Abstract
Purpose
To describe the burden, and characteristics, of influenza-like illness (ILI) associated with non-influenza respiratory viruses (NIRV).
Methods
We performed a prospective, multicenter, observational study of adults admitted with ILI during three influenza seasons (2012–2015). Patients were screened for picornavirus, respiratory syncytial virus (RSV), coronavirus, human metapneumovirus, adenovirus, bocavirus, parainfluenza virus, and influenza, by PCR on nasopharyngeal samples. We excluded patients coinfected with NIRV and influenza.
Results
Among 1421 patients enrolled, influenza virus was detected in 535 (38%), and NIRV in 215 (15%), mostly picornavirus (n = 61), RSV (n = 53), coronavirus 229E (n = 48), and human metapneumovirus (n = 40). In-hospital mortality was 5% (NIRV), 4% (influenza), and 5% (no respiratory virus). As compared to influenza, NIRV were associated with age (median, 73 years vs. 68, P = 0.026), chronic respiratory diseases (53% vs. 45%, P = 0.034), cancer (14% vs. 9%, P = 0.029), and immunosuppressive drugs (21% vs. 14%, P = 0.028), and inversely associated with diabetes (18% vs. 25%, P = 0.038). On multivariable analysis, only chronic respiratory diseases (OR 1.5 [1.1–2.0], P = 0.008), and diabetes (OR 0.5 [0.4–0.8], P = 0.01) were associated with NIRV detection.
Conclusions
NIRV are common in adults admitted with ILI during influenza seasons. Outcomes are similar in patients with NIRV, influenza, or no respiratory virus.
Keywords: Influenza-like illness, Influenza, Picornavirus, Respiratory syncytial virus, Coronavirus, Human metapneumovirus
Introduction
Non-influenza respiratory viruses (NIRV) are responsible for a substantial proportion of influenza-like illness (ILI), and pneumonia [1, 2], although epidemiological data on their burden are scarce. The pathogenicity of viruses recently discovered, such as human metapneumovirus in 2001, or bocavirus in 2005 [3, 4], remains poorly characterized. Even NIRV discovered earlier (e.g. parainfluenza virus, respiratory syncytial virus (RSV), or adenovirus) have attracted limited clinical interest thus far, as a result of the absence of rapid tests routinely available, and the lack of vaccine or antiviral treatment with proven clinical efficacy [1, 2, 5, 6]. The advent of highly sensitive and specific point-of-care tests for simultaneous detection of the main respiratory viruses is an opportunity to investigate the role of NIRV in common acute respiratory febrile illness, including the risk factors for, and the characteristics of, NIRV-associated ILI. These data may have important implications, including (1) screening and early implementation of respiratory isolation for patients with NIRV to prevent nosocomial transmission; (2) identification of the major NIRV that should be targeted for development of antivirals or vaccines. We aimed to determine the characteristics of, and the risk factors for NIRV among adults admitted with ILI during influenza seasons in France.
Methods
Study design and population
We analyzed cases of laboratory-confirmed NIRV infection during three consecutive influenza seasons (2012/2013, 2013/2014, and 2014/2015), in a post hoc analysis of patients hospitalized with community-acquired ILI, and enrolled in the FLUVAC study. FLUVAC is a prospective observational study of influenza vaccine efficacy conducted in six university hospitals in France (Cochin Hospital, Paris; Bichat Hospital, Paris; Pontchaillou Hospital, Rennes; Dupuytren Hospital, Limoges; Montpellier University Hospital; Edouard Herriot Hospital, Lyon). Patients were invited to participate during periods of influenza circulation (from December to March). We collected data on non-institutionalized adults (> 18 years), who were hospitalized for at least 24 h with ILI, with symptoms onset less than 7 days prior to sampling, through an active surveillance system staffed by trained healthcare professionals. ILI was defined as a combination of two criteria: (1) at least one of the following symptoms: fever (≥ 38 °C), headache, myalgia or malaise, and (2) at least one of the following respiratory symptoms: cough, sore throat, or shortness of breath (dyspnea) [7]. The characteristics and outcome of patients with influenza [8, 9] and RSV [10] in this cohort have been previously reported.
Clinical data
We collected data on demographic, chronic underlying diseases, hospital admission during the previous 12 months, smoking status, hospitalization ward, and main characteristics of current ILI, including date of onset, date of admission, length of hospital stay, and outcome (i.e. complications and in-hospital mortality). Data were collected on a standardized questionnaire from medical records and face-to-face interviews with patients.
Virology
Tests for respiratory viruses were performed in nasopharyngeal swabs from all patients by means of multiplex reverse transcription-polymerase chain reaction (mRT-PCR). Bronchoalveolar lavage fluid samples and tracheal aspirates ordered by the physician in charge were also tested. Samples were initially tested in the virology laboratory of each participating hospital by means of real-time influenza A & B PCR. All samples were then sent to the French National Influenza Reference Center (CNR-Lyon) for confirmation. RNA and DNA were extracted with the automated Easymag system from BioMérieux (Marcy l’Etoile, France), and influenza viruses were detected with an in-house real-time RT-PCR protocol [11]. Samples were also screened for a panel of NIRV: adenovirus (52 serotypes), human bocaviruses 1–4, human coronaviruses (229E, NL63, OC43, HKU1), human metapneumoviruses 1–4, parainfluenza viruses 1–4, picornavirus, and RSV, by real-time PCR, using the Respiratory Multiwell System (MWS) r-gene® on an ABI 7300 analyzer.
Ethics
The FLUVAC study (clinicaltrials.gov NCT02027233) respected Good Epidemiological and Clinical Practices in clinical research and the Declaration of Helsinki, and was approved by institutional review board. All study participants provided informed consent for respiratory viruses’ testing and data collection.
Statistical analysis
We first described the characteristics of all patients hospitalized with ILI who tested positive for at least one NIRV. Results were expressed as mean and standard deviation (SD), or median and interquartile range (IQR) for quantitative variables, and n (%) for qualitative variables. Missing data for each variable were excluded from the denominator. Factors associated with NIRV infection were analyzed using two different comparison groups: (1) patients with negative tests for both NIRV and influenza; (2) patients with documented influenza. The Wilcoxon rank sum test or Fisher’s exact test were used, as appropriate, for univariable comparisons. For multivariable analysis, we used a backward stepwise logistic regression model, using NIRV test results (positive/negative) as the dependent variable. Covariables tested in the multivariable model were all variables with a P value < 0.2 in the univariable analysis. Results were expressed as odds ratios (OR) and adjusted OR (aOR) with 95% confidence intervals (CI). A P value of 0.05 or less was considered statistically significant. All analyses were performed using R software (v3, R Foundation for Statistical Computing, Vienna, Austria) [12].
Results
Characteristics of patients with influenza-like illness, and virus distribution
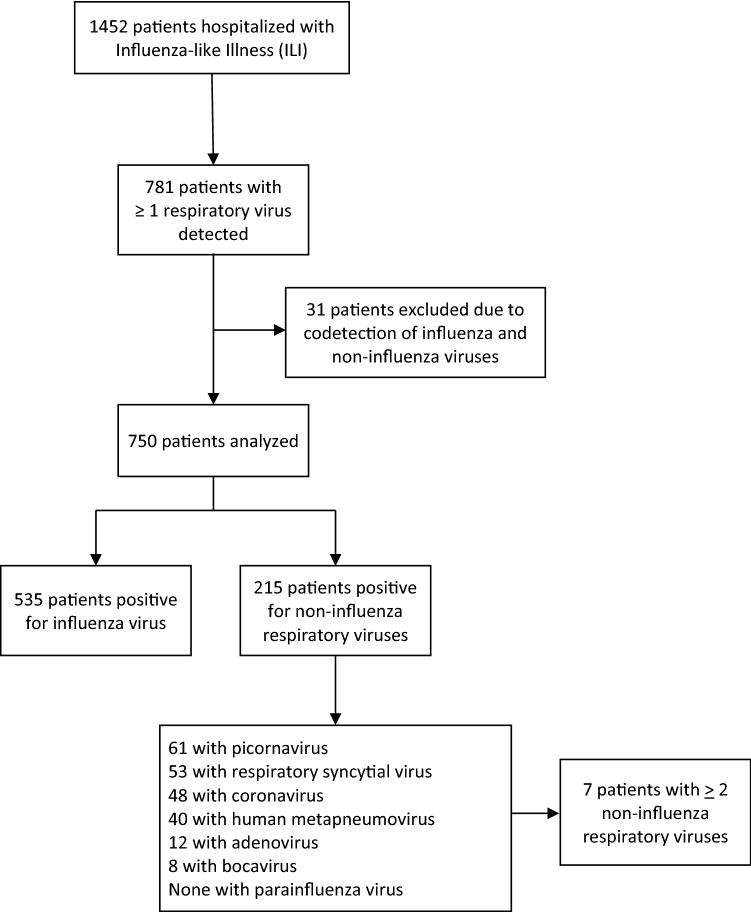
Overall, 1452 patients hospitalized with ILI were enrolled. Median age was 70 years [IQR, 54–82], 780 patients were male (54%), 1155 (80%) had at least one chronic underlying disease (mostly respiratory or heart diseases), 661 (46%) had been hospitalized in the previous 12 months, and 644 (44%) had been vaccinated against influenza. Among the 1452 patients tested, 781 (54%) were positive for at least 1 respiratory virus. We excluded the 31 patients (2% of total), who had simultaneous detection of influenza virus, and NIRV. Among the 1421 remaining patients, influenza virus was detected in 535 (38%), and NIRV in 215 patients (15%), including 7 with 2 NIRV. The NIRV detected were picornavirus, n = 61 (27%), RSV, n = 53 (24%), coronavirus 229E, n = 48 (22%), human metapneumovirus, n = 40 (18%), adenovirus, n = 12 (5%), and bocavirus, n = 8 (4%). No parainfluenza virus was detected. Flowchart is presented in Fig. 1. The proportion of positive tests for influenza and for NIRV remained similar during the first two influenza seasons; a significant increase of influenza cases was noted in 2014–2015 winter (Table 1).
Fig. 1.
Flowchart of patients hospitalized with influenza-like illness, and viruses detected in respiratory sample
Table 1.
Number and percentage of patients hospitalized with influenza-like illness who tested positive for non-influenza respiratory viruses, and for influenza, by year
| Influenza season | Non-influenza respiratory virus (NIRV), n (%) | Influenza, n (%) | Co-infection influenza + NIRV, n (%) | No respiratory virus, n (%) | Total |
|---|---|---|---|---|---|
| 2012/2013 | 64 (14%)ref | 149 (33%)ref | 13 (3%)ref | 222 (50%)ref | 448 |
| 2013/2014 | 76 (19%)NS | 101 (25%)NS | 11 (3%)NS | 219 (54%)NS | 407 |
| 2014/2015 | 75 (13%)NS | 285 (48%)** | 7 (1%)* | 230 (38%)** | 597 |
| Total | 215 (15%) | 535 (37%) | 31 (2%) | 671 (46%) | 1452 |
Chi square test was done to compare the evolution, with 2012/2013 as the reference season
NIRV non-influenza respiratory virus, Ref reference, NS non significant
*P < 0.05, **P < 0.001
Characteristics of patients with non-influenza respiratory viruses (Table 2)
Table 2.
Comparison between patients who tested positive for non-influenza respiratory virus (NIRV), patients with influenza, and patients with no respiratory virus
| NIRV n = 215 (15%) |
Influenza n = 535 (38%) |
P value for comparison between NIRV and influenza | No respiratory virus n = 671 (47%) |
P value for comparison between NIRV and no virus | |
|---|---|---|---|---|---|
| Men | 116 (54%) | 269 (50%) | 0.36 | 380 (57%) | 0.47 |
| Median age, years (IQR) | 73 (60–83) | 68 (53–81) | 0.026 | 70 (54–83) | 0.11 |
| Age ≥ 65 years | 141 (66%) | 310 (58%) | 0.053 | 398 (59%) | 0.10 |
| Median BMI, kg/m2 (IQR) | 24.5 (21–28) | 24.9 (22–28) | 0.25 | 24,8 (21–29) | 0.32 |
| Chronic diseases | 174 (81%) | 423 (79%) | 0.56 | 537 (80%) | 0.77 |
| Chronic respiratory disease | 114 (53%) | 237 (45%) | 0.034 | 296 (44%) | 0.024 |
| Chronic heart disease | 90 (42%) | 222 (42%) | 0.93 | 287 (43%) | 0.81 |
| Cancer | 31 (14%) | 48 (9%) | 0.029 | 84 (13%) | 0.48 |
| Diabetes | 39 (18%) | 135 (25%) | 0.038 | 179 (27%) | 0.011 |
| Immunosuppressive drugs | 44 (21%) | 75 (14%) | 0.028 | 98 (15%) | 0.041 |
| Pregnancy | 4 (27%) | 8 (16%) | 0.45 | 3 (5%) | 0.021 |
| Current smokers | 52 (24%) | 121 (23%) | 0.38 | 138 (21%) | 0.16 |
| Median time from symptom onset to admission, days (IQR) | 3.0 (2–4) | 3.5 (2–4) | 0.46 | 3.0 (2–5) | 0.27 |
| Symptoms | |||||
| Fever (≥ 38 °C) | 169 (79%) | 480 (90%) | < 0.001 | 520 (78%) | 0.78 |
| Weakness/malaise | 55 (26%) | 165 (31%) | 0.14 | 219 (33%) | 0.047 |
| Headache | 47 (22%) | 134 (25%) | 0.33 | 167 (25%) | 0.32 |
| Myalgia | 44 (21%) | 116 (22%) | 0.66 | 153 (23%) | 0.44 |
| Cough | 167 (78%) | 434 (81%) | 0.28 | 506 (76%) | 0.54 |
| Dyspnea | 160 (74%) | 393 (73%) | 0.78 | 517 (77%) | 0.36 |
| Complications | 94 (44%) | 248 (47%) | 0.50 | 266 (40%) | 0.28 |
| Pneumonia | 68 (32%) | 152 (29%) | 0.96 | 160 (24%) | 0.019 |
| Respiratory failure | 47 (22%) | 125 (23%) | 0.69 | 135 (20%) | 0.54 |
| ARDS | 20 (9%) | 55 (10%) | 0.78 | 50 (7%) | 0.38 |
| Heart failure | 30 (14%) | 78 (15%) | 0.85 | 80 (12%) | 0.41 |
| ICU admission | 16 (11%) | 26 (10%) | 1.0 | 39 (9%) | 0.50 |
| Median length of stay, days (IQR) | 8 (5–17) | 10 (4–23) | 0.15 | 8 (3–16) | 0.47 |
| Mortality | 11 (5%) | 23 (4%) | 0.69 | 32 (5%) | 0.85 |
NIRV non-influenza respiratory virus, BMI body mass index, IQR interquartile range, SD standard deviation, ARDS, acute respiratory distress syndrome, ICU intensive care unit
The 215 patients with at least 1 NIRV detected, had a median age of 73 years [60–83], 116 (54%) were male, and 174 (81%) had at least 1 chronic underlying disease, mostly respiratory (n = 114, 53%), or heart diseases (n = 90, 42%). 52 patients (24%) were current smokers, 44 (21%) were taking immunosuppressive drugs, and 4 were pregnant (27% of women less than 50 years old). Mean duration of ILI at the time of admission was 3.0 day [2–4]. Main symptoms were fever (78%), cough (78%), dyspnea (74%), weakness or malaise (26%), headache (22%), and myalgia (21%). 94 patients developed at least 1 complication during hospital stay, including pneumonia, n = 68 (32%), and respiratory failure, n = 47 (22%). Median length of stay was 8 days [5–17]. ICU admission was required for 16 patients (11%). Eleven patients died during hospitalization (5%).
Comparison of patients who tested positive for non-influenza respiratory viruses and (1) patients with influenza; (2) patients with no respiratory virus detected (Table 2)
Patients who tested positive for NIRV were older than patients with influenza (median, 73 years versus 68, P = 0.026), more likely to have chronic respiratory diseases (53% vs 45%, P = 0.034), solid cancer (14% vs 9%, P = 0.029), and to be on immunosuppressive drugs (21% vs 14%, P = 0.028), but less likely to have diabetes (18% vs 25%, P = 0.038). Fever was less common with NIRV than influenza (79% vs 90%, P < 0.001). Outcomes were similar, including complications, median length of stay, and in-hospital mortality. On multivariable analysis, only chronic respiratory disease (OR 1.5 [1.1–2.0], P = 0.008), and diabetes (OR 0.5 [0.4–0.8], P = 0.01), were significantly associated with NIRV.
When compared to patients with no detection of respiratory virus, patients who tested positive for NIRV were more likely to have chronic respiratory diseases (53% vs 44%, P = 0.024), to be pregnant (27% vs 5%, P = 0.021), and on immunosuppressive drugs (21% vs 15%, P = 0.041), but less likely to have diabetes (18% vs 27%, P = 0.011). Weakness was less common in patients with NIRV than in patients who tested negative for all respiratory viruses (26% vs 33%, P = 0.047). Regarding outcome, pneumonia was more common in patients who tested positive for NIRV (32% vs 24%, P = 0.019).
Discussion
In this prospective multicenter study performed during three influenza seasons in France, at least one NIRV was found in 15% of patients admitted with ILI, the major players being picornavirus (27%), RSV (24%), coronavirus 229E (22%), and human metapneumovirus (18%). NIRV were more common in patients with chronic respiratory diseases, and less common in patients with diabetes, whatever the comparison group (patients with influenza, or patients with no respiratory virus), and these associations remained significant in multivariable analysis. Although patients with NIRV were more likely to develop pneumonia than patients with no respiratory virus, mortality was similar in patients with NIRV, in patients with influenza, and in patients with no respiratory virus, at 4–5%.
Few studies have evaluated the epidemiology of NIRV in patients with symptoms suggestive of acute respiratory tract infection. Tanner et al. [13] performed a prospective study during the 2009–2010 winter season in Central England, both in hospitals and in general practitioner offices. The two main NIRV were RSV (31%), and picornavirus (24%), as in our study, but the authors did not test for coronavirus, and bocavirus. Ambrosioni et al. [14] conducted a 2-year prospective study in one referral center in Switzerland, and their findings were in line with ours, with two main differences: (1) coronaviruses were more heterogeneous, and included 229E, NL63, OC43, and HKU1 strains; (2) human metapneumovirus was rare, at around 5%, with no significant difference between upper and lower respiratory tract samples, and between adults younger than 65 years, and elderlies.
Previous studies have identified age > 65 years as a risk factor for NIRV, as well as chronic respiratory diseases [1, 6, 14, 15]. For the latter, experimental studies suggested that NIRV may trigger acute exacerbation of chronic obstructive pulmonary diseases (COPD) [16–18]. Interestingly, chronic respiratory diseases remained associated with NIRV in our study, even when the comparison group was patients with influenza, and even in multivariable analysis. This suggests a specific pathogenicity of NIRV in this population, that may be more prone to decompensate during NIRV infection than during influenza. Treatment with immunosuppressive drugs was also associated with NIRV in our study, whatever the comparison group, but the association was no longer significant on multivariable analysis, which suggests that confounding factors are involved. The lower prevalence of diabetes mellitus in patients with NIRV, as compared to patients with influenza, and to patients with no respiratory virus, in our study, was not expected. Diabetes has been associated with a broad range of infectious diseases [19, 20], including upper respiratory tract infection in some studies [19], but not all [21]. Although the lower prevalence of diabetes in patients with NIRV as compared to patients with influenza could be related to stronger association between diabetes and influenza than between diabetes and NIRV, we have no explanation for the lower prevalence of diabetes in patients with NIRV as compared to patients with no respiratory virus.
Our study has limitations. First, we have no data to support causality between the presence of NIRV in upper respiratory samples and the ILI that required admission. Other pathogens may be involved (e.g. bacteria), and NIRV may merely be a bystander infectious agent with no role in the symptoms reported. The lack of a control population with no respiratory symptoms is another limitation that precludes any conclusion on the pathogenicity of NIRV. Second, we only performed systematic tests for influenza, and a selection of NIRVs, although other pathogens have been associated with acute respiratory infections [22]. Third, our study was limited to influenza seasons, in one country, and its findings may not apply to other settings. However, our study has strengths, including its prospective, multicenter design, and the standardization of viral tests for all adult patients admitted with predefined ILI, during three consecutive influenza seasons. These strengths, and the limited number of missing data, reduce the risk of potential biases. Our study adds another brick in the wall by contributing to better characterization of the burden of NIRV in adult patients with suspicion of acute respiratory tract infections. The identification of transmissible, NIRV, in 15% of adult patients admitted with ILI would advocate for more systematic testing of these patients, especially those with chronic respiratory diseases. This would allow early respiratory isolation, as to prevent nosocomial transmission of NIRV, which may have severe consequences on patients with chronic respiratory diseases and patients on immunosuppressive drugs.
Acknowledgements
The current work received no funding. However, the study sites received funding from Sanofi Pasteur and Sanofi Pasteur MSD for the FLUVAC study. Vaccine producers had no role in the study design, data analysis, decision to publish or preparation of the manuscript. The FLUVAC Study group. Hôpital Cochin, Paris: P. Loulergue, S. Momcilovic, JP Mira, N. Marin, A. Regent, Kanaan, F. Dumas, B. Doumenc. Hôpital Bichat Claude Bernard, Paris: J.F. Alexandra, H. Becheur, K. Belghalem, J. Bernard, A. Bleitreu, M. Boisseau, R. Bories, O. Brugiere, F. Brunet, C. Burdet, E. Casalino, M. Caseris, Chansiaux, M. Chauchard, P. Chavance, C. Choquet, Cloppet-Fontaine, L. Colosi, B. Couset, B. Crestani, F. Crocket, A. Debit, Delanoe, V. Descamps, P. Dieude, A. Dossier, N. Douron, E. Dupeyrat, N. Emeyrat, Fernet, T. Goulenok, S. Harent, Jouenne, A. Justet, M. Lachatre, A. Leleu, I. Lerat, M. Lilamand, H. Mal, A. Marceau, A-C Metivier, K. Oplelatora, T. Papo, A-L. Pelletier, L. Pereira, P. Pradere, Prommier, P. Ralainnazava, M. Ranaivoision, A. Raynaud-Simon, C. Rioux, K. Sacre, V. Verry, V. Vuong, Y. Yazdapanah. CHU de Montpellier: P. Géraud, V. Driss, V. Maugueret, M. Ray, F. Letois, T. Mura, C. Merle, A. Bourdin, A. Konaté, X. Capdevilla, G. Du Cailar, A. Terminet, H. Blain, M.-S. Leglise, A. Le Quellec, P. Corne, L. Landreau, K. Klouche, A. Bourgeois, M. Sebbane, G. Mourad, H. Leray, M. Maarouf. CHU Dupuytren, Limoges: D. Postil, S. Alcolea, E. Couve Deacon, S. Rogez. Hôpital Edouard Herriot, Lyon: L. Argaud, K. Tazarourte, R. Hernu, M. Cour, M. Simon, T. Baudry, L. Jacquin. CHU Pontchaillou, Rennes: F. Lainé, B. Laviolle, J.-S. Allain, N. Belhomme, V. Thibault, S. Rochas, S. Cochennec, E. Ouamara-Digue, C. Lepape, M. Revest, S. Simon, J. Fouchard, C. Gautier, N. Nouredine, E. Thébault.
Compliance with ethical standards
Conflict of interest
The authors declare no competing interest related to the study. O Launay is an investigator for clinical trials sponsored by Janssen and other companies and received travel support to attend scientific meetings from pharmaceutical companies.
Footnotes
Florence Galtier and Charlotte Pronier equally contributed to this work.
Contributor Information
Pierre Tattevin, Email: pierre.tattevin@chu-rennes.fr.
The FLUVAC Study Group:
P. Loulergue, S. Momcilovic, J. P. Mira, N. Marin, A. Regent, Kanaan, F. Dumas, B. Doumenc, J. F. Alexandra, H. Becheur, K. Belghalem, J. Bernard, A. Bleitreu, M. Boisseau, R. Bories, O. Brugiere, F. Brunet, C. Burdet, E. Casalino, M. Caseris, Chansiaux, M. Chauchard, P. Chavance, C. Choquet, Cloppet-Fontaine, L. Colosi, B. Couset, B. Crestani, F. Crocket, A. Debit, Delanoe, V. Descamps, P. Dieude, A. Dossier, N. Douron, E. Dupeyrat, N. Emeyrat, Fernet, T. Goulenok, S. Harent, Jouenne, A. Justet, M. Lachatre, A. Leleu, I. Lerat, M. Lilamand, H. Mal, A. Marceau, A.-C. Metivier, K. Oplelatora, T. Papo, A.-L. Pelletier, L. Pereira, P. Pradere, Prommier, P. Ralainnazava, M. Ranaivoision, A. Raynaud-Simon, C. Rioux, K. Sacre, V. Verry, V. Vuong, Y. Yazdapanah, P. Géraud, V. Driss, V. Maugueret, M. Ray, F. Letois, T. Mura, C. Merle, A. Bourdin, A. Konaté, X. Capdevilla, G. Du Cailar, A. Terminet, H. Blain, M.-S. Leglise, A. Le Quellec, P. Corne, L. Landreau, K. Klouche, A. Bourgeois, M. Sebbane, G. Mourad, H. Leray, M. Maarouf, D. Postil, S. Alcolea, E. Couve Deacon, S. Rogez, L. Argaud, K. Tazarourte, R. Hernu, M. Cour, M. Simon, T. Baudry, L. Jacquin, F. Lainé, B. Laviolle, J.-S. Allain, N. Belhomme, V. Thibault, S. Rochas, S. Cochennec, E. Ouamara-Digue, C. Lepape, M. Revest, S. Simon, J. Fouchard, C. Gautier, N. Nouredine, and E. Thébault
References
- 1.Ruuskanen O, Lahti E, Jennings LC, Murdoch DR. Viral pneumonia. Lancet Lond Engl. 2011;377:1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luchsinger V, Ruiz M, Zunino E, Martínez MA, Machado C, Piedra PA, et al. Community-acquired pneumonia in Chile: the clinical relevance in the detection of viruses and atypical bacteria. Thorax. 2013;68:1000–1006. doi: 10.1136/thoraxjnl-2013-203551. [DOI] [PubMed] [Google Scholar]
- 3.van den Hoogen BG, de Jong JC, Groen J, Kuiken T, de Groot R, Fouchier RAM, et al. A newly discovered human pneumovirus isolated from young children with respiratory tract disease. Nat Med. 2001;7:719–724. doi: 10.1038/89098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allander T, Tammi MT, Eriksson M, Bjerkner A, Tiveljung-Lindell A, Andersson B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc Natl Acad Sci USA. 2005;102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pavia AT. What is the role of respiratory viruses in community-acquired pneumonia? What is the best therapy for influenza and other viral causes of community-acquired pneumonia? Infect Dis Clin North Am. 2013;27:157–175. doi: 10.1016/j.idc.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galván JM, Rajas O, Aspa J. Review of non-bacterial infections in respiratory medicine: viral pneumonia. Arch Bronconeumol Engl Ed. 2015;51:590–597. doi: 10.1016/j.arbr.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Centre for Disease Prevention and Control. Influenza case definitions. 2019. https://ecdc.europa.eu/en/healthtopics/influenza/surveillance/Pages/influenza_case_definitions.aspx. Accessed 9 Jan 2020.
- 8.Rondy M, Puig-Barbera J, Launay O, Duval X, Castilla J, Guevara M, et al. 2011–12 seasonal influenza vaccines effectiveness against confirmed A(H3N2) influenza hospitalisation: pooled analysis from a European network of hospitals. A pilot study. PLoS One. 2013;8:e59681. doi: 10.1371/journal.pone.0059681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loubet P, Samih-Lenzi N, Galtier F, Vanhems P, Loulergue P, Duval X, et al. Factors associated with poor outcomes among adults hospitalized for influenza in France: a three-year prospective multicenter study. J Clin Virol. 2016;79:68–73. doi: 10.1016/j.jcv.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Loubet P, Lenzi N, Valette M, Foulongne V, Krivine A, Houhou N, et al. Clinical characteristics and outcome of respiratory syncytial virus infection among adults hospitalized with influenza-like illness in France. Clin Microbiol Infect. 2017;23:253–259. doi: 10.1016/j.cmi.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bouscambert Duchamp M, Casalegno JS, Gillet Y, Frobert E, Bernard E, Escuret V, et al. Pandemic A(H1N1)2009 influenza virus detection by real time RT-PCR: is viral quantification useful? Clin Microbiol Infect. 2010;16:317–321. doi: 10.1111/j.1469-0691.2010.03169.x. [DOI] [PubMed] [Google Scholar]
- 12.R: The R Project for Statistical Computing. 2019. https://www.r-project.org/.
- 13.Tanner H, Boxall E, Osman H. Respiratory viral infections during the 2009–2010 winter season in Central England, UK: incidence and patterns of multiple virus co-infections. Eur J Clin Microbiol Infect Dis Off Publ Eur Soc Clin Microbiol. 2012;31:3001–3006. doi: 10.1007/s10096-012-1653-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ambrosioni J, Bridevaux P-O, Wagner G, Mamin A, Kaiser L. Epidemiology of viral respiratory infections in a tertiary care centre in the era of molecular diagnosis, Geneva, Switzerland, 2011–2012. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2014;20:O578–584. doi: 10.1111/1469-0691.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 16.Zwaans WAR, Mallia P, van Winden MEC, Rohde GGU. The relevance of respiratory viral infections in the exacerbations of chronic obstructive pulmonary disease—a systematic review. J Clin Virol Off Publ Pan Am Soc Clin Virol 2014;61:181–8. 10.1016/j.jcv.2014.06.025 [DOI] [PMC free article] [PubMed]
- 17.Gunawardana N, Finney L, Johnston SL, Mallia P. Experimental rhinovirus infection in COPD: implications for antiviral therapies. Antiviral Res. 2014;102:95–105. doi: 10.1016/j.antiviral.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mallia P, Message SD, Gielen V, Contoli M, Gray K, Kebadze T, et al. Experimental rhinovirus infection as a human model of chronic obstructive pulmonary disease exacerbation. Am J Respir Crit Care Med. 2011;183:734–742. doi: 10.1164/rccm.201006-0833OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26:510–513. doi: 10.2337/diacare.26.2.510. [DOI] [PubMed] [Google Scholar]
- 20.Pearson-Stuttard J, Blundell S, Harris T, Cook DG, Critchley J. Diabetes and infection: assessing the association with glycaemic control in population-based studies. Lancet Diabetes Endocrinol. 2016;4:148–158. doi: 10.1016/S2213-8587(15)00379-4. [DOI] [PubMed] [Google Scholar]
- 21.Muller LMAJ, Gorter KJ, Hak E, Goudzwaard WL, Schellevis FG, Hoepelman AIM, et al. Increased risk of common infections in patients with type 1 and type 2 diabetes mellitus. Clin Infect Dis Off Publ Infect Dis Soc Am 2005;41:281–8. 10.1086/431587 [DOI] [PubMed]
- 22.Jain S, Self WH, Wunderink RG, Fakhran S, Balk R, Bramley AM, et al. Community-Acquired Pneumonia Requiring Hospitalization among US Adults. N Engl J Med 2015;373:415–27. 10.1056/NEJMoa1500245 [DOI] [PMC free article] [PubMed]