Abstract
Here we show, in a double-blind, randomized, placebo-controlled trial in 37,107 fully immunized infants in Soweto, South Africa, that a 9-valent pneumococcal conjugate vaccine, PncCV, prevents 31% (95% confidence interval = 15–43%) of pneumonias associated with any of seven respiratory viruses in children in hospital. These data suggest that the pneumococcus has a major role in the development of pneumonia associated with these viruses and that viruses contribute to the pathogenesis of bacterial pneumonia. NOTE: In the version of this article originally published online, the species name was misspelled Streptococcus pnemoniae in the title of the article. The name should be Streptococcus pneumoniae . This error has been corrected for the HTML and print versions of the article.
Supplementary information
The online version of this article (doi:10.1038/nm1077) contains supplementary material, which is available to authorized users.
Main
We previously showed that PncCV reduces invasive pneumococcal disease caused by vaccine serotypes by 72% and radiologically confirmed pneumonia by 17% in a population of both HIV-infected and HIV-uninfected African infants1. As the fraction of pneumonia attributable to pneumococcus may be reduced by seasonal respiratory syncytial virus (RSV) or influenza epidemics2, we sought evidence of viral infection in these infants.
Ecological studies have shown that temporal associations have occurred between peaks of influenza and peaks of bacterial pneumonia, for example, in 1918 and 1957 (ref. 3); however, no randomized study has examined the hypothesis that bacteria and viruses are important copathogens in the etiology of pneumonia. Although there are no sensitive techniques available to diagnose pneumococcal pneumonia, the demonstration that, at least in children without HIV infection, PncCV prevents 85–97% of invasive disease caused by vaccine serotypes1,4 provides a sensitive probe that can be used to explore the role of a bacterium (the pneumococcus) in the etiology of viral pneumonia.
Details of the demographics of the study population have been reported1. Of 39,836 children, 18,245 received all three doses of study vaccine and 18,268 received placebo, according to the per protocol analysis (see Supplementary Methods online for the trial method).
We showed previously that children without HIV who received PncCV had 25% less pneumonia with alveolar consolidation, as assessed by the World Health Organization definition (WHO-AC)1,5. We now extend those data to show a 20% reduction in all-cause first episodes of clinical pneumonia among all children (95% confidence interval (CI) = 10–28%, P = 0.00009) (Table 1) with a similar reduction (14%; 95% CI = 2–44%) in pneumonias with which no virus was identified. Table 1 also shows similar reductions in all-cause pneumonias among HIV-infected (14%; 95% CI = 4–28%) and HIV-uninfected (23%; 95% CI = 11–33%) children. In all children, PncCV also reduced pneumonias associated with any of the identified viruses by 31% (95% CI = 15–43%; P = 0.0004), with similar point estimates of efficacy and CI associated with influenza A virus (45%; 95% CI = 14–64%), parainfluenza viruses (PIVs) types 1–3 (44%; 95% CI = 8–66%) and RSV (22%; 95% CI = −3 to +41%).
Table 1.
Percentage efficacy of pneumococcal conjugate vaccine by per protocol analysis in fully immunized infants
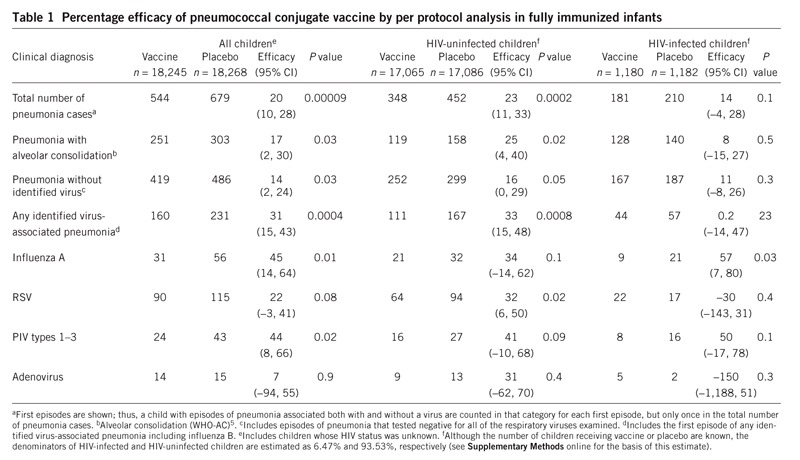
The results of intent to treat analyses are shown in Table 2. The frequency of Streptococcus pneumoniae isolated from blood associated with viral pneumonia is shown in Supplementary Table 1 online. No differences were found in the frequency of all-cause or virus-specific bronchiolitis between children who received the vaccine and those who received placebo (data not shown).
Table 2.
Percentage efficacy of pneumococcal conjugate vaccine by intent-to-treat analysis
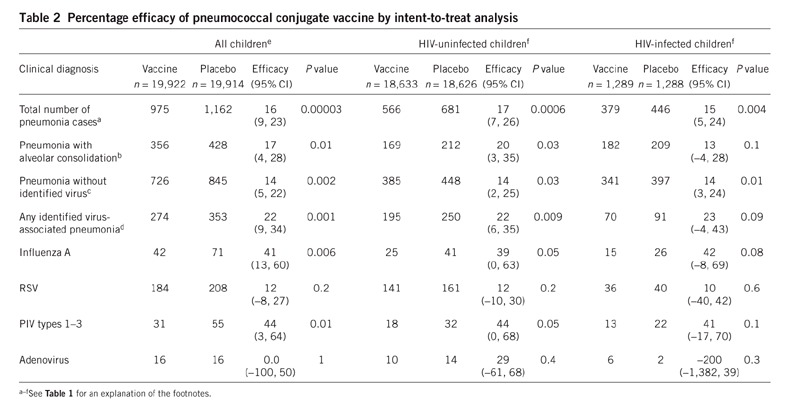
This study provides quantitative evidence of the importance of S. pneumoniae superinfection in virus-associated pneumonias in children in hospital and underscores the limited value of blood cultures to identify this association. The reduction in pneumonias associated with RSV, influenza A and PIV types 1–3 in children without HIV (Table 1) suggests that most of the pneumonias associated with these viruses in hospitalized children are due to concurrent bacterial infections. Conversely, most vaccine-preventable pneumococcal pneumonias in hospitalized children may require a viral respiratory infection.
Although the 9-valent PncCV provides coverage against 87% of serogroups of pneumococci in the study community6, the vaccine cannot be expected to protect against bacterial pathogens such as Staphylococcus aureus. All children received Haemophilus influenzae type B conjugate vaccine, and serotype replacement carriage with non-vaccine pneumococcal serotypes occurs in this population7. Our data thus provide a minimum estimate of the burden of virus-associated pneumonia that may be due to bacteria.
Abundant epidemiological and biological evidence indicates that respiratory viruses contribute to bacterial infections (reviewed in ref. 8) through viral destruction of respiratory epithelium, viral upregulation of bacterial adhesion molecules such as the PAF receptor and (for influenza and PIV) the effect of viral neuraminidase on bacterial adhesion8. In addition to the viruses examined in our study, rhinovirus may upregulate pneumococcal adherence to respiratory epithelial cells9, and it is possible that coronavirus and human metapneumovirus (an important cause of virus-associated pneumonia in this population10) may be also involved in the pathogenesis of bacterial pneumonia. The effect of whole-cell killed, split virus, or live attenuated influenza vaccines, in addition to PncCV, on pneumonia in children deserves study. We have shown that PncCV reduces pneumonia associated with respiratory viral infections, presumably by preventing superimposed bacterial coinfection.
Thus, pneumonia after acquisition of a new pneumococcal serotype during an upper respiratory viral infection may be prevented by opsonophagocytic antibody induced by conjugate vaccine. During the 7 d before the pneumococcal capsule-induced antibody response, there may be a temporary increase in susceptibility to virus-associated pneumococcal pneumonia, the mechanism of which remains speculative1. We would caution that our data supporting empirical antibiotic use for virus-associated pneumonia apply only to infants in hospital, most of whom would be already receiving antibiotics. Conjugate vaccine has been shown to reduce antibiotic use in outpatient settings11. The lesser impact of PncCV on virus-associated pneumonias in HIV-infected children may be due to the high frequency of concurrent Pneumocystis jiroveci infections (42%) and bacterial infections other than pneumococcal among these children12.
In conclusion, PncCV not only prevents invasive disease and radiologically confirmed pneumonia1, but also reduces all-cause clinically diagnosed pneumonia. The data in Table 1 suggest that the population-based effect of the vaccine on total pneumonia, including virus-associated pneumonias, should be considered in terms of total cases prevented and the cost-effectiveness of the vaccine. We have also shown that the vaccine is a useful probe that has established, for the first time to our knowledge, that a significant fraction of viral pneumonia is attributable to bacterial coinfection and is preventable by a bacterial vaccine. Because immunization of children has been shown to reduce invasive pneumococcal disease in adults13, our data suggest that studies should be developed to investigate the strategy of infant immunization with pneumococcal conjugate vaccine to reduce morbidity and mortality associated with influenza and other viral pneumonias in both children and adults.
Note: Supplementary information is available on the Nature Medicine website.
Supplementary information
Pneumococcal bacteremia and respiratory viral isolation in intent-to-treat analysis. (PDF 20 kb)
Competing interests
Vaccine trial was funded by Wyeth.
References
- 1.Klugman KP, et al. N. Engl. J. Med. 2003;349:1341–1348. doi: 10.1056/NEJMoa035060. [DOI] [PubMed] [Google Scholar]
- 2.Madhi SA, Schoub B, Simmank K, Blackburn N, Klugman KP. J. Pediatr. 2000;137:78–84. doi: 10.1067/mpd.2000.105350. [DOI] [PubMed] [Google Scholar]
- 3.Hament JM, Kimpen JL, Fleer A, Wolfs TF. FEMS Immunol. Med. Microbiol. 1999;26:189–195. doi: 10.1111/j.1574-695X.1999.tb01389.x. [DOI] [PubMed] [Google Scholar]
- 4.Black S, et al. Pediatr. Infect. Dis. J. 2000;19:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Pneumonia Vaccine Trial Investigators' Group. Standardization of Interpretation of Chest Radiographs for the Diagnosis of Pneumonia in Children (Department of Vaccines and Biologicals, WHO, Geneva, 2001).
- 6.Madhi SA, Petersen K, Madhi A, Wasas A, Klugman KP. Pediatr. Infect. Dis. J. 2000;19:1141–1147. doi: 10.1097/00006454-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 7.Mbelle N, et al. J. Infect. Dis. 1999;180:1171–1176. doi: 10.1086/315009. [DOI] [PubMed] [Google Scholar]
- 8.Peltola VT, McCullers JA. Pediatr. Infect. Dis. J. 2004;23:S87–S97. doi: 10.1097/01.inf.0000108197.81270.35. [DOI] [PubMed] [Google Scholar]
- 9.Ishizuka S, et al. J. Infect. Dis. 2003;188:1928–1939. doi: 10.1086/379833. [DOI] [PubMed] [Google Scholar]
- 10.Madhi SA, Ludewick H, Abed Y, Klugman KP, Boivin G. Clin. Infect. Dis. 2003;37:1705–1710. doi: 10.1086/379771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dagan R, et al. Pediatr. Infect. Dis. J. 2001;20:951–958. doi: 10.1097/00006454-200110000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Madhi SA, et al. Clin. Infect. Dis. 2002;35:1120–1126. doi: 10.1086/343049. [DOI] [PubMed] [Google Scholar]
- 13.Whitney CG, et al. N. Engl. J. Med. 2003;348:1737–1746. doi: 10.1056/NEJMoa022823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Pneumococcal bacteremia and respiratory viral isolation in intent-to-treat analysis. (PDF 20 kb)


