Abstract
Objective
To determine the epidemiological features, course, and outcomes of critically ill pediatric patients with Influenza A (H1N1) virus.
Design
Prospective cohort of children in pediatric intensive care units (PICUs) due to Influenza A (H1N1) virus infection.
Setting
Seventeen medical-surgical PICUs in tertiary care hospital in Argentina.
Patients
All consecutive patients admitted to the PICUs with influenza A (H1N1) viral infection from 15 June to 31 July 2009.
Measurements and main results
Of 437 patients with acute lower respiratory infection in PICUs, 147 (34%) were diagnosed with influenza A (H1N1) related to critical illness. The median age of these patients was 10 months (IQR 3–59). Invasive mechanical ventilation was used in 117 (84%) on admission. The rate of acute respiratory distress syndrome (ARDS) was 80% (118 of 147 patients). Initial non-invasive ventilation failed in 19 of 22 attempts (86%). Mortality at 28 days was 39% (n = 57). Chronic complex conditions (CCCs), acute renal dysfunction (ARD) and ratio PaO2/FiO2 at day 3 on MV were independently associated with a higher risk of mortality. The odds ratio (OR) for CCCs was 3.06, (CI 95% 1.36–6.84); OR for ARD, 3.38, (CI 95% 1.45–10.33); OR for PaO2/FiO2, 4 (CI 95% 1.57–9.59). The administration of oseltamivir within 24 h after admission had a protective effect: OR 0.2 (CI 95% 0.07–0.54).
Conclusions
In children with ARDS, H1N1 as an etiologic agent confers high mortality, and the presence of CCCs in such patients increases the risk of death.
Keywords: Critically ill, Influenza A (H1N1), Infants and children, Mechanical ventilation, Survey
Introduction
Severe acute respiratory failure due to influenza A virus (H1N1) is a newly recognized illness that has spread quickly all over the world, and the morbidity and mortality associated with this virus have caused international concern.
In Argentina, 5,710 cases (both adults and children) with 337 deaths were confirmed as of 31 July 2009 [1]. The outbreak began in June, which in this country is a winter month when pediatric intensive care units (PICUs) generally find their resources strained by a seasonal increase in acute lower respiratory infection (ALRI). In view of the impact of this illness in Mexico, it was thought that the number of patients here would be even greater than usual, and measures were taken to prepare PICUs for the possibility of an epidemic. The effectiveness of these measures, however, was potentially limited by a lack of information on the incidence of severe respiratory disease requiring mechanical ventilation (MV) concurrent with the circulation of the H1N1 virus. Though this information already existed for adult patients, to this day it has still not been described in pediatric ICU populations.
Faced with this situation, we decided to perform a prospective multicenter study in order to characterize the epidemiology, clinical aspects, and 28-day outcomes of pediatric patients with influenza A (H1N1) admitted to PICUs.
Methods
Study design
Anticipating that most patients admitted to PICUs with influenza A H1N1 would probably need MV as well, we decided to activate the protocol from a previous multicenter study of the characteristics of ventilated children during outbreaks of ALRI in 2007 and 2008 [2]. This protocol had been reviewed and approved by the review boards and ethical committees of all the participating hospitals. We were thus able to rapidly survey consecutive critically ill pediatric patients with confirmed influenza A (H1N1) admitted to 17 PICUs from 15 June to 31 July 2009 (Fig. 1).
Fig. 1.
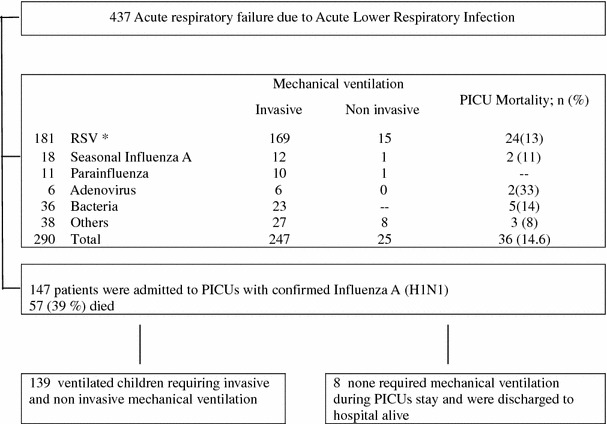
Identification of infants and children who would be eligible for studies of severe acute respiratory failure by H1N1 influenza A across 17 pediatric ICUs from 15 June 2009 to 31 July 2009. *RSV respiratory syncytial virus
Confirmed influenza A (H1N1) was defined as an individual with the symptoms of suspected cases [3] with confirmation by real-time reverse polymerase chain reaction. We defined critically ill patients as those admitted to the PICUs and requiring invasive or non-invasive MV, fraction of inspired oxygen (FiO2) on face mask greater than or equal to 60%, and/or administration of intropic or vasopressors.
Study site personnel entered the data into an Internet database, and these data were then sent to the coordinating center in Buenos Aires where they were checked daily during the study period by the coordination center team (JAF, AF, EM, PA), which identified and corrected omissions and inconsistencies.
Data on age, weight, and sex were collected for each patient diagnosed with influenza A (H1N1). The PIM II score was used to evaluate the severity of illness upon admission to the PICUs [4]. Prematurity, presence of complex chronic conditions (CCCs) [5], the acquisition of H1N1 (community or nosocomial), and the presence of co-infections (defined as positive microbial culture obtained in blood or pleural effusion or bronchial aspiration samples during the first 24 h after admission to a PICU, and/or positive indirect immunofluorescens to other viral agents) were also registered. Dates of hospital and PICU admissions, dates of initiation of MV (invasive or non invasive), and liberation from MV were recorded in due time. For each of the first 28 days after a patient’s admission to the PICU, physiological markers of organ dysfunction, ventilatory parameters, X-rays, blood gases, and treatment-related variables were also registered. All of these variables included: mode of ventilation, FiO2, positive end expiratory pressure, peak airway pressure, plateau airway pressure, mean airway pressure, and adjuncts to MV such as prone positioning, inhaled nitric oxide, or extra-corporeal membrane oxygenation (ECMO). Daily arterial blood gas values included pH, PaCO2, PaO2, and oxygen saturation. When multiple daily measurements were performed, those closest to 0800 hours were recorded. The presence of barotrauma, previously defined, was documented [6]. Acute respiratory distress syndrome (ARDS) was defined according to the American-European Consensus Conference on ARDS [7], and ventilator-associated pneumonia was defined using the criteria of the CDC [8]. Sepsis and organ dysfunctions were defined according to the International Consensus Conference on Sepsis in Pediatric Patients [9]. Specific treatments were recorded, including the administration of oseltamivir, inotropic or vasopressor drugs, and antibiotics. The primary outcome was mortality at 28 days after admission to the PICU.
Statistical analysis
Data are shown as mean and standard deviation, median with the interquartile range (IQR), and proportions as appropriate. All categorical variables were analyzed with the chi-square test, except where small sizes required the use of Fisher’s exact test. The comparison of continuous variables was made with the Student’s t test for variables with normal distribution or the Mann-Whitney U test for variables with non-normal distribution. The Kruskal-Wallis test was used to compare continuous variables among more than two groups. Multivariate logistic-regression analysis was used to identify factors independently associated with mortality, and stepwise selection and backward elimination techniques were used to construct the multivariate model. We included the following variables that were significant in the univariate analysis: hospital acquisition of H1N1, presence of at least one chronic condition, presence of cardiovascular, renal, or hematological dysfunctions, use of oseltamivir within the 24 h of hospital admission, and a PaO2 to FiO2 ratio at the 3rd day of less than 100 (we chose this cutoff point because a previous study showed that a PaO2 to FiO2 ratio value of <100 was that which could best be used to discriminate between surviving and non-surviving mechanically ventilated pediatric patients [6]). Goodness of fit was determined using the Hosmer-Lemeshow statistic. A two-sided p value below 0.05 was considered statistically significant, except in the multivariate model, where a p-value of less than 0.01 was considered statistically significant.
Results
Characteristics of the population
During the study period, 437 critically ill patients with acute respiratory failure due to ALRI were admitted to the participating PICUs, and 147 of these (34%) were confirmed cases of influenza A (H1N1). The median number of patients with ALRI admitted to each PICU was 24 (IQR 15–39), of which the median number of patients with confirmed influenza A (H1N1) was 5.5 (IQR 4–11).
Ventilatory requirements
MV was used in 139 (94%) of the patients with confirmed influenza A (H1N1). One hundred seventeen (84%) received invasive MV and 22 (16%), NIV. Of these, 19 (86%) subsequently received invasive MV. Prone position and high frequency oscillatory ventilation were employed in 50 and 11 patients, respectively. None of the patients received inhaled nitric oxide, and only two were given extra-corporeal membrane oxygenation.
Seventy-nine patients were weaned from MV. The methods most frequently used were the spontaneous breathing trial (SBT) and the gradual reduction of ventilatory support (GRVS). The SBT (T-piece, pressure support ≤10 cm of H2O or CPAP = 5 cm H2O) was used in 58 patients (73%) and GRSV in 6 patients (7%). The overall rate of reintubation was 24% (19/79). Reintubated patients had higher mortality than successfully extubated patients: 16 versus 2%, p = 0.04.
Comparison between survivor and non-survivors (Tables 1, 2)
Table 1.
Comparison of the main characteristics of survivors and non-survivors upon admission to the PICUs
| Survivors at 28 days after PICU admission (n = 90) | Non-survivors at 28 days after PICU admission (n = 57) | p Value | |
|---|---|---|---|
| Age, median (IQR) | 9 (3–36) | 15 (4–70) | 0.26 |
| Weight, kg, median (IQR) | 7.3 (5–14.2) | 8.9 (4.9–20) | 0.83 |
| Sex, female/male, n (%) | 34 (38)/56 (62) | 28 (49)/29 (51) | 0.17 |
| PIM 2 score, median (IQR) | 7 (3.6–11.8) | 12.9 (6–44) | <0.01 |
| Nosocomial acquisition; n (%) | 13 (15) | 16 (28) | <0.043 |
| At least one complex chronic condition, n (%) | 34 (38) | 34 (59) | <0.01 |
| Complex chronic condition, n (%) | |||
| Neuromuscular | 12 (13) | 12 (21) | 0.21 |
| Cardiovascular | 5 (12) | 5 (13) | 0.45 |
| Renal/metabolic | – | 2 (3.5) | – |
| Respiratory | 10 (11) | 6 (10.5) | 0.91 |
| Genetic defect | 3 (3) | 5 (9) | 0.26 |
| Malignancy-immunodeficiency | 4 (4) | 4 (7) | 0.7 |
| Multiple complex chronic conditions, n (%) | 10 (11) | 7 (12) | 0.82 |
| Ex-premature, n (%) | 7 (8) | 6 (10.5) | 0.56 |
Table 2.
Comparison of the complications, treatments, and outcomes of survivors and non-survivors
| Survivor at 28 days after PICU admission (n = 90) | Non-survivors at 28 days after PICU admission (n = 57) | p Value | |
|---|---|---|---|
| Complications during PICU stay; n (%) | |||
| Cardiovascular dysfunction | 48 (55) | 46 (77) | <0.01 |
| Hepatic dysfunction | 5 (6) | 7 (12) | 0.21 |
| Renal dysfunction | 10 (11) | 20 (35) | <0.01 |
| Hematologic dysfunction | 6 (7) | 16 (28) | <0.01 |
| Barotrauma | 11 (13) | 16 (28) | 0.03 |
| Ventilation-associated pneumonia | 15 (17) | 13 (23) | 0.54 |
| Acute respiratory distress syndrome | 59 (65) | 53 (93) | <0.01 |
| Treatments; n (%) | |||
| Antibiotic | 89 (99) | 54 (95) | 0.3 |
| Oseltamivir | 83 (92) | 52 (91) | 1 |
| Oseltamivir ≤24 h | 77 (86) | 39 (68) | 0.01 |
| Inotropes or vasopressors | 56 (62) | 49 (86) | <0.01 |
| Outcomes; days, median (IQR) | |||
| Length of mechanical ventilation | 11.5 (6–22) | 8 (3–15) | 0.01 |
| Length of stay in the hospital | 19 (13–31) | 12 (5–19) | <0.01 |
| Length of stay in the PICU | 12 (8–25) | 8 (3–15.5) | <0.01 |
The PIM score was significantly higher in non-survivors (22.8 ± 22 vs. 10.6 ± 11.3, p = 0.01), as was also the incidence of nosocomial acquired H1N1 influenza A (28 vs. 15%, p = 0.04). The rate of co-infections for the whole population upon admission was 34% (50 out of 147 patients), and there were no differences between the two groups. The agents most frequently isolated were respiratory syncytial virus (48%), Staphylococcus aureus (10%), Streptococcus pneumoniae (6%), and Haemophilus (6%).
We also found that the rate of at least one CCC in the group of non-survivors was higher than in the survivor group (59 vs. 38%, p = < 0.01). There was a high rate of ARDS in the entire population (80%), and this condition was more frequent in non-survivors: 93 versus 65% (p = <0.01). These patients also had a lower ratio of PaO2 to FiO2 in the first 15 days of MV and needed higher inspiratory pressures (peak and plateau), applied PEEP, and mean airway pressure, (Table 3). The rates of renal, cardiovascular, and hematological dysfunctions were significantly higher in non- survivors.
Table 3.
Ventilatory parameters during the first 15 days of MV, median [interquartile range (IQR)]
| Day 1 | Day 3 | Day 7 | Day 15 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Survivors n = 80 | Non-survivors n = 56 | p Value | Survivors n = 75 | Non-survivors n = 43 | p Value | Survivors n = 58 | Non-survivors n = 28 | p Value | Survivors n = 31 | Non-survivors n = 11 | p Value | |
| Peak inspiratory pressure (cm H2O) | 32 (25–35.2) | 33 (28–39.2) | 0.10 | 30 (25–33) | 34 (27.5–41) | <0.01 | 28.5 (25–34.5) | 38.5 (33.7–45) | <0.01 | 26 (22.2–30.5) | 35 (16.5–41) | 0.29 |
| Plateau pressure (cm H2O) | 29 (23–32) | 32 (28–36) | <0.01 | 25 (22–30) | 32 (28–37.7) | <0.01 | 26 (20.7–30) | 32.5 (28.2–39.7) | <0.01 | 24 (20.2–27.7) | 32.5 (18.7–38.2) | 0.23 |
| Mean airway pressure (cm H2O) | 12 (9.7–15) | 20 (14.5–22) | <0.01 | 12.5 (10–14) | 20 (15–23) | <0.01 | 12 (10–15) | 21 (19–23) | <0.01 | 13 (10–15) | 19.5 (15.7–21.5) | 0.06 |
| Applied positive end expiratory pressure (cm H2O) | 7 (5–10) | 10 (7–12) | <0.01 | 7 (5–10) | 12 (8.5–13) | <0.01 | 8.5 (5–10) | 13 (8.7–15) | <0.01 | 7.5 (5–9.7) | 14 (11.5–15) | <0.01 |
| PaO2 to FiO2 | 121.5 (101–169) | 80.5 (63–122) | <0.01 | 150 (100–202) | 85 (57–114) | <0.01 | 144 (118–181) | 85 (52–120) | <0.01 | 164 (130–220) | 60 (50–118) | <0.01 |
| Oxygenation index | 9 (7–13) | 27 (16–35) | <0.01 | 9 (5.5–11) | 25 (12–41) | <0.01 | 8 (5–15) | 25 (11–43) | <0.01 | 5 (4–12) | 38 (32–40) | 0.01 |
| pH | 7.33 (7.25–7.40) | 7.27 (7.20–7.38) | 0.15 | 7.37 (7.30–7.41) | 7.27 (7.22–7.40) | 0.10 | 7.40 (7.36–7.42) | 7.35 (7.32–7.38) | 0.10 | 7.40 (7.33–7.44) | 7.28 (7.26–7.39) | 0.05 |
| PCO2 (mmHg) | 48 (42–62) | 57 (38–74) | 0.45 | 50 (41–65) | 55 (43–66) | 0.57 | 58 (50–62) | 58 (45–64) | 0.95 | 50 (44–0.56) | 76 (68–87) | 0.02 |
More patients in the non-survivor group needed inotropic infusion (86 vs. 62%, p = < 0.001). In this group, 39 (68%) received oseltamivir within 24 h after admission to the hospital, while in the survivor group the number was 77 (86%) p = 0.01, and the medians of the lengths of MV, PICU, and hospital stays were higher.
The mortality rate for influenza A (H1N1) patients admitted to the PICUs was 39% (57/147). The most common causes of death were refractory hypoxia in 27 patients (47%), followed by multiple organ failure (16/57:28%) and refractory septic shock (11/57:19%). Of those patients who died of refractory hypoxia, 16 (59%) were ventilated in prone position, and 6 (22%) received HFOV. Both of the two patients on ECMO for refractory hypoxia after receiving HFOV died of multiple organ dysfunctions. In all those patients who died or were discharged alive, four factors were found to be independently associated with mortality: CCCs [odds ratio (OR) 3.06, CI 95% 1.36–6.84, p < 0.01)]; acute renal dysfunction (OR 3.88, CI 95% 1.45–10.33, p > 001); ratio PaO2 to FiO2 ≤100 at day 3 on MV (OR: 4, CI 95% 1.7–9.59, p < 0.01; the administration of oseltamivir, which had a protective effect when administered within 24 h after hospital admission (OR 0.20, CI 95% 0.07–0.54; p < 0.01). A Hosmer-Lemeshow test showed that the data were well fitted for the model (p = 0.82).
Discussion
This prospective and multicenter study presents the largest cohort to date of critically ill pediatric patients with confirmed influenza A (H1N1) infection in the 2009 pandemic in South America. It describes the epidemiology, clinical features, and mortality-associated risk factors of the sickness in children in PICUs.
Our study shows that most patients (94%) admitted to PICUs with influenza A (H1N1) were submitted to MV. The use of NIV was unsuccessful in 19 of 22 (86%) attempts, and this corroborates the scarce evidence of the usefulness of NIV in ARDS [10, 11]. The rate of utilization of MV reported in adult patients with this infection varies from 65 to 96% [12–14]. In Australia and New Zealand, the ANZIC Influenza Investigators recently found that 456 of 706 (65%) adult patients underwent MV [12], and in Mexico, Dominguez-Cherit et al. [14] have reported an even higher rate of 96% (56 out of 58) in adults, a rate that is similar to that of the children in our study. Such figures give us reason to fear that, at least in winter, this illness could result in a worrisome strain on the resources of PICUs.
The overall mortality rate of 39% in this study is higher than that reported for pediatric ARDS, and it is evident that the most common cause of death is not multi-organ dysfunction or septic shock but rather refractory hypoxia. Of 147 patients with severe acute respiratory failure during their PICU stay, 118 (80%) met the criteria for ARDS. Hence, the non-survivors had more serious lung injury, characterized by a lower PaO2/FiO2 ratio and a higher oxygenation index. The mortality rate of pediatric patients with H1N1 infection is clearly higher than the rate for those with ALI of other causes [15, 16]. Our findings are comparable to previously reported rates of 42 and 50%, respectively, of ventilated children with H1N1 infection [17, 18], though a recent Canadian study has reported a lower mortality rate of almost 8% [13]. These differences in mortality rates could be well explained, at least in part, by the fact that the studies were carried out in different seasons of the year and had different inclusion criteria that might have been reflected in the incidences of ARDS and the use of MV.
Patients more seriously ill upon admission with a higher PIM score made up the majority of non-survivors. Many of the patients in our study had histories of CCCs. Of the non-survivors, these conditions were present in 34 (59%). Although this rate is lower than those reported for adult patients, which vary from 50 to 90% [19–21], as well as those reported for children, which vary from 67 to 77% [17, 22], it is nonetheless clearly associated with higher mortality. Influenza A (H1N1) infection-related acute renal dysfunction has been said to affect up to 38% of mechanically ventilated adult patients [23–25], 90% of whom require renal replacement therapy [24, 25]. As we reported in another study, acute renal dysfunction (ARD) is an independent factor associated with lower survival in ventilated pediatric patients [6]. ARD in ICUs is seen less frequently in children than in adults, and this study, not unexpectedly, shows a lower need for renal replacement therapies.
There are some studies on adults and children in ambulatory settings reporting reduced intensity and/or duration of symptoms after the administration of oseltamivir in avian influenza [26] and influenza A or B [27–30], but this benefit was found to be very small in a meta-analysis that included three randomized controlled trials [31]. In critically ill adults with influenza A, treatment with oseltamivir was associated with a significant reduction in mortality [32]. Our study shows a positive impact in patients receiving the drug within 24 h of hospital admission. Nevertheless, this finding should be taken with caution since our study was not designed to evaluate the effectiveness of oseltamivir in H1N1 patients.
Libster and coworkers [33] have recently reported the epidemiological aspects of H1N1 infection in pediatric patients in Argentina. Our present study complements their findings by providing a more complete picture of the course of this illness in the PICU with a much larger cohort of critically ill patients (147 vs. 47) in a greater number of PICUs (17 vs. 5). Since only three of the PICUs that participated in the Libster study were included in this study, any possible overlap between the two studies is minimal. Both studies show similar rates of MV utilization (89% in [33] vs. 94%) and different mortality rates in spite of lacking statistical significance (29% in [33] vs. 39%). It is worth noting that both of these rates are much higher than the mortality rates for seasonal influenza.
Summary
When mechanical ventilation is necessary in critically ill H1N1 infants and children, it is best to use invasive MV at the onset. Mortality is high in children with severe acute respiratory failure due to influenza A (H1N1). The risk of death is higher in pediatric H1N1 patients with a history of CCCs.
Acknowledgment
We are grateful to Brian Kavanagh for his comments and suggestions on the manuscript. This study was in part supported by the Sociedad Argentina de Terapia Intensiva (SATI).
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Author Affiliations and a list of the members of the Mechanical Ventilation in Pediatric Critical Care Group of the Argentine Society of Critical Care Medicine
Hospital de Niños R Gutiérrez, Buenos Aires (Drs. J. A. Farias, A. Fernández, E. Monteverde, D. Turina, C. Chávez); Hospital A. Posadas, Haedo, Provincia de Buenos Aires (Drs. L. Albano, N. Vidal); Hospital de Niños “De la Santísima Trinidad”, Córdoba (Drs. M. Montes, P. Capocasa, Irma Azar); Sanatorio Franchin, Buenos Aires (Dr. P. Arias, R. Chiabrando); Hospital F Abete, Malvinas Argentinas (Drs. M. G. Rodríguez, G. Caprotta, A. Esen); Hospital de Niños, Santa Fe “O Allasia” (Dr. M. Allasia); Hospital de Niños “Sor Ludovica” La Plata (Dr. M. E. Ratto, F. Podestá); Hospital de Clínicas José de San Martin, Buenos Aires (Drs. R. Jaén, V. Fulco); Hospital de Niños “P de Elizalde”, Buenos Aires (C. Meregalli, G. Debaisi); Hospital “El Cruce”, F. Varela, Provincia de Buenos Aires (Dr. K. Fiquepron); Hospital “Austral”, Pilar, Provincia de Buenos Aires (Drs. A. Siaba, E. Schniltzer); Hospital de Niños “J Pablo II”, Corrientes (Drs. R. Jabornisky, G. Abreo); Hospital Churruca-Visca, Buenos Aires (Dr. G. González); Sanatorio Anchorena, Buenos Aires (Drs. R. Poterala, A. Cairnie); Sanatorio UOM, Buenos Aires (Dr. E. Koch); Sanatorio Corporación General San Martin, San Martin, Provincia de Buenos Aires (Dr. P. Neira); Hospital de Niños “Niño Jesús”, Tucumán (Dr. A. Rodríguez Calvo, L. Marcos); Hospital Italiano, Buenos Aires (Drs. S. Díaz, P. Minces); Clínica Bazterrica, Buenos Aires (Dr. F. Olazarri); Hospital Universitario de Getafe, and CIBER de Enfermedades Respiratorias in Madrid, Spain (Dr. A. Esteban).
Footnotes
The Mechanical Ventilation in Pediatric Critical Care Group of the Argentine Society of Critical Care Medicine
References
- 1.Ministerio de Salud de Argentina (2009) Influenza pandémica H1N1. http://municipios.msal.gov.ar/h1n1/parte_influenza/parte-65-fecha-05-08-09.pdf
- 2.Monteverde E, Fernandez A, Farias JA. Segundo Estudio Internacional de la Ventilación Mecanica en Pediatría. Medicina Intensiva (Argentina) 2009;26:32. [Google Scholar]
- 3.WHO (2009) Human infection with pandemic (H1N1) 2009 virus: updated interim WHO guidance on global surveillance. http://www.who.int/csr/disease/swineflu/WHO_case_definition_swine_flu_2009_04_29.pdf
- 4.Slater A, Shann F, Pearson G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29:278–285. doi: 10.1007/s00134-002-1601-2. [DOI] [PubMed] [Google Scholar]
- 5.Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980–1997. Pediatrics. 2000;106:205–209. [PubMed] [Google Scholar]
- 6.Farias JA, Frutos-Vivar F, Casado Flores J, Siaba A, Retta A, Fernandez A, Baltodano A, Ko IJ, Johnson M, Esteban A. Factors associated with the prognosis of mechanically ventilated infants and children. An international study. Med Intensiva. 2006;30:425–431. doi: 10.1016/S0210-5691(06)74565-X. [DOI] [PubMed] [Google Scholar]
- 7.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, Lamy M, Legall JR, Morris A, Spragg R. The American–European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 8.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med. 2005;6:2–8. doi: 10.1097/01.PCC.0000149131.72248.E6. [DOI] [PubMed] [Google Scholar]
- 10.Rana S, Jenad H, Gay P, Buck C, Hubmayr R, Gajic O. Failure of non-invasive ventilation in patients with acute lung injury: observational cohort study. Crit Care. 2006;10:R 79. doi: 10.1186/cc4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Festic E, Gajic O, Limper AH, Aksamit TR. Acute respiratory failure due to pneumocystis pneumonia in patients without human immunodeficiency virus infection. Chest. 2005;128:573–579. doi: 10.1378/chest.128.2.573. [DOI] [PubMed] [Google Scholar]
- 12.The ANZIC Influenza Investigators Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361:1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 13.Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, Stelfox T, Bagshaw S, Choong K, Lamontagne F, Turgeon AF, Lapinsky S, Ahern SP, Smith O, Siddiqui F, Jouvet P, Khwaja K, McIntyre L, Menon K, Hutchison J, Hornstein D, Joffe A, Lauzier F, Singh J, Karachi T, Wiebe K, Olafson K, Ramsey C, Sharma S, Dodek P, Meade M, Hall R, Fowler R. Critically ill patients with 2009 Influenza A (H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 14.Dominguez-Cherit G, Lapinsky SE, Macias AE, Pinto R, Espinosa-Perez L, de la Torre A, Poblano-Morales M, Baltazar-Torres JA, Bautista E, Martinez A, Martinez MA, Rivero E, Valdez R, Ruiz-Palacios G, Hernandez M, Stewart TE, Fowler RA. Critically ill patients with 2009 Influenza A (H1N1) in Mexico. JAMA. 2009;302:1880–1887. doi: 10.1001/jama.2009.1536. [DOI] [PubMed] [Google Scholar]
- 15.Dahlem P, van Aalderen WM, Hamaker ME, Dijkgraaf MG, Bos AP. Incidence and short-term outcome of acute lung injury in mechanically ventilated children. Eur Respir J. 2003;22:980–985. doi: 10.1183/09031936.03.00003303. [DOI] [PubMed] [Google Scholar]
- 16.Flori HR, Glidden DV, Rutherford GW, Matthay MA. Pediatric acute lung injury: prospective evaluation of risk factors associated with mortality. Am J Respir Crit Care Med. 2005;171:995–1001. doi: 10.1164/rccm.200404-544OC. [DOI] [PubMed] [Google Scholar]
- 17.Lister P, Reynolds F, Parslow R, Chan A, Cooper M, Plunkett A, Riphagen S, Peters M. Swine-origin influenza virus H1N1, seasonal influenza virus, and critical illness in children. Lancet. 2009;374:605–607. doi: 10.1016/S0140-6736(09)61512-9. [DOI] [PubMed] [Google Scholar]
- 18.Caprotta G, Gonzalez Crotti P, Primucci Y, Alesio H, Esen A. Influenza A H1N1 respiratory infection in an intensive care unit in Argentina. An Pediatr (Barc) 2010;72:62–66. doi: 10.1016/j.anpedi.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 19.Rello J, Rodriguez A, Ibanez P, Socias L, Cebrian J, Marques A, Guerrero J, Ruiz-Santana S, Marquez E, Del Nogal-Saez F, Alvarez-Lerma F, Martinez S, Ferrer M, Avellanas M, Granada R, Maravi-Poma E, Albert P, Sierra R, Vidaur L, Ortiz P, Prieto Del Portillo I, Galvan B, Leon-Gil C. Intensive care adult patients with severe respiratory failure caused by Influenza A (H1N1) in Spain. Crit Care. 2009;13:R148. doi: 10.1186/cc8044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention (CDC) (2009) Intensive-care patients with severe novel Influenza A (H1N1) virus infection—Michigan, June 2009. MMWR Morb Mortal Wkly Rep 58:749–752 [PubMed]
- 21.Vaillant L, La Ruche G, Tarantola A, Barboza P. Epidemiology of fatal cases associated with pandemic H1N1 influenza 2009. Euro Surveill. 2009;14:pii–19309. doi: 10.2807/ese.14.33.19309-en. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC) Surveillance for pediatric deaths associated with pandemic Influenza A H1N1 virus infection—United States, April–August 2009. MMWR Morb Mortal Wkly Rep. 2009;58:941–947. [PubMed] [Google Scholar]
- 23.Perez-Padilla R, de la Rosa-Zamboni D, Ponce de Leon S, Hernandez M, Quinones-Falconi F, Bautista E, Ramirez-Venegas A, Rojas-Serrano J, Ormsby CE, Corrales A, Higuera A, Mondragon E, Cordova-Villalobos JA. Pneumonia and respiratory failure from swine-origin Influenza A (H1N1) in Mexico. N Engl J Med. 2009;361:680–689. doi: 10.1056/NEJMoa0904252. [DOI] [PubMed] [Google Scholar]
- 24.Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, Garten RJ, Gubareva LV, Xu X, Bridges CB, Uyeki TM. Emergence of a novel swine-origin Influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. doi: 10.1056/NEJMoa0903810. [DOI] [PubMed] [Google Scholar]
- 25.Raffo L. Influenza A (H1N1) epidemic in Argentina. Experience in a National General Hospital (Hospital Nacional Alejandro Posadas) Medicina (B Aires) 2009;69:393–423. [PubMed] [Google Scholar]
- 26.Kandun IN, Tresnaningsih E, Purba WH, Lee V, Samaan G, Harun S, Soni E, Septiawati C, Setiawati T, Sariwati E, Wandra T. Factors associated with case fatality of human H5N1 virus infections in Indonesia: a case series. Lancet. 2008;372:744–749. doi: 10.1016/S0140-6736(08)61125-3. [DOI] [PubMed] [Google Scholar]
- 27.Sato M, Saito R, Sato I, Tanabe N, Shobugawa Y, Sasaki A, Li D, Suzuki Y, Sato M, Sakai T, Oguma T, Tsukada H, Gejyo F, Suzuki H. Effectiveness of oseltamivir treatment among children with Influenza A or B virus infections during four successive winters in Niigata City, Japan. Tohoku J Exp Med. 2008;214:113–120. doi: 10.1620/tjem.214.113. [DOI] [PubMed] [Google Scholar]
- 28.Kawai N, Ikematsu H, Iwaki N, Maeda T, Satoh I, Hirotsu N, Kashiwagi S. A comparison of the effectiveness of oseltamivir for the treatment of Influenza A and Influenza B: a Japanese multicenter study of the 2003–2004 and 2004–2005 influenza seasons. Clin Infect Dis. 2006;43:439–444. doi: 10.1086/505868. [DOI] [PubMed] [Google Scholar]
- 29.Lin JT, Yu XZ, Cui DJ, Chen XY, Zhu JH, Wang YZ, Wu XD. A multicentre, randomized, controlled trial of oseltamivir in the treatment of influenza in a high-risk Chinese population. Curr Med Res Opin. 2006;22:75–82. doi: 10.1185/030079906X80297. [DOI] [PubMed] [Google Scholar]
- 30.Kaiser L, Wat C, Mills T, Mahoney P, Ward P, Hayden F. Impact of oseltamivir treatment on influenza-related lower respiratory tract complications and hospitalizations. Arch Intern Med. 2003;163:1667–1672. doi: 10.1001/archinte.163.14.1667. [DOI] [PubMed] [Google Scholar]
- 31.Shun-Shin M, Thompson M, Heneghan C, Perera R, Harnden A, Mant D. Neuraminidase inhibitors for treatment and prophylaxis of influenza in children: systematic review and meta-analysis of randomised controlled trials. BMJ. 2009;339:b3172. doi: 10.1136/bmj.b3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McGeer A, Green KA, Plevneshi A, Shigayeva A, Siddiqi N, Raboud J, Low DE. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis. 2007;45:1568–1575. doi: 10.1086/523584. [DOI] [PubMed] [Google Scholar]
- 33.Libster R, Bugna J, Coviello S, Hijano DR, Dunaiewsky M, Reynoso N, Cavalieri ML, Guglielmo MC, Areso MS, Gilligan T, Santucho F, Cabral G, Gregorio GL, Moreno R, Lutz MI, Panigasi AL, Saligari L, Caballero MT, Egues Almeida RM, Gutierrez Meyer ME, Neder MD, Davenport MC, Del Valle MP, Santidrian VS, Mosca G, Garcia Dominguez M, Alvarez L, Landa P, Pota A, Bolonati N, Dalamon R, Sanchez Mercol VI, Espinoza M, Peuchot JC, Karolinski A, Bruno M, Borsa A, Ferrero F, Bonina A, Ramonet M, Albano LC, Luedicke N, Alterman E, Savy V, Baumeister E, Chappell JD, Edwards KM, Melendi GA, Polack FP. Pediatric Hospitalizations Associated with 2009. N Engl J Med. 2010;362:45–55. doi: 10.1056/NEJMoa0907673. [DOI] [PubMed] [Google Scholar]


