Abstract
The coronavirus disease (COVID-19) worldwide outbreak has led to a dramatic challenge for all healthcare systems, including inflammatory bowel disease (IBD) centres. Here, we describe the fast changes and clinical issues that IBD specialists could face during this SARS-CoV-2 infection pandemic, highlighting the potential rearrangements of care and resetting of clinical priorities.
Subject terms: Inflammatory bowel disease, Infection
Since December 2019 when the 2019 novel coronavirus (2019-nCoV as it was then termed, now named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by the WHO) outbreak had been described in Wuhan, Hubei, China, the situation has dramatically evolved1. The pandemic, as declared by the WHO, has led to >300,000 cases worldwide reported as of March 2020, in all continents, excluding Antarctica, spreading on a logarithmic scale in Europe. Italy is currently the second most affected country after China and, as of 13 March 2020, Europe was declared the centre of the pandemic2. Because of the very high transmission capacity, the WHO declared the outbreak of coronavirus disease (COVID-19) caused by SARS-CoV-2 infection a public health emergency of international concern.
As the need for hospitalization is very high among symptomatic cases (~10%), with an increased need to have access to intensive care units and mortality in the order of 3% globally2,3, European hospitals have started to intensively reduce elective activities, including surgery, to prepare for the high numbers of admissions. In addition, action by governments to contain the outbreak and slow the spread of COVID-19 has restricted regions and nations (the entire country of Italy, for example) by reducing their mobility within countries and across borders.
Patients with IBD
But what are the implications of COVID-19 for patients with inflammatory bowel disease (IBD)? With >5,000 patients with Crohn’s disease and ulcerative colitis, our IBD center in Milan, Italy, has been flooded by requests from the patients themselves inquiring about the risk of infection in patients with IBD, and asking what precautions to take, particularly regarding, but not limited to, their immunosuppressive treatment. The COVID-19 outbreak is a fast and evolving situation, and information on the incidence and/or risk of infection in patients with IBD is not yet available. However, it is important to counsel patients to inform them that >80% of reported cases of COVID-19 have been mild in published studies, and the proportion of fatal cases might be an overestimate as many asymptomatic cases are not identified3.
In our opinion, the best advice for patients with IBD is to try to minimize the risk of infection by following good hand hygiene (frequent washing with soap and water), covering the mouth and nose with a tissue or your sleeve (not hands) when coughing or sneezing, avoiding close contact with anyone with influenza-like and/or upper respiratory symptoms, and staying home or isolated if possible. In addition to these measures, emerging reports state that patients might have viral RNA present in their faeces and live virus has been isolated from faecal samples4–6. Thus, caution should be taken when using public toilets given the implications for the potential route of faecal–oral transmission.
An increasing number of patients with IBD treated with immunomodulators or biologic agents in our centre have asked whether a pause in their immunosuppressive therapy would be justified during the COVID-19 outbreak. At the moment, there are no formal evidence-based recommendations from clinical societies or governments for patients on immunosuppression, such as those with IBD. However, a study in a tertiary care population of 2,600 patients with IBD followed for >15,000 patient-years described an exhaustive characterization and validation (hospitalization reports) of all serious viral infections (for example, all that required hospitalization) including varicella zoster virus (VZV), herpes simplex virus (HSV), cytomegalovirus (CMV) and Epstein–Barr virus (EBV) but not SARS-CoV-2 (ref.7). The follow-up included >3,800 patient-years of exposure to anti-TNF agents, >4,800 patient-years of exposure to immunosuppressants, and >1,200 patient-years of follow-up in those >65 years. The researchers identified 31 cases of serious viral infections related to EBV, CMV, VZV and HSV infection. The two independent drivers of the risk were clinically active IBD and exposure to thiopurines. No cases of severe seasonal flu and no deaths by seasonal flu were observed. Although many of our patients have been vaccinated against seasonal flu, as recommended by European Crohn’s and Colitis Organisation (ECCO) guidelines, vaccination strategy adoption is very low and the protective effect of the vaccine is moderate, particularly in patients with an immunosuppression status. Thus, at the moment, it does not seem appropriate to recommend the pause of immunosuppressive treatment in patients with IBD, as stated by International Organization of IBD8 (Fig. 1), Crohn’s & Colitis UK and Crohn’s & Colitis Foundation in their guidance for patients. Moreover, although thiopurines have been associated with risk of serious viral infection in IBD7, the IOIBD recommends they continue to be taken as these agents take months to leave the body and so stopping these medications will not help in the short term. In addition, a survey from the European Federation of Crohn’s and Colitis association and ECCO is ongoing to explore the need for education about COVID-19.
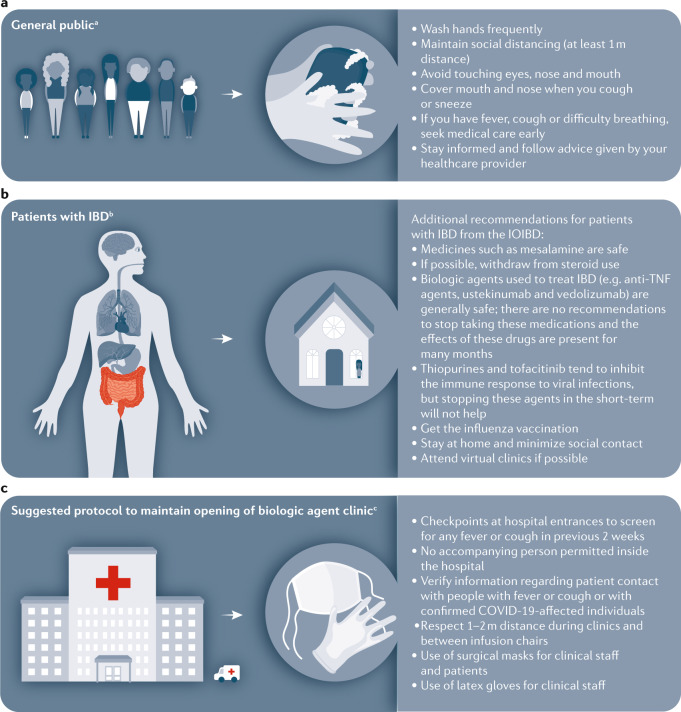
Fig. 1. Approaches to minimize spread of infectious disease for patients with IBD during COVID-19.
a | The WHO recommends basic protective measures against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for the general public, including washing hands frequently and maintaining social distancing. b | The International Organization for the study of Inflammatory Bowel Disease (IOIBD) have made several recommendations for patients with inflammatory bowel disease (IBD) related to coronavirus disease (COVID-19), including the continuation of immunosuppressants. c | Strategies to enable maintenance of our biologic agent clinic during the COVID-19 outbreak in Italy include checkpoints at hospital entrances for symptom screening and use of surgical masks for clinical staff and patients. aBasic protective measures for the public according to the WHO. bRecommendations by IOIBD correct as of 16 Mar 2020. cExperience at the IBD Center, Humanitas University, Milan, Italy.
Changing priorities
As priorities and resources increasingly shift towards the COVID-19 pandemic, will it be possible to maintain the high standard and quality of care for patients with IBD? Multiple layers of complexity are faced during this challenging scenario. The interruption of any elective or routine follow-up clinic has caused anxiety among patients and clinicians. However, patients should be reminded that this interruption is temporary, and a shift towards virtual clinics can help patients and healthcare providers to avoid any potential loss of follow-up in case of clinical issues. This approach has also been initiated by IBD specialists in China during the COVID-19 outbreak9.
The running of biologic agent clinics and drug administration during travel restrictions in tandem with recommendations by health authorities to stay at home have been of major concern. So far, IBD biologic agent clinics have been maintained in our centre following rigorous actions to avoid infection outbreaks (Fig. 1), such as: checkpoints at the hospital entrance to screen and ask if there has been any cough or fever in the previous 2 weeks; verification of patient contact information with people with similar symptoms such as cough and fever; respecting 1–2 m distance between chairs where patients sit for their infusions and during clinics; use of surgical masks for clinical staff and patients, and latex gloves for clinical staff.
A topic of discussion is whether wearing surgical masks is a requirement, which is still a matter of debate. The WHO does not recommend wearing masks in the community as evidence is lacking10. However, facing such a novel situation with limited options, the use of masks could lead to benefit. Indeed, for influenza it has long been recommended that affected patients should wear masks to limit droplet spread and, therefore, the very same action could be used for immunosuppressed patients to reduce the risk of spread of infection. We have chosen to use surgical masks for both the clinical staff and the patients, but a global shortage of disposable surgical masks is creating challenges and this issue continues.
As timely surgery is the other mainstay of IBD care, it is of deep concern that stopping scheduled surgery completely for several weeks for patients with IBD (allowing only oncological cases to undergo surgery) will soon result in increased numbers of emergency presentations and more complications from treatment delay. In the region of Milan, Italy, where elective surgery for every benign indication (including IBD) has been substantially slowed or stopped over the past 3 weeks, with the prospect of further weeks of delays or cancellations, notable concerns have been raised for possible disease progression and poor outcomes of IBD surgery once performed.
Conclusions
The COVID-19 pandemic will continue to spread worldwide with increasing burden on our health-care systems and care of all patients with disease, not just those affected by COVID-19. To face these challenging circumstances, as clinicians we need to support political decision-making to rapidly adapt priorities, to reset temporarily the standards of quality of care and to help to communicate relevant information to patients.
Competing interests
S.D. has served as a speaker, a consultant and an advisory board member for Abbvie, Ferring, Hospira, Johnson & Johnson, Merck, Millennium Takeda, Mundipharma, Pfizer, Tigenix, UCB Pharma and Vifor. A.S. has served as a Consultant or speaker for Ethicon, Frankenman, Oasis, Pfizer, Takeda and Sofar. M.C. has served as a consultant or speaker for Cheetah Medical, Directed Systems and Edwards Lifesciences.
Footnotes
Related links
Crohn’s & Colitis Foundation: https://www.crohnscolitisfoundation.org/what-ibd-patients-should-know-about-2019-novel-coronavirus-covid-19
Crohn’s & Colitis UK: https://www.crohnsandcolitis.org.uk/news/updated-wuhan-novel-coronavirus-advice
European Crohn’s Colitis Organization: https://www.ecco-ibd.eu/
International Organization for the study of Inflammatory Bowel Disease: https://www.ioibd.org/
World Health Organization: https://www.who.int/
Change history
5/6/2020
A Correction to this paper has been published: 10.1038/s41575-020-0311-y
References
- 1.Wang D, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johns Hopkins University & Medicine. Coronavirus COVID-19 global cases. Coronavirus resource centerhttps://coronavirus.jhu.edu/map.html (2020).
- 4.Ling, Y. et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin. Med. J. (Engl.)10.1097/CM9.0000000000000774 (2020). [DOI] [PMC free article] [PubMed]
- 5.Xu Y, et al. Characteristics of pediatric SARS-CoV-2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020 doi: 10.1038/s41591-020-0817-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang W, et al. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020 doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wisniewski A, et al. Increased incidence of systemic serious viral infections in patients with inflammatory bowel disease associates with active disease and use of thiopurines. United Eur. Gastroenterol. J. 2020 doi: 10.1177/2050640619889763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.IOIBD. IOIBD Update on COVID19 for Patients with Crohn’s Disease and Ulcerative Colitis. IOIBDhttps://www.ioibd.org/ioibd-update-on-covid19-for-patients-with-crohns-disease-and-ulcerative-colitis/ (2020).
- 9.Mao R, et al. Implications of COVID-19 for patients with pre-existing digestive diseases. Lancet Gastroenterol Hepatol. 2020 doi: 10.1016/S2468-1253(20)30076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.WHO. Advice on the use of masks in the community, during home care and in health care settings in the context of the novel coronavirus (2019-nCoV) outbreak: interim guidance. WHOhttps://apps.who.int/iris/handle/10665/330987 (2020).