Abstract
Concussive brain injury (CBI) is encountered by clinicians in sports medicine, pediatrics, neurosurgery, neurology, physiatry, and primary care. There is no gold standard diagnostic test for CBI, nor is there consensus on what neuro-musculoskeletal physical examination (PE) tests should be performed on patients who have sustained CBI. This paper presents an approach to the history and PE of the patient who has sustained a CBI that is based on a review of the literature evidence and the authors’ extensive experience with this patient population. Suggested components include an elemental neurological exam that emphasizes the oculomotor/ophthalmologic and vestibular systems, as well as appropriate musculoskeletal assessment of the craniocervical and upper shoulder girdle complex. The use of supplementary tests for CBI, including assessment of exercise tolerance using the Buffalo Concussion Treadmill Test and tests of neurocognitive function, can aid in the differential diagnosis of CBI. The proposed protocol is envisioned for initial and follow-up assessments in the clinic after CBI, as well as for those with more protracted signs or symptoms. If symptoms persist beyond 2 weeks in adults or 4 weeks in adolescents, then referral to a multi-disciplinary center that focuses on CBI is recommended.
Keywords: concussion, concussive brain injury, assessment, physical examination
Introduction
Concussive brain injury (CBI) typically results in reversible neurological dysfunction caused by a direct blow to the head, neck, or elsewhere on the body, with an accelerative or de-accelerative force transmitted to the head.2 CBI is common in everyday life and is encountered by clinicians in sports medicine, pediatrics, neurosurgery, neurology, physiatry, and primary care.3 Outcomes are optimized when patients are seen earlier rather than later and when they are supported throughout their recovery.4 It is of paramount importance to identify symptom generators given that these patients often have different etiologies for their complaints that may evolve over time.5–7 Of equal importance is avoiding use of inappropriate terminology in documenting post-CBI symptoms and signs.8 In this context, we discourage the use of the term post-concussion syndrome (PCS) given the following facts:
A syndrome is a consistent set of findings associated with a condition with symptom linkage and coupling of symptom resolution.9
There is no consistency to the signs or symptoms of concussion.
There is no symptom or set of symptoms that are a-priori diagnostic of CBI.2
After initial assessment, patients with CBI ideally should be seen every few weeks and as clinically necessary to monitor recovery.10 If they develop persistent symptoms, which some have referred to as Persistent Post-Concussive Symptoms (PPCS), the frequency can be reduced provided symptomology and functional status remain stable or are improving. There is no symptom burden threshold for the diagnostic label of PPCS and careful differential diagnosis is important so as to not label someone with non-cerebral based impairments as concussed if their symptoms have an alternate explanation such as cranial trauma or cervical whiplash. The 5th Conference In Sport Group (CISG) guidelines defined PPCS as symptoms persisting for more than 2 weeks in adults and for more than 1 month in children and adolescents.2 This timeframe, however, has been defined inconsistently across various studies published on this topic.
There is no gold standard diagnostic test for CBI and no consensus on what tests should be performed for the neuro-musculoskeletal examination of patients who have sustained CBI.11 Recent literature emphasizes the need for holistic assessment, including musculoskeletal, given the increased risk of these injuries in the concussion population.12 This paper presents a detailed assessment methodology for persons with CBI, or claimed CBI, for use in the outpatient setting by physicians and advance practice providers. Evidence-based medicine from the published literature has been integrated into the protocol where it is relevant and available. The proposed protocol is envisioned for initial and follow-up assessments in the outpatient setting after CBI but not in the acute setting (i.e. sideline or emergency room).
Classification
It is useful to identify predominant signs and symptoms of CBI to begin to understand the underlying generators and help direct specific treatments4 (Figure 1). CBI is a spectrum disorder, as are the comorbidities that often accompany it. It is therefore not possible to place all patients with CBI into specific categories due to the heterogeneity of the mechanisms of injury (both cerebral and otherwise) as well as the high rate of co-morbid conditions (i.e., cervical whiplash, depression, anxiety/PTSD, pain, and sleep disruption). Clinicians must understand that symptoms may be due to more than one post-traumatic insult. One classification system defines Autonomic/Physiological CBI as persistent autonomic dysfunction and altered control of cerebral blood flow secondary to the global cerebral metabolic disturbance that occurs after CBI.13 These patients typically present with minimal physical examination (PE) abnormalities although they may demonstrate oculomotor and/or vestibular impairments.14 Autonomic/Physiological CBI patients frequently experience early exercise intolerance on graded exercise testing (i.e., exercise limited by symptom exacerbation at < 70% of age-predicted maximum heart rate (HR) on the Buffalo Concussion Treadmill Test, BCTT).15 Vestibulo-ocular and cervicogenic/cervicocephalic post-traumatic disorders (PTD) mimic post-CBI symptomatology. These disorders are not labelled as true post-concussive disorders because they are not considered to represent ongoing global metabolic and cerebrovascular disturbance of brain function but rather injuries to the central oculomotor and vestibular sub-systems and/or to the upper cervical spine, respectively.16 These patients may demonstrate exercise intolerance during exertion testing but symptom exacerbation typically occurs at a significantly greater workload (beyond 70% of age-predicted maximum HR) than in Autonomic/Physiological CBI.17,18 Some patients have primarily affective and/or cognitive symptoms that may be related to CBI, although other etiologies should be considered. Some of these etiologies include reactive affective states, sleep-wake cycle disruption, nocebo effects of the diagnosis, or post-traumatic pain issues. In the authors’ experience, PPCS patients with primarily affective and/or cognitive impairments are capable of exercising to exhaustion during graded exercise testing without symptom exacerbation. These patients require an interdisciplinary team approach to treatment that may include a physician, physical therapist, psychologist and/or a speech-language pathologist.19,20
Figure 1.
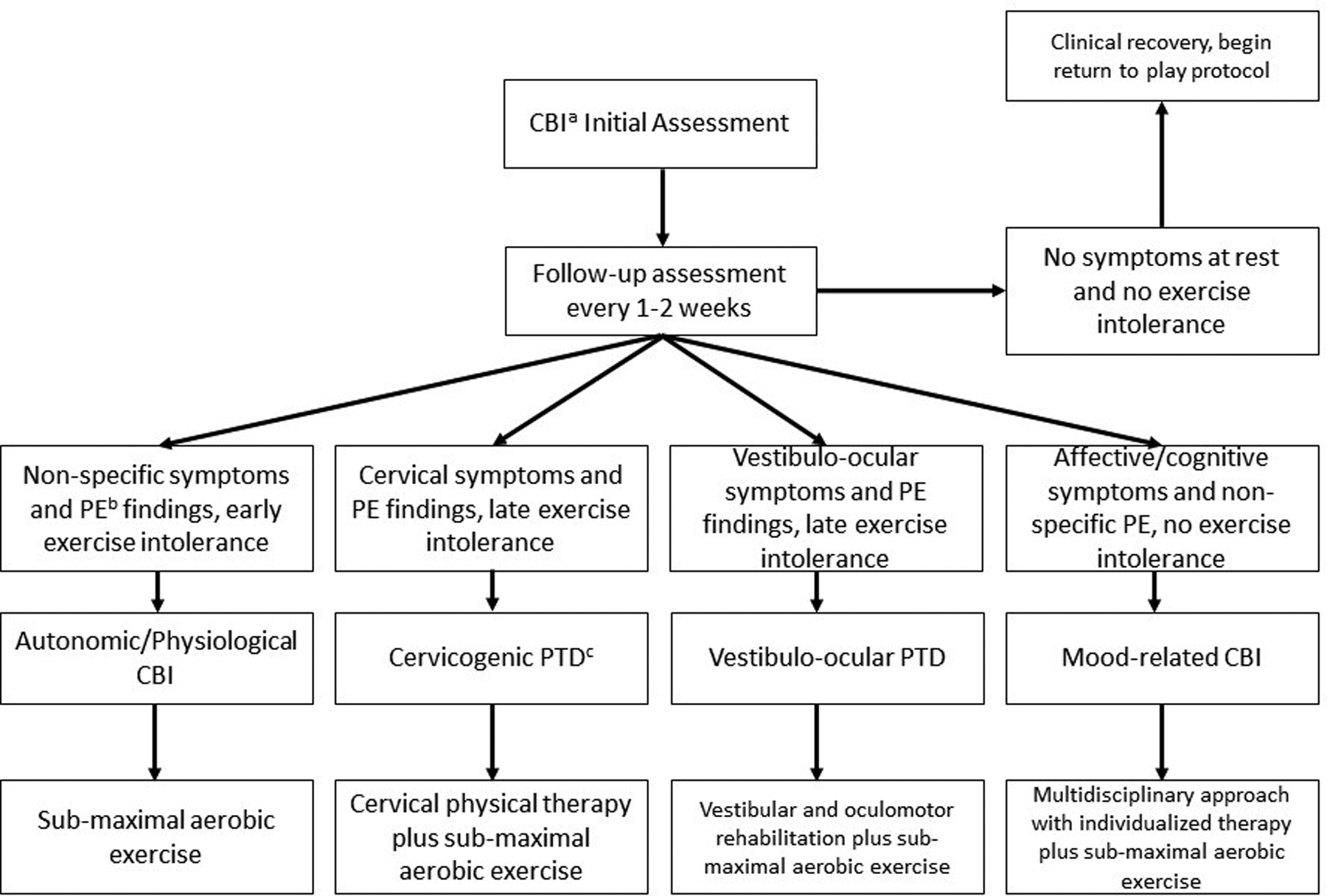
Overview of classification and management
a: concussive brain injury
b: physical examination
c: post-traumatic disorder
History
The first step in CBI assessment in the outpatient setting is a detailed account of the injury and a comprehensive medical history. The history should ideally be taken from multiple sources and should include a validated concussion symptom questionnaire [e.g., the Post-Concussion Symptom Scale (PCSS)21 and the Post-Concussion Symptom Inventory (PCSI)22] and assessment of neurocognitive (NC) function and behavior. Symptom questionnaires have been advocated to facilitate a more thorough systems review and focus subsequent assessment, but some have questioned the potential downside of symptom questionnaires since they may reinforce illness behavior and/or create reporting bias that encourages over-endorsement of symptoms that might not otherwise have been reported on free recall inquiry.18,23 Information about symptoms is essential to CBI management, but relying solely on subjective reports is problematic because there can be significant variation in symptom reporting, from athletes who may under-report symptoms to persons with potential secondary gain incentives who are more likely to over-report symptom severity and frequency.24 It is important to document pre-existing medical conditions that may put a patient at increased risk of developing PPCS or confound assessment relative to apportionment of impairment. These conditions include a history of previous CBI, migraine headaches, learning disorders, psychiatric conditions such as depression and anxiety, and pre-existing ophthalmological conditions such as strabismus or convergence insufficiency.4,25 Other risk factors for developing PPCS include female sex, pre-injury genetic susceptibility, type of forces applied to the head/brain (rotational is worse than linear), endocrine influences, secondary gain incentives, degree of exercise intolerance on systematic testing in the first week after injury, vestibular and oculomotor PE abnormalities, and the nature and burden of early post-concussive symptoms.26–32
A neurocognitive screen should be performed within the first two weeks, as clinically indicated, using a standardized assessment tool. The most common is the Standardized Assessment of Concussion (SAC) of the Sport Concussion Assessment Tool 5 (SCAT5),33 which briefly measures orientation, memory, and concentration. Results of the neurocognitive screen can be compared with concussion symptom questionnaires for validation. Neurocognitive assessment is further described under Supplementary Testing.
Depending on the circumstances surrounding the injury, a determination should be made regarding the need for behavioral assessment as related to risk factors for post-traumatic psychologic or psychiatric impairment. The more common behavioral changes reported in the evidence-based literature include acute stress disorder (diagnosable only within the first 30 days post-event), post-traumatic stress disorder (diagnosable only after 30 days post-event), and/or other anxiety disorders and depression.19,34 There are many different methods to assess behavioral change following CBI; some are brief without high levels of specificity and sensitivity whereas others are considered gold standards, such as the Minnesota Multiphasic Personality Inventory or Personality Assessment Inventory. These tests must be interpreted in context and with familiarity to how persons with post-traumatic impairments may respond given that these tests were originally designed for psychiatric populations.35 Some behavioral measures are diagnosis-specific, such as the Trauma Symptom Inventory36 or Detailed Assessment of Post-Traumatic Stress.37 When possible, measures that include information on reporting validity and response bias should be utilized.
Physical Examination
Following the history and NC/behavioral assessment, clinicians should perform a focused PE at the initial outpatient visit. PE findings, combined with history and supplementary testing where indicated, will facilitate differential diagnosis. A comprehensive PE is always indicated at the initial encounter because some trauma-related abnormalities may develop in the 24 hours after injury that were not present during initial assessment.2
General Assessment Overview
Assessment in the outpatient setting is different from assessment in the acute setting. In the acute setting of a suspected CBI (for example, on the field or emergency room), a thorough and organized physical should first establish the degree of injury to all parts of the body and with additional focus on the cervical spine. With any unconscious or obtunded patient, clinicians should consider that the patient has sustained a cervical injury until it can be safely ruled out. For the conscious patient, the assessment, ideally, should be performed in a quiet place to optimize concentration and minimize distractions.
For the sub-acute setting in a multidisciplinary clinic, a general survey should inspect for bruising, swelling, and/or deformities with special attention to the face, scalp, neck and shoulder girdles. Persistent rhinorrhea or otorrhea, as well as racoon’s eyes or Battle’s sign, may suggest a basilar skull fracture and CSF leak, which should provoke neurosurgical consultation.38 The survey should make note of any musculoskeletal findings including body asymmetries (e.g., head tilt, shoulder droop, tilted pelvis, leg length discrepancy or asymmetric gait), postural abnormalities (e.g., forward head posture, rounded shoulders, stance or pelvic alignment), and observation of cervical and lumbar lordosis. The elemental neurological assessment should include cognitive-behavioral screening, CN I – XII, reflexes, sensory exam, visual field exam, cerebellar exam, motor assessment including manual muscle testing of the upper and lower extremities, and assessment of drift.22 The peripheral neurological examination should include assessment of neuralgic and/or neuritic headache pain generators such as supra-orbital, auriculotemporal and occipital neuralgia. Trauma to the neck is a known etiology of occipital neuralgia (which may involve the lesser, greater and/or third occipital nerves).39 Although there is no consensus on the specifics of the craniofacial or upper cervical peripheral neurological exam, assessment for trigeminocervical involvement is critical in the context of diagnosing specific post-traumatic pain generators, including headache. Tenderness to palpation in any portion of the nerve branch, and/or a positive Tinel’s sign over the nerve, is strongly suggestive of nerve sensitization and a neuritic or neuralgic source of pain. Additional evidence of nerve hypersensitivity (including central sensitization) is pain that refers into the sensory distribution of the nerve when the more proximal end of the nerve is stimulated.
Buffalo Concussion Physical Exam
The Buffalo Concussion Physical Examination (BCPE) is a brief and focused PE for CBI.10 A sample BCPE assessment form, along with directions on how to perform, is presented as a supplementary table. After vital signs are measured, the BCPE takes about 5 minutes to perform and is convenient enough to perform at every visit.
Orthostatic Vital Signs:
Autonomic dysregulation is common after CBI and may present with symptoms of orthostatic hypotension, dizziness/vestibular dysfunction, postural orthostatic tachycardia syndrome (POTS), or altered HR and blood pressure (BP) responses at rest and during exercise.40 According to the American Autonomic Society, orthostatic hypotension (OH) is defined as a 20 mmHg or greater reduction in systolic BP or a >10 mmHg reduction in diastolic BP after 1 and 3 minutes of standing from the supine position.41 Since the prevalence of OH is between 5–30% in the non-concussed population, this change in BP is clinically significant when it is accompanied by symptoms of dizziness or lightheadedness.42 Patients with symptoms upon standing may have orthostasis and/or central vestibular dysfunction.43,44 HR response is useful since a rise in HR (>40 bpm standing vs. supine) with a drop in BP usually indicates hypovolemia whereas lack of HR response is more consistent with a central neurogenic cause. Patients with symptoms of dizziness or vertigo while supine are more likely to have a peripheral vestibular injury and performance of the Dix-Hallpike maneuver45 and otoscopic examination may be indicated. Orthostatic vital signs are measured only supine to standing since two-thirds of cases can be missed if performed in the seated to standing position.46 Common sources of error for measuring BP include not maintaining the arm at the level of the heart, using an improperly sized cuff, and rapid cuff deflation in those with a slow HR.47 To save time in clinical practice, this can be measured by an allied health professional prior to seeing the physician.
Cervical Examination:
The neck and sub-occipital regions are frequently involved in trauma associated with CBI and/or in isolated cervical whiplash injuries which present with similar symptoms to CBI, including headache, dizziness, blurred vision, tinnitus, reduced concentration, and balance difficulties.48–50 Physical tests can help differentiate between cervical injury and CBI to direct appropriate evidence-based treatment. The BCPE includes range of motion and palpation of the neck and sub-occipital regions for muscle tenderness and spasm. Apart from this, there are several additional tests for cervical examination that can be performed when clinically indicated. Given the high incidence of headache in this population, clinicians need to be aware of the evidence supporting the most useful assessment techniques. A 2016 Delphi study51 concluded that the most useful techniques used by physical therapists for patients with headache includes the cranio-cervical flexion test, cervical flexion rotation test, active range of cervical movement, trigger point palpation, muscle tests of the shoulder girdle, passive physiological inter-vertebral movements, thoracic spine screening, and combined movement tests. Table 1 presents a comprehensive list of cervical tests, with directions and reliability, which may be used to assess the neck when clinically indicated. The cervical flexion rotation test, smooth-pursuit neck torsion test and cervical-joint reposition error test have been shown to have high reliability and strong diagnostic accuracy for diagnosing cervicogenic headache, which is often a comorbid contributor or sole cause of headache in this patient population.52–54 They attempt to minimize visual and vestibular factors while targeting cervical position and movement-sensory information to help isolate cervical pathology. The cervical-joint reposition error test identifies damage to muscle spindles in the neck while the smooth-pursuit neck torsion test is for identifying cervicogenic disturbances.55 The right and left alar ligament tests assesses the integrity of the upper cervical spine using lateral flexion, which has been validated to significantly increase the length of the contralateral alar ligament.56 The transverse ligament integrity of the cervical spine can be assessed using the Sharp Purser test.57,58 Spurling’s test for cervical radiculopathy is best viewed as confirmatory rather than as a screening test due to its high specificity and low sensitivity.59 In patients with cervical instability, Spurling’s test should be performed with caution due to the provocative stress required.
Table 1.
Sensitivity and Specificity of Supplementary Cervical Tests
| Test | Method | Sensitivity* | Specificity* |
|---|---|---|---|
| Right alar ligament test93 | Place one hand on the occiput and use the other hand to palpate the spinous process/lamina of C2. Laterally flex or rotate the head to the contralateral side of the alar ligament to be tested. Movement of the spinous process to the opposite side should be palpated. Absence of the spinous process moving to the opposite side may indicate alar ligament injury. | 0.69 | 1.0 |
| If you block the spinous process of C2 from moving, you may stress the ligament. You should encounter a firm end-feel in this case. Significant movement may indicate ligamentous injury. | |||
| Left alar ligament test93 | Place one hand on the occiput and use the other hand to palpate the spinous process/lamina of C2. Laterally flex or rotate the head to the contralateral side of the alar ligament to be tested. Movement of the spinous process to the opposite side should be palpated. Absence of the spinous process moving to the opposite side may indicate alar ligament injury. | 0.72 | 0.96 |
| If the C2 spinous process is blocked from moving, the ligament may be stressed. A firm end-feel should be encountered in this case. Significant movement may indicate ligamentous injury. | |||
| Sharp purser test57 | Place one hand on the occiput with the index finger on the space between C2 spinous process and the occipital protuberance (where the posterior arch of C1 lies). Place the other hand on the forehead. Lift the head straight up in a vertical plane (protracting not flexing motion). | 0.69 | 0.96 |
| The test is positive if the patient experiences weakness, dizziness, numbness, nystagmus, or an odd feeling in the back of the throat. There is normally a firm end-feel. | |||
| Spurling test59 | This cervical compression test involves the examiner turning the patient’s head to the affected side while extending and applying downward pressure to the top of the patient’s head. | 0.30 | 0.93 |
| A positive Spurling’s sign is when the pain arising in the neck radiates in the direction of the corresponding ipsilateral dermatome. It is a type of cervical compression test. | |||
| Patients with a positive Spurling’s sign can present with a variety of symptoms, including pain, numbness and weakness. In addition to the clinical history, the neurological examination may show signs suggesting a cervical radiculopathy. | |||
| Smooth-pursuit neck torsion test55 | The patient sits in neutral position in front of a screen with a red dot moving horizontally to and fro. The task is to follow the target visually without moving the head. The chair is then turned 45 degrees to the one side so the torso is turned away, but the head is still pointing forward, supported by an assistant, at a maximum angle of 45 degrees–or at some angle which did not increase pain, stress and/or other discomfort. The eye-tracking test is then repeated. Next, the procedure is repeated to the opposite side. There should be a short pause between each change of posture with the purpose to eliminate vestibular influence. |
0.90 | 0.91 |
| In all three test situations (i.e., neutral torso position, torso turned to the right, and turned to the left) the patient’s ability to follow the red dot is registered and the gain is calculated. Normally, the eyes do not keep pace, then they needs to catch-up to the target which is done by very fast corrective eye movements called corrective saccades. These saccades in turn decrease the gain from 1.00 to about 0.80–0.90 in a healthy individual. | |||
| Cervical-joint reposition error test55 | The cervical joint position error test is a method to assess proprioception. This test is particularly relevant for people with neck pain and whiplash associated disorder, and it is of potential interest for people with neurological disorders. In clinical practice, patients are asked to move their head and match the original position while wearing a laser pointer on their head. The error is measured manually as the distance between the projection of the laser on a target before and after neck movement. | 0.82 | 0.92 |
| Cervical flexion rotation52,54 | The patient should be relaxed in supine position. The examiner fully flexes the cervical spine with the occiput resting against the examiner’s abdomen. The patient’s head is then rotated to the left and the right. | 0.91 | 0.90 |
| If a firm resistance is encountered, pain provoked, and range is limited before the expected end range, then the test is considered positive, with a presumptive diagnosis of limited rotation of C1 on C2. |
Sensitivity and specificity for recognizing cervicogenic disturbances
Head and Face Examination:
Palpation of the face, head, pericranial musculature, temporomandibular joints (TMJ), muscles of mastication, craniocervical junction, cervical and thoracic spine, and the shoulders should be performed. To attempt to assess activated trigger points and referred pain patterns, the palpation must be carefully performed in a layer-by-layer fashion. Jaw range of motion and palpation will help evaluate malocclusion, myofascial involvement of masticatory muscles, TMJ pain or, in rare cases, jaw fracture.60 The TMJ should also be auscultated, as clinically indicated, for abnormal articular sounds (clicking, popping, grinding)61,62 Auscultation for bruits should be done as appropriate over the carotids, temporal arteries, closed eyes, and mastoids to assess for vascular turbulence that might be due to post-traumatic vascular anomalies such as fistulas or dissections.
Cranial Nerve Examination:
The initial evaluation should entail a neurological examination of all twelve cranial nerves (CN). This part of the BCPE contains those nerves not assessed during the oculomotor portion of the examination. Isolated abnormalities may suggest a brainstem lesion and should prompt further investigation including cerebral MRI with thin cuts of the posterior fossa. Re-examination of initially normal CN findings on follow-up visits can be omitted; however, abnormal CN exam findings should be serially assessed. The most commonly63 affected CN after CBI is CN I; yet, it is frequently not assessed. These patients may present with complaints of altered taste as opposed to altered smell per se. Standardized tests for olfaction include the Doty Smell Identification Tests (ideally 40 item) and Green’s Alberta Smell Test.64 However, these tests may not be available so a good history and a readily available non-irritating aromatic substance (e.g. coffee grounds) can provide the information clinicians need without the expense and time of standardized tests.
Oculomotor and Ophthalmological Examination:
Fundoscopy should be performed at the first visit using a standard ophthalmoscope to assess blurring of optic nerve borders that could indicate increased intracranial pressure (although this phenomenon is of very low frequency after CBI).65,66 If there are any concerns about the presence of optic disc pallor (suggesting increased intracranial pressure), the patient should be immediately referred to ophthalmology for a dilated examination.67 Abnormal results should prompt immediate neurosurgical referral. New symptoms of floaters and flashing lights may also be an indication for referral to ophthalmology. Abnormal smooth pursuits, repetitive saccades, vestibulo-ocular reflex (VOR), near point of convergence (NPC, binocular vision), and abnormal accommodation (monocular vision) are common after CBI and should be documented.68 Abnormal and/or symptomatic repetitive saccades and smooth pursuits (complaints of blurred vision, headache, dizziness and/or non-smooth motion) may be associated with prolonged recovery.69 Due to age-related changes in the eye and face, normal NPC values are lower in children (diplopia reported >6 cm from the forehead) than in adults (diplopia reported >10 cm from the forehead).70 Accurate performance of these tests requires examiner experience as the specific tests involve observing for relatively subtle impairments.
Vestibular Examination:
Postural control and motor coordination problems are common after CBI and could lead to further injuries during sport or work.71 Objective signs of vestibular pathology may not be detectable at rest but only upon provocation, potentially pointing to subtle vestibular pathology.69 The Balance Error Scoring System (BESS) test and the modified BESS are validated tests that are typically used to assess balance on the sport sideline.72 For clinicians in a busy clinic, tandem gait and tandem stance (also referred to as a Sharpened Romberg) are more useful tests because of their convenience and high inter-rater reliability.73 Other tests for vestibular assessment may be considered, including the Nylen-Barany or Hallpike maneuver,45 head shaking test,74 and the Hennebert’s test.75 Detailed vestibular assessment76 is indicated if the patient complains of dizziness/lightheadedness in the supine position. It is important for clinicians to remember that there can be multiple reasons for post-traumatic dizziness and that a thorough line of questioning can help guide the clinician to what the generators are for this symptom. Some patients will report true vertigo whereas others will report a general feeling of wooziness or imbalance. The aforementioned symptoms should be differentiated from those that suggest a dysautonomic problem such as lightheadedness on arising. Dizziness complaints can be seen with labyrinthine concussion, benign paroxysmal positional vertigo, perilymphatic fistulas, post-traumatic endolymphatic hydrops, and cervicogenic vertigo.
Supplementary Testing
Exercise Tolerance Testing:
Exercise tolerance after CBI may be assessed using a graded exercise test such as the Buffalo Concussion Treadmill Test (BCTT)77 or the Buffalo Concussion Bike Test (BCBT).78 The BCTT is safe to perform within the first week in adolescents after CBI77 and early exercise intolerance at the initial exam is a sensitive indicator of Autonomic/Physiological CBI.77 The HR at symptom exacerbation will improve as the patient recovers so classification is based on the initial test in the sub-acute phase. The ability to exercise to maximum without symptom exacerbation and patient report of a baseline level of symptoms at rest correlates with cardiovascular and cerebrovascular physiological recovery from CBI.77 The BCBT is recommended for significant vestibular complaints or other injuries that prevent them from walking safely on a treadmill. Exercise that rapidly raises HR, for example, weight lifting or sprinting, is not recommended for assessing CBI patients because the concussed brain does not tolerate a rapid increase in BP.
Neurocognitive Testing:
As mentioned above, complete CBI assessment must include neurocognitive assessment. Formal neurocognitive testing performed by a neuropsychologist is not always recommended but should be considered if the patient complains of persistent cognitive difficulties. For example, if significant deficiencies are identified at the initial evaluation, then serial neurocognitive screening should be performed. Computerized neurocognitive testing (CNT), such as Concussion Vital Signs or the Immediate Post-concussion Assessment and Cognitive Testing (ImPACT), are an alternative to more comprehensive and formal neuropsychological evaluation that have been shown to be sensitive to measuring subtle cognitive impairments after CBI and tracking of same over time.79,80 CNT has the advantage of being time efficient, self-scoring, and with immediate report feedback. Baseline pre-injury CNT provides a comparison tool that affords even greater accuracy in detecting post-CBI neurocognitive impairments.81 However, CNT should not be the sole determinant in clinical decision-making as there are several concerns regarding invalid performances82 and re-test reliability.83 A systematic review82 of ImPACT’s invalidity indicators suggests that these measures miss invalid performance approximately 20% of the time when individuals purposefully underperform. It has been shown that remarkably high rates of athletic trainers, who often utilize these tests, do not double check for baseline validity and may be inadequately trained to conduct or interpret CNT.81 No matter the neurocognitive test chosen, clinicians should inform patients of their results and their functional implications. As appropriate, compensatory strategies for identified cognitive impairments should be discussed. Advice should be provided based in part on neurocognitive testing regarding issues such as return to school or work. Formal neurocognitive testing done by a neuropsychologist should be considered if significant cognitive difficulties persist beyond 2 months post-injury.84 Such testing can help to more clearly identify the range of cognitive difficulties as well as the contribution of affective, pain, and other factors to the profile generated, and can assist with recommending specifically tailored cognitive compensatory strategies and specialized counseling.85 Formal neurocognitive testing represents the gold standard for delineating cognitive status but is time consuming, expensive and requires a trained neuropsychologist.
Recovery
There is lack of scientific consensus on the definition of recovery from CBI. The latest CISG guidelines2 define clinical recovery from sport-related CBI as a return to normal activities without any exacerbation of symptoms during step-wise return-to-play and return-to-learn protocols. The earlier CISG guideline86 stated that a return-to-play protocol should begin when there is resolution of all post-injury symptoms. Several studies,87 however, have shown that non-injured people commonly report symptoms on concussion symptom checklists so return to baseline level of symptoms is preferred.88 There is also inconsistency between clinical and physiological recovery, with physiological recovery outlasting clinical recovery in most cases.89 This is due to the variety of measures used to define physiological recovery from CBI, which include cardiovascular metrics, electrophysiology, and advanced functional imaging.2 An evidence-based definition of recovery from CBI is important given the risk of more severe consequences should repeat injury occur before recovery90 and increased awareness of possible long-term effects.91 A recent systematic review92 found that the most common criteria to define recovery from CBI were self-reported symptoms and neurocognitive assessment. Physical exertion assessment was less commonly used. This review concluded that it was most clinically useful to use a combination of criteria to define recovery from CBI, including return to baseline level of symptoms, baseline level of cognition, a normal PE (including assessment of cervical, oculomotor, and vestibular subsystems) and, in certain cases, exercise tolerance testing.
Conclusions
This paper presents a suggested paradigm for a pertinent and practical assessment for patients following CBI that includes history, neurocognitive and behavioral screen, PE that includes an elemental neurological exam, oculomotor/ophthalmologic and vestibular systems, and musculoskeletal assessment of the cervical region. The proposed evaluation combines elements of the SCAT5,33 Vestibular/Ocular Motor Screening,69 and BCPE10 while adding greater emphasis to the cervical spine and adjunct tests such as the BCTT/BCBT. It is recommended that patients should be evaluated every 1–2 weeks until recovery. If symptoms persist beyond 2 weeks for adults and 4 weeks for adolescents, then referral to a multi-disciplinary center that focuses on CBI is recommended.2
Supplementary Material
Funding:
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number 1R01NS094444 and by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001412 to the University at Buffalo. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: The authors report no conflicts of interest.
References
- 1.Chen J, Oddson B, Gilbert HC. Differential Effect of Recurrent Concussions on Symptom Clusters in Sport Concussion Assessment Tool. Journal of sport rehabilitation. 2018:1–18. [DOI] [PubMed] [Google Scholar]
- 2.McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med. 2017;51(11):838–847. [DOI] [PubMed] [Google Scholar]
- 3.Arbogast KB, Curry AE, Pfeiffer MR, et al. Point of health care entry for youth with concussion within a large pediatric care network. JAMA pediatrics. 2016;170(7):e160294–e160294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellis MJ, Leddy JJ, Willer B. Physiological, vestibulo-ocular and cervicogenic post-concussion disorders: an evidence-based classification system with directions for treatment. Brain injury. 2015;29(2):238–248. [DOI] [PubMed] [Google Scholar]
- 5.Asken BM, Snyder AR, Clugston JR, Gaynor LS, Sullan MJ, Bauer RM. Concussion-Like Symptom Reporting in Non-Concussed Collegiate Athletes. Arch Clin Neuropsychol. 2017;32(8):963–971. [DOI] [PubMed] [Google Scholar]
- 6.Zasler ND. Post-traumatic Sensory Disorders in TBI In: Arcineigas D, Vanderploeg R, Zasler ND, Jaffee MS, eds. Management of adults with traumatic brain injury. American Psychiatric Publishing, Inc.; 2013. [Google Scholar]
- 7.Hammond FM, Masel T. Cranial nerve disorders In: Zasler N, Katz D, Zafonte R, eds. Brain injury medicine: principles and practice. 2 ed: Demos Medical Publishing; 2012. [Google Scholar]
- 8.Goldberg G, Zasler ND, Watanabe T. Management of a Patient With Slow Recovery From a Mild Traumatic Brain Injury. PM&R. 2013;5(10):890–899. [DOI] [PubMed] [Google Scholar]
- 9.Arciniegas DB, Anderson CA, Topkoff J, McAllister TW. Mild traumatic brain injury: a neuropsychiatric approach to diagnosis, evaluation, and treatment. Neuropsychiatr Dis Treat. 2005;1(4):311–327. [PMC free article] [PubMed] [Google Scholar]
- 10.Haider MN, Leddy JJ, Du W, Viera K, Willer B. Practical Management: Brief Physical Examination for Sport-Related Concussion in the Outpatient Setting. Clinical journal of sport medicine: official journal of the Canadian Academy of Sport Medicine. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.King D, Brughelli M, Hume P, Gissane C. Assessment, Management and Knowledge of Sport-Related Concussion: Systematic Review. Sports Medicine. 2014;44(4):449–471. [DOI] [PubMed] [Google Scholar]
- 12.McPherson AL, Nagai T, Webster KE, Hewett TE. Musculoskeletal injury risk after sport-related concussion: a systematic review and meta-analysis. The American journal of sports medicine. 2018:0363546518785901. [DOI] [PubMed] [Google Scholar]
- 13.Soustiel JF, Glenn TC, Shik V, Boscardin J, Mahamid E, Zaaroor M. Monitoring of cerebral blood flow and metabolism in traumatic brain injury. J Neurotrauma. 2005;22(9):955–965. [DOI] [PubMed] [Google Scholar]
- 14.Cheever KM, McDevitt J, Tierney R, Wright WG. Concussion Recovery Phase Affects Vestibular and Oculomotor Symptom Provocation. Int J Sports Med. 2018;39(2):141–147. [DOI] [PubMed] [Google Scholar]
- 15.Leddy JJ, Willer B. Use of graded exercise testing in concussion and return-to-activity management. Current sports medicine reports. 2013;12(6):370–376. [DOI] [PubMed] [Google Scholar]
- 16.Silver JM, McAllister TW, Archiniegas DB. Textbook of traumatic brain injury. Third Edition ed: American Psychiatric Pub; 2019. [Google Scholar]
- 17.Leddy J, Baker JG, Haider MN, Hinds A, Willer B. A Physiological Approach to Prolonged Recovery From Sport-Related Concussion. J Athl Train. 2017;52(3):299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leddy JJ, Kozlowski K, Donnelly JP, Pendergast DR, Epstein LH, Willer B. A preliminary study of subsymptom threshold exercise training for refractory post-concussion syndrome. Clinical Journal of Sport Medicine. 2010;20(1):21–27. [DOI] [PubMed] [Google Scholar]
- 19.Brent DA, Max J. Psychiatric Sequelae of Concussions. Curr Psychiatry Rep. 2017;19(12):108. [DOI] [PubMed] [Google Scholar]
- 20.Prince C, Bruhns ME. Evaluation and Treatment of Mild Traumatic Brain Injury: The Role of Neuropsychology. Brain Sci. 2017;7(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen JK, Johnston KM, Collie A, McCrory P, Ptito A. A validation of the post concussion symptom scale in the assessment of complex concussion using cognitive testing and functional MRI. J Neurol Neurosurg Psychiatry. 2007;78(11):1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Randolph C, Millis S, Barr WB, et al. Concussion symptom inventory: an empirically derived scale for monitoring resolution of symptoms following sport-related concussion. Arch Clin Neuropsychol. 2009;24(3):219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Althubaiti A Information bias in health research: definition, pitfalls, and adjustment methods. J Multidiscip Healthc. 2016;9:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kroshus E, Garnett B, Hawrilenko M, Baugh CM, Calzo JP. Concussion under-reporting and pressure from coaches, teammates, fans, and parents. Social Science & Medicine. 2015;134:66–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iverson GL, Gardner AJ, Terry DP, et al. Predictors of clinical recovery from concussion: a systematic review. Br J Sports Med. 2017;51(12):941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fehr SD, Nelson LD, Scharer KR, et al. Risk factors for prolonged symptoms of mild traumatic brain injury: a pediatric sports concussion clinic cohort. Clinical journal of sport medicine. 2019;29(1):11–17. [DOI] [PubMed] [Google Scholar]
- 27.Cnossen MC, van der Naalt J, Spikman JM, et al. Prediction of Persistent Post-Concussion Symptoms after Mild Traumatic Brain Injury. J Neurotrauma. 2018;35(22):2691–2698. [DOI] [PubMed] [Google Scholar]
- 28.Ponsford J, Nguyen S, Downing M, et al. Factors associated with persistent post-concussion symptoms following mild traumatic brain injury in adults. J Rehabil Med. 2018;51(1):32–39. [DOI] [PubMed] [Google Scholar]
- 29.Ewing-Cobbs L, Cox CS Jr, Clark AE, Holubkov R, Keenan HT. Persistent Postconcussion Symptoms After Injury. Pediatrics. 2018;142(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Howell DR, O’Brien MJ, Beasley MA, Mannix RC, Meehan WP 3rd., Initial somatic symptoms are associated with prolonged symptom duration following concussion in adolescents. Acta paediatrica (Oslo, Norway : 1992). 2016;105(9):e426–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leddy J, Baker JG, Haider MN, Hinds A, Willer B. A physiological approach to prolonged recovery from sport-related concussion. Journal of athletic training. 2017;52(3):299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carney N, Ghajar J, Jagoda A, et al. Concussion guidelines step 1: systematic review of prevalent indicators. Neurosurgery. 2014;75(suppl_1):S3–S15. [DOI] [PubMed] [Google Scholar]
- 33.Echemendia RJ, Meeuwisse W, McCrory P, et al. The Sport Concussion Assessment Tool 5th Edition (SCAT5). Br J Sports Med. 2017:bjsports-2017–097506. [DOI] [PubMed] [Google Scholar]
- 34.Pineau H, Marchand A, Guay S. Specificity of cognitive and behavioral complaints in post-traumatic stress disorder and mild traumatic brain injury. Behav Sci (Basel). 2015;5(1):43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Slavin-Mulford J, Sinclair SJ, Stein M, Malone J, Bello I, Blais MA. External validity of the personality assessment inventory (PAI) in a clinical sample. J Pers Assess. 2012;94(6):593–600. [DOI] [PubMed] [Google Scholar]
- 36.Snyder JJ, Elhai JD, North TC, Heaney CJ. Reliability and validity of the Trauma Symptom Inventory with veterans evaluated for posttraumatic stress disorder. Psychiatry Res. 2009;170(2–3):256–261. [DOI] [PubMed] [Google Scholar]
- 37.Briere J, Agee E, Dietrich A. Cumulative trauma and current posttraumatic stress disorder status in general population and inmate samples. Psychol Trauma. 2016;8(4):439–446. [DOI] [PubMed] [Google Scholar]
- 38.Kutcher JS. Management of the complicated sports concussion patient. Sports health. 2010;2(3):197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Society HCSotIH. The international classification of headache disorders. cephalalgia. 2004;24(1):9–160. [DOI] [PubMed] [Google Scholar]
- 40.Matuszak JM, McVige J, McPherson J, Willer B, Leddy J. A Practical Concussion Physical Examination Toolbox Evidence-Based Physical Examination for Concussion. Sports Health: A Multidisciplinary Approach. 2016;8(3):260–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clinical Autonomic Research. 2011;21(2):69–72. [DOI] [PubMed] [Google Scholar]
- 42.Low PA. Prevalence of orthostatic hypotension. Clinical Autonomic Research. 2008;18(1):8–13. [DOI] [PubMed] [Google Scholar]
- 43.Akin FW, Murnane OD, Hall CD, Riska KM. Vestibular consequences of mild traumatic brain injury and blast exposure: a review. Brain Inj. 2017;31(9):1188–1194. [DOI] [PubMed] [Google Scholar]
- 44.Pertab JL, Merkley TL, Cramond AJ, Cramond K, Paxton H, Wu T. Concussion and the autonomic nervous system: An introduction to the field and the results of a systematic review. NeuroRehabilitation. 2018;42(4):397–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Viirre E, Purcell I, Baloh RW. The Dix‐Hallpike Test and The Canalith Repositioning Maneuver. The Laryngoscope. 2005;115(1):184–187. [DOI] [PubMed] [Google Scholar]
- 46.Cooke J, Carew S, O’connor M, Costelloe A, Sheehy T, Lyons D. Sitting and standing blood pressure measurements are not accurate for the diagnosis of orthostatic hypotension. QJM: An International Journal of Medicine. 2009;102(5):335–339. [DOI] [PubMed] [Google Scholar]
- 47.Kallioinen N, Hill A, Horswill MS, Ward HE, Watson MO. Sources of inaccuracy in the measurement of adult patients’ resting blood pressure in clinical settings: a systematic review. J Hypertens. 2017;35(3):421–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marshall CM, Vernon H, Leddy JJ, Baldwin BA. The role of the cervical spine in post-concussion syndrome. The Physician and sportsmedicine. 2015;43(3):274–284. [DOI] [PubMed] [Google Scholar]
- 49.Morin M, Langevin P, Fait P. Cervical Spine Involvement in Mild Traumatic Brain Injury: A Review. J Sports Med (Hindawi Publ Corp). 2016;2016:1590161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Leddy JJ, Baker JG, Merchant A, et al. Brain or strain? Symptoms alone do not distinguish physiologic concussion from cervical/vestibular injury. Clinical journal of sport medicine : official journal of the Canadian Academy of Sport Medicine. 2015;25(3):237–242. [DOI] [PubMed] [Google Scholar]
- 51.Luedtke K, Boissonnault W, Caspersen N, et al. International consensus on the most useful physical examination tests used by physiotherapists for patients with headache: A Delphi study. Manual therapy. 2016;23:17–24. [DOI] [PubMed] [Google Scholar]
- 52.Hall TM, Robinson KW, Fujinawa O, Akasaka K, Pyne EA. Intertester reliability and diagnostic validity of the cervical flexion-rotation test. Journal of manipulative and physiological therapeutics. 2008;31(4):293–300. [DOI] [PubMed] [Google Scholar]
- 53.Rubio-Ochoa J, Benítez-Martínez J, Lluch E, Santacruz-Zaragozá S, Gómez-Contreras P, Cook C. Physical examination tests for screening and diagnosis of cervicogenic headache: A systematic review. Manual therapy. 2016;21:35–40. [DOI] [PubMed] [Google Scholar]
- 54.Ogince M, Hall T, Robinson K, Blackmore A. The diagnostic validity of the cervical flexion–rotation test in C1/2-related cervicogenic headache. Manual therapy. 2007;12(3):256–262. [DOI] [PubMed] [Google Scholar]
- 55.Cheever K, Kawata K, Tierney R, Galgon A. Cervical Injury Assessments for Concussion Evaluation: A Review. J Athl Train. 2016;51(12):1037–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Osmotherly PG, Rivett DA, Rowe LJ. Construct validity of clinical tests for alar ligament integrity: an evaluation using magnetic resonance imaging. Physical therapy. 2012;92(5):718–725. [DOI] [PubMed] [Google Scholar]
- 57.Hutting N, Scholten-Peeters GG, Vijverman V, Keesenberg MD, Verhagen AP. Diagnostic accuracy of upper cervical spine instability tests: a systematic review. Phys Ther. 2013;93(12):1686–1695. [DOI] [PubMed] [Google Scholar]
- 58.Uitvlugt G, Indenbaum S. Clinical assessment of atlantoaxial instability using the Sharp-Purser test. Arthritis Rheum. 1988;31(7):918–922. [DOI] [PubMed] [Google Scholar]
- 59.Tong HC, Haig AJ, Yamakawa K. The Spurling test and cervical radiculopathy. Spine (Phila Pa 1976). 2002;27(2):156–159. [DOI] [PubMed] [Google Scholar]
- 60.McCambridge TM, Small E, Bernhardt DT. Concussion. N Engl J Med. 2007;356(17):1788; author reply 1789. [PubMed] [Google Scholar]
- 61.Meyer RA. The Temporomandibular Joint Examination In: rd, Walker HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. Boston: 1990. [PubMed] [Google Scholar]
- 62.HUDDLESTON-SLATER JJR VAN SELMS MKA, LOBBEZOO F, NAEIJE M. The clinical assessment of TMJ sounds by means of auscultation, palpation or both. Journal of Oral Rehabilitation. 2002;29(9):873–873. [Google Scholar]
- 63.Reiter ER, Costanzo RM. Chemosensory Impairment after Traumatic Brain Injury: Assessment and Management. International neurotrauma letter. 2012;23:3. [PMC free article] [PubMed] [Google Scholar]
- 64.Fortin A, Lefebvre MB, Ptito M. Traumatic brain injury and olfactory deficits: the tale of two smell tests! Brain injury. 2010;24(1):27–33. [DOI] [PubMed] [Google Scholar]
- 65.Whiting AS, Johnson LN. Papilledema: clinical clues and differential diagnosis. Am Fam Physician. 1992;45(3):1125–1134. [PubMed] [Google Scholar]
- 66.Haider MN, Leddy JJ, Hinds AL, et al. Intracranial pressure changes after mild traumatic brain injury: a systematic review. Brain injury. 2018;32(7):809–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shawkat FS, Kriss A, Thompson D, Russell-Eggitt I, Taylor D, Harris C. Vertical or asymmetric nystagmus need not imply neurological disease. British journal of ophthalmology. 2000;84(2):175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Master CL, Scheiman M, Gallaway M, et al. Vision Diagnoses Are Common After Concussion in Adolescents. Clinical Pediatrics. 2016;55(3):260–267. [DOI] [PubMed] [Google Scholar]
- 69.Anzalone AJ, Blueitt D, Case T, et al. A Positive Vestibular/Ocular Motor Screening (VOMS) Is Associated With Increased Recovery Time After Sports-Related Concussion in Youth and Adolescent Athletes. The American journal of sports medicine. 2017;45(2):474–479. [DOI] [PubMed] [Google Scholar]
- 70.Ostadimoghaddam H, Hashemi H, Nabovati P, Yekta A, Khabazkhoob M. The distribution of near point of convergence and its association with age, gender and refractive error: a population‐based study. Clinical and Experimental Optometry. 2017;100(3):255–259. [DOI] [PubMed] [Google Scholar]
- 71.Kontos AP, Elbin R, Schatz P, et al. A revised factor structure for the post-concussion symptom scale: baseline and postconcussion factors. The American journal of sports medicine. 2012;40(10):2375–2384. [DOI] [PubMed] [Google Scholar]
- 72.Iverson GL, Kaarto ML, Koehle MS. Normative data for the balance error scoring system: implications for brain injury evaluations. Brain injury. 2008;22(2):147–152. [DOI] [PubMed] [Google Scholar]
- 73.Schneiders AG, Sullivan SJ, Gray AR, Hammond-Tooke GD, McCrory PR. Normative values for three clinical measures of motor performance used in the neurological assessment of sports concussion. Journal of Science and medicine in Sport. 2010;13(2):196–201. [DOI] [PubMed] [Google Scholar]
- 74.Kamei T Two types of head-shaking tests in vestibular examination. Acta Oto-Laryngologica. 1988;105(sup458):108–112. [DOI] [PubMed] [Google Scholar]
- 75.Pearlman RC. The fistula and Hennebert tests. Journal of the American Audiology Society. 1976;2(1):1–2. [PubMed] [Google Scholar]
- 76.Feddermann-Demont N, Echemendia RJ, Schneider KJ, et al. What domains of clinical function should be assessed after sport-related concussion? A systematic review. Br J Sports Med. 2017;51(11):903–918. [DOI] [PubMed] [Google Scholar]
- 77.Leddy JJ, Hinds AL, Miecznikowski J, et al. Safety and prognostic utility of provocative exercise testing in acutely concussed adolescents: a randomized trial. Clinical journal of sport medicine. 2018;28(1):13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leddy JJ, Haider MN, Ellis M, Willer BS. Exercise is medicine for concussion. Current sports medicine reports. 2018;17(8):262–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schatz P, Pardini JE, Lovell MR, Collins MW, Podell K. Sensitivity and specificity of the ImPACT Test Battery for concussion in athletes. Archives of Clinical Neuropsychology. 2006;21(1):91–99. [DOI] [PubMed] [Google Scholar]
- 80.Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Archives of Clinical Neuropsychology. 2006;21(7):623–643. [DOI] [PubMed] [Google Scholar]
- 81.Covassin T, Elbin RJ 3rd, Stiller-Ostrowski JL, Kontos AP. Immediate post-concussion assessment and cognitive testing (ImPACT) practices of sports medicine professionals. J Athl Train. 2009;44(6):639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gaudet CE, Weyandt LL. Immediate Post-Concussion and Cognitive Testing (ImPACT): a systematic review of the prevalence and assessment of invalid performance. The Clinical Neuropsychologist. 2017;31(1):43–58. [DOI] [PubMed] [Google Scholar]
- 83.Broglio SP, Ferrara MS, Macciocchi SN, Baumgartner TA, Elliott R. Test-retest reliability of computerized concussion assessment programs. J Athl Train. 2007;42(4):509–514. [PMC free article] [PubMed] [Google Scholar]
- 84.Lovell MR. Neuropsychological assessment of the professional athlete. Sports neuropsychology: Assessment and management of traumatic brain injury. 2006:176–190. [Google Scholar]
- 85.Connery AK, Peterson RL, Baker DA, Randolph C, Kirkwood MW. The Role of Neuropsychological Evaluation in the Clinical Management of Concussion. Physical medicine and rehabilitation clinics of North America. 2016;27(2):475–486. [DOI] [PubMed] [Google Scholar]
- 86.McCrory P, Meeuwisse WH, Aubry M, et al. Consensus statement on concussion in sport: the 4th International Conference on Concussion in Sport held in Zurich, November 2012. British journal of sports medicine. 2013;47(5):250–258. [DOI] [PubMed] [Google Scholar]
- 87.McCrory P, Meeuwisse W, Johnston K, et al. SCAT2. British Journal of Sports Medicine. 2009;43(Suppl_1):i85–i88. [DOI] [PubMed] [Google Scholar]
- 88.Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA pediatrics. 2015;169(12):1132–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kamins J, Bigler E, Covassin T, et al. What is the physiological time to recovery after concussion? Systematic review. Br J Sports Med. 2017:bjsports-2016–097464. [DOI] [PubMed] [Google Scholar]
- 90.Dessy AM, Rasouli J, Yuk F, Choudhri TF. Second Impact Syndrome: A Rare, Devastating Consequence of Repetitive Concussions. Contemporary Neurosurgery. 2015;37(20):1–5.27175042 [Google Scholar]
- 91.Control CfD Prevention. Nonfatal traumatic brain injuries related to sports and recreation activities among persons aged≤ 19 years---United States, 2001–-2009. MMWR: Morbidity and mortality weekly report. 2011;60(39):1337–1342. [PubMed] [Google Scholar]
- 92.Haider MN, Leddy JJ, Pavlesen S, et al. A systematic review of criteria used to define recovery from sport-related concussion in youth athletes. Br J Sports Med. 2017:bjsports-2016–096551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaale BR, Krakenes J, Albrektsen G, Wester K. Clinical assessment techniques for detecting ligament and membrane injuries in the upper cervical spine region--a comparison with MRI results. Man Ther. 2008;13(5):397–403. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


