Novel human infections continue to appear all over the world, but the risk is higher in some regions than others. Identification of emerging-disease 'hotspots' will help target surveillance work.
The next new disease
Emerging infectious diseases are a major threat to health: AIDS, SARS, drug-resistant bacteria and Ebola virus are among the more recent examples. By identifying emerging disease 'hotspots', the thinking goes, it should be possible to spot health risks at an early stage and prepare containment strategies. An analysis of over 300 examples of disease emerging between 1940 and 2004 suggests that these hotspots can be accurately mapped based on socio-economic, environmental and ecological factors. The data show that the surveillance effort, and much current research spending, is concentrated in developed economies, yet the risk maps point to developing countries as the more likely source of new diseases.
The steady stream of outbreaks of new or unexpected infectious diseases is a much-discussed issue in the field of public health1,2 and has even acquired its own dedicated scientific journal3. But for many years research has generally taken a case-by-case approach to understanding why new infections emerge. Now, Jones et al.4 (page 990 of this issue) have published a systematic, quantitative analysis of recent global patterns of disease emergence. Their work provides insight into the ecology of emerging diseases, and has practical implications, providing pointers for the design of international surveillance programmes.
Jones and her colleagues began by collating data on what they call emerging infectious disease 'events' — that is, outbreaks of human disease associated with a new species or variant of an infectious agent (which could be any type of pathogen, from a virus to a parasitic worm). A painstaking review of the literature going back to 1940 turned up more than 300 such events, most of them involving bacteria (Box 1). (The database is published in full as supplementary information to the paper4 and is itself a valuable resource.) The authors then quantified variation in the frequency of these events decade by decade across the world, and carried out a series of statistical analyses to look for relationships with other variables, ranging from human population growth to rainfall patterns.
Before discussing their results, several issues that bedevil this kind of analysis should be acknowledged: how to define 'emerging'5; choosing the appropriate taxonomic unit of study6; making statistical allowance for groups of closely related pathogens sharing characteristics7; and ascertainment or reporting bias8. There are no definitive solutions to these problems, but Jones et al. fully explain and justify their approach, and are careful not to over-interpret their data.
The frequency of events rose to a peak in the 1980s and has since fallen (despite rising reporting effort). Jones and colleagues suggest that the peak might reflect the onset of the AIDS pandemic, creating a large (and still expanding) population that is highly susceptible to concomitant infections. The raw data also suggest that most events occur at higher latitudes — and particularly in Europe and North America (Box 1). This initially unexpected result is explained partly as an artefact of greater reporting effort, which, in turn, implies significant under-reporting in other parts of the world. Once reporting bias is accounted for, it becomes clear that, in general, most emerging infections are found where there are most people (rather than, as might have been supposed, on the remote fringes of human society).
Beyond these general patterns, there were some variations between different types of infection. Zoonoses (human infections shared with other vertebrates) were the most important category, accounting for 60% of events. This conclusion echoes that of earlier studies on the animal origins of human disease over both ecological and evolutionary timescales9,10. Jones et al. also confirm reports that zoonoses associated with wildlife were particularly important11, but go on to show that the frequency of such events correlated with mammalian species richness (by contrast, no such correlation was found for infections associated with domestic animals). This result is neatly consistent with two previous observations: first, that many emerging pathogens have a broad host range12; and second, that a wide range of other mammals (and some birds) is associated with novel human pathogens13.
Jones et al.4 also stress the importance of drug-resistant infections. These account for more than 20% of events, mostly involving bacteria, and are especially common at higher latitudes — that is, in more developed regions where the use of antimicrobials is presumed to be greatest. Another major category is vector-borne infections. These also account for more than 20% of events and are currently on the increase, possibly linked to climate change, although other explanations cannot be ruled out.
Clearly, we must expect more infectious diseases to emerge in the near future. Jones and colleagues extrapolate their statistical analysis to generate risk maps that correct for current biases in reporting effort. The maps suggest that there are potential 'hotspots' of disease emergence — particularly in central America, tropical Africa and south Asia — that warrant greater surveillance. These findings support calls for international investment in the capacity to detect, identify and monitor infectious diseases, targeted at regions of the world where the need is greatest14. The benefits would not just be felt locally: in an era of increasing globalization, emerging infectious diseases are everybody's problem.
Box 1: Emerging diseases: the pathogens and the places.
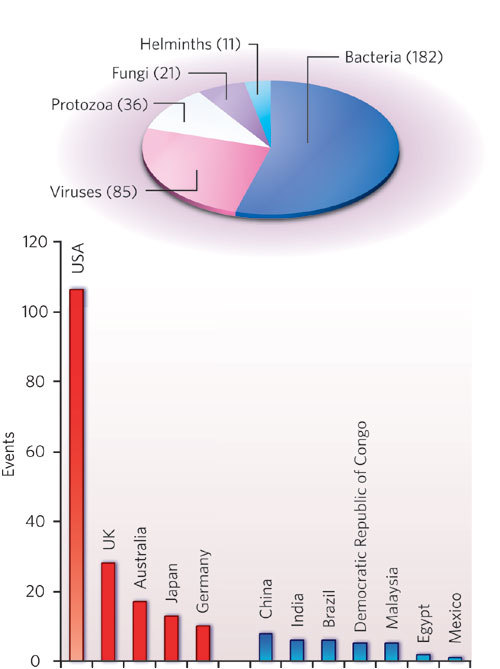
In the work discussed here, Jones et al.4 identified 335 emerging-disease 'events' reported worldwide between 1940 and 2004. The pathogens involved could be novel species or strains, including drug-resistant strains, of known species. Just over half of the events were associated with bacteria, as shown in the pie chart.
An example is Escherichia coli serotype O157:H7, first reported in the 1970s. This strain of the usually benign E. coli group is a food-borne pathogen that can cause fatal renal illness in the young and the elderly. It turned out to be just one type of verocytotoxigenic E. coli (VTEC): other VTECs have since been reported in the United States, the United Kingdom, Japan and other, mostly industrialized, countries.
In general, most reports of emerging-disease events come from developed countries; the bar chart shows the five countries with the most reports in red, with selected others in blue. In the United States alone, there have been more than 100 events reported (almost one-third of the total). Examples are infections with several species of hantavirus (such as Sin Nombre virus), fungal infections in hospital patients (including different species of Candida) and a range of bacterial infections acquired from animal reservoirs (for instance, Bartonella henselae, the cause of cat-scratch disease). Jones et al. suggest that this pattern reflects reporting bias. Often, the United States or another developed country can be merely the site of discovery of pathogens with wider distributions. This implies that there is still significant under-reporting of emerging infectious diseases from other regions of the world.
M.E.J.W.
References
- 1.Institute of Medicine Emerging Infections: Microbial Threats to Health in the United States (National Academies Press, Washington DC, 1992). [PubMed]
- 2.Institute of Medicine & Board on Global Health Microbial Threats to Health: Emergence, Detection, and Response (National Academies Press, Washington DC, 2003). [PubMed]
- 3.Satcher D. Emerg. Infect. Dis. 1995;1:1–6. doi: 10.3201/eid0101.950101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jones KE, et al. Nature. 2008;451:990–993. doi: 10.1038/nature06536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolhouse MEJ, Dye C. Phil. Trans. R. Soc. Lond. B. 2001;356:981–982. doi: 10.1098/rstb.2001.0899. [DOI] [Google Scholar]
- 6.Tibayrenc M. Trends Parasitol. 2006;22:66–70. doi: 10.1016/j.pt.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 7.Harvey PH, Pagel MD. The Comparative Method in Evolutionary Biology. 1991. [Google Scholar]
- 8.Stephens DS, et al. Am. J. Med. Sci. 1998;315:64–75. doi: 10.1097/00000441-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Taylor LH, Latham SM, Woolhouse MEJ. Phil. Trans. R. Soc. Lond. B. 2001;356:983–989. doi: 10.1098/rstb.2001.0888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolfe ND, Dunavan CP, Diamond J. Nature. 2007;447:279–283. doi: 10.1038/nature05775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cleaveland S, Laurenson MK, Taylor LH. Phil. Trans. R. Soc. Lond. B. 2001;356:991–999. doi: 10.1098/rstb.2001.0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Woolhouse MEJ, Gowtage-Sequeria S. Emerg. Infect. Dis. 2005;11:1842–1847. doi: 10.3201/eid1112.050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woolhouse M, Gaunt E. Crit. Rev. Microbiol. 2007;33:231–242. doi: 10.1080/10408410701647560. [DOI] [PubMed] [Google Scholar]
- 14.King DA, Peckham C, Waage JK, Brownlie J, Woolhouse MEJ. Science. 2006;313:1392–1393. doi: 10.1126/science.1129134. [DOI] [PubMed] [Google Scholar]


