Abstract
Active transport of sodium across the alveolar epithelium, undertaken in part by the Na,K-adenosine triphosphatase (Na,K-ATPase), is critical for clearance of pulmonary edema fluid and thus the outcome of patients with acute lung injury. Acute lung injury results in disruption of the alveolar epithelial barrier and leads to impaired clearance of edema fluid and altered Na,K-ATPase function. There has been significant progress in the understanding of mechanisms regulating alveolar edema clearance and signaling pathways modulating Na,K-ATPase function during lung injury. The accompanying review by Morty et al. focuses on intact organ and animal models as well as clinical studies assessing alveolar fluid reabsorption in alveolar epithelial injury. Elucidation of the mechanisms underlying regulation of active Na+ transport, as well as the pathways by which the Na,K-ATPase regulates epithelial barrier function and edema clearance, are of significance to identify interventional targets to improve outcomes of patients with acute lung injury.
Keywords: Edema clearance, Hypoxia, Coagulation, Catecholamines, Dopamine, Protein trafficking
Alveolar epithelial barrier function in acute lung injury
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are associated with disruption of the alveolo-capillary barrier, which results in edema fluid accumulation and impaired gas exchange [1]. Injury of the alveolo-capillary barrier alters active Na+ transport, leading to impaired edema fluid clearance from the alveolar spaces. Failure to return to normal clearance is associated with poor prognosis [2, 3].
The monolayer of the alveolar epithelium consists of squamous type I cells (ATI) and cuboidal type II cells (ATII). While the number of ATI and ATII cells in the alveolar epithelium is equal, ATI cells represent ∼95% of the alveolar surface area [4]. Under physiological conditions, the alveolo-capillary barrier is highly impermeable, primarily due to the tight epithelial barrier, while the capillary endothelium is more leaky [5]. Interestingly, there appears to be a cross-talk between these monolayers. In an elegant series of experiments Kuebler and coworkers have demonstrated that inflammatory stimuli to the alveoli initiate rapid alveolo-capillary signaling leading to propagation of the inflammation [6]. Thus the alveolar epithelium might play a central role in modulating endothelial cell biology.
The apical surface of the alveolar epithelium is lined by a thin layer of fluid, which facilitates the maintenance of surface tension, host defense properties, and gas exchange [7]. During lung injury, pulmonary edema accumulates, leading to a life-threatening impairment of gas exchange. Thus, clearing the excess liquid from the alveolar space is of critical importance.
Active Na+ transport is the primary force of alveolar fluid reabsorption
The primary force driving fluid reabsorption from the alveolar space into the interstitium and the pulmonary circulation is active Na+ transport. Sodium is taken up on the apical surface of the alveolar epithelium by amiloride-sensitive and -insensitive Na+ channels [8, 9] and is subsequently “pumped” out of the cell by the Na,K-adenosine triphosphatase (Na,K-ATPase) on the basolateral side [10, 11] (Fig. 1). This process generates an osmotic gradient, which drives passive movement of water from the apical side of the epithelium (the alveolar space) to the basolateral side (the interstitium) paracellularly and through aquaporin-5 water channels which are located in ATI cells [12]. Extensive research on human lungs and in animal models has demonstrated that alveolar fluid clearance is inhibited by amiloride, an inhibitor of epithelial Na+ channels (ENaC), as well as by the Na,K-ATPase inhibitor, ouabain [12]. Moreover, in ENaCα(-/-) mice Na+ transport was found to be defective, resulting in fatal respiratory distress of the neonates [13]. Furthermore, it has been well established that up-regulation of ENaC and Na,K-ATPase increases active Na+ transport, leading to increased ability of the lungs to clear edema [14, 15].
Fig. 1.

Schematic representation of the alveolo-capillary barrier and the channels and transporters involved in ion and fluid transport through the alveolar type I (ATI) and alveolar type II (ATII) cells
Na,K-ATPase
While Na+ uptake is driven by several Na+ transport mechanisms (apically located amiloride-sensitive and -insensitive sodium channels and cotransporters of Na+) in the alveolar epithelium (and notably in many other epithelia as well), the only transporter by which alveolar epithelial cells actively extrude sodium out of the cell is the Na,K-ATPase [4].
The Na,K-ATPase is a transmembrane protein expressed on the basolateral surface of most mammalian epithelial cells. In an ATP-consuming process, it maintains gradients of Na+and K+across the plasma membrane by pumping three sodium ions out of the cell in exchange for two ions of potassium into the cell, accounting for up to 40% of a cell's energy expenditure [10]. The Na,K-ATPase is a heterodimeric holoenzyme composed of an α- and a β-subunit. The catalytic α-subunit is a large polypeptide comprising approximately 1,000 amino acid residues, spanning the plasma membrane 10 times. This subunit contains sites for cleavage of high-energy phosphate bonds, binding of Na+, K+ and nucleotides, and binding of the classic Na,K-ATPase inhibitor, ouabain [16]. Four α-isoforms have been identified (α1 – α4), each with a unique tissue distribution [17, 18]. The α1 and α2 Na,K-ATPase subunits are expressed in the alveolar epithelium [19–21]. The glycosylated β-subunit, a smaller polypeptide comprising approximately 370 amino acid residues, has a single membrane-spanning domain and regulates the membrane insertion and activity of the enzyme [16, 22]. Four β-isoforms have been reported (β1 – β4), which are also expressed in a tissue- and cell-specific manner [23]. Recently a small, transmembrane protein of the FXYD family (characterized by the short invariant sequence of phenylalanine-X-tyrosine-aspartate) has been identified as a γ-subunit, which associates with the Na,K-ATPase in some tissues [24]. Though it may have an important role in regulating the affinity of the sodium pump for ATP, recent evidence suggests that this γ-subunit is not an integral part of the holoenzyme complex [25].
Role of Na,K-ATPase in epithelial barrier integrity
The Na,K-ATPase is known for its function in maintaining a transepithelial osmotic gradient, secondary to the establishment of vectorial sodium transport, and its role in the clearance of lung edema fluid [11]. However, it is less widely recognized for its critical role in epithelial barrier function. In fact, Na,K-ATPase expression and function is intimately coupled to that of junctional complexes, including tight junctions [26]. Through the action of tight junctions, alveolar epithelial cells form a barrier, restricting the passage of lipid-insoluble molecules from the interstitial to the alveolar space (and vice versa). Disruption of this barrier results in altered lung permeability, accumulation of pulmonary edema, and impaired gas exchange [27]. Therefore, through its role in barrier integrity, the Na,K-ATPase is crucial for the maintenance of a selectively permeable epithelium and for the prevention of lung edema formation.
Structure and function of tight junctions
The relative impermeability of alveolar epithelia to paracellular solute diffusion depends upon tight junctions. These tight junctions have a dual role in regulating the passage of molecules between alveolar and interstitial spaces, and in separating the apical and basolateral surfaces of the epithelium, allowing the cells to polarize [26–28].
Tight junctions form intimate intercellular contacts, which are best viewed by electron microscopy [29]. These tight junctions are comprised of three integral membrane protein types: occludins, claudins, and junctional adhesion molecules [30, 31]. These protein complexes associate with cytoplasmic plaque proteins, zona occludens (ZO-1), which in turn bind cytoplasmic signaling molecules and the actin cytoskeleton [31, 32]. Effective tight junction assembly allows for the polarized phenotype exhibited by normal epithelial cells.
Regulation of tight junction integrity by Na,K-ATPase
Rajasekaran and others demonstrated an important role for the Na,K-ATPase β1-subunit in the establishment of functional tight junctions and epithelial polarity [26]. Maloney sarcoma virus-transformed Madin–Darby canine kidney (MDCK) cells, which express low levels of E-cadherin (a transmembrane protein central to adherens junctions, thus representing the main mediator of intercellular adhesion) and Na,K-ATPase β1-subunit, were used in their experiments. These cells do not form effective tight junctions, and therefore have lost the polarized phenotype. However, reconstitution of both E-cadherin and β1-subunit induced the formation of tight junctions as well as epithelial polarization. In contrast, overexpression of E-cadherin alone failed to do so, though it did restore the presence of tight junction proteins in the plasma membrane [26]. This finding implicated the Na,K-ATPase in barrier function, separate from its role in vectorial sodium transport.
Effects of Na,K-ATPase on tight junction formation were shown to correlate with the absolute concentration of Na+ in the cell, rather than with the Na+ gradient across the plasma membrane. Removal of extracellular Na+, though it did disturb the transmembrane gradient, did not prevent formation of tight junctions or the establishment of polarity [33]. Therefore, aspects of cell function which depend on a transmembrane Na+gradient, such as cytoplasmic pH or calcium concentration, are not likely to be involved in Na,K-ATPase regulation of tight junctions. Alternative signaling mechanisms as a modulator of barrier function have been suggested, including normal intracellular Na+ homeostasis, a decrease in cell K+ concentration, depolarization of the plasma membrane potential, and small cell volume changes [32]. Recently, signaling pathways involved in this tight junction regulation have been described. For example, a role for RhoA GTPase, a Ras-related small GTP-binding protein, in the formation of functional tight junctions [29], where overexpression of RhoA GTPase partially interrupted the negative effect of Na,K-ATPase inhibition on tight junction integrity. Conversely, endogenous RhoA activity was found to be significantly decreased in Na,K-ATPase-inhibited cells, suggesting that RhoA GTPase acts downstream of Na,K-ATPase to regulate tight junction formation [33].
A role for RhoA in regulating the assembly of filamentous actin in intestinal epithelial cells, which subsequently influences the integrity of tight junctions, has also been implicated [29]. Transient formation of these bundled stress fibers in MDCK cells correlates with tight junction assembly. Inhibition of Na,K-ATPase activity with ouabain, K+ depletion, or gramicidin prevented the formation of bundled stress fibers [33]. Taken together, these studies suggest that Na,K-ATPase interacts with RhoA GTPase to affect actin polymerization (i.e. the formation of stress fibers), which in turn influences tight junction formation.
Molecular mechanisms of impaired alveolar edema clearance in ALI
Barrier integrity of the alveolar epithelium, created in large part by tight junctions, is not only required to prevent the formation of pulmonary edema; it is also essential for fluid clearance. Creation of a sodium gradient by vectorial transport (which then allows for passive flux of water across the epithelium) depends upon the integrity of tight junctions, which separate the alveolar and interstitial spaces and form a fence between apical and basolateral domains, allowing for the asymmetric distribution of Na+ channels and sodium Na+ pumps to the apical and basolateral membranes, respectively [34].
Effective alveolar fluid reabsorption is impaired in the majority of patients with ALI/ARDS, in part as a consequence of disrupted alveolo-capillary barrier integrity, and is associated with worse clinical outcomes [2]. Interestingly, fluid reabsorption is often altered in models of lung injury, even in the absence of a gross barrier leak [35–39].
Effects of hypoxia on alveolar edema clearance
Alveolar hypoxia commonly develops during ALI/ARDS, when damage to the alveolar–capillary barrier results in airspace flooding. This edema impairs normal oxygen transfer from the alveolar airspaces into the circulation [40]. It is well established that hypoxia significantly reduces active Na+ transport across the alveolar epithelium [41–43], in a process that is reversible by β-adrenergic stimulation of the Na,K-ATPase [35, 44].
Mechanisms of hypoxia-induced inhibition of Na+ transport depend on the duration and severity of the hypoxic exposure. Transepithelial Na+ transport is inhibited in both A549 human epithelial adenocarcinoma cells and in primary rat epithelial ATII cells upon exposure to hypoxia (3% O2) [43]. Short-term exposure of alveolar epithelial cells (as early as 15 min) to severe hypoxia (1.5% O2) induced a time-dependent decrease in the number of Na,K-ATPase molecules at the plasma membrane of alveolar epithelial cells. Hypoxia caused a rapid increase in levels of mitochondrial reactive oxygen species (ROS), leading to activation and translocation of PKC-ζ from cytosolic to membrane compartments of the cells. PKC-ζ directly phosphorylated the Na,K-ATPase α1-subunit at the Ser-18 residue, thereby promoting Na,K-ATPase endocytosis [45]. In contrast, long-term exposure to severe hypoxia down-regulated both ENaC and Na,K-ATPase at both mRNA and protein levels [43, 46]. However, a recent report suggested that prolonged exposure of alveolar epithelial cells to severe hypoxia (1.5% O2) resulted in preferential degradation of the plasma membrane Na,K-ATPase, via the ubiquitin-conjugating system. This degradation led to decreases in Na+ pump activity and oxygen consumption [47]. Plasma membrane-associated Na,K-ATPase, which represents the active, ATP-consuming Na+ pump population, was most vulnerable to hypoxic effects. In contrast, intracellular pools, representing an inactive form of the Na,K-ATPase, required 24–30 h exposure to hypoxia before degradation was observed. These findings suggested that cellular adaptation to hypoxia involves decreased ATP consumption, particularly by conserving ATP utilization by the Na,K-ATPase. More detailed reviews on hypoxia-mediated effects of alveolar fluid reabsorption are available [27, 48, 49].
Effects of coagulation on alveolar fluid reabsorption
Coagulation is an emerging area of interest in the pathogenesis of ALI/ARDS. In particular, concentrations of the edemagenic coagulation factor thrombin are elevated in plasma and broncho-alveolar lavage fluids of patients with ALI/ARDS [50]. Elevated levels of thrombin in the pulmonary circulation significantly reduced active Na+ transport and fluid clearance in the lung. Thrombin decreased Na,K-ATPase activity by promoting its endocytosis from the plasma membrane, which interestingly, just as in hypoxic exposure, was mediated by ROS and PKC-ζ [51], suggesting a central role for these signaling pathways in the regulation of edema clearance. Understanding the role of signaling induced by elements of the enhanced coagulation cascade in ALI/ARDS has yielded, and will likely continue to yield, new therapeutic approaches, including antithrombin III, thrombomodulin, heparin, activated protein C and tissue factor pathway inhibitor. More information regarding these therapeutic approaches is provided in the accompanying review by Morty et al.
Ventilator-induced lung injury
Morbidity and mortality associated with respiratory failure is in part iatrogenic. While mechanical ventilation must be used to support severely ill patients with ALI/ARDS, deleterious physiologic and morphologic alterations may occur as a consequence of mechanical ventilation with high tidal volumes [52]. The resulting alveolo-capillary barrier damage, altered lung permeability, and pulmonary edema are defined as ventilator-induced lung injury (VILI) [52]. However, VILI not only increases lung permeability to small and large solutes, but also decreases Na,K-ATPase activity and active Na+ transport, and therefore lung edema clearance [53]. Furthermore, Frank and coworkers demonstrated that ventilation with high tidal volumes for 2 h resulted in inhibition of cAMP-dependent alveolar fluid reabsorption, through activation of nitric oxide synthase 2 and increased formation of reactive nitrogen species [54]. Thus ventilation with high tidal volume not only promotes the formation of alveolar edema but also impairs its resolution.
While hypoxia, coagulation defects, and VILI have been included as insults relevant to patients with ALI/ARDS, additional pathophysiologic perturbations resulting in altered Na,K-ATPase function in ALI are numerous and may represent potential therapeutic targets as the signaling pathways become elucidated. These include, though are certainly not limited to, hyperoxia [55], hypocapnic alkalosis [56], hypercapnia [57], ischemia/reperfusion injury [58] and inflammatory events [59] (Fig. 2).
Fig. 2.

Mechanisms that lead to endocytosis of Na,K-ATPase from the cell surface to intracellular compartments alter sodium pump activity and thus alveolar fluid clearance during acute lung injury. These pathways include, though are certainly not limited to alveolar hypoxia (O 2 ↓) or hyperoxia (O 2 ↑), elevated levels of the coagulation factor thrombin (THR), ventilator-induced lung injury (VILI), hypocapnic alkalosis (CO 2 ↓), hypercapnia (CO 2 ↑) and ischemia/reperfusion injury (I/R)
Signaling pathways activating Na,K-ATPase: potential new therapeutic approaches in ALI/ARDS
Under physiological conditions the Na,K-ATPase is not saturated by the intracellular Na+ concentration and thus entry of excess Na+ into the epithelium can lead to activation of the Na+ pump [60]. Thus, mechanisms that enhance activity of the apical Na+ channels may indirectly increase Na,K-ATPase function. In the following sections of this review, mechanisms that directly modulate Na,K-ATPase function will be discussed.
β-Adrenergic receptor-mediated up-regulation of Na,K-ATPase
Beta-adrenergic agonists have been proposed as a potential therapy for ALI/ARDS and were among the first agents shown to accelerate Na+ transport by increasing activity of Na+ and Cl-- channels and the Na,K-ATPase in the alveolar epithelium [12, 61–65]. In particular, signaling pathways involved in the β-adrenergic stimulation of Na,K-ATPase activity have been investigated in detail [11, 44]. Short-term stimulation of β2-adrenergic receptors by isoproterenol (ISO) has been found to result in receptor-dependent activation of the G protein Gs, which successively stimulates adenylyl cyclase, resulting in enhanced cAMP generation and consequent activation of PKA, which leads to phosphorylation of the β2-adrenergic receptor, and switching of the receptor's G protein coupling specificity from Gs to Gi. This switch in turn activates RhoA and its downstream effector RhoA-associated kinase, leading to reorganization of the actin cytoskeleton necessary for Na,K-ATPase exocytosis and consequent increase in Na,K-ATPase activity [66].
Long-term β-adrenergic stimulation (96–164 h) of Na,K-ATPase by ISO also results in increased abundance of Na,K-ATPase α1- and β1-subunits in the plasma membrane and enhanced sodium pump activity. Interestingly, this increase in protein abundance following ISO incubation was not found to be associated with changes in mRNA levels, suggesting that the increase in sodium pump protein abundance is regulated post-transcriptionally [67]. The signaling mechanisms in this post-transcriptional regulation of Na,K-ATPase by ISO involve phosphatidylinositol 3-kinase by utilizing the epidermal growth factor receptor as a scaffold protein, which in turn activates the mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) and rapamycin-sensitive pathways [mammalian target of rapamycin (mTOR)]. The mTOR pathway has been implicated in the regulation of Na,K-ATPase translation, which involves activation of the protein S6 kinase (p70S6k) enzymatic complex [68]. Thus, both short- and long-term β-adrenergic stimulation up-regulate Na,K-ATPase function, representing a potential therapy in the resolution of pulmonary edema.
Dopamine-mediated regulation of Na,K-ATPase
Dopamine increases fluid reabsorption by up-regulating both the apical Na+ channels [69] and Na,K-ATPase in the alveolar epithelium [70, 71]. Treatment of alveolar epithelial cells with dopamine for only minutes results in translocation of Na,K-ATPase from intracellular pools to the basolateral membrane of the cell. This short-term effect is mediated by D1 receptors and activation of protein phosphatase 2A (PP2A), a serine/threonine protein phosphatase that plays an important role in the trafficking of transporters [72]. PP2A was suggested to be involved in dephosphorylation of the sodium pump, an initial signal in its trafficking [73]. Recently, it has been reported that PP2A directly interacts with the N-terminus of the Na,K-ATPase α1-subunit, leading to its dephosphorylation at the Ser-18 residue and its recruitment to the plasma membrane from intracellular compartments in alveolar epithelial cells [74]. It has also been shown that PKC-δ and PKC-ε were essential for dopamine-induced Na,K-ATPase exocytosis and increased activity [75]. These PKCs are differentially compartmentalized, and activation of PKC-δ precedes that of PKC-ε. Whether PKC-δ and -ε phosphorylate intermediate proteins, such as actin or other adapter proteins, that subsequently regulate Na,K-ATPase function in the lung remains unresolved.
The MAPK/ERK cascade is an important signaling system by which cells transduce extracellular signals into intracellular responses. Activation of ERK classically involves ligand binding to a receptor tyrosine kinase, although ERK proteins can also be activated by G protein-coupled receptors, such as the dopaminergic receptors. Treatment of alveolar epithelial cells with dopamine for long periods of time (24 h) activated the MAPK pathway via D2 receptors, resulting in increased Na,K-ATPase activity. This effect is mediated by Ras, which associates with the serine/threonine kinase Raf-1. Raf-1 subsequently phosphorylates and activates the downstream kinase MAPK kinase MEK1, the direct activator of ERK proteins [76]. This dopamine-induced activation of ERK leads to a significant increases in Na-K-ATPase β1-subunit mRNA and protein levels and thus increases abundance of functional Na,K-ATPase molecules in the plasma membrane [77]. Therefore, application of dopamine (both short- and long-term) stimulates Na,K-ATPase function and appears to be beneficial in the resolution of alveolar edema.
Corticosteroids and growth factors in Na,K-ATPase regulation
Since inflammation is one of the hallmarks of ALI/ARDS, corticosteroids have been proposed as a potential anti-inflammatory therapeutic modality. Furthermore, it has been reported that a single dexamethasone injection increased alveolar fluid reabsorption in rats by 48 h [78]. The Na,K-ATPase has been shown to be modulated by corticosteroids in different organs. Dexamethasone increased Na,K-ATPase β1 mRNA transcript levels after 6 h of incubation, resulting in elevated Na,K-ATPase protein abundance in alveolar epithelial cells and β- and γ-ENaC mRNA and protein levels without affecting the expression of the α-subunit of the channel [79–81].
As ALI/ARDS is often associated with alveolar epithelial cell death, potential new therapeutic approaches to restore the alveolo-capillary barrier integrity focused on growth factors. Exposing alveolar epithelial monolayers to keratinocyte growth factor (KGF) resulted in a sustained increase of active transcellular Na+ transport [82]. Similarly, epidermal growth factor (EGF) also increased lung liquid clearance [83]. While EGF increased both Na,K-ATPase α1- and β1-subunit mRNA and protein levels it did not enhance ENaC expression [84], but induced non-selective cation channels in ATII cells [85]. Fibroblast growth factor-10 (FGF-10), a protein structurally similar to KGF, is also a potent mitogen that promotes epithelial cell differentiation and wound healing [86]. Treatment of ATII cells with FGF-10 increased Na,K-ATPase activity and membrane protein abundance within 30 min without changing the Na,K-ATPase α1-subunit protein levels in total cell lysates (in contrast to the long-term effects of KGF or EGF), thus demonstrating recruitment of Na,K-ATPase from intracellular pools [87]. FGF-10 effects were prevented by the MAPK kinase inhibitor U0126, suggesting that the FGF-10-induced activation of the Na+ pump was mediated by ERK-1/2 [87]. Thus, growth factors not only facilitate re-epithelialization of the alveolar barrier but may also have beneficial effects in edema resolution. A schematic representation of mechanisms up-regulating cell surface abundance of Na,K-ATPase, and thus alveolar edema clearance, is depicted in Fig. 3.
Fig. 3.
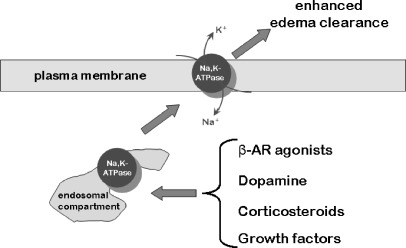
Signaling pathways resulting in recruitment of Na,K-ATPase molecules from intracellular pools to the basolateral membrane of the alveolar epithelium enhance Na,K-ATPase function and thus alveolar edema clearance and might represent new therapeutic approaches in acute lung injury. In particular, β-adrenergic receptor (β-AR) agonists, dopamine, mineralo- and glucocorticosteroids and growth factors have been implicated
Gene therapy to overexpress Na,K-ATPase increases alveolar edema clearance
In normal adult rats, overexpression of the β1-subunit gene by utilizing a replication-incompetent human type 5 adenovirus expressing Na,K-ATPase β1-subunit cDNA increased alveolar edema clearance over twofold compared with controls [14]. Similarly, gene transfer of the Na,K-ATPase β1-subunit using electroporation increased alveolar fluid reabsorption [88]. Furthermore, while rats exposed to 100% oxygen develop ALI and impaired alveolar fluid clearance; overexpression of the Na,K-ATPase β1-subunit in the alveolar epithelium of rats increased lung liquid clearance and, most importantly, overexpression of the Na,K-ATPase β1-subunit resulted in 100% survival over 14 days of hyperoxia (compared with 25–31% survival in the non-treated or null virus-treated control groups) [55]. Also, overexpression of the β2-adrenergic receptor leads to increased alveolar fluid clearance in rats by increasing both membrane-bound amiloride-sensitive Na+ channel expression and Na,K-ATPase function, probably by enhancing responsiveness to endogenous catecholamines in the alveolar epithelium [89].
Since oxidative stress has been accredited with a key role in hypoxemic respiratory failure, antioxidant therapy can have a role in the management of ALI/ARDS. Most recently, it has been reported that adenoviral overexpression of the ROS scavenger superoxide dismutase might be protective and prevents alteration in alveolar fluid reabsorption in rats exposed to prolonged hypoxia [35].
Summary
Acute lung injury is characterized by the disruption of the alveolar epithelial barrier, which results in increased edema formation, as well as impaired edema clearance. Impairment of Na,K-ATPase function appears to be a hallmark during lung injury even in a preclinical stage. Therefore, elucidation of the mechanisms by which lung injury alters Na,K-ATPase activity may provide novel therapeutic targets and warrants further studies.
Acknowledgements
The authors are grateful to Drs. Laura A. Dada and Emilia Lecuona for their advice and for the valuable discussions and to Dr. Alejandro M. Bertorello for his help with the preparation of the figures. This work was supported in part by grants from the National Institutes of Health (HL-71643, HL-65161 and HL-48129) and by the Deutsche Forschungsgemeinschaft (SFB547).
Footnotes
This article refers to the articles available at: http://dx.doi.org/10.1007/s00134-007-0625-z and http://dx.doi.org/10.1007/s00134-007-0662-7.
References
- 1.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 2.Ware LB, Matthay MA. Alveolar fluid clearance is impaired in the majority of patients with acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1376–1383. doi: 10.1164/ajrccm.163.6.2004035. [DOI] [PubMed] [Google Scholar]
- 3.Sznajder JI. Alveolar edema must be cleared for the acute respiratory distress syndrome patient to survive. Am J Respir Crit Care Med. 2001;163:1293–1294. doi: 10.1164/ajrccm.163.6.ed1801d. [DOI] [PubMed] [Google Scholar]
- 4.Mutlu GM, Sznajder JI. Mechanisms of pulmonary edema clearance. Am J Physiol Lung Cell Mol Physiol. 2005;289:L685–695. doi: 10.1152/ajplung.00247.2005. [DOI] [PubMed] [Google Scholar]
- 5.Gorin AB, Stewart PA. Differential permeability of endothelial and epithelial barriers to albumin flux. J Appl Physiol. 1979;47:1315–1324. doi: 10.1152/jappl.1979.47.6.1315. [DOI] [PubMed] [Google Scholar]
- 6.Kuebler WM, Parthasarathi K, Wang PM, Bhattacharya J. A novel signaling mechanism between gas and blood compartments of the lung. J Clin Invest. 2000;105:905–913. doi: 10.1172/JCI8604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weibel ER. Morphological basis of alveolar–capillary gas exchange. Physiol Rev. 1973;53:419–495. doi: 10.1152/physrev.1973.53.2.419. [DOI] [PubMed] [Google Scholar]
- 8.Kellenberger S, Schild L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol Rev. 2002;82:735–767. doi: 10.1152/physrev.00007.2002. [DOI] [PubMed] [Google Scholar]
- 9.Matalon S, O'Brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annu Rev Physiol. 1999;61:627–661. doi: 10.1146/annurev.physiol.61.1.627. [DOI] [PubMed] [Google Scholar]
- 10.Skou JC. Nobel Lecture. The identification of the sodium pump. Biosci Rep. 1998;18:155–169. doi: 10.1023/A:1020196612909. [DOI] [PubMed] [Google Scholar]
- 11.Sznajder JI, Factor P, Ingbar DH. Invited review: lung edema clearance: role of Na(+)-K(+)-ATPase. J Appl Physiol. 2002;93:1860–1866. doi: 10.1152/japplphysiol.00022.2002. [DOI] [PubMed] [Google Scholar]
- 12.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 13.Hummler E, Barker P, Gatzy J, Beermann F, Verdumo C, Schmidt A, Boucher R, Rossier BC. Early death due to defective neonatal lung liquid clearance in alpha-ENaC-deficient mice. Nat Genet. 1996;12:325–328. doi: 10.1038/ng0396-325. [DOI] [PubMed] [Google Scholar]
- 14.Factor P, Saldias F, Ridge K, Dumasius V, Zabner J, Jaffe HA, Blanco G, Barnard M, Mercer R, Perrin R, Sznajder JI. Augmentation of lung liquid clearance via adenovirus-mediated transfer of a Na,K-ATPase beta1 subunit gene. J Clin Invest. 1998;102:1421–1430. doi: 10.1172/JCI3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan JH. Biochemistry of Na,K-ATPase. Annu Rev Biochem. 2002;71:511–35. doi: 10.1146/annurev.biochem.71.102201.141218. [DOI] [PubMed] [Google Scholar]
- 17.Shull MM, Pugh DG, Lingrel JB. Characterization of the human Na,K-ATPase alpha 2 gene and identification of intragenic restriction fragment length polymorphisms. J Biol Chem. 1989;264:17532–17543. [PubMed] [Google Scholar]
- 18.Woo AL, James PF, Lingrel JB. Characterization of the fourth alpha isoform of the Na,K-ATPase. J Membr Biol. 1999;169:39–44. doi: 10.1007/PL00005899. [DOI] [PubMed] [Google Scholar]
- 19.Ridge KM, Rutschman DH, Factor P, Katz AI, Bertorello AM, Sznajder JL. Differential expression of Na-K-ATPase isoforms in rat alveolar epithelial cells. Am J Physiol. 1997;273:L246–255. doi: 10.1152/ajpcell.1997.273.1.C246. [DOI] [PubMed] [Google Scholar]
- 20.Borok Z, Liebler JM, Lubman RL, Foster MJ, Zhou B, Li X, Zabski SM, Kim KJ, Crandall ED. Na transport proteins are expressed by rat alveolar epithelial type I cells. Am J Physiol Lung Cell Mol Physiol. 2002;282:L599–608. doi: 10.1152/ajplung.00130.2000. [DOI] [PubMed] [Google Scholar]
- 21.Ridge KM, Olivera WG, Saldias F, Azzam Z, Horowitz S, Rutschman DH, Dumasius V, Factor P, Sznajder JI. Alveolar type 1 cells express the alpha2 Na,K-ATPase, which contributes to lung liquid clearance. Circ Res. 2003;92:453–460. doi: 10.1161/01.RES.0000059414.10360.F2. [DOI] [PubMed] [Google Scholar]
- 22.Therien AG, Blostein R. Mechanisms of sodium pump regulation. Am J Physiol Cell Physiol. 2000;279:C541–566. doi: 10.1152/ajpcell.2000.279.3.C541. [DOI] [PubMed] [Google Scholar]
- 23.Orlowski J, Lingrel JB. Tissue-specific and developmental regulation of rat Na,K-ATPase catalytic alpha isoform and beta subunit mRNAs. J Biol Chem. 1988;263:10436–10442. [PubMed] [Google Scholar]
- 24.Therien AG, Goldshleger R, Karlish SJ, Blostein R. Tissue-specific distribution and modulatory role of the gamma subunit of the Na,K-ATPase. J Biol Chem. 1997;272:32628–32634. doi: 10.1074/jbc.272.51.32628. [DOI] [PubMed] [Google Scholar]
- 25.Therien AG, Karlish SJ, Blostein R. Expression and functional role of the gamma subunit of the Na, K-ATPase in mammalian cells. J Biol Chem. 1999;274:12252–12256. doi: 10.1074/jbc.274.18.12252. [DOI] [PubMed] [Google Scholar]
- 26.Rajasekaran SA, Palmer LG, Quan K, Harper JF, Ball WJ, Jr., Bander NH, Peralta Soler A, Rajasekaran AK. Na,K-ATPase beta-subunit is required for epithelial polarization, suppression of invasion, and cell motility. Mol Biol Cell. 2001;12:279–295. doi: 10.1091/mbc.12.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain M, Sznajder JI. Effects of hypoxia on the alveolar epithelium. Proc Am Thorac Soc. 2005;2:202–205. doi: 10.1513/pats.200501-006AC. [DOI] [PubMed] [Google Scholar]
- 28.Cavanaugh KJ, Jr., Oswari J, Margulies SS. Role of stretch on tight junction structure in alveolar epithelial cells. Am J Respir Cell Mol Biol. 2001;25:584–591. doi: 10.1165/ajrcmb.25.5.4486. [DOI] [PubMed] [Google Scholar]
- 29.Nusrat A, von Eichel-Streiber C, Turner JR, Verkade P, Madara JL, Parkos CA. Clostridium difficile toxins disrupt epithelial barrier function by altering membrane microdomain localization of tight junction proteins. Infect Immun. 2001;69:1329–1336. doi: 10.1128/IAI.69.3.1329-1336.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ebnet K, Aurrand-Lions M, Kuhn A, Kiefer F, Butz S, Zander K, Meyer zu Brickwedde MK, Suzuki A, Imhof BA, Vestweber D. The junctional adhesion molecule (JAM) family members JAM-2 and JAM-3 associate with the cell polarity protein PAR-3: a possible role for JAMs in endothelial cell polarity. J Cell Sci. 2003;116:3879–3891. doi: 10.1242/jcs.00704. [DOI] [PubMed] [Google Scholar]
- 31.Mehta D, Malik AB. Signaling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367. doi: 10.1152/physrev.00012.2005. [DOI] [PubMed] [Google Scholar]
- 32.Rajasekaran AK, Rajasekaran SA. Role of Na-K-ATPase in the assembly of tight junctions. Am J Physiol Renal Physiol. 2003;285:F388–396. doi: 10.1152/ajprenal.00439.2002. [DOI] [PubMed] [Google Scholar]
- 33.Rajasekaran SA, Palmer LG, Moon SY, Peralta Soler A, Apodaca GL, Harper JF, Zheng Y, Rajasekaran AK. Na,K-ATPase activity is required for formation of tight junctions, desmosomes, and induction of polarity in epithelial cells. Mol Biol Cell. 2001;12:3717–3732. doi: 10.1091/mbc.12.12.3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Litvan J, Briva A, Wilson MS, Budinger GR, Sznajder JI, Ridge KM. beta-Adrenergic Receptor Stimulation and Adenoviral Overexpression of Superoxide Dismutase Prevent the Hypoxia-mediated Decrease in Na,K-ATPase and Alveolar Fluid Reabsorption. J Biol Chem. 2006;281:19892–19898. doi: 10.1074/jbc.M602064200. [DOI] [PubMed] [Google Scholar]
- 36.Vadasz I, Morty RE, Kohstall MG, Olschewski A, Grimminger F, Seeger W, Ghofrani HA. Oleic acid inhibits alveolar fluid reabsorption: a role in acute respiratory distress syndrome? Am J Respir Crit Care Med. 2005;171:469–479. doi: 10.1164/rccm.200407-954OC. [DOI] [PubMed] [Google Scholar]
- 37.Comellas AP, Pesce LM, Azzam Z, Saldias FJ, Sznajder JI. Scorpion venom decreases lung liquid clearance in rats. Am J Respir Crit Care Med. 2003;167:1064–1067. doi: 10.1164/rccm.200207-688OC. [DOI] [PubMed] [Google Scholar]
- 38.Saldias FJ, Azzam ZS, Ridge KM, Yeldandi A, Rutschman DH, Schraufnagel D, Sznajder JI. Alveolar fluid reabsorption is impaired by increased left atrial pressures in rats. Am J Physiol Lung Cell Mol Physiol. 2001;281:L591–597. doi: 10.1152/ajplung.2001.281.3.L591. [DOI] [PubMed] [Google Scholar]
- 39.Azzam ZS, Adir Y, Welch L, Chen J, Winaver J, Factor P, Krivoy N, Hoffman A, Sznajder JI, Abassi Z. Alveolar fluid reabsorption is increased in rats with compensated heart failure. Am J Physiol Lung Cell Mol Physiol. 2006;291:L1094–1100. doi: 10.1152/ajplung.00180.2005. [DOI] [PubMed] [Google Scholar]
- 40.Sznajder JI, Wood LD. Beneficial effects of reducing pulmonary edema in patients with acute hypoxemic respiratory failure. Chest. 1991;100:890–892. doi: 10.1378/chest.100.4.890. [DOI] [PubMed] [Google Scholar]
- 41.Suzuki S, Noda M, Sugita M, Ono S, Koike K, Fujimura S. Impairment of transalveolar fluid transport and lung Na(+)-K(+)-ATPase function by hypoxia in rats. J Appl Physiol. 1999;87:962–968. doi: 10.1152/jappl.1999.87.3.962. [DOI] [PubMed] [Google Scholar]
- 42.Vivona ML, Matthay M, Chabaud MB, Friedlander G, Clerici C. Hypoxia reduces alveolar epithelial sodium and fluid transport in rats: reversal by beta-adrenergic agonist treatment. Am J Respir Cell Mol Biol. 2001;25:554–561. doi: 10.1165/ajrcmb.25.5.4420. [DOI] [PubMed] [Google Scholar]
- 43.Wodopia R, Ko HS, Billian J, Wiesner R, Bartsch P, Mairbaurl H. Hypoxia decreases proteins involved in epithelial electrolyte transport in A549 cells and rat lung. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1110–1119. doi: 10.1152/ajplung.2000.279.6.L1110. [DOI] [PubMed] [Google Scholar]
- 44.Bertorello AM, Ridge KM, Chibalin AV, Katz AI, Sznajder JI. Isoproterenol increases Na+-K+-ATPase activity by membrane insertion of alpha-subunits in lung alveolar cells. Am J Physiol. 1999;276:L20–27. doi: 10.1152/ajplung.1999.276.1.L20. [DOI] [PubMed] [Google Scholar]
- 45.Dada LA, Chandel NS, Ridge KM, Pedemonte C, Bertorello AM, Sznajder JI. Hypoxia-induced endocytosis of Na,K-ATPase in alveolar epithelial cells is mediated by mitochondrial reactive oxygen species and PKC-zeta. J Clin Invest. 2003;111:1057–1064. doi: 10.1172/JCI16826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Planes C, Escoubet B, Blot-Chabaud M, Friedlander G, Farman N, Clerici C. Hypoxia downregulates expression and activity of epithelial sodium channels in rat alveolar epithelial cells. Am J Respir Cell Mol Biol. 1997;17:508–518. doi: 10.1165/ajrcmb.17.4.2680. [DOI] [PubMed] [Google Scholar]
- 47.Comellas AP, Dada LA, Lecuona E, Pesce LM, Chandel NS, Quesada N, Budinger GR, Strous GJ, Ciechanover A, Sznajder JI. Hypoxia-mediated degradation of Na,K-ATPase via mitochondrial reactive oxygen species and the ubiquitin-conjugating system. Circ Res. 2006;98:1314–1322. doi: 10.1161/01.RES.0000222418.99976.1d. [DOI] [PubMed] [Google Scholar]
- 48.Hardiman KM, Matalon S. Modification of sodium transport and alveolar fluid clearance by hypoxia: mechanisms and physiological implications. Am J Respir Cell Mol Biol. 2001;25:538–541. doi: 10.1165/ajrcmb.25.5.f219. [DOI] [PubMed] [Google Scholar]
- 49.Vadasz I, Sznajder JI. Hypoxia-induced alveolar epithelial dysfunction. J Organ Dysfunction. 2006;2:244–249. doi: 10.1080/17471060600763377. [DOI] [Google Scholar]
- 50.Idell S. Coagulation, fibrinolysis, and fibrin deposition in acute lung injury. Crit Care Med. 2003;31:S213–220. doi: 10.1097/01.CCM.0000057846.21303.AB. [DOI] [PubMed] [Google Scholar]
- 51.Vadasz I, Morty RE, Olschewski A, Konigshoff M, Kohstall MG, Ghofrani HA, Grimminger F, Seeger W. Thrombin impairs alveolar fluid clearance by promoting endocytosis of Na+,K+-ATPase. Am J Respir Cell Mol Biol. 2005;33:343–354. doi: 10.1165/rcmb.2004-0407OC. [DOI] [PubMed] [Google Scholar]
- 52.Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294–323. doi: 10.1164/ajrccm.157.1.9604014. [DOI] [PubMed] [Google Scholar]
- 53.Lecuona E, Saldias F, Comellas A, Ridge K, Guerrero C, Sznajder JI. Ventilator-associated lung injury decreases lung ability to clear edema in rats. Am J Respir Crit Care Med. 1999;159:603–609. doi: 10.1164/ajrccm.159.2.9805050. [DOI] [PubMed] [Google Scholar]
- 54.Frank JA, Pittet JF, Lee H, Godzich M, Matthay MA. High tidal volume ventilation induces NOS2 and impairs cAMP- dependent air space fluid clearance. Am J Physiol Lung Cell Mol Physiol. 2003;284:L791–798. doi: 10.1152/ajplung.00331.2002. [DOI] [PubMed] [Google Scholar]
- 55.Factor P, Dumasius V, Saldias F, Brown LA, Sznajder JI. Adenovirus-mediated transfer of an Na+/K+-ATPase beta1 subunit gene improves alveolar fluid clearance and survival in hyperoxic rats. Hum Gene Ther. 2000;11:2231–2242. doi: 10.1089/104303400750035753. [DOI] [PubMed] [Google Scholar]
- 56.Myrianthefs PM, Briva A, Lecuona E, Dumasius V, Rutschman DH, Ridge KM, Baltopoulos GJ, Sznajder JI. Hypocapnic but not metabolic alkalosis impairs alveolar fluid reabsorption. Am J Respir Crit Care Med. 2005;171:1267–1271. doi: 10.1164/rccm.200408-998OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Briva A, Lecuona E, Welch L, Dada LA, Vadasz I, Chen J, Gruenbaum J, Gelfand V, Sznajder JI. PKC-zeta Is necessary for hypercapnia-mediated Na,K-ATPase endocytosis in alveolar epithelial cells. Proc Am Thorac Soc. 2006;3:A436. [Google Scholar]
- 58.Sugita M, Ferraro P, Dagenais A, Clermont ME, Barbry P, Michel RP, Berthiaume Y. Alveolar liquid clearance and sodium channel expression are decreased in transplanted canine lungs. Am J Respir Crit Care Med. 2003;167:1440–1450. doi: 10.1164/rccm.200204-312OC. [DOI] [PubMed] [Google Scholar]
- 59.Cher CD, Armugam A, Lachumanan R, Coghlan MW, Jeyaseelan K. Pulmonary inflammation and edema induced by phospholipase A2: global gene analysis and effects on aquaporins and Na+/K+-ATPase. J Biol Chem. 2003;278:31352–31360. doi: 10.1074/jbc.M302446200. [DOI] [PubMed] [Google Scholar]
- 60.Ewart HS, Klip A. Hormonal regulation of the Na(+)-K(+)-ATPase: mechanisms underlying rapid and sustained changes in pump activity. Am J Physiol. 1995;269:C295–311. doi: 10.1152/ajpcell.1995.269.2.C295. [DOI] [PubMed] [Google Scholar]
- 61.Berthiaume Y, Staub NC, Matthay MA. Beta-adrenergic agonists increase lung liquid clearance in anesthetized sheep. J Clin Invest. 1987;79:335–343. doi: 10.1172/JCI112817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saldias F, Lecuona E, Friedman E, Barnard ML, Ridge KM, Sznajder JI. Modulation of lung liquid clearance by isoproterenol in rat lungs. Am J Physiol. 1998;274:L694–701. doi: 10.1152/ajplung.1998.274.5.L694. [DOI] [PubMed] [Google Scholar]
- 63.Pittet JF, Wiener-Kronish JP, McElroy MC, Folkesson HG, Matthay MA. Stimulation of lung epithelial liquid clearance by endogenous release of catecholamines in septic shock in anesthetized rats. J Clin Invest. 1994;94:663–671. doi: 10.1172/JCI117383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ghofrani HA, Kohstall MG, Weissmann N, Schmehl T, Schermuly RT, Seeger W, Grimminger F. Alveolar epithelial barrier functions in ventilated perfused rabbit lungs. Am J Physiol Lung Cell Mol Physiol. 2001;280:L896–904. doi: 10.1152/ajplung.2001.280.5.L896. [DOI] [PubMed] [Google Scholar]
- 65.Mutlu GM, Adir Y, Jameel M, Akhmedov AT, Welch L, Dumasius V, Meng FJ, Zabner J, Koenig C, Lewis ER, Balagani R, Traver G, Sznajder JI, Factor P. Interdependency of beta-adrenergic receptors and CFTR in regulation of alveolar active Na+ transport. Circ Res. 2005;96:999–1005. doi: 10.1161/01.RES.0000164554.21993.AC. [DOI] [PubMed] [Google Scholar]
- 66.Lecuona E, Ridge K, Pesce L, Batlle D, Sznajder JI. The GTP-binding protein RhoA mediates Na,K-ATPase exocytosis in alveolar epithelial cells. Mol Biol Cell. 2003;14:3888–3897. doi: 10.1091/mbc.E02-12-0781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pesce L, Guerrero C, Comellas A, Ridge KM, Sznajder JI. beta-agonists regulate Na,K-ATPase via novel MAPK/ERK and rapamycin-sensitive pathways. FEBS Lett. 2000;486:310–314. doi: 10.1016/S0014-5793(00)02298-5. [DOI] [PubMed] [Google Scholar]
- 68.Pesce L, Comellas A, Sznajder JI. Beta-adrenergic agonists regulate Na-K-ATPase via p70S6k. Am J Physiol Lung Cell Mol Physiol. 2003;285:L802–807. doi: 10.1152/ajplung.00266.2002. [DOI] [PubMed] [Google Scholar]
- 69.Helms MN, Chen XJ, Ramosevac S, Eaton DC, Jain L. Dopamine regulation of amiloride-sensitive sodium channels in lung cells. Am J Physiol Lung Cell Mol Physiol. 2006;290:L710–L722. doi: 10.1152/ajplung.00486.2004. [DOI] [PubMed] [Google Scholar]
- 70.Barnard ML, Olivera WG, Rutschman DM, Bertorello AM, Katz AI, Sznajder JI. Dopamine stimulates sodium transport and liquid clearance in rat lung epithelium. Am J Respir Crit Care Med. 1997;156:709–714. doi: 10.1164/ajrccm.156.3.9610013. [DOI] [PubMed] [Google Scholar]
- 71.Adir Y, Azzam ZS, Lecuona E, Leal S, Pesce L, Dumasius V, Bertorello AM, Factor P, Young JB, Ridge KM, Sznajder JI. Augmentation of endogenous dopamine production increases lung liquid clearance. Am J Respir Crit Care Med. 2004;169:757–763. doi: 10.1164/rccm.200207-744OC. [DOI] [PubMed] [Google Scholar]
- 72.Mukhopadhyay S, Webster CR, Anwer MS. Role of protein phosphatases in cyclic AMP-mediated stimulation of hepatic Na+/taurocholate cotransport. J Biol Chem. 1998;273:30039–30045. doi: 10.1074/jbc.273.45.30039. [DOI] [PubMed] [Google Scholar]
- 73.Lecuona E, Garcia A, Sznajder JI. A novel role for protein phosphatase 2A in the dopaminergic regulation of Na,K-ATPase. FEBS Lett. 2000;481:217–220. doi: 10.1016/S0014-5793(00)02009-3. [DOI] [PubMed] [Google Scholar]
- 74.Lecuona E, Dada LA, Sun H, Butti ML, Zhou G, Chew TL, Sznajder JI. Na,K-ATPase α1-subunit dephosphorylation by protein phosphatase 2A is necessary for its recruitment to the plasma membrane. FASEB J. 2006;20:2618–2620. doi: 10.1096/fj.06-6503fje. [DOI] [PubMed] [Google Scholar]
- 75.Ridge KM, Dada L, Lecuona E, Bertorello AM, Katz AI, Mochly-Rosen D, Sznajder JI. Dopamine-induced exocytosis of Na,K-ATPase is dependent on activation of protein kinase C-epsilon and -delta. Mol Biol Cell. 2002;13:1381–1389. doi: 10.1091/mbc.01-07-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guerrero C, Pesce L, Lecuona E, Ridge KM, Sznajder JI. Dopamine activates ERKs in alveolar epithelial cells via Ras-PKC-dependent and Grb2/Sos-independent mechanisms. Am J Physiol Lung Cell Mol Physiol. 2002;282:L1099–1107. doi: 10.1152/ajplung.00178.2001. [DOI] [PubMed] [Google Scholar]
- 77.Guerrero C, Lecuona E, Pesce L, Ridge KM, Sznajder JI. Dopamine regulates Na-K-ATPase in alveolar epithelial cells via MAPK-ERK-dependent mechanisms. Am J Physiol Lung Cell Mol Physiol. 2001;281:L79–85. doi: 10.1152/ajplung.2001.281.1.L79. [DOI] [PubMed] [Google Scholar]
- 78.Noda M, Suzuki S, Tsubochi H, Sugita M, Maeda S, Kobayashi S, Kubo H, Kondo T. Single dexamethasone injection increases alveolar fluid clearance in adult rats. Crit Care Med. 2003;31:1183–1189. doi: 10.1097/01.CCM.0000059640.77535.29. [DOI] [PubMed] [Google Scholar]
- 79.Barquin N, Ciccolella DE, Ridge KM, Sznajder JI. Dexamethasone upregulates the Na-K-ATPase in rat alveolar epithelial cells. Am J Physiol. 1997;273:L825–830. doi: 10.1152/ajplung.1997.273.4.L825. [DOI] [PubMed] [Google Scholar]
- 80.Noguchi S, Higashi K, Kawamura M. A possible role of the beta-subunit of (Na,K)-ATPase in facilitating correct assembly of the alpha-subunit into the membrane. J Biol Chem. 1990;265:15991–15995. [PubMed] [Google Scholar]
- 81.Lazrak A, Samanta A, Venetsanou K, Barbry P, Matalon S. Modification of biophysical properties of lung epithelial Na(+) channels by dexamethasone. Am J Physiol Cell Physiol. 2000;279:C762–770. doi: 10.1152/ajpcell.2000.279.3.C762. [DOI] [PubMed] [Google Scholar]
- 82.Borok Z, Danto SI, Dimen LL, Zhang XL, Lubman RL. Na(+)-K(+)-ATPase expression in alveolar epithelial cells: upregulation of active ion transport by KGF. Am J Physiol. 1998;274:L149–158. doi: 10.1152/ajpcell.1998.274.1.C149. [DOI] [PubMed] [Google Scholar]
- 83.Sznajder JI, Ridge KM, Yeates DB, Ilekis J, Olivera W. Epidermal growth factor increases lung liquid clearance in rat lungs. J Appl Physiol. 1998;85:1004–1010. doi: 10.1152/jappl.1998.85.3.1004. [DOI] [PubMed] [Google Scholar]
- 84.Danto SI, Borok Z, Zhang XL, Lopez MZ, Patel P, Crandall ED, Lubman RL. Mechanisms of EGF-induced stimulation of sodium reabsorption by alveolar epithelial cells. Am J Physiol. 1998;275:C82–92. doi: 10.1152/ajpcell.1998.275.1.C82. [DOI] [PubMed] [Google Scholar]
- 85.Kemp PJ, Borok Z, Kim KJ, Lubman RL, Danto SI, Crandall ED. Epidermal growth factor regulation in adult rat alveolar type II cells of amiloride-sensitive cation channels. Am J Physiol. 1999;277:C1058–1065. doi: 10.1152/ajpcell.1999.277.6.C1058. [DOI] [PubMed] [Google Scholar]
- 86.Igarashi M, Finch PW, Aaronson SA. Characterization of recombinant human fibroblast growth factor (FGF)-10 reveals functional similarities with keratinocyte growth factor (FGF-7) J Biol Chem. 1998;273:13230–13235. doi: 10.1074/jbc.273.21.13230. [DOI] [PubMed] [Google Scholar]
- 87.Upadhyay D, Lecuona E, Comellas A, Kamp DW, Sznajder JI. Fibroblast growth factor-10 upregulates Na,K-ATPase via the MAPK pathway. FEBS Lett. 2003;545:173–176. doi: 10.1016/S0014-5793(03)00527-1. [DOI] [PubMed] [Google Scholar]
- 88.Machado-Aranda D, Adir Y, Young JL, Briva A, Budinger GR, Yeldandi AV, Sznajder JI, Dean DA. Gene transfer of the Na+,K+-ATPase beta1 subunit using electroporation increases lung liquid clearance. Am J Respir Crit Care Med. 2005;171:204–211. doi: 10.1164/rccm.200403-313OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dumasius V, Sznajder JI, Azzam ZS, Boja J, Mutlu GM, Maron MB, Factor P. beta(2)-adrenergic receptor overexpression increases alveolar fluid clearance and responsiveness to endogenous catecholamines in rats. Circ Res. 2001;89:907–914. doi: 10.1161/hh2201.100204. [DOI] [PubMed] [Google Scholar]


