Abstract
Objective:
The study aimed to explore the seroprevalence of some bacterial (Brucella spp., Chlamydia abortus) and viral [Rift Valley fever virus (RVFV), foot and mouth disease virus (FMDV)] zoonoses in domestic ruminants in Medina.
Materials and Methods:
A total of 1,000 blood samples from 665 sheep, 228 goats, and 107 camels were collected from the central slaughterhouse, private farms, and veterinary clinics affiliated to the Ministry of Agriculture. The samples were screened using the Enzyme-Linked Immunosorbent Assay (ELISA). The results were statistically analyzed using Statistical Package for the Social Sciences version 20.
Results:
Brucella was found in 7.7%, 8.8%, and 6.5% of sheep, goats, and camel’s sera, respectively. In humans, Brucella abortus and Brucella melitensis had higher frequencies in males (61.3%, 59.8%) than in females (38.7%, 40.2%). Chlamydia abortus was reported only in sheep at a rate of 0.75%. RVFV was prevalent in sheep (10.7%) and goats (17.9%). FMDV was reported in 27.8% of sheep and 7.9% of goats. There was a highly statistical significance between Brucella, RVFV, and FMDV seropositivity and locally bred animals (p < 0.01). Multiple seropositivities have been reported among sheep and goats. Brucella was commonly observed in mixed infection with other bacterial and viral agents under study.
Conclusion:
The surveyed viral and bacterial agents were prevalent in ruminants in the Medina region. Since Medina is an important destination for pilgrims from all over the world, therefore, an integrated approach involving strict control measures and routine vaccination programs should be adopted to reduce the possibility of global epidemics.
Keywords: Brucella, C. abortus, RVFV, FMDV, ELISA
Introduction
Pathogens that can transmit among different host species have special public health importance and significance [1,2]. The World Health Organization defined Zoonoses as infections which are transmitted between man and vertebrate animals [3]. Approximately, 75% of recently emerging human infectious diseases is of animal origin, and about 60% of human pathogens are zoonotic. Transmission of zoonotic diseases to humans would occur directly, by infected animals contact, or indirectly by consumption of contaminated food or water, inhalation, arthropod vectors and pests [4].
Sheep, goats, and camels are economically the most imperative farm animals in Saudi Arabia, and serve as major sources of meat; milk and income for a large sector of the population [5]. They are imported from elsewhere, and it may be infected by several bacterial and viral diseases which may cause economic losses. Also, it causes a devastating effect on human health.
Brucellosis remains one of the world’s major zoonosis that constitutes a major economic burden and public health problem, especially in Saudi Arabia [6]. It is caused by Brucella species, a Gram-negative, non-motile, non-spore forming, and small coccobacilli bacteria. Brucella infection may occur by ingestion of undercooked meat or unpasteurized milk from infected animals, or by direct contact with their secretions. Humans can be infected by Brucella abortus (cattle), Brucella canis (dogs), Brucella suis (pigs), and Brucella melitensis (sheep and goats) [7]. In animals, brucellosis is manifested mainly by abortion, the genitalia and fetal membranes inflammation, lesions in the lymphatics and joints, and sterility. Human Brucellosis common symptoms are undulating fever, weakness, headache, joint pain, and night sweats [8].
Chlamydiosis is an endemic disease of ruminants that is responsible for abortion in humans, birds, and animals. The causative agent is Chlamydia abortus, a Gram-negative, and an obligatory intracellular bacterium [9]. In ruminants, it is characterized by stillbirth or weak new-borns which die within the first 2 days, placental retention, and failure of reproduction, arthritis, recurrent respiratory symptoms, and inflammation of eyes [10]. It is thought to be responsible for 20%–50% of stillbirths and abortions in sheep [5]. In humans, the patient may show no symptoms, or have influenza-like symptoms with headaches, fever, chills, joint pains, light sensitivity, sore throat, and vomiting [10]. However, in pregnant women, it can develop life-threatening illnesses and abortion, pneumonia, and urogenital sign [11].
Rift Valley fever is a mosquito-borne viral disease, caused by Rift Valley fever virus (RVFV). It is a member of the Bunyaviridae family that causes abortion in ruminants, especially cattle and sheep [12]. The infected animal shows fever, loss of appetite, abortions, bloody diarrhea, vomiting, dullness, unsteady gait, skin necrosis on udder or scrotum, and/or death. The infected human shows mild fever, weakness, liver abnormalities, headaches, vision loss, weight loss, back pain, and may progress into inflammations of the brain [13].
Foot-and-mouth disease is a kind of constantly re-emerging at the interface between humans, animals, and the ecosystems. Accordingly, it has serious consequences for animal and human health [12,14]. It is caused by foot and mouth disease virus (FMDV), a member of the family Picornaviridae [15]. It is manifested by the formation of vesicles (blisters) in the mouth and feet of cattle, pigs, sheep, and goats. It is highly infectious and the main plague of animal farming [16]. Infected humans show red ulcerative lesions of the oral tissues, fever, malaise, vomiting, and sometimes vesicles (blisters) of the skin [17].
The present study aimed to survey the seroprevalence of some bacterial (Brucella spp., C. abortus) and viral (RVFV, FMDV) zoonoses in ruminants in the Medina region, and give an insight on the ruminant’s role as a reservoir for such public health threats.
Materials and Methods
Study design
A prospective study was conducted between May 2016 and December 2017 to investigate the serprevalence of some viral and bacterial zoonotic agents in domestic ruminants in Medina, Kingdom of Saudi Arabia (KSA). The animals reared under semi-extensive husbandry for their milk and/or meat. Ethical approval for this study was obtained from Taibah University ethics committee (1438/13).
Samples collection and serum separation
A total of 1,000 blood sample from 665 sheep, 228 goats, and 107 camels were collected. Of these samples, 617 samples were from the central slaughterhouse, 124 samples were from private farms, and 259 samples were kindly supplied from veterinary clinics affiliated to the Ministry of Agriculture. Animal’s bio-data, such as type, gender, locality, and source, were recorded. Historical data showed that the sampled animals were not vaccinated against any of the studied microbes. The blood samples were obtained from the jugular vein of each animal by sterile syringes and stored in 6-ml vacutainer tubes that had no anticoagulant and allowed to coagulate in the laboratory for 30–45 min. Then, the clots were removed by centrifugation at 6,000 rpm for 10 min, sera were separated and transferred to clean and sterile Eppendorf tubes and stored at −20°C till use.
Enzyme-Linked Immunosorbent Assay (ELISA) assay
Multi-species kits were purchased from ID.VET Company (ID.VET Diagnostics—France); ID Screen® Brucellosis Serum Indirect kit, ID Screen® C. abortus Indirect Enzyme-Linked Immunosorbent Assay (ELISA) kit, ID Screen® Rift Valley fever Competitive Elisa kit and ID Screen® FMDV Type O Competitive Elisa kit, following the manufacturer instructions. The optical density was measured at 450 nm with an automated ELISA reader (SIRIOS Elisa Reader, Indonesia).
Detection of Brucella
Indirect ELISA test was used for the detection of antibodies against B. abortus, B. melitensis, and B. suis in serum specimens. The diluted serum samples and controls were added to the microwells which were coated with purified B. abortus lipopolysaccharides. If serum contained anti-Brucella antibodies, an antibody-antigen complex would be formed. Then, a horseradish peroxidase conjugate was added, and an antigen-antibody-conjugate was formed. After washing, a substrate was added, and the developed blue color relied on the concentration of antibodies in the tested serum. In the presence of antibodies, the blue solution turned into yellow after adding the stop solution. While no color developed if there were no antibodies in the serum sample.
Detection of Chlamydia
The ID Screen® C. abortus Indirect ELISA kit contains a major outer-membrane protein (MOMP) antigen specific to C. abortus, it reduces the frequency of non-specific reactions. If the tested serum contained anti-Chlamydia antibodies, an antigen-antibody complex would be formed. Then, a horseradish peroxidase conjugate was added, and an antigen-antibody-conjugate was formed. After washing, a substrate was added, and the developed blue color relied on the concentration of antibodies in the tested serum. In the presence of antibodies, the blue solution turned into yellow after adding the stop solution. While no color developed if there were no antibodies in the serum sample.
Detection of Rift Valley fever virus
The competitive ELISA diagnostic kit was designed to detect antibodies specific for the RVFV nucleoprotein in sera or plasma specimens. ELISA microplates were coated with RVFV nucleoprotein. If the serum samples contained antibodies to RVFV, it would inhibit the binding of an anti-nucleoprotein-peroxidase (horseradish peroxidase)-labeled monoclonal antibody to the RVFV nucleoprotein coated on the plastic wells. While if there were no antibodies in the sample, a blue solution would appear and it turned yellow after adding the stop solution.
Detection of foot and mouth disease virus
The competitive ELISA kit is used to detect FMDV serotype O antibodies in serum specimens. ELISA microplates were coated with non-infectious FMDV type O antigen. If the antibodies exist in the serum samples, it will inhibit the binding of an anti-serotype O horseradish peroxidase-labeled monoclonal antibody to the non-infectious FMDV type O antigen coated on the plastic wells. If there were antibodies in the sample, no color would appear. While if there were no antibodies in the sample, a blue solution would appear and it turned yellow after adding the stop solution.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences version 20.0 (SPSS Inc., Chicago, IL, USA). The association between the prevalence of the studied agents with animal’s species, age, gender and/or locality was determined by Pearson’s correlation coefficient. A p-value of 0.05 or less was considered as statistically significant.
Results
Basic demographic characteristics of the examined animals
As shown in Table 1, a total of 1,000 blood samples were collected from sheep (n = 665), goats (n = 228), and camels (n = 107). Concerning the animal’s gender, a total of 482 males and 518 females were examined, in more details 406 males and 259 female’s sheep, 76 males and 152 female’s goats, and 107 female’s camels. Regarding the animal’s locality, 360 sheep were local and 305 were imported, 187 local goats and 41 imported ones, and 107 local camels. Animals were from different sources, 369 samples were kindly supplied from a veterinary clinic affiliated to the Ministry of Agriculture, and 190 from the slaughterhouse and 441 samples were collected from private farms.
Table 1. Basic demographic characteristics of the examined animals.
| Animals species | Gender | Locality | Source of samples |
|---|---|---|---|
| Sheep (n = 665) | Male (n = 406) | Local (n = 360) | Veterinary clinic (n = 162) |
| Female (n = 259) | Imported (n = 305) | Slaughterhouse (n = 116) | |
| Private farms (n = 387) | |||
| Goat (n = 228) | Male (n = 76) | Local (n =187) | Veterinary clinic(n = 100) |
| Female (n = 152) | Imported (n = 41) | Slaughterhouse (n = 74) | |
| Private farms (n = 54) | |||
| Camel (n = 107) | Male (n = 0) | Local (n = 107) | Veterinary clinic (n = 107) |
| Female (n = 107) | Imported (n = 0) | Slaughterhouse (n = 0) | |
| Private farms (n = 0) | |||
| Total (n = 1000) | Male (n = 482) | Local (n = 654) | Veterinary clinic (n = 369) |
| Female (n = 518) | Imported (n = 346) | Slaughterhouse (n = 190) | |
| Private farms (n = 441) |
Seroprevalence of the bacterial agents
Brucella species
As shown in Table 2, the incidence of Brucella species was 7.7%, 8.8%, and 6.5% in sheep, goats, and camels, respectively. Regarding the animal’s gender, higher frequencies of Brucella spp. were detected among females than males. Although the prevalence rate among females was higher than males, there was a highly significant correlation with male animals (p < 0.01). Similarly, local animals had a higher frequency of Brucella spp. than the imported with a significant correlation (p < 0.05). Animals belonged to different sources: a veterinary clinic, the local slaughterhouse, and private farms. Brucella spp. was at higher frequencies among the animals of the veterinary clinic with a highly significant correlation (p < 0.01).
Table 2. Prevalence of bacterial agents among animals.
| Host | Demographic factor | Brucella | C. abortus | |
|---|---|---|---|---|
| Sheep (n = 665) | Gender | Male (n = 406) | 24 (3.6%)** | 4 (0.6%) |
| Female (n = 259) | 27 (4.1%) | 1 (0.15%) | ||
| Locality | Local (n = 360) | 39 (5.9%)* | 3 (0.45%) | |
| Imported (n = 305) | 12 (1.8%) | 2 (0.3%) | ||
| Source | Veterinary clinic | 24 (3.6%)** | 3 (0.45%) | |
| Slaughterhouse | 6 (0.9%) | 0 (0%) | ||
| Private farms | 21 (3.1%) | 2 (0.3%) | ||
| Total | 51 (7.7%) | 5 (0.75%) | ||
| Goats (n = 228) | Gender | Male (n = 76) | 2 (0.9%)** | 0 (0%) |
| Female (n = 152) | 18 (7.9%) | 0 (0%) | ||
| Locality | Local (n = 187) | 19 (8.3%)* | 0 (0%) | |
| Imported (n = 41) | 1 (0.43%) | 0 (0%) | ||
| Source | Veterinary clinic | 17 (7.4%)** | 0 (0%) | |
| Slaughterhouse | 3 (1.3%) | 0 (0%) | ||
| Private farms | 0 (0%) | 0 (0%) | ||
| Total | 20 (8.8%) | 0 (0%) | ||
| Camels (n = 107) | Gender | Male (n = 0) | 0 (0%) | 0 (0%) |
| Female (n = 107) | 7 (6.5%) | 0 (0%) | ||
| Locality | Local (n = 107) | 7 (6.5%) | 0 (0%) | |
| Imported (n = 0) | 0 (0% | 0 (0%) | ||
| Source | Veterinary clinic | 7 (6.5%) | 0(0%) | |
| Slaughterhouse | 0 (0%) | 0(0%) | ||
| Private farms | 0 (0%) | 0 (0%) | ||
| Total | 7 (6.5%) | 0 (0%) | ||
Correlation is significant at the 0.01 level (2-tailed)
Correlation is significant at the 0.05 level (2-tailed)
Chlamydia abortus
C. abortus was recorded only in sheep (5; 0.75%) with no significant correlation (p > 0.05). Its prevalence was higher among males (4; 0.6%) than females (1; 0.15%). Also, it was higher among local (3; 0.45%) than (2; 0.3%) imported ones, with no significant correlation (p > 0.05). The prevalence of C. abortus accordingly the animal’s source was (3; 0.45%) in sheep from the veterinary clinic and (2; 0.3%) from the private farms (Table 2).
Prevalence of Brucella species among the human population in Medina
Prevalence of Brucella species among the human population from 2015 to 2017 according to King Fahad Hospital was shown in Table 3. Brucella abortus and B. melitensis had higher frequencies among males (61.3%, 59.8%) than females (38.7%, 40.2%). Patients were divided into six age groups, B. abortus and B. melitensis were at similar frequencies in the first three age groups. In the age group 4 and 6, B. abortus was at a higher rate (13.97%, 24.7%), while B. melitensis was more frequent (22.8%) in group 5. There was no statistical significance between the prevalence of B. abortus and/or B. melitensis with patient’s gender and/or age. Most of the examined patients had a mixed infection of both B. abortus and B. melitensis. Out of the 112 male patients, (55; 49.1%) had a mixed infection. Female patients had a mixed infection at a lower rate (35, 47.9%). Mixed infection in the first three age groups had the same rate (50%). Patients of age group 4, 5, and 6 had mixed infection at a rate of 48%, 47.5%, and 47.7%, respectively.
Table 3. Prevalence of Brucella species among the human population.
| Demographic Factor | B. abortus (n = 93) | B. melitensis (n = 92) | Mixed infection | |
|---|---|---|---|---|
| Gender | Male (n = 112) | 57 (61.3%) | 55 (59.8%) | 55 (49.1%) |
| Female (n = 73) | 36 (38.7%) | 37 (40.2%) | 35 (47.9%) | |
| Age | Group 1 (Less than 20) | 3 (3.2%) | 3 (3.3%) | 3 (50%) |
| Group 2 (Less than 30) | 16 (17.2%) | 16 (17.4%) | 16 (50%) | |
| Group 3 (Less than 40) | 19 (20.4%) | 19 (20.6%) | 19 (50%) | |
| Group 4 (Less than 50) | 13 (13.97%) | 12 (13%) | 12 (48%) | |
| Group 5 (Less than 60) | 19 (20.4%) | 21 (22.8%) | 19 (47.5%) | |
| Group 6 (60 and more) | 23 (24.7%) | 21 (22.8%) | 21 (47.7%) | |
Prevalence of the viral agents
Rift Valley fever virus
The incidence of RVFV among animals was shown in Table 4. The highest prevalence rate of RVFV was recorded in sheep (71: 10.7%), then in goats (41: 17.9%), while there was no prevalence recorded among camels. Concerning the animal’s gender, the prevalence rate among male animals was higher than females, with a highly significant correlation (p < 0.01). Although the prevalence rate was higher in the imported animals, there was a highly significant correlation between the prevalence of RVFV and locally bred animals (p < 0.01). Animals from the slaughterhouse had higher frequencies in both sheep and goats (10.7%, 17.1%) without any statistical significance (p > 0.05).
Table 4. Prevalence of viral agents among animals.
| Host | Demographic factor | RVFV | FMDV | |
|---|---|---|---|---|
| Sheep (n =665) ** | Gender | Male (n = 406) | 67 (10.1%)** | 151 (22.7%)** |
| Female (n = 259) | 4 (0.6%) | 34 (5.1%) | ||
| Locality | Local (n = 360) | 25 (3.75%)** | 42 (6.3%)** | |
| Imported (n = 305) | 46 (6.9%) | 143 (21.5%) | ||
| Source | Veterinary clinic | 0 (0%) | 38 (5.7%)** | |
| Slaughterhouse | 71 (10.7%) | 0 (0%) | ||
| Private farms | 0 (0%) | 147 (22.1%) | ||
| Total | 71 (10.7%) | 185 (27.8%) | ||
| Goats (n = 228) | Gender | Male (n = 76) | 35 (15.3%)** | 0 (0%) |
| Female (n = 152) | 6 (2.6%) | 18 (7.9%) | ||
| Locality | Local (n = 187) | 22 (9.6%)** | 18 (7.9%)** | |
| Imported (n = 41) | 19 (8.3%) | 0 (0%) | ||
| Source | Veterinary clinic | 2 (0.9%) | 15 (6.6%) ** | |
| Slaughterhouse | 39 (17.1%) | 0 (0%) | ||
| Private farms | 0 (0%) | 3 (1.3%) | ||
| Total | 41 (17.9%) | 18 (7.9%) | ||
Correlation is significant at the 0.01 level (2-tailed)
Correlation is significant at the 0.05 level (2-tailed).
Foot and mouth disease virus
The highest prevalence rate of FMDV was recorded in sheep (185; 27.8%) with a highly significant correlation (p < 0.01), then in goats (18; 7.9%). There was no seropositivity was detected among camels. FMDV had a higher frequency in male sheep (151; 22.7%) than females (34; 5.1%) with a highly significant correlation (p < 0.01). FMDV was highly correlated to locally bred animals (p < 0.01). According to animal’s source, FMDV frequency was the highest in animals of the veterinary clinic with a highly significant correlation (p < 0.01) (Table 4).
Bacterial and Viral Combination Patterns
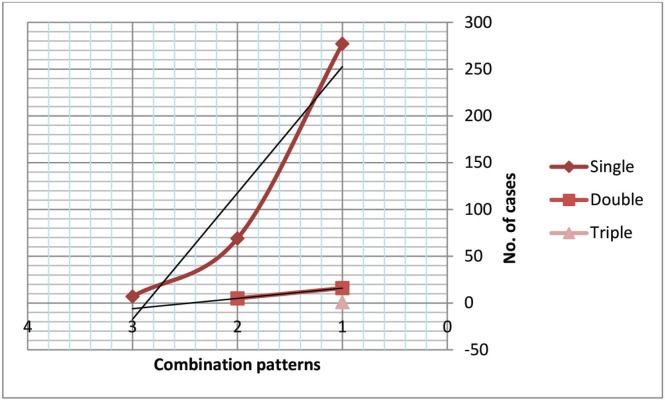
Although the single infection pattern was the most common (42%), viral and/or bacterial agents sometimes were in combinations. In sheep, the double infection pattern presented as a combination Brucella and FMDV, Brucella and RVFV, and C. abortus and FMDV. Triple infection pattern included combination between Brucella, FMDV, and C. abortus. In goats, the double infection pattern presented as a combination between two zoonotic pathogens, such as Brucella and RVFV cases, and Brucella and FMDV. In camels, no combinations were detected (Fig. 1, Table 5).
Figure 1. Bacterial and viral combination patterns.
Table 5. Bacterial and viral combination patterns.
| Infection Patterns | Pattern Percentage | The combinations in sheep | Pattern Percentage | The combinations in goats |
|---|---|---|---|---|
| Single | 277 (42%) | 36 (Brucella) 2 (C. abortus) 65 (RVFV) 174 (FMDV) |
69 (30.3%) | 15 (Brucella) 38 (RVFV) 16 (FMDV) |
| Double | 16 (2.4%) | 6 (Brucella + RVFV) 8 (Brucella + FMDV) 2 (C. abortus + FMDV) |
5 (2.2%) | 3 (Brucella + RVFV) 2 (Brucella+ FMDV) |
| Triple | 1 (0.15%) | 1 (Brucella + FMDV+ C. abortus) | 0 (0%) | 0 |
Discussion
Zoonoses are the infections that are naturally transmitted between vertebrate animals and humans [3]. The link between animals and human populations is very close in regions where peoples retain traditional lifestyles. Such contact, especially in absence of proper care can lead to a serious risk to public health with great economic consequences. Zoonotic diseases may occur through direct contact with the animal, through vectors, or water or food contamination [4]. Zoonoses constitute a diverse group of viral, bacterial, fungal, and parasitic diseases.
The present study was aimed to investigate the prevalence rates of Brucellosis, Chlamydiosis, RVFV, and FMDV in 665 sheep, 228 goats, and 107 camels in Medina, KSA. Among the livestock population in the KSA, sheep, goats, and camels accounted for 7.4 million, 4.2 million, and half a million, respectively [3]. They are considered as very important economic and food sources practically under certain religious circumstances (a period of pilgrimage), as its meat is the most preferred choice among people. Collecting data on the prevalence of these diseases is important not only for the economic impact of these diseases on the livestock but also because of its zoonotic nature, which initiates a series of events that constitute the pathogenesis of the infection. Since the animals were not vaccinated against the surveyed pathogens, the results may indicate that these bacterial and viral agents are circulating within the animal populations in Medina.
Kingdom of Saudi Arabia and the neighboring countries faced an extensive ascent in brucellosis incidence during 1991 [18]. Brucellosis poses serious economic losses because of its effect both on animal production and human health. In the present study, brucellosis was detected in sheep, goats, and camel’s sera by ELISA assay as followed in many studies [19–22]. All of them stated that ELISA was valid, sensitive, and reliable to diagnose brucellosis infection in domestic ruminants. The overall prevalence rates of brucellosis in this study were 7.7%, 8.8%, and 6.5% in sheep, goats, and camel’s sera, respectively. Al-Sekait [23] reported a higher seroprevalence in sheep (15.3%) and goats (20.1%) in Medina. Nearby in Mecca, the occurrence of brucellosis was 0.5% in sheep, 0.8% in goats, 3.6% in cows, and 2.8% in camels [24]. Furthermore, Abd El-rahim and Asghar [25] recorded an incidence of 15.6% in sheep and 3.9% in goats in Mecca and Medina. In Alkamil Province of Saudi Arabia, brucellosis prevalence were 5.88% in sheep and 4.87% in goats [19]. The serological evidence for Brucella infection in camels was 8% in Riyadh and Al-Kharj cities [24]. In the present study, brucellosis seroprevalence in the locally bred animals was higher than in imported ones with a highly significant correlation (p > 0.01). Also, it was much higher in females than in males with a highly significant correlation with male animals (p > 0.01). Higher seroprevalence in females could be attributed to the physiological stresses as pregnancy and suckling which affect their resistance to infection and also the females staying longer due to their reproduction role in the herd. Lower seroprevalence in males could also be attributed to the fact that male sheep and goats are usually provided with adequate feed and water. This disagreed with previous studies in Saudi Arabia, where male and female animals showed no significant difference in brucellosis seroprevalence [19].
Brucellosis is the most reportable communicable disease according to Saudi Arabian National Guard communities. There are more than 8000 human cases reported each year to Saudi Arabian public health authorities [26]. Brucellosis was reported all over the kingdom, but with the highest incidence at Al-Jouf, Qassim and Aseer, with a marked increase in summer and spring. The rise in brucellosis frequency in Saudi Arabia is due to the inefficient animal husbandry and overall propensity for ingesting raw unpasteurized dairy products from infected camels or goats [27]. In general, most cases of brucellosis are treated like other diseases and usually labeled as fever of unknown causes. The real number of brucellosis cases is uncertain and is thought to be far more than the officially announced numbers [28]. King Fahad Hospital is the main referral health center in Medina. People in Medina keep traditional lifestyles, where people communities live in close association with livestock such as sheep, goats, and camels, and consume their unpasteurized milk. In the period between 2015 and 2017, B. abortus was detected at a rate of 61.3% and 38.7% in males and females patients, respectively, while B. melitensis was at a rate of 59.8% in males and 40.2% in females. This was following several studies that have indicated male gender as a significant risk factor for brucellosis for several reasons, including those men had more close contact with animals [28–30]. Regarding the age of the patients, our results showed that old-age patients are particularly at risk of B. Abortus and B. melitensis sole or mixed infection. Following other reports, the prevalence of brucellosis increased with age [27,31], this may be as a result of the weak immune system due to aging.
Chlamydiosis is a major ruminant infectious disease that may result in sudden abortion outbreaks, thereby affecting herd production and reproduction [5,32]. In humans, only a few infection cases were reported, however, chlamydiosis during pregnancy causes a life-threatening risk for the fetus and the mother as well [14]. ELISA assay as a highly specific, sensitive, easy to perform for the detection of chlamydiosis [9–36]. Moreover, Baud et al. [37] stated that the MOMP-based ELISA ideal for large-scale serological studies, because of its high throughput and excellent specificity. In our study, the prevalence of chlamydiosis was 0.75% in sheep, while goats and camels did not show any positivity. There were few studies reported the prevalence of C. abortus in Saudi Arabia; 19.4% in camels [35], 10.05% in Al-Ahsa camels [38], 7.52% and 34.50% in sheep and goats, respectively in AL-Riyadh [5]. We found C. abortus was more frequent in males than in females with no significant correlation (p > 0.01), the reason for the frequency difference is unclear but sex may be a factor affecting the prevalence of chlamydiosis [39].
In the year 2000, Saudi Arabian Ministry of Health in received reports of unexplained humans hemorrhagic fever and associated animal deaths from the southwestern border of Saudi Arabia [40,41]. The key aspect of RVFV in this epidemic was acute hepatitis associated with complications this epidemic renal failure, Central Nervous System involvement, thrombocytopenia, severe anemia, vision loss, and hemorrhagic manifestations. The outbreak occurs in climatic conditions favoring the breeding of mosquito and is most severe in goats, sheep, and cattle. It causes animals abortions and a high mortality rate in the new-borns. Non-pregnant older animals are susceptible to infection too, but they are more resistant to clinical disease [42,43]. Competitive ELISA can be used as an efficient and highly accurate diagnostic tool in RVFV control programs and surveillance [44]. There was no evidence of serological cross-reactivity of RVFV with other African Phlebovirus which could conceal the diagnosis of RVFV [45,46]. In this study, RVFV was detected in sheep (10.7%) and goats (17.9%) only. Nearby in Mecca, Mohamed et al. [45] tested sheep and goats and found that 16.8% were seropositive using competitive ELISA. In Jizan, RVFV was reported in 22 animals from 17 herds [42]. Al-Ahsa Oasis, Al-Qabati, and Al-Afaleq found that only 2 out of 225 sheep sera were seropositive for RVFV, while goats, cattle, and camels did not show any positivity [47]. Furthermore, Boshra et al. [48] found only a single positive sample among goats and sheep in both Aseer and Al Riyadh, while the Al-Ahsa had no seropositive animals. The variation in seroprevalences between reports is influenced by the animal’s age, sampling season, sampling time, mixing of animals under same husbandry practices, the diagnostic test used, and virus maintenance and persistence of ecological stressors [49].
Foot and mouth disease is one of the enzootic diseases in Saudi Arabia [50,51]. Every year, Saudi Arabia imports millions of ruminants for slaughter and many of them are imported from countries where FMDV is enzootic. These imported animals are either subclinically infected animals that might actively excrete FMDV virus or a carrier animal which might act as a possible source of infection [52]. In humans, FMDV is considered very rare, but it has been reported mainly in connection with direct contact with infected animals or as a result of consumption of unpasteurized milk, dairy, or unprocessed meat products from infected animals. It is one of the diseases which impacted badly on farmers in most African countries [53]. In the current study, FMDV serotype O was detected, the predominant serotype of the FMDV in Saudi Arabia [51,54]. The overall prevalence rate of FMDV serotype O was 27.8% and 7.9% in sheep and goats, respectively, while the camel’s sera didn’t show any positivity. There was a highly significant correlation for the presence of FMDV antibodies among sheep (p < 0.01). In other parts of Saudi Arabia, there have been a few studies investigating the spread of FMDV among ruminants. Woodbury et al. [55] isolated type O viruses from cattle and found out that 16 out of 31 samples collected were seropositive. Moreover, Hafez et al. [56] detected antibodies against serotypes O in 92 reactors (38 sheep, 35 goats, and 19 cattle) in different regions of Saudi Arabia. In Riyadh and AL Qassim Province, Yousef [51] found that only 24 (6.3%) out of 376 serum samples were positive for antibodies against serotype O in camels. Furthermore, in Riyadh, Mahmoud and Galbat [54] reported FMDV serotype O in 38 (67%) sheep of a Marino flock. This variation in FMDV seroprevalence among different regions could be attributed to differences in climatic conditions, ecology, and overall exposure to the virus [7,57]. In the present study, FMDV seroprevalence was much higher in male sheep (22.7%) than in females (5.1%) with a highly significant correlation (p < 0.01), while the infected goats were all females (7.9%). Higher seroprevalence in male sheep could be attributed to the behavior of males that are constantly sneaking from one herd seeking a mating partner. It is widely accepted that contact is one of the common ways in which FMDV is spread between susceptible and infected animals [53,58].
Conclusion
The viral agents (RVFV and FMDV) were more prevalent than the bacterial agents (Brucella spp. and C. abortus) in single and multiple patterns. Species susceptibility, locality, source, age, and gender had an impact on the prevalence rates. Brucella abortus and B. melitensis were reported among the human population with a higher prevalence among males and old aged patients. Finding a link between human health and pathogens that circulate in domestic ruminants in Medina will help us to deal with such serious conditions.
Statement of Animal Rights
This study approved by the appropriate ethics committee and performed following the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Conflict of interest
The authors declare that there is no conflict of interest
Authors’ contributions
Shabana, I.I.: Contributed to the design and the establishment of experiment scheme, the performance of the ELISA assay, data modelling, analysis, and interpretation. She contributed also to the orientation of statistical graphics, the writing of the manuscript and important review contributions.
Krimly, R. A.: Contributed to the design and the establishment of experiment scheme. She contributed also to the sample collection, serum separation, and the writing of the manuscript.
Ethics
This article is original and contains unpublished material. The corresponding author confirms that all of the other authors have read and approved the manuscript and no ethical issues involved.
References
- [1].Cleaveland S, Laurenson M, Taylor L. Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Philos Trans R Soc Lond B Biol Sci. 2001;356:991–9. doi: 10.1098/rstb.2001.0889. https://doi.org/10.1098/rstb.2001.0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McDaniel CJ, Cardwell DM, Moeller RB, Gray GC. Humans and cattle: a review of bovine zoonoses. Vector Borne Zoonotic Dis. 2014;14:1–19. doi: 10.1089/vbz.2012.1164. https://doi.org/10.1089/vbz.2012.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wernery U. Zoonoses in the Arabian Peninsula. Saudi Med J. 2013;35:1455–62. [PMC free article] [PubMed] [Google Scholar]
- [4].Ganter M. Zoonotic risks from small ruminants. Vet Microbiol. 2015;181:53–65. doi: 10.1016/j.vetmic.2015.07.015. https://doi.org/10.1016/j.vetmic.2015.07.015. [DOI] [PubMed] [Google Scholar]
- [5].Aljumaah Rs, Hussein M. Serological prevalence of ovine and caprine Chlamydophilosis in Riyadh region, Saudi Arabia. Afr J Microbiol Res. 2012;6:26548. https://doi.org/10.5897/AJMR11.1056. [Google Scholar]
- [6].Jokhdar H. Brucellosis in Saudi Arabia: review of literature and an alarming case report in a hospital in Jeddah. Med J Cairo Univ. 2009;77:47–55. [Google Scholar]
- [7].Ogugua AJ, Akinseye VO, Ayoola MC, Oyesola OO, Shima FK, Tijjani AO, et al. Seroprevalence and risk factors of Brucellosis in goats in selected states in Nigeria and the public health implications. Afr J Med Med Sci. 2014;43:121–9. [PMC free article] [PubMed] [Google Scholar]
- [8].Dean AS, Crump L, Greter H, Hattendorf J, Schelling E, Zinsstag J. Clinical manifestations of human Brucellosis: a systematic review and Meta-Analysis. PLoS Negl Trop Dis. 2012;6:e1929. doi: 10.1371/journal.pntd.0001929. https://doi.org/10.1371/journal.pntd.0001929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Didugu H, Reddy CEN, Ramanipushpa RN, Ramaraju SSB, Reddy MV, Satyanarayana M, et al. Serological investigation of Chlamydial infection among ruminants in Krishna district of Andhra Pradesh, India. J Livest Sci. 2016;7:187–91. [Google Scholar]
- [10].Chen Q, Gong X, Zheng F, Cao X, Li Z, Zhou J. Seroprevalence of Chlamydophila abortus infection in yaks (bos grunniens) in Qinghai, China. Trop Anim Health Prod. 2014;46:503–7. doi: 10.1007/s11250-013-0519-8. https://doi.org/10.1007/s11250-013-0519-8. [DOI] [PubMed] [Google Scholar]
- [11].El-Razik K. Investigations on non Brucella abortifacients in small ruminants in Saudi Arabia with emphasis on zoonotic causes. Glob Vet. 2011;6:25–32. [Google Scholar]
- [12].Blomström AL, Scharin I, Stenberg H, Figueiredo J, Nhambirre O, Abílio A, et al. Seroprevalence of Rift Valley fever virus in sheep and goats in Zambézia, Mozambique. Infect Ecol Epidemiol. 2016;6:31343. doi: 10.3402/iee.v6.31343. https://doi.org/10.3402/iee.v6.31343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Reperant L, Brown I, Haenen O, de Jong M, Osterhaus AD, Papa A, et al. Companion animals as a source of viruses for human beings and food production animals. J Comp Pathol. 2016;155:S41–53. doi: 10.1016/j.jcpa.2016.07.006. https://doi.org/10.1016/j.jcpa.2016.07.006. [DOI] [PubMed] [Google Scholar]
- [14].Bouchot A, Bordier M. The OIEstrategy to address threats at the interface between humans, animals and ecosystems. In: Morand S, Dujardin J-P, Lefait-Robin R, Apiwathnasorn C, editors. Socio-ecological dimensions of infectious diseases in Southeast Asia. Springer; 2015. pp. 275–91. https://doi.org/10.1007/978-981-287-527-3_16. [Google Scholar]
- [15].Weaver GV, Domenech J, Thiermann AR, Karesh WB. Foot and mouth disease: a look from the wild side. J Wildl Dis. 2013;49:759–85. doi: 10.7589/2012-11-276. https://doi.org/10.7589/2012-11-276. [DOI] [PubMed] [Google Scholar]
- [16].Carrillo C, Tulman E, Delhon G, Lu Z, Carreno A, Vagnozzi A, et al. High throughput sequencing and comparative genomics of foot-and-mouth disease virus. Dev Biol. 2006;126:23–30. doi: 10.1128/jvi.79.10.6487. [DOI] [PubMed] [Google Scholar]
- [17].Knight-Jones T, Rushton J. The economic impacts of foot and mouth disease–what are they, how big are they and where do they occur? Prev Vet Med. 2013;112:161–73. doi: 10.1016/j.prevetmed.2013.07.013. https://doi.org/10.1016/j.prevetmed.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Bilal NE, Jamjoom GA, Bobo RA, Aly OF, el-Nashar NM. A study of the knowledge, attitude and practice (KAP) of a Saudi Arabian community towards the problem of Brucellosis. J Egypt Public Health Assoc. 1991;66:227–38. [PubMed] [Google Scholar]
- [19].Kandeel A, Jamal MA, Sedik AS, Salauldin H, Fadlelmoula A. Seroprevalence of Brucellosis within sheep and goat flocks in Alkamil Province in Saudi Arabia. Bothalia J. 2014;44:131–40. [Google Scholar]
- [20].Osoro EM, Munyua P, Omulo S, Ogola E, Ade F, Mbatha P, et al. Strong association between human and animal Brucella seropositivity.in a linked study in Kenya, 2012–2013. Am J Trop Med Hyg. 2015;93:224–31. doi: 10.4269/ajtmh.15-0113. https://doi.org/10.4269/ajtmh.15-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Khaldi M, Touil AT, Ahmed EK, Bendahmane Epidemiology and spatial distribution of Brucellosis in cattle and sheep in West Algerian Regions from 2002–2014. J Appl Environ Biol Sci. 2016;6:1–6. [Google Scholar]
- [22].Shahzad A, Khan A, Khan MZ, Saqib M. Seroprevalence and molecular investigation of Brucellosis in camels of selected districts of Punjab, Pakistan. The Thai J Vet Med. 2017;47:207. [Google Scholar]
- [23].Al-Sekait MA. Epidemiology of Brucellosis in Al-Medina Region, Saudi Arabia. J Family Community Med. 2000;7:47–53. [PMC free article] [PubMed] [Google Scholar]
- [24].Radwan AI, Bekairi SI, Prasad PV. Serological and bacteriological study of Brucellosis in camels in Central Saudi Arabia. Rev Sci Tech. 1992;11:837–44. doi: 10.20506/rst.11.3.612. https://doi.org/10.20506/rst.11.3.612. [DOI] [PubMed] [Google Scholar]
- [25].Abd El-rahim I, Asghar A. Brucellosis in ruminant animals and their close contact humans in Western Region of Saudi Arabia in 2012. Assiut Vet Med J. 2014;60:140. [Google Scholar]
- [26].Memish Z, Mah M. Brucellosis in laboratory workers at a Saudi Arabian hospital. Am J Infect Control. 2001;29:8–52. doi: 10.1067/mic.2001.111374. https://doi.org/10.1067/mic.2001.111374. [DOI] [PubMed] [Google Scholar]
- [27].Alhoshani R, Ali S, Irfan UM. Brucellosis seropositivity among adults in Al Rass City, Qassim Province, Saudi Arabia. Int J Med Investig. 2016;5:158–64. [Google Scholar]
- [28].Al-Tawfiq JA, AbuKhamsin A. A 24-year study of the epidemiology of human Brucellosis in a health-care system in Eastern Saudi Arabia. J Infect Public Health. 2009;2:81–5. doi: 10.1016/j.jiph.2009.03.003. https://doi.org/10.1016/j.jiph.2009.03.003. [DOI] [PubMed] [Google Scholar]
- [29].Aloufi AD, Memish ZA, Assiri AM, McNabb SJ. Trends of reported human cases of Brucellosis, Kingdom of Saudi Arabia, 2004–2012. J Epidemiol Glob Health. 2016;6:11–18. doi: 10.1016/j.jegh.2015.09.001. https://doi.org/10.1016/j.jegh.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Meky F, Hassan E, Abdel Hafez A, Aboul Fetouh A, El Ghazali S. Epidemiology and risk factors of Brucellosis in Alexandria Governorate. World Vet J. 2007;5:74–81. [PubMed] [Google Scholar]
- [31].Al Dahouk S, Neubauer H, Hensel A, Schöneberg I, Nöckler K, Alpers K, et al. Changing epidemiology of human Brucellosis, Germany, 1962–2005. Emerg Infect Dis. 2007;13:1895. doi: 10.3201/eid1312.070527. https://doi.org/10.3201/eid1312.070527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Elzlitne R, Elhafi G. Seroprevalence of Chlamydia abortus in camel in the Western region of Libya. J Adv Vet Anim Res. 2016;3:178. https://doi.org/10.5455/javar.2016.c151. [Google Scholar]
- [33].Borel N, Doherr MG, Vretou E, Psarrou E, Thoma R, Pospischil A. Seroprevalences for ovine enzootic abortion in Switzerland. Prev Vet Med. 2004;65:205–16. doi: 10.1016/j.prevetmed.2004.08.005. https://doi.org/10.1016/j.prevetmed.2004.08.005. [DOI] [PubMed] [Google Scholar]
- [34].Hany AMZ, Swelum AAA, Sawsan AMA, Alkablawy AH, Ismael AB. Seroprevalence of chlamydiosis in Abu Dhabi dromedary camel (camelus dromedarius) and its association with hematobiochemical responses towards the infection. J Adv Vet Anim Res. 2017;4:175–80. https://doi.org/10.5455/javar.2017.d208. [Google Scholar]
- [35].Hussein M, Alshaikh M, El-Rab G, Aljumaah RS, Gar El Nabi AR, Abdel Bagi AM. Serological prevalence of Q fever and chlamydiosis in camels in Saudi Arabia. J Anim Vet Adv. 2008;7:685–8. [Google Scholar]
- [36].Krkalić L, Šatrović E, Goletić T, Džaja P, Severin K. Chlamydophila abortus infection in a flock of goats in Bosnia and Herzegovina. Veterinarski Arhiv. 2015;85:359–68. [Google Scholar]
- [37].Baud D, Regan L, Greub G. Comparison of five commercial serological tests for the detection of anti-chlamydiatrachomatis antibodies. Eur J Clin Microbiol Infect Dis. 2010;29:669–75. doi: 10.1007/s10096-010-0912-4. https://doi.org/10.1007/s10096-010-0912-4. [DOI] [PubMed] [Google Scholar]
- [38].Khalifa IA. Serological prevalence of abortifacient agents in female mijaheem camels (camelus dromedarius) in Saudi Arabia. J Anim Res. 2018;8:335–43. https://doi.org/10.30954/2277-940X.06.2018.1. [Google Scholar]
- [39].Pinheiro Junior JW, Mota RA, Piatti RM, Oliveira AA, Silva AM, Abreu SR, et al. Seroprevalence of antibodies to Chlamydophila abortus in ovine in the state of Alagoas, Brazil. Braz J Microbiol. 2010;41:358–64. doi: 10.1590/S1517-838220100002000015. https://doi.org/10.1590/S1517-83822010000200015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Davies FG. Risk of a Rift Valley Fever epidemic at the Haj in Mecca, Saudi Arabia Rift Valley fever. Revue Scientifique Et Technique De L Office International Des Epizooties. 2006;25:137–47. doi: 10.20506/rst.25.1.1648. https://doi.org/10.20506/rst.25.1.1648. [DOI] [PubMed] [Google Scholar]
- [41].Memish ZA, Masri MA, Anderson BD, Heil GL, Merrill HR, K, et al. Elevated antibodies against Rift Valley fever virus among humans with exposure to ruminants in Saudi Arabia. Am J Trop Med Hyg. 2015;92:739–43. doi: 10.4269/ajtmh.14-0575. https://doi.org/10.4269/ajtmh.14-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Elfadil A, Hasab-Allah K, Dafa-Allah O, Elmanea A. The persistence of Rift Valley fever in the Jazan region of Saudi Arabia. Revue scientifique et technique-office international des epizooties. 2006;25:1131–6. https://doi.org/10.20506/rst.25.3.1716. [PubMed] [Google Scholar]
- [43].Mbotha D, Bett B, Kairu-Wanyoike S, Grace D, Kihara A, Wainaina M, et al. Inter-epidemic Rift Valley fever virus seroconversions in an Irrigation Scheme in Bura, South-East Kenya. Transbound Emerg Dis. 2018;65:e55–62. doi: 10.1111/tbed.12674. https://doi.org/10.1111/tbed.12674. [DOI] [PubMed] [Google Scholar]
- [44].Paweska JT, Mortimer E, Leman PA, Swanepoel R. An inhibition enzyme-linked immunosorbent assay for the detection of antibody to Rift Valley fever virus in humans, domestic and wild ruminants. J Virol Methods. 2005;127:10–8. doi: 10.1016/j.jviromet.2005.02.008. https://doi.org/10.1016/j.jviromet.2005.02.008. [DOI] [PubMed] [Google Scholar]
- [45].Mohamed AM, Ashshi AM, Asghar AH, Abd El-Rahim IHA, El-Shemi AG, Zafar T. Seroepidemiological survey on Rift Valley fever among small ruminants and their close human contacts in Makkah, Saudi Arabia, in 2011. Revue Scientifique et Technique de l’OIE. 2014;33:903–15. doi: 10.20506/rst.33.3.2328. https://doi.org/10.20506/rst.33.3.2328. [DOI] [PubMed] [Google Scholar]
- [46].Mroz C, Gwida M, El-Ashker M, El-Diasty M, El-Beskawy M, Ziegler U, et al. Seroprevalence of Rift Valley Fever virus in livestock during inter-epidemic period in Egypt, 2014/15. BMC Vet Res. 2017;13:87. doi: 10.1186/s12917-017-0993-8. https://doi.org/10.1186/s12917-017-0993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Al-Qabati AG, Al-Afaleq AI. Crossectional, longitudinaland prospective epidemiologic studyof Rift Valley fever in Al-Hassa Oasis, Saudi Arabia. J Anim Vet Adv. 2010;9:258–65. https://doi.org/10.3923/javaa.2010.258.265. [Google Scholar]
- [48].Boshra H, Truong T, Babiuk S, Hemida MG. Seroprevalence of sheep and goat pox, peste des petits ruminants and Rift Valley fever in Saudi Arabia. PLoS One. 2015;10:e0140328. doi: 10.1371/journal.pone.0140328. https://doi.org/10.1371/journal.pone.0140328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Swai ES, Sindato C. Seroprevalence of Rift Valley fever virus infection in camels (dromedaries) in Northern Tanzania. Trop Anim Health Prod. 2015;47:347–52. doi: 10.1007/s11250-014-0726-y. https://doi.org/10.1007/s11250-014-0726-y. [DOI] [PubMed] [Google Scholar]
- [50].Aidaros HA. Regional status and approaches to control and eradication of foot and mouth disease in the Middle East and North Africa. Rev Sci Tech. 2002;21:451–8. doi: 10.20506/rst.21.3.1348. https://doi.org/10.20506/rst.21.3.1348. [DOI] [PubMed] [Google Scholar]
- [51].Yousef MR, Mazloum KS, Al-Nakhli HM. Serological evidence of natural exposure of camels to foot and mouth disease virus. Vet World. 2012;5:197–200. https://doi.org/10.5455/vetworld.2012.197-200. [Google Scholar]
- [52].Hafez S, Farag M, Al-Sukayran A, Al-Mujalli D. Epizootiology of foot and mouth disease in Saudi Arabia: I. Analysis of data obtained through district field veterinarians. Revue scientifique et technique (International Office of Epizootics) 1993;12:807–16. doi: 10.20506/rst.12.3.715. https://doi.org/10.20506/rst.12.3.715. [DOI] [PubMed] [Google Scholar]
- [53].Raletobana JG. Sero-prevalence and risk factors of foot and mouth disease in goats in Ngamiland and North East Districts of Botswana. University of Botswana, Gaborone; South Africa: 2013. (Unpublished master’s thesis) [Google Scholar]
- [54].Mahmoud MA, Galbat SA. Outbreak of foot and mouth disease and peste des petits ruminants in sheep flock imported for immediate slaughter in Riyadh. Vet World. 2017;10:238–43. doi: 10.14202/vetworld.2017.238-243. https://doi.org/10.14202/vetworld.2017.238-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Woodbury E, Samuel A, Knowles N, Hafez S, Kitching R. Analysis of mixed foot-and-mouth disease virus infections in Saudi Arabia: prolonged circulation of an exotic serotype. Epidemiol Infect. 1994;112:201–12. doi: 10.1017/s0950268800057575. https://doi.org/10.1017/S0950268800057575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hafez S, Farag M, Mazloum K, Al-Bokmy A. Serological survey of Foot and Mouth Disease in Saudi Arabia. Revue scientifique et technique-office international des epizooties. 1994;13:711–9. doi: 10.20506/rst.13.3.791. https://doi.org/10.20506/rst.13.3.791. [DOI] [PubMed] [Google Scholar]
- [57].Balinda SN, Tjørnehøj K, Muwanika VB, Sangula A, Mwiine FN, Ayebazibwe C, et al. Prevalence estimates of antibodies towards foot-and-mouth disease virus in small ruminants in Uganda. Transbound Emerg Dis. 2009;56:362–71. doi: 10.1111/j.1865-1682.2009.01094.x. https://doi.org/10.1111/j.1865-1682.2009.01094.x. [DOI] [PubMed] [Google Scholar]
- [58].Ur-Rehman S, Arshad M, Hussain I, Iqbal Z. Detection and seroprevalence of foot and mouth disease in sheep and goats in Punjab, Pakistan. Transbound Emerg Dis. 2014;61(Suppl 1):25–30. doi: 10.1111/tbed.12194. https://doi.org/10.1111/tbed.12194. [DOI] [PubMed] [Google Scholar]