Oseltamivir is a neuraminidase inhibitor licensed for treatment of influenza A and B. The recommended dosage is 75 mg BID for 5 days. However higher dosage of oseltamivir (150 mg BID) and increased duration of therapy may be applied in severe cases with evidence of clinical progression [1]. The French Drug Agency (AFSSAPS) also recommended this dosage for severe influenza A/H1N1.
Extracorporeal membrane oxygenation (ECMO) is an important rescue therapy for severe cases of influenza A/H1N1 infection which include rapid respiratory failure and development of acute respiratory distress syndrome (ARDS) [2]. Adhesion of drugs to ECMO circuit components can occur, and drugs may be sequestrated within the circuit, leading to sub-therapeutic drug levels [3]. Oseltamivir is efficiently removed by haemodialysis, with a 70% decrease in plasma concentrations after haemodialysis [4].
We report the case of a 24-year-old woman admitted to our intensive care unit for ARDS related to influenza A/H1N1 virus infection. She had been well until 3 days before admission, when she rapidly developed non-productive cough and acute chest pain. Body temperature was 37.8°C, and room air partial pressure of oxygen and oxygen saturation were 42 mmHg and 77%, respectively.
On arrival, her body mass index was 31.9 kg/m2, her blood pressure was 114/66 mmHg and respiratory rate was 6 breaths/min. Because of worsening respiratory distress, the patient was intubated and 150 mg BID oseltamivir was added to broad-spectrum antibacterial therapy. Veno-venous ECMO (Jostra-Maquet, France) was initiated on day 1 with percutaneous venous femoral and jugular cannulae. Reverse-transcription polymerase chain reaction (RT-PCR) on respiratory sample obtained by broncho-alveolar lavage was positive for 2009 pandemic influenza A (H1N1) virus.
While the patient was anuric, continuous veno-venous haemodiafiltration was started on day 1. Blood flow was set at 250 mL/min, dialysate flow at 1,500 mL/h and ultrafiltration flow at 1,500 mL/h. Oseltamivir carboxylate (OC), the active metabolite of oseltamivir, blood levels were measured on the fourth day of treatment, before and 1, 2, 3, 4, 5, 6, 8, 10 and 12 h after oseltamivir administration. OC was quantified using liquid chromatography tandem mass spectrometry (LC–MS/MS).
Levels of OC in plasma (Fig. 1) and pharmacokinetic parameters suggested drug accumulation in comparison with healthy volunteers [5]. Fivefold higher C max (3,900 ng/mL) and AUC0-12 (39.6 μg h/mL) were observed, mainly as a consequence of lower apparent clearance (25 versus 400 mL/min) which, despite the decreased V d (32 versus 153 L), conditioned an increase in the elimination half-life (15 versus 7 h).
Fig. 1.
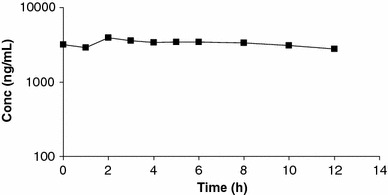
Concentrations of oseltamivir carboxylate (ng/mL) in a patient treated by 150 mg BID oseltamivir, extracorporeal membrane oxygenation and continuous veno-venous haemodiafiltration
RT-PCR for H1N1 virus was negative from day 15. Renal function recovered by day 30, and ECMO was withdrawn on day 39, as the patient’s respiratory function had significantly improved.
This case report shows that OC accumulation can occur even when continuous haemodiafiltration and ECMO are applied. Since half-maximal inhibitory concentrations (IC50s) for oseltamivir are very low, and since in this patient AUC0–12 appeared to be very high when compared with healthy volunteers, standard high dosage of oseltamivir might even be excessive in treatment of patients with acute renal failure simultaneously undergoing continuous haemodiafiltration and ECMO. Nevertheless, further studies are warranted.
References
- 1.Napolitano LM, Park PK, Sihler KC, Papadimos T, Chenoweth C, Cinti S, Zalewski C, Sharangpani R, Somsel P, Wells E, Fry AM, Fiore AE, Villanueva JM, Lindstrom S, Uyeki TM, Center for Disease Control Intensive-care patients with severe novel influenza A (H1N1) virus infection. MMWR Morb Mortal Wkly Rep. 2009;58:749–752. [Google Scholar]
- 2.Firstenberg MS, Blais D, Louis LB, Stevenson KB, Sun B, Mangino JE. Extracorporeal membrane oxygenation for pandemic (H1N1) 2009. Emerg Infect Dis. 2009;15:2059–2060. doi: 10.3201/eid1512.091434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ruiz S, Papy E, Da Silva D, Nataf P, Massias L, Wolff M, Bouadma L. Potential voriconazole and caspofungin sequestration during extracorporeal membrane oxygenation. Intensive Care Med. 2009;35:183–184. doi: 10.1007/s00134-008-1269-3. [DOI] [PubMed] [Google Scholar]
- 4.Robson R, Buttimore A, Lynn K, Brewster M, Ward P. The pharmacokinetics and tolerability of oseltamivir suspension in patients on haemodialysis and continuous ambulatory peritoneal dialysis. Nephrol Dial Transplant. 2006;21:2556–2562. doi: 10.1093/ndt/gfl267. [DOI] [PubMed] [Google Scholar]
- 5.Dutkowski R, Smith JR, Davies BE (2010) Safety and pharmacokinetics of oseltamivir at standard and high dosages. Int J Antimicrob Agents. doi:10.1016/j.ijantimicag.2009.12.023 [DOI] [PubMed]


