Abstract
Objective:
To incorporate blood pressure (BP), diagnoses codes, and medication fills from electronic medical records (EMR) to identify pregnant women with hypertension.
Study design:
a retrospective cohort study of singleton pregnancies at three US integrated health delivery systems during 2005–2014.
Main outcome measures:
Women were considered hypertensive if they had any of the following: (1) ≥2 high BPs (≥ 140/90 mmHg) within 30 days during pregnancy (High BP); (2) an antihypertensive medication fill in the 120 days before pregnancy and a hypertension diagnosis from 1 year prior to pregnancy through 20 weeks gestation (Treated Chronic Hypertension); or (3) a high BP, a hypertension diagnosis, and a prescription fill within 7 days during pregnancy (Rapid Treatment). We described characteristics of these pregnancies and conducted medical record review to understand hypertension presence and severity.
Results:
Of 566,624 pregnancies, 27,049 (4.8%) met our hypertension case definition: 24,140 (89.2%) with High BP, 5,409 (20.0%) with Treated Chronic Hypertension, and 5,363 (19.8%) with Rapid Treatment (not mutually exclusive). Of hypertensive pregnancies, 19,298 (71.3%) received a diagnosis, 9,762 (36.1%) received treatment and 11,226 (41.5%) had a BP ≥ 160/110. In a random sample (n=55) of the 7,559 pregnancies meeting the High BP criterion with no hypertension diagnosis, clinical statements about hypertension were found in medical records for 58% of them.
Conclusion:
Incorporating EMR BP identified many pregnant women with hypertension who would have been missed by using diagnosis codes alone. Future studies should seek to incorporate BP to study treatment and outcomes of hypertension in pregnancy.
Introduction
Hypertensive disorders, including preeclampsia, chronic and gestational hypertension, affects nearly 300,000 (6–8%) pregnancies annually in the US, increasing risks of adverse outcomes including maternal and perinatal death and medically indicated preterm birth.1–7 The guideline from American College of Obstetricians and Gynecologists (ACOG), which informs U.S. clinical practice, defines hypertension in pregnancy as a systolic blood pressure (BP) ≥ 140 mm Hg, and/or a diastolic BP ≥90 mm Hg, typically on 2 occasions at least 4 hours apart.8 Administrative claims data have been used to evaluate prevalence2,9–13 and outcomes14 of hypertension during pregnancy. In contrast to the ACOG clinical definition, such studies define hypertension in pregnancy based on diagnosis codes,2,9–14 often relying solely on codes recorded at the delivery hospitalization.2,9–11,13,14 However, this approach has relatively low sensitivity:in non-pregnant adult populations, defining hypertension based on 2 or more diagnosis codes within 3 years only achieved 73% sensitivity and 95% specificity, and additionally requiring pharmacy claims improved specificity but decreased sensitivity.15,16 Hence, studies based on claims data may substantially underestimate the true burden of hypertensive disease in pregnancy.
Increasingly available large scale electronic medical record (EMR) data containing richer clinical data than claims data provides opportunities for improved ascertainment of various diseases17,18 including hypertension.19 A hypertension case definition based on a combination of diagnosis codes, medication fills and BP has been used in studies of non-pregnant populations.20–22 We aimed to identify hypertension during pregnancy using an approach that integrates BP with diagnoses and antihypertensive medication fills. We also sought to better understand discrepancies in cases identified by this approach versus other methods.
Methods
This study used data from a cohort study of singleton pregnancies within three healthcare systems: Kaiser Permanente Northern California (KPNC), Southern California (KPSC) and Washington (KPWA), who together provide medical coverage and care to over 10 million members. The goal of the parent study was to examine the impact of antihypertensive treatments on maternal and neonatal outcomes within singleton pregnancies. The current study describes the approach used to identify the study cohort. Research activities were approved by the institutional review boards at each Kaiser Permanente region and by state institutional review boards (governing the use of birth certificate data).
Study Population
We included singleton live or stillbirth pregnancies among women between ages 15 and 49 years during 2007–2014 at KPSC or KPWA, and 2005–2014 at KPNC (based on the time periods when EMR BPs became available). Pregnancies were identified via hospital pregnancy registries supplemented with diagnosis and procedure codes. Gestational age was ascertained primarily from clinical data in the EMR such as the estimated due date (EDD), which is based on results of first trimester ultrasound or the date of last menstrual period if the former was missing. We then estimated the start of pregnancy as the EDD minus 280 days. To ensure sufficient data for ascertaining hypertension, we restricted to pregnancies that had: a non-missing gestational age at delivery, continuous enrollment in the health plan from no later than 16 weeks gestation through delivery, and at least 1 recorded BP prior to 20 weeks gestation. The parent study made several exclusions to reduce confounding in studying hypertension treatment effect on birth outcomes, including: multiple gestations, certain rare conditions or exposure to teratogenic medications or medications indicative of severe maternal conditions (see Appendix 1).
Data source
All three health systems maintain a Virtual Data Warehouse (VDW) with common variables and definitions to facilitate data sharing and harmonization across systems.24 From the VDW, we extracted women’s enrollment information, BP, height, weight, diagnosis and procedure codes from health care encounters, and outpatient pharmacy fills. For women with multiple BPs recorded on a single day, the highest systolic and diastolic BP were used. Women’s EMR were linked to state birth certificates to supplement information on maternal race/ethnicity, education, tobacco use, infant sex and parity (see Appendix 1).
Hypertension case definition
Because the parent study aimed to study pregnancies that may be candidates for oral antihypertensive treatment, we focused on hypertension that emerged by 35 weeks 6 days gestation so that there would be an opportunity for the mother to receive treatment and for the treatment to have an effect. We operationalized the ACOG definition of hypertension as 2 BPs ≥140/90 mm Hg recorded on separate days within 30 days from the start of the pregnancy through 35 weeks 6 days gestation (“High BP” criterion).8 We then created 2 other criteria to identify women who were hypertensive but might not have repeated high BPs recorded in the EMR due to effective treatment. These were women: 1) with “Treated chronic hypertension” (defined as ≥1 fills of an antihypertensive medication in the 120 days before pregnancy plus a hypertension diagnosis code within 1 year prior to pregnancy through 20 weeks gestation); and 2) who received “Rapid Treatment” (defined as a BP of 140/90 or higher from start of the pregnancy through 35 weeks 6 days accompanied by 1 hypertension diagnosis plus 1 antihypertensive medication fill within 7 days of the high BP). Pregnancies could meet multiple criteria.
Some prior studies have defined hypertension based on outpatient BP alone, as inpatient BP may be influenced by acute illness.20,25 Given the short time window to identify and treat hypertension in pregnancy, the ACOG guideline does not require using only outpatient BP.8 Thus, our primary analyses did not distinguish BP by clinical setting.
Indicators and severity of hypertension
Among pregnancies identified as hypertensive by 35 weeks 6 days gestation, we then used EMR data collected throughout the pregnancy to examine various clinical characteristics that could be indicators for the presence and severity of hypertension. We first examined receipt of a hypertension diagnosis code in 2 ways: (1) during pregnancy —having 1 hypertension diagnosis code recorded from the start of pregnancy through delivery, or 1 inpatient diagnosis of preeclampsia or eclampsia26 from 20 weeks gestation through delivery; and (2) at the delivery hospitalization— having 1 inpatient diagnosis code for hypertension, preeclampsia or eclampsia from three days prior to the delivery date through delivery date. We defined BP ≥140/90 mm Hg as high BP and BP≥160/110 mm Hg as severe high BP.8 We calculated the proportion of women with at least 1 high BP and 1 severe high BP any time during pregnancy and from 20 weeks gestation to delivery. Lastly, we examined receipt of treatment, defined as filling ≥1 outpatient prescriptions of antihypertensive medications during pregnancy.
Medical record review
To better understand discrepancies in hypertensive cases according to our definition compared to cases according to other approaches, we reviewed medical records for a sample of pregnancies in three groups: (1) among women with multiple hypertension diagnosis codes, those who did not meet our hypertension case definition (Set A); (2) among women meeting our High BP criterion, those without hypertension diagnosis (Set B); and (3) among women filling antihypertensive medications, those did not meet our hypertension case definition (Set C). Because in our settings, labetalol and methyldopa are rarely used for other indications, we evaluated users of these 2 medications separately. Across all health systems, we selected random samples and reviewed 45–55 charts from each group. We searched medical records, including clinical notes, for evidence of high BP and antihypertensive prescriptions. For Set B, we also searched for recorded statements about hypertension in the clinical notes (e.g., mentions of “hypertension”, “white coat hypertension”, or “labile” or “elevated blood pressure”). Medical record review was conducted by trained abstractors at each site, who followed the same medical review manual of operations and met regularly as a group with study investigators to ensure consistency in interpreting and applying the manual.
Statistical analyses
We grouped pregnancies according to which criterion of our hypertension case definition they met. Pregnancies meeting multiple criteria were included in each group for which they met criteria. We calculated the proportion of total pregnancies meeting each criterion, examined overlap between these groups and described maternal demographic and clinical characteristics. Analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC).
Results
We identified 566,624 eligible pregnancies, 30,669 were hypertensive according to our case definition. We further excluded 1,768 pregnancies with multiple gestations and 1852 with rare conditions or certain teratogenic medication exposures, leaving a final analytic sample of 27,049 (Figure 1). In our cohort, 24,140 pregnancies (89.2%) were identified as hypertensive via the High BP criterion, and 75.5% of these were identified solely by this criterion (Figure 2). The Treated chronic Hypertension criterion identified 5,409 (20.0%) pregnancies as hypertensive and the Rapid Treatment criterion identified 5,363 (19.8%), and many pregnancies in these 2 groups also met the High BP criterion (52.9% and 87.9%, respectively).
Figure 1. Identification of the study cohort.
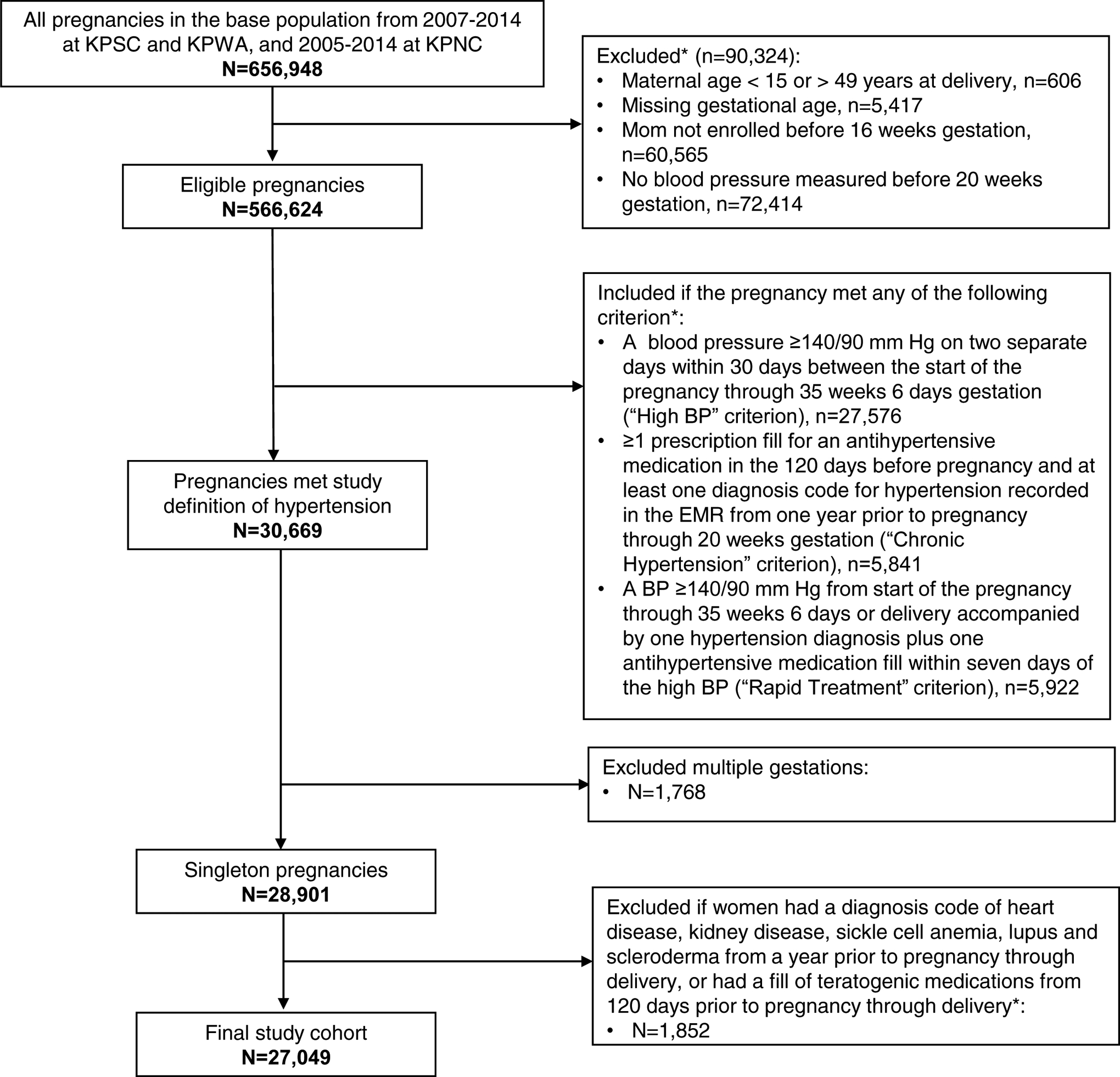
* Criteria not mutually exclusive. KPNC: Kaiser Permanente Northern California; KPSC: Kaiser Permanente Southern California; KPWA: Kaiser Permanente Washington.
Figure 2. Groups identified by each criterion in study case definition for hypertension.
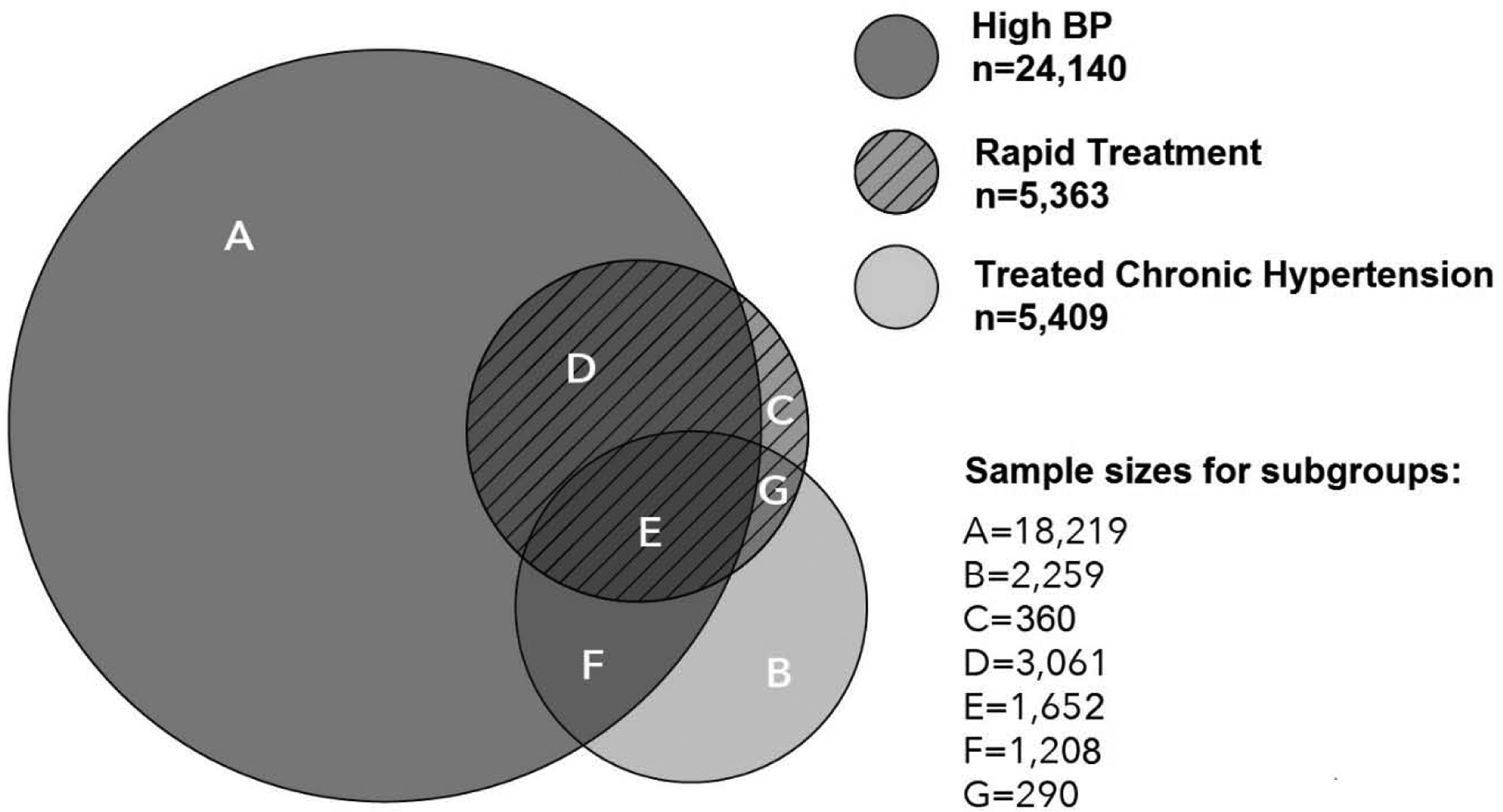
High BP criterion: 2 or more BP ≥ 140/90 within 30 days from the start of pregnancy through 35 weeks 6 days; Treated chronic Hypertension criterion: 1 antihypertensive medication fill within 120 days prior to pregnancy and 1 hypertension diagnosis code from 1 year prior to pregnancy through 20 weeks gestation; Rapid Treatment criterion: 1 high BP (≥ 140/90) from the start of pregnancy through 35 weeks 6 days accompanied by 1 diagnosis code and 1 antihypertensive medication fill all within 7 days.
In our hypertensive pregnancy cohort, the mean maternal age was 31.7 years, 63.6% of women were non-white, 7.7% had pre-existing diabetes, and only 18.7% had normal weight or were underweight prior to pregnancy (Table 1).
Table 1.
Characteristics of women with hypertension in pregnancy, 2005–2014
| Hypertensive Cohort | Pregnancies Meeting Each Hypertension Case Criterion* | |||||||
|---|---|---|---|---|---|---|---|---|
| Maternal Characteristics | High BP | Chronic Hypertension | Rapid Treatment | |||||
| n= 27,049 | n=24,140 | n=5,409 | n=5,363 | |||||
| n | % | n | % | n | % | n | % | |
| Age [in years, mean, (Standard deviation)] | 31.7 (5.9) | 31.4 (5.9) | 34.7 (4.9) | 33.3 (5.4) | ||||
| Race/Ethnicity | ||||||||
| White | 9,801 | (36.4) | 8,887 | (37.0) | 1,597 | (29.7) | 1,715 | (32.2) |
| Hispanic | 8,531 | (31.7) | 7,650 | (31.9) | 1,624 | (30.2) | 1,534 | (28.8) |
| Asian | 4,483 | (16.7) | 3,858 | (16.1) | 1,219 | (22.6) | 1,141 | (21.4) |
| African American | 3,905 | (14.5) | 3,446 | (14.3) | 903 | (16.8) | 888 | (16.7) |
| Other | 192 | (0.7) | 173 | (0.7) | 39 | (0.7) | 48 | (0.9) |
| Missing or unknown † | 137 | 126 | 27 | 37 | ||||
| At least college education | ||||||||
| Yes | 10,607 | (40.9) | 9,348 | (40.4) | 2,352 | (45.5) | 2,094 | (40.9) |
| Missing † | 1,118 | 990 | 237 | 247 | ||||
| Smoked during pregnancy | 1,551 | (5.7) | 1,406 | (5.8) | 256 | (4.7) | 273 | (5.1) |
| Parous | 14,190 | (54.8) | 12,295 | (53.3) | 3,463 | (65.7) | 3,139 | (60.6) |
| Missing parity † | 1,169 | 1,089 | 141 | 181 | ||||
| Had pre-existing diabetes | 2,082 | (7.7) | 1,713 | (7.1) | 860 | (15.9) | 539 | (10.1) |
| Had Medicaid insurance | 1,879 | (6.9) | 1,710 | (7.1) | 355 | (6.6) | 376 | (7.0) |
| Pre-pregnancy BMI | ||||||||
| <25 kg/m2 | 4,688 | (18.7) | 4,211 | (18.8) | 740 | (15.1) | 677 | (13.8) |
| 25–29 kg/m2 | 6,142 | (24.6) | 5,491 | (24.5) | 1,132 | (23.2) | 1,133 | (23.1) |
| ≥ 30 kg/m2 | 14,180 | (56.7) | 12,744 | (56.8) | 3,014 | (61.7) | 3,086 | (63.0) |
| Missing † | 2,039 | 1,694 | 523 | 467 | ||||
| Infant sex | ||||||||
| Male | 13,938 | (52.1) | 12484 | (52.3) | 2743 | (51.3) | 2753 | (52.1) |
| Missing a | 288 | 262 | 64 | 83 | ||||
Women meeting multiple criteria of the hypertension case definition were included in each group.
High BP criterion: 2 or more BP ≥ 140/90 within 30 days from the start of pregnancy through 35 weeks 6 days; Treated chronic Hypertension criterion: 1 antihypertensive medication fill within 120 days prior to pregnancy and 1 hypertension diagnosis code from 1 year prior to pregnancy through 20 weeks gestation; Rapid Treatment criterion: 1 high BP (≥ 140/90) from the start of pregnancy through 35 weeks 6 days accompanied by 1 diagnosis code and 1 antihypertensive medication fill all within 7 days.
Women with missing value were not included in the denominator for percentage calculation.
Clinical characteristics of the cohort overall and by subgroups are shown in Table 2. In our cohort, 71.3% received a hypertension diagnosis code recorded at any time during pregnancy with 33% having a hypertension diagnosis assigned prior to 20 weeks gestation. At delivery, 59.3% received a hypertension diagnosis code. This was largely driven by patterns seen in women identified by the High BP criterion, of whom 68.7% received a diagnosis code during pregnancy and 57.0% at delivery. Overall, 36.1% of hypertensive pregnancies were treated and 94.6% had at least 2 separate visits with recorded high BP. Severe high BP occurred to 41.5% of all pregnancies at some point during pregnancy and 20.8% between 20 weeks gestation and delivery. The proportion of experiencing a severe high BP varied across groups, with the highest proportion in the rapid treatment group.
Table 2.
Diagnosis, treatment and blood pressure of the hypertensive cohort by case definition
| Hypertensive cohort | Pregnancies Meeting Each Hypertension Case Criterion* | |||||||
|---|---|---|---|---|---|---|---|---|
| High BP | Treated chronic Hypertension | Rapid Treatment | ||||||
| n= 27,049 | n=24,140 | n=5,409 | n=5,363 | |||||
| n | % | n | % | n | % | n | % | |
| Received a hypertension diagnosis during pregnancy | 19,298 | (71.3) | 16,581 | (68.7) | 5,140‡ | (95.0) | 5,363 | (100.0) |
| First diagnosis recorded prior to 20 weeks† | 8,938 | (33.0) | 6,602 | (27.4) | 4,647 | (85.9) | 4,056 | (75.6) |
| First diagnosis recorded after 20 weeks | 10,360 | (38.3) | 9,979 | (41.3) | 493 | (9.1) | 1,307 | (24.4) |
| At the delivery hospitalization§ | 16,039 | (59.3) | 13,754 | (57.0) | 4,397 | (81.3) | 4,679 | (87.2) |
| Filled antihypertensive medications during pregnancy | 9,762 | (36.1) | 7,498 | (31.1) | 4,472 | (82.7) | 5,362 | (100) |
| Median gestational age at first prescription (interquartile range, in weeks) | 10.4 (5.3,24.1) | 12.2 (6.1,28.4) | 6.3 (3.6, 10.3) | 11.0 (5.9,25.4) | ||||
| Visits with high BP during pregnancy¶ | ||||||||
| Anytime during pregnancy | ||||||||
| ≥1 visit with high BP | 26,355 | (97.4) | 24,140 | (100) | 4,715 | (87.2) | 5,363 | (100) |
| ≥2 visits with high BP | 25,593 | (94.6) | 24,140 | (100) | 4,035 | (74.6) | 5,226 | (97.5) |
| Median time between first and last visits with high BP (interquartile range, in weeks) | 31.4 (28.3,34.0) | 31.3 (28.1,34.0) | 31.9 (29.1,34.4) | 30.9 (27.6, 33.7) | ||||
| ≥1 visit with severe high BP | 11,226 | (41.5) | 10,774 | (44.6) | 1,981 | (36.6) | 3,322 | (61.9) |
| From 20 weeks gestation to delivery | ||||||||
| ≥1 visit with high BP | 20,889 | (77.2) | 19,922 | (82.5) | 3,265 | (60.4) | 4,705 | (87.7) |
| ≥1 visit with severe high BP | 5,639 | (20.8) | 5,588 | (23.1) | 9,24 | (17.1) | 1,989 | (37.1) |
Women meeting multiple criteria of the hypertension case definition were included in each group.
High BP criterion: 2 or more BP ≥ 140/90 within 30 days from the start of pregnancy through 35 weeks 6 days; Treated chronic Hypertension criterion: 1 antihypertensive medication fill within 120 days prior to pregnancy and 1 hypertension diagnosis code from 1 year prior to pregnancy through 20 weeks gestation; Rapid Treatment criterion: 1 high BP (≥ 140/90) from the start of pregnancy through 35 weeks 6 days accompanied by 1 diagnosis code and 1 antihypertensive medication fill all within 7 days.
This means the earliest time in pregnancy a diagnosis was recorded, which may not be the first time a woman’s hypertension was recognized in her lifetime.
All pregnancies that met the Treated chronic hypertension criterion had a diagnosis between 1 year prior to pregnancy through 20 weeks. When restricted to time during pregnancy, some (n=269) did not have a diagnosis code, and some had a first code after 20 weeks.
Defined as a hypertension diagnosis in an inpatient setting from three days before delivery through delivery.
High blood pressure (BP) defined as BP ≥ 140/90 mm Hg and severe high BP defined as BP ≥ 160/110 mm Hg. Excluding BP measured on the delivery day.
Table 3 shows characteristics of 7,559 pregnancies (27.9% of our cohort) that met the High BP criterion without receiving a hypertension diagnosis code during pregnancy. Of these, only 7.7% were treated, 76.5% had at least 3 separate visits with recorded high BP, and 20.3% had at least 1 severe high BP.
Table 3.
Pregnancies with multiple high blood pressures without a diagnosis (Set B)-Treatment and Blood pressure
| All n= 7,559 |
||
|---|---|---|
| n | % | |
| Received antihypertensive medication fills during pregnancy | 585 | (7.7) |
| Gestational age at first prescription (median, interquartile range in weeks)* | 20.7 (9.4,31.7) | |
| Visits with high BP during pregnancy† | ||
| Anytime during pregnancy | ||
| ≥2 visits with high BP | 7,559 | (100) |
| Median time between first and last visits with high BP (interquartile range, in weeks) | 32.3 (29.4, 35.0) | |
| ≥3 visits with high BP | 5,786 | (76.5) |
| ≥1 visit with severe high BP | 1,537 | (20.3) |
| From 20 weeks gestation to delivery | ||
| ≥1 visit with high BP | 4,990 | (66.0) |
| ≥1 visit with severe high BP | 480 | (6.4) |
Calculation of median and interquartile was restricted to pregnancies with prescription fill respectively.
High blood pressure (BP) defined as BP ≥ 140/90 mm Hg and severe high BP defined as BP ≥ 160/110 mm Hg. Excluding BP measured on the delivery day.
Medical record reviews were conducted for a total of 150 pregnancies to elucidate discrepancies in cases identified by different methods (Table 4). Of pregnancies with multiple hypertension diagnosis code during pregnancy, 22.2% did not meet our case definition of hypertension (Set A). Of the 50 Set A cases reviewed, 14 (28.0%) had multiple high BPs and 8 (16.0%) filled antihypertensive medications during pregnancy. They, however, did not meet our definition either because prescriptions were filled outside of our facility or timing of fills or high BP were not in the specified time window. We also reviewed 55 pregnancies meeting our High BP criterion but did not have a hypertension diagnosis code (Set B) and found clinical notes with statements about hypertension, labile BP or white coat hypertension in 32 charts (58.1%). Set C cases were women who filled antihypertensive medications during pregnancy but did not meet our case definition, which accounted for 9.6% of those treated with labetalol or methyldopa and 46.4% of those treated with other medications. According to chart notes, 75.0% of the labetalol/methyldopa-treated group (n=15 out of 20 charts reviewed) was using the medication to treat hypertension, compared to 16.0% in the group treated with other medication (n=4 out of 25 chats reviewed).
Table 4.
Medical record review on discrepant cases meeting different hypertension identification algorithms
| Set A: Among pregnancies with multiple hypertension diagnosis codes during pregnancy and thus would have been identified by a diagnosis-based approach, those that did not meet our hypertension case definition |
|---|
|
| Set B: Among pregnancies met our High BP criterion, those did not have a hypertension diagnosis code. They would have been missed by a diagnosis-based approach. |
|
| Set C: Among pregnancies treated with antihypertensive medications, those did not meet our hypertension case definition. |
|
In an ad-hoc analysis, we examined gestational ages at delivery. The mean (standard deviation) gestational age at delivery was 37.6 (3.0) weeks overall and ranged from 37.0 (3.3) in women identified by the Rapid Treatment criterion to 37.8 (2.9) weeks in women identified by the Treated Chronic Hypertension criterion. The mean gestational age at delivery was 37.5 (2.8) weeks among women with a hypertension diagnosis code at delivery and 37.7 (3.4) weeks among those without.
Discussion
In this large population-based study, we developed a comprehensive approach that integrated EMR BP with diagnosis codes and medication fills to identify pregnant women with hypertension. One of our key findings is that using BP identifies a large group of pregnant women (n=7,559, 28% of the cohort) who meet the clinical definition of hypertension according to EMR BP but would have been missed by using claims data alone. This finding further confirms the low sensitivity for claims-based hypertension definitions previously reported in non-pregnant populations.15,16 One possible explanation for this finding could be low rates of clinical recognition of new-onset hypertension in the general population20 including in younger women.25 A previous EMR-based study found that over 2-thirds of non-pregnant younger women with multiple BPs of 140/90 mm Hg or higher did not receive a hypertension diagnosis or treatment within a year.25 Although we cannot determine with absolute certainty whether a woman had hypertension based solely on EMR data, we observed evidence suggestive of hypertension in many women with repeated high BPs who did not receive a diagnosis: 76.5% had 3 or more separate visits with recorded high BPs, 20% experienced a severely elevated BP, and for 58% there was a clinical statement in their medical record about hypertension. Given that few women in this group were treated, it is possible that a diagnosis may be more likely to be coded when a clinician decides to prescribe antihypertensive medications but more likely to be absent for untreated women. This finding suggests that studies relying on diagnosis codes alone may miss a substantial group of untreated hypertensive women, potentially resulting in an underestimate of prevalence of hypertension in pregnancy.
In pregnancies identified as hypertensive in this study, 36% were treated. This is not surprising given that US guidelines do not recommend treatment for women with mild to moderate hypertension;8 in contrast to a number of international guidelines,27,28 including from the International Society for the Study of Hypertension in Pregnancy (ISSHP),29 current US guidelines recommend treatment for women with BP 160/110 mmHg or above. A prior study among publicly insured U.S. pregnancies reported that only 45% of women treated pre-existing hypertension received any outpatient hypertension treatment during pregnancy.12 The treatment rate was lower in our study as we included both new and preexisting hypertension and women with newly developed hypertension may be less likely to initiate an antihypertensive medication.
The goal of our study was not to assess prevalence of hypertensive disorders in pregnancy. Rather, we presented a hypertension definition used in our parent study to identify hypertensive pregnancies for whom a treatment decision could influence pregnancy outcomes. We attempted to achieve high specificity through requiring strict timing of diagnoses, BP and receipt of pharmacologic treatment. This strategy resulted in exclusion of some pregnancies that would have been identified as hypertensive using diagnosis or treatment-based approach. This case definition, however, can be modified to identify a broader group of hypertensive pregnancies to suit new research objectives. Based on our chart review results, some possible strategies include allowing: (1) women with multiple diagnosis codes to qualify if they also had high BP or fills of antihypertensive medications; (2) women on methyldopa or labetalol to qualify if they also had a hypertension diagnosis in pregnancy.
The study has several limitations. First, we used BP from routine clinical care, which may not be measured according to the optimal (research) standard.30 KP staff have received proper training and, at many sites, certification in BP measurement. Equipment is professionally monitored and recalibrated as needed. However, we recognize that some shortcomings may persist. These BP data reflect the best available data in real-world settings and are used for making clinical decisions. Also because we relied on clinical BP, we may have included women with repeated high BP at clinic who had normal BP at home (known as white-coat or transient gestational hypertension depending on timing of detection).28 Due to uses of diagnosis codes and medication fills, misclassification may also occur when a provider misdiagnosed and/or started treating a woman who was not truly hypertensive. In the general population, white-coat hypertension accounts for 15–30% of people with elevated office BP and whether to treat these patients is controversial.31 However, high BP can rapidly progress to more serious hypertension in an ongoing pregnancy and the U.S. clinical guideline from the ACOG did not attempt to distinguish white-coat hypertension or transient gestational hypertension from other hypertensive disorders in pregnancy.8 The ISSHP guideline while acknowledging these 2 categories also cautioned that they can convey risk of preeclampsia.29 Hence, from a research point of view, we believe it is still worthwhile to identify women with repeated high BP values in the clinic, including those who may have white-coat hypertension or transient gestational hypertension, to better understand their risk of adverse pregnancy outcomes. Second, we included women delivering in three U.S. large integrated healthcare delivery systems, most of whom were privately insured. Results may be different in other settings and populations. Third, we limited our study to hypertension that emerged by 35 weeks 6 days gestation and thus characteristics of our cohort, including likelihood of receiving a diagnosis code at delivery, may not represent pregnancies with late onset hypertension.
Our study is among the first to evaluate an approach including BP from the EMR to identify women with hypertension during pregnancy. Other strengths include a large population with diverse race/ethnicity background, drawing on data from three healthcare systems, and conducting medical record review to understand reasons that women with some evidence of hypertension did not meet our case definition.
In summary, using EMR data, we found it feasible to implement a case definition for hypertension in pregnancy based on a combination of BP, diagnosis codes and pharmacy fills. Notably, 28% of women identified as hypertensive using this approach would have been missed by approaches using diagnosis codes alone. Many women identified by our method experienced severely elevated BP, showing the importance of including these women in future studies. Future research should also examine pregnancy outcomes among women who would be overlooked by approaches only using diagnosis codes.
Supplementary Material
Highlights:
Blood pressure (BP) was used with claims data to identify hypertensive pregnancies.
28% of pregnancies with ≥2 high BPs did not have a hypertension diagnosis code.
Future studies should seek to incorporate BP to study hypertension in pregnancy.
Funding:
This study was funded by the Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD) R01HD082141 (PI: Dublin S.). Dr. Chen’s time was partly funded by Group Health Foundation Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Dr. Shortreed has worked on grants awarded to Kaiser Permanente Washington Health Research Institute (KPWHRI) by Pfizer. She has also received support as a co-Investigator on grants awarded to KPWHRI from Syneos Health, who is representing a consortium of pharmaceutical companies carrying out FDA-mandated studies regarding the safety of extended-release opioids. All other authors declare no conflict of interest.
References:
- 1.Lennestal R, Otterblad Olausson P, Kallen B. Maternal use of antihypertensive drugs in early pregnancy and delivery outcome, notably the presence of congenital heart defects in the infants. Eur J Clin Pharmacol. 2009;65(6):615–625. [DOI] [PubMed] [Google Scholar]
- 2.Bateman BT, Bansil P, Hernandez-Diaz S, Mhyre JM, Callaghan WM, Kuklina EV. Prevalence, trends, and outcomes of chronic hypertension: a nationwide sample of delivery admissions. Am J Obstet Gynecol. 2012;206(2):134 e131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orbach H, Matok I, Gorodischer R, et al. Hypertension and antihypertensive drugs in pregnancy and perinatal outcomes. Am J Obstet Gynecol. 2013;208(4):301 e301–306. [DOI] [PubMed] [Google Scholar]
- 4.Zetterstrom K, Lindeberg SN, Haglund B, Hanson U. Maternal complications in women with chronic hypertension: a population-based cohort study. Acta Obstet Gynecol Scand. 2005;84(5):419–424. [DOI] [PubMed] [Google Scholar]
- 5.Su CY, Lin HC, Cheng HC, Yen AM, Chen YH, Kao S. Pregnancy outcomes of anti-hypertensives for women with chronic hypertension: a population-based study. PLoS One. 2013;8(2):e53844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American College of Obstetricians Gynecologists. ACOG Practice Bulletin No. 125: Chronic hypertension in pregnancy. Obstet Gynecol. 2012;119(2 Pt 1):396–407. [DOI] [PubMed] [Google Scholar]
- 7.Talge NM, Holzman C, Van Egeren LA, et al. Late-preterm birth by delivery circumstance and its association with parent-reported attention problems in childhood. J Dev Behav Pediatr. 2012;33(5):405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Committee on Hypertension in Pregnancy. Hypertension in Pregnancy. Washington, DC: American College of Obstetricians and Gynecologists;2013. [Google Scholar]
- 9.Baraban E, McCoy L, Simon P. Increasing prevalence of gestational diabetes and pregnancy-related hypertension in Los Angeles County, California, 1991–2003. Prev Chronic Dis. 2008;5(3):A77. [PMC free article] [PubMed] [Google Scholar]
- 10.Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21(5):521–526. [DOI] [PubMed] [Google Scholar]
- 11.Kuklina EV, Ayala C, Callaghan WM. Hypertensive disorders and severe obstetric morbidity in the United States. Obstet Gynecol. 2009;113(6):1299–1306. [DOI] [PubMed] [Google Scholar]
- 12.Bateman BT, Hernandez-Diaz S, Huybrechts KF, et al. Patterns of outpatient antihypertensive medication use during pregnancy in a Medicaid population. Hypertension. 2012;60(4):913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Savitz DA, Danilack VA, Engel SM, Elston B, Lipkind HS. Descriptive epidemiology of chronic hypertension, gestational hypertension, and preeclampsia in New York State, 1995–2004. Matern Child Health J. 2014;18(4):829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savitz DA, Danilack VA, Elston B, Lipkind HS. Pregnancy-induced hypertension and diabetes and the risk of cardiovascular disease, stroke, and diabetes hospitalization in the year following delivery. Am J Epidemiol. 2014;180(1):41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tu K, Campbell NR, Chen ZL, Cauch-Dudek KJ, McAlister FA. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1(1):e18–26. [PMC free article] [PubMed] [Google Scholar]
- 16.Rector TS, Wickstrom SL, Shah M, et al. Specificity and sensitivity of claims-based algorithms for identifying members of Medicare+Choice health plans that have chronic medical conditions. Health Serv Res. 2004;39(6 Pt 1):1839–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Richesson RL, Rusincovitch SA, Wixted D, et al. A comparison of phenotype definitions for diabetes mellitus. J Am Med Inform Assoc. 2013;20(e2):e319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spratt SE, Pereira K, Granger BB, et al. Assessing electronic health record phenotypes against gold-standard diagnostic criteria for diabetes mellitus. J Am Med Inform Assoc. 2017;24(e1):e121–e128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teixeira PL, Wei WQ, Cronin RM, et al. Evaluating electronic health record data sources and algorithmic approaches to identify hypertensive individuals. J Am Med Inform Assoc. 2017;24(1):162–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Selby JV, Lee J, Swain BE, et al. Trends in time to confirmation and recognition of new-onset hypertension, 2002–2006. Hypertension. 2010;56(4):605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selby JV, Peng T, Karter AJ, et al. High rates of co-occurrence of hypertension, elevated low-density lipoprotein cholesterol, and diabetes mellitus in a large managed care population. Am J Manag Care. 2004;10(2 Pt 2):163–170. [PubMed] [Google Scholar]
- 22.Sandhu A, Ho PM, Asche S, et al. Recidivism to uncontrolled blood pressure in patients with previously controlled hypertension. Am Heart J. 2015;169(6):791–797. [DOI] [PubMed] [Google Scholar]
- 23.Baldwin E, Johnson K, Berthoud H, Dublin S. Linking mothers and infants within electronic health records: a comparison of deterministic and probabilistic algorithms. Pharmacoepidemiol Drug Saf. 2015;24(1):45–51. [DOI] [PubMed] [Google Scholar]
- 24.Ross TR, Ng D, Brown JS, et al. The HMO Research Network Virtual Data Warehouse: A Public Data Model to Support Collaboration. EGEMS (Wash DC). 2014;2(1):1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmittdiel J, Selby JV, Swain B, et al. Missed opportunities in cardiovascular disease prevention?: low rates of hypertension recognition for women at medicine and obstetrics-gynecology clinics. Hypertension. 2011;57(4):717–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmsten K, Huybrechts KF, Kowal MK, Mogun H, Hernandez-Diaz S. Validity of maternal and infant outcomes within nationwide Medicaid data. Pharmacoepidemiol Drug Saf. 2014;23(6):646–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butalia S, Audibert F, Cote AM, et al. Hypertension Canada’s 2018 Guidelines for the Management of Hypertension in Pregnancy. Can J Cardiol. 2018;34(5):526–531. [DOI] [PubMed] [Google Scholar]
- 28.National Institute for Health and Care Excellence. Hypertension in pregnancy: diagnosis and management. 2019; https://www.nice.org.uk/guidance/ng133/chapter/Recommendations#management-of-gestational-hypertension. Accessed October 17, 2019. [PubMed]
- 29.Brown MA, Magee LA, Kenny LC, et al. Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension. 2018;72(1):24–43. [DOI] [PubMed] [Google Scholar]
- 30.Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697–716. [DOI] [PubMed] [Google Scholar]
- 31.Franklin SS, Thijs L, Hansen TW, O’Brien E, Staessen JA. White-coat hypertension: new insights from recent studies. Hypertension. 2013;62(6):982–987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


