Abstract
The vaccines developed over the first two hundred years since Jenner's lifetime have accomplished striking reductions of infection and disease wherever applied. Pasteur's early approaches to vaccine development, attenuation and inactivation, are even now the two poles of vaccine technology. Today, purification of microbial elements, genetic engineering and improved knowledge of immune protection allow direct creation of attenuated mutants, expression of vaccine proteins in live vectors, purification and even synthesis of microbial antigens, and induction of a variety of immune responses through manipulation of DNA, RNA, proteins and polysaccharides. Both noninfectious and infectious diseases are now within the realm of vaccinology. The profusion of new vaccines enables new populations to be targeted for vaccination, and requires the development of routes of administration additional to injection. With all this come new problems in the production, regulation and distribution of vaccines.
Main
“The Circassians [a Middle Eastern people] perceived that of a thousand persons hardly one was attacked twice by full blown smallpox; that in truth one sees three or four mild cases but never two that are serious and dangerous; that in a word one never truly has that illness twice in life.”
Voltaire, “On Variolation,” Philosophical Letters, 1734
Early successes
The beginnings of vaccination, defined as an overt attempt to use part or all of a microbial pathogen to protect against that microbe, are lost in the proverbial mists of time. Vaccination probably originated in homeopathic beliefs about small doses of disease protecting against severe disease, verified empirically by ingestion of small doses of poison to prevent fatal intentional poisoning of rulers by rivals. By the eleventh century there were hints in Chinese literature of the use of variola scabs insufflated into the nose to immunize against smallpox, perhaps based on observations that prior smallpox protected against subsequent exposure1,2.
Whereas the Chinese are generally given credit for the invention of variolation, support for that view comes only in writings of the seventeenth century. The other candidate region for the origin of variolation is India, where a scarification procedure was invented either separately or imported from China. From there cutaneous variolation passed to the Middle East and Africa, and as is well known, from Turkey to Great Britain, the rest of Europe and elsewhere, as the epigraph above from Voltaire suggests.
Although variolation was a success (for example, as confirmed during the American Revolution by the immunity of British troops to smallpox outbreaks and Washington's later decision to inoculate his army3), significant and even fatal reactions acted as a brake on its use. No doubt this was the impetus for Jenner's epochal observation that cowpox, a mild illness in humans, could prevent smallpox. This discovery not only led to the eradication of smallpox in the twentieth century, but also gave cachet to the idea of deliberate protection against exposure to infectious diseases.
The history of vaccination as a deliberate endeavor began in the laboratory of Louis Pasteur. His aphorism that 'chance favors the prepared mind' was never more aptly illustrated than by his own discovery of attenuation. Pasteur was on vacation in the summer of 1881, and returned in the autumn to studies of chicken cholera, caused by what we call today Pasteurella multocida. A culture left on the bench during the summer was inoculated into chickens but did not cause disease. Pasteur then made a fresh culture and inoculated the same chickens, whether through parsimony or purpose we do not know. In any case, the chickens were resistant to the fresh challenge, and Pasteur realized that the aged culture had rendered them immune4.
From these observations Pasteur constructed the hypothesis that pathogens could be attenuated by exposure to environmental insults such as high temperature, oxygen and chemicals. His ensuing work on anthrax and rabies confirmed the hypothesis5. Table 1 outlines the strategies used subsequently for the development of live vaccines. In the next century, Calmette and Guérin used passage in artificial media to attenuate Mycobacterium bovis6, and Theiler used passage in mice and chick embryos to attenuate yellow fever virus7.
Table 1.
Live vaccines and their approximate times of availability
| Development strategy | Date | Vaccine or target |
|---|---|---|
| Use of related animal virus | 1798 | Smallpox |
| Chemical attenuation | 1885 | Rabies |
| 1881 | Anthrax | |
| Passage in vitro | 1927 | BCG |
| 1935 | Yellow fever | |
| Cell culture passage | 1962 | OPV |
| 1963 | Measles | |
| 1971 | Adenoviruses | |
| 1995 | Varicella | |
| 2005 | Rotavirus 89-12 | |
| Cell culture passage with cold adaptation | 1969 | Rubella |
| 2003 | Live influenza | |
| Auxotrophy | 1989 | Ty21a typhoid |
| Use of reassortants | 1970s | Inactivated influenza seed |
| 2003 | Live influenza | |
| 2005 | Rotavirus bovine-human |
OPV, oral polio vaccine.
Meanwhile, the concept of antibodies and cellular immune responses had developed from the original work of Paul Ehrlich and Ilya Metchnikoff, respectively, and measurement thereof by relatively primitive methods established the dual nature of the adaptive immune system. Vaccinologists consequently focused on stimulating these responses.
The cell-culture revolution
In the middle of the twentieth century, cell culture was adapted to growth of viruses8, and it was not long before it was realized that passage in cell culture was also a means of attenuation, presumably by fortuitous selection of mutants better adapted to replication in vitro than in the living host. Cell culture also permitted conscious selection of mutants by isolation of single clones and by incubation at temperatures below the normal temperature of the host. Thus, the period between 1950 and 1980 saw the development of numerous attenuated virus vaccines, including those for polio (Sabin oral), measles, rubella, mumps and varicella.
An important technology applicable to viruses with segmented genomes has been reassortment in cell culture. The development of influenza and rotavirus vaccines is greatly aided by the ability to mix RNA segments from attenuated strains with RNA encoding protective antigens from circulating wild strains. Both live and killed influenza vaccines are dependent on reassortment: live vaccine contains replicating reassortants, whereas inactivated vaccines are produced from live reassortant seeds9. Two of the three rotavirus vaccines developed so far have depended on reassortants containing the vp7 genes of human strains with the genes from animal rotaviruses nonpathogenic for humans10,11.
Inactivated vaccines
The idea of complete inactivation as a means of vaccine development also started in the nineteenth century, not long after Pasteur's original insight. Here priority probably goes to Daniel Salmon and Theobald Smith in the United States, although Pasteur's team, led by Emile Roux, made an independent discovery of the same principle2,12.
Table 2 outlines the subsequent strategies for the development of inactivated vaccines, the first ones being directed against the typhoid and cholera bacilli. More recently, the inactivated polio vaccine (Salk type), which together with oral vaccine has almost eradicated polio from the world, and the hepatitis A vaccine contain whole inactivated viruses.
Table 2.
Nonliving vaccines and their approximate times of availability
| Vaccine strategy | Date | Vaccine or target |
|---|---|---|
| Inactivated whole organisms | 1896 | Typhoid |
| 1896 | Cholera | |
| 1897 | Plague | |
| 1926 | Whole-cell pertussis | |
| 1938 | Influenza | |
| 1955 | IPV | |
| 1995 | Hepatitis A | |
| Use of extracts and subunits | 1944 | Japanese encephalitis |
| 1970s | Influenza | |
| 1960 | Anthrax | |
| 1976 | Cell-culture rabies | |
| Use of toxoids | 1923 | Diphtheria |
| 1927 | Tetanus | |
| 2008 (?) | New anthrax | |
| Use of capsular polysaccharides | 1977 | Pneumococcal |
| 1974 | Meningococcal | |
| 1995 | Typhoid | |
| Use of protein-conjugated capsular | 1987 | H. influenzae type b |
| polysaccharides | 2002 | Pneumococcal |
| 2002 | Meningococcal | |
| Future | Staphylococcal | |
| Use of purified or recombinant proteins | 1986 | Hepatitis Ba |
| 1996 | Acellular pertussisb | |
| 1998 | Lyme disease |
aPlasma-derived vaccine in 1981.
bEarlier in Japan. IPV, inactivated polio vaccine.
The recognition of extracellular bacterial toxins by Roux, Yersin, Behring and Kitasato permitted the development of toxoids (inactivated toxins) by Ramon for diphtheria and tetanus13,14. As technology advanced, it became possible to separate and use subunits of organisms in the form of extracts of infected tissues (e.g., rabies), capsular polysaccharides (e.g., typhoid Vi and pneumococci) and proteins (e.g., acellular pertussis). Late in the twentieth century, conjugation of proteins by polysaccharides became a powerful weapon against encapsulated bacteria (e.g., Haemophilus influenzae type b), when it was realized that infants would not respond to stimulation of B cells without a concomitant T-cell stimulation15. The use of peptides as vaccines has been slowed by the need for strong adjuvants, but peptides do have a role in experimental cancer vaccines16.
Genetic engineering
The advent of molecular biology and genetic engineering, as in every other domain of biology, has had a dramatic effect on vaccine development, providing greater opportunities for construction of inactivated antigens and for rational attenuation of organisms through directed mutation. Table 3 lists some of the newer strategies that depend on molecular biology.
Table 3.
Newer strategies for vaccine development starting from microbial DNA, cDNA or RNA
| Strategy | Examples of pathogens targeted |
|---|---|
| Recombinant protein production | Hepatitis B S Ag, pertussis toxin, Lyme outer surface protein A, CMV gB protein |
| Live recombinants carrying genes from related agents | Dengue genes in yellow fever 17D, parainfluenza 1 + 2 genes in parainfluenza 3, M. tuberculosis genes in BCG |
| Recombinant vectors recombining genes from pathogens | HIV, CMV |
| Alpha virus replicons | HIV, Hemorrhagic Fevers |
| Replication-defective particles | HPV, SARS |
| 'Naked' DNA plasmids | HIV and many others |
| Prime boost using DNA and/or vectors | HIV, malaria, tuberculosis |
| Reverse vaccinology | Meningococcus B |
| Microarrays for expression of virulence genes | Mainly bacteria |
| Synthetic peptides | Cancer, CTL vaccines |
| Synthetic capsular polysaccharides | Hib |
| Reverse genetics | Influenza, parainfluenza, RSV |
Hib, H. influenza type b; IPV, inactivated polio vaccine; T, tetanus; d, adult diphtheria dose; CMV, cytomegalovirus; HPV, human papillomavirus; HSV, herpes simplex virus; RSV, respiratory syncytial virus; HIV, human immunodeficiency virus; CTL, cytotoxic T lymphocyte.
The first success of genetic engineering was a hepatitis B vaccine manufactured in a yeast recombinant carrying the gene for the S protein17, which replaced a vaccine based on purification of S particles from plasma of infected individuals18. Subsequently, insertion of genes into yeast, Escherichia coli or Chinese hamster ovary cells enabled production of a variety of recombinant proteins, such as Lyme OspA19, cytomegalovirus gB20 and pertussis toxin21.
Recombinants of viruses and bacteria also may be used as live vaccines, on condition that they are apathogenic. For example, bovine or attenuated human parainfluenza 3 viruses can serve as the backbone for insertion of genes from other parainfluenza viruses or from respiratory syncytial virus22, and attenuated yellow fever virus can serve as the carrier for genes from dengue or West Nile viruses23. Protein expression by the inserted genes is immunizing.
The term 'vectored vaccine' is often used for live recombinants, as the key issue is to have a vector, or carrier, that will incorporate and express the gene for a pathogen without itself causing illness. Many viral and bacterial vectors have been proposed, but those most favored have been poxviruses, adenoviruses and Bacille Calmette-Guérin (BCG)24. All of these, as well as others, have been employed in an effort to develop HIV and malaria vaccines, often in a prime-boost configuration, so called because the immune system is primed with proteins expressed by injected DNA plasmid or vector, and then boosted with the same proteins in soluble form or expressed by another vector25,26,27. Alphavirus replicons are a special case of vectors, in which the replicon particle carries no viral genome but expresses foreign genes in a single cycle of replication28.
The production of some viral proteins in vitro results in self-assembly of structures resembling the whole virus, so-called virus-like particles (Fig. 1). The particles are more immunogenic than the soluble proteins, and have led to highly effective papillomavirus vaccines29,30 and other vaccines still to be tested in humans, such as one against SARS virus31. A widely used technology in modern vaccinology, both for antigen discovery and for vaccine development is the fruit of a discovery as fully serendipitous as that of Pasteur: that bacterial DNA plasmids containing genes from viral pathogens would express the corresponding proteins after intramuscular or intradermal injection in vivo32. The antigens thus produced are carried to the bone marrow, where antibody and cellular immunity are elicited33. Unfortunately, this technology has not proven reliable in humans, although induction of cellular immunity is more frequent than that of antibodies. Nevertheless, DNA plasmids have interesting properties, such as the ability to induce responses despite the presence of passive maternal antibodies34, and are still much studied.
Figure 1. Pseudo-particles of human papillomavirus type 16 formed by self-assembly of the L1 viral protein.
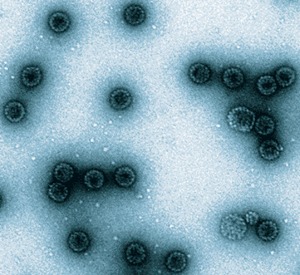
Courtesy of Drs. John Schiller and Susana Pang.
One particular application of DNA plasmids has been reverse genetics35,36, which has allowed the construction of novel negative-strand segmented RNA viruses by mutation of their cDNA and then introduction of multiple cDNA plasmids containing the entire viral genome into cell culture, together with other plasmids expressing enzymes for reconstitution of the virus. This technique is currently being incorporated into the manufacture of influenza vaccines, and will allow, for example, the more rapid production of seed virus for an H5 avian influenza vaccine if, as feared, the virus adapts to humans37.
The ability to sequence microbial genomes has permitted the identification of new protective factors. The predicted genes from a nucleotide sequence are expressed in E. coli, and the resulting proteins used to immunize mice. If antibodies are produced against the organism of interest, especially bactericidal or neutralizing antibodies that protect against a wide range of strains, the protein becomes of interest for further development. This process has been called 'reverse vaccinology'38. Similarly, microarray analysis can be used to identify the microbial genes that are turned on during invasive infection, and thus the proteins that may be virulence factors to be countered by prior immunization39,40.
Immunology finally helps vaccinology
It must be admitted that until recently immunology has not contributed much to the development of vaccines. As emphasized in an accompanying article41, most successes in immunization have been mediated through the induction of protective antibodies42, whereas the major challenges now facing us (e.g., HIV, malaria, tuberculosis) will require the induction of T cell immunity as well. Fortunately, several of the new strategies, including vectors, plasmid DNA and lipidated peptides43, are capable of inducing both CD4+ and CD8+ cellular responses. In addition, the paucity of adjuvants for vaccines, until recently essentially limited to aluminum salts that stimulate a T helper type 2 (TH2) response, is at last being corrected by the creation of new oil-in-water emulsions, liposomes, Toll-like receptor agonists, cytokines and other substances that push the immune system in a T helper type 1 (TH1) direction44.
Moreover, immunologists have recently provided us with tests for cellular immunity that can be done on a large scale, such as ELISPOT assays for cytokine induction and tetramer staining for CD8+ cell peptide specificity45. The recent rediscovery of T regulatory cells may also have an impact on vaccines for pathogens that try to evade the immune system46.
New means and new ends
In the early days of the twenty-first century, one can descry several notable tendencies in vaccine development. Combinations of vaccines have become ever more necessary as new components become part of routine vaccination. Already hexavalent combinations containing diphtheria, tetanus, pertussis, H. influenzae type b (Hib), hepatitis B and inactivated polio vaccines are used in Europe, and pentavalent combinations in many other parts of the world47. Varicella vaccine has been incorporated into measles-mumps-rubella vaccines, and various combinations of H. influenzae type b, pneumococcal and meningococcal conjugated bacterial polysaccharide vaccines will become available.
Another easily discerned tendency is toward the stimulation of innate as well as adaptive immune responses. This can be accomplished by the choice of proper adjuvants such as CpG oligonucleotides48, which stimulate both types of responses. Proteomics will probably advance to the point of allowing construction in vitro of proteins with more natural conformations, and polysaccharide synthesis is just beginning to be practical.
Whereas vaccination is usually considered as prophylaxis, serious attempts are being made to develop therapeutic vaccines for chronic infections49. The basic idea is to induce cellular immune responses that suppress infection, even when the host has been unable to mount those responses naturally. Examples include immunization against the E6 and E7 oncogenes of papillomaviruses for the treatment of cervical cancer, and against the gag and tat genes of HIV for the suppression of viral replication in AIDS50,51,52,53.
A very important part of the future is the enlargement of routes of immunization (Table 4). Most vaccines today are given by parenteral injection, which induces systemic immune responses expressed by B and T cells in the blood. But the need for mucosal immune responses has become increasingly obvious. The new live, attenuated influenza vaccine is given intranasally, induces both systemic and local responses and gives a broader protection against antigenically drifted strains54. Aerosol administration of measles and rubella vaccines implants the attenuated viruses at the natural sites of replication and elicits immunity equivalent to that after injection55. The aerosol route could lend itself to mass immunization using inhalation devices. Oral immunization has been used for some time to immunize with living organisms that replicate in the intestine, such as oral polio and typhoid Ty21a vaccines. Now attempts are being made to induce mucosal responses with nonliving antigens56. One approach is to develop oral vaccines from plants made transgenic for vaccine antigens57,58. Demonstration of adequate immune responses in human is awaited. Immunization by rectal or vaginal application of antigens is also under investigation59.
Table 4.
Nonparenteral routes of administration
| Route | Example of use |
|---|---|
| Intranasal | Live influenza |
| Aerosol | Measles |
| Rubella | |
| Oral | Plants transgenic for Hepatitis BsAg |
| Transcutaneous (patches, microneedles, powder) | Hepatitis B, anthrax |
Closest to actual use is vaccination by transdermal application60,61,62,63,64. Many devices have been developed to deliver antigens across the skin. These include patches containing adjuvant applied to lightly abraded skin and microneedles to pierce the stratum corneum. Figure 2 shows one such microneedle device. Once past the superficial layer, the antigen comes in contact with dendritic antigen-presenting cells, which travel to lymph nodes and initiate immune responses61. If transdermal immunization works well, vaccination practice could be revolutionized.
Figure 2. Scanning electron photomicrograph of a microprojection array used to deliver antigen to the skin.
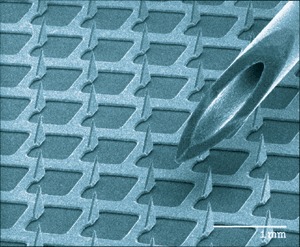
A 25-gauge needle is shown (at right) for size comparison. Figure reprinted from ref. 87 courtesy of J. Matriano (ALZA Corporation) with kind permission of Springer Science and Business Media.
Extension to noninfectious diseases
Active immunization has heretofore been largely confined to infectious diseases, with some use of desensitization to treat allergies. Now consideration is being given to immunization against a wide variety of noninfectious diseases. Most effort is being directed against cancers, in which novel cellular antigens are often present65,66. Vaccine incorporating proteins or peptides from cancer antigens are in advanced trials, with promising results measured by prolongation of life. It is also intriguing that individuals with inherited mutations that predispose to cancer might be immunized prophylactically before cancer develops.
Tolerization to autoantigens is being attempted in many autoimmune diseases, such as multiple sclerosis67 and diabetes mellitus68. Better antigens for inducing IgG rather than IgE antibodies against allergens are in development69. Contraception can be maintained by immunization against hormones70. Atherosclerosis and Alzheimer disease can perhaps be controlled by immunization against cholesterol fractions or amyloid, respectively71,72. Lastly, drug addictions, including nicotine, methamphetamine and cocaine, may be controllable by inducing antibodies that rapidly remove the drugs from the body73.
New targets
New populations are being targeted for vaccination, as summarized in Table 5. Until now, most vaccination has been directed at infants and children; but it has become increasingly clear that adolescents and adults also need universal immunizations. Aside from new recommendations for booster immunization with diphtheria-tetanus-acellular pertussis vaccine74, the possible incorporation of vaccines against meningococci, papillomaviruses, Herpes simplex75 and cytomegalovirus76 into routine vaccination will require an adolescent immunization date to prevent, respectively, sepsis, cervical cancer, genital herpes and congenital infection. Adults currently receive influenza and pneumococcal vaccines, but vaccination may also come into play against varicella virus to prevent reactivation in the form of zoster77. Also, during the course of their lives, adults may need vaccination during pregnancy, hospitalization and travel. An experimental Group B streptococcal vaccine is available to prevent transmission of the bacteria from mothers to neonates78 and pregnant women could be immunized against a number of other pathogens (e.g., pneumococci, respiratory syncytial virus) in order to transmit protective antibodies that will protect their newborns for some months79. Antibiotic-resistant nosocomial bacteria are an increasing problem and a staphylococcal capsular polysaccharide vaccine is in a later stage of development for patients susceptible to secondary infections80.
Table 5.
New target groups for vaccination
| Groups | Vaccine targets |
|---|---|
| Infants (combination vaccines) | Diphtheria, tetanus, acellular pertussis, Hameophilus influenzae type b, hepatitis B, inactivated polio vaccine |
| Adolescents | Tetanus, adult diphtheria dose, acellular pertussis, CMV, HPV, HSV-2 |
| Adults | Zoster, HSV-2 |
| Hospital patients | Staphylococcal, Candida |
| Pregnant women | Group B Streptococcus, RSV |
| Civil defense workers | New vaccinia, anthrax, plague, Ebola, etc. |
| Individuals with noninfectious diseases | Cancer, Alzheimer disease, dental caries, autoimmune disorders, drug addiction |
| Individuals with chronic infections (therapeutic vaccines) | HIV, HPV |
CMV, cytomegalovirus; HPV, human papillomavirus; HSV, herpes simplex virus; RSV, respiratory syncytial virus; HIV, human immunodeficiency virus.
Although bioterrorism is unpleasant to think about, augmented financial support has stimulated development of new vaccines against anthrax, plague and smallpox, to name but a few. In the case of anthrax and plague, purified antigens will afford better protection and safety, whereas poxvirus research has generated attenuated strains of vaccinia to protect against smallpox81,82.
New problems
The prospects for control of diseases by vaccination are thus quite bright, but it must be admitted that several problems loom large and darken the picture. First, vaccine supply is insufficient. Even in industrialized countries, shortages of vaccines occur because there are too few manufacturers, and regulatory pressures render production ever more difficult. In the event of an emergency, such as an influenza pandemic, it is difficult to see how demand could be satisfied or access provided to developing countries. The growth of new manufacturers in developing countries like India, China, Indonesia and Brazil may fill this gap, but the solution to supply shortage is not yet clear.
Cost of vaccines is also now a problem, because new vaccines require $300 to $800 million to develop and those companies that do research and development must recoup the costs. If vaccines are to be applied broadly throughout the world, several circumstances must be maintained: higher price in developed countries, recognition by governments that the financial savings because of vaccination justify expenditures to buy vaccines, and support by donor agencies of vaccine purchases for poor countries. When the vaccine target is one that concerns only developing countries, the problem becomes even more difficult. The support of the Bill and Melinda Gates Foundation for the development of vaccines against those targets has been crucial, but at a certain point industrial manufacture will be necessary. This will require vaccine production facilities outside of developed countries or subsidized facilities at major manufacturers.
There is a growing demand for vaccine safety, fueled in part by antivaccination groups. As disease recedes, the need for vaccination becomes less evident to the public, and more people opt out of the social contract to be vaccinated, depending instead on the herd immunity of surrounding vaccinated persons. Of course, herd immunity will fail if too many refuse to be vaccinated. But there are real safety problems associated with vaccines, such as paralysis after oral polio vaccine83 and disseminated infections after Bacille Calmette-Guérin84. For that reason, older vaccines need to be reexamined to see whether safety can be improved, as was done through replacement of whole-cell pertussis vaccine by acellular pertussis vaccines and replacement of rabies vaccine made in brain by vaccine made in cell culture. In the near future, Jenner's vaccinia will be replaced by further attenuated vaccinia82 and Bacille Calmette-Guérin by engineered vaccines for tuberculosis85. Indeed, one of the advantages of the newer molecular technologies is improved safety. As risk-benefit ratios become more controversial when disease presence declines, it will be important to reduce vaccine-associated reactions to a minimum. On the other hand, zero risk is impossible to attain, and there will always be tension between the needs of public health and the regulatory impulse to guard against even remote and theoretical risks. The latter tendency acts as a brake on the rapid application of new public health measures. Thus, there is disagreement as to whether to err on the side of safety or of disease prevention.
As vaccines are key tools for maintenance of public health, governments have a major role in their dissemination through recommendations and purchase. Although governmental agencies (particularly the US National Institutes of Health) importantly support the basic research that provides candidate vaccines, their direct involvement in industrial development and production has decreased. It is doubtless more efficient for industry to take vaccines from concept to license, but governments should advise about the choice of targets for vaccine development and guarantee markets for products developed at their request. Moreover, it has become obvious that governments must be proactive in preventing vaccine shortages by inducements for multiple suppliers.
There are many diseases as yet uncontrolled by vaccination, and new diseases are sure to emerge through evolution by mutation and gene exchange, interspecies transfer or human exposure to new environments86. Fortunately, we have many new tools with which to produce protective antigens. Two hundred years of research have enabled us to turn the immune system to our advantage, and increased understanding of microbial pathogenesis and host responses should allow us to extend control of disease by vaccination.
Competing interests
Stanley A. Plotkin is an employee of Sanofi Pasteur.
References
- 1.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. History of International Public Health, No. 6. 1988. Early efforts at control: variolation, vaccination, and isolation and quarantine; pp. 245–276. [Google Scholar]
- 2.Plotkin SL, Plotkin SA. Vaccines. 2004. A short history of vaccination; pp. 1–15. [Google Scholar]
- 3.Fenn, E.A. Pox Americana: The Great Smallpox Epidemic of 1775-82. 1 (Hill and Wang, New York, 2001). [PubMed]
- 4.Pasteur L. De l'attenuation du virus du cholera des poules. C. R. Acad. Sci. Paris. 1880;91:673–680. [Google Scholar]
- 5.Pasteur L, Chamberland C-E. Sur la vaccination charbonneuse. C. R. Acad. Sci. Paris. 1881;92:1378–1383. [Google Scholar]
- 6.Calmette A, Guerin C, Breton M. Contribution a l'etude de la tuberculose experimental du cobaye (infection et essais de vaccination par la voie digestive) Ann. Inst. Pasteur Paris. 1907;21:401–416. [Google Scholar]
- 7.Theiler M, Smith HH. The use of yellow fever virus by in vitro cultivation for human immunization. J. Exp. Med. 1937;65:787–800. doi: 10.1084/jem.65.6.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weller TH. Cultivation of poliomyelitis virus in cultures of human foreskin and embryonic tissues. Proc. Soc. Exp. Biol. Med. 1949;72:153–155. doi: 10.3181/00379727-72-17359. [DOI] [PubMed] [Google Scholar]
- 9.Kilbourne ED, Murphy JS. Genetic studies of influenza viruses. I. Viral morphology and growth capacity as exchangeable genetic traits. Rapid in ovo adaptation of early passage Asian strain isolates by combination with PR8. J. Exp. Med. 1960;111:387–406. doi: 10.1084/jem.111.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clark HF, Offit P, Glass RI, Ward RL. Vaccines. 2004. Rotavirus vaccines; pp. 1327–1345. [Google Scholar]
- 11.Bernstein DI, et al. Safety and immunogenicity of live, attenuated human rotavirus vaccine 89-12. Vaccine. 1998;16:381–387. doi: 10.1016/S0264-410X(97)00210-7. [DOI] [PubMed] [Google Scholar]
- 12.Salmon DE, Smith T. On a new method of producing immunity from infectious diseases. Am. Vet. Rev. 1886;10:63–69. [Google Scholar]
- 13.Ramon G. Sur le pouvoir floculant et sur les proprietes immunisantes d'une toxine diphterique rendu anatoxique (anatosine) C. R. Acad. Sci. Paris. 1923;177:1338–1340. [Google Scholar]
- 14.Ramon G, Zeller C. De la valeur antigenique de l'anatoxine tetanique chez l'homme. C. R. Acad. Sci. Paris. 1926;182:245–247. [Google Scholar]
- 15.Schneerson R, Barrera O, Sutton A, Robbins JB. Preparation, characterization, and immunogenicity of Haemophilus influenzae type b polysaccharide-protein conjugates. J. Exp. Med. 1980;152:361–376. doi: 10.1084/jem.152.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moingeon P. Cancer vaccines. Vaccine. 2001;9:1305–1326. doi: 10.1016/S0264-410X(00)00372-8. [DOI] [PubMed] [Google Scholar]
- 17.McAleer WJ, Buynak EB, Maigetter RZ, Wampler DE, Miller WJ, Hilleman MR. Human hepatitis B vaccine from recombinant yeast. Nature. 1984;307:178–180. doi: 10.1038/307178a0. [DOI] [PubMed] [Google Scholar]
- 18.Szmuness W, Stevens CE, Zang EA, Harley EJ, Kellner A. A controlled clinical trial of the efficacy of the hepatitis B vaccine (Heptavax B): a final report. Hepatology. 1981;1:377–385. doi: 10.1002/hep.1840010502. [DOI] [PubMed] [Google Scholar]
- 19.Fikrig E, Barthold SW, Kantor FS, Flavell RA. Protection of mice against the Lyme disease agent by immunizing with recombinant OspA. Science. 1990;250:553–556. doi: 10.1126/science.2237407. [DOI] [PubMed] [Google Scholar]
- 20.Spaete RR. A recombinant subunit vaccine approach to HCMV vaccine development. Transplant. Proc. 1991;23:90–96. [PubMed] [Google Scholar]
- 21.Pizza M, et al. Mutants of pertussis toxin suitable for vaccine development. Science. 1989;246:497–500. doi: 10.1126/science.2683073. [DOI] [PubMed] [Google Scholar]
- 22.Durbin AP, Karron RA. Progress in the development of respiratory syncytial virus and parainfluenza virus vaccines. Clin. Infect. Dis. 2003;37:1668–1677. doi: 10.1086/379775. [DOI] [PubMed] [Google Scholar]
- 23.Guirakhoo F, et al. Safety and efficacy of chimeric yellow Fever-dengue virus tetravalent vaccine formulations in nonhuman primates. J. Virol. 2004;78:4761–4775. doi: 10.1128/JVI.78.9.4761-4775.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plotkin SA. Six revolutions in vaccinology. Pediatr. Infect. Dis. J. 2005;24:1–9. doi: 10.1097/01.inf.0000148933.08301.02. [DOI] [PubMed] [Google Scholar]
- 25.Excler JL, Plotkin S. The prime-boost concept applied to HIV preventive vaccines. AIDS. 1997;11(Suppl A):S127–S137. [PubMed] [Google Scholar]
- 26.Amara RR, et al. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Vaccine. 2002;20:1949–1955. doi: 10.1016/S0264-410X(02)00076-2. [DOI] [PubMed] [Google Scholar]
- 27.Moore AC, Hill AV. Progress in DNA-based heterologous prime-boost immunization strategies for malaria. Immunol. Rev. 2004;199:126–143. doi: 10.1111/j.0105-2896.2004.00138.x. [DOI] [PubMed] [Google Scholar]
- 28.Rayner JO, Dryga SA, Kamrud KI. Alphavirus vectors and vaccination. Rev. Med. Virol. 2002;12:279–296. doi: 10.1002/rmv.360. [DOI] [PubMed] [Google Scholar]
- 29.Schiller JT, Davies P. Delivering on the promise: HPV vaccines and cervical cancer. Nat. Rev. Microbiol. 2004;2:343–347. doi: 10.1038/nrmicro867. [DOI] [PubMed] [Google Scholar]
- 30.Koutsky LA, et al. A controlled trial of a human papillomavirus type 16 vaccine. N. Engl. J. Med. 2002;347:1645–1651. doi: 10.1056/NEJMoa020586. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y, Yang Z-Y, Kong WP, Nabel GJ. Generation of synthetic severe acute respiratory syndrome coronavirus pseudoparticles: implications for assembly and vaccine production. J. Virol. 2004;78:12557–12565. doi: 10.1128/JVI.78.22.12557-12565.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ulmer JB, et al. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 33.Robinson HL. Nucleic acid vaccines: an overview. Vaccine. 1997;15:785–787. doi: 10.1016/S0264-410X(96)00249-6. [DOI] [PubMed] [Google Scholar]
- 34.Reddy ST, Ertl HC. The potential use of DNA vaccines for neonatal immunization. Curr. Opin. Mol. Ther. 1999;1:22–29. [PubMed] [Google Scholar]
- 35.Neumann G, et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc. Natl. Acad. Sci. USA. 1999;96:9345–9350. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palese P, Garcia SA. Influenza vaccines: present and future. J. Clin. Invest. 2002;110:9–13. doi: 10.1172/JCI0215999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wood JM, Robertson JS. From lethal virus to life-saving vaccine: developing inactivated vaccines for pandemic influenza. Nat. Rev. Microbiol. 2004;2:842–847. doi: 10.1038/nrmicro979. [DOI] [PubMed] [Google Scholar]
- 38.Mora M, Veggi D, Santini L, Pizza M, Rappuoli R. Reverse vaccinology. Drug Discov. Today. 2003;8:459–464. doi: 10.1016/S1359-6446(03)02689-8. [DOI] [PubMed] [Google Scholar]
- 39.Dhiman N, Bonilla R, O'Kane DJ, Poland GA. Gene expression microarrays: a 21st century tool for directed vaccine design. Vaccine. 2001;20:22–30. doi: 10.1016/S0264-410X(01)00319-X. [DOI] [PubMed] [Google Scholar]
- 40.Serruto D, Adu-Bobie J, Capecchi B, Rappuoli R, Pizza M, Masignani V. Biotechnology and vaccines: application of functional genomics to Neisseria meningitidis and other bacterial pathogens. J. Biotechnol. 2004;113:15–32. doi: 10.1016/j.jbiotec.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 41.Lambert P-H. Companion paper. Nat. Med. 2005;11(Suppl 1):S54–S62. doi: 10.1038/nm1216. [DOI] [PubMed] [Google Scholar]
- 42.Plotkin SA. Immunologic correlates of protection induced by vaccination. Pediatr. Infect. Dis. J. 2001;20:63–75. doi: 10.1097/00006454-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 43.BenMohamed L, Wechsler SL, Nesburn AB. Lipopeptide vaccines—yesterday, today, and tomorrow. Lancet Infect. Dis. 2002;2:425–431. doi: 10.1016/S1473-3099(02)00318-3. [DOI] [PubMed] [Google Scholar]
- 44.Moingeon P, Haensler J, Lindberg A. Towards the rational design of Th1 adjuvants. Vaccine. 2001;19:4363–4372. doi: 10.1016/S0264-410X(01)00193-1. [DOI] [PubMed] [Google Scholar]
- 45.Autran B, Molet L, Lederman MM. Practical Guidelines in Antiviral Therapy. 2002. Host defenses against viral infection; pp. 65–94. [Google Scholar]
- 46.O'Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat. Med. 2004;10:801–805. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 47.Decker MD, Edwards KM, Bogaerts HH. Vaccine. 2004. Combination vaccines; pp. 825–861. [Google Scholar]
- 48.Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat. Rev. Immunol. 2004;4:249–258. doi: 10.1038/nri1329. [DOI] [PubMed] [Google Scholar]
- 49.Sela M, Hilleman MR. Therapeutic vaccines: realities of today and hopes for tomorrow. Proc. Natl. Acad. Sci. USA. 2004;101(Suppl 2):14559. doi: 10.1073/pnas.0405924101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vandepapeliere P. Therapeutic vaccination against chronic viral infections. Lancet Infect. Dis. 2002;2:353–367. doi: 10.1016/S1473-3099(02)00289-X. [DOI] [PubMed] [Google Scholar]
- 51.Hallez S, et al. Phase I/II trial of immunogenicity of a human papillomavirus (HPV) type 16 E7 protein-based vaccine in women with oncogenic HPV-positive cervical intraepithelial neoplasia. Cancer Immunol. Immunother. 2004;53:642–650. doi: 10.1007/s00262-004-0501-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Markowitz M, et al. Discontinuation of antiretroviral therapy commenced early during the course of human immunodeficiency virus type 1 infection, with or without adjunctive vaccination. J. Infect. Dis. 2002;186:634–643. doi: 10.1086/342559. [DOI] [PubMed] [Google Scholar]
- 53.Buckner C, et al. Priming B cell-mediated anti-HIV envelope responses by vaccination allows for the long-term control of infection in macaques exposed to a R5-tropic SHIV. Virology. 2004;320:167–180. doi: 10.1016/j.virol.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 54.Belshe RB, et al. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J. Pediatr. 2000;136:168–175. doi: 10.1016/S0022-3476(00)70097-7. [DOI] [PubMed] [Google Scholar]
- 55.Sepulveda-Amor J, et al. A randomized trial demonstrating successful boosting responses following simultaneous aerosols of measles and rubella (MR) vaccines in school age children. Vaccine. 2002;20:2790–2795. doi: 10.1016/S0264-410X(02)00179-2. [DOI] [PubMed] [Google Scholar]
- 56.Jones T, et al. A nasal Proteosome influenza vaccine containing baculovirus-derived hemagglutinin induces protective mucosal and systemic immunity. Vaccine. 2003;21:3706–3712. doi: 10.1016/S0264-410X(03)00387-6. [DOI] [PubMed] [Google Scholar]
- 57.Tacket CO. Garden-variety vaccines: antigens derived from transgenic plants. Expert Rev. Vaccines. 2004;3:529–531. doi: 10.1586/14760584.3.5.529. [DOI] [PubMed] [Google Scholar]
- 58.Webster DE, et al. Successful boosting of a DNA measles immunization with an oral plant-derived measles virus vaccine. J. Virol. 2002;76:7910–7912. doi: 10.1128/JVI.76.15.7910-7912.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stevceva L, Strober W. Mucosal HIV vaccines: where are we now? Curr. HIV Res. 2004;2:1–10. doi: 10.2174/1570162043485004. [DOI] [PubMed] [Google Scholar]
- 60.Hammond SA, Walwender D, Alving CR, Glenn GM. Transcutaneous immunization: T cell responses and boosting of existing immunity. Vaccine. 2001;19:2701–2707. doi: 10.1016/S0264-410X(00)00506-5. [DOI] [PubMed] [Google Scholar]
- 61.Glenn GM, Kenney RT, Ellingsworth LR, Frech SA, Hammond SA, Zoeteweij JP. Transcutaneous immunization and immunostimulant strategies: capitalizing on the immunocompetence of the skin. Expert Rev. Vaccines. 2003;2:253–267. doi: 10.1586/14760584.2.2.253. [DOI] [PubMed] [Google Scholar]
- 62.Matyas GR, Friedlander AM, Glenn GM, Little S, Yu J, Alving CR. Needle-free skin patch vaccination method for anthrax. Infect. Immun. 2004;72:1181–1183. doi: 10.1128/IAI.72.2.1181-1183.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen D, et al. Epidermal powder immunization of mice and monkeys with an influenza vaccine. Vaccine. 2003;21:2830–2836. doi: 10.1016/S0264-410X(03)00175-0. [DOI] [PubMed] [Google Scholar]
- 64.Mikszta JA, Alarcon JB, Brittingham JM, Sutter DE, Pettis RJ, Harvey NG. Improved genetic immunization via micromechanical disruption of skin-barrier function and targeted epidermal delivery. Nat. Med. 2002;8:415–419. doi: 10.1038/nm0402-415. [DOI] [PubMed] [Google Scholar]
- 65.Finn OJ. Cancer vaccines: between the idea and the reality. Nat. Rev. Immunol. 2003;3:630–641. doi: 10.1038/nri1150. [DOI] [PubMed] [Google Scholar]
- 66.Lewis JJ. Therapeutic cancer vaccines: using unique antigens. Proc. Natl. Acad. Sci. USA. 2004;101(Suppl 2):14653–14656. doi: 10.1073/pnas.0404839101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hohlfeld R, Wekerle H. Autoimmune concepts of multiple sclerosis as a basis for selective immunotherapy: from pipe dreams to (therapeutic) pipelines. Proc. Natl. Acad. Sci. USA. 2004;101:14599–14606. doi: 10.1073/pnas.0404874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petrovsky N, Silva D, Schatz DA. Vaccine therapies for the prevention of type 1 diabetes mellitus. Paediatr. Drugs. 2003;5:575–582. doi: 10.2165/00148581-200305090-00001. [DOI] [PubMed] [Google Scholar]
- 69.Niederberger V, et al. Vaccination with genetically engineered allergens prevents progression of allergic disease. Proc. Natl. Acad. Sci. USA. 2004;101(Suppl 2):14677–14682. doi: 10.1073/pnas.0404735101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh M, Das SK, Suri S, Singh O, Talwar GP. Regain of fertility and normality of progeny born during below protective threshold antibody titers in women immunized with the HSD-hCG vaccine. Am. J. Reprod. Immunol. 1998;39:395–398. doi: 10.1111/j.1600-0897.1998.tb00376.x. [DOI] [PubMed] [Google Scholar]
- 71.Mettens P, Monteyne P. Life-style vaccines. Br. Med. Bull. 2002;62:175–186. doi: 10.1093/bmb/62.1.175. [DOI] [PubMed] [Google Scholar]
- 72.Gelinas DS, DaSilva K, Fenili D, George-Hyslop P, McLaurin J. Immunotherapy for Alzheimer's disease. Proc. Natl. Acad. Sci. USA. 2004;101(Suppl 2):14657–14662. doi: 10.1073/pnas.0404866101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kantak KM, Collins SL, Lipman EG, Bond J, Giovanoni K, Fox BS. Evaluation of anti-cocaine antibodies and a cocaine vaccine in a rat self-administration model. Psychopharmacology (Berl.) 2000;148:251–262. doi: 10.1007/s002130050049. [DOI] [PubMed] [Google Scholar]
- 74.Forsyth KD, et al. New pertussis vaccination strategies beyond infancy: recommendations by the Global Pertussis Initiative. Clin. Infect. Dis. 2004;39:1802–1809. doi: 10.1086/426020. [DOI] [PubMed] [Google Scholar]
- 75.Stanberry LR. Herpes. 2004. pp. 161A–169A. [PubMed] [Google Scholar]
- 76.Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin. Infect. Dis. 2004;39:233–239. doi: 10.1086/421999. [DOI] [PubMed] [Google Scholar]
- 77.Levin MJ, et al. Decline in varicella-zoster virus (VZV)-specific cell-mediated immunity with increasing age and boosting with a high-hose VZV vaccine. J. Infect. Dis. 2003;188:1336–1344. doi: 10.1086/379048. [DOI] [PubMed] [Google Scholar]
- 78.Baker CJ, Paoletti LC, Rench MA, Guttormsen HK, Edwards MS, Kasper DL. Immune response of healthy women to 2 different group B streptococcal type V capsular polysaccharide-protein conjugate vaccines. J. Infect. Dis. 2004;189:1103–1112. doi: 10.1086/382193. [DOI] [PubMed] [Google Scholar]
- 79.Munoz FM, Piedra PA, Glezen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine. 2003;21:3465–3467. doi: 10.1016/S0264-410X(03)00352-9. [DOI] [PubMed] [Google Scholar]
- 80.Fattom AI, Horwith G, Fuller S, Propst M, Naso R. Development of StaphVAX, a polysaccharide conjugate vaccine against S. aureus infection: from the lab bench to phase III clinical trials. Vaccine. 2004;22:880–887. doi: 10.1016/j.vaccine.2003.11.034. [DOI] [PubMed] [Google Scholar]
- 81.Hassani M, Patel MC, Pirofski LA. Vaccines for the prevention of diseases caused by potential bioweapons. Clin. Immunol. 2004;111:1–15. doi: 10.1016/j.clim.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 82.McCurdy LH, Larkin BD, Martin JE, Graham BS. Modified vaccinia Ankara: potential as an alternative smallpox vaccine. Clin. Infect. Dis. 2004;38:1749–1753. doi: 10.1086/421266. [DOI] [PubMed] [Google Scholar]
- 83.WHO collaborative study group: The relationship between persisting spinal paralysis and poliomyelitis vaccine—results of a ten-year enquiry. Bull WHO60, 231–242 (1982). [PMC free article] [PubMed]
- 84.Hoft DF, et al. Clinical reactogenicity of intradermal bacille Calmette-Guerin vaccination. Clin. Infect. Dis. 1999;28:785–790. doi: 10.1086/515201. [DOI] [PubMed] [Google Scholar]
- 85.Horwitz MA, Harth G. A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infect. Immun. 2003;71:1672–1679. doi: 10.1128/IAI.71.4.1672-1679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morse SS. The Evolutionary Biology of Viruses. 1994. The viruses of the future? Emerging viruses and evolution; pp. 325–335. [Google Scholar]
- 87.Matriano JA, et al. Macroflux microprojection array patch technology: a new and efficient approach for intracutaneous immunization. Pharm. Res. 2002;19:63–70. doi: 10.1023/A:1013607400040. [DOI] [PubMed] [Google Scholar]


