Abstract
The accumulation of chondroitin sulfate proteoglycans (CSPGs) in the glial scar following acute damage to the central nervous system (CNS) limits the regeneration of injured axons. Given the rich diversity of CSPG core proteins and patterns of GAG sulfation, identifying the composition of these CSPGs is essential for understanding their roles in injury and repair. Differential expression of core proteins and sulfation patterns have been characterized in the brain and spinal cord of mice and rats, but a comprehensive study of these changes following optic nerve injury has not yet been performed. Here, we show evidence that the composition of CSPGs in the optic nerve and retina following optic nerve crush (ONC) in mice and rats exhibits an increase in aggrecan, brevican, phosphacan, neurocan and versican, similar to changes following spinal cord injury. We also observe an increase in inhibitory 4-sulfated (4S) GAG chains, which suggests that the persistence of CSPGs in the glial scar opposes the growth of CNS axons, thereby contributing to the failure of regeneration and recovery of function.
Keywords: Extracellular matrix, optic nerve crush, immunocytochemistry, glycosaminoglycan sulfation, chondroitin sulfate proteoglycans
1. Introduction
The extracellular matrix (ECM) undergoes dynamic changes in response to injuries of the central nervous system (CNS). Many of these responses reduce damage and promote recovery, such as the formation of a glial scar that limits the spread of inflammation (Faulkner et al., 2004; Fawcett and Asher, 1999; Silver and Miller, 2004) and the deposition of chondroitin sulfate proteoglycans (CSPGs), which are believed to help organize infiltrating immune cells (Rolls et al., 2008; Shechter et al., 2009; Shechter et al., 2011). However, the persistence of these modifications beyond the acute phase of injury can impede long-term recovery: for instance, the presence of CSPGs in the glial scar obstructs the regeneration of damaged neurons (Asher et al., 2000; Asher et al., 2002; Davies et al., 1997; Snow et al., 1990). In particular, the elevation of CSPGs following spinal cord injury (SCI) (Jones et al., 2003) has been shown to play a critical role in regeneration failure, and experimental therapies such as digestion of GAG chains with the enzyme chondroitinase ABC (ChABC) after SCI have shown axonal regeneration and improvements in motor function in rats (Bradbury and Carter, 2011; Bradbury et al., 2002). An increase in CSPGs has also been detected in the optic nerve after a crush injury (Becker and Becker, 2002), and ChABC treatment enhances optic nerve regeneration (Brown et al., 2012). Moreover, we recently reported that modifying the sulfation of glycosaminoglycan (GAG) chains on CSPGs produces an equivalent effect (Pearson et al., 2018).
These studies emphasize the importance of identifying how the changing composition of the ECM, particularly modifications to proteoglycan sulfation and differential expression of CSPG core proteins, influences the survival and growth of axons. Previous studies have characterized the injury-induced changes to proteoglycans and GAG chains in the brain (Yi et al., 2012) and spinal cord (Jones et al., 2003; Tang et al., 2003), but despite the observation by several groups that CSPGs accumulate in the glial scar after optic nerve crush (ONC) in rodents (Brown et al., 2012; Park et al., 2008; Pearson et al., 2018; Qu and Jakobs, 2013; Selles-Navarro et al., 2001; Sengottuvel et al., 2011), a comprehensive examination of the compositional changes in proteoglycans and GAG sulfation has not been performed. Here, we describe the spatial and temporal regulation of CSPG deposition in the optic nerves of mice and rats, and compare their expression following dorsal column crush. We demonstrate that ONC dynamically modifies proteoglycan composition and sulfation both at the lesion site and in the retina, in the layer containing the cell bodies of injured neurons. Finally, we find a consistent elevation of the 4S motif, which is essential for axonal inhibition (Pearson et al., 2018; Wang et al., 2008) in lesioned tissue, suggesting that these changes in proteoglycans are opposed to the regeneration of injured axons.
2. Materials and Methods
2.1. Laboratory Animals
Experiments and procedures performed for this paper were in accordance with protocols approved by the Institutional Animal Care and Use Committee (IACUC) at the National Institutes of Health and the United Kingdom Animal (Scientific Procedure) Act of 1986. Female, 6–8 week old C57Bl/6 mice and female Sprague-Dawley rats weighing 250–275 g (Charles River) were housed in a pathogen free facility with standard 12 h light/dark cycle and unlimited access to food.
2.2. Optic nerve crush and dorsal column crush
Optic nerve crush (ONC) was performed as described previously (Park et al., 2008) on 12 mice and 11 rats. After exposing the optic never intraorbitally, curved forceps were inserted beneath the external ocular muscle taking care to avoid the ophthalmic artery and retrobulbar sinus. The nerve was crushed for 10s approximately 1 mm caudal to the eye. Blood flow in the eyes was monitored fundoscopically immediately following optic nerve crush. Additionally, animals continued to be observed in the hours following ONC for any signs of bleeding. For pain, animals received an analgesic (1 mg/kg buprenorphrine, subcutaneous). To prevent the cornea from drying, animals received a topical application of ophthalmic ointment.
Dorsal column crush was performed as described previously (Cheah et al., 2016) on 6 animals. First, the skin above the spinal cord was shaved. This was followed by an incision above the dorsal spinal column. Skin and muscle were pulled away and held apart using sterile retractors allowing the vertebrae to be exposed. A laminectomy removed the T10 vertebral bone. After exposing the spinal cord, the meninges were carefully removed. Jeweler’s forceps were closed on the dorsal column for 10 s crushing the spinal cord. After suturing the muscle and skin, animals received a subcutaneous injection of 1 mg/kg buprenorphrine and were carefully monitored for signs of infection in the days following surgery. Mobility and bladder function were assessed daily.
2.3. CTB injection
Two days prior to harvesting of optic nerve tissue by perfusion, intravitreal injections of CTB (1.0 μg/μL, Sigma) were administered. The injecting solution was drawn into a sterile 5 μL Hamilton syringe with a 33-gauge removable needle. 2 μL of the solution was then slowly injected through the superior nasal sclera at a 45° angle, being careful to avoid the lens, external ocular muscle, and blood vessels. The cornea was punctured by a sterile 33-gauge needle and the anterior chamber was drained before removing the injecting needle. This draining was to reduce intraocular pressure and prevent reflux of the injected solution. The syringe was rinsed with ethanol followed by sterile PBS between injections.
2.4. Immunohistochemistry
Optic pathway tissue
Animals were anesthetized (1–2% isoflurane) and perfused transcardially with PBS followed by 4% paraformaldehyde (PFA). Optic nerves or whole optic pathway tissue were dissected, laid flat on 13 mm filter paper (Millipore AABG01300), and post-fixed overnight in 4% PFA. Next, the tissue was cryoprotected by immersion for at least 24h in 30% sucrose. Prior to cryosectioning, the tissue was embedded in OCT J (Tissue-Tek) and snap-frozen. Cryosectioning using a Leica CM3050 cryostat yielded 14 μm longitudinal sections on charged Superfrost microscope slides, which were dried and then stored at −80°C until staining.
Retinas
Whole eyes were dissected from perfused mice and post-fixed (4% PFA) for 2 h followed by immersion in 1X PBS. To obtain whole mounts of the retina, the cornea and lens were removed, four exterior cuts at half the radius of the retina were made, and the sclera was gently peeled away. This allowed isolation of the intact retina. The retina was carefully cleaned of any remaining vitreous body, placed in 1X PBS and stored at 4 °C.
Spinal cord
After perfusion with 4% PFA, isolation of the spinal column and removal of the vertebral bones, intact spinal cord tissue was dissected and post-fixed (4% PFA) overnight. Cryoprotection was accomplished by immersing spinal cord tissue 30% sucrose for 24 h after which the tissue was embedded in Tissue-Tek OCT, and snap-frozen for cryosectioning.
Immunostaining
To detect CSPGs and glial cell activation, optic nerve, spinal cord or retinal sections from at least three different animals for each antibody and condition were stained with antibodies as follows: slides were incubated for 1 h in blocking solution (PBS containing 3% goat serum and 0.2% Triton X-100 followed by overnight incubation at 4°C in blocking solution containing diluted primary antibodies. For detection of core proteins (neurocan, aggrecan and versican), a pretreatment step in which slides were treated with chondroitinase ABC overnight at 37°C was required. Following chondroitinase ABC digestion, slides were washed twice with PBS, and then followed previously described blocking and primary antibody treatment. Next, slides were washed three times for 5 min (PBS), incubated for 2 h in blocking solution containing diluted secondary antibodies, washed, and mounted using glass coverslips with Fluoromount medium (Sigma). Imaging was performed using a Zeiss 780 confocal microscope. For fluorescence localization comparison, image capture settings were held constant, and samples from within each experimental group were imaged at the same time.
3. Results
3.1. 4-sulfated GAGs are elevated following injuries to the optic nerve and spinal cord
To assess the time course and spatial distribution of CSPG and 4S GAG deposition after injury, optic nerve crush or dorsal column crush surgery was performed on adult mice and rats (Fig. 1), and tissue was collected 1, 3, 7, 14, and 21 dpc. The composition of GAGs in the lesion area was assessed using immunohistochemistry with either CS-56, an antibody that reacts with GAG chains of various sulfation patterns (Sugiura et al., 2012), or 2H6, an antibody that reacts predominantly with 4S (Yamamoto et al., 1995), and to a lesser degree, with 6S (Sugiura et al., 2012) and 2,6S (Matsushita et al., 2018). The intensity of GAG immunoreactivity was increased at the lesion during the scar-forming phase at 7 dpc in all conditions (Fig. 2a–d). In particular, the 4S motif was also elevated after both optic nerve and spinal cord injuries in the two species (Fig. 2a–d). The distribution of GAGs in non-lesioned sham control tissue was uniform, with low levels observed in the tissue and meninges. In lesioned mouse optic nerves, where GAGs were measured over a period of 7 days, detection of total GAG and 4S GAG peaked at 7 dpc (Fig. 2a). Together, these results illustrate that optic nerve and spinal cord injuries in mice and rats lead to a similar elevation of GAGs, including 4S, as early as 7 days.
Fig. 1.
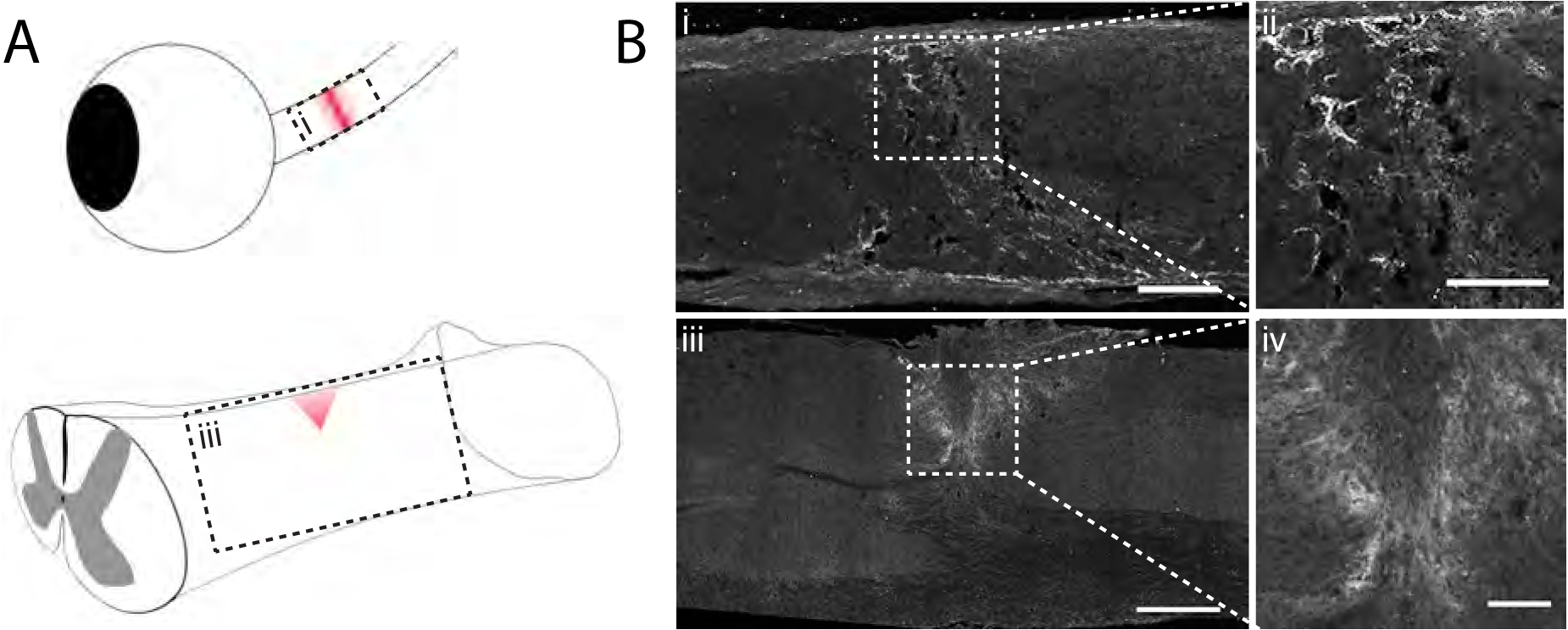
4-sulfated CSPGs accumulate in the glial scar after injury. A) Schematic diagram depicting optic nerve crush and dorsal column crush surgeries. Red shading indicates the area of the lesion following injury. B) Micrographs from the boxed regions in A showing injured mouse tissue 7 days after injury analyzed by immunohistochemistry with CS-56. Scale bar = (i) 100 μm, (ii) 50 μm, (iii) 400 μm, (iv) 100 μm
Fig. 2.
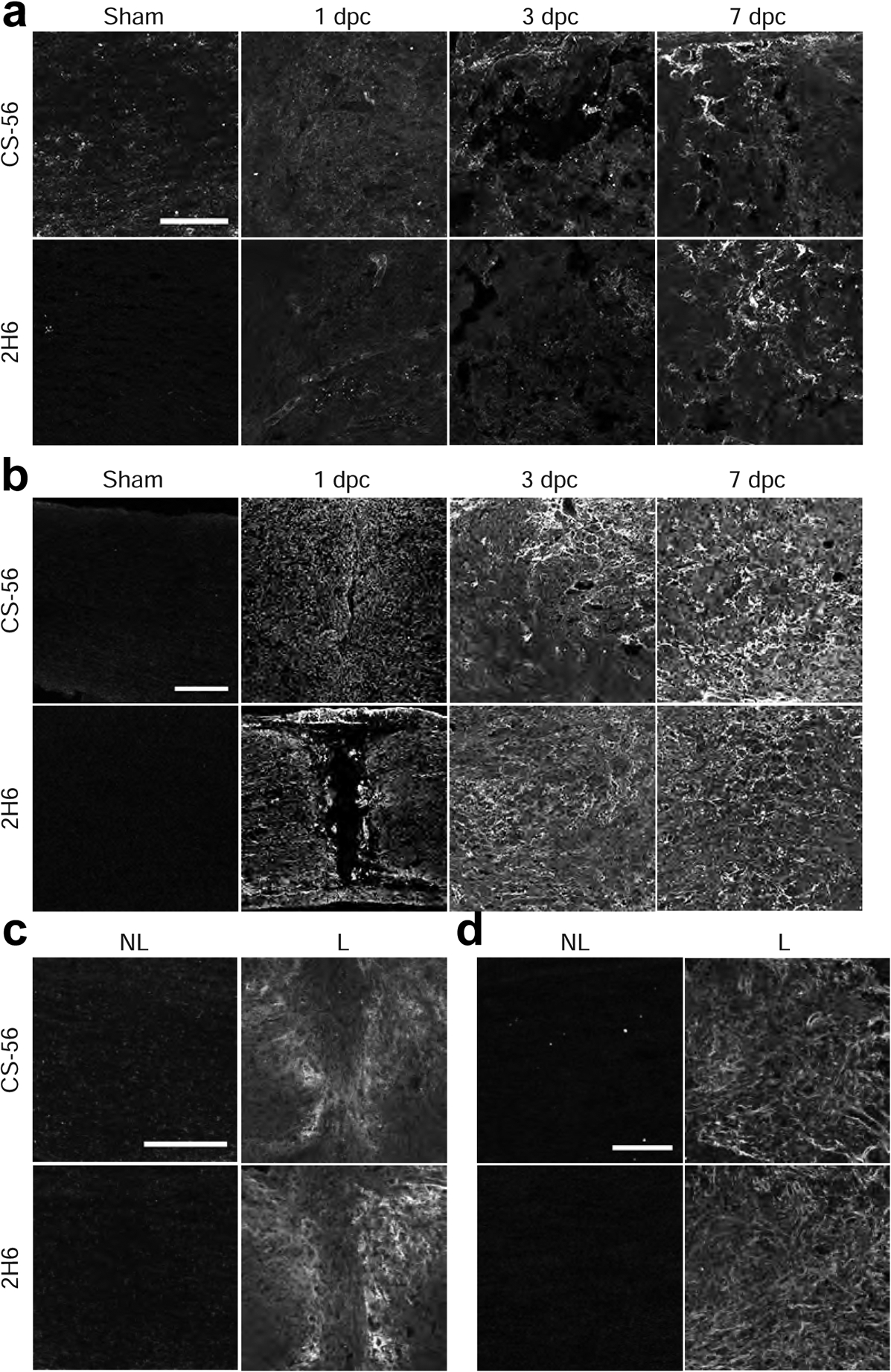
4-sulfated CSPGs accumulate in the glial scar after injury. Micrographs showing (a) mouse optic nerve crush, (b) rat optic nerve crush, and (c) mouse dorsal column crush and (d) rat dorsal column crush tissue. Sections were analyzed by immunohistochemistry with antibodies detecting CSPGs (CS-56) and 4S (2H6). Scale bars = (a) 50 μm, (b) 100 μm, and (c, d) 200 μm. Western blot analysis showing elevation of CS-56 signal within lesioned (e) rat optic nerve and (f) rat spinal cord tissue. L = lesioned, N-L = non-lesioned.
3.2. CSPG elevation corresponds with the acute phase of astrogliosis
The sources of CSPG deposition have been well characterized in the injured brain, where CSPGs have been observed to associate with astrocytes, oligodendrocyte progenitor cells (OPCs), microglia, macrophages, and meningeal fibroblasts, implying that multiple cell types contribute to their production after injury (Yi et al., 2012). To assess how glial cells contribute to CSPG deposition following ONC, the time course of astrocyte reactivity and microglia activation were examined by immunohistochemistry with GFAP (to detect astrocytes), Iba1 (to detect microglia and macrophages), and NG2 (which primarily detects OPCs (Dimou and Gallo, 2015) and some activated astrocytes after injury (Hackett et al., 2018)) (Fig. 3), as well as CS-56 (Fig. 4).
Fig. 3.
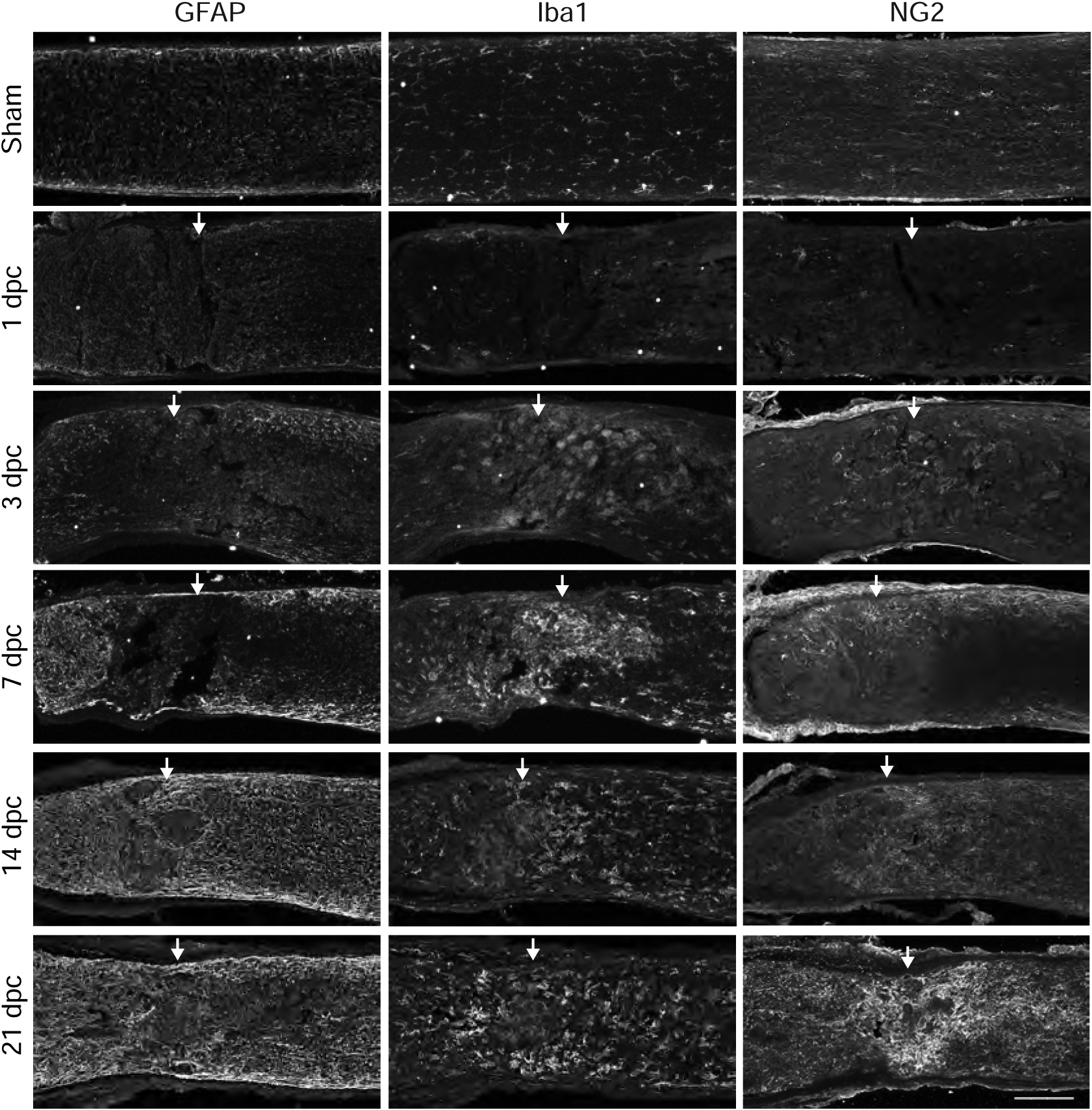
Time course of reactive astrocytes, activated microglia and NG2+ cells after optic nerve crush. Micrographs showing lesioned and non-lesioned sham mouse optic nerve tissue analyzed by immunohistochemistry with GFAP, Iba1, and NG2 at 1, 3, 7, 14, and 21 dpc. Arrows indicate lesion site. Scale bar = 100 μm.
Fig. 4.
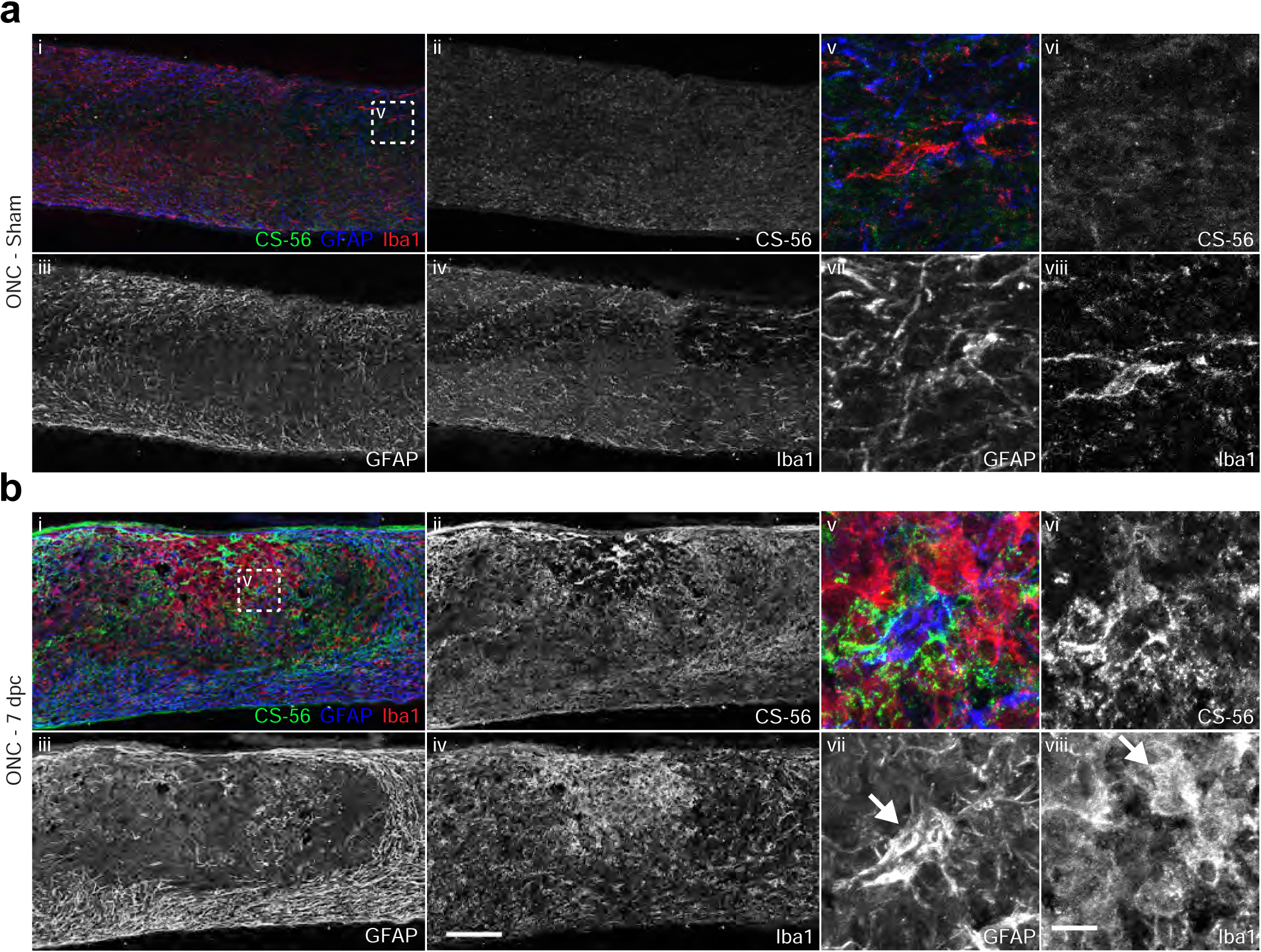
Elevation of CSPGs corresponds to peak of astrogliosis. Micrographs showing (a) sham or (b) 7 dpc mouse optic nerve tissue analyzed by immunohistochemistry with antibodies detecting reactive astrocytes (GFAP), activated microglia and macrophages (Iba1), and CSPGs (CS-56). Arrows indicate GFAP+ astrocytes (b-vii) and Iba1+ microglia (b-viii). Scale bar = 100 μm, insets = 10 μm.
At 7 dpc, GFAP immunoreactivity was enhanced, with reactive astrocytes withdrawing from the lesioned area to form a cavity that was filled with Iba1+ activated microglia and macrophages (Fig. 3, Fig. 4b–i). Many GFAP+ cells displayed morphological changes, becoming hypertrophic and extending elongated processes that defined the lesion boundary (Fig. 4b–iii). Some GFAP+ cells were also found within the lesion core (Fig. 4b–vii, arrow). Likewise, Iba1+ cells displayed more intense immunoreactivity and were larger and rounder, with retracted processes (Fig. 4b–viii, arrow), distinct from cells in the distal optic nerve or in the non-lesioned sham condition, which exhibited a striated morphology (Fig. 4a–viii). CSPGs were found in close proximity with reactive astrocytes and microglia, both around the boundary of the lesion and within the lesion core (Fig. 4b–v). Glial cells that were associated with elevated levels of CSPG typically displayed reactive or activated morphologies (Fig. 4b–vii, viii). At later time points through 21 dpc, GFAP immunoreactivity remained high, while Iba1+ cells withdrew from the lesion core. At this time, NG2+ cells were observed within the lesion core (Fig. 3).
To examine the interactions between injured axons and the accumulated glial cells and CSPGs within the lesion, a group of mice that underwent ONC also received injections of fluorescently tagged choleratoxin B (CTB), a retrograde axon tracer. Tissue was collected at 21 dpc and analyzed by immunohistochemistry with GFAP, Iba1, NG2, and CS-56 (Fig. 5). The CTB+ lesioned axons failed to traverse the lesion. In many cases, axons appeared to terminate in areas of high CSPG deposition (Fig. 5a–inset); endbulbs were also found associated with NG2+ cells (Fig. 5a–ii, arrowhead, iv), while axon endbulbs did not exhibit a comparable association with GFAP+ or Iba1+ cells (Fig. 5b).
Fig. 5.
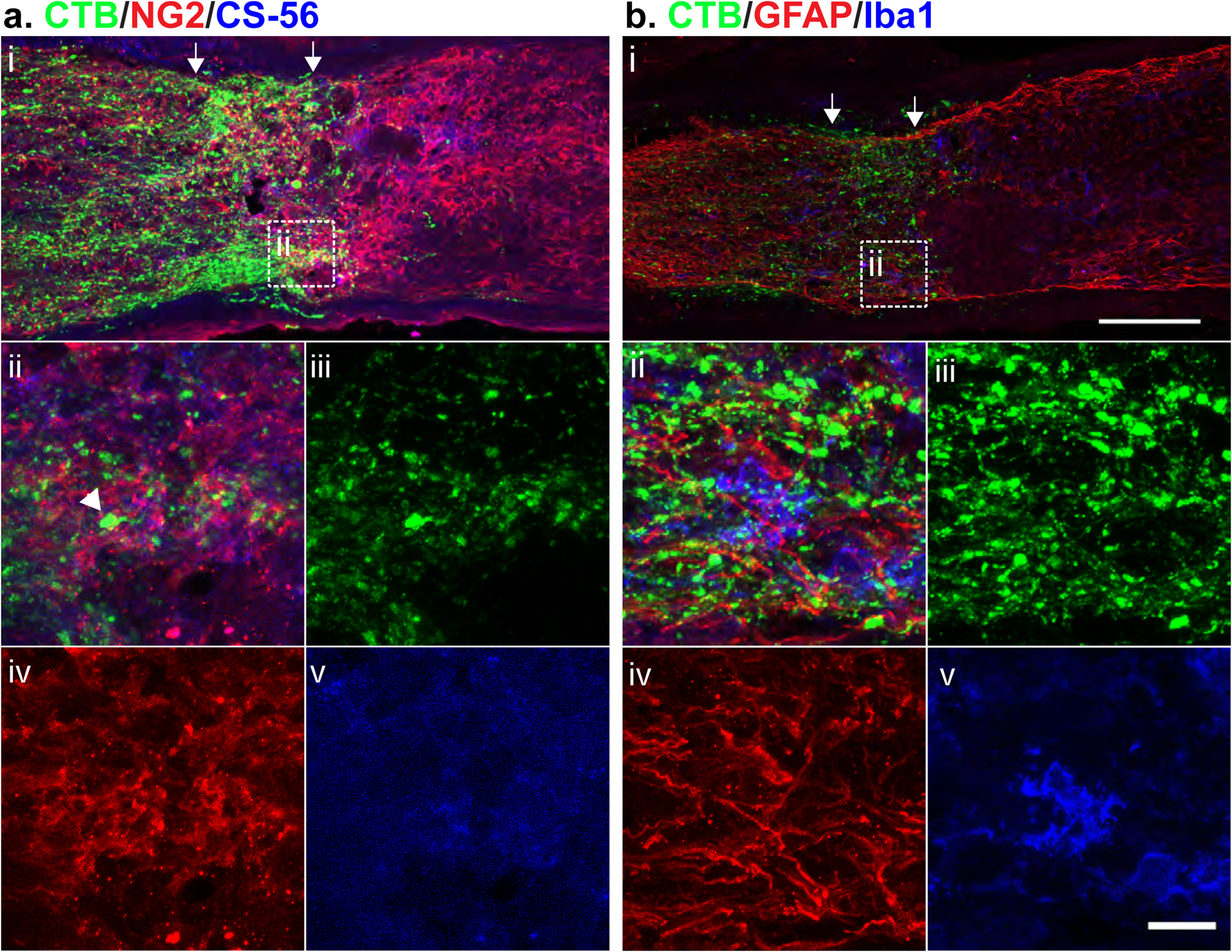
Injured axons associate with CSPGs and NG2+ cells. Micrographs showing 21 dpc lesioned mouse optic nerve tissue, analyzed by immunohistochemistry with (a) NG2 and CS-56, and (b) GFAP and Iba1. The lesioned area is indicated by arrows. Injured axons were visualized with CTB injected intravitreally 1 d prior to tissue collection. Scale bar = 100 μm, insets ii-v = 20 μm.
3.3. Proteoglycan core proteins are elevated following a crush injury to the optic nerve
Sulfated GAG chains are attached to several different proteoglycan core proteins (Schwartz and Domowicz, 2018). Because GAG levels reached maximal levels at 7 dpc (Fig. 2), we evaluated the changes in the expression and localization of proteoglycan core proteins at that time point in both mouse and rat. We first examined the localization of the proteoglycan core proteins aggrecan, neurocan, versican, brevican and phosphacan in the rat optic nerve at 7 dpc (Fig. 6). There were very low levels of proteoglycan staining in the optic nerves of both uninjured and sham injured animals (data not shown). However, there were obvious localized increases following injury. Aggrecan (Fig. 6a) and versican (Fig. 6b) staining was most prominent in the GFAP-rich area proximal to the lesion, with a somewhat lower level of staining in the GFAP-rich area distal to the lesion, and low or no staining found in the lesion core. In contrast, neurocan (Fig. 6a) staining was strongest distal to the lesion, while brevican (Fig. 6b) and phosphacan (Fig. 6c) staining showed an inverse distribution to versican and aggrecan, with the highest levels observed in the lesion core, and lower levels both proximal and distal to the lesion.
Fig. 6.
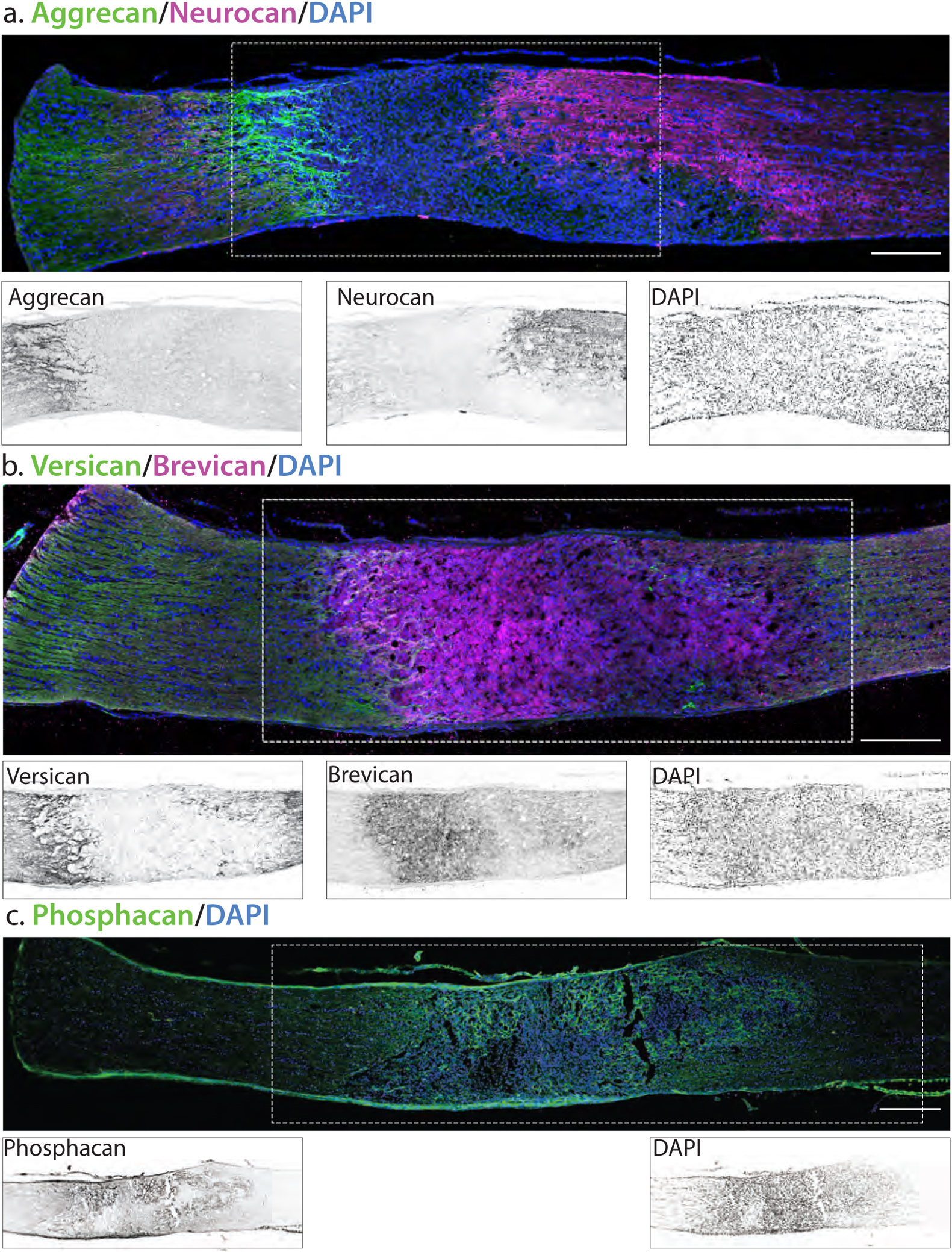
Proteoglycan core proteins in injured rat optic nerve. Sections of optic nerve were obtained 7 days post crush. The localization of the crush is demarked by arrows. Insets are inverted images of the boxed area. (A) Aggrecan staining is strongest proximal to the lesion area, while neurocan staining is strongest distal to the lesion area. Insets are inverted images of the boxed area. (B) Versican is highest both distal and proximal to the lesion area, while brevican is highest intensity in the lesion core. (C) Phosphacan is increased in the lesion core. Scale bar = 250 μm.
We then examined the localization of these proteoglycans in the mouse optic nerve at 7 dpc (Fig. 7). The pattern of staining in the mouse was very similar to that of the rat optic nerve, with very low levels of proteoglycan staining in the optic nerves of sham injured animals (data not shown). As in the rat, versican and aggrecan staining was most prominent in the GFAP-rich area proximal to the lesion, with a somewhat lower level of staining in the GFAP-rich area distal to the lesion, and low or no staining found in the lesion core (Fig 4a). In contrast, neurocan staining was strongest distal to the lesion, while phosphacan and brevican staining showed an inverse distribution to versican and aggrecan, with the highest levels observed in the lesion core, and lower levels both proximal and distal to the lesion.
Fig. 7.
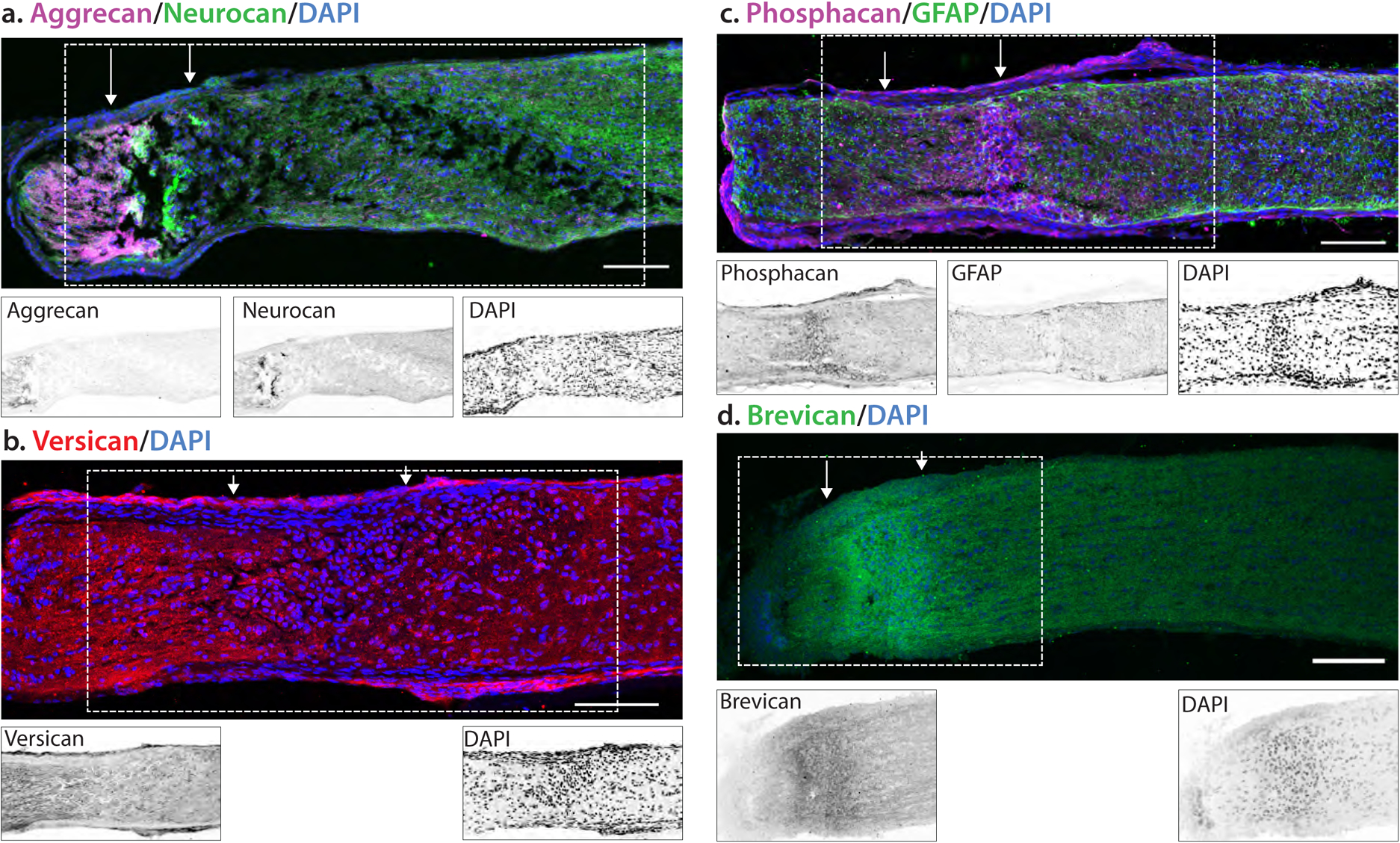
Localization of proteoglycan core proteins in 7 dpc injured mouse optic nerve. Mice were subject to a crush injury and tissues were obtained 7 days post crush. The localization of the crush is demarked by arrows. Insets are inverted images of the boxed area. (A) Aggrecan staining increased in the area proximal to the lesion, with low levels of staining in the lesion core devoid of GFAP. In contrast, neurocan staining was highest in the lesion core. (B) Versican levels were increased both proximal and distal to the lesion, with lower levels in the lesion core. (C) Phosphacan and (D) brevican staining were increased in the lesion core. Scale bar = 100 μm.
3.4. Optic nerve crush elevates CSPG expression in the retina
ONC severs RGC axons in the optic nerve, which causes Wallerian degeneration of the distal axon segments, leaving behind cellular debris including myelin (Bignami et al., 1981), which can be highly inhibitory to the regeneration of injured axons. Axonal damage also causes the progressive death of RGCs (Grafstein and Ingoglia, 1982; Misantone et al., 1984), which undergo apoptosis in the days and weeks following the lesion (Berkelaar et al., 1994). To assess whether there are changes to CSPGs or glial cells in the retina following ONC, retinas were collected from mice at 21 dpc and probed with antibodies against CSPGs, GFAP (expressed by astrocytes and Müller cells), and microglia. As previously reported (Fisher et al., 1995; Hippert et al., 2015), GFAP in the sham injured mouse retina is confined to the inner retinal margin, adjacent to the ganglion cell layer (Fig. 8a). Similarly, CS-56 and 2H6 immunoreactivity were virtually undetectable (Fig. 8a, c). Iba1+ microglia were distributed throughout the retina (Fig. 8c). These microglia had striated morphologies, suggesting they were not activated (Fig. 8c, arrows). In lesioned samples at 21 dpc, there was a robust increase in GFAP+ immunoreactivity (Fig. 8b). This reactivity was largely localized with the astrocytes in the layer adjacent to the GCL, but was also found in processes in the IPL and INL (Fig. 8b, arrows), likely activated Müller cells. Iba1+ cells were rounder and many had withdrawn their processes, suggesting an activated state (Fig. 8d, arrows). GFAP+ cells adjacent to the GCL were associated with regions of elevated CS-56 immunoreactivity (Fig. 8b), while there was no observed CS reactivity in the INL or OPL. Similarly, elevation of 4S GAGs was also observed adjacent to the GCL (Fig. 8d). Together, these observations suggest that the reactive gliosis and deposition of CSPGs observed in the optic nerve also occurs within the retina, primarily affecting the astrocytes adjacent to the GCL.
Fig. 8.
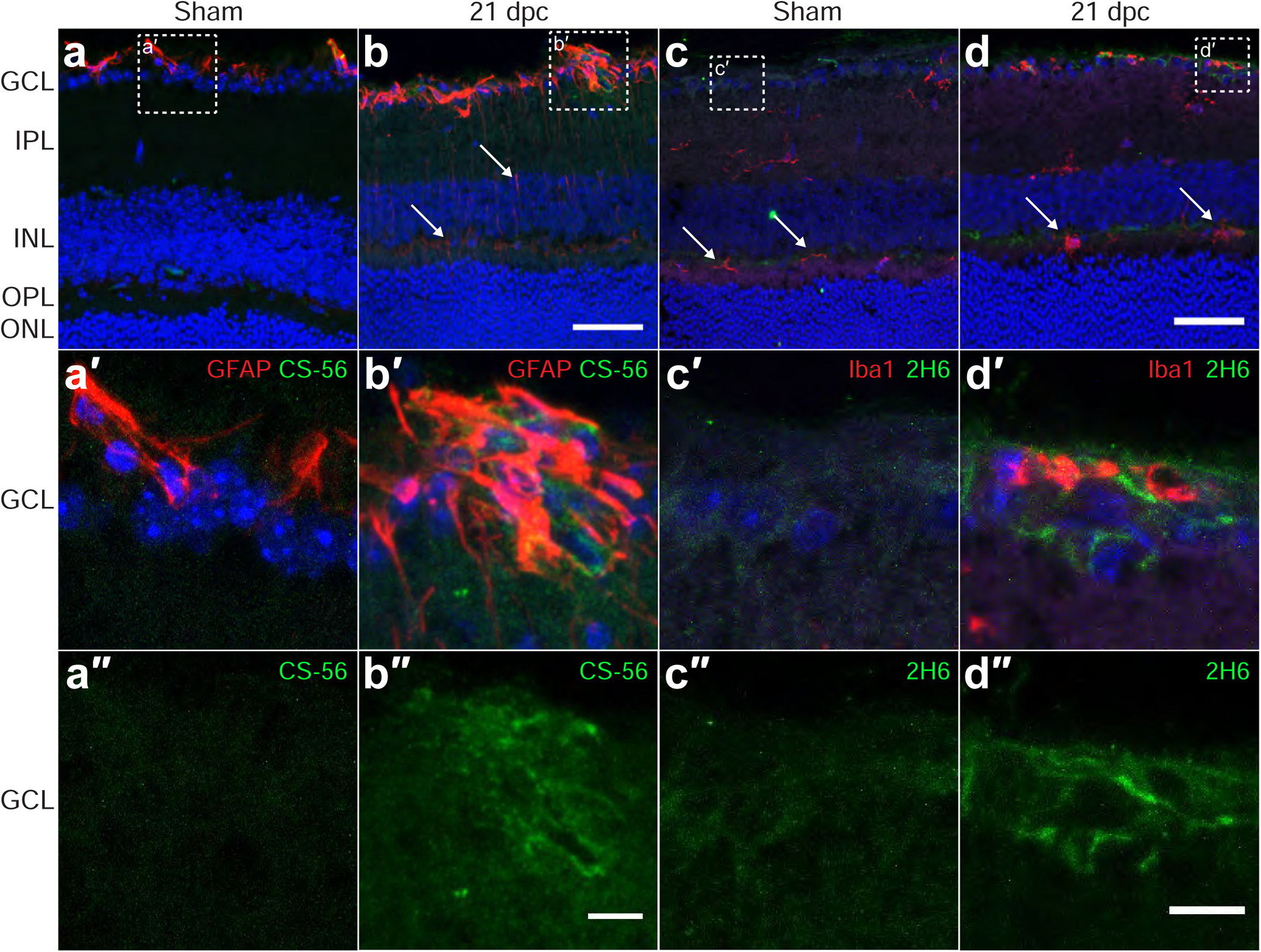
Optic nerve crush stimulates astrocyte reactivity and upregulates CSPGs in the retina. Micrographs showing mouse retina sections from non-lesioned sham controls and 21 days post ONC, analyzed by immunohistochemistry with antibodies detecting CSPG (CS-56), 4S CSPG (2H6), reactive astrocytes and Müller cells (GFAP), and microglia (Iba1). (a-b) CS-56 and GFAP are elevated in the GCL, with CSPGs detected in areas of high GFAP expression. Arrows in (b) indicate elongated processes of GFAP+ cells. (c-d) 4S GAG is also elevated in the GCL. Arrows in (c) indicate resting microglia. Arrows in (d) indicate activated microglia. Scale bars = 50 μm, insets = 10 μm. Abbreviations: GCL = ganglion cell layer; INL = inner nuclear layer; ONL = outer nuclear layer.
4. Discussion
Here, we report that a crush injury to the optic nerve of mice and rats leads to a glial scar which exhibits sustained increases in the production of chondroitin sulfate proteoglycans in both the optic nerve and the retina. This is very similar to the result of injury to the brain and spinal cord, where the glial scar has been thought to limit the ability of damaged neurons to regenerate their axons. In recent years, several studies have questioned the long-held notion that the formation of a glial scar is unilaterally opposed to axon regeneration (Liddelow and Barres, 2017; Silver, 2016; Sofroniew, 2018). For example, preventing astrogliosis in the spinal cord by using transgenic mice engineered to kill proliferating scar-forming astrocytes successfully attenuates astrocytic scar formation but fails to promote axon regeneration (Anderson et al., 2016). Reactive astrocytes also exhibit different phenotypes over time, with only the scar-forming astrocytes, which appear at 14 days following spinal cord injury and express elevated levels of several CSPG-related mRNA transcripts, inhibiting regeneration (Hara et al., 2017). From these studies, it can be inferred that CSPGs, rather than reactive astrocytes or other glial cells, may be instrumental in the obstruction of axonal regeneration after injury.
In our study, the timing and spatial distribution of GFAP+ reactive astrocytes and Iba1+ microglia aligns with previously published data (Podhajsky et al., 1997; Qu and Jakobs, 2013). Detection of GFAP and Iba1 increased in the acute phase and remained elevated for several weeks, but CTB+ axons did not preferentially associate with either astrocytes or microglia. These findings confirm that the glial scar itself likely plays an important role in limiting the spread of damage following CNS injury, and that reactive astrocytes may support the regeneration of injured axons. Instead, it is the production of CSPGs by these cells that appears to undermine this supportive role. The precise duration of CSPG-mediated inhibition of axonal regeneration remains to be studied. In the rat spinal cord, for instance, CSPG immunoreactivity persists at the lesion site as late as 40 days post-injury (Lemons et al., 1999). Our study detects CSPGs in the mouse optic nerve as late as 21 days post-injury, but a longer-scale study would be required to elucidate whether CSPG expression declines, or whether sulfation patterns are further modified, at later times. Evidence exists that some RGC axon regeneration can occur with therapies given late after injury (Yungher et al., 2017), but it is not clear whether altering CSPGs or GAG sulfation might enhance such therapies.
The inhibitory actions of CSPGs are largely attributed to their GAG chains, as this activity can be reduced by enzymatic digestion of the CS chains with chondroitinase ABC (Snow et al., 1990) or by inhibition of their synthesis (Fichard et al., 1991; Laabs et al., 2007). GAGs were elevated in both types of injury, reaching peak levels at 7 dpc. Elevated levels of GAG and 4S GAG were first evident at earlier time points in the rat optic nerve than in the mouse, indicating that the precise timing of CSPG deposition differs somewhat between these species. The association of CSPGs with astrocytes and microglia was similar across conditions, suggesting that the multicellular sources of these inhibitory proteins may be conserved throughout the CNS.
Retinal gliosis is also observed after optic nerve crush, which has been attributed to a secondary effect of RGC death (Mac Nair et al., 2016). ONC stimulated an increase in the detection of GFAP+ reactive astrocytes and Müller cells, Iba1+ microglia, and CS-56+ GAGs and 2H6+ 4S GAGs. The increases in GFAP and CSPGs are typical for a gliotic response in many injury models, with astrocytes being the primary cell types (Silver, 2016), but Müller cells have also been identified as sources of CSPGs (Aquino et al., 1984; Siddiqui et al., 2009). During retinal development, localized CSPGs create inhibitory boundaries that direct RGC axons (Brittis et al., 1992), and it is likely that the increased CSPGs after injury serve a similar function to inhibit sprouting after injury. Müller cells may also act as stem cells (Das et al., 2006). Because CSPGs inhibit grafted Müller cell integration into the retina (Singhal et al., 2008), they may also affect the function of endogenous Müller cells as well. While our data were obtained from the mouse, gliosis in the retina following optic nerve crush has been recently been reported for rats (Wang and Li, 2017), and it is likely that rats will express CSPGs in a similar way.
CSPG GAG chains are comprised of a series of disaccharides of N-Acety-Galactosamine (GalNAc) and glucuronic acid (GlcA). These sugars may be modified by sulfation in any of several different positions: the 4- or 6- position on GalNAc, or the 2-position on GlcA. Each disaccharide in the chain may have a combination of sulfation on these potential sites, i.e., 0S would have no sulfation, 4S would have a single sulfate on the 4-position of GalNAc, etc. The predominant form of sulfation in mammalian CNS is 4S, with lower amounts of 6S (Yu et al., 2018). The role of GAG sulfation in governing CSPG function has been demonstrated using reagents which block sulfation, such as sodium chlorate, which broadly eliminates GAG sulfation (Smith-Thomas et al., 1995), or by modulating the activity of specific sulfotransferase enzymes (Wang et al., 2008). Recent studies have characterized the behaviors of specific sulfation motifs. For instance, axons grow profusely on surfaces coated with 6S GAGs (Wang et al., 2008), while axonal regeneration in mice is reduced after deleting the enzyme that adds 6S to GAGs (Lin et al., 2011). In contrast, axons turn at boundaries of 4S GAGs, but continue to grow onto 4S GAGs treated with 4-sulfatase (Wang et al., 2008). Elevation of 4S has been observed after traumatic brain injury (Yi et al., 2012) and spinal cord injury (Yoo et al., 2013). Recently, we identified an increase in 4S in optic nerve lesions and showed that removing 4S from the terminal ends of GAG chains using arylsulfatase B (ARSB) enhances the regeneration of RGC axons (Pearson et al., 2018). Additional sulfation motifs, such as 4,6S, are also implicated in the inhibition of axonal growth in vivo and in vitro (Brown et al., 2012). The case of 4,6S is particularly interesting, as it may be possible that GAG chains with terminal 4,6S could be converted to growth-permissive 6S motifs in the presence of ARSB.
While GAG chains are the major signaling moiety of proteoglycans, they are always secreted into the matrix attached to specific proteins which are produced in a cell type-specific manner. Some evidence also supports an inhibitory action of the proteins themselves (Dou and Levine, 1994; Milev et al., 1994). Neurons express aggrecan and versican (Asher et al., 2000), while reactive astrocytes are known produce brevican, neurocan, and phosphacan (Beggah et al., 2005; Jones et al., 2003; McKeon et al., 1999; Yamada et al., 1997). Microglia and NG2+ OPCs are known to produce versican (Asher et al., 2002; Beggah et al., 2005), while OPCs also produce phosphacan and neurocan (Chen et al., 2002). Following spinal cord injury, levels of neurocan, brevican, and versican are increased (Jones et al., 2003; Mukhamedshina et al., 2019), while aggrecan expression is reduced (Lemons et al., 2001), and phosphacan is transiently reduced before increasing (Jones et al., 2003; Morgenstern et al., 2002). The localization of these proteoglycans in the injured optic nerve in both the mouse and rat are somewhat consistent with these sources, as aggrecan and versican were found in the area proximal to the lesion, which contains uninjured nerve fibers. Neurocan was found in areas adjacent to the lesion with high GFAP reactivity, consistent with astrocytes as the source. On the other hand, both brevican and phosphacan were very high in the lesion core which is devoid of both neurons and astrocytes. These increases are similar to those reported for injured spinal cord (Jones et al., 2003). While production of either of these proteoglycans by non-neural cells has not been reported, the staining of the optic nerve sheath with these antibodies in both injured and sham-injured nerves suggests that meningeal cells may also produce brevican and phosphacan, as they do versican (Beggah et al., 2005; Shearer et al., 2003). Proteoglycans are degraded by matrix metalloproteases, and the time course of expression of several different MMPs in the injured optic nerve is similar to that of the proteoglycans, with increased expression at 6 dpc, and continuing for at least 21 days (Ahmed et al., 2005; Liu et al., 2008), which would argue for a homeostatic mechanism supporting regeneration.
The mechanisms by which CSPGs inhibit axonal growth are not entirely understood. During neuronal development, CSPGs play the role of repulsive guidance cues, inducing growth cone turning away from areas of high CSPG concentration to prevent growing axons from deviating from their intended paths (Erskine and Herrera, 2007). This process appears to be largely mediated by the binding of CSPGs to surface receptors on neurons, such as receptor tyrosine phosphatase σ (Coles et al., 2011; Katagiri et al., 2018) and the Nogo receptors (Dickendesher et al., 2012). These interactions activate signaling cascades that result in cytoskeletal reorganization and growth cone turning (Dergham et al., 2002; Yu et al., 2012), and their inhibition can promote optic nerve regeneration (Lingor et al., 2007). We have previously demonstrated that selectively reducing the 4-sulfation on CSPGs can promote optic nerve regeneration (Pearson et al., 2018). The current study demonstrates the spatiotemporal increase in 4-sulfated GAGs in the injured optic nerve and shows that these increases are associated with several different core proteins. This knowledge, combined with an increased understanding of the effects of modification of intracellular signaling on axonal regeneration (Curcio and Bradke, 2018) provides potential pathways for promoting recovery of function after axonal injury.
Table 1.
List of antibodies used.
| Primary antibodies | Dilution | Source |
|---|---|---|
| CS-56 (mouse) | 1:500 | Sigma (C8035) |
| 2H6 (mouse) | 1:500 | Amsbio (370710-IEC) |
| Iba1 (rabbit) | 1:500 | Wako (019–19747) |
| GFAP (rabbit) | 1:500 | Dako (Z0334) |
| GFAP (chicken) | 1:500 | Abcam (ab4674) |
| Phosphacan (mouse) | 1:50 | Developmental Studies Hybridoma Bank (3F8) |
| Neurocan (biotinylated sheep) | 1:100 | R&D systems (BAF5800) |
| Aggrecan (rabbit) | 1:200 | Chemicon (AB1031) |
| Brevican (sheep) | 1:10 | R&D systems (AF4009) |
| Versican (rabbit) | 1:100 | Chemicon (AB1032) |
| Secondary antibodies | 1:1,000 | Thermo Fisher (O-6381) |
| goat anti-rabbit, Oregon Green 488 | 1:1,000 | Thermo Fisher (A-21070) |
| goat anti-rabbit, Alexa Fluor 633 | 1:1,000 | Thermo Fisher (A-11039) |
| goat anti-chicken, Alexa Fluor 488 | 1:500 | Abcam (ab98749) |
| goat anti-mouse, Alexa Fluor 633 | 1:1000 | Thermo Fisher (A-21050) |
| streptavidin-488 | 1:200 | Thermo Fisher (S11223) |
| donkey anti-sheep, Alexa Fluor 488 | 1:2500 | Thermo Fisher (A-11015) |
Acknowledgments
This research was supported by the Division of Intramural Research of the National Heart, Lung, and Blood Institute, the Cambridge Eye Trust, Fight for Sight UK, and the Jukes Glaucoma Research Fund. We appreciate the assistance of the NHLBI DIR Light Microscopy Core.
Footnotes
Conflicts of interest
The authors declare no conflict of interest.
References
- Ahmed Z, Dent RG, Leadbeater WE, Smith C, Berry M, Logan A, 2005. Matrix metalloproteases: degradation of the inhibitory environment of the transected optic nerve and the scar by regenerating axons. Mol. Cell. Neurosci 28, 64–78. 10.1016/j.mcn.2004.08.013 [DOI] [PubMed] [Google Scholar]
- Anderson MA, Burda JE, Ren Y, Ao Y, O’Shea TM, Kawaguchi R, Coppola G, Khakh BS, Deming TJ, Sofroniew MV, 2016. Astrocyte scar formation aids central nervous system axon regeneration. Nature 532, 195–200. 10.1038/nature17623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aquino DA, Margolis RU, Margolis RK, 1984. Immunocytochemical localization of a chondrotin sulfate proteoglycan in nervous tissue. II. Studies in developing brain. J. Cell Biol 99, 1130–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher RA, Morgenstern DA, Fidler PS, Adcock KH, Oohira A, Braistead JE, Levine JM, Margolis RU, Rogers JH, Fawcett JW, 2000. Neurocan is upregulated in injured brain and in cytokine-treated astrocytes. J. Neurosci 20, 2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asher RA, Morgenstern DA, Shearer MC, Adcock KH, Pesheva P, Fawcett JW, 2002. Versican is upregulated in CNS injury and is a product of oligodendrocyte lineage cells. J. Neurosci 22, 2225–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CG, Becker T, 2002. Repellent guidance of regenerating optic axons by chondroitin sulfate glycosaminoglycans in zebrafish. J. Neurosci 22, 842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beggah AT, Dours-Zimmermann MT, Barras FM, Brosius A, Zimmermann DR, Zurn AD, 2005. Lesion-induced differential expression and cell association of Neurocan, Brevican, Versican V1 and V2 in the mouse dorsal root entry zone. Neuroscience 133, 749–762. [DOI] [PubMed] [Google Scholar]
- Berkelaar M, Clarke DB, Wang YC, Bray GM, Aguayo AJ, 1994. Axotomy results in delayed death and apoptosis of retinal ganglion cells in adult rats. J. Neurosci 14, 4368–4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami A, Dahl D, Nguyen BT, Crosby CJ, 1981. The fate of axonal debris in Wallerian degeneration of rat optic and sciatic nerves. Electron microscopy and immunofluorescence studies with neurofilament antisera. J. Neuropathol. Exp. Neurol 40, 537–550. 10.1097/00005072-198109000-00005 [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Carter LM, 2011. Manipulating the glial scar: Chondroitinase ABC as a therapy for spinal cord injury. Brain Res. Bull 84, 306–316. 10.1016/j.brainresbull.2010.06.015 [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, Fawcett JW, McMahon SB, 2002. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416, 636–640. 10.1038/416636a [DOI] [PubMed] [Google Scholar]
- Brittis PA, Canning DR, Silver J, 1992. Chondroitin sulfate as a regulator of neuronal patterning in the retina. Science 255, 733–736. [DOI] [PubMed] [Google Scholar]
- Brown JM, Xia J, Zhuang B, Cho KS, Rogers CJ, Gama CI, Rawat M, Tully SE, Uetani N, Mason DE, Tremblay ML, Peters EC, Habuchi O, Chen DF, Hsieh-Wilson LC, 2012. A sulfated carbohydrate epitope inhibits axon regeneration after injury. Proc. Natl. Acad. Sci. U. S. A 109, 4768–4773. 10.1073/pnas.1121318109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah M, Andrews MR, Chew DJ, Moloney EB, Verhaagen J, Fassler R, Fawcett JW, 2016. Expression of an activated integrin promotes long-distance sensory axon regeneration in the spinal cord. J. Neurosci 36, 7283–7297. 10.1523/JNEUROSCI.0901-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, Ughrin Y, Levine JM, 2002. Inhibition of axon growth by oligodendrocyte precursor cells. Mol. Cell. Neurosci 20, 125–139. 10.1006/mcne.2002.1102 [DOI] [PubMed] [Google Scholar]
- Coles CH, Shen Y, Tenney AP, Siebold C, Sutton GC, Lu W, Gallagher JT, Jones EY, Flanagan JG, Aricescu AR, 2011. Proteoglycan-specific molecular switch for RPTPsigma clustering and neuronal extension. Science 332, 484–488. 10.1126/science.1200840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curcio M, Bradke F, 2018. Axon regeneration in the central nervous system: facing the challenges from the inside. Annu. Rev. Cell Dev. Biol 34, 495–521. 10.1146/annurev-cellbio-100617-062508 [DOI] [PubMed] [Google Scholar]
- Das AV, Mallya KB, Zhao X, Ahmad F, Bhattacharya S, Thoreson WB, Hegde GV, Ahmad I, 2006. Neural stem cell properties of Muller glia in the mammalian retina: regulation by Notch and Wnt signaling. Dev. Biol 299, 283–302. 10.1016/j.ydbio.2006.07.029 [DOI] [PubMed] [Google Scholar]
- Davies SJ, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J, 1997. Regeneration of adult axons in white matter tracts of the central nervous system. Nature 390, 680–683. [DOI] [PubMed] [Google Scholar]
- Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L, 2002. Rho signaling pathway targeted to promote spinal cord repair. J. Neurosci 22, 6570–6577. 10.1523/JNEUROSCI.22-15-06570.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickendesher TL, Baldwin KT, Mironova YA, Koriyama Y, Raiker SJ, Askew KL, Wood A, Geoffroy CG, Zheng B, Liepmann CD, Katagiri Y, Benowitz LI, Geller HM, Giger RJ, 2012. NgR1 and NgR3 are receptors for chondroitin sulfate proteoglycans. Nat. Neurosci 15, 703–712. 10.1038/nn.3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimou L, Gallo V, 2015. NG2-glia and their functions in the central nervous system. Glia 63, 1429–1451. 10.1002/glia.22859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou CL, Levine JM, 1994. Inhibition of neurite growth by the NG2 chondroitin sulfate proteoglycan. J. Neurosci 14, 7616–7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine L, Herrera E, 2007. The retinal ganglion cell axon’s journey: insights into molecular mechanisms of axon guidance. Dev. Biol 308, 1–14. 10.1016/j.ydbio.2007.05.013 [DOI] [PubMed] [Google Scholar]
- Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV, 2004. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J. Neurosci 24, 2143–2155. 10.1523/JNEUROSCI.3547-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JW, Asher RA, 1999. The glial scar and central nervous system repair. Brain Res. Bull 49, 377–391. [DOI] [PubMed] [Google Scholar]
- Fichard A, Verna JM, Olivares J, Saxod R, 1991. Involvement of a chondroitin sulfate proteoglycan in the avoidance of chick epidermis by dorsal-root ganglia fibers - a study using β-D-xyloside. Dev. Biol 148, 1–9. [DOI] [PubMed] [Google Scholar]
- Fisher SK, Lewis GP, Linberg KA, Barawid E, Verardo MR, 1995. Cellular remodeling in mammalian retina induced by retinal detachment, in: Kolb H, Fernandez E, Nelson R (Eds.), Webvision: The Organization of the Retina and Visual System. University of Utah Health Sciences Center, Salt Lake City (UT). [PubMed] [Google Scholar]
- Grafstein B, Ingoglia NA, 1982. Intracranial transection of the optic nerve in adult mice: preliminary observations. Exp. Neurol 76, 318–330. [DOI] [PubMed] [Google Scholar]
- Hackett AR, Yahn SL, Lyapichev K, Dajnoki A, Lee DH, Rodriguez M, Cammer N, Pak J, Mehta ST, Bodamer O, Lemmon VP, Lee JK, 2018. Injury type-dependent differentiation of NG2 glia into heterogeneous astrocytes. Exp. Neurol 308, 72–79. 10.1016/j.expneurol.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M, Kobayakawa K, Ohkawa Y, Kumamaru H, Yokota K, Saito T, Kijima K, Yoshizaki S, Harimaya K, Nakashima Y, Okada S, 2017. Interaction of reactive astrocytes with type I collagen induces astrocytic scar formation through the integrin-N-cadherin pathway after spinal cord injury. Nat. Med 23, 818–828. 10.1038/nm.4354 [DOI] [PubMed] [Google Scholar]
- Hippert C, Graca AB, Barber AC, West EL, Smith AJ, Ali RR, Pearson RA, 2015. Muller glia activation in response to inherited retinal degeneration is highly varied and disease-specific. PLoS One 10, e0120415 10.1371/journal.pone.0120415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LL, Margolis RU, Tuszynski MH, 2003. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp. Neurol 182, 399–411. [DOI] [PubMed] [Google Scholar]
- Katagiri Y, Morgan AA, Yu P, Bangayan NJ, Junka R, Geller HM, 2018. Identification of novel binding sites for heparin in receptor protein-tyrosine phosphatase (RPTPsigma): Implications for proteoglycan signaling. J. Biol. Chem 293, 11639–11647. 10.1074/jbc.RA118.003081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laabs TL, Wang H, Katagiri Y, McCann T, Fawcett JW, Geller HM, 2007. Inhibiting glycosaminoglycan chain polymerization decreases the inhibitory activity of astrocyte-derived chondroitin sulfate proteoglycans. J. Neurosci 27, 14494–14501. 10.1523/JNEUROSCI.2807-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemons ML, Howland DR, Anderson DK, 1999. Chondroitin sulfate proteoglycan immunoreactivity increases following spinal cord injury and transplantation. Exp. Neurol 160, 51–65. 10.1006/exnr.1999.7184 [DOI] [PubMed] [Google Scholar]
- Lemons ML, Sandy JD, Anderson DK, Howland DR, 2001. Intact aggrecan and fragments generated by both aggrecanse and metalloproteinase-like activities are present in the developing and adult rat spinal cord and their relative abundance is altered by injury. J. Neurosci 21, 4772–4781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liddelow SA, Barres BA, 2017. Reactive astrocytes: Production, function, and therapeutic potential. Immunity 46, 957–967. 10.1016/j.immuni.2017.06.006 [DOI] [PubMed] [Google Scholar]
- Lin R, Rosahl TW, Whiting PJ, Fawcett JW, Kwok JC, 2011. 6-Sulphated chondroitins have a positive influence on axonal regeneration. PLoS One 6, e21499 10.1371/journal.pone.0021499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingor P, Teusch N, Schwarz K, Mueller R, Mack H, Bahr M, Mueller BK, 2007. Inhibition of Rho kinase (ROCK) increases neurite outgrowth on chondroitin sulphate proteoglycan in vitro and axonal regeneration in the adult optic nerve in vivo. J. Neurochem 103, 181–189. 10.1111/j.1471-4159.2007.04756.x [DOI] [PubMed] [Google Scholar]
- Liu LY, Zheng H, Xiao HL, She ZJ, Zhao SM, Chen ZL, Zhou GD, 2008. Comparison of blood-nerve barrier disruption and matrix metalloprotease-9 expression in injured central and peripheral nerves in mice. Neurosci. Lett 434, 155–159. 10.1016/j.neulet.2007.12.052 [DOI] [PubMed] [Google Scholar]
- Mac Nair CE, Schlamp CL, Montgomery AD, Shestopalov VI, Nickells RW, 2016. Retinal glial responses to optic nerve crush are attenuated in Bax-deficient mice and modulated by purinergic signaling pathways. J. Neuroinflammation 13, 93 10.1186/s12974-016-0558-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita K, Nakata T, Takeda-Okuda N, Nadanaka S, Kitagawa H, Tamura JI, 2018. Synthesis of chondroitin sulfate CC and DD tetrasaccharides and interactions with 2H6 and LY111. Bioorg. Med. Chem 26, 1016–1025. 10.1016/j.bmc.2018.01.011 [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Jurynec MJ, Buck CR, 1999. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. J. Neurosci 19, 10778–10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milev P, Friedlander DR, Sakurai T, Karthikeyan L, Flad M, Margolis RK, Grumet M, Margolis RU, 1994. Interactions of the chondroitin sulfate proteoglycan phosphacan, the extracellular domain of a receptor-type protein tyrosine phosphatase, with neurons, glia, and neural cell adhesion molecules. J. Cell Biol 127, 1703–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misantone LJ, Gershenbaum M, Murray M, 1984. Viability of retinal ganglion cells after optic nerve crush in adult rats. J. Neurocytol 13, 449–465. [DOI] [PubMed] [Google Scholar]
- Morgenstern DA, Asher RA, Fawcett JW, 2002. Chondroitin sulphate proteoglycans in the CNS injury response. Prog. Brain Res 137, 313–332. [DOI] [PubMed] [Google Scholar]
- Mukhamedshina YO, Povysheva TV, Nikolenko VN, Kuznecov MS, Rizvanov AA, Chelyshev YA, 2019. Upregulation of proteoglycans in the perilesion perimeter in ventral horns after spinal cord injury. Neurosci. Lett 704, 220–228. 10.1016/j.neulet.2019.04.006 [DOI] [PubMed] [Google Scholar]
- Park KK, Liu K, Hu Y, Smith PD, Wang C, Cai B, Xu B, Connolly L, Kramvis I, Sahin M, He Z, 2008. Promoting axon regeneration in the adult CNS by modulation of the PTEN/mTOR pathway. Science 322, 963–966. 10.1126/science.1161566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson CS, Mencio CP, Barber AC, Martin KR, Geller HM, 2018. Identification of a critical sulfation in chondroitin that inhibits axonal regeneration. Elife 7 10.7554/eLife.37139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podhajsky RJ, Bidanset DJ, Caterson B, Blight AR, 1997. A quantitative immunohistochemical study of the cellular response to crush injury in optic nerve. Exp. Neurol 143, 153–161. 10.1006/exnr.1996.6354 [DOI] [PubMed] [Google Scholar]
- Qu J, Jakobs TC, 2013. The time course of gene expression during reactive gliosis in the optic nerve. PLoS One 8, e67094 10.1371/journal.pone.0067094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls A, Shechter R, London A, Segev Y, Jacob-Hirsch J, Amariglio N, Rechavi G, Schwartz M, 2008. Two faces of chondroitin sulfate proteoglycan in spinal cord repair: a role in microglia/macrophage activation. PLoS Med. 5, e171 10.1371/journal.pmed.0050171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz NB, Domowicz MS, 2018. Proteoglycans in brain development and pathogenesis. FEBS Lett. 592, 3791–3805. 10.1002/1873-3468.13026 [DOI] [PubMed] [Google Scholar]
- Selles-Navarro I, Ellezam B, Fajardo R, Latour M, McKerracher L, 2001. Retinal ganglion cell and nonneuronal cell responses to a microcrush lesion of adult rat optic nerve. Exp. Neurol 167, 282–289. 10.1006/exnr.2000.7573 [DOI] [PubMed] [Google Scholar]
- Sengottuvel V, Leibinger M, Pfreimer M, Andreadaki A, Fischer D, 2011. Taxol facilitates axon regeneration in the mature CNS. J. Neurosci 31, 2688–2699. 10.1523/JNEUROSCI.4885-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer MC, Niclou SP, Brown D, Asher RA, Holtmaat AJ, Levine JM, Verhaagen J, Fawcett JW, 2003. The astrocyte/meningeal cell interface is a barrier to neurite outgrowth which can be overcome by manipulation of inhibitory molecules or axonal signalling pathways. Mol. Cell. Neurosci 24, 913–925. [DOI] [PubMed] [Google Scholar]
- Shechter R, London A, Varol C, Raposo C, Cusimano M, Yovel G, Rolls A, Mack M, Pluchino S, Martino G, Jung S, Schwartz M, 2009. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 6, e1000113 10.1371/journal.pmed.1000113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter R, Raposo C, London A, Sagi I, Schwartz M, 2011. The glial scar-monocyte interplay: a pivotal resolution phase in spinal cord repair. PLoS One 6, e27969 10.1371/journal.pone.0027969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui S, Horvat-Broecker A, Faissner A, 2009. Comparative screening of glial cell types reveals extracellular matrix that inhibits retinal axon growth in a chondroitinase ABC-resistant fashion. Glia 57, 1420–1438. [DOI] [PubMed] [Google Scholar]
- Silver J, 2016. The glial scar is more than just astrocytes. Exp. Neurol 286, 147–149. 10.1016/j.expneurol.2016.06.018 [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH, 2004. Regeneration beyond the glial scar. Nat. Rev. Neurosci 5, 146–156. 10.1038/nrn1326 [DOI] [PubMed] [Google Scholar]
- Singhal S, Lawrence JM, Bhatia B, Ellis JS, Kwan AS, Macneil A, Luthert PJ, Fawcett JW, Perez MT, Khaw PT, Limb GA, 2008. Chondroitin sulfate proteoglycans and microglia prevent migration and integration of grafted Muller stem cells into degenerating retina. Stem Cells 26, 1074–1082. 10.1634/stemcells.2007-0898 [DOI] [PubMed] [Google Scholar]
- Smith-Thomas LC, Stevens J, Fok-Seang J, Faissner A, Rogers JH, Fawcett JW, 1995. Increased axon regeneration in astrocytes grown in the presence of proteoglycan synthesis inhibitors. J. Cell Sci 108, 1307–1315. [DOI] [PubMed] [Google Scholar]
- Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J, 1990. Sulfated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp. Neurol 109, 111–130. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, 2018. Dissecting spinal cord regeneration. Nature 557, 343–350. 10.1038/s41586-018-0068-4 [DOI] [PubMed] [Google Scholar]
- Sugiura N, Shioiri T, Chiba M, Sato T, Narimatsu H, Kimata K, Watanabe H, 2012. Construction of a chondroitin sulfate library with defined structures and analysis of molecular interactions. The Journal of biological chemistry 287, 43390–43400. 10.1074/jbc.M112.412676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Davies JE, Davies SJ, 2003. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J. Neurosci. Res 71, 427–444. 10.1002/jnr.10523 [DOI] [PubMed] [Google Scholar]
- Wang H, Katagiri Y, McCann TE, Unsworth E, Goldsmith P, Yu ZX, Tan F, Santiago L, Mills EM, Wang Y, Symes AJ, Geller HM, 2008. Chondroitin-4-sulfation negatively regulates axonal guidance and growth. J. Cell Sci 121, 3083–3091. 10.1242/jcs.032649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li P, 2017. Expressions of nestin and glial fibrillary acidic protein in rat retina after optic nerve transection. Int J Ophthalmol 10, 1510–1515. 10.18240/ijo.2017.10.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Fredette B, Shitara K, Hagihara K, Miura R, Ranscht B, Stallcup WB, Yamaguchi Y, 1997. The brain chondroitin sulfate proteoglycan brevican associates with astrocytes ensheathing cerebellar glomeruli and inhibits neurite outgrowth from granule neurons. J. Neurosci 17, 7784–7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Atoji Y, Oohira A, Suzuki Y, 1995. Immunohistochemical localization of chondroitin sulfate in the forestomach of the sheep. Eur. J. Histochem 39, 265–272. [PubMed] [Google Scholar]
- Yi JH, Katagiri Y, Susarla B, Figge D, Symes AJ, Geller HM, 2012. Alterations in sulfated chondroitin glycosaminoglycans following controlled cortical impact injury in mice. J. Comp. Neurol 520, 3295–3313. 10.1002/cne.23156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo M, Khaled M, Gibbs KM, Kim J, Kowalewski B, Dierks T, Schachner M, 2013. Arylsulfatase B improves locomotor function after mouse spinal cord injury. PLoS One 8, e57415 10.1371/journal.pone.0057415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Pearson CS, Geller HM, 2018. Flexible roles for proteoglycan sulfation and receptor signaling. Trends Neurosci. 41, 47–61. 10.1016/j.tins.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Santiago LY, Katagiri Y, Geller HM, 2012. Myosin II activity regulates neurite outgrowth and guidance in response to chondroitin sulfate proteoglycans. J. Neurochem 120, 1117–1128. 10.1111/j.1471-4159.2011.07638.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yungher BJ, Ribeiro M, Park KK, 2017. Regenerative responses and axon pathfinding of retinal ganglion cells in chronically injured mice. Invest. Ophthalmol. Vis. Sci 58, 1743–1750. 10.1167/iovs.16-19873 [DOI] [PMC free article] [PubMed] [Google Scholar]


