prevention strategies [97].
Unless and until we develop sensitive and specific markers for the presence or absence of VAP, providers are advised to consider more objective outcomes when evaluating the potential merits of proposed prevention strategies [98]. These include duration of mechanical ventilation, ICU length-of-stay, ventilator-associated events, antibiotic utilization, and mortality. Comparing prevention measures’ impacts on VAP rates versus more objective outcomes can sometimes lead to surprising discrepancies. For example, meta-analyses of randomized trials of oral care with chlorhexidine suggest this intervention may lower VAP rates but increase mortality [99, 100]. We will use this lens to briefly review common VAP prevention strategies.
Several recent trials evaluated the potential benefits of modifying endotracheal tube cuff shapes and/or materials to minimize seepage of microbe-laden fluids across the cuff and into the lungs. Unfortunately, neither tapered cuffs nor ultrathin polyurethane proved to be any better than conventional cylindrical cuffs or polyvinyl chloride at preventing VAP or improving objective outcomes [101–104]. Likewise, manually monitoring cuff pressures every 8 h to minimize inadvertent drops in endotracheal tube cuff pressure was no better at preventing VAP, decreasing length-of-stay, or lowering mortality in a recent single center study compared to checking cuff pressures only at intubation, following frank tube migration, or detection of a cuff pressure leak [105]. A meta-analysis of three randomized trials of automated cuff pressure monitoring did report significantly lower VAP rates with automated cuff pressure systems, but the analysis was limited by small numbers, substantial heterogeneity, and limited evaluation of secondary outcomes [106].
Subglottic secretion drainage has repeatedly been associated with lower VAP rates in both individual randomized trials and meta-analyses but does not appear to shorten the time to extubation, ICU length-of-stay, prevent ventilator-associated events, or lower mortality rates [94]. Earlier meta-analyses did suggest a possible impact on time to extubation and ICU discharge but were confounded by ambiguous study results and high levels of heterogeneity [94, 107]. Two studies have reported an association between subglottic secretion drainage and less antibiotic utilization, but a third did not [108–110].
Recent studies have also called into question the effectiveness and safety of oral chlorhexidine. There is no association between oral care with chlorhexidine and lower VAP rates on meta-analysis of double-blind randomized trials [99]. More concerningly, some meta-analyses and observational studies have reported that oral care with chlorhexidine may increase mortality rates, perhaps because some patients may aspirate some of the antiseptic triggering acute lung injury [91, 95, 99, 100, 111, 112]. A cluster randomized de-adoption study is currently underway to better characterize the safety and effectiveness of oral chlorhexidine for ventilated patients [113].
Elevating the head of the bed to prevent reflux of gastric secretions into the lungs is the most commonly practiced intervention to prevent VAP [114, 115] but is supported by surprisingly few randomized trials. A Cochrane review of 8 randomized trials enrolling 759 patients did report collectively fewer clinically suspected VAPs in patients randomized to head-of-bed elevation, but no effect on microbiologically confirmed VAP and no effect on objective outcomes [116]. Some investigators have hypothesized that putting patients in the lateral Trendelenburg may be a better way to prevent VAP by recruiting gravity to carry oral secretions away from the lungs. A recent study confirmed this hypothesis, but the trial was terminated early due to a surfeit of adverse events among patients randomized to lateral Trendelenburg [117].
Selective oral and digestive decontamination is one of the very few preventative strategies in critical care that has repeatedly been associated with lower mortality rates [118, 119]. This strategy is widely practiced in the Netherlands, but practitioners elsewhere have been loath to adopt antibiotic decontamination for fear that it might promote antibiotic resistance, particularly in ICUs with high baseline rates of antibiotic-resistant bacteria and antibiotic utilization. Ironically, oral and digestive decontamination may actually decrease overall antibiotic utilization presumably by decreasing the incidence of infections requiring treatment [120]. Nonetheless, a recent cluster randomized trial of oral and digestive decontamination in ICUs with high baseline rates of antibiotic resistance and antibiotic utilization found no significant impact on bloodstream infections or mortality [93].
Probiotics may protect patients from VAP by modulating the microbiome and inhibiting colonization with invasive pathogens. Some randomized trials have reported lower VAP rates, but this signal is not present on meta-analysis restricted to double-blinded studies [121]. A large multicenter study is currently underway [122].
Stress ulcer prophylaxis has been associated with higher VAP rates in some observational studies and in a recent meta-analysis of randomized trials [92, 123, 124]. A large randomized trial of pantoprazole vs placebo, however, reported no difference between arms in pneumonia rates [125]. At the same time, stress ulcer prophylaxis had a relatively modest effect on gastrointestinal bleeding rates (2.5% vs 4.2%) and no impact on transfusion requirements or mortality rates. Additional large randomized trials are underway.
The prevention practices that have most consistently been associated with improving objective outcomes for ventilated patients have been those focused on avoiding intubation and minimizing exposure to invasive ventilation by using high flow oxygen or noninvasive ventilation as alternatives to intubation, lightening sedation, using spontaneous breathing trials to prompt early extubation, and early mobilization [126, 127]. These interventions appear to be synergistic insofar as minimizing sedation facilitates mobilization and early extubation. Observational studies of quality improvement collaboratives have reported that bundling these practices together is associated with earlier extubation and lower mortality rates [128–131]. It will be important, however, to confirm these findings in randomized trials given the many potential sources of bias in observational studies [132]. Table 2 summarizes current knowledge about VAP prevention.
Table 2.
Summary of the current knowledge about VAP prevention [162]
| Intervention | Probable impact on VAP rates | Comments |
|---|---|---|
| Head-of-bed elevation [116] | May lower rates | Understudied, few and contradictory randomized trials |
| Tapered endotracheal tube cuffs and ultrathin polyurethane [102, 104] | No impact | In vivo studies document persistently high rates of subclinical aspiration despite the theoretical advantages of these designs |
| Automated endotracheal tube cuff pressure monitoring [106] | May lower rates | Understudied, merits further evaluation |
| Subglottic secretion drainage [94] | May lower rates | Extensively studied but despite lower VAP rates no impact on duration of mechanical ventilation, ICU length-of-stay, ventilator-associated events, or mortality. Unclear impact on antibiotic utilization |
| Oral care with chlorhexidine [99, 100, 112] | Unclear | Extensively studied. Most individual studies negative. Meta-analysis of open-label studies suggest lower VAP rates but meta-analysis of double-blind studies find no impact. May increase mortality rates. Oral care with sterile water preferred |
| Selective oral and digestive decontamination [93, 119] | Likely lowers VAP rates | Extensively studied. Less net antibiotic utilization and lower mortality rates in Dutch studies. No impact on mortality in units with high baseline rates of antibiotic resistance and antibiotic utilization |
| Probiotics [163] | Unclear | Many studies but most of limited quality, mixed results. Lower VAP rates on meta-analysis but no signal when restricting to double-blind studies |
| Stress ulcer prophylaxis [92, 123, 125] | May increase VAP rates | Observational studies and some meta-analyses suggest higher VAP rates but a recent large randomized trial found no impact |
| VAP prevention bundles [128] | Likely lower VAP rates | Extensively studied, almost exclusively in before–after and time-series analyses. May be associated with lower mortality rates. Most benefit likely from minimizing sedation and encouraging early extubation |
Treatment of VAP
Intravenous (IV) antimicrobial therapy is the cornerstone of VAP treatment. Physicians face a dilemma, however, between avoiding ineffective treatment, inappropriate initial antimicrobial treatment being associated with increased mortality [133]; and on the other hand, reducing the consumption of broad-spectrum antibiotics, the latter being associated with increased bacterial resistance [134]. Therefore, treatment of VAP should be a two-step process: the first step is empiric treatment, the choice and immediacy of treatment being driven by disease severity (i.e., mortality risk) and risk factors of MDR pathogens; and the second step is definitive treatment, for which clinicians should try to avoid overuse of antibiotics.
Empirical treatment
The choice and timing of antimicrobial agents used should take into account four parameters: severity of the current illness, type and number of underlying diseases and their severity, risk factors for MDR pathogens, and the local pattern of antimicrobial susceptibility. Risk factors for MDR pathogens include high (> 25%) local prevalence of pathogen resistance, antibiotic therapy in the previous 90 days, hospital stay > 5 days, septic shock at VAP onset, ARDS prior to VAP onset, acute renal replacement therapy prior to VAP onset and previous colonization with MDR pathogens [34, 65]. In non-immunocompromised patients with early-onset VAP and no risk factors for MDR pathogens (as defined above), monotherapy with narrow-spectrum antibiotic (non-pseudomonal third generation cephalosporin) can be used (Table 3) [38] (this situation is not mentioned in the IDSA/ATS guidelines [34]). In other situations, initial empiric treatment should include a broad-spectrum β-lactam targeting Pseudomonas aeruginosa and/or ESBL-producing Enterobacteriaceae (ceftazidime, cefepime, piperacillin–tazobactam or a carbapenem) plus a non-β-lactam antipseudomonal agent, such as aminoglycosides (amikacin or tobramycin) or fluoroquinolones (ciprofloxacin or levofloxacin) (Table 3). The choice of the β-lactam agent should take into account previously used antibiotics, local pattern of susceptibilities and patient colonization with MDR pathogen. For example, a carbapenem should be preferred in patients colonized with ESBL-producing Enterobacteriaceae. Indeed, although carbapenem are overprescribed in ESBL carriers, 7–10% of VAP episodes in these patients are due to an ESBL-producing Enterobacteriaceae, and it seems difficult not to take into account this pathogen in the empirical antimicrobial treatment [135, 136]. Moreover, it has been shown that 63% of infection-related ventilator-associated complications were neither VAP nor attributable to a documented ICU infection [136], indicating that efforts should be concentrated on the diagnostic strategy, to use carbapenems only in patients with true infection, and to withhold carbapenems when the likelihood of infection is low.
Table 3.
Suggested initial empirical antimicrobial treatment of ventilator-associated pneumonia.
| Situation | Therapeutic class | Agent |
|---|---|---|
| Early VAP (< 5 days), without MDR bacteria risk factor* | Non-antipseudomonal β-lactam |
Amoxicillin/clavulanic acid† OR Third generation cephalosporin |
|
Late VAP (≥ 5 days), OR Risk factors for MDR bacteria |
β-lactam active against Pseudomonas aeruginosa AND Non β-lactam antipseudomonal agent |
Cefepime 2 g q 8 h OR Ceftazidime 2 g q 8 h OR Piperacillin–tazobactam 4 g q 6 h OR Meropenem 2 g q 8 h Amikacin 25 mg/kg/day OR Ciprofloxacin 1200 mg/day |
| Known MRSA colonization, or high (> 20%) MRSA prevalence in the unit | Agent active against MRSA |
Vancomycin 30–45 mg/kg/day OR Linezolid 600 mg/12 h |
| Known colonization with carbapenem-resistant Enterobacteriaceae or Pseudomonas aeruginosa susceptible only to new beta-lactam agents | New β-lactam agent |
Ceftolozane–tazobactam 3 g q 8 h‡ OR Ceftazidime–avibactam 2.5 g q 8 h‡ OR Meropenem–vaborbactam 4 g q 8 h‡ OR Imipenem–relebactam 1.5 g q 6 h‡ |
MDR risk factors include antibiotic therapy in the previous 90 days, hospital stay > 5 days, septic shock at VAP onset, acute respiratory distress syndrome prior to VAP onset, acute renal replacement therapy prior to VAP onset, previous colonization with MDR pathogen
VAP Ventilator-associated pneumonia, MDR multidrug resistant, MRSA methicillin-resistant Staphylococcus aureus
*This situation and the corresponding antimicrobial agents are not mentioned in IDSA/ATS guidelines [34]
†According to [65]
‡The empirical use of these agents should be restricted to patients colonized by specific pathogens (carbapenem-resistant Enterobacteriaceae or extensively drug-resistant Pseudomonas aeruginosa), according to previous susceptibility testing showing that the pathogen is susceptible to the agent
The use of new beta-lactam agents (ceftazidime–avibactam, ceftolozane–tazobactam, meropenem–vaborbactam, imipenem–relebactam) in the empirical treatment of VAP should probably be reserved in patients colonized with MDR/XDR pathogens, such as carbapenem-resistant Enterobacteriaceae or XDR Pseudomonas aeruginosa susceptible only to these drugs.
The 2017 IDSA/ATS guidelines recommend empiric coverage of methicillin-resistant Staphylococcus aureus (MRSA) in patients who received antibiotics in the preceding 90 days or those hospitalized in units with high (> 20%) or unknown MRSA prevalence among VAP patients [34]. European guidelines state that MRSA coverage should be considered if the unit has > 25% of Staphylococcus aureus respiratory isolates as MRSA [38]. A recent study performed in the USA showed that among 3562 patients with hospital-acquired pneumonia (not specifically VAP) in hospitals with MRSA prevalence > 20%, only 5% grew MRSA on respiratory specimen culture, indicating that potentially 95% would have been over treated by using the hospital-wide prevalence of MRSA instead of the VAP-specific prevalence of MRSA [34, 137]. Moreover, MRSA VAP prevalence is low in several countries [65]. Therefore, the empiric use of an anti-MRSA agent should be restricted to units with high (> 20%) incidence of VAP secondary to MRSA, or in patients already colonized by MRSA.
Pathogen-specific treatment
One of the goals for clinicians should be to avoid overuse of antibiotics. First, antibiotics should be stopped if no pathogen is retrieved. Indeed, many episodes of suspected VAP are not VAP [64]. Second, in patients with bacteriologically proven VAP, antibiotics should be narrowed once culture results and susceptibility tests are available, including the following measures: withdrawing of anti-MRSA antibiotics if no MRSA is recovered; restriction of carbapenems to carbapenem-only susceptible pathogens (ESBL-producing Enterobacteriaceae infection, carbapenem-only susceptible Pseudomonas aeruginosa or Acinetobacter spp.); and use of narrow-spectrum agents in patients infected with susceptible strains [82]. In patients with ESBL-producing Enterobacteriaceae VAP susceptible to piperacillin–tazobactam, the use of this drug could be discussed as an alternative to carbapenem since results of the Merino trial may be disputable [138–141]. Moreover, the place of new beta-lactam agents (ceftolozane–tazobactam, ceftazidime–avibactam) as carbapenem-sparing agents remains to be determined, since their impact on emergence of antimicrobial resistance as compared to carbapenem is not known. Their use should be reserved as last resort agents in MDR/XDR difficult to treat pathogens (carbapenem-resistant Enterobacteriaceae, XDR Pseudomonas aeruginosa…). Last, antimicrobial therapy can be safely switched to monotherapy once pathogens responsible for infection are identified and susceptibility results have been obtained, even for non-fermenting Gram-negative bacilli such as Pseudomonas aeruginosa [142]. Indeed, the usefulness of combination therapy is mostly to increase the likelihood of appropriateness of treatment rather than improving the prognosis of patients. Therefore, double antipseudomonal coverage in patients with Pseudomonas aeruginosa VAP with uncomplicated course should be avoided once susceptibility tests are available [143, 144].
Duration of treatment
Both European and US guidelines recommend that the duration of antimicrobial treatment for VAP should not exceed 7 days in most patients, including those infected with non-fermenting Gram-negative bacilli (Pseudomonas aeruginosa, Acinetobacter spp….) [34, 38, 65, 145]. Longer course may be appropriate for immunocompromised patients and are likely necessary for patients with empyema, lung abscess, or necrotising pneumonia [38]. Shortening duration of antimicrobial below 7 days is currently not recommended [34, 38, 65, 145], but some authors have demonstrated that treatment duration can be customized based on procalcitonin kinetics and have been able to treat some patients for < 7 days using this strategy [80, 81, 146].
Nebulisation
Nebulisation of antibiotics has grown in recent years, but the ideal candidates to receive this treatment are not well defined [147]. To date, nebulized antibiotics cannot be recommended as an alternative to the intravenous route, partly because data are lacking on this indication, partly because 10–20% of patients with VAP have concurrent bacteraemia, and partly because multiple and repeated daily use of nebulisation may prolong duration of mechanical ventilation [148, 149]. The use of nebulised antibiotics as an adjunctive treatment (i.e., in addition to effective intravenous therapy) is also not recommended; two recent randomized-controlled trials failed to demonstrate superiority of nebulised antibiotics (amikacin alone or combined with fosfomycin) over placebo in patients with VAP due to “traditional pathogens” [150, 151]. The use of nebulised antibiotics should therefore be restricted to patients with VAP to XDR-Gram-negative pathogens susceptible only to colistin or aminoglycosides [149]. Indeed, three meta-analyses found that in patients infected with such pathogens, the use of nebulised colistin combined with IV colistin led to better outcomes compared to IV colistin alone [152–154]. Whether or not nebulised antibiotics may decrease emergence of bacterial resistance, as suggested by two studies performed in patients with ventilator-associated tracheobronchitis, remains to be determined [155, 156].
Future treatments
The use of pathogen-specific antibodies as an adjunctive or preventive treatment is currently under investigation. Aerucin (Aridis®) is an IgG mAb that binds to the Pseudomonas alginate exopolysaccharide involved in cellular adhesion. A phase 2, placebo-controlled, double-blind study to assess its safety and efficacy as adjunctive therapy to standard antibiotics in patients with P aeruginosa HAP/VAP (NCT00851435) has been performed recently, but results are not yet available.
Recent studies have evaluated the usefulness of antibodies to neutralize or inhibit specific S. aureus or P. aeruginosa virulence factors [157]. The purpose of this kind of strategy is to reduce the risk for developing VAP in patients colonized by these pathogens. Results of these studies are promising, since a phase 2 trial targeting Pseudomonas aeruginosa virulence factors showed trend toward lower rate of infection due to this pathogen [158], and the recently released results of the SAATELITE study, that evaluated an antibody against Staphylococcus aureus virulence factor, found also trend toward lower incidence of Staphylococcus aureus pneumonia [159]. Although this strategy is more preventive than curative, the usefulness of these anti-virulence agents as adjuvant to antibiotics remains to be evaluated.
Should viruses reactivations be treated?
There is no definitive answer to this question. The recent PTH trial suggested that acyclovir does not change the number of ventilator-free days in patients presenting with HSV oropharyngeal reactivation disease [160]. Intriguingly, however, the same study reported a near significant decrease in mortality among patients randomized to acyclovir [160]. Antiviral prophylaxis using valganciclovir or valacyclovir was able to decrease blood reactivation in a randomized study involving 124 patients [161]. However, the valacyclovir arm was halted prematurely because of higher mortality by 28 days without clear explanation [161]. In another interventional trial, ganciclovir prophylaxis did not reduce plasma interleukin 6 levels in critically ill CMV-seropositive adults [57]. The ganciclovir group did, however, have more ventilator-free days in both the intention-to-treat population and in the sepsis subgroup [57]. More research is needed to evaluate the precise clinical consequences of viral reactivations and whether and how they should be managed.
Conclusions
Despite a lot of research, VAP remains one of the most frequent ICU-acquired infections and is associated with an increased mortality. Which sampling method to use is still a matter of controversy. Emerging microbiological tools will likely modify our routine approach to diagnosing and treating VAP in the next future. Large randomized trials are needed to confirm that bundles that combine multiple prevention strategies may improve outcomes. Treatment should be limited to 7 days in the vast majority of the cases. Further research is needed to identify and assess new therapeutic approaches.
Abbreviations
- MALDI-TOF MS
Matrix-assisted laser desorption ionization time-of-flight mass spectrometry
- PCR
Polymerase chain reaction
Funding
No financial support.
Compliance with ethical standards
Conflicts of interest
LP received consultancy fees from Air Liquide MS, Faron, and MSD. CEL received fees from Bayer Healthcare in relationship with the current work (advisory board on inhaled amikacin), and fees from Merck, ThermoFischer Brahms, Biomérieux, Carmat, and Faron outside the current work. MK has received royalties from UpToDate Inc. for articles on ventilator-associated pneumonia.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, Keohane C, Denham CR, Bates DW. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013;173:2039–2046. doi: 10.1001/jamainternmed.2013.9763. [DOI] [PubMed] [Google Scholar]
- 2.American Thoracic Society, Infectious Diseases Society of America Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am J Respir Crit Care Med. 2005;171:388–416. doi: 10.1164/rccm.200405-644ST. [DOI] [PubMed] [Google Scholar]
- 3.Reignier J, Mercier E, Le Gouge A, Boulain T, Desachy A, Bellec F, Clavel M, Frat JP, Plantefeve G, Quenot JP, Lascarrou JB, Clinical Research in Intensive C, Sepsis G (2013) Effect of not monitoring residual gastric volume on risk of ventilator-associated pneumonia in adults receiving mechanical ventilation and early enteral feeding: a randomized controlled trial. JAMA 309: 249–256 [DOI] [PubMed]
- 4.Seguin P, Laviolle B, Dahyot-Fizelier C, Dumont R, Veber B, Gergaud S, Asehnoune K, Mimoz O, Donnio PY, Bellissant E, Malledant Y, Study of Povidone Iodine to Reduce Pulmonary Infection in Head T, Cerebral Hemorrhage Patients ICUSG, AtlanRea G (2014) Effect of oropharyngeal povidone-iodine preventive oral care on ventilator-associated pneumonia in severely brain-injured or cerebral hemorrhage patients: a multicenter, randomized controlled trial. Crit Care Med 42: 1–8 [DOI] [PubMed]
- 5.Dudeck MA, Horan TC, Peterson KD, Allen-Bridson K, Morrell G, Anttila A, Pollock DA, Edwards JR. National healthcare safety network report, data summary for 2011, device-associated module. Am J Infect Control. 2013;41:286–300. doi: 10.1016/j.ajic.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koulenti D, Tsigou E, Rello J. Nosocomial pneumonia in 27 ICUs in Europe: perspectives from the EU-VAP/CAP study. Eur J Clin Microbiol Infect Dis. 2017;36:1999–2006. doi: 10.1007/s10096-016-2703-z. [DOI] [PubMed] [Google Scholar]
- 7.Bonell A, Azarrafiy R, Huong VTL, Viet TL, Phu VD, Dat VQ, Wertheim H, van Doorn HR, Lewycka S, Nadjm B. A systematic review and meta-analysis of ventilator-associated pneumonia in adults in Asia: an analysis of national income level on incidence and etiology. Clin Infect Dis. 2019;68:511–518. doi: 10.1093/cid/ciy543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ego A, Preiser JC, Vincent JL. Impact of diagnostic criteria on the incidence of ventilator-associated pneumonia. Chest. 2015;147:347–355. doi: 10.1378/chest.14-0610. [DOI] [PubMed] [Google Scholar]
- 9.Cook D, Walter S, Cook R, Griffith L, Guyatt G, Leasa D, Jaeschke R, Brun -BC. Incidence of and risk factors for ventilator-associated pneumonia in critically ill patients. Ann Intern Med. 1998;129:433–440. doi: 10.7326/0003-4819-129-6-199809150-00002. [DOI] [PubMed] [Google Scholar]
- 10.Forel JM, Voillet F, Pulina D, Gacouin A, Perrin G, Barrau K, Jaber S, Arnal JM, Fathallah M, Auquier P, Roch A, Azoulay E, Papazian L. Ventilator-associated pneumonia and ICU mortality in severe ARDS patients ventilated according to a lung-protective strategy. Crit Care. 2012;16:R65. doi: 10.1186/cc11312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dudeck MA, Edwards JR, Allen-Bridson K, Gross C, Malpiedi PJ, Peterson KD, Pollock DA, Weiner LM, Sievert DM. National healthcare safety network report, data summary for 2013, device-associated module. Am J Infect Control. 2015;43:206–221. doi: 10.1016/j.ajic.2014.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Metersky ML, Wang Y, Klompas M, Eckenrode S, Bakullari A, Eldridge N. Trend in ventilator-associated pneumonia rates between 2005 and 2013. JAMA. 2016;316:2427–2429. doi: 10.1001/jama.2016.16226. [DOI] [PubMed] [Google Scholar]
- 13.Magill SS, Klompas M, Balk R, Burns SM, Deutschman CS, Diekema D, Fridkin S, Greene L, Guh A, Gutterman D, Hammer B, Henderson D, Hess D, Hill NS, Horan T, Kollef M, Levy M, Septimus E, Vanantwerpen C, Wright D, Lipsett P. Developing a new, national approach to surveillance for ventilator-associated events: executive summary. Clin Infect Dis. 2013;57:1742–1746. doi: 10.1093/cid/cit577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoclin A, Rotolo F, Hicheri Y, Mons M, Chachaty E, Gachot B, Pignon JP, Wartelle M, Blot F. Ventilator-associated pneumonia and bloodstream infections in intensive care unit cancer patients: a retrospective 12-year study on 3388 prospectively monitored patients. Support Care Cancer. 2020;28:193–200. doi: 10.1007/s00520-019-04800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cook A, Norwood S, Berne J. Ventilator-associated pneumonia is more common and of less consequence in trauma patients compared with other critically ill patients. J Trauma. 2010;69:1083–1091. doi: 10.1097/TA.0b013e3181f9fb51. [DOI] [PubMed] [Google Scholar]
- 16.Rouze A, Cottereau A, Nseir S. Chronic obstructive pulmonary disease and the risk for ventilator-associated pneumonia. Curr Opin Crit Care. 2014;20:525–531. doi: 10.1097/MCC.0000000000000123. [DOI] [PubMed] [Google Scholar]
- 17.Grasselli G, Scaravilli V, Di Bella S, Biffi S, Bombino M, Patroniti N, Bisi L, Peri AM, Pesenti A, Gori A, Alagna L. Nosocomial infections during extracorporeal membrane oxygenation: incidence, etiology, and impact on patients’ outcome. Crit Care Med. 2017;45:1726–1733. doi: 10.1097/CCM.0000000000002652. [DOI] [PubMed] [Google Scholar]
- 18.Blot S, Koulenti D, Dimopoulos G, Martin C, Komnos A, Krueger WA, Spina G, Armaganidis A, Rello J, Investigators E-VS. Prevalence, risk factors, and mortality for ventilator-associated pneumonia in middle-aged, old, and very old critically ill patients. Crit Care Med. 2014;42:601–609. doi: 10.1097/01.ccm.0000435665.07446.50. [DOI] [PubMed] [Google Scholar]
- 19.Dananche C, Vanhems P, Machut A, Aupee M, Bervas C, L’Heriteau F, Lepape A, Lucet JC, Stoeckel V, Timsit JF, Berger-Carbonne A, Savey A, Benet T, Healthcare-Associated Infections Surveillance Network of I Trends of incidence and risk factors of ventilator-associated pneumonia in elderly patients admitted to French ICUs between 2007 and 2014. Crit Care Med. 2018;46:869–877. doi: 10.1097/CCM.0000000000003019. [DOI] [PubMed] [Google Scholar]
- 20.Fagon J, Chastre J, Hance A, Montravers P, Novara A, Gibert C. Nosocomial pneumonia in ventilated patients: a cohort study evaluating attributable mortality and hospital stay. Am J Med. 1993;94:281–288. doi: 10.1016/0002-9343(93)90060-3. [DOI] [PubMed] [Google Scholar]
- 21.Papazian L, Bregeon F, Thirion X, Gregoire R, Saux P, Denis J, Perin G, Charrel J, Dumon J, Affray J, Gouin F. Effect of ventilator-associated pneumonia on mortality and morbidity. Am J Respir Crit Care Med. 1996;154:91–97. doi: 10.1164/ajrccm.154.1.8680705. [DOI] [PubMed] [Google Scholar]
- 22.Melsen WG, Rovers MM, Bonten MJ. Ventilator-associated pneumonia and mortality: a systematic review of observational studies. Crit Care Med. 2009;37:2709–2718. doi: 10.1097/ccm.0b013e3181ab8655. [DOI] [PubMed] [Google Scholar]
- 23.Bekaert M, Timsit JF, Vansteelandt S, Depuydt P, Vesin A, Garrouste-Orgeas M, Decruyenaere J, Clec’h C, Azoulay E, Benoit D, Outcomerea Study G (2011) Attributable mortality of ventilator-associated pneumonia: a reappraisal using causal analysis. Am J Respir Crit Care Med 184: 1133–1139 [DOI] [PubMed]
- 24.Li Y, Liu C, Xiao W, Song T, Wang S. Incidence, risk factors, and outcomes of ventilator-associated pneumonia in traumatic brain injury: a meta-analysis. Neurocrit Care. 2019 doi: 10.1007/s12028-019-00773-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Melsen WG, Rovers MM, Koeman M, Bonten MJ. Estimating the attributable mortality of ventilator-associated pneumonia from randomized prevention studies. Crit Care Med. 2011;39:2736–2742. doi: 10.1097/CCM.0b013e3182281f33. [DOI] [PubMed] [Google Scholar]
- 26.Melsen WG, Rovers MM, Groenwold RH, Bergmans DC, Camus C, Bauer TT, Hanisch EW, Klarin B, Koeman M, Krueger WA, Lacherade JC, Lorente L, Memish ZA, Morrow LE, Nardi G, van Nieuwenhoven CA, O’Keefe GE, Nakos G, Scannapieco FA, Seguin P, Staudinger T, Topeli A, Ferrer M, Bonten MJ. Attributable mortality of ventilator-associated pneumonia: a meta-analysis of individual patient data from randomised prevention studies. Lancet Infect Dis. 2013;13:665–671. doi: 10.1016/S1473-3099(13)70081-1. [DOI] [PubMed] [Google Scholar]
- 27.Martin-Loeches I, Torres A, Rinaudo M, Terraneo S, de Rosa F, Ramirez P, Diaz E, Fernandez-Barat L, Li Bassi GL, Ferrer M. Resistance patterns and outcomes in intensive care unit (ICU)-acquired pneumonia. Validation of European Centre for Disease Prevention and Control (ECDC) and the Centers for Disease Control and Prevention (CDC) classification of multidrug resistant organisms. J Infect. 2015;70:213–222. doi: 10.1016/j.jinf.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Di Pasquale M, Ferrer M, Esperatti M, Crisafulli E, Giunta V, Li Bassi G, Rinaudo M, Blasi F, Niederman M, Torres A. Assessment of severity of ICU-acquired pneumonia and association with etiology. Crit Care Med. 2014;42:303–312. doi: 10.1097/CCM.0b013e3182a272a2. [DOI] [PubMed] [Google Scholar]
- 29.Esperatti M, Ferrer M, Theessen A, Liapikou A, Valencia M, Saucedo LM, Zavala E, Welte T, Torres A. Nosocomial pneumonia in the intensive care unit acquired during mechanical ventilation or not. Am J Respir Crit Care Med. 2010;182:1533–1539. doi: 10.1164/rccm.201001-0094OC. [DOI] [PubMed] [Google Scholar]
- 30.Rello J, Ollendorf DA, Oster G, Vera-Llonch M, Bellm L, Redman R, Kollef MH. Epidemiology and outcomes of ventilator-associated pneumonia in a large US database. Chest. 2002;122:2115–2121. doi: 10.1378/chest.122.6.2115. [DOI] [PubMed] [Google Scholar]
- 31.Bailey KL, Kalil AC. Ventilator-associated pneumonia (VAP) with multidrug-resistant (MDR) pathogens: optimal treatment? Curr Infect Dis Rep. 2015;17:494. doi: 10.1007/s11908-015-0494-5. [DOI] [PubMed] [Google Scholar]
- 32.Luyt CE, Hekimian G, Koulenti D, Chastre J. Microbial cause of ICU-acquired pneumonia: hospital-acquired pneumonia versus ventilator-associated pneumonia. Curr Opin Crit Care. 2018;24:332–338. doi: 10.1097/MCC.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, Jiao Y, Zhang J, Xu J, Cheng Q, Li Y, Liang S, Li H, Gong J, Zhu Y, Song L, Rong Z, Liu B, Jie Z, Sun S, Li P, Wang G, Qu J, Infection Assembly of Shanghai Respiratory S Microbial etiology and prognostic factors of ventilator-associated pneumonia: a multicenter retrospective study in Shanghai. Clin Infect Dis. 2018;67:S146–S152. doi: 10.1093/cid/ciy686. [DOI] [PubMed] [Google Scholar]
- 34.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O’Grady NP, Bartlett JG, Carratala J, El Solh AA, Ewig S, Fey PD, File TM, Jr, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American thoracic society. Clin Infect Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan R, Al-Dorzi HM, Tamim HM, Rishu AH, Balkhy H, El-Saed A, Arabi YM. The impact of onset time on the isolated pathogens and outcomes in ventilator associated pneumonia. J Infect Public Health. 2016;9:161–171. doi: 10.1016/j.jiph.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 36.Martin-Loeches I, Deja M, Koulenti D, Dimopoulos G, Marsh B, Torres A, Niederman MS, Rello J, Investigators E-VS. Potentially resistant microorganisms in intubated patients with hospital-acquired pneumonia: the interaction of ecology, shock and risk factors. Intensive Care Med. 2013;39:672–681. doi: 10.1007/s00134-012-2808-5. [DOI] [PubMed] [Google Scholar]
- 37.Restrepo MI, Peterson J, Fernandez JF, Qin Z, Fisher AC, Nicholson SC. Comparison of the bacterial etiology of early-onset and late-onset ventilator-associated pneumonia in subjects enrolled in 2 large clinical studies. Respir Care. 2013;58:1220–1225. doi: 10.4187/respcare.02173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Torres A, Niederman, Chastre J, Ewig S, Fernandez-Vandellos P, Hanberger H, Kollef M, Li Bassi G, Luna CM, Martin-Loeches I, Paiva JA, Read RC, Rigau D, Timsit JF, Welte T, Wunderink R. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia: Guidelines for the management of hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) of the European Respiratory Society (ERS), European Society of Intensive Care Medicine (ESICM), European Society of Clinical Microbiology and Infectious Diseases (ESCMID) and Asociacion Latinoamericana del Torax (ALAT) Eur Respir J. 2017 doi: 10.1183/13993003.00582-2017. [DOI] [PubMed] [Google Scholar]
- 39.Russo A, Giuliano S, Ceccarelli G, Alessandri F, Giordano A, Brunetti G, Venditti M. Comparison of septic shock due to multidrug-resistant Acinetobacter baumannii or Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in Intensive Care Unit Patients. Antimicrob Agents Chemother. 2018 doi: 10.1128/AAC.02562-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Denis JB, Lehingue S, Pauly V, Cassir N, Gainnier M, Leone M, Daviet F, Coiffard B, Baron S, Guervilly C, Forel JM, Roch A, Papazian L. Multidrug-resistant Pseudomonas aeruginosa and mortality in mechanically ventilated ICU patients. Am J Infect Control. 2019;47:1059–1064. doi: 10.1016/j.ajic.2019.02.030. [DOI] [PubMed] [Google Scholar]
- 41.Chung DR, Song JH, Kim SH, Thamlikitkul V, Huang SG, Wang H, So TM, Yasin RM, Hsueh PR, Carlos CC, Hsu LY, Buntaran L, Lalitha MK, Kim MJ, Choi JY, Kim SI, Ko KS, Kang CI, Peck KR, Asian Network for Surveillance of Resistant Pathogens Study G High prevalence of multidrug-resistant nonfermenters in hospital-acquired pneumonia in Asia. Am J Respir Crit Care Med. 2011;184:1409–1417. doi: 10.1164/rccm.201102-0349OC. [DOI] [PubMed] [Google Scholar]
- 42.Perez A, Gato E, Perez-Llarena J, Fernandez-Cuenca F, Gude MJ, Oviano M, Pachon ME, Garnacho J, Gonzalez V, Pascual A, Cisneros JM, Bou G. High incidence of MDR and XDR Pseudomonas aeruginosa isolates obtained from patients with ventilator-associated pneumonia in Greece, Italy and Spain as part of the MagicBullet clinical trial. J Antimicrob Chemother. 2019;74:1244–1252. doi: 10.1093/jac/dkz030. [DOI] [PubMed] [Google Scholar]
- 43.Combes A, Figliolini C, Trouillet JL, Kassis N, Wolff M, Gibert C, Chastre J. Incidence and outcome of polymicrobial ventilator-associated pneumonia. Chest. 2002;121:1618–1623. doi: 10.1378/chest.121.5.1618. [DOI] [PubMed] [Google Scholar]
- 44.Niederman MS. Antibiotic treatment of hospital-acquired pneumonia: is it different from ventilator-associated pneumonia? Curr Opin Crit Care. 2018;24:353–360. doi: 10.1097/MCC.0000000000000531. [DOI] [PubMed] [Google Scholar]
- 45.Fabregas N, Torres A, El-Ebiary M, Ramirez J, Hernandez C, Gonzalez J, de la Bellacasa J, de Anta J, Rodriguez R-R. Histopathologic and microbiologic aspects of ventilator-associated pneumonia. Anesthesiology. 1996;84:760–771. doi: 10.1097/00000542-199604000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Delisle MS, Williamson DR, Albert M, Perreault MM, Jiang X, Day AG, Heyland DK. Impact of Candida species on clinical outcomes in patients with suspected ventilator-associated pneumonia. Can Respir J. 2011;18:131–136. doi: 10.1155/2011/827692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azoulay E, Timsit JF, Tafflet M, de Lassence A, Darmon M, Zahar JR, Adrie C, Garrouste-Orgeas M, Cohen Y, Mourvillier B, Schlemmer B, Outcomerea Study G Candida colonization of the respiratory tract and subsequent pseudomonas ventilator-associated pneumonia. Chest. 2006;129:110–117. doi: 10.1378/chest.129.1.110. [DOI] [PubMed] [Google Scholar]
- 48.Timsit JF, Schwebel C, Styfalova L, Cornet M, Poirier P, Forrestier C, Ruckly S, Jacob MC, Souweine B. Impact of bronchial colonization with Candida spp. on the risk of bacterial ventilator-associated pneumonia in the ICU: the FUNGIBACT prospective cohort study. Intensive Care Med. 2019;45:834–843. doi: 10.1007/s00134-019-05622-0. [DOI] [PubMed] [Google Scholar]
- 49.Schauwvlieghe A, Rijnders BJA, Philips N, Verwijs R, Vanderbeke L, Van Tienen C, Lagrou K, Verweij PE, Van de Veerdonk FL, Gommers D, Spronk P, Bergmans D, Hoedemaekers A, Andrinopoulou ER, van den Berg C, Juffermans NP, Hodiamont CJ, Vonk AG, Depuydt P, Boelens J, Wauters J, Dutch-Belgian Mycosis study group Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6:782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 50.Blot SI, Taccone FS, Van den Abeele AM, Bulpa P, Meersseman W, Brusselaers N, Dimopoulos G, Paiva JA, Misset B, Rello J, Vandewoude K, Vogelaers D, Asp ICUSI. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186:56–64. doi: 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- 51.Cassir N, Hraiech S, Nougairede A, Zandotti C, Fournier PE, Papazian L. Outbreak of adenovirus type 1 severe pneumonia in a French intensive care unit, September–October 2012. Euro Surveill. 2014 doi: 10.2807/1560-7917.es2014.19.39.20914. [DOI] [PubMed] [Google Scholar]
- 52.Hong HL, Hong SB, Ko GB, Huh JW, Sung H, Do KH, Kim SH, Lee SO, Kim MN, Jeong JY, Lim CM, Kim YS, Woo JH, Koh Y, Choi SH. Viral infection is not uncommon in adult patients with severe hospital-acquired pneumonia. PLoS ONE. 2014;9:e95865. doi: 10.1371/journal.pone.0095865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loubet P, Voiriot G, Houhou-Fidouh N, Neuville M, Bouadma L, Lescure FX, Descamps D, Timsit JF, Yazdanpanah Y, Visseaux B. Impact of respiratory viruses in hospital-acquired pneumonia in the intensive care unit: a single-center retrospective study. J Clin Virol. 2017;91:52–57. doi: 10.1016/j.jcv.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.van Someren Greve F, Juffermans NP, Bos LDJ, Binnekade JM, Braber A, Cremer OL, de Jonge E, Molenkamp R, Ong DSY, Rebers SPH, Spoelstra-de Man AME, van der Sluijs KF, Spronk PE, Verheul KD, de Waard MC, de Wilde RBP, Winters T, de Jong MD, Schultz MJ. Respiratory viruses in invasively ventilated critically ill patients-a prospective multicenter observational study. Crit Care Med. 2018;46:29–36. doi: 10.1097/CCM.0000000000002752. [DOI] [PubMed] [Google Scholar]
- 55.Luyt CE, Combes A, Deback C, Aubriot-Lorton MH, Nieszkowska A, Trouillet JL, Capron F, Agut H, Gibert C, Chastre J. Herpes simplex virus lung infection in patients undergoing prolonged mechanical ventilation. Am J Respir Crit Care Med. 2007;175:935–942. doi: 10.1164/rccm.200609-1322OC. [DOI] [PubMed] [Google Scholar]
- 56.Limaye AP, Kirby KA, Rubenfeld GD, Leisenring WM, Bulger EM, Neff MJ, Gibran NS, Huang ML, Santo Hayes TK, Corey L, Boeckh M. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA. 2008;300:413–422. doi: 10.1001/jama.300.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Limaye AP, Stapleton RD, Peng L, Gunn SR, Kimball LE, Hyzy R, Exline MC, Files DC, Morris PE, Frankel SK, Mikkelsen ME, Hite D, Enfield KB, Steingrub J, O’Brien J, Parsons PE, Cuschieri J, Wunderink RG, Hotchkin DL, Chen YQ, Rubenfeld GD, Boeckh M. Effect of ganciclovir on IL-6 levels among cytomegalovirus-seropositive adults with critical illness: a randomized clinical trial. JAMA. 2017;318:731–740. doi: 10.1001/jama.2017.10569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Papazian L, Doddoli C, Chetaille B, Gernez Y, Thirion X, Roch A, Donati Y, Bonnety M, Zandotti C, Thomas P. A contributive result of open-lung biopsy improves survival in acute respiratory distress syndrome patients. Crit Care Med. 2007;35:755–762. doi: 10.1097/01.CCM.0000257325.88144.30. [DOI] [PubMed] [Google Scholar]
- 59.Papazian L, Fraisse A, Garbe L, Zandotti C, Thomas P, Saux P, Pierrin G, Gouin F. Cytomegalovirus. An unexpected cause of ventilator-associated pneumonia. Anesthesiology. 1996;84:280–287. doi: 10.1097/00000542-199602000-00005. [DOI] [PubMed] [Google Scholar]
- 60.Papazian L, Hraiech S, Lehingue S, Roch A, Chiche L, Wiramus S, Forel JM. Cytomegalovirus reactivation in ICU patients. Intensive Care Med. 2016;42:28–37. doi: 10.1007/s00134-015-4066-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Papazian L, Thomas P, Bregeon F, Garbe L, Zandotti C, Saux P, Gaillat F, Drancourt M, Auffray J, Gouin F. Open-lung biopsy in patients with acute respiratory distress syndrome. Anesthesiology. 1998;88:935–944. doi: 10.1097/00000542-199804000-00013. [DOI] [PubMed] [Google Scholar]
- 62.Ong DSY, Bonten MJM, Spitoni C, Verduyn Lunel FM, Frencken JF, Horn J, Schultz MJ, van der Poll T, Klein Klouwenberg PMC, Cremer OL, Molecular D, Stratification Risk, Risk Stratification of Sepsis C Epidemiology of multiple herpes viremia in previously immunocompetent patients with septic shock. Clin Infect Dis. 2017;64:1204–1210. doi: 10.1093/cid/cix120. [DOI] [PubMed] [Google Scholar]
- 63.Walton AH, Muenzer JT, Rasche D, Boomer JS, Sato B, Brownstein BH, Pachot A, Brooks TL, Deych E, Shannon WD, Green JM, Storch GA, Hotchkiss RS. Reactivation of multiple viruses in patients with sepsis. PLoS ONE. 2014;9:e98819. doi: 10.1371/journal.pone.0098819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chastre J, Luyt CE. Does this patient have VAP? Intensive Care Med. 2016;42:1159–1163. doi: 10.1007/s00134-016-4239-1. [DOI] [PubMed] [Google Scholar]
- 65.Leone M, Bouadma L, Bouhemad B, Brissaud O, Dauger S, Gibot S, Hraiech S, Jung B, Kipnis E, Launey Y, Luyt CE, Margetis D, Michel F, Mokart D, Montravers P, Monsel A, Nseir S, Pugin J, Roquilly A, Velly L, Zahar JR, Bruyere R, Chanques G, Adarpef Gfrup. Brief summary of French guidelines for the prevention, diagnosis and treatment of hospital-acquired pneumonia in ICU. Ann Intensive Care. 2018;8:104. doi: 10.1186/s13613-018-0444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Klompas M. Does this patient have ventilator-associated pneumonia? JAMA. 2007;297:1583–1593. doi: 10.1001/jama.297.14.1583. [DOI] [PubMed] [Google Scholar]
- 67.Pugin J, Auckenthaler R, Mili N, Janssens J, Lew P, Suter P. Diagnosis of ventilator-associated pneumonia by bacteriologic analysis of bronchoscopic and nonbronchoscopic “blind” bronchoalveolar lavage fluid. Am Rev Respir Dis. 1991;143:1121–1129. doi: 10.1164/ajrccm/143.5_Pt_1.1121. [DOI] [PubMed] [Google Scholar]
- 68.Singh N, Rogers P, Atwood C, Wagener M, Yu V. Short-course empiric antibiotic therapy for patients with pulmonary infiltrates in the intensive care unit. A proposed solution for indiscriminate antibiotic prescription. Am J Respir Crit Care Med. 2000;162:505–511. doi: 10.1164/ajrccm.162.2.9909095. [DOI] [PubMed] [Google Scholar]
- 69.Luyt CE, Chastre J, Fagon JY. Value of the clinical pulmonary infection score for the identification and management of ventilator-associated pneumonia. Intensive Care Med. 2004;30:844–852. doi: 10.1007/s00134-003-2125-0. [DOI] [PubMed] [Google Scholar]
- 70.Wunderink R, Woldenberg L, Zeiss J, Day C, Ciemins J, Lacher D. The radiologic diagnosis of autopsy-proven ventilator-associated pneumonia. Chest. 1992;101:458–463. doi: 10.1378/chest.101.2.458. [DOI] [PubMed] [Google Scholar]
- 71.Bouhemad B, Dransart-Raye O, Mojoli F, Mongodi S. Lung ultrasound for diagnosis and monitoring of ventilator-associated pneumonia. Ann Transl Med. 2018;6:418. doi: 10.21037/atm.2018.10.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gibot S, Cravoisy A, Levy B, Bene MC, Faure G, Bollaert PE. Soluble triggering receptor expressed on myeloid cells and the diagnosis of pneumonia. N Engl J Med. 2004;350:451–458. doi: 10.1056/NEJMoa031544. [DOI] [PubMed] [Google Scholar]
- 73.Labenne M, Poyart C, Rambaud C, Goldfarb B, Pron B, Jouvet P, Delamare C, Sebag G, Hubert P. Blind protected specimen brush and bronchoalveolar lavage in ventilated children. Crit Care Med. 1999;27:2537–2543. doi: 10.1097/00003246-199911000-00035. [DOI] [PubMed] [Google Scholar]
- 74.Luyt CE, Combes A, Reynaud C, Hekimian G, Nieszkowska A, Tonnellier M, Aubry A, Trouillet JL, Bernard M, Chastre J. Usefulness of procalcitonin for the diagnosis of ventilator-associated pneumonia. Intensive Care Med. 2008;34:1434–1440. doi: 10.1007/s00134-008-1112-x. [DOI] [PubMed] [Google Scholar]
- 75.Povoa P, Martin-Loeches I, Ramirez P, Bos LD, Esperatti M, Silvestre J, Gili G, Goma G, Berlanga E, Espasa M, Goncalves E, Torres A, Artigas A. Biomarker kinetics in the prediction of VAP diagnosis: results from the BioVAP study. Ann Intensive Care. 2016;6:32. doi: 10.1186/s13613-016-0134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Salluh JIF, Souza-Dantas VC, Povoa P. The current status of biomarkers for the diagnosis of nosocomial pneumonias. Curr Opin Crit Care. 2017;23:391–397. doi: 10.1097/MCC.0000000000000442. [DOI] [PubMed] [Google Scholar]
- 77.Fagon J, Chastre J, Wolff M, Gervais C, Parer -AS, Stephan F, Similowski T, Mercat A, Diehl J, Sollet J, Tenaillon A. Invasive and noninvasive strategies for management of suspected ventilator-associated pneumonia. A randomized trial. Ann Intern Med. 2000;132:621–630. doi: 10.7326/0003-4819-132-8-200004180-00004. [DOI] [PubMed] [Google Scholar]
- 78.Berton DC, Kalil AC, Teixeira PJ. Quantitative versus qualitative cultures of respiratory secretions for clinical outcomes in patients with ventilator-associated pneumonia. Cochrane Database Syst Rev. 2014 doi: 10.1002/14651858.CD006482.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berton DC, Kalil AC, Teixeira PJ. Quantitative versus qualitative cultures of respiratory secretions for clinical outcomes in patients with ventilator-associated pneumonia. Cochrane Database Syst Rev. 2012;1:Cd006482. doi: 10.1002/14651858.CD006482.pub3. [DOI] [PubMed] [Google Scholar]
- 80.Schuetz P, Wirz Y, Sager R, Christ-Crain M, Stolz D, Tamm M, Bouadma L, Luyt CE, Wolff M, Chastre J, Tubach F, Kristoffersen KB, Burkhardt O, Welte T, Schroeder S, Nobre V, Wei L, Bucher HC, Annane D, Reinhart K, Falsey AR, Branche A, Damas P, Nijsten M, de Lange DW, Deliberato RO, Oliveira CF, Maravic-Stojkovic V, Verduri A, Beghe B, Cao B, Shehabi Y, Jensen JS, Corti C, van Oers JAH, Beishuizen A, Girbes ARJ, de Jong E, Briel M, Mueller B. Effect of procalcitonin-guided antibiotic treatment on mortality in acute respiratory infections: a patient level meta-analysis. Lancet Infect Dis. 2018;18:95–107. doi: 10.1016/S1473-3099(17)30592-3. [DOI] [PubMed] [Google Scholar]
- 81.Schuetz P, Beishuizen A, Broyles M, Ferrer R, Gavazzi G, Gluck EH, Gonzalez Del Castillo J, Jensen JU, Kanizsai PL, Kwa ALH, Krueger S, Luyt CE, Oppert M, Plebani M, Shlyapnikov SA, Toccafondi G, Townsend J, Welte T, Saeed K. Procalcitonin (PCT)-guided antibiotic stewardship: an international experts consensus on optimized clinical use. Clin Chem Lab Med. 2019;57:1308–1318. doi: 10.1515/cclm-2018-1181. [DOI] [PubMed] [Google Scholar]
- 82.Luyt CE, Brechot N, Trouillet JL, Chastre J. Antibiotic stewardship in the intensive care unit. Crit Care. 2014;18:480. doi: 10.1186/s13054-014-0480-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thomas LC, Gidding HF, Ginn AN, Olma T, Iredell J. Development of a real-time Staphylococcus aureus and MRSA (SAM-) PCR for routine blood culture. J Microbiol Methods. 2007;68:296–302. doi: 10.1016/j.mimet.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 84.Fullston EF, Doyle MJ, Higgins MF, Knowles SJ. Clinical impact of rapid polymerase chain reaction (PCR) test for group B Streptococcus (GBS) in term women with ruptured membranes. Ir J Med Sci. 2019;188:1269–1274. doi: 10.1007/s11845-019-01977-x. [DOI] [PubMed] [Google Scholar]
- 85.Jamal W, Al Roomi E, AbdulAziz LR, Rotimi VO. Evaluation of curetis unyvero, a multiplex PCR-based testing system, for rapid detection of bacteria and antibiotic resistance and impact of the assay on management of severe nosocomial pneumonia. J Clin Microbiol. 2014;52:2487–2492. doi: 10.1128/JCM.00325-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gadsby NJ, McHugh MP, Forbes C, MacKenzie L, Hamilton SKD, Griffith DM, Templeton KE. Comparison of Unyvero P55 Pneumonia Cartridge, in-house PCR and culture for the identification of respiratory pathogens and antibiotic resistance in bronchoalveolar lavage fluids in the critical care setting. Eur J Clin Microbiol Infect Dis. 2019;38:1171–1178. doi: 10.1007/s10096-019-03526-x. [DOI] [PubMed] [Google Scholar]
- 87.Kunze N, Moerer O, Steinmetz N, Schulze MH, Quintel M, Perl T. Point-of-care multiplex PCR promises short turnaround times for microbial testing in hospital-acquired pneumonia—an observational pilot study in critical ill patients. Ann Clin Microbiol Antimicrob. 2015;14:33. doi: 10.1186/s12941-015-0091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Papan C, Meyer-Buehn M, Laniado G, Nicolai T, Griese M, Huebner J. Assessment of the multiplex PCR-based assay Unyvero pneumonia application for detection of bacterial pathogens and antibiotic resistance genes in children and neonates. Infection. 2018;46:189–196. doi: 10.1007/s15010-017-1088-y. [DOI] [PubMed] [Google Scholar]
- 89.Brusselaers N, Labeau S, Vogelaers D, Blot S. Value of lower respiratory tract surveillance cultures to predict bacterial pathogens in ventilator-associated pneumonia: systematic review and diagnostic test accuracy meta-analysis. Intensive Care Med. 2013;39:365–375. doi: 10.1007/s00134-012-2759-x. [DOI] [PubMed] [Google Scholar]
- 90.Michel F, Franceschini B, Berger P, Arnal JM, Gainnier M, Sainty JM, Papazian L. Early antibiotic treatment for BAL-confirmed ventilator-associated pneumonia: a role for routine endotracheal aspirate cultures. Chest. 2005;127:589–597. doi: 10.1378/chest.127.2.589. [DOI] [PubMed] [Google Scholar]
- 91.Klompas M. Oropharyngeal decontamination with antiseptics to prevent ventilator-associated pneumonia: rethinking the benefits of chlorhexidine. Semin Respir Crit Care Med. 2017;38:381–390. doi: 10.1055/s-0037-1602584. [DOI] [PubMed] [Google Scholar]
- 92.Huang HB, Jiang W, Wang CY, Qin HY, Du B. Stress ulcer prophylaxis in intensive care unit patients receiving enteral nutrition: a systematic review and meta-analysis. Crit Care. 2018;22:20. doi: 10.1186/s13054-017-1937-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wittekamp BH, Plantinga NL, Cooper BS, Lopez-Contreras J, Coll P, Mancebo J, Wise MP, Morgan MPG, Depuydt P, Boelens J, Dugernier T, Verbelen V, Jorens PG, Verbrugghe W, Malhotra-Kumar S, Damas P, Meex C, Leleu K, van den Abeele AM, Pimenta Gomes, de Matos AF, Fernandez Mendez S, Vergara Gomez A, Tomic V, Sifrer F, Villarreal Tello E, Ruiz Ramos J, Aragao I, Santos C, Sperning RHM, Coppadoro P, Nardi G, Brun-Buisson C, Bonten MJM. Decontamination strategies and bloodstream infections with antibiotic-resistant microorganisms in ventilated patients: a randomized clinical trial. JAMA. 2018;320:2087–2098. doi: 10.1001/jama.2018.13765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Caroff DA, Li L, Muscedere J, Klompas M. Subglottic secretion drainage and objective outcomes: a systematic review and meta-analysis. Crit Care Med. 2016;44:830–840. doi: 10.1097/CCM.0000000000001414. [DOI] [PubMed] [Google Scholar]
- 95.Harris BD, Thomas GA, Greene MH, Spires SS, Talbot TR. Ventilator bundle compliance and risk of ventilator-associated events. Infect Control Hosp Epidemiol. 2018;39:637–643. doi: 10.1017/ice.2018.30. [DOI] [PubMed] [Google Scholar]
- 96.Legoff J, Zucman N, Lemiale V, Mokart D, Pene F, Lambert J, Kouatchet A, Demoule A, Vincent F, Nyunga M, Bruneel F, Contejean A, Mercier-Delarue S, Rabbat A, Lebert C, Perez P, Meert AP, Benoit D, Schwebel C, Jourdain M, Darmon M, Resche-Rigon M, Azoulay E. Clinical significance of upper airway virus detection in critically ill hematology patients. Am J Respir Crit Care Med. 2019;199:518–528. doi: 10.1164/rccm.201804-0681OC. [DOI] [PubMed] [Google Scholar]
- 97.Klompas M. Eight initiatives that misleadingly lower ventilator-associated pneumonia rates. Am J Infect Control. 2012;40:408–410. doi: 10.1016/j.ajic.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 98.Klompas M, Branson R, Eichenwald EC, Greene LR, Howell MD, Lee G, Magill SS, Maragakis LL, Priebe GP, Speck K, Yokoe DS, Berenholtz SM. Strategies to prevent ventilator-associated pneumonia in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35(Suppl 2):S133–S154. doi: 10.1017/s0899823x00193894. [DOI] [PubMed] [Google Scholar]
- 99.Klompas M, Speck K, Howell MD, Greene LR, Berenholtz SM. Reappraisal of routine oral care with chlorhexidine gluconate for patients receiving mechanical ventilation: systematic review and meta-analysis. JAMA Intern Med. 2014;174:751–761. doi: 10.1001/jamainternmed.2014.359. [DOI] [PubMed] [Google Scholar]
- 100.Price R, MacLennan G, Glen J, Su DC. Selective digestive or oropharyngeal decontamination and topical oropharyngeal chlorhexidine for prevention of death in general intensive care: systematic review and network meta-analysis. BMJ. 2014;348:g2197. doi: 10.1136/bmj.g2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Philippart F, Gaudry S, Quinquis L, Lau N, Ouanes I, Touati S, Nguyen JC, Branger C, Faibis F, Mastouri M, Forceville X, Abroug F, Ricard JD, Grabar S, Misset B, Group TO-CS Randomized intubation with polyurethane or conical cuffs to prevent pneumonia in ventilated patients. Am J Respir Crit Care Med. 2015;191:637–645. doi: 10.1164/rccm.201408-1398OC. [DOI] [PubMed] [Google Scholar]
- 102.Jaillette E, Girault C, Brunin G, Zerimech F, Behal H, Chiche A, Broucqsault-Dedrie C, Fayolle C, Minacori F, Alves I, Barrailler S, Labreuche J, Robriquet L, Tamion F, Delaporte E, Thellier D, Delcourte C, Duhamel A, Nseir S, BestCuff Study G, the BoReal N (2017) Impact of tapered-cuff tracheal tube on microaspiration of gastric contents in intubated critically ill patients: a multicenter cluster-randomized cross-over controlled trial. Intensive Care Med 43: 1562–1571 [DOI] [PubMed]
- 103.Deem S, Yanez D, Sissons-Ross L, Broeckel JA, Daniel S, Treggiari M. Randomized pilot trial of two modified endotracheal tubes to prevent ventilator-associated pneumonia. Ann Am Thorac Soc. 2016;13:72–80. doi: 10.1513/AnnalsATS.201506-346OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maertens B, Blot K, Blot S. Prevention of ventilator-associated and early postoperative pneumonia through tapered endotracheal tube cuffs: a systematic review and meta-analysis of randomized controlled trials. Crit Care Med. 2018;46:316–323. doi: 10.1097/CCM.0000000000002889. [DOI] [PubMed] [Google Scholar]
- 105.Letvin A, Kremer P, Silver PC, Samih N, Reed-Watts P, Kollef MH. Frequent versus infrequent monitoring of endotracheal tube cuff pressures. Respir Care. 2018;63:495–501. doi: 10.4187/respcare.05926. [DOI] [PubMed] [Google Scholar]
- 106.Nseir S, Lorente L, Ferrer M, Rouze A, Gonzalez O, Bassi GL, Duhamel A, Torres A. Continuous control of tracheal cuff pressure for VAP prevention: a collaborative meta-analysis of individual participant data. Ann Intensive Care. 2015;5:43. doi: 10.1186/s13613-015-0087-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Muscedere J, Rewa O, McKechnie K, Jiang X, Laporta D, Heyland DK. Subglottic secretion drainage for the prevention of ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care Med. 2011;39:1985–1991. doi: 10.1097/CCM.0b013e318218a4d9. [DOI] [PubMed] [Google Scholar]
- 108.Bouza E, Perez MJ, Munoz P, Rincon C, Barrio JM, Hortal J. Continuous aspiration of subglottic secretions in the prevention of ventilator-associated pneumonia in the postoperative period of major heart surgery. Chest. 2008;134:938–946. doi: 10.1378/chest.08-0103. [DOI] [PubMed] [Google Scholar]
- 109.Damas P, Frippiat F, Ancion A, Canivet JL, Lambermont B, Layios N, Massion P, Morimont P, Nys M, Piret S, Lancellotti P, Wiesen P, D’Orio V, Samalea N, Ledoux D. Prevention of ventilator-associated pneumonia and ventilator-associated conditions: a randomized controlled trial with subglottic secretion suctioning. Crit Care Med. 2015;43:22–30. doi: 10.1097/CCM.0000000000000674. [DOI] [PubMed] [Google Scholar]
- 110.Lacherade JC, De Jonghe B, Guezennec P, Debbat K, Hayon J, Monsel A, Fangio P, Appere de Vecchi C, Ramaut C, Outin H, Bastuji-Garin S. Intermittent subglottic secretion drainage and ventilator-associated pneumonia: a multicenter trial. Am J Respir Crit Care Med. 2010;182:910–917. doi: 10.1164/rccm.200906-0838OC. [DOI] [PubMed] [Google Scholar]
- 111.Klompas M, Li L, Kleinman K, Szumita PM, Massaro AF. Associations between ventilator bundle components and outcomes. JAMA Intern Med. 2016;176:1277–1283. doi: 10.1001/jamainternmed.2016.2427. [DOI] [PubMed] [Google Scholar]
- 112.Deschepper M, Waegeman W, Eeckloo K, Vogelaers D, Blot S. Effects of chlorhexidine gluconate oral care on hospital mortality: a hospital-wide, observational cohort study. Intensive Care Med. 2018;44:1017–1026. doi: 10.1007/s00134-018-5171-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dale CM, Rose L, Carbone S, Smith OM, Burry L, Fan E, Amaral ACK, McCredie VA, Pinto R, Quinonez CR, Sutherland S, Scales DC, Cuthbertson BH. Protocol for a multi-centered, stepped wedge, cluster randomized controlled trial of the de-adoption of oral chlorhexidine prophylaxis and implementation of an oral care bundle for mechanically ventilated critically ill patients: the CHORAL study. Trials. 2019;20:603. doi: 10.1186/s13063-019-3673-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Saint S, Greene MT, Fowler KE, Ratz D, Patel PK, Meddings J, Krein SL. What US hospitals are currently doing to prevent common device-associated infections: results from a national survey. BMJ Qual Saf. 2019;28:741–749. doi: 10.1136/bmjqs-2018-009111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Krein SL, Greene MT, Apisarnthanarak A, Sakamoto F, Tokuda Y, Sakihama T, Fowler KE, Ratz D, Saint S. Infection prevention practices in Japan, Thailand, and the United States: results from national surveys. Clin Infect Dis. 2017;64:S105–S111. doi: 10.1093/cid/cix073. [DOI] [PubMed] [Google Scholar]
- 116.Wang L, Li X, Yang Z, Tang X, Yuan Q, Deng L, Sun X. Semi-recumbent position versus supine position for the prevention of ventilator-associated pneumonia in adults requiring mechanical ventilation. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD009946.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Li Bassi G, Panigada M, Ranzani OT, Zanella A, Berra L, Cressoni M, Parrini V, Kandil H, Salati G, Selvaggi P, Amatu A, Sanz-Moncosi M, Biagioni E, Tagliaferri F, Furia M, Mercurio G, Costa A, Manca T, Lindau S, Babel J, Cavana M, Chiurazzi C, Marti JD, Consonni D, Gattinoni L, Pesenti A, Wiener-Kronish J, Bruschi C, Ballotta A, Salsi P, Livigni S, Iotti G, Fernandez J, Girardis M, Barbagallo M, Moise G, Antonelli M, Caspani ML, Vezzani A, Meybohm P, Gasparovic V, Geat E, Amato M, Niederman M, Kolobow T, Torres A, Gravity VAPN. Randomized, multicenter trial of lateral Trendelenburg versus semirecumbent body position for the prevention of ventilator-associated pneumonia. Intensive Care Med. 2017;43:1572–1584. doi: 10.1007/s00134-017-4858-1. [DOI] [PubMed] [Google Scholar]
- 118.Santacruz CA, Pereira AJ, Celis E, Vincent JL. Which multicenter randomized controlled trials in critical care medicine have shown reduced mortality? A systematic review. Crit Care Med. 2019;47:1680–1691. doi: 10.1097/CCM.0000000000004000. [DOI] [PubMed] [Google Scholar]
- 119.Plantinga NL, de Smet A, Oostdijk EAN, de Jonge E, Camus C, Krueger WA, Bergmans D, Reitsma JB, Bonten MJM. Selective digestive and oropharyngeal decontamination in medical and surgical ICU patients: individual patient data meta-analysis. Clin Microbiol Infect. 2018;24:505–513. doi: 10.1016/j.cmi.2017.08.019. [DOI] [PubMed] [Google Scholar]
- 120.Daneman N, Sarwar S, Fowler RA, Cuthbertson BH, Su DCSG. Effect of selective decontamination on antimicrobial resistance in intensive care units: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13:328–341. doi: 10.1016/S1473-3099(12)70322-5. [DOI] [PubMed] [Google Scholar]
- 121.Su M, Jia Y, Li Y, Zhou D, Jia J. Probiotics for the prevention of ventilator-associated pneumonia: a meta-analysis of randomized controlled trials. Respir Care. 2020 doi: 10.4187/respcare.07097. [DOI] [PubMed] [Google Scholar]
- 122.Cook DJ, Johnstone J, Marshall JC, Lauzier F, Thabane L, Mehta S, Dodek PM, McIntyre L, Pagliarello J, Henderson W, Taylor RW, Cartin-Ceba R, Golan E, Herridge M, Wood G, Ovakim D, Karachi T, Surette MG, Bowdish DM, Lamarche D, Verschoor CP, Duan EH, Heels-Ansdell D, Arabi Y, Meade M, Investigators P, the Canadian Critical Care Trials G Probiotics: prevention of severe pneumonia and endotracheal colonization Trial-PROSPECT: a pilot trial. Trials. 2016;17:377. doi: 10.1186/s13063-016-1495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Alhazzani W, Alshamsi F, Belley-Cote E, Heels-Ansdell D, Brignardello-Petersen R, Alquraini M, Perner A, Moller MH, Krag M, Almenawer S, Rochwerg B, Dionne J, Jaeschke R, Alshahrani M, Deane A, Perri D, Thebane L, Al-Omari A, Finfer S, Cook D, Guyatt G. Efficacy and safety of stress ulcer prophylaxis in critically ill patients: a network meta-analysis of randomized trials. Intensive Care Med. 2018;44:1–11. doi: 10.1007/s00134-017-5005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sasabuchi Y, Matsui H, Lefor AK, Fushimi K, Yasunaga H. Risks and benefits of stress ulcer prophylaxis for patients with severe sepsis. Crit Care Med. 2016;44:e464–e469. doi: 10.1097/CCM.0000000000001667. [DOI] [PubMed] [Google Scholar]
- 125.Krag M, Marker S, Perner A, Wetterslev J, Wise MP, Schefold JC, Keus F, Guttormsen AB, Bendel S, Borthwick M, Lange T, Rasmussen BS, Siegemund M, Bundgaard H, Elkmann T, Jensen JV, Nielsen RD, Liboriussen L, Bestle MH, Elkjaer JM, Palmqvist DF, Backlund M, Laake JH, Badstolokken PM, Gronlund J, Breum O, Walli A, Winding R, Iversen S, Jarnvig IL, White JO, Brand B, Madsen MB, Quist L, Thornberg KJ, Moller A, Wiis J, Granholm A, Anthon CT, Meyhoff TS, Hjortrup PB, Aagaard SR, Andreasen JB, Sorensen CA, Haure P, Hauge J, Hollinger A, Scheuzger J, Tuchscherer D, Vuilliomenet T, Takala J, Jakob SM, Vang ML, Paelestik KB, Andersen KLD, van der Horst ICC, Dieperink W, Fjolner J, Kjer CKW, Solling C, Solling CG, Karttunen J, Morgan MPG, Sjobo B, Engstrom J, Agerholm-Larsen B, Moller MH, group S-It Pantoprazole in Patients at Risk for Gastrointestinal Bleeding in the ICU. N Engl J Med. 2018;379:2199–2208. doi: 10.1056/NEJMoa1714919. [DOI] [PubMed] [Google Scholar]
- 126.Devlin JW, Skrobik Y, Gelinas C, Needham DM, Slooter AJC, Pandharipande PP, Watson PL, Weinhouse GL, Nunnally ME, Rochwerg B, Balas MC, van den Boogaard M, Bosma KJ, Brummel NE, Chanques G, Denehy L, Drouot X, Fraser GL, Harris JE, Joffe AM, Kho ME, Kress JP, Lanphere JA, McKinley S, Neufeld KJ, Pisani MA, Payen JF, Pun BT, Puntillo KA, Riker RR, Robinson BRH, Shehabi Y, Szumita PM, Winkelman C, Centofanti JE, Price C, Nikayin S, Misak CJ, Flood PD, Kiedrowski K, Alhazzani W. Executive summary: clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:1532–1548. doi: 10.1097/CCM.0000000000003259. [DOI] [PubMed] [Google Scholar]
- 127.Tipping CJ, Harrold M, Holland A, Romero L, Nisbet T, Hodgson CL. The effects of active mobilisation and rehabilitation in ICU on mortality and function: a systematic review. Intensive Care Med. 2017;43:171–183. doi: 10.1007/s00134-016-4612-0. [DOI] [PubMed] [Google Scholar]
- 128.Pileggi C, Mascaro V, Bianco A, Nobile CGA, Pavia M. Ventilator bundle and its effects on mortality among ICU patients: a meta-analysis. Crit Care Med. 2018;46:1167–1174. doi: 10.1097/CCM.0000000000003136. [DOI] [PubMed] [Google Scholar]
- 129.Barnes-Daly MA, Phillips G, Ely EW. Improving hospital survival and reducing brain dysfunction at Seven California Community Hospitals: implementing PAD guidelines via the ABCDEF bundle in 6,064 patients. Crit Care Med. 2017;45:171–178. doi: 10.1097/CCM.0000000000002149. [DOI] [PubMed] [Google Scholar]
- 130.Pun BT, Balas MC, Barnes-Daly MA, Thompson JL, Aldrich JM, Barr J, Byrum D, Carson SS, Devlin JW, Engel HJ, Esbrook CL, Hargett KD, Harmon L, Hielsberg C, Jackson JC, Kelly TL, Kumar V, Millner L, Morse A, Perme CS, Posa PJ, Puntillo KA, Schweickert WD, Stollings JL, Tan A, D’Agostino McGowan L, Ely EW. Caring for critically ill patients with the ABCDEF bundle: results of the ICU liberation collaborative in over 15,000 adults. Crit Care Med. 2019;47:3–14. doi: 10.1097/CCM.0000000000003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hsieh SJ, Otusanya O, Gershengorn HB, Hope AA, Dayton C, Levi D, Garcia M, Prince D, Mills M, Fein D, Colman S, Gong MN. Staged implementation of awakening and breathing, coordination, delirium monitoring and management, and early mobilization bundle improves patient outcomes and reduces hospital costs. Crit Care Med. 2019;47:885–893. doi: 10.1097/CCM.0000000000003765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Klompas M, Kalil AC. Rethinking ventilator bundles. Crit Care Med. 2018;46:1201–1203. doi: 10.1097/CCM.0000000000003173. [DOI] [PubMed] [Google Scholar]
- 133.Iregui M, Ward S, Sherman G, Fraser VJ, Kollef MH. Clinical importance of delays in the initiation of appropriate antibiotic treatment for ventilator-associated pneumonia. Chest. 2002;122:262–268. doi: 10.1378/chest.122.1.262. [DOI] [PubMed] [Google Scholar]
- 134.Barbier F, Luyt CE. Understanding resistance. Intensive Care Med. 2016;42:2080–2083. doi: 10.1007/s00134-016-4543-9. [DOI] [PubMed] [Google Scholar]
- 135.Razazi K, Mekontso Dessap A, Carteaux G, Jansen C, Decousser JW, de Prost N, Brun-Buisson C. Frequency, associated factors and outcome of multi-drug-resistant intensive care unit-acquired pneumonia among patients colonized with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Ann Intensive Care. 2017;7:61. doi: 10.1186/s13613-017-0283-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Barbier F, Bailly S, Schwebel C, Papazian L, Azoulay E, Kallel H, Siami S, Argaud L, Marcotte G, Misset B, Reignier J, Darmon M, Zahar JR, Goldgran-Toledano D, de Montmollin E, Souweine B, Mourvillier B, Timsit JF, Group OS Infection-related ventilator-associated complications in ICU patients colonised with extended-spectrum beta-lactamase-producing Enterobacteriaceae. Intensive Care Med. 2018;44:616–626. doi: 10.1007/s00134-018-5154-4. [DOI] [PubMed] [Google Scholar]
- 137.Bostwick AD, Jones BE, Paine R, Goetz MB, Samore M, Jones M. Potential Impact of Hospital-acquired Pneumonia Guidelines on Empiric Antibiotics. An Evaluation of 113 Veterans Affairs Medical Centers. Ann Am Thorac Soc. 2019;16:1392–1398. doi: 10.1513/AnnalsATS.201902-162OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Harris PNA, Tambyah PA, Lye DC, Mo Y, Lee TH, Yilmaz M, Alenazi TH, Arabi Y, Falcone M, Bassetti M, Righi E, Rogers BA, Kanj S, Bhally H, Iredell J, Mendelson M, Boyles TH, Looke D, Miyakis S, Walls G, Al Khamis M, Zikri A, Crowe A, Ingram P, Daneman N, Griffin P, Athan E, Lorenc P, Baker P, Roberts L, Beatson SA, Peleg AY, Harris-Brown T, Paterson DL, Investigators MT, the Australasian Society for Infectious Disease Clinical Research N Effect of Piperacillin-Tazobactam vs Meropenem on 30-day mortality for patients with E. coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA. 2018;320:984–994. doi: 10.1001/jama.2018.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rodriguez-Bano J, Gutierrez-Gutierrez B, Kahlmeter G. Antibiotics for ceftriaxone-resistant gram-negative bacterial bloodstream infections. JAMA. 2019;321:612–613. doi: 10.1001/jama.2018.19345. [DOI] [PubMed] [Google Scholar]
- 140.Luyt CE, Faure M, Bonnet I, Besset S, Huang F, Junot H, Hekimian G, Schmidt M, Brechot N, Combes A, Aubry A, Mayaux J, Chastre J. Use of non-carbapenem antibiotics to treat severe extended-spectrum beta-lactamase-producing Enterobacteriaceae infections in intensive care unit patients. Int J Antimicrob Agents. 2019;53:547–552. doi: 10.1016/j.ijantimicag.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 141.Gutierrez-Gutierrez B, Perez-Galera S, Salamanca E, de Cueto M, Calbo E, Almirante B, Viale P, Oliver A, Pintado V, Gasch O, Martinez-Martinez L, Pitout J, Akova M, Pena C, Molina J, Hernandez A, Venditti M, Prim N, Origuen J, Bou G, Tacconelli E, Tumbarello M, Hamprecht A, Giamarellou H, Almela M, Perez F, Schwaber MJ, Bermejo J, Lowman W, Hsueh PR, Mora-Rillo M, Natera C, Souli M, Bonomo RA, Carmeli Y, Paterson DL, Pascual A, Rodriguez-Bano J. A multinational, preregistered cohort study of beta-lactam/beta-lactamase inhibitor combinations for treatment of bloodstream infections due to extended-spectrum-beta-lactamase-producing enterobacteriaceae. Antimicrob Agents Chemother. 2016;60:4159–4169. doi: 10.1128/AAC.00365-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pena C, Suarez C, Ocampo-Sosa A, Murillas J, Almirante B, Pomar V, Aguilar M, Granados A, Calbo E, Rodriguez-Bano J, Rodriguez F, Tubau F, Oliver A, Martinez-Martinez L, Spanish Network for Research in Infectious D Effect of adequate single-drug vs combination antimicrobial therapy on mortality in Pseudomonas aeruginosa bloodstream infections: a post Hoc analysis of a prospective cohort. Clin Infect Dis. 2013;57:208–216. doi: 10.1093/cid/cit223. [DOI] [PubMed] [Google Scholar]
- 143.Martinez JA, Cobos-Trigueros N, Soriano A, Almela M, Ortega M, Marco F, Pitart C, Sterzik H, Lopez J, Mensa J. Influence of empiric therapy with a beta-lactam alone or combined with an aminoglycoside on prognosis of bacteremia due to gram-negative microorganisms. Antimicrob Agents Chemother. 2010;54:3590–3596. doi: 10.1128/AAC.00115-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Brunkhorst FM, Oppert M, Marx G, Bloos F, Ludewig K, Putensen C, Nierhaus A, Jaschinski U, Meier-Hellmann A, Weyland A, Grundling M, Moerer O, Riessen R, Seibel A, Ragaller M, Buchler MW, John S, Bach F, Spies C, Reill L, Fritz H, Kiehntopf M, Kuhnt E, Bogatsch H, Engel C, Loeffler M, Kollef MH, Reinhart K, Welte T, German Study Group Competence Network S Effect of empirical treatment with moxifloxacin and meropenem vs meropenem on sepsis-related organ dysfunction in patients with severe sepsis: a randomized trial. JAMA. 2012;307:2390–2399. doi: 10.1001/jama.2012.5833. [DOI] [PubMed] [Google Scholar]
- 145.Chastre J, Wolff M, Fagon JY, Chevret S, Thomas F, Wermert D, Clementi E, Gonzalez J, Jusserand D, Asfar P, Perrin D, Fieux F, Aubas S, Pneum ATG. Comparison of 8 vs 15 days of antibiotic therapy for ventilator-associated pneumonia in adults: a randomized trial. JAMA. 2003;290:2588–2598. doi: 10.1001/jama.290.19.2588. [DOI] [PubMed] [Google Scholar]
- 146.Bouadma L, Luyt CE, Tubach F, Cracco C, Alvarez A, Schwebel C, Schortgen F, Lasocki S, Veber B, Dehoux M, Bernard M, Pasquet B, Regnier B, Brun-Buisson C, Chastre J, Wolff M, group Pt Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): a multicentre randomised controlled trial. Lancet. 2010;375:463–474. doi: 10.1016/S0140-6736(09)61879-1. [DOI] [PubMed] [Google Scholar]
- 147.Rello J, Sole-Lleonart C, Rouby JJ, Chastre J, Blot S, Poulakou G, Luyt CE, Riera J, Palmer LB, Pereira JM, Felton T, Dhanani J, Bassetti M, Welte T, Roberts JA. Use of nebulized antimicrobials for the treatment of respiratory infections in invasively mechanically ventilated adults: a position paper from the European Society of clinical microbiology and infectious diseases. Clin Microbiol Infect. 2017;23:629–639. doi: 10.1016/j.cmi.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 148.Lu Q, Yang J, Liu Z, Gutierrez C, Aymard G, Rouby JJ, Nebulized Antibiotics Study G Nebulized ceftazidime and amikacin in ventilator-associated pneumonia caused by Pseudomonas aeruginosa. Am J Respir Crit Care Med. 2011;184:106–115. doi: 10.1164/rccm.201011-1894OC. [DOI] [PubMed] [Google Scholar]
- 149.Luyt CE, Hekimian G, Brechot N, Chastre J. Aerosol therapy for pneumonia in the intensive care unit. Clin Chest Med. 2018;39:823–836. doi: 10.1016/j.ccm.2018.08.005. [DOI] [PubMed] [Google Scholar]
- 150.Kollef MH, Ricard JD, Roux D, Francois B, Ischaki E, Rozgonyi Z, Boulain T, Ivanyi Z, Janos G, Garot D, Koura F, Zakynthinos E, Dimopoulos G, Torres A, Danker W, Montgomery AB. A randomized trial of the amikacin fosfomycin inhalation system for the adjunctive therapy of Gram-Negative ventilator-associated pneumonia: IASIS trial. Chest. 2017;151:1239–1246. doi: 10.1016/j.chest.2016.11.026. [DOI] [PubMed] [Google Scholar]
- 151.Niederman, Alder J, Bassetti M, Boateng F, Cao B, Corkery K, Dhand R, Kaye KS, Lawatscheck R, McLeroth P, Nicolau DP, Wang C, Wood GC, Wunderink RG, Chastre J. Inhaled amikacin adjunctive to intravenous standard-of-care antibiotics in mechanically ventilated patients with Gram-negative pneumonia (INHALE): a double-blind, randomised, placebo-controlled, phase 3, superiority trial. Lancet Infect Dis Dec. 2019 doi: 10.1016/S1473-3099(19)30574-2. [DOI] [PubMed] [Google Scholar]
- 152.Zampieri FG, Nassar AP, Jr, Gusmao-Flores D, Taniguchi LU, Torres A, Ranzani OT. Nebulized antibiotics for ventilator-associated pneumonia: a systematic review and meta-analysis. Crit Care. 2015;19:150. doi: 10.1186/s13054-015-0868-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu D, Zhang J, Liu HX, Zhu YG, Qu JM. Intravenous combined with aerosolised polymyxin versus intravenous polymyxin alone in the treatment of pneumonia caused by multidrug-resistant pathogens: a systematic review and meta-analysis. Int J Antimicrob Agents. 2015;46:603–609. doi: 10.1016/j.ijantimicag.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 154.Valachis A, Samonis G, Kofteridis DP. The role of aerosolized colistin in the treatment of ventilator-associated pneumonia: a systematic review and metaanalysis. Crit Care Med. 2015;43:527–533. doi: 10.1097/CCM.0000000000000771. [DOI] [PubMed] [Google Scholar]
- 155.Palmer LB, Smaldone GC, Chen JJ, Baram D, Duan T, Monteforte M, Varela M, Tempone AK, O’Riordan T, Daroowalla F, Richman P. Aerosolized antibiotics and ventilator-associated tracheobronchitis in the intensive care unit. Crit Care Med. 2008;36:2008–2013. doi: 10.1097/CCM.0b013e31817c0f9e. [DOI] [PubMed] [Google Scholar]
- 156.Palmer LB, Smaldone GC. Reduction of bacterial resistance with inhaled antibiotics in the intensive care unit. Am J Respir Crit Care Med. 2014;189:1225–1233. doi: 10.1164/rccm.201312-2161OC. [DOI] [PubMed] [Google Scholar]
- 157.Francois B, Luyt CE, Stover CK, Brubaker JO, Chastre J, Jafri HS. New strategies targeting virulence factors of staphylococcus aureus and Pseudomonas aeruginosa. Semin Respir Crit Care Med. 2017;38:346–358. doi: 10.1055/s-0037-1602715. [DOI] [PubMed] [Google Scholar]
- 158.Francois B, Luyt CE, Dugard A, Wolff M, Diehl JL, Jaber S, Forel JM, Garot D, Kipnis E, Mebazaa A, Misset B, Andremont A, Ploy MC, Jacobs A, Yarranton G, Pearce T, Fagon JY, Chastre J. Safety and pharmacokinetics of an anti-PcrV PEGylated monoclonal antibody fragment in mechanically ventilated patients colonized with Pseudomonas aeruginosa: a randomized, double-blind, placebo-controlled trial. Crit Care Med. 2012;40:2320–2326. doi: 10.1097/CCM.0b013e31825334f6. [DOI] [PubMed] [Google Scholar]
- 159.François B, Sanchez MG, Eggimann P, Dequin P-F, Laterre P-F, Huberlant V, Escudero D, Boulain T, Bretonniere C, Pugin J, Alvarez JT, Ali O, Shoemaker K, Ruzin A, Vandamme D, Colbert S, Bellamy T, Dubovsky F, Jafri H, Group SS Suvratoxumab Reduces Staphylococcus Aureus Pneumonia in high-risk ICU patients: results of the SAATELLITE study. Am J Respir Crit Care Med. 2019;199:A7358–A7358. [Google Scholar]
- 160.Luyt CE, Forel JM, Hajage D, Jaber S, Cayot-Constantin S, Rimmele T, Coupez E, Lu Q, Diallo MH, Penot-Ragon C, Clavel M, Schwebel C, Timsit JF, Bedos JP, Hauw-Berlemont C, Bourenne J, Mayaux J, Lefrant JY, Mira JP, Combes A, Wolff M, Chastre J, Papazian L. Acyclovir for mechanically ventilated patients with herpes simplex virus oropharyngeal reactivation: a randomized clinical trial. JAMA Intern Med Dec. 2019;16:pii: 2757496. doi: 10.1001/jamainternmed.2019.5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cowley NJ, Owen A, Shiels SC, Millar J, Woolley R, Ives N, Osman H, Moss P, Bion JF. Safety and efficacy of antiviral therapy for prevention of cytomegalovirus reactivation in immunocompetent critically ill patients: a randomized clinical trial. JAMA Intern Med. 2017;177:774–783. doi: 10.1001/jamainternmed.2017.0895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Klompas M. Prevention of intensive care unit-acquired pneumonia. Semin Respir Crit Care Med. 2019;40:548–557. doi: 10.1055/s-0039-1695783. [DOI] [PubMed] [Google Scholar]
- 163.Weng H, Li JG, Mao Z, Feng Y, Wang CY, Ren XQ, Zeng XT. Probiotics for preventing ventilator-associated pneumonia in mechanically ventilated patients: a meta-analysis with trial sequential analysis. Front Pharmacol. 2017;8:717. doi: 10.3389/fphar.2017.00717. [DOI] [PMC free article] [PubMed] [Google Scholar]