Abstract
Background
Breathing exercises have been widely used worldwide as a non‐pharmacological therapy to treat people with asthma. Breathing exercises aim to control the symptoms of asthma and can be performed as the Papworth Method, the Buteyko breathing technique, yogic breathing, deep diaphragmatic breathing or any other similar intervention that manipulates the breathing pattern. The training of breathing usually focuses on tidal and minute volume and encourages relaxation, exercise at home, the modification of breathing pattern, nasal breathing, holding of breath, lower rib cage and abdominal breathing.
Objectives
To evaluate the evidence for the efficacy of breathing exercises in the management of people with asthma.
Search methods
To identify relevant studies we searched The Cochrane Library, MEDLINE, Embase, PsycINFO, CINAHL and AMED and performed handsearching of respiratory journals and meeting abstracts. We also consulted trials registers and reference lists of included articles.
The most recent literature search was on 4 April 2019.
Selection criteria
We included randomised controlled trials of breathing exercises in adults with asthma compared with a control group receiving asthma education or, alternatively, with no active control group.
Data collection and analysis
Two review authors independently assessed study quality and extracted data. We used Review Manager 5 software for data analysis based on the random‐effects model. We expressed continuous outcomes as mean differences (MDs) with confidence intervals (CIs) of 95%. We assessed heterogeneity by inspecting the forest plots. We applied the Chi2 test, with a P value of 0.10 indicating statistical significance, and the I2 statistic, with a value greater than 50% representing a substantial level of heterogeneity. The primary outcome was quality of life.
Main results
We included nine new studies (1910 participants) in this update, resulting in a total of 22 studies involving 2880 participants in the review. Fourteen studies used Yoga as the intervention, four studies involved breathing retraining, one the Buteyko method, one the Buteyko method and pranayama, one the Papworth method and one deep diaphragmatic breathing. The studies were different from one another in terms of type of breathing exercise performed, number of participants enrolled, number of sessions completed, period of follow‐up, outcomes reported and statistical presentation of data. Asthma severity in participants from the included studies ranged from mild to moderate, and the samples consisted solely of outpatients. Twenty studies compared breathing exercise with inactive control, and two with asthma education control groups. Meta‐analysis was possible for the primary outcome quality of life and the secondary outcomes asthma symptoms, hyperventilation symptoms, and some lung function variables. Assessment of risk of bias was impaired by incomplete reporting of methodological aspects of most of the included studies. We did not include adverse effects as an outcome in the review.
Breathing exercises versus inactive control
For quality of life, measured by the Asthma Quality of Life Questionnaire (AQLQ), meta‐analysis showed improvement favouring the breathing exercises group at three months (MD 0.42, 95% CI 0.17 to 0.68; 4 studies, 974 participants; moderate‐certainty evidence), and at six months the OR was 1.34 for the proportion of people with at least 0.5 unit improvement in AQLQ, (95% CI 0.97 to 1.86; 1 study, 655 participants). For asthma symptoms, measured by the Asthma Control Questionnaire (ACQ), meta‐analysis at up to three months was inconclusive, MD of ‐0.15 units (95% CI −2.32 to 2.02; 1 study, 115 participants; low‐certainty evidence), and was similar over six months (MD −0.08 units, 95% CI −0.22 to 0.07; 1 study, 449 participants). For hyperventilation symptoms, measured by the Nijmegen Questionnaire (from four to six months), meta‐analysis showed less symptoms with breathing exercises (MD −3.22, 95% CI −6.31 to −0.13; 2 studies, 118 participants; moderate‐certainty evidence), but this was not shown at six months (MD 0.63, 95% CI −0.90 to 2.17; 2 studies, 521 participants). Meta‐analyses for forced expiratory volume in 1 second (FEV1) measured at up to three months was inconclusive, MD −0.10 L, (95% CI −0.32 to 0.12; 4 studies, 252 participants; very low‐certainty evidence). However, for FEV1 % of predicted, an improvement was observed in favour of the breathing exercise group (MD 6.88%, 95% CI 5.03 to 8.73; five studies, 618 participants).
Breathing exercises versus asthma education
For quality of life, one study measuring AQLQ was inconclusive up to three months (MD 0.04, 95% CI ‐0.26 to 0.34; 1 study, 183 participants). When assessed from four to six months, the results favoured breathing exercises (MD 0.38, 95% CI 0.08 to 0.68; 1 study, 183 participants). Hyperventilation symptoms measured by the Nijmegen Questionnaire were inconclusive up to three months (MD −1.24, 95% CI −3.23 to 0.75; 1 study, 183 participants), but favoured breathing exercises from four to six months (MD −3.16, 95% CI −5.35 to −0.97; 1 study, 183 participants).
Authors' conclusions
Breathing exercises may have some positive effects on quality of life, hyperventilation symptoms, and lung function. Due to some methodological differences among included studies and studies with poor methodology, the quality of evidence for the measured outcomes ranged from moderate to very low certainty according to GRADE criteria. In addition, further studies including full descriptions of treatment methods and outcome measurements are required.
Plain language summary
Breathing exercises for asthma
Background
Asthma is a lung disease. Asthma works in two ways. It that causes the airways to become inflamed (the body's response to injury and infection) and it causes the small tubes of the airways to tighten (called airway obstruction). The tightening of the tubes can happen in response to asthma triggers, such as animal fur or feathers, dust or pollen.
Asthma is very common worldwide and is a major public health problem due to the high healthcare costs associated with hospitalisation and medication. Breathing exercises have been used to treat people with asthma as a way of controlling the symptoms of asthma without medication. People use various breathing techniques to change their breathing pattern.
Review question
We wanted to find out how effective breathing exercises are for adults with asthma. We were most interested to know if breathing exercises improved people's quality of life (our primary outcome), and also if they helped improve asthma symptoms, hyperventilation (over‐breathing), and lung function (our secondary outcomes).
Key results
We searched for randomised controlled trials. This means people were selected at random to try either breathing exercises or a control. We included education about asthma or usual asthma care as the controls.
We found 22 studies involving 2880 adults with mild to moderate asthma. The studies used different breathing exercises. Fourteen studies used yoga, four studies used breathing retraining, one study used Buteyko method, one study used Buteyko method and pranayama, one study used Papworth method and one study used deep diaphragmatic breathing. Twenty studies compared breathing exercises with normal asthma care and two compared breathing exercises with asthma education. Studies assessed quality of life, asthma symptoms and hyperventilation symptoms, number of acute exacerbations (flare‐ups), lung function (breathing tests), and general practitioner (GP) appointments.
Several studies looked at our primary outcome, quality of life. The results showed an improvement in quality of life after three months in the breathing‐exercises group. We found that breathing exercises probably did not help to improve asthma symptoms. However, breathing exercises did improve hyperventilation symptoms, when measured from four months after starting the exercises to six months. One lung function test, percentage of predicted FEV1 (the amount of air you can force from your lungs in one second) showed some improvement in the people who did breathing exercises.
Certainty of the evidence
We are moderately certain about the benefits of breathing exercises. However, we found some differences between the studies in terms of type of breathing exercises performed, number of participants enrolled, number and duration of sessions completed, outcomes reported and statistical presentation of data.
Conclusion
Breathing exercises may have positive effects on quality of life, hyperventilation symptoms, and lung function in adults with mild to moderate asthma.
The evidence is current to April 2019.
Summary of findings
Summary of findings for the main comparison. Breathing exercises compared to inactive control for adults with asthma.
| Breathing exercises compared to inactive control for adults with asthma | ||||||
| Patient or population: adults with asthma Setting: outpatient Intervention: breathing exercises (yoga, breathing retraining and Buteyko) Comparison: inactive control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with inactive control | Risk with breathing exercises | |||||
|
Change in AQLQ Scores Range 1‐7, with higher scores indicating better quality of life. Follow‐up: < 3 months |
The mean change in AQLQ was 0.14 units | MD 0.42 higher (0.17 higher to 0.68 higher) | ‐ | 974 (4 RCTs) |
⊕⊕⊕⊝ Moderatea | MCID for AQLQ is 0.5 units (Juniper 2004) |
|
Change in ACQ Scores Range 0‐6 with lower scores indicating better control of asthma symptoms. Follow‐up: < 3 months |
The mean change in ACQ was minus 0.11 units | MD 0.15 lower (2.32 lower to 2.02 higher) | ‐ | 115 (1 RCT) | ⊕⊕⊝⊝ Lowa,b | MCID for ACQ is 0.5 units (Juniper 2005) |
|
Nijmegen Questionnaire score (4‐6 months) Scores range 1‐5 with lower scores indicating better control of asthma symptoms. Follow‐up: between 4 and 6 months |
The mean Nijmegen Questionnaire score was 15.7 points | MD 3.22 points lower (6.31 lower to 0.13 lower) | ‐ | 118 (2 RCTs) | ⊕⊕⊕⊝ Moderatec | MCID has not been established for the Nijmegen Questionnaire (van Dixhoorn 2015) |
|
Number of acute exacerbations Follow‐up: between 2 and 54 months |
See comment | See comment | ‐ | 952 (4 RCTs) |
⊕⊝⊝⊝ Very lowd | The studies did not report sufficient data to allow us to include them in meta‐analysis. |
| Inpatient hospitalisation episodes | See comment | See comment | ‐ | ‐ | ‐ | No studies reported this outcome |
|
Lung function (FEV1, L ) Follow up: < 3 months |
The mean FEV1 was 2.07 to 4.19 L | MD 0.1 L lower (0.32 lower to 0.12 higher) | ‐ | 252 (4 RCTs) | ⊕⊝⊝⊝ Very lowe | The accepted variability of change from 0.1 to 0.2 L is likely to have clinical relevance (Enright 2004; Tepper 2012) |
| Days off work | See comment | See comment | ‐ | ‐ | ‐ | No studies reported this outcome |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACQ: asthma control questionnaire; AQLQ: asthma quality of life questionnaire; CI: confidence interval; FEV1: forced expiratory volume in one second; MCID: minimal clinically important difference; MD: mean difference | ||||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aDowngraded one level because one study was high risk of bias for selective reporting. bDowngraded one level because we included only one study with a small sample size in the analysis. cDowngraded one level because of the small sample size and wide confidential interval presented. dGraded as very low due to the impossibility of pooling the data into meta‐analysis. eDowngraded two levels because the included studies showed in general a high risk of bias and one level because of small sample size.
Background
Description of the condition
Asthma is a chronic airway inflammatory disorder of the lungs that can lead to structural and functional changes, resulting in bronchial hyperresponsiveness and airflow obstruction (Zhang 2010; Brightling 2012; GINA 2018). Symptoms of asthma include recurrent episodes of wheeze, cough, breathlessness and chest tightness, together with episodes of marked worsening of symptoms, known as exacerbations (Zhang 2010; Brightling 2012; GINA 2018). Exacerbations can be fatal and they are more frequent and more serious in high‐risk patients or patients with uncontrolled asthma (GINA 2018). Factors such as viral infections, allergens, tobacco smoke, physical exercise, stress, certain medications (non‐steroidal anti‐inflammatory drugs and beta‐blockers) may trigger or worsen asthma symptoms (GINA 2018; WHO 2018). Some phenotypes are already identified, such as allergic asthma, non‐allergic asthma, and late‐onset asthma (GINA 2018).
The diagnosis of asthma is based on the individual's medical history, physical examination findings and lung function and laboratory test results (Sveum 2012; National Asthma Council Australia 2016; Gillis 2017). Measurement of lung function provides an assessment of the severity of airflow limitation. These measures yield complementary information about different aspects of asthma control and are obtained by spirometry and by peak expiratory flow measurement (GINA 2018). Assessment of airway responsiveness to factors that can cause asthma symptoms, evaluation of airway inflammation and measurement of allergic status may facilitate the diagnosis and management of people with asthma (GINA 2018).
Asthma is a serious public health problem that is a major cause of disability and health resource utilisation for those affected, which may need emergency care including hospital admission (Bateman 2008; Eisner 2012; To 2012; Nunes 2017). Asthma affects around 1% to 18% of the population worldwide. Annually, the number of asthma deaths is about 180,000 with a wide variation between age, economic groups, continents and regions (GINA 2018; WHO 2018).
Some chronic respiratory diseases, such as asthma, have been commonly associated with dysfunctional breathing patterns (Veidal 2017). The prevalence of dysfunctional breathing in people with asthma was reported as ranging from 29% to 64% (Courtney 2017). Some of the mechanisms regarding the dysfunctional breathing include multiple dimensions. These dimensions are biochemical, biomechanical and psychophysiological and refer to hyperventilation, to breathing pattern disorders and to interactions of physiology with cognitive and emotional factors, respectively (Courtney 2017). Dysfunctional breathing may occur in different forms, with hyperventilation syndrome one of the most well known forms, affecting a third of people with asthma (Grammatopoulou 2014; Boulding 2016; Vidotto 2019). One of the major symptoms is breathlessness that may occur associated with hyperventilation and respiratory alkalosis. However, the breathlessness is not always caused only by hyperventilation or the presence of an abnormal breathing pattern. The changes in breathing pattern are a result of a physiological response (Thomas 2001; Morgan 2002). In addition, psychological symptoms may interfere with the severity of the respiratory symptoms and may influence patients' quality of life (Rimington 2001; Juniper 2004; Lavoie 2005). Thus, an important component of asthma management is identifying individual issues that impair health‐related quality of life and treating them (Rimington 2001; Juniper 2004). So, the control of asthma may be achieved by an individualised plan, considering the factors that contribute to attaining and maintaining asthma control (Braido 2013).
Description of the intervention
Although no cure for asthma is known, there are various pharmacological and non‐pharmacological interventions that may help people control their asthma symptoms (GINA 2018; Beasley 2016). For example, avoiding triggers (such as pollen or cold temperatures) and asthma education can also help people to control their asthma symptoms (Burgess 2011; Welsh 2011; Kew 2016; GINA 2018).
Medications to treat asthma can be broadly divided into long‐term controllers and short‐term relievers (Arun 2012). Controller medications are taken daily on a long‐term basis, and the relievers are used to rapidly decrease bronchoconstriction and relieve its symptoms (GINA 2018). Such treatment can be administered in different ways (by inhalation, orally or parenterally; GINA 2018).
Non‐pharmacological interventions have gained attention in the treatment of asthma. Those interventions include breathing exercises, physical activity, and other strategies such as cessation of smoking, avoidance of occupational exposure and indoor allergens, and weight reduction, among others (GINA 2018). Another approach comprises complementary and alternative medicine that includes non‐conventional therapies such as homeopathy, acupuncture, aromatherapy, reflexology, massage, hypnotherapy, dietary supplements, and Alexander technique (Blanc 2001; Torres‐Llenza 2010; Dennis 2012; Mark 2015). Breathing exercises have been used by physiotherapists and other professionals to control the symptoms of asthma (Bruton 2005; James 2016) and can be performed as the Papworth method, Buteyko breathing technique, yoga or any other similar intervention that manipulates the breathing pattern (Ram 2003; Denehy 2016).
How the intervention might work
In people with asthma, the presence of dysfunctional breathing independently of hyperventilation can contribute to dyspnoea. Breathing exercises are a commonly used approach for correcting dysfunctional breathing. The breathing retraining programme aims to help people with asthma in their daily life or when experiencing dyspnoea by teaching them to breathe using a better breathing pattern. The protocols for training of breathing usually pay attention to tidal and minute volume and encourage relaxation, exercise at home, the modification of breathing pattern, nasal breathing, holding of breath, and lower rib cage and abdominal breathing (Courtney 2017; Sankar 2018). Breathing training is usually a multi‐component intervention that aims at behavioural change and involves many different methods and techniques of breathing exercises such as Buteyko method, yogic breathing, Papworth method and deep diaphragmatic breathing (Bailey 2016). When breathing retraining appropriately targets the biochemical, biomechanical or psychophysiological dimensions of dysfunctional breathing, asthma control, medication usage, dysfunctional breathing symptoms and quality of life can be improved (Courtney 2019). The biochemical and biomechanical dimensions can respond to breathing protocols when they target hyperventilation, control of breathing volume, relaxation of hypertonic respiratory muscles, and teach patients to adopt a more normal breathing pattern, whether they have dyspnoea or not. Regarding the psychophysiological dimension of dysfunctional breathing, breathing retraining can cover important aspects involving relaxation techniques, and emotional and mental self‐regulations tools to decrease hyperarousal and anxiety (Courtney 2017; Courtney 2019).
Why it is important to do this review
The worldwide high prevalence of asthma has become a public health problem because of the high healthcare costs resulting from hospitalisation and medication. It causes a high number of missed work days and can result in early permanent disability and premature death. In general, asthma‐related costs are very high (Giavina‐Bianchi 2010; Nunes 2017). Breathing exercises have been widely used as an adjunct therapy in the treatment of people with asthma, generating considerable interest among researchers to develop studies that seek to show evidence of the effectiveness of this intervention.
This is an update of a review last published in 2013, in which the review authors concluded that no conclusive evidence was provided to support or refute the benefits of breathing exercises in people with asthma. Since 2013, new studies have been conducted to evaluate the effects of breathing exercises on quality of life, symptom control and lung function in people with asthma. Thus, within this review update, we aim to summarise and assess evidence from randomised controlled trials (RCT) showing the efficacy of breathing exercises in the treatment of adults with asthma.
Objectives
To evaluate the evidence for the efficacy of breathing exercises in the management of people with asthma.
Methods
Criteria for considering studies for this review
Types of studies
RCTs of breathing exercises in adults with asthma.
Types of participants
Adults with physician‐diagnosed asthma or diagnosis by internationally established criteria, or both: American Thoracic Society (ATS), European Respiratory Society (ERS) or British Thoracic Society (BTS). Participants may be either community‐ or hospital‐based.
To operationalise the age criteria, the mean age of the participants should be over 18 years old.
Types of interventions
Intervention: adults with asthma who have been assigned to treatment comprising breathing retraining
Comparison: control group receiving asthma education or, alternatively, no active control group (e.g. waiting list control)
Types of outcome measures
Primary outcomes
Quality of life
Secondary outcomes
Asthma symptoms and hyperventilation symptoms (e.g. measures of dyspnoea or breathlessness with Borg score or visual analogue scale)
Number of acute exacerbations (mean number and number of participants experiencing one or more exacerbations)
Inpatient hospitalisation episodes
Physiological measures: lung function and functional capacity
General practitioner (GP) or hospital outpatient appointments or both
Days off work
Participant's subjective evaluation of the intervention
Search methods for identification of studies
Electronic searches
We identified studies from the Cochrane Airways Trials Register, which is maintained by the Information Specialist for the Group. The Cochrane Airways Trials Register contains studies identified from several sources:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies;
weekly searches of MEDLINE Ovid SP 1946 to date;
weekly searches of Embase Ovid SP 1974 to date;
Monthly searches of PsycINFO Ovid SP 1967 to date;
Monthly searches of CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature) 1937 to date;
Monthly searches of AMED EBSCO (Allied and Complementary Medicine) all years to date;
handsearches of the proceedings of major respiratory conferences.
Studies contained in the Trials Register are identified through search strategies based on the scope of Cochrane Airways. Details of these strategies, as well as a list of handsearched conference proceedings are in Appendix 2. See Appendix 3 for search terms used to identify studies for this review.
For the previous version of this review (Freitas 2013), searches were conducted up to January 2013. For this version, the literature search has been updated to 4 April 2019. This review update includes searches conducted in April 2003, February 2012, January 2013, December 2016, December 2017, and April 2019.
Searching other resources
We looked for additional studies by consulting reference lists of relevant articles found by the above methods. We searched the US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov), and the World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch), to look for planned, ongoing and unpublished studies.
Data collection and analysis
Selection of studies
Two review authors (KMPPM and TAS) independently assessed studies for the possibility of inclusion in this review. We retrieved full‐text articles and reviewed them to determine eligibility. We resolved final decisions and disagreements by consultation with a third review author (DAF). We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009), and 'Characteristics of excluded studies' table.
Data extraction and management
Two review authors (KMPPM and GAAF) independently extracted data into Review Manager 5 (Review Manager 2014), by using a standard data collection form. According to methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), we collected information from the studies, including the following.
Methods (design, method of randomisation, method of allocation concealment, outcome assessor blinding, withdrawal and dropouts)
Participants (country, health status, mean age, gender, total sample and exclusion criteria)
Interventions (methods and types of intervention, including number and duration of sessions and methods used for control group comparisons)
Outcomes (improvement in quality‐of‐life indices, asthma symptoms, number of acute exacerbations, inpatient hospitalisation episodes, etc)
We resolved disagreements by discussion and consensus with a third review author (GSSC).
Assessment of risk of bias in included studies
Two review authors (KMPPM and TAS) independently assessed the risk of bias using the Cochrane tool for assessing risk of bias, which includes the following items: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias. We classified risk of bias as high, low or unclear, according to the methods described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2017). We resolved disagreements by discussion and consensus with a third review author (GSSC).
Measures of treatment effect
We expressed continuous outcomes as mean difference (MD) with 95% confidence interval (CI) when outcome measurements were performed on the same scale. We planned to use standardised mean difference (SMD) with 95% CI if studies assessed an outcome by using different methods. We expressed dichotomous outcomes as odds ratio (OR) with 95% CI.
We used intention‐to‐treat (ITT) analyses where they are reported instead of per‐protocol/completer analyses.
Unit of analysis issues
We did not include studies with a cross‐over or cluster‐randomised design in the review.
Dealing with missing data
We wrote to authors of included studies to request additional data as required.
Assessment of heterogeneity
We assessed heterogeneity by inspecting the forest plots to detect non‐overlapping CIs, while applying the Chi2 test with a P value of 0.10 indicating statistical significance. We also implemented the I2 statistic, with a value of 50% denoting moderate levels of heterogeneity and above 50% indicating a substantial level of heterogeneity (Deeks 2017).
Assessment of reporting biases
If we had been able to meta‐analyse sufficient data (10 studies or more), we planned to assess reporting bias among the studies using the funnel plot method discussed in the Cochrane Handbook for Systematic Reviews of Interventions (Sterne 2017). If asymmetry was noted, we planned to explore possible causes, including publication bias, poor methodological quality and true heterogeneity.
Data synthesis
We used Cochrane's statistical package, Review Manager 5, to combine outcomes when possible (Review Manager 2014). We used the random‐effects method, which considers that different studies are estimating different, yet related, intervention effects (DerSimonian 1986). For studies with more than two arms, we split the control group to avoid double counting.
Subgroup analysis and investigation of heterogeneity
If we were able to combine sufficient data and identify substantial heterogeneity (an I2 statistic value greater than 50%), we planned to conduct the following subgroup analyses.
Degree of asthma severity
Age groups (adult versus elderly)
Duration of treatment
Type of breathing exercise
Sensitivity analysis
If we had been able to combine sufficient data, sensitivity analysis would have been performed to explore the influence on the results of the following factors.
Study quality (RCTs with poor methodology)
Study size (stratified by sample size)
Allocation concealment (high risk of bias versus low risk of bias versus unclear risk of bias)
Assessor blinding (high risk of bias versus low risk of bias versus unclear risk of bias)
Summary of findings and assessment of the certainty of the evidence
We created a 'Summary of findings' table that included the following outcomes, according to the methods described in Chapter 11 of the Cochrane Handbook for Systematic Reviews of Interventions: change in quality of life measured by the Asthma Quality of Life Questionnaire (AQLQ), change in asthma symptoms measured by the Asthma Control Questionnaire (ACQ), change in hyperventilation symptoms measured by the Nijmegen Questionnaire, number of acute exacerbations, inpatient hospitalisation episodes, and lung function (Forced Expiratory Volume in 1 second (FEV1)), and days off work.
We determined the quality of the evidence using the GRADE approach and GRADEproGDT software (GRADEpro GDT). We justified all decisions to downgrade the quality of studies in footnotes, and made comments to aid readers' understanding of the review where necessary.
Results
Description of studies
Results of the search
The previous version of the review (Freitas 2013), included 13 studies (Nagarathna 1985; Girodo 1992; Fluge 1994; Vedanthan 1998; Thomas 2003; Holloway 2007; Sodhi 2009; Thomas 2009; Vempati 2009; Grammatopoulou 2011; Bidwell 2012; Singh 2012; Prem 2013). For this 2019 update, the most recent search was 4 April 2019. The database searching returned 245 references, resulting in 176 references after removing duplicates. Four additional references were obtained by searching other sources, resulting in 180 references. Of these, we identified 19 as potentially relevant, and we retrieved the full‐text articles for closer inspection. We added 11 of these (relating to nine studies) as new additions in the 2019 update (Aggarwal 2013; Gupta 2015; Prasanna 2015; Agnihotri 2016; Satpathy 2016; Thomas 2017; Agnihotri 2018; Malarvizhi 2018; Pushpa 2018). See 'Figure 1' for full details on the results of the search.
1.
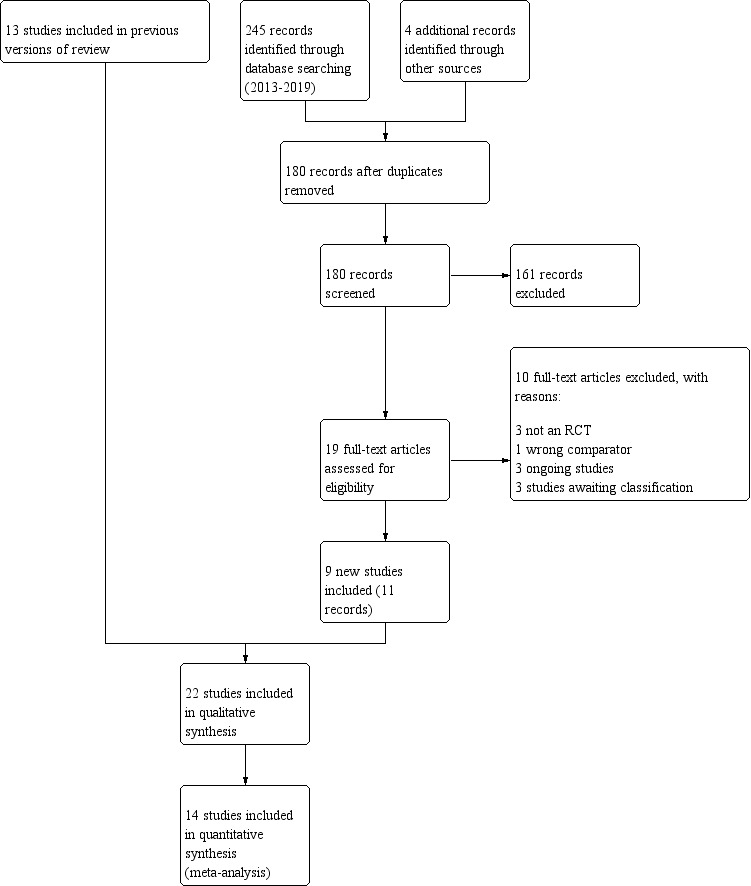
Study flow diagram
Included studies
In total, 22 studies are included in this review (Nagarathna 1985; Girodo 1992; Fluge 1994; Vedanthan 1998; Thomas 2003; Holloway 2007; Sodhi 2009; Thomas 2009; Vempati 2009; Grammatopoulou 2011; Bidwell 2012; Singh 2012; Aggarwal 2013; Prem 2013; Gupta 2015; Prasanna 2015; Agnihotri 2016; Satpathy 2016; Thomas 2017; Agnihotri 2018; Malarvizhi 2018; Pushpa 2018). Of these, two included studies have one additional report that was collected under a single study ID (Sodhi 2009; Thomas 2017). Therefore, we included 22 studies reported in 24 reports. We included 14 studies in the quantitative synthesis (Vedanthan 1998; Holloway 2007; Sodhi 2009; Thomas 2009; Vempati 2009; Grammatopoulou 2011; Bidwell 2012; Singh 2012; Aggarwal 2013; Prem 2013; Gupta 2015; Agnihotri 2016; Thomas 2017; Pushpa 2018). See 'Characteristics of included studies' for full details on each study and Table 2 for an overview of the characteristics of the included studies.
1. Characteristics of the included studies.
| Study (country) | N randomised | Intervention | Comparator | Outcomes |
Duration of intervention |
Follow‐up | Participants' baseline characteristics | ||
| Mean agea (years) | Male (%) | Asthma severity | |||||||
| Aggarwal 2013 (India) | 100 | Yoga | Inactive control (not practising yoga) | LF | 3 months | NF | NR | NR | Mild to moderate persistent |
| Agnihotri 2016 (India) | 276 | Yoga | Usual care | LF | 6 months | NF | NR | NR | Mild to moderate persistent |
| Agnihotri 2018 (India) | 300 | Yoga | Usual care | QoL | 6 months | NF | NR | NR | Mild to moderate persistent |
| Bidwell 2012 (USA) | 19 | Yoga | Inactive control (not practising yoga or any breathing exercise) | QoL, LF | 10 weeks | NF | 40.0 to 43.0 | 0 | Mild to moderate |
| Fluge 1994 (Germany) | 36 | Yoga | Inactive control (no additional treatment) | LF | 3 weeks | NF | 48.8 | 38.9 | Mild |
| Girodo 1992 (Canada) | 55 | Deep diaphragmatic breathing | Inactive control (waiting list) | Asthma symptoms | 16 weeks | 8 weeks | 28.6 to 32.9 | 40.0 | NR |
| Grammatopoulou 2011 (Greece) | 40 | Breathing retraining | Inactive control (no additional treatment) | QoL, asthma control, hyperventilation symptoms, LF, capnography | 6 months | NF | 45.4 to 48.1 | 57.5 | Mild to moderate |
| Gupta 2015 (India) | 100 | Yoga | Usual care | LF | 3 months | NF | NR | NR | NR |
| Holloway 2007 (UK) | 85 | Papworth | Usual care | QoL, hyperventilation symptoms, LF, capnography | 6 months | 6 months | 49.3 to 50.2 | 42.3 | Mild to moderate |
| Malarvizhi 2018 (India) | 250 | Yoga | Usual care | QoL | 6 months | NF | NR | 55.6 | Mild to moderate |
| Nagarathna 1985 (India) | 106 | Yoga | Usual care | LF, exacerbations | 54 months | NF | 26.4 | ||
| Prasanna 2015 (India) | 100 | Buteyko | Usual care | LF, asthma symptoms | 2 months | NF | 37.4 to 40.4 | 38.0 | NR |
| Prem 2013 (India) | 120 | Buteyko and pranayama | Usual care | QoL, asthma symptoms, LF | 3 months | NF | 35 to 41 | 39.2 | Mild to moderate |
| Pushpa 2018 (India) | 60 | Yoga | Usual care | LF | 8 weeks | NF | 31.0 to 32.7 | 33.3 | Mild to moderate |
| Satpathy 2016 (India) | 71 | Yoga | Usual care | Exacerbations, dyspnoea, asthma symptoms | 4 months | NF | 25.0 to 25.3 | 100.0 | Persistent, chronic |
| Singh 2012 (India) | 60 | Yoga | Inactive control (no additional treatment) | QoL, LF | 2 months | NF | NR | NR | Mild to moderate |
| Sodhi 2009 (India) | 120 | Yoga | Usual care | QoL, LF | 8 weeks | NF | 35.5 to 38.8 | 59.2 | Mild to moderate |
| Thomas 2003 (UK) | 33 | Breathing retraining | Asthma education | QoL, hyperventilation symptoms | 6 months | NF | 48.8 to 48.9 | 21.2 | NR |
| Thomas 2009 (UK) | 183 | Breathing retraining | Asthma education | QoL, asthma control, hyperventilation symptoms, LF, capnography | 6 months | NF | 46.0b | 45.9 | Mild to moderate |
| Thomas 2017 (UK) | 655 | Breathing retraining | Usual care | QoL, asthma control, airway inflammation, hyperventilation symptoms | 12 months | NF | 57.0 | 36.0 | Mild to moderate |
| Vedanthan 1998 (USA) | 17 | Yoga | Usual care | LF, asthma symptoms | 16 weeks | NF | 26.5 | 47.0 | Mild to moderate |
| Vempati 2009 (India) | 57 | Yoga | Usual care | QoL, LF | 8 weeks | NF | 33.4 to 33.5 | 36.8 | Mild to moderate |
| LF: lung function; N: number of participants; NF: no follow‐up; NR: not reported; QoL: quality of life | |||||||||
aRange across treatment arms reported where overall data were not reported. bMedian and IQR.
Setting and populations
Thirteen studies were conducted in India (Nagarathna 1985; Sodhi 2009; Vempati 2009; Singh 2012; Aggarwal 2013; Prem 2013; Gupta 2015; Prasanna 2015; Agnihotri 2016; Satpathy 2016; Agnihotri 2018; Malarvizhi 2018; Pushpa 2018), one in Canada (Girodo 1992), one in Germany (Fluge 1994), four in the UK (Thomas 2003; Holloway 2007; Thomas 2009; Thomas 2017), two in the USA (Vedanthan 1998; Bidwell 2012) and one in Greece (Grammatopoulou 2011). All papers were written in English with the exception of Fluge 1994, which was written in German. Seven studies were conducted between 2014 and 2019, 11 studies were conducted between 2003 and 2013, three were conducted between 1992 and 1998 and one was conducted in 1985. The studies varied in size from 17 to 655 participants. Participants in the included studies were older than 18 years of age, with the exception of Nagarathna 1985 (aged 9 to 47), Thomas 2003 (aged 17 to 65) and Holloway 2007 and Thomas 2017 (aged 16 to 70), Agnihotri 2016 and Agnihotri 2018 (aged 12 to 60). We included all studies as the mean age was over 18.
Interventions and control groups
In fourteen studies (Nagarathna 1985; Fluge 1994; Vedanthan 1998; Sodhi 2009; Vempati 2009; Bidwell 2012; Singh 2012; Aggarwal 2013; Gupta 2015; Agnihotri 2016; Satpathy 2016; Agnihotri 2018; Malarvizhi 2018; Pushpa 2018), participants undertook yoga exercises that involved pranayama breathing exercises as the major component, and the control groups did not undergo yoga training but continued taking their usual medication. In Nagarathna 1985, participants in the intervention group underwent training for two weeks and were told to practise these exercises for 65 minutes daily. In Fluge 1994, participants underwent 15 yoga sessions over three weeks. In Vedanthan 1998, yoga sessions were performed three times a week over 16 weeks. In Sodhi 2009, each yoga training session was of 45 minutes' duration per week with a trained instructor for a period of eight weeks. In Vempati 2009, the intervention consisted of two‐week supervised training in lifestyle modification and stress management based on yoga followed by closely monitored continuation of these practices at home for six weeks. In Bidwell 2012, yoga training consisted of two, one‐hour supervised yoga sessions per week for 10 weeks. In Singh 2012, participants attended yoga training provided by a yoga expert for five to six days. Thereafter, participants were told to practise yoga for an average of 40 to 50 minutes daily at home for two months. Participants were called to the yoga centre regularly (about every seven days) so investigators could see whether they were doing the yoga exercises properly. In Aggarwal 2013 and Gupta 2015, participants performed yoga for three months. In Satpathy 2016, participants in intervention group performed yogic exercises daily early in the morning under the guidance of a yoga instructor over four months. In Agnihotri 2016 and Agnihotri 2018, participants performed yoga sessions for 30 minutes per day, five days a week over six months. In Malarvizhi 2018, participants received 30 minutes of yoga training for a week under a trained yoga teacher and were advised to practise at home daily once a day for six months. In Pushpa 2018, participants practised yoga exercises for 45 minutes a day over two weeks and were instructed to practise at home for 45 minutes twice daily, regularly for the remaining six weeks and were instructed to maintain a diary record of each day of yoga practice.
In the Girodo 1992 study, participants undertook a 16‐week programme of deep diaphragmatic breathing exercises and were compared against a group of controls that were on a waiting list. Thomas 2003 compared participants who completed three short breathing retraining sessions (total contact time 75 minutes), taught by a physiotherapist, with a control group that received asthma education from a nurse. In Holloway 2007, the intervention group completed five 60‐minute individual sessions on the Papworth method provided by a respiratory physiotherapist. The control group received no additional treatment. In Thomas 2009, the breathing training group attended three sessions (one small group session and two individual sessions) that provided an explanation of normal breathing and possible effects of abnormal 'dysfunctional breathing'. During individual sessions, participants were taught diaphragmatic and nasal breathing techniques and were encouraged to practise these exercises for at least 10 minutes per day. The control group received three sessions of nurse‐provided asthma education. The intervention group in the Grammatopoulou 2011 study received 12 individual breathing retraining sessions, and the control group received usual asthma care. Prem 2013 divided 120 participants into three groups: Buteyko, yoga and control. Participants assigned to Buteyko or yoga groups received three to five days of sessions totaling 60 minutes each day. Participants in the control group followed routine physician care involving pharmacological management. Prasanna 2015 instructed participants to perform Buteyko breathing exercises at least twice a day for a period of two months, and the control group used only inhaled corticosteroids. Thomas 2017 divided participants into three groups: breathing retraining group using DVD and booklet format, face‐to‐face physiotherapy and a usual care group.
Outcomes
The primary outcome in seven studies (Holloway 2007; Thomas 2009; Bidwell 2012; Prem 2013; Thomas 2017; Agnihotri 2018; Malarvizhi 2018), was quality of life, although the studies used different instruments (SGRQ in Holloway 2007 and Bidwell 2012, AQLQ in Thomas 2009, Prem 2013, Thomas 2017, and Malarvizhi 2018, and MiniAQLQ in Agnihotri 2018). Asthma symptoms as measured by the Asthma Control Test (ACT) score were the main outcome in Grammatopoulou 2011. In Vempati 2009, pulmonary function was the primary outcome.
Lung function was the secondary outcome for Holloway 2007, Thomas 2009, Grammatopoulou 2011, and Thomas 2017. Asthma symptoms measured by the Asthma Control Questionnaire (ACQ) were a secondary outcome in Thomas 2009 and Thomas 2017. In addition, Thomas 2003, Thomas 2009, Holloway 2007 and Thomas 2017assessed hyperventilation symptoms by the Nijmegen Questionnaire. Grammatopoulou 2011 and Vempati 2009 assessed quality of life as a secondary outcome, measured by the Short Form (SF)‐36 v2 Health Survey and by the AQLQ, respectively.
The other included studies did not specify primary and secondary outcomes, but the study authors reported several main outcome measures, including pulmonary function (Fluge 1994; Aggarwal 2013; Gupta 2015; Agnihotri 2016; Pushpa 2018), asthma symptoms (Girodo 1992), number of acute exacerbations and pulmonary function (Nagarathna 1985), quality of life and asthma symptoms (Thomas 2003), asthma symptoms and lung function (Vedanthan 1998; Prasanna 2015), and lung function and quality of life (Sodhi 2009; Singh 2012).
Excluded studies
After we had retrieved the full text of potentially eligible studies, we excluded a total of 48 studies from the review. Two previously included studies had been excluded in the 2012 update (Bowler 1998; Opat 2000). We have described reasons for exclusion in the Characteristics of excluded studies.
Risk of bias in included studies
Full details of 'Risk of bias' judgments can be found in Characteristics of included studies and in Figure 2.
2.
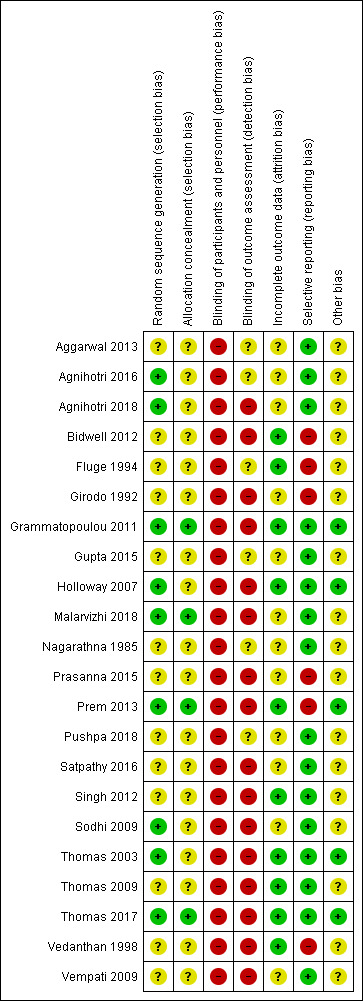
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Allocation
Nine studies reported adequate sequence generation and we judged them to have low risk of bias (Thomas 2003; Holloway 2007; Sodhi 2009Grammatopoulou 2011; Prem 2013; Agnihotri 2016; Thomas 2017; Agnihotri 2018; Malarvizhi 2018).
Thomas 2003 recruited individuals with high Nijmegen Questionnaire scores who were currently being treated for asthma at a general practice. They assigned volunteers randomly by numbering them alphabetically and using random number tables to assign them to study groups. Holloway 2007, undertook randomisation by a computer‐generated number sequence that assigned consecutive participant ID numbers a 1 or a 2 to denote intervention or a control condition. Sodhi 2009 randomised participants in two groups (Yoga and control) using permuted block randomisation. Grammatopoulou 2011 undertook random allocation with sealed envelopes. Prem 2013 assigned participants to three groups (Buteyko, yoga and control) through block randomisation. Agnihotri 2016 and Agnihotri 2018 undertook randomisation by a computer‐generated random number table. Thomas 2017 used the web based Tenalea randomisation system. Malarvizhi 2018 randomised participants by using a random allocation software.
Thirteen studies reported that they were randomised but gave no description of the methods used, and we therefore judged them to be at unclear risk of bias (Nagarathna 1985; Girodo 1992; Fluge 1994; Vedanthan 1998; Thomas 2009; Vempati 2009; Bidwell 2012; Singh 2012; Aggarwal 2013; Gupta 2015; Prasanna 2015; Satpathy 2016; Pushpa 2018).
Only four studies described adequate allocation concealment and we judged them to have low risk of bias (Grammatopoulou 2011; Prem 2013; Thomas 2017; Malarvizhi 2018). Grammatopoulou 2011 concealed allocation with sealed envelopes, and Prem 2013 concealed allocation in sequentially numbered, sealed, opaque envelopes. Thomas 2017 randomly allocated participants to one of the three study arms by using a telephone call service. Malarvizhi 2018 randomly allocated participants to one of the two study arms by using random allocation software. The other eighteen studies gave no description of the methods of allocation concealment used and we therefore judged them to have unclear risk of bias (Nagarathna 1985; Girodo 1992; Fluge 1994; Vedanthan 1998; Thomas 2003; Holloway 2007; Thomas 2009; Vempati 2009; Sodhi 2009; Bidwell 2012; Singh 2012; Aggarwal 2013; Gupta 2015; Prasanna 2015; Agnihotri 2016; Satpathy 2016; Agnihotri 2018; Pushpa 2018).
Blinding
Blinding of the investigator and the participant involved is not possible or practical in these studies. Participants in these studies must know whether or not they are undertaking breathing training or asthma education, as compliance is critical to the study; therefore we judged these studies to have a high risk of bias. However, it is possible to blind the assessor who is analysing the results of the study. One study reported that participants were blinded, however due to the type of intervention used in the study arms, it is likely that the blinding could have been broken. We therefore judged this study to have a high risk of bias (Malarvizhi 2018).
Only one study reported blinded outcome assessment, however the outcome was patient‐rated, which could introduce high risk of detection bias (Malarvizhi 2018). Six studies said that blinding was not possible; we judged these studies to have a high risk of bias, as we determined that the outcomes may have been influenced by the lack of blinding (Thomas 2003; Holloway 2007; Thomas 2009; Vempati 2009; Grammatopoulou 2011; Thomas 2017). Nine studies did not describe blinding and we considered the assessed outcomes to be patient‐rated, so we judged them to have a high risk of bias (Girodo 1992; Vedanthan 1998; Sodhi 2009; Bidwell 2012; Singh 2012; Prem 2013; Prasanna 2015; Satpathy 2016; Agnihotri 2018). Six studies did not report any procedures intended to blind the participants and outcome assessors (Nagarathna 1985; Fluge 1994; Aggarwal 2013; Gupta 2015; Agnihotri 2016; Pushpa 2018), so we judged them to have unclear risk of bias.
Incomplete outcome data
Seven studies did not describe the occurrence of withdrawals and dropouts and we judged them to be at unclear risk of bias (Girodo 1992; Sodhi 2009; Aggarwal 2013; Gupta 2015; Prasanna 2015; Satpathy 2016; Pushpa 2018). Nagarathna 1985 affirmed that in total 25 participants dropped out of the study, however, we judged this study to have an unclear risk of bias because it did not describe the reasons, nor how many participants dropped out of the study in each group (intervention and control). Three studies did not report any withdrawals or dropouts; we judged these studies to have a low risk of bias (Vedanthan 1998; Grammatopoulou 2011; Bidwell 2012). Seven studies described withdrawals and dropouts and we judged them to have a low risk of bias too, because the missing outcome data were balanced in numbers across intervention groups and the reasons were similar (Thomas 2003; Holloway 2007; Thomas 2009; Thomas 2017) or because the reasons for missing outcome data were unlikely to be related to true outcomes (Fluge 1994; Singh 2012; Prem 2013). Four studies described withdrawals and dropouts. However, the reasons for missing outcome data were not clearly described. Thus, we judged them to be at unclear risk of bias (Vempati 2009; Agnihotri 2016; Agnihotri 2018; Malarvizhi 2018).
Selective reporting
Two studies were registered on clinicaltrials.gov, another one on ISRCTN register, and all of the prespecified primary and secondary outcomes were reported in the prespecified way (Holloway 2007; Vempati 2009; Thomas 2017). We judged these studies to have a low risk of selective reporting bias. Thirteen studies adequately reported outcome data for all outcomes as listed in the methods and were therefore assessed as low risk of bias, although none of the protocols for these studies are available (Nagarathna 1985; Thomas 2003; Sodhi 2009; Thomas 2009; Grammatopoulou 2011; Singh 2012; Aggarwal 2013; Gupta 2015; Agnihotri 2016; Satpathy 2016; Agnihotri 2018; Malarvizhi 2018; Pushpa 2018). We judged six studies to be at high risk of bias because they reported one or more outcomes of interest in the review incompletely (Girodo 1992; Fluge 1994; Vedanthan 1998; Bidwell 2012; Prem 2013; Prasanna 2015).
Other potential sources of bias
We were unable to identify any other potential biases in five studies (Thomas 2003; Holloway 2007; Grammatopoulou 2011; Prem 2013; Thomas 2017). We judged 17 studies to be at unclear risk of bias, as they did not provide sufficient information to allow assessment of whether an important risk of bias is present (Nagarathna 1985; Girodo 1992; Fluge 1994; Vedanthan 1998; Sodhi 2009; Thomas 2009; Vempati 2009; Bidwell 2012; Singh 2012; Aggarwal 2013; Gupta 2015; Prasanna 2015; Agnihotri 2016; Satpathy 2016; Agnihotri 2018; Malarvizhi 2018; Pushpa 2018).
Effects of interventions
See: Table 1
The included studies had different durations, with multiple time points. So, to deal with methodological differences, we performed the meta‐analysis for the assessed outcomes by pooling the available data by the last time point of assessments after their baseline. For all outcomes, we pooled data at up to three months, from four to six months, and over six months.
Breathing exercises versus inactive control
Primary outcome: quality of life
Ten studies involving 1706 participants reported quality of life (Holloway 2007; Sodhi 2009; Vempati 2009; Grammatopoulou 2011; Bidwell 2012; Singh 2012; Prem 2013; Thomas 2017; Agnihotri 2018; Malarvizhi 2018). We included six of these studies in meta‐analysis (Holloway 2007; Vempati 2009; Bidwell 2012; Prem 2013; Thomas 2017; Agnihotri 2018).
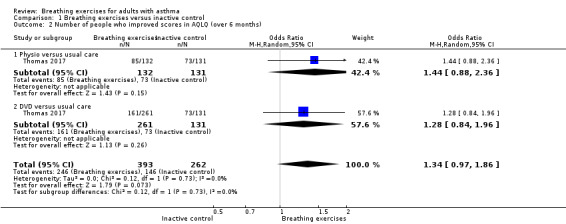
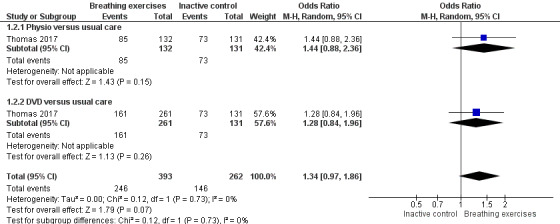
For the outcome 'Change in AQLQ', which included Vempati 2009, Prem 2013, Thomas 2017 and Agnihotri 2018, meta‐analysis showed significant differences favouring the intervention group (MD 0.42, 95% CI 0.17 to 0.68; 4 studies, 974 participants; Analysis 1.1; Figure 3), however we observed substantial heterogeneity (Chi2 = 20.85, df = 5 (P = 0.0009); I2 = 76%). Thomas 2017 reported that 64.4% of participants in the physiotherapy group, 61.7% in the DVD group and 55.7% in the control group reported a clinically significant improvement on AQLQ assessment after six months, higher than the minimal clinically important difference (MCID) of 0.5 (Juniper 2004). However, due to an improvement in both the intervention and control groups, there was uncertainty in the between‐group difference (OR 1.34, 95% CI 0.97 to 1.86, P = 0.07; 1 study, 655 participants; Analysis 1.2; Figure 4).
1.1. Analysis.
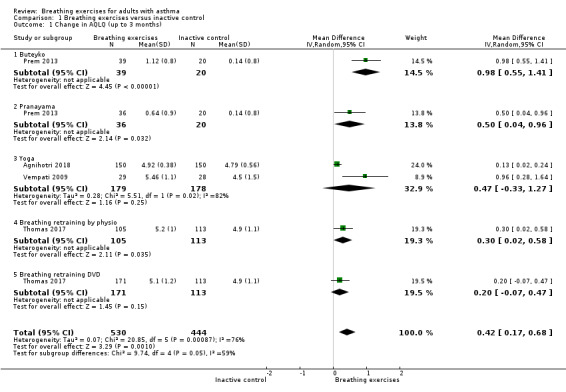
Comparison 1 Breathing exercises versus inactive control, Outcome 1 Change in AQLQ (up to 3 months).
3.
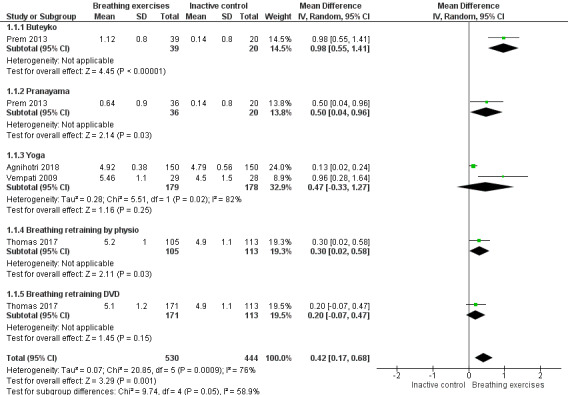
Forest plot of comparison 1. Breathing exercises versus inactive control, outcome 1.1: change in AQLQ (up to 3 months)
1.2. Analysis.
Comparison 1 Breathing exercises versus inactive control, Outcome 2 Number of people who improved scores in AQLQ (over 6 months).
4.
Forest plot of comparison 1. Breathing exercises versus inactive control, outcome 1.2: change in AQLQ (over 6 months)
Thomas 2017 also reported the number needed to treat for one participant to have a clinically relevant improvement (NNTB: number needed to treat for an additional beneficial outcome) in quality of life of 7 and 8 for the physiotherapy and DVD arm, respectively, compared with the usual‐care arm.
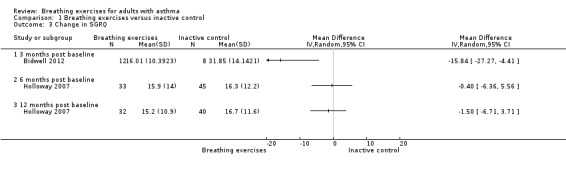
The outcome 'Change in SGRQ', included Holloway 2007 and Bidwell 2012 in two different time points (six months and three months, respectively). However, due to the high heterogeneity, we could not pool data. Higher scores in SGRQ indicate worse quality of life and a change of 4 units was established as a MCID (Jones 2002). In Bidwell 2012, the mean score change after three months from baseline was very large and was higher than the MCID (16.01 units in the breathing exercises group and 31.85 units in the control group). In order to visualise the data, we included the second time point of 12 months, evaluated in Holloway 2007, in the graphic and it shows a tendency to an improvement of quality of life favouring the breathing exercises group (Analysis 1.3).
1.3. Analysis.
Comparison 1 Breathing exercises versus inactive control, Outcome 3 Change in SGRQ.
We were not able to include the other four studies in the meta‐analysis (Sodhi 2009; Grammatopoulou 2011; Singh 2012; Malarvizhi 2018). Of these, Sodhi 2009 found improvement in three domains (symptoms, activities and environment) and in total scores of the AQLQ in the yoga group compared with the control group (P < 0.01). Grammatopoulou 2011 showed that the group that performed breathing exercises improved the physical component of the SF‐36 quality‐of‐life questionnaire compared with controls in all assessments (two, three and six months after intervention, with P value of 0.003, 0.0002 and 0.066, respectively). Singh 2012 observed a significant difference favouring the group submitted to the intervention, with P < 0.001 for all four domains of the AQLQ. Agnihotri 2018 reported AQLQmini at six months as well as three months, but we could not combine data from Agnihotri 2018 with Thomas 2017 at six months. Agnihotri 2018 found an improvement between breathing exercise and control group (mean 5.72 (standard deviation (SD) 0.38) and mean 5.43 (SD 0.34) respectively). Finally, we could not include Malarvizhi 2018 in the meta‐analysis due to a discrepancy between the graphical presentation and the data provided by correspondence.
Secondary outcome: asthma symptoms and hyperventilation symptoms
Six studies involving 1055 participants reported asthma symptoms (Girodo 1992; Vedanthan 1998; Prem 2013; Prasanna 2015; Satpathy 2016; Thomas 2017). Of these, two studies used the Asthma Control Questionnaire (ACQ) (Prem 2013; Thomas 2017), and we included them in the meta‐analysis. Three studies involving 780 participants reported hyperventilation symptoms (Holloway 2007; Grammatopoulou 2011; Thomas 2017), by using the Nijmegen Questionnaire.
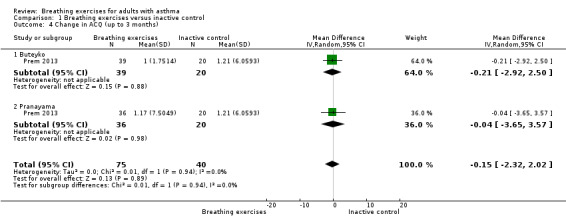
For the outcome 'Change in ACQ', we performed meta‐analysis including data from up to three months and over six months. However, we included only one study for each analysis (Prem 2013 and Thomas 2017, respectively); note that each study has three arms. The differences between the intervention and control groups from up to three months (MD −0.15, 95% CI −2.32 to 2.02; 1 study, 115 participants; Analysis 1.4), and over six months (MD −0.08, 95% CI −0.22 to 0.07; 1 study, 449 participants; Analysis 1.5) were both uncertain.
1.4. Analysis.
Comparison 1 Breathing exercises versus inactive control, Outcome 4 Change in ACQ (up to 3 months).
1.5. Analysis.
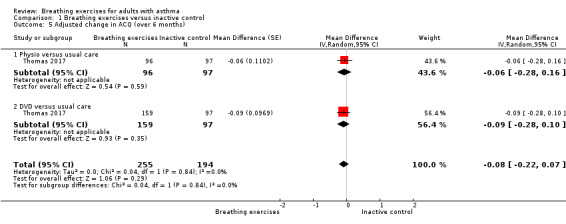
Comparison 1 Breathing exercises versus inactive control, Outcome 5 Adjusted change in ACQ (over 6 months).
For the outcome 'Change in Nijmegen', we performed meta‐analysis including data from four to six months (Holloway 2007; Grammatopoulou 2011), and over six months (Holloway 2007; Thomas 2017). Meta‐analysis showed differences favouring the intervention group for hyperventilation symptoms from baseline to four to six months (MD −3.22, 95% CI −6.31 to −0.13; 2 studies, 118 participants; Analysis 1.6). For over six months, improvement was not shown (MD 0.63, 95% CI −0.90 to 2.17; 2 studies, 521 participants; Analysis 1.7).
1.6. Analysis.
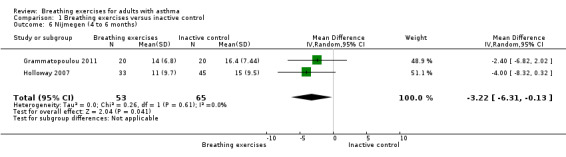
Comparison 1 Breathing exercises versus inactive control, Outcome 6 Nijmegen (4 to 6 months).
1.7. Analysis.
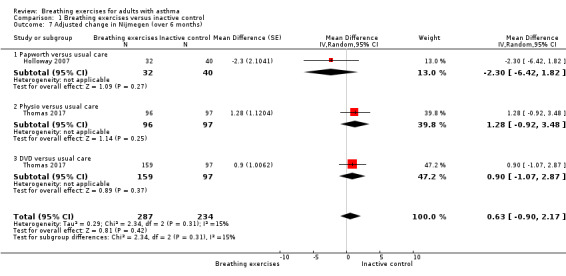
Comparison 1 Breathing exercises versus inactive control, Outcome 7 Adjusted change in Nijmegen (over 6 months).
Four studies (Girodo 1992; Vedanthan 1998; Prasanna 2015; Satpathy 2016), did not report sufficient data to enter the meta‐analysis. Of these, one study reported no clear difference in asthma symptoms between yoga and control groups mean 7.0 (SD 10.16) and mean 1.75 (SD 24.24); P > 0.05 (Vedanthan 1998). Girodo 1992 did not observe significant changes in frequency of symptoms on the Asthma Symptom Checklist in any group. Prasanna 2015 reported improvement of asthma symptoms between the intervention and control groups (P < 0.05). Satpathy 2016 showed a difference in the proportion of participants, in both groups, who presented lower frequency of asthma symptoms assessed by the Asthma Symptom Score.
Secondary outcome: number of acute exacerbations
Four studies reported this outcome (Nagarathna 1985; Sodhi 2009; Satpathy 2016; Thomas 2017), however they did not report sufficient data to enter the meta‐analysis. Nagarathna 1985 showed a decrease in the number of exacerbations per week between intervention and control groups, with mean 0.83 (SD 2.49) and mean 2.1 (SD 2.7), (P < 0.005). Sodhi 2009 found a decrease in the number of acute exacerbations per week between intervention and control groups from baseline up to eight weeks, with mean 0.38 (SD 0.48) and mean 0.58 (SD 0.53); P < 0.05. Satpathy 2016 showed a decrease (P < 0.01) in the number of participants who had acute exacerbation between baseline and four months post‐intervention in the intervention group. Thomas 2017 assessed asthma exacerbations by the use of oral corticosteroids. Results from Thomas 2017 showed no statistically significant difference between the intervention and control groups. In Thomas 2017, the percentage of participants of the physiotherapy, DVD and control groups that showed more than one oral corticosteroid courses (asthma exacerbations) after 12 months was 11.36%, 9.20% and 14.89%, respectively.
Secondary outcome: physiological measures
Sixteen studies involving 1951 participants assessed lung function (Nagarathna 1985; Fluge 1994; Vedanthan 1998; Holloway 2007; Sodhi 2009; Vempati 2009; Grammatopoulou 2011; Bidwell 2012; Singh 2012; Aggarwal 2013; Prem 2013; Gupta 2015; Prasanna 2015; Agnihotri 2016; Thomas 2017; Pushpa 2018). Of these, we included 11 studies in meta‐analysis (Vedanthan 1998; Holloway 2007; Sodhi 2009; Vempati 2009; Singh 2012; Aggarwal 2013; Prem 2013; Gupta 2015; Agnihotri 2016; Thomas 2017; Pushpa 2018). Five studies did not report sufficient data to enter the meta‐analysis (Nagarathna 1985; Fluge 1994; Grammatopoulou 2011; Bidwell 2012; Prasanna 2015).
For FEV1 (L) measured at up to three months, we observed no clear difference between intervention and control groups (MD −0.10 L, 95% CI −0.32 to 0.12; I2 = 61%; 4 studies, 252 participants; Analysis 1.8). Although the MCID in FEV1 has not been rigorously established for asthma, the magnitude of the MD found of 0.1 L is likely to have a clinical relevance (Enright 2004; Tepper 2012). For FEV1 % of predicted, we found a difference favouring the intervention group (MD 6.88 % predicted, 95% CI 5.03 to 8.73; 5 studies, 618 participants; Analysis 1.9). When measured at over six months, no clear differences were observed in FEV1 in litres (MD −0.02 L, 95% CI −0.08 to 0.04; 2 studies, 491 participants; Analysis 1.10), and in % of predicted (MD 0.49 % predicted, 95% CI −2.48 to 3.46; 1 study, 424 participants; Analysis 1.11).
1.8. Analysis.
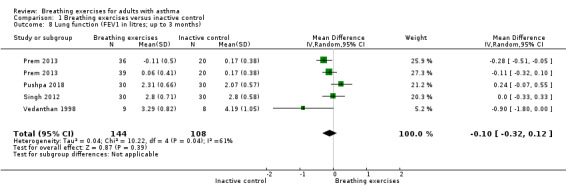
Comparison 1 Breathing exercises versus inactive control, Outcome 8 Lung function (FEV1 in litres; up to 3 months).
1.9. Analysis.
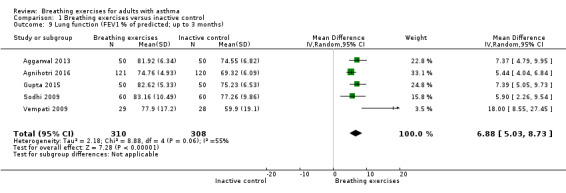
Comparison 1 Breathing exercises versus inactive control, Outcome 9 Lung function (FEV1 % of predicted; up to 3 months).
1.10. Analysis.
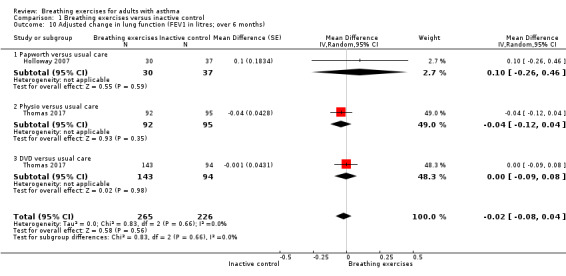
Comparison 1 Breathing exercises versus inactive control, Outcome 10 Adjusted change in lung function (FEV1 in litres; over 6 months).
1.11. Analysis.
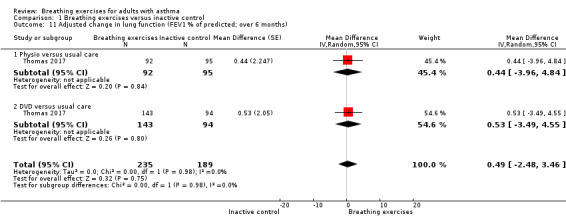
Comparison 1 Breathing exercises versus inactive control, Outcome 11 Adjusted change in lung function (FEV1 % of predicted; over 6 months).
For the outcome peak expiratory flow rate, measured at up to three months, we observed a difference favouring the intervention group, however there was substantial heterogeneity among the studies (Analysis 1.12). We were able to perform a meta‐analysis including two studies with data measured at over six months (Holloway 2007; Thomas 2017). Results from this analysis showed no clear difference favouring the intervention group comparing to control group (MD −1.07, 95% CI −14.89 to 12.74; 2 studies, 491 participants; Analysis 1.13).
1.12. Analysis.
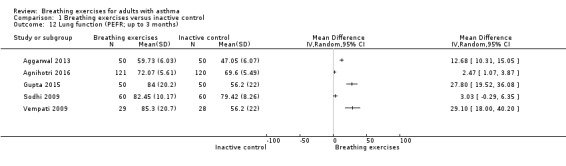
Comparison 1 Breathing exercises versus inactive control, Outcome 12 Lung function (PEFR; up to 3 months).
1.13. Analysis.
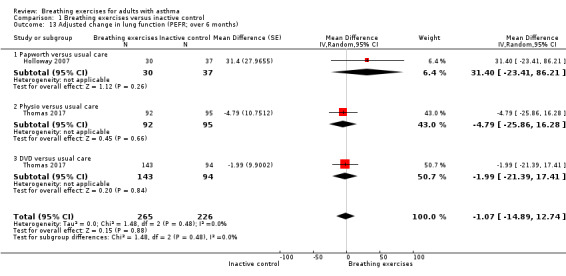
Comparison 1 Breathing exercises versus inactive control, Outcome 13 Adjusted change in lung function (PEFR; over 6 months).
Two studies also assessed capnography (Holloway 2007; Grammatopoulou 2011). Holloway 2007 did not find differences between intervention and control groups regarding end‐tidal carbon dioxide. However, values for relaxed breathing rate over a 10‐minute period, in breaths per minute (bpm), showed better results in the intervention group than in the control group: mean 10.0 bpm (SD 3.0) and mean 15.3 bpm (SD 2.4); P < 0.001, at six months post‐baseline, respectively; and mean 9.6 bpm (SD 3.7) and mean 15.3 bpm (SD 2.7); P < 0.001 at 12 months post‐baseline assessment, respectively. In Grammatopoulou 2011, the intervention group compared with the control group showed increased end‐tidal carbon dioxide (mean 37.95 mmHg (SD 2.70) and mean 34.90 mmHg (SD 2.91); P = 0.002 for one month post baseline; mean 38.50 mmHg (SD 1.88) and mean 35.15 mmHg (SD 2.58); P < 0.0001 for two months post‐baseline; and mean 37.90 mmHg (SD 3.54) and mean 34.60 mmHg (SD 2.91); P = 0.003 for six months post‐baseline. The intervention group showed a decreased respiratory rate compared with the control group (P < 0.0001) in all time point assessments.
One study (Thomas 2017), also assessed airway inflammation by the fraction of exhaled nitric oxide (FeNO) measured in parts per billion (ppb). In Thomas 2017, there was no clear difference in the comparison between physiotherapy and control groups regarding baseline and 12‐month values (P = 0.28; median 19 ppb (interquartile range (IQR) 13 to 33) and median 20 ppb (IQR 13 to 31). However, a small difference was observed between DVD and control groups (P = 0.02; median 20 ppb (IQR 13 to 33) and median 20 ppb (IQR 13 to 31), respectively).
Secondary outcomes: general practitioner (GP) appointments
Only one study (Thomas 2017), assessed GP appointments, from baseline to a 12‐month follow‐up. Thomas 2017 found no clear difference in consultation rates between the physiotherapy intervention group (P = 0.87) or the DVD intervention group than the control group (P = 0.69).
Secondary outcomes: inpatient hospitalisation episodes, days off work and subjective evaluation of the intervention
None of the included studies reported these outcomes.
Breathing exercises versus asthma education
Primary outcome: quality of life
Two studies involving 216 participants assessed this outcome (Thomas 2003; Thomas 2009). Both studies had follow‐up periods of one and six months.
Thomas 2003 showed that the median (IQR) changes in overall asthma quality‐of‐life score at one month were 0.6 (IQR 0.05 to 1.12) and 0.09 (IQR −0.25 to 0.26) for the breathing retraining and education groups, respectively (P = 0.018) after one month. After six months, only the improvement in the activities domain of the AQLQ was clearly greater in the breathing retraining than in the education group (0.83 (IQR −0.10 to 1.71) and −0.05 (IQR −0.74 to 0.34), P = 0.018).
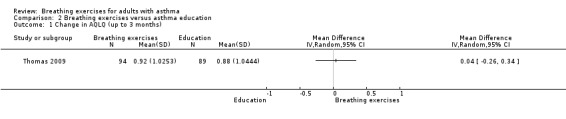
Thomas 2009 showed no clear between‐group differences in four subdomains, symptoms (MD 0.18, 95% −0.19 to 0.55, P = 0.34), activities (MD 0.10, 95% CI −0.22 to 0.43, P = 0.53), emotion (MD −0.07, 95% CI −0.46 to 0.32, P = 0.72), and environment (MD −0.10, 95% CI −0.46 to 0.25, P = 0.56), and for the total score of the AQLQ at up to three months' assessment (MD 0.04, 95% CI −0.26 to 0.34; 1 study, 183 participants; Analysis 2.1). From four to six months, there were greater improvements in the intervention group in terms of the subdomains of symptoms (P = 0.01), activities (P = 0.01) and emotions (P = 0.05) but not in the environment subdomain (P = 0.40) compared with controls, with a between‐group difference favouring the intervention group (P = 0.01) for the total score (MD 0.38, 95% CI 0.08 to 0.68; 1 study, 183 participants; Analysis 2.2).
2.1. Analysis.
Comparison 2 Breathing exercises versus asthma education, Outcome 1 Change in AQLQ (up to 3 months).
2.2. Analysis.
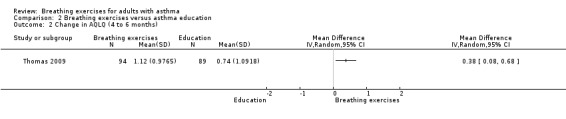
Comparison 2 Breathing exercises versus asthma education, Outcome 2 Change in AQLQ (4 to 6 months).
Secondary outcome: asthma symptoms and hyperventilation symptoms
One study involving 183 participants assessed asthma symptoms (Thomas 2009). Thomas 2009 carried out assessment of symptoms at baseline and one month and six months after the intervention which was inconclusive for the ACQ (MD −0.17, 95% CI −0.38 to 0.04, P = 0.12).
Two studies, involving 216 participants, used the Nijmegen Questionnaire to assess hyperventilation symptoms (Thomas 2003; Thomas 2009). In Thomas 2003, the between‐group difference favouring the intervention was statistically significant only after six months (median −9.50 (IQR −11.75 to 0), 1.00 (IQR −5.75 to 2), P = 0.01, breathing exercise and education group, respectively). We could not use data from Thomas 2003 in the meta‐analysis because only median and interquartile range were available. Thomas 2009 found no clear difference favouring the intervention group up to three months (MD −1.24, 95% CI −3.23 to 0.75; 1 study, 183 participants; Analysis 2.3), whereas a difference favoured the intervention group from four to six months (MD −3.16, 95% CI −5.35 to −0.97; 1 study; 183 participants; Analysis 2.4).
2.3. Analysis.
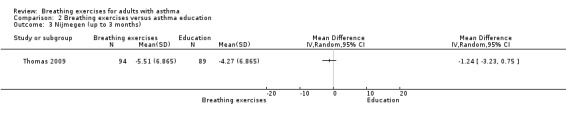
Comparison 2 Breathing exercises versus asthma education, Outcome 3 Nijmegen (up to 3 months).
2.4. Analysis.
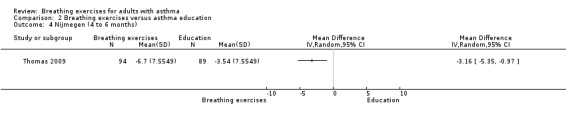
Comparison 2 Breathing exercises versus asthma education, Outcome 4 Nijmegen (4 to 6 months).
Secondary outcome: physiological measures
Only one study assessed spirometric values (Thomas 2009). This study assessed FEV1 (L) and found no clear difference (MD −0.06 L, 95% CI −0.13 to 0.00; P = 0.07) between the intervention and control groups.
Thomas 2009 also assessed resting end‐tidal carbon dioxide concentration, showing that values for this outcome were uncertain within and between groups (MD 0.08 mmHg, 95% CI −0.15 to 0.30; P = 0.51).
Secondary outcomes: numbers of acute exacerbations, inpatient hospitalisation episodes, general practice (GP) appointments, days off work, and subjective evaluation of the intervention
Neither of these two studies reported these outcomes.
Discussion
Summary of main results
This Cochrane Review assessed available evidence for the efficacy of breathing exercises in the treatment of adults with asthma. A total of 22 studies involving 2880 participants satisfied the inclusion criteria. Although these studies met the inclusion criteria, they differed in terms of intervention characteristics, such as type of breathing exercise, number of participants, number and duration of sessions, reported outcomes and statistical presentation of data. These differences limited the inclusion of several studies in our meta‐analyses.
We found a probable effect favouring the breathing exercises over inactive control in quality of life assessed by AQLQ up to three months. The mean difference for AQLQ showed in this review was slightly lower than the MCID of 0.5 (Juniper 2004). For asthma symptoms, we found no clear difference between the breathing exercises and control group. However, the very large confidence interval includes both clinically relevant benefits and harm. We also found a probable effect for the breathing exercises over inactive control and asthma education in symptoms of hyperventilation, measured by the Nijimegen Questionnaire from four to six months. However, the MCID has not been established for the Nijmegen Questionnaire (van Dixhoorn 2015). For lung function parameter, we found a possible effect for the breathing exercises over inactive control in FEV1 % of predicted up to three months. No clear effect was found between breathing exercises and inactive control in FEV1 (L) up to three months.
Overall completeness and applicability of evidence
Despite the broad spectrum of breathing exercises, the findings of this review were based on the techniques that were used in the included studies, which were some of the most commonly used techniques. Those studies included the Papworth method, Buteyko, diaphragmatic breathing, yoga and breathing retraining exercises. We found that breathing exercises showed some probable improvements in quality of life and hyperventilation symptoms. The types of delivery differed between face‐to‐face or by using an audio‐visual media self‐guided programme. The self‐guided programme is convenient and low‐cost (Arden‐Close 2017). Some included studies involved group sessions in which participants were able to talk to each other and share their experiences. This can also be considered as a therapeutic procedure that may affect the sensation of well‐being (Evans 1993). Awareness of participation in the study, the sensation of increased care and cure and the specialists' recommendations to continue regular asthma medication, and educational approaches are characteristics that must be considered when the findings of an experimental study are interpreted (Grammatopoulou 2011).
Moreover, asthma severity of participants from the included studies ranged from mild to moderate, so it was not possible to assess the effects of breathing exercises on participants with severe asthma. The samples from studies consisted solely of outpatients. Besides that, three of the eight outcomes proposed by this review were not addressed: inpatient hospitalisation episodes, days off work and participants' subjective evaluation of the intervention.
Certainty of the evidence
The certainty of the evidence ranges from moderate to very low according to GRADE criteria and we have presented our GRADE judgements in the 'Summary of findings' table. We downgraded the evidence for quality of life due to one study being at high risk of bias for selective reporting. For the outcome asthma symptoms, we downgraded the certainty of evidence because we included in the analysis only one study with a small sample size, and that we considered to be at high risk of bias for selective reporting. We downgraded the certainty of evidence for symptoms of hyperventilation due to the small sample size and wide confidence interval presented. For the outcome lung function, we downgraded the certainty of evidence because the included studies showed in general a high risk of bias and small sample size.
Potential biases in the review process
Although we attempted to apply a systematic process for including and excluding studies in this review, alongside following the criteria prespecified in the protocol, with robust methods for data collection and 'Risk of bias' assessment, final decisions are open to interpretation or criticism. It is also not clear whether some of the participants in Agnihotri 2016 were included in the quality of life reporting in Agnihotri 2018, so although there is no risk of double counting individuals for the outcomes, the total number of individuals studied may have been overstated.
Incomplete outcome data may be considered a potential source of bias of this review. It is difficult to quantify the impact of this potential bias as we were unable to enter the data from these studies into a meta‐analysis. Also related to meta‐analysis, sensitivity analysis was not possible because we were unable to obtain sufficient data. This would have allowed us to investigate possible effect modifiers such as degree of asthma severity, age groups and duration of treatment. Moreover, sensitivity analysis could have identified the influence of some factors (such as study quality and study size) on the results; thus, revealing the source of the substantial heterogeneity that we found among studies on quality of life and lung function.
Agreements and disagreements with other studies or reviews
The current review update included nine new RCTs. Although there is a large number of available studies on this topic, it is important to emphasise that we found no recent or updated systematic review. A previous review performed by Bruurs 2013 assessed the effectiveness of breathing exercises as physiotherapy in the treatment of people with asthma. The outcomes assessed by Bruurs 2013 were based on those measures used in the Cochrane Reviews, Dennis 2000 and Holloway 2004, which they classified as subjective and objective patient‐relevant outcomes for asthma. Bruurs 2013 reported improvement in quality of life and symptoms, and reduction of medication use, but the breathing exercises did not affect lung function. The findings of this current review update, based on meta‐analysis results, are consistent with the improvement in asthma symptoms favouring the breathing exercises groups. However, the review of Bruurs 2013 presented some methodological differences, regarding the age of participants and the inclusion of other types of physiotherapy, such as inspiratory muscle training, physical exercises, and airway clearance techniques. Moreover, Bruurs 2013 did not assess the risk of bias, perform meta‐analysis or assess the quality of the evidence. One Cochrane Review that assessed the effects of yoga in people with asthma analysed data from 15 RCTs, where five of them included yogic breathing alone and the other studies assessed yoga interventions that included breathing exercises, posture, and meditation (Yang 2016). Similar to our findings, despite the limitations in the quality of the included studies, Yang 2016 found improvements in quality of life and symptoms of asthma.
Authors' conclusions
Implications for practice.
Breathing exercises may have positive effects on quality of life, hyperventilation symptoms, and lung function. Due to some methodological differences among included studies and studies with poor methodology, the certainty of evidence for the measured outcomes ranged from moderate to very low according to GRADE criteria. In addition, no data are available regarding the effects of breathing exercises on inpatient hospitalisation episodes, days off work and participants' subjective evaluation of the intervention.
Implications for research.
Well conducted randomised controlled trials are still needed to assess the clinical benefit of breathing exercises in the management of asthma, including people with severe asthma, and those outcomes that were not assessed by the studies included in this review such as inpatient hospitalisation episodes, days off work and participants' subjective evaluation of the intervention. It is also important to emphasise the need for studies with a clear report of the sample age, asthma severity, and a specific description of the breathing exercise used rather than the combined interventions. As the Nijimegen Questionnaire is an important outcome‐reported measurement instrument to assess hyperventilation symptoms, further research is needed to determine a minimal clinically important difference for this questionnaire in people with asthma.
Researchers are encouraged to conduct studies to investigate in the asthma population the effects of other interventions that were not covered in this review, such as pursed‐lip breathing. The effects of the breathing exercises on different mechanisms (hyperventilation, breathing pattern disorders, cognitive and emotional factors related to biochemical, biomechanical and psychophysiological dimensions of dysfunctional breathing in asthma) should also be investigated. Furthermore, in the future, much more attention needs to be paid to good reporting and high‐quality study design, including items such as adequate random sequence generation and allocation concealment, blinding of outcome assessor and determination of the study sample size before the study is begun.
What's new
| Date | Event | Description |
|---|---|---|
| 4 April 2019 | New citation required and conclusions have changed | Nine studies and 1910 participants added. The background and review text has been updated partially throughout. Added details of study funder to characteristics included studies. |
| 4 April 2019 | New search has been performed | Literature search run |
History
Protocol first published: Issue 4, 1998 Review first published: Issue 3, 2000
| Date | Event | Description |
|---|---|---|
| 30 January 2013 | New citation required and conclusions have changed | Eight new studies included; two formerly included studies excluded. New author team. Title changed to specify that the review pertains to adults only. Summary of findings table added. |
| 21 July 2008 | Amended | Converted to new review format |
| 16 September 2003 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The review authors would like to thank Emma Dennett (the Managing Editor of Cochrane Airways) for providing assistance throughout the review process and Elizabeth Stovold (the Information Specialist of Cochrane Airways) for performing the search. We acknowledge the assistance of Christopher Cates in relation to the statistical support.
We would also like to thank all the study authors who responded to our enquiries.
The current review authors would like to thank Elizabeth Holloway for the development of the original review and her clinical expertise, and Selma S Bruno for her previous input into the development of the 2013 update of this review.
The review authors and Cochrane Airways editorial team are grateful to the following peer reviewers for their time and comments:
Amanda Roberts, UK (consumer);
Eleanor Fairbank, UK (consumer);
Negar Jamshidi, RMIT University, Australia (consumer);
Johannes C van der Wouden, Amsterdam UMC, Vrije Universiteit, The Netherlands; and
Alice Jones, The University of Sydney and The University of Queensland, Australia.
The Background and Methods sections of this review are based on a standard template used by Cochrane Airways.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to Cochrane Airways. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health.
Appendices
Appendix 1. Description of terms
Buteyko method is a breathing exercise that uses a combination of slow and reduced‐volume breathing with breath control and breath‐holding exercises to normalise the breathing pattern (Bruton 2005; Courtney 2008). This method aims to help people normalise their breathing patterns and to avoid their tendency to hyperventilate (Austin 2013; Chaitow 2014).
Diaphragmatic breathing emphasises the contraction of the diaphragm, expansion of the belly, and a deep inhale and exhale of breath (Ma 2017). Initially, people should be trained in this technique by 'tactile stimulation' by placing the therapist's (or patient's) hand over the patient's abdomen and on the upper chest, and by 'visual stimulation' by looking at abdominal and chest movement. The patient should be in a comfortable and appropriate position, and the therapist must tell the patient to breathe deeply, and then exhale. During the inhale, the hand placed on the abdomen should rise. In the exhale, the patient should be instructed to actively contract their abdominal muscles. Pursed‐lip breathing can be included during the exhalation. Diaphragmatic breathing aims to improve ventilation and decrease the activity of the respiratory muscle by minimising the work of breathing (Watchie 2010; Bandy 2012). This technique is also referred to as belly, deep or abdominal breathing (Varvogli 2011; Ma 2017).
Papworth method is focused on a sequence of components that includes integrated breathing and relaxation exercises combined with education. This method aims to improve breathing patterns and minimise hyperventilation by using diaphragmatic breathing, with a slow nasal expiration (Holloway 2007).
Spirometry is a non‐invasive physiological test that measures the maximal volume of air inspired and expired with maximal effort (Graham 2019). The test can be performed by using a spirometer with different techniques. The person is asked to take a complete breath in, press their lips around a mouthpiece, then perform a full fast expiration, and follow with an inspiration. To ensure no air escape, a nose clip must be used. The spirometry test has been used to assess ventilatory function and as a tool to detect the presence of lung disease (Barreiro 2004; Moore 2012). The forced vital capacity (FVC) is the total volume of air exhaled during a forced and complete expiration. The expiratory volume in the first second (FEV1) is the volume of air exhaled in the first second under force after a maximal inhalation. The percentage of the FVC expired in one second is expressed as FEV1/FVC. The vital capacity (VC) is the total volume of air that can be exhaled as fast as possible. The forced expiratory flow (FEF), also known as mid‐expiratory flow, is the average flow that can be recorded at 25%, 50% and 75% of the FVC (Graham 2019).
Yoga has been described as techniques used for relaxation and is known as therapy for mind and body improvements (Bernardi 2000). Yoga includes a combination of body postures (asanas), breathing exercises (pranayamas or yogic breathing exercises), and meditation (dhyana) (Riley 2004; Hakked 2017). Many yoga breathing exercises have been described in the literature. These exercises can have different pace (rate and depth of respiration), alteration of nostrils or breath‐hold, and they also combine abdominal, thoracic and clavicular breathing phases (Muktibodhananda 2002; Lopes 2018).
Appendix 2. Sources and search methods for the Cochrane Airways Specialised Register (CAGR)
Electronic searches: core databases
| Cochrane Central Register of Controlled Trials (CENTRAL) via the Cochrane Register of Studies (CRS) | From inception | Monthly |
| MEDLINE (Ovid SP) | 1946 onwards | Weekly |
| Embase (Ovid SP) | 1974 onwards | Weekly |
| PsycINFO (Ovid SP) | 1967 onwards | Monthly |
| CINAHL (EBSCO) | 1937 onwards | Monthly |
| AMED (EBSCO) | From inception | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify studies for the Cochrane Airways Specialised Register
Asthma search
1. exp Asthma/
2. asthma$.mp.
3. (antiasthma$ or anti‐asthma$).mp.
4. Respiratory Sounds/
5. wheez$.mp.
6. Bronchial Spasm/
7. bronchospas$.mp.
8. (bronch$ adj3 spasm$).mp.
9. bronchoconstrict$.mp.
10. exp Bronchoconstriction/
11. (bronch$ adj3 constrict$).mp.
12. Bronchial Hyperreactivity/
13. Respiratory Hypersensitivity/
14. ((bronchial$ or respiratory or airway$ or lung$) adj3 (hypersensitiv$ or hyperreactiv$ or allerg$ or insufficiency)).mp.
15. ((dust or mite$) adj3 (allerg$ or hypersensitiv$)).mp.
16. or/1‐15
Filter to identify randomised controlled trials
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and randomised controlled trial filter are adapted to identify studies in other electronic databases.
Appendix 3. Search strategy to identify relevant studies from the Cochrane Airways Specialised Register
Via the Cochrane Register of Studies (CRS)
#1 AST:MISC1 #2 MeSH DESCRIPTOR Asthma Explode All #3 asthma*:ti,ab #4 #1 or #2 or #3 #5 MeSH DESCRIPTOR Breathing Exercises #6 (breath*) NEAR5 (technique* or exercise* or re‐train* or train* or re‐educat* or educat* or physiotherap* or "physical therapy" or "respiratory therapy") #7 buteyko or "qigong yangsheng" or pranayama* OR yoga* #8 "breathing control" #9 #5 or #6 or #7 or #8 #10 #4 and #9
In search line #1, MISC1 denotes the field in the record where the reference has been coded for condition, in this case, asthma.
Data and analyses
Comparison 1. Breathing exercises versus inactive control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in AQLQ (up to 3 months) | 4 | 974 | Mean Difference (IV, Random, 95% CI) | 0.42 [0.17, 0.68] |
| 1.1 Buteyko | 1 | 59 | Mean Difference (IV, Random, 95% CI) | 0.98 [0.55, 1.41] |
| 1.2 Pranayama | 1 | 56 | Mean Difference (IV, Random, 95% CI) | 0.5 [0.04, 0.96] |
| 1.3 Yoga | 2 | 357 | Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.33, 1.27] |
| 1.4 Breathing retraining by physio | 1 | 218 | Mean Difference (IV, Random, 95% CI) | 0.30 [0.02, 0.58] |
| 1.5 Breathing retraining DVD | 1 | 284 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.07, 0.47] |
| 2 Number of people who improved scores in AQLQ (over 6 months) | 1 | 655 | Odds Ratio (M‐H, Random, 95% CI) | 1.34 [0.97, 1.86] |
| 2.1 Physio versus usual care | 1 | 263 | Odds Ratio (M‐H, Random, 95% CI) | 1.44 [0.88, 2.36] |
| 2.2 DVD versus usual care | 1 | 392 | Odds Ratio (M‐H, Random, 95% CI) | 1.28 [0.84, 1.96] |
| 3 Change in SGRQ | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 3 months post baseline | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 6 months post baseline | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 12 months post baseline | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Change in ACQ (up to 3 months) | 1 | 115 | Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐2.32, 2.02] |
| 4.1 Buteyko | 1 | 59 | Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐2.92, 2.50] |
| 4.2 Pranayama | 1 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐3.65, 3.57] |
| 5 Adjusted change in ACQ (over 6 months) | 1 | 449 | Mean Difference (Random, 95% CI) | ‐0.08 [‐0.22, 0.07] |
| 5.1 Physio versus usual care | 1 | 193 | Mean Difference (Random, 95% CI) | ‐0.06 [‐0.28, 0.16] |
| 5.2 DVD versus usual care | 1 | 256 | Mean Difference (Random, 95% CI) | ‐0.09 [‐0.28, 0.10] |
| 6 Nijmegen (4 to 6 months) | 2 | 118 | Mean Difference (IV, Random, 95% CI) | ‐3.22 [‐6.31, ‐0.13] |
| 7 Adjusted change in Nijmegen (over 6 months) | 2 | 521 | Mean Difference (Random, 95% CI) | 0.63 [‐0.90, 2.17] |
| 7.1 Papworth versus usual care | 1 | 72 | Mean Difference (Random, 95% CI) | ‐2.3 [‐6.42, 1.82] |
| 7.2 Physio versus usual care | 1 | 193 | Mean Difference (Random, 95% CI) | 1.28 [‐0.92, 3.48] |
| 7.3 DVD versus usual care | 1 | 256 | Mean Difference (Random, 95% CI) | 0.9 [‐1.07, 2.87] |
| 8 Lung function (FEV1 in litres; up to 3 months) | 4 | 252 | Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.32, 0.12] |
| 9 Lung function (FEV1 % of predicted; up to 3 months) | 5 | 618 | Mean Difference (IV, Random, 95% CI) | 6.88 [5.03, 8.73] |
| 10 Adjusted change in lung function (FEV1 in litres; over 6 months) | 2 | 491 | Mean Difference (Random, 95% CI) | ‐0.02 [‐0.08, 0.04] |
| 10.1 Papworth versus usual care | 1 | 67 | Mean Difference (Random, 95% CI) | 0.1 [‐0.26, 0.46] |
| 10.2 Physio versus usual care | 1 | 187 | Mean Difference (Random, 95% CI) | ‐0.04 [‐0.12, 0.04] |
| 10.3 DVD versus usual care | 1 | 237 | Mean Difference (Random, 95% CI) | ‐0.00 [‐0.09, 0.08] |
| 11 Adjusted change in lung function (FEV1 % of predicted; over 6 months) | 1 | 424 | Mean Difference (Random, 95% CI) | 0.49 [‐2.48, 3.46] |
| 11.1 Physio versus usual care | 1 | 187 | Mean Difference (Random, 95% CI) | 0.44 [‐3.96, 4.84] |
| 11.2 DVD versus usual care | 1 | 237 | Mean Difference (Random, 95% CI) | 0.53 [‐3.49, 4.55] |
| 12 Lung function (PEFR; up to 3 months) | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 13 Adjusted change in lung function (PEFR; over 6 months) | 2 | 491 | Mean Difference (Random, 95% CI) | ‐1.07 [‐14.89, 12.74] |
| 13.1 Papworth versus usual care | 1 | 67 | Mean Difference (Random, 95% CI) | 31.4 [‐23.41, 86.21] |
| 13.2 Physio versus usual care | 1 | 187 | Mean Difference (Random, 95% CI) | ‐4.79 [‐25.86, 16.28] |
| 13.3 DVD versus usual care | 1 | 237 | Mean Difference (Random, 95% CI) | ‐1.99 [‐21.39, 17.41] |
Comparison 2. Breathing exercises versus asthma education.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in AQLQ (up to 3 months) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2 Change in AQLQ (4 to 6 months) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3 Nijmegen (up to 3 months) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Nijmegen (4 to 6 months) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aggarwal 2013.
| Methods | Design: RCT | |
| Participants | Country: India Setting: outpatient ‐ department of physiology at SN Medical College Health status: not described Diagnosis criteria: not described Total sample: 100 participants, 50 in each group Mean age, years: not described Age range, years: not described Gender: men or women Inclusion criteria: men or women aged between 20‐45 years; people with bronchial asthma practising pranayama with medical treatment; people with bronchial asthma under medical treatment but not practising pranayama Exclusion criteria: unwilling to participate in study; history of cigarette smoking, hypertension, diabetes and chronic chest infections like T.B. (X‐ ray and sputum examination done, if needed) and chest deformity |
|
| Interventions | Intervention group: pranayama (anuloma‐viloma and kapalbhati pranayama) for 3 months. Anuloma‐Viloma: Done 3‐3 round by both nostril and one round of anuloma‐viloma include: inhale through left nostril, closing the right with the thumb, to the count of 4; hold the breath, closing both nostrils to the count of 16; exhale through the right nostril, closing the left with the ring and little finger to the count of 8; inhale through the right nostril, closing the left nostril with ring and little finger to the count of 4; hold the breath, closing both nostrils, to the count of 16; exhale through the left nostril, keeping the right closed with the thumb, to the count of 8. Kapalbhati pranayama: also k/as breath of fire is used as a 'cleaning breathing exercise'. The technique uses a forced exhalation with the premise of ridding lower lungs of stale air allowing the intake of O2‐rich air, thereby purifying the body. Frequency of intervention: not described Control group: not practising pranayama |
|
| Outcomes | Lung function (FVC, FEV1, FEV1/FVC, PEFR) | |
| Notes | Funding: not described Register number: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This study has not reported any procedures intended to blind the participants and personnel, and blinding was probably not possible due to the nature of the intervention, so we judged it to have high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available, but the published reports included all expected outcomes, including those that were prespecified |
| Other bias | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
Agnihotri 2016.
| Methods | Design: RCT | |
| Participants | Country: India Setting: outpatient Health status: mild to moderate persistent bronchial asthma Diagnosis criteria: not described Total sample: 276 (138 in intervention group and 138 in control group) Mean age, years: not described Age range, years: 12‐60 Gender: not described Inclusion criteria: mild to moderate persistent bronchial asthma severity according to GINA 2009, with age ranging from 12‐60 years. They were nonsmokers or ex smokers who had not smoked for at least 6 months with reversible airflow limitation of > 12% and > 200 mL (post bronchodilator FEV1 > 12% and > 200 mL) Exclusion criteria: severe airflow limitation or more (FEV1 < 60%), pregnant or lactating women, any associated chronic respiratory diseases and having major psychiatric illnesses and current smokers |
|
| Interventions | Intervention group: participants in the yoga group received yogic intervention (asanas, pranayama, and meditation) for 30 min/day, 5 days/week for a period of 6 months along with standard medical treatment Control group: standard medical treatment |
|
| Outcomes | Lung function (FVC, FEV1, FEV1/FVC, PEFR) | |
| Notes | Funding: ICMR, New Delhi Register number: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation was undertaken by a computer‐generated random number table |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This study has not reported any procedures intended to blind the participants and personnel, and blinding was probably not possible due to the nature of the intervention, so we judged it to have high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing outcome data balanced in numbers across intervention groups, however the reasons for the missing data were not clearly described |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available, but the published reports include all expected outcomes |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Agnihotri 2018.
| Methods | Design: RCT | |
| Participants | Country: India
Setting: outpatient
Health status: mild‑to‑moderate persistent asthma (FEV1 > 60%) Total sample: 300 (150 in intervention group and 150 in control group) Mean age, years: not described Age range, years: 12‐60 Gender: not described Inclusion criteria: mild to moderate persistent bronchial asthma severity according to GINA, with age ranging from 12‐60 years. They were nonsmokers or ex smokers who had not smoked for at least 6 months with reversible airflow limitation of > 12% and > 200 mL (post bronchodilator FEV1 > 12% and > 200 mL). Exclusion criteria: severe airflow limitation or more (FEV1 > 60%), pregnant or lactating women, any associated chronic respiratory diseases and having major psychiatric illnesses and current smokers |
|
| Interventions | Intervention group: participants in the yoga group received yogic intervention (asanas, pranayama, and meditation) for 30 min/day, 5 days/week for a period of 6 months along with standard medical treatment Control group: standard medical treatment | |
| Outcomes | QoL (assessed by the MiniAQLQ at baseline and then after 3 and 6 months from baseline) | |
| Notes | Funding: ICMR, New Delhi Register number: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generation was undertaken by a computer‐generated random number table |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel, and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some outcomes were participant‐rated, which could introduce high risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing outcome data balanced in numbers across intervention groups, however the reasons for the missing data were not clearly described |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available, but the published reports include all expected outcomes |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Bidwell 2012.
| Methods | Design: RCT | |
| Participants | Country: USA
Setting: Human Performance Laboratory
Health status: mild to moderate asthma Diagnosis criteria: not described This trial included 2 arms (yoga and control group) Total sample: 19 female participants Mean age, years: 40 ± 4 (control group) and 43 ± 4 (yoga group) Age range, years: 20‐65 Gender: not described Inclusion criteria: not described Exclusion criteria: participants were excluded if they were smokers, participated in yoga therapy in the previous 12 months, were diagnosed as having hypertension or major orthopaedic injuries prohibiting the performance of various yoga postures and/or were currently taking any medications that would alter autonomic function |
|
| Interventions | Intervention group: yoga training consisted of 2 x 1‐h supervised yoga sessions/week for 10 weeks. Additionally, participants were required to perform 1 x 30‐min session/week at home, which was based on a written lesson plan (5 min of deep breathing, 20 min of asanas and 5 min of meditation and relaxation) Control group: participants were instructed not to participate in yoga or related breathing practices for the duration of the study |
|
| Outcomes | QoL (SGRQ) Lung function |
|
| Notes | Funding: not described Register number: not described We have written to the study author for further clarification regarding total sample size and the values of the SGRQ and the pulmonary function test |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation is not described |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel, and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some outcomes were participant‐rated, which could introduce high risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | High risk | ≥ 1 outcomes of interest in the review are reported incompletely, so that they cannot be entered into a meta‐analysis |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Fluge 1994.
| Methods | Design: RCT over 3 weeks (This paper was translated from German) | |
| Participants | Country: Germany
Setting: community
Health status: mild asthma
This trial included 3 arms (yoga, physiotherapy and physical exercises, and control group). 2 arms were included in the review (yoga and control groups)
Total sample: 36 participants (12 participants in each group)
Mean age, years: 48.8 ± 1.8 Age range, years: 21‐55 Gender: 14 men and 22 women Exclusion criteria: cardiopulmonary complications due to asthma, exacerbation 8 weeks before the beginning of the study, smoke |
|
| Interventions | Intervention group: yoga consisted of asana, mudra, pranayama, kriya and yoga nidra Frequency of intervention: 3 weeks' training of 3 h, 15 times total Control group received no additional treatment Participants were re‐evaluated after 15 sessions |
|
| Outcomes | Lung function | |
| Notes | Register number: not described We have written to the study author for further clarification |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation is not described |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This study has not reported any procedures intended to blind the participants and personnel, and blinding was probably not possible due to the nature of the intervention, so we judged it to have high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | High risk | One outcome of interest in the review is reported incompletely |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Girodo 1992.
| Methods | Design: RCT over 16 weeks | |
| Participants | Country: Canada
Setting: community Health status: not described Diagnosis criteria: not described Total sample: 92 participants to 4 groups 2 deep diaphragmatic breathing (DDB) groups, 1 taught by a singing instructor, the other by a 25‐year‐old participant with asthma. Physical education (PE) group led by student with PE experience. Control group: waiting list participants. 3 arms were included in the review (the deep diaphragmatic breathing groups and the control groups) Mean age, years: 28.61 ± 11.21 (DDB group); 34.92 ± 10.53 (PE group); 32.9 ± 6.55 (control group) Age range, years: not described Gender: 20 (62%) female (DDB group); 8 (66%) female (PE group); 13 (56%) female (control group) Inclusion criteria: detailed examination of the history of their condition ‐ doctor's approval and informed consent Exclusion criteria: history of allergies, severe asthma, chest disease, diabetes, inability to make 26‐week commitment |
|
| Interventions | Intervention group (breathing groups): physical and respiratory exercises to enlarge the thoracic cage and increase the capacity for maximum lung efficiency during expiration Frequency of intervention: 16 weeks' training of 1 h three times/week PE group: physical exercises with no emphasis on deep diaphragmatic breathing Control group: waiting list controls had pretest assessment of 'chronicled' medication use and asthma symptoms Re‐evaluated after 8 and 16 weeks Co‐intervention: no information given We combined data from the 2 breathing groups |
|
| Outcomes | Asthma symptom checklist | |
| Notes | Funding: not described Register number: not described We have written to the study author to ask for information on randomisation methods and further data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel, and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some outcomes were participant‐rated, which could introduce high risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Selective reporting (reporting bias) | High risk | One outcome of interest in the review is reported incompletely, so that it cannot be entered into a meta‐analysis |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Grammatopoulou 2011.
| Methods | Design: RCT over 6 months | |
| Participants | Country: Greece Setting: outpatient Health status: mild to moderate asthma Diagnosis criteria: not described Total sample: 40 participants (20 in each group) Mean age, years: 45.45 ± 12.67 (control group) and 48.15 ± 14.63 (intervention group) Age range, years: 18‐60 Gender: 10 men and 10 women (control group), 13 men and 7 women (intervention group) Inclusion criteria: not described Exclusion criteria: individuals > 60 years, smokers, use of oral corticosteroids in the previous 3 months, heart disease, participation in a prior asthma education programme |
|
| Interventions | The intervention consisted of 2 phases:
Control group: no additional treatment |
|
| Outcomes | QoL (as measured by the SF‐36 questionnaire) Asthma control was measured by the ACT, whose score ranges from 5 (poorly controlled) to 25 (completely controlled) NQ (used to screen for the hyperventilation syndrome) Lung function Capnography |
|
| Notes | Funding: not described Register number: not described Study author responded to our request regarding further clarification about the scores of the SF‐36 questionnaire |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random allocation was undertaken with sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was undertaken with sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel, and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some outcomes were participant‐rated, which could introduce high risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing outcome data |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but the published reports include all expected outcomes, including those that were prespecified |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Gupta 2015.
| Methods | Design: RCT over 3 months | |
| Participants | Country: India Setting: outpatient Health status: not described Diagnosis criteria: not described Total sample: 100 participants (50 in each group) Mean age, years: not described Age range, years: not described Gender: not described Inclusion criteria: either sex, aged between 20‐55 years; bronchial asthma patients practising yoga with medical treatment; bronchial asthma patients under medical treatment but not practising yoga. Exclusion criteria: unwilling to participate in study; history of cigarette smoking, hypertension, diabetes and chronic chest infections like TB (X‐ray and sputum examination done, if needed) and chest deformity |
|
| Interventions | Intervention group: various yogic exercises, which included anuloma‐viloma and kapalbhati bhramari pranayama suryanamaskar Control group: not practising yoga |
|
| Outcomes | Lung function (FVC, FEV1, FEV1/FVC, PEFR) | |
| Notes | Funding: not described Register number: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation is not described |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This study has not reported any procedures intended to blind the participants and personnel, and blinding was probably not possible due to the nature of the intervention, so we judged it to have high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available, but the published report included all expected outcomes, including those that were prespecified |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Holloway 2007.
| Methods | Design: RCT over 5 sessions | |
| Participants | Country: England, UK Setting: semi‐rural GP practice Health status: mild to moderate asthma Diagnosis criteria: not described Total sample: 85 participants (46 in control group and 39 in intervention group) Mean age, years: 49.3 ± 14.2 (control group) and 50.2 ± 14.0 (intervention group) Gender: 18 men and 28 women (control group), 18 men and 21 women (intervention group) Inclusion criteria: participants aged 16‐70 years; able to understand, read and write English, with a commitment to participate for possibly 8 attendances; willing to give written informed consent and with no serious comorbidity Exclusion criteria: not described |
|
| Interventions | Intervention group: 5 x 60‐min individual sessions on the Papworth method from a respiratory physiotherapist. The Papworth method consisted of 5 components: breathing training, education, relaxation training, integration of 'appropriate' breathing and relaxation techniques into daily living activities and home exercises (audiotape or CD containing reminders of the breathing and relaxation techniques) Control group: no additional treatment Assessments took place at baseline and at 6 and 12 months after baseline |
|
| Outcomes | QoL as measured by the SGRQ, which assesses impaired respiratory symptoms and QoL related to these Hypocapnic symptoms as assessed by the NQ HADS Lung function (VC, FEV1, FVC, PEFR) Capnography |
|
| Notes | Funding: this study was not sponsored but was undertaken as part fulfilment of a PhD degree at University College London. A study author's post was funded by Cancer Research UK Register number: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was undertaken by a computer‐generated number sequence assigning consecutive participant ID numbers a 1 or a 2 to denote intervention or control condition |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel, and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some outcomes were participant‐rated, which could introduce high risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups (7 from the intervention group and 6 from the control group), with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Malarvizhi 2018.
| Methods | Design: RCT | |
| Participants | Country: India Setting: outpatient Department of Respiratory Medicine in Sri Ramachandra Medical hospital Severity of asthma: mild or moderate bronchial asthma Diagnosis criteria: GINA criteria Total sample: 250 participants (125 in each group) Mean age, years: not described Age range, years: 21‐60 Gender: 69 men and 56 women (control group); 70 men and 55 women (yoga group) Inclusion criteria: the inclusion criteria consisted of either sex, aged 21‐60 years, who met GINA criteria, minimum of 2 years, able to understand either English or Tamil and willing to participate in the study. Exclusion criteria: participants were excluded if they had severe airflow limitation (FEV1 > 60%), smokers, history of co‐morbid illness (medical, neurologic and psychiatric, orthopaedics) associated chronic respiratory diseases such as TB, autoimmune lung diseases and practised yoga or any other similar discipline. | |
| Interventions | Intervention group: yoga group received 30 min of yoga training for a week under a trained yoga teacher and advised to practise at home daily once a day for 6 months. It consisted of basic asanas (posture) like bhujangasana (cobra pose), tadasana (tree pose) and gomukhasana (cow face pose) for 10 min and simple pranayama (breathing exercise), nadi sudhi pranayama and bhastrika for 10 min followed by relaxation ('om' chanting and shavasana). A yoga checklist booklet was given to the participants to assess the level of performance and monitor the practice. On the regular visits to the outpatient's department days, participants were asked to show return demonstration and their doubts were clarified. During this period at 3 and 6 months, participants were assessed through yoga performance checklist for their level of practice on yoga techniques. Lacunas made during the practice were corrected and reinforced. Control Group: conventional care Frequency of intervention: daily once a day for 6 months |
|
| Outcomes | QoL (using AQLQ at baseline, 3 and after 6 months interval) | |
| Notes | Funding: not described
Register number: not described The study author responded to our request regarding additional data from AQLQ total scores. However, due to a discrepancy between the graphical presentation and the data provided by correspondence, we were unable to include the data in the analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was undertaken by using random allocation software. Randomisation was performed by 1 of the authors who was not involved in any part of the assessment |
| Allocation concealment (selection bias) | Low risk | Participants were randomly allocated to 1 of the 2 study arms by using random allocation software |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | The presence of participant blinding was reported and is likely that the blinding could have been broken due the type of intervention used in the study arms |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | The outcome was participant‐rated, which could introduce high risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing outcome data balanced in numbers across intervention groups, however the reasons for the missing data were not clearly described |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available, but the published reports included all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Nagarathna 1985.
| Methods | Design: RCT over 2 weeks | |
| Participants | Country: India Setting: Yoga Therapy and Research Centre Health status: not described Diagnosis criteria: satisfying the clinical criteria of Crotton, Douglas and Shivpuri Total sample: 106 participants (53 in each group) Mean age, years: 26.41 (control group) and 26.36 (intervention group) Age range, years: 9‐47 (control group) and 9‐47 (intervention group) Gender: 15 (28%) women (control group) and 15 (28%) women (intervention group) Inclusion criteria: established bronchial asthma Exclusion criteria: not described |
|
| Interventions | Intervention group: training programme over 2 weeks with 2.5 h daily. Included were breathing exercises associated with simple movements; yoga loosening exercises; various physical postures combined with slow breathing and exercise; relaxation and slow deep breathing; meditation and devotional session including chanting; weekly traditional voluntary nose and stomach wash techniques; yoga philosophy lectures and discussions Participants were instructed to continue the practices daily during the follow‐up period of 30 months Control group: continued taking the usual drugs during the study. Participants in this group reported for checkups every 6 months Co‐intervention: no inhalers used; continued with usual, self‐regulated bronchodilators and injections. The doctor decided whether a change in the 'brand' of drug was required Participants kept diaries recording drug usage, number and severity of attacks of airway obstruction |
|
| Outcomes | Lung function (PEF) Number and severity of attacks (1 = mild, but did not disturb sleep or daily routine; 2 = moderate, disturbed sleep and daily routine and relieved by oral drugs; 3 = severe, required injection or admission to hospital) | |
| Notes | Funding: not described Register number: not described Wrote to author for clarification |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation is not described |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This study has not reported any procedures intended to blind the participants and personnel, and blinding was probably not possible due to the nature of the intervention, so we judged it to have high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient reporting of attrition/exclusions to permit judgement of 'low risk' or 'high risk' |
| Selective reporting (reporting bias) | Low risk | Published reports include all expected outcomes |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists Blinding of participants and personnel (performance bias) Days off work |
Prasanna 2015.
| Methods | Design: RCT over 2 months | |
| Participants | Country: India Setting: outpatient Health status: not described Diagnosis criteria: not described Total sample: 100 participants Mean age, years: 40.4 ± 9.1 (intervention group) and 37.36 ± 7.9 (control group) Age range, years: 31‐40 Gender: 64% of women in the intervention group and 60% in the control group Inclusion criteria: newly diagnosed patients of both sexes in the age group of 25‐60 years were selected Exclusion criteria: smokers and people with chronic asthma were excluded |
|
| Interventions | Intervention group: Buteyko breathing exercise. The participants were instructed to do the breathing exercise at least twice in a day (morning and evening) for 2 months. Control group: ICS |
|
| Outcomes | Lung function (FEV1 and PEFR at the beginning and at the of the 1st and 2nd month) Subjective improvements of symptoms at the beginning and at the end of 1st and 2nd month of the study measured using a pretested, structured, close‐ended questionnaire in the local language. The questionnaire assessed the severity the severity of symptoms, frequency of exacerbations, changes in the lung capacity, and side effects of medication. |
|
| Notes | Funding: ICMR Register number: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation is not described |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel, and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some outcomes were participant‐rated, which could introduce high risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Selective reporting (reporting bias) | High risk | One outcome of interest in the review is reported incompletely, so that it cannot be entered into a meta‐analysis |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Prem 2013.
| Methods | Design: RCT | |
| Participants | Country: India
Setting: conducted at an outpatient department of chest medicine
Health status: mild to moderate asthma Diagnosis criteria: not described 3 arms in this trial (Buteyko, pranayama and control group). We included all 3 arms in the review Total sample: 120 participants, 40 in each group Mean age, years: 38 ± 13 (Buteyko group), 35 ± 13 (pranayama group) and 41 ± 14 (control group) Age range, years: 18‐60 Inclusion criteria: participants aged 18‐60 years, AQLQ 24 < 5.5, FEV1 increase by 12% following bronchodilator administration, usage of bronchodilator for 6 months and patients without exacerbation in the preceding 8 weeks Exclusion criteria: participants were excluded if they had medical conditions that impaired the performance of breathing techniques, had a previous history of breathing retraining, were pregnant and non‐compliant with exercise for more than 15% of study period |
|
| Interventions | Intervention group: Buteyko and pranayama Frequency of intervention: 3‐5 days with a session of 60 min/day. Participants were then followed up for 3 months and were instructed to practise the exercise for 15 min twice daily Control group received no additional treatment |
|
| Outcomes | QoL (AQLQ) Asthma symptoms (ACQ) Lung function (FEV1, FEV1/FVC) |
|
| Notes | Funding: this research received no specific grant from any funding agency in the public, commercial, or not‐for‐profit sectors Register number: not described The study author responded to our request regarding asthma severity of participants and the values on AQLQ, ACQ and pulmonary function tests |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to 3 groups through block randomisation |
| Allocation concealment (selection bias) | Low risk | The method of allocation was concealed by sequentially numbered, sealed, opaque envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel, and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some outcomes were participant‐rated, which could introduce high risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | High risk | ≥ 1 outcomes of interest in the review are reported incompletely, so that we cannot enter them into a meta‐analysis |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Pushpa 2018.
| Methods | Design: RCT | |
| Participants | Country: India
Setting: outpatient − Department of Physiology, Bangalore Medical College and Research Institute, Bengaluru
Severity of asthma: mild‐moderate bronchial asthma
Diagnosis criteria: not described
Total sample: 60 participants, 30 in each group
Mean age, years: 31.00 ± 9.03 (control group) and 32.67 ± 8.69 (intervention group)
Age range, years: 18‐50 years
Gender: 10 men and 20 women (in each group)
Inclusion criteria: the study included diagnosed cases of bronchial asthma, aged 18−50 years with an established diagnosis for at least 6 months, mild‐moderate cases meeting NAEPP classification. Participants on inhaled beta2‐agonist (short‐acting and long‐acting) with stable medication dose for past 1 month. Exclusion criteria: study excluded smokers, patients with concomitant lung disease, those who practised yoga or any other similar discipline during 6 months preceding the study, pregnancy, any chronic medical condition that required a treatment with oral/systemic steroids in the past months, any medical condition that contraindicated exercise, history of TB, diabetes mellitus, renal failure, coronary artery disease, musculoskeletal deformities, and status asthmaticus. |
|
| Interventions | Intervention group: yoga training group practised yoga exercises along with the medication, yogic exercises used by the participants included pranayamas (deep breathing exercises), kapalabhati (cleaning breath), bhastrika (rapid and deep respiratory movements like that of the bellows), ujjayi (loud sound producing pranayama) and sukhapurvaka pranayama (easy comfortable breathing), meditation, and shavasana (relaxation technique) under the guidance of trained yoga teacher.
Control group: only pharmacological therapy Frequency of intervention: 45 min/day for 2 weeks and instructed to practise at home for 45 min twice daily, regularly for remaining 6 weeks and were instructed to maintain a diary record of each day of yoga practice. |
|
| Outcomes | Lung function (FVC, FEV1, FEV1/FVC, PEFR, FEF25‐75%) Airway resistance (RAW) Specific airway conductance (sGAW) | |
| Notes | Funding: none Register number: not described | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | This study did not report any procedures intended to blind the participants and personnel, and blinding was probably not possible due to the nature of the intervention, so we judged it to have high risk of bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available, but the published reports included all expected outcomes, including those that were prespecified |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Satpathy 2016.
| Methods | Design: RCT over 4 months | |
| Participants | Country: India Setting: outpatient Health status: persistent, chronic asthma Diagnosis criteria: bronchial asthma confirmed by the physician/chest physician as mentioned in diagnostic criteria Total sample: 71 participants Mean age, years: 24.98 ± 2.92 (control group) and 25.27 ± 2.28 (intervention group) Age range, years: not described Gender: 71 men Inclusion criteria: cases of bronchial asthma confirmed by the physician/chest physician as mentioned in diagnostic criteria; with symptoms of asthma persisting for at least 6 months Exclusion criteria: history of smoking within the last 1 year; acute infection or infections within the past 6 weeks; serious systemic illness − hepatic, renal, cardiac or CNS diseases; cardiovascular diseases including hypertension |
|
| Interventions | Intervention group: yoga including 5 yogic exercises daily, along with the same medication. The yogic exercises included were tadashana, tiryaka tadasana, simhagarjanashana, kastatakshyasana and bhastrika pranayama, followed by relaxation or shavasana. Initially breathing exercises were performed 10 times and then extended to 15‐20 times after 2 weeks. Simhagarjanasana was started with 3‐4 times at first and then extended after 2 weeks as per comfort. Control group: only drugs Initially they were stabilised on drugs (ICS and bronchodilators) till no further symptomatic improvement occurred |
|
| Outcomes | Acute exacerbation of asthma Dyspnoea (assessed by dyspnoea grading system) Asthma symptom score Use of bronchodilators Use of ICS |
|
| Notes | Funding: not described Register number: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation is not described |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel, and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some outcomes were participant‐rated, which could introduce high risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available, but the published report included all expected outcomes, including those that were pre‐specified |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Singh 2012.
| Methods | Design: RCT | |
| Participants | Country: India
Setting: Department of Physiology, University College of Medical Sciences Health status: mild to moderate asthma Diagnosis criteria: the diagnosis was based on paroxysms of dyspnoea, wheezing and cough, which improved either spontaneously or with drug therapy. 2 arms in this trial (yoga and control groups) Total sample: 60 participants (30 participants in each group) Mean age, years: not described Age range, years: 18‐60 Inclusion criteria: non smokers, in the age group of 18‐60 years with mild to moderate grades of bronchial asthma as per GINA guidelines Exclusion criteria: individuals with a history of an exacerbation or respiratory tract infection, current smokers, pregnant or lactating women and those with any other disorder were excluded |
|
| Interventions | Intervention group: yoga sessions included pranayama (30‐35 min), asanas (10 min), meditation (10 min) and lifestyle modification for 5‐6 days Frequency of intervention: participants were practising yoga for an average of 40‐50 min daily at home for 2 months Control group: no additional treatment |
|
| Outcomes | QoL (AQLQ) Lung function (FVC, FEV1, FEV1/FVC, PEFR, MVV, SVC) |
|
| Notes | Funding: not described Register number: not described We have written to the study author to ask for further clarification regarding total sample size and total score on the AQLQ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation is not described |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel, and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some outcomes were participant‐rated, which could introduce high risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for missing outcome data unlikely to be related to true outcome |
| Selective reporting (reporting bias) | Low risk | Published reports include all expected outcomes |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Sodhi 2009.
| Methods | Design: RCT over 8 weeks | |
| Participants | Country: India Setting: Departments of Medicine and Physiology, Christian Medical College & Hospital Health status: mild to moderate asthma Diagnosis criteria: not described Total sample: 120 participants Mean age, years: 35.55 ± 10.62 (control group) and 38.77 ± 9.92 (intervention group) Age range, years: 17‐50 (control group) and 20‐50 (intervention group) Gender: 37 men and 23 women (control group) and 34 men and 26 women (intervention group) Inclusion criteria: non‐smokers in the age group of 17‐50 years with mild to moderate asthma Exclusion criteria: individuals with a history of TB, COPD, diabetes, renal failure, coronary artery disease and musculoskeletal chest deformities, respiratory tract infection within the previous 6 weeks and engagement in any regular exercise/training |
|
| Interventions | Intervention group: 45‐min yoga training sessions/week with a trained instructor, which included pranayamas (deep breathing exercises), kapalabhati (cleaning breath), bhastrika (rapid and deep respiratory movements), ujjayi (loud sound‐producing pranayama) and sukha purvaka pranayama (easy comfortable breathing). Participants were instructed to practise at home, 45 min twice daily, on all days of the week. Participants maintained a diary record of each day of the yoga practice Control group: conventional treatment Pulmonary function tests were performed on all participants at baseline and after 4 and 8 weeks |
|
| Outcomes | Lung function (FVC, FEV1, FEV1/FVC, PEFR) QoL (AQLQ) |
|
| Notes | Funding: not described Register number: not described The study author responded to our request regarding control group treatment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was undertaken by using permuted block randomisation |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel, and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some outcomes were participant‐rated, which could introduce high risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement of 'low risk' or 'high risk' |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available, but published reports include all expected outcomes |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Thomas 2003.
| Methods | Design: RCT | |
| Participants | Country: UK
Setting: semi‐rural general practice Health status: Diagnosis criteria: study authors searched medical notes of participants with a diagnosis of asthma made by a GP and at least 1 prescription for an inhaled or oral bronchodilator or a prophylactic anti‐asthma medication in the previous year Total sample: 33 participants (17 in intervention group and 16 in control group) Age range, years: 17‐65 Mean age, years: 48.8 ± 10.9 (intervention group) and 48.9 ±15.6 (control group) Gender: 7 (21%) male participants Inclusion criteria: patients aged 17−65 years with a diagnosis of asthma who had received at least 1 prescription for an inhaled or oral bronchodilator or prophylactic anti‐asthma medication in the previous year were identified from the medical records; with NQ > 23, which suggests a diagnosis of dysfunctional breathing. Exclusion criteria: not described |
|
| Interventions | Intervention group: breathing retraining with a chartered physiotherapist. Initial group treatment for 45 min followed by 2 individual training sessions lasting 15 min each, which were 1 and 2 weeks apart. Explanation and training given in relaxed diaphragmatic breathing. Participants were assessed at 1 and 6 months post‐intervention Control group: 60‐min group session with asthma nurse teaching asthma education. Participants invited to attend for further individual asthma review if they wished | |
| Outcomes | QoL (AQLQ) Hyperventilation symptoms measured by the NQ |
|
| Notes | Funding: Royal College of GPs' Scientific Foundation Board. Minchinhampton Surgery is a funded R&D practice under the NHS Executive South & West R&D General Practice Scheme Register number: not described The study author responded with further information regarding non‐parametric data, method of randomisation, etc |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Volunteers were randomly assigned by alphabetical numbering and by use of random number tables for assignment to trial groups |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was supervised by a statistician, but the paper does not fully describe the method of allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel or of the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some outcomes were participant‐rated which could introduce high risk of detection bias. The questionnaires were scored blind by the investigator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data were balanced in numbers across intervention groups, with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Published reports include all expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Thomas 2009.
| Methods | Design: single‐blind RCT over 3 sessions | |
| Participants | Country: UK Setting: not described Health status: mild to moderate asthma Diagnosis criteria: not described Total sample: 183 Median age, years: 46.0 (35‐57) (control group) and 46.0 (33‐57.3) (intervention group) Age range, years: not described Gender: 29 men and 60 women (control group) and 42 men and 52 women (intervention group) Inclusion criteria: non‐smokers, patients treated for asthma at 10 UK primary care general practices in Leicester, UK, and having moderate impairment of asthma‐related health status (AQLQ score < 5.5) Exclusion criteria: not described |
|
| Interventions | Both groups consisted of an initial 60‐min small group session (2‐4 participants) followed by 2 individual sessions of 30‐45 min with 2‐4 weeks between attendances Intervention group: explanation of normal breathing and possible effects of abnormal 'dysfunctional breathing'. During individual sessions, participants were taught diaphragmatic and nasal breathing techniques and were encouraged to practise these exercises for at least 10 min each day Control group: 3 sessions of nurse‐provided asthma education |
|
| Outcomes | QoL (AQLQ) Asthma control questionnaire (ACQ) and asthma symptoms measured by the NQ HADS Spirometry Capnography |
|
| Notes | Funding: this study was funded by a grant from Asthma UK (03/014). 1 study author is in receipt of an Asthma UK Senior Research Fellowship Register number: not described The study author has responded to our enquiries with further details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation was not described |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment was not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel, and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some outcomes were participant‐rated, which could introduce high risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | The study protocol is not available, but the published reports include all expected outcomes |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Thomas 2017.
| Methods | Design: parallel‐group randomised trial over 12 months | |
| Participants | Country: UK Setting: outpatient Health status: mild and moderate asthma Diagnosis criteria: a physician diagnosis of asthma Total sample: 655 Median age, years: 57 (46−64) Age range, years: 16‐70 Gender: female (419 ‐ 64%), male (236 ‐ 36%) Inclusion criteria: 16–70 years, be registered at a medical practice for at least 12 months, have asthma diagnosed by a physician, have been prescribed at least 1 asthma medication in the previous year, achieve an AQLQ < 5.5, and to provide written informed consent Exclusion criteria: concomitant COPD diagnosis with FEV1 < 60% predicted |
|
| Interventions | Intervention group
Control group: usual medical care, with no additional attention to the baseline assessment Frequency of intervention: for face‐to‐face physiotherapy group, 3 x one‐to‐one sessions, approximately once every 2 weeks after randomisation Intensity and timing of intervention: for face‐to‐face physiotherapy group, each about 40‐min duration |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Funding: UK National Institute of Health Research, Health Technology Assessment Register number: ISRCTN88318003 The study author responded to our enquiries with further details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was undertaken by a telephone randomisation service using random number generators |
| Allocation concealment (selection bias) | Low risk | Participants were randomly allocated to 1 of the 3 study arms by using a telephone call service |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel, and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some outcomes were participant‐rated, which could introduce high risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Missing outcome data balanced in numbers across intervention groups with similar reasons for missing data across groups |
| Selective reporting (reporting bias) | Low risk | Published reports include all expected outcomes |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Vedanthan 1998.
| Methods | Design: RCT over 3 weeks | |
| Participants | Country: USA Setting: University, Allergy and Asthma Clinic. Health centre Health status: not described Diagnosis criteria: based on guidelines established by the National Asthma Education Panel Total sample: 17 students Mean age, years: 25.11 (control group) and 28.12 (yoga group) Age range, years: 19‐52 Gender: 6 women (75%) (control group) and 3 women (33%) (yoga group) Recruitment: student volunteers from a university asthma and allergy clinic Inclusion criteria: mild to moderate asthma Exclusion criteria: not described |
|
| Interventions | Intervention group: training programme of yoga techniques, including various breath‐slowing exercises (pranayama) together with physical exercises; these were performed without breath holding: also physical postures, meditation, exercises and lectures on yoga philosophy Participants given audio cassettes and written information for home practice Duration: 45 min, 3 times a week for 16 weeks Control group: no information given Both groups were given peak flow meters to record daily am and pm readings Participants from both groups regularly attended the Health Center | |
| Outcomes | Lung function (FVC, FEV1) Symptom questionnaire, which included severity and frequency of attack scores | |
| Notes | Funding: not described Register number: not described The author has responded to our enquiries with further details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation is not described |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel, and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some outcomes were participant‐rated, which could introduce high risk of detection bias. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts reported |
| Selective reporting (reporting bias) | High risk | ≥ 1 outcomes of interest in the review are reported incompletely, so they cannot be entered into a meta‐analysis |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
Vempati 2009.
| Methods | Design: RCT over 8 weeks | |
| Participants | Country: India Setting: Integral Health Clinic, All India Institute of Medical Sciences (2 weeks) and intervention at home (6 weeks) Health status: mild to moderate asthma Diagnosis criteria: the ATS spirometry criteria Total sample: 57 participants (28 in control group and 29 in intervention group) Mean age, years: 33.4 ± 11.5 (control group) and 33.5 ± 11.4 (intervention group) Age range, years: not described Gender: 20 men and 8 women (control group) and 13 men and 16 women (intervention group) Inclusion criteria: age ≥ 18 years; an established diagnosis of mild to moderate asthma for at least 6 months (meeting ATS spirometry criteria for mild to moderate asthma; taking at least 1 of the following: inhaled β‐agonists, methyl‐methylxanthines, anticholinergics, ICS; and stable medication dosing for the past month Exclusion criteria: individuals who smoked currently (or in the past year) or had a smoking history of > 5 pack‐years; had a concomitant lung disease; were taking leukotriene inhibitors or receptor antagonists, or mast cell–stabilising agents for at least 6 months; practised yoga or any other similar discipline during 6 months preceding the study; were pregnant; had a chronic medical condition that required treatment with oral or systemic corticosteroids in the past month; had a medical condition that contraindicated exercise; or had an unstable medical condition |
|
| Interventions | Intervention: yoga group underwent a yoga‐based lifestyle modification and stress management programme for 4 h/day over 2 weeks and an additional 6 weeks of home practice. The programme consisted of lectures and practical sessions on asanas (postures), pranayamas (breathing techniques), kriyas (cleansing techniques), meditation and shavasana (a relaxation technique) Control group: conventional care Outcome measures were performed on all participants at baseline and after 2, 4 and 8 weeks |
|
| Outcomes | QoL (AQLQ) Lung function (FVC, FEV1, FEV1/FVC, PEFR) |
|
| Notes | Funding: Central Council for Research in Yoga and Naturopathy (CCRYN), Department of AYUSH, Ministry of Health, Government of India and Wellpark College of Natural Therapies, New Zealand Register number: not described |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of sequence generation is not described |
| Allocation concealment (selection bias) | Unclear risk | The method of concealment is not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of participants and personnel, and the outcome is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Some outcomes were participant‐rated, which could introduce high risk of detection bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing outcome data were balanced in numbers across intervention groups, however the reasons for the missing data were not clearly described |
| Selective reporting (reporting bias) | Low risk | The study protocol is available, and all of the study's prespecified (primary and secondary) outcomes that are of interest in the review have been reported in the prespecified way |
| Other bias | Unclear risk | Insufficient information to assess whether an important risk of bias exists |
ACT: Asthma Control Test; QOL: Asthma Quality of Life; AQLQ: Asthma Quality of Life Questionnaire; ATS: American Thoracic society; CNS: central nervous system; COPD: chronic obstructive airway disease; FEF25‐75: forced expiratory flow averaged over the middle portion of forced vital capacity; FENO: fraction of exhaled nitric oxide; FEV1: forced expiratory volume during the first second; FVC: forced vital capacity; GINA: global initiative for asthma; GP: general practitioner; HADS: Hospital Anxiety and Depression Scale; ICMR: Indian Council of Medical Research; ICS: inhalational corticosteroids; MMV: maximum voluntary ventilation; NAEPP: National Asthma Education and Prevention Program; NHS: National Health Service; NQ: Nijmegen (Hyperventilation) Questionnaire; PEFR: peak expiratory flow rate; QoL: quality of life; R&D: research and development; RCT: randomised controlled trial; SF‐36: 36‐Item Short Form; SGRQ: St George's Respiratory Questionnaire; SVC: slow vital capacity; TB: tuberculosis; VC: vital capacity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aleksandrov 1990 | Not an RCT |
| Anonymous 1968 | Investigation comparing the active group treated with hypnosis and the control group treated with breathing plus relaxation training |
| Asher 1990 | Each treatment preceded by nebulised salbutamol. Multiple intervention treatments |
| Berlowitz 1995 | Not an RCT |
| Bobokhodzhaev 1984 | Not an RCT |
| Bowler 1998 | The control group was given relaxation techniques |
| Cambach 1997 | Mixed population of COPD and asthma in programme Multiple interventions |
| Cambach 1999 | Not an RCT |
| Coll 1994 | Journal letter only. Not an RCT |
| Cooper 2003 | Device used |
| Cowie 2008 | Comparison between Buteyko and chest physiotherapy. No control group |
| Emtner 1998 | Not an RCT. 3 studies included in dissertation. Physical training principal component in all studies |
| Erskine 1979 | No breathing training. Comparison of muscular relaxation with muscular and mental relaxation training in participants with asthma. No control group |
| Falkenbach 1993 | Not an RCT. Participants acted as their own control in a before‐after trial. A control group was not established for satisfactory statistical analyses because of the small numbers involved |
| Gallefoss 1999 | Breathing training not a major component of the physiotherapy intervention |
| Gosselink 1993 | Not an RCT |
| Hoang 2015 | Not an RCT |
| Holmes 1990 | Not an RCT |
| Johansson 2017 | Not an RCT |
| Joseph 1999 | Not an RCT |
| Karam 2017 | Not an RCT |
| Khanam 1996 | Not an RCT |
| Kotses 1978 | No breathing training. Examining muscle tension relationships with bronchial airways resistance |
| Kurabayashi 1998 | Not an RCT, no control group, COPD not asthma |
| Lacasse 1997 | Not an RCT |
| Loew 1996 | Not RCT. Comparison of the effects of treatment with terbutaline and relaxation. Breathing plus movement therapy in 2 groups of asthmatic participants |
| Manocha 2002 | Breathing retraining not included as part of intervention |
| Mass 1991 | Device used for breathing training |
| McFadden 1986 | Not a study of breathing exercises. Study was set up to observe thermal effects on the airways in exercise‐induced asthma |
| McHugh 2003 | The control group was given relaxation techniques |
| Mussell 1986 | Device used for reducing bronchospasm |
| Opat 2000 | The control group was given a placebo relaxing video |
| Paleev 1988 | Not an RCT |
| Pryor 1979 | Thesis. Treatments randomly assigned, not participants. Evaluation of postural drainage time for evacuation of secretions with and without the addition of FET, in addition to other physiotherapeutic modalities |
| Raghavendra 2016 | Comparison between yoga and deep breathing |
| Redchits 1986 | Not an RCT |
| Sabina 2005 | Comparison between yoga and stretching. No control group |
| Saxena 2009 | Comparison between yoga and meditation. No control group |
| Schulze 2000 | Not an RCT. Children |
| Shaw 2011 | Device used for diaphragmatic breathing training |
| Singh 1987 | A device was used to alter the breathing pattern. Description of device amended in 2003 update in response to researcher's comment that the device does not increase muscle strength but imposes components of pranayamic breathing |
| Singh 1990 | Device used |
| Slader 2006 | Comparison of 2 breathing techniques. No control group |
| Smyth 1999 | Not an RCT. Relaxation training, not breathing, as principal component |
| Tandon 1978 | Study involved participants with COPD, not asthma |
| Van der Schans 1997 | No breathing exercises. Study used propranolol to induce bronchoconstriction and then reversed its effect using pursed‐lip breathing |
| Weiner 1992 | Device used |
| Wilson 1975 | Transcendental meditation, not breathing training |
COPD: chronic obstructive pulmonary disease; FET: forced expiratory technique; RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Agnihotri 2013.
| Methods | Design: RCT Method of randomisation: not described Method of allocation concealment: not described Total number of withdrawals/dropouts: 35 participants Total duration of study: not described Outcome assessor blinding: not described |
| Participants | Country: India Setting: not described Diagnosis criteria: not described Severity of asthma: moderate asthma Total sample: 206 Mean age, years: not described Age range, years: not described Gender: not described Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Intervention group: yoga (asanas, pranayama and meditation), in addition to standard medical treatment Frequency of intervention: not described Control group: standard medical treatment |
| Outcomes | Asthma QoL scores in both groups over the period of 6 months and in yoga group in comparison to control group |
| Notes | Funding: not described Register number: not described |
Divya 2013.
| Methods | Design: not described Method of randomisation: not described Method of allocation concealment: not described Total number of withdrawals/dropouts: not described Total duration of study: 8 weeks Outcome assessor blinding: not described |
| Participants | Country: not described Setting: not described Total sample: 60 participants Mean age: not described Age range: not described Gender: not described Diagnosis criteria: not described Severity of asthma: mild to moderate bronchial asthma Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Intervention group: yoga along with pharmacological treatment Frequency of intervention: not described Intensity and timing of intervention: not described Control group: only pharmacological treatment |
| Outcomes | Airway resistance and specific airway conductance were measured by using a body plethysmograph |
| Notes | Funding: not described Register number: not described We have written to the study author to ask for further clarification, however have not had an answer. |
Kant 2013.
| Methods | Design: RCT Method of randomisation: not described Method of allocation concealment: not described Blinding/masking: not described |
| Participants | Country: India Setting: outpatients ‐ Department of Pulmonary Medicine, King George's Medical University, Lucknow, UP Total sample: 276 participants Mean age: not described Age range: not described Gender: not described Diagnosis criteria: not described Severity of asthma: mild or moderate bronchial asthma Inclusion criteria: not described Exclusion criteria: not described |
| Interventions | Intervention group: yoga (asanas, pranayama and meditation), in addition to standard medical treatment Control group: only standard medical treatment Both groups were assessed at baseline, 3rd and 6th month |
| Outcomes | Asthma QoL scores Pulmonary function Bio‐chemical changes Asthma symptom scores Rescue medication uses |
| Notes | Funding: not described Register number: not described We have written to the study author to ask for further clarification, however we have not had an answer. |
QoL: quality of life; RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
Andreasson 2017.
| Trial name or title | Breathing exercises in asthma targeting dysfunctional breathing (BEAT_DB) |
| Methods | Design: multicentre RCT Method of randomisation: not described Method of allocation concealment: parallel assignment Total duration of study: 12 months Outcome assessor blinding: double (investigator, outcomes assessor) |
| Participants | Country: Denmark Setting: Naestved Hospital Total sample: 220 Age range: ≥ 18 years Diagnosis criteria: not described Severity of asthma: not described Inclusion criteria: pulmonologist‐diagnosed asthma, ≥ 2 consultations at pulmonologist previously ACQ6 ≥ 1.5, able to provide written informed consent Exclusion criteria: patients who have had breathing exercises treatment or similar before |
| Interventions | Intervention: breathing exercises (Papworth method; Buteyko technique) Frequency of intervention: 10 min of home exercise twice daily with 3 physiotherapist‐sessions of breathing exercises (BrEX) with duration of 60 min (the initial) and 30 min (other sessions) at week 1, 4, and 9 Intensity and timing of intervention: 6 months' follow‐up Secondary endpoints will be 3 and 12 months' follow‐up |
| Outcomes | Primary outcome: QoL measured by MiniAQLQ at 6 months' follow‐up Secondary outcomes: changes in ACQ6, NQ, HADS, accelerometry (physical activity level, number of steps), 6 min walk distance, and FEV1, besides response of GPE rate in asthma‐related QOL and asthma control |
| Starting date | 25 April 2017 |
| Contact information | Karen Hjerrild Andreasson, Ph.D.‐Student, PT, Naestved Hospital |
| Notes | Funding: not described Register number: NCT03127059 |
Elahi 2016 .
| Trial name or title | Effect of breathing exercises on indicators of clinical, spirometric parameters and quality of life of patients with asthma referred to allergic asthma clinic of Imam Khomeini in Ahwaz |
| Methods | Design: quasi‐experimental study Method of randomisation: not described Method of allocation concealment: parallel Total duration of study: 4 weeks Outcome assessor blinding: not blinded |
| Participants | Country: Iran Setting: Imam Khomeini Hospital Total sample: 60 Age range, years: 50‐25 Diagnosis criteria: specialist physician diagnosis of asthma Severity of asthma: not described Inclusion criteria: age range 50‐25 years, specialist physician diagnosis of asthma, passing at least a year of diagnosis, willingness to co‐operate in the study Exclusion criteria: patients with cardiovascular, neuromuscular or chronic diseases (cancer and diabetes), and patients with other respiratory diseases. Patients with communication problems and with 3 or 4 consecutive days of intermittent breathing exercises will not be excluded. |
| Interventions | Intervention group: breathing exercises Frequency of intervention: 20 min daily for 4 weeks Control group did not receive any training |
| Outcomes | Primary outcomes: spirometric parameters and QoL Secondary outcomes: blood pressure, heart beat, respiratory rate |
| Starting date | 10 August 2016 (expected date) |
| Contact information | Nasrin Elahi, Ahvaz Jundishapur University of Medical Sciences elahi‐n@ajums.ac.ir |
| Notes | Funding: Ahvaz University Of Medical Sciences Register number: RCT2016072629086N1 |
Murthy 2010.
| Trial name or title | Effect of naturopathy interventions in bronchial asthma |
| Methods | Design: randomised wait‐listed control clinical study Method of randomisation: computer‐generated randomisation Method of allocation concealment: open list of random numbers Blinding/masking: not applicable |
| Participants | Individuals aged 18‐65 years, with mild to moderate persistent asthma; non‐smokers/stopped smoking 6 months previously |
| Interventions | Intervention: naturopathy and yoga interventions Control Intervention: waiting list control |
| Outcomes | Primary outcomes
Secondary outcomes
|
| Starting date | 01 October 2009 (date of first enrolment) |
| Contact information | INYS‐Medical Research Society. Jindal Nagar |
| Notes | N/A |
Vagedes 2017.
| Trial name or title | Effectiveness of a Buteyko‐based breathing technique for asthma patients |
| Methods | Design: randomised clinical trial Method of randomisation: not described Method of allocation concealment: cross‐over assignment Total duration of study: 6 months Outcome assessor blinding: none (open‐label) |
| Participants | Country: Germany Setting: ARCIM Institute Academic Research in Complementary and Integrative Medicine University Hospital Tuebingen Total sample: 64 Age range, years: 18‐80 Diagnosis criteria: not described Severity of asthma: not described Inclusion criteria: written informed consent, regular intake of prescribed asthma medication according to the AWMF scheme level 1, native speaker of German Exclusion criteria: participation in another study, comorbidities (heart diseases, cancer still under treatment, psychiatric diseases) |
| Interventions | Intervention group: Buteyko breathing technique Frequency of intervention: not described Control group: standard care as usual |
| Outcomes | Primary outcomes: change in end‐tidal CO2, change in NQ score, change in Buteyko Control Pause Secondary outcomes: spirometry, heart rate variability, vascular stiffness, blood pressure, haemodynamics stroke volume, haemodynamics pleth variability, oxygen saturation, ACQ, AQLQ, ARCIM Questionnaire, STAI Questionnaire, ASF questionnaire, asthma medication |
| Starting date | 25 January 2017 |
| Contact information | Jan Vagedes, Dr. MD. ARCIM Institute Academic Research in Complementary and Integrative Medicine. University Hospital Tuebingen |
| Notes | Funding: ARCIM Institute Academic Research in Complementary and Integrative Medicine Register number: NCT03098849 |
ACQ: Asthma Control Questionnaire; AQLQ: Asthma Quality of Life Questionnaire; ARCIM: Institute of Academic Research in Complementary and Integrative Medicine; ASF: Asthma Short Form; FEV1: forced expiratory volume in first second; GPE: Global perceived effect; HADS: Hospital Anxiety and Depression Scale: NQ: Nijmegen (Hyperventilation) Questionnaire; QoL: quality of life; RCT: randomised controlled trial; STAI: State‐Trait Anxiety Inventory
Differences between protocol and review
For the 2019 update, we have included one new author in the current version of this review. We have amended the 'Type of interventions' description, to make clearer that all participants assigned to treatment comprising breathing retraining were included. We included studies with participants with a mean age of over 18 years old, rather than studies enrolling only people aged over 18 years. We felt that the studies were reflective of a relevant, adult population. We have included an additional description regarding the age in the 'Type of participants' section. We have introduced the type of breathing exercise as a subgroup analyses criteria.
We included the change in asthma symptoms measured by asthma control questionnaire (ACQ), change in hyperventilation symptoms measured by the Nijmegen Questionnaire in the 'Summary of findings' table. We have added 'hyperventilation symptoms' as a complementary information to the original secondary outcome 'asthma symptoms'.
We initially stated that we used a fixed‐effect model unless there was substantial heterogeneity and for this 2019 update we used the random‐effects method, which considers that different studies are estimating different, yet related, intervention effects. We included a statement to make clearer that we used intention‐to‐treat analyses instead of per‐protocol/completer analyses, when both were reported.
Contributions of authors
Thayla Santino: selected the studies, assessed the risk of bias, entered data into Review Manager 5, interpreted data and drafted the final review.
Gabriela Chaves: extracted data, assessed the risk of bias, entered data into Review Manager 5, carried out the analysis, interpreted data and drafted the final review.
Diana Freitas: selected the studies, entered data into Review Manager 5, carried out the analysis, interpreted data and drafted the final review.
Guilherme Fregonezi: extracted data
Karla Mendonça: co‐ordinated the review, made an intellectual contribution, extracted data, assessed the risk of bias, interpreted data and drafted the final review.
Contributions of editorial team
Rebecca Fortescue (Co‐ordinating Editor): edited the review. Chris Cates (Co‐ordinating Editor) checked the data entry prior to the full write‐up of the review, advised on methodology, interpretation and content; approved the final review prior to publication. Anne Holland (Editor): edited the review; advised on methodology, interpretation and content. Emma Dennett (Managing Editor): co‐ordinated the editorial process; advised on interpretation and content; edited the review. Emma Jackson (Assistant Managing Editor): conducted peer review; edited the Plain language summary and reference sections of the protocol and the review. Elizabeth Stovold (Information Specialist): designed the search strategy; ran the searches; edited the search methods section.
Sources of support
Internal sources
The review authors declare that no such funding was received for this systematic review, Other.
External sources
-
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Brazil.
TAS is a PhD scholarship holder funded by CAPES (Finance Code 001).
Declarations of interest
TAS: none known GSSC: none known DAF: none known GAFF: none known KMPPM: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Aggarwal 2013 {published data only}
- Aggarwal T, Khatri A, Siddiqui SS, Hasan SN, Deepankar, Kulshreshtha M, et al. Pranayama has additive beneficial effects along with medication in bronchial asthma patients. Journal of Physiology and Pharmacology Advances 2013;3(12):292‐7. [Google Scholar]
Agnihotri 2016 {published data only}
- Agnihotri S, Kant S, Kumar S, Mishra RK, Mishra SK. The assessment of effects of yoga on pulmonary functions in asthmatic patients: a randomized controlled study. Journal of Medical Society 2016;30(2):98‐102. [Google Scholar]
Agnihotri 2018 {published data only}
- Agnihotri S, Kant S, Mishra SK, Verma A. Assessment of significance of yoga on quality of life in asthma patients: a randomized controlled study. Ayu Journal 2017;38:28‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bidwell 2012 {published data only}
- Bidwell AJ, Yazel B, Davin D, Fairchild TJ, Kanaley JA. Yoga training improves quality of life in women with asthma. Journal of Alternative and Complementary Medicine 2012;18(8):749‐55. [DOI] [PubMed] [Google Scholar]
Fluge 1994 {published data only}
- Fluge T, Ritcher H, Fabel H, Zysno E, Wehner E, Wagner IU. Long term effects of breathing exercises and yoga in patients with asthma [Langzeiteffekte von atemgymnastik und yoga bei patienten mit asthma bronchiale]. Pneumologie 1994;48:485‐90. [PubMed] [Google Scholar]
Girodo 1992 {published data only}
- Girodo M, Ekstrand KA, Metiver GJ. Deep diaphragmatic breathing: rehabilitation exercises for the asthmatic patient. Archives of Physiology, Medicine and Rehabilitation 1992;73:717‐20. [PubMed] [Google Scholar]
Grammatopoulou 2011 {published data only}
- Grammatopoulou EP, Skordilis EK, Stavrou N, Myrianthefs P, Karteroliotis K, Baltopoulos G, et al. The effect of physiotherapy‐based breathing retraining on asthma control. Journal of Asthma 2011;48(6):593‐601. [DOI] [PubMed] [Google Scholar]
Gupta 2015 {published data only}
- Gupta N, Kumar A, Joshi HS, Sharma D. Effect of yoga on spirometric value in bronchial asthma patients. Global Journal for Research Analysis 2015;4(7):231‐3. [DOI: 10.15373/22778160] [DOI] [Google Scholar]
Holloway 2007 {published data only}
- Holloway EA, West RJ. Integrated breathing and relaxation training (the Papworth method) for adults with asthma in primary care: a randomised controlled trial. Thorax 2007;62(12):1039–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Malarvizhi 2018 {published data only}
- Malarvizhi M, Maheshkumar K, Bhavani H, Hariprasadd B. Effect of 6 months of yoga practice on quality of life among patient with asthma: a randomized control trial. Advances in Integrative Medicine 2018;6(4):163‐6. [Google Scholar]
Nagarathna 1985 {published data only}
- Nagarathna R, Nagendra HR. Yoga for bronchial asthma: a controlled study. British Medical Journal 1985;291:1077‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prasanna 2015 {published data only}
- Prasanna KB, Sowmiya KR, Dhileeban CM. Effect of Buteyko breathing exercise in newly diagnosed asthmatic patients. International Journal of Medicine and Public Health 2015;5(1):77‐81. [Google Scholar]
Prem 2013 {published data only}
- Prem V, Sahoo RC, Adhikari P. Comparison of the effects of Buteyko and pranayama breathing techniques on quality of life in patients with asthma: a randomized controlled trial. Clinical Rehabilitation 2013;27(2):133‐41. [DOI] [PubMed] [Google Scholar]
Pushpa 2018 {published data only}
- Pushpa K, Sharma D. Yoga as a complementary therapy improves pulmonary functions in patients of bronchial asthma: a randomized controlled trial. National Journal of Physiology, Pharmacy and Pharmacology 2018;8(12):1704‐08. [Google Scholar]
Satpathy 2016 {published data only}
- Satpathy S, Kar A, Purohit KC, Manik R. A comparative study of effect of Yoga on symptoms and drug use in bronchial asthma. IOSR Journal of Dental and Medical Sciences 2016;15(8):41‐4. [DOI: 10.9790/0853-1508074144] [DOI] [Google Scholar]
Singh 2012 {published data only}
- Singh S, Soni R, Singh KP, Tandon OP. Effect of yoga practices on pulmonary function tests including transfer factor of lung for carbon monoxide (TLCO) in asthma patients. Indian Journal of Physiology and Pharmacology 2012;56(1):63‐8. [PubMed] [Google Scholar]
Sodhi 2009 {published and unpublished data}
- Sodhi C, Singh S, Bery A. Assessment of the quality of life in patients with bronchial asthma, before and after yoga: a randomised trial. Iranian Journal of Allergy, Asthma and Immunology 2014;13(1):55‐60. [PubMed] [Google Scholar]
- Sodhi C, Singh S, Dandona PK. A study of the effect of yoga training on pulmonary functions in patients with bronchial asthma. Indian Journal of Physiology and Pharmacology 2009;53(2):169‐74. [PubMed] [Google Scholar]
Thomas 2003 {published data only}
- Thomas M, McKinley RK, Freeman E, Foy C, Prodger P, Price D. Breathing retraining for dysfunctional breathing in asthma: a randomised controlled trial. Thorax 2003;58(2):110‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2009 {published data only}
- Thomas M, McKinley RK, Mellor S, Watkin G, Holloway E, Scullion J, et al. Breathing exercises for asthma: a randomised controlled trial. Thorax 2009;64(1):55‐61. [DOI] [PubMed] [Google Scholar]
Thomas 2017 {published data only}
- Bruton A, Lee A, Yardley L, Raftery J, Arden‐Close E, Kirby S, et al. Physiotherapy breathing retraining for asthma: a randomised controlled trial. Lancet Respiratory Medicine 2017;6(1):19‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M, Bruton A, Little P, Holgate S, Lee A, Yardley L, et al. A randomised controlled study of the effectiveness of breathing retraining exercises taught by a physiotherapist either by instructional DVD or in face‐to‐face sessions in the management of asthma in adults. Health Technology Assessment 2017;21(53):1‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vedanthan 1998 {published data only}
- Vedanthan PK, Kasavalu LN, Mutthy KC, Duvall K, Hall MJ, Baker S, et al. Clinical study of yoga techniques in university students with asthma: a controlled study. Allergy and Asthma Proceedings 1998;19(1):3‐9. [DOI] [PubMed] [Google Scholar]
Vempati 2009 {published data only}
- Vempati R, Bijlani RL, Deepak KK. The efficacy of a comprehensive lifestyle modification programme based on yoga in the management of bronchial asthma: a randomized controlled trial. BMC Pulmonary Medicine 2009;30(9):10.1186/1471‐2466‐9‐37. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aleksandrov 1990 {published data only}
- Aleksandrov OV, Struchkov PV, Fedechkin VV, et al. The use of a respiratory regulator in the combined therapy of obstructive lung diseases. Terapevticheskii Arkhiv 1990;62:73‐75. [PubMed] [Google Scholar]
Anonymous 1968 {published data only}
- Anonymous. Hypnosis for asthma. British Medical Journal 1968;4:71‐6. [PMC free article] [PubMed] [Google Scholar]
Asher 1990 {published data only}
- Asher MI, Douglas C, Airy M, Andrews D, Trenholme A. Effects of chest physical therapy on lung function in children recovering from acute severe asthma. Pediatric Pulmonology 1990;3(9):146‐51. [DOI] [PubMed] [Google Scholar]
Berlowitz 1995 {published data only}
- Berlowitz D, Denehy L, Johns DP, Bish RM, Walters EH. The Buteyko asthma breathing technique [letter]. Medical Journal of Australia 1995;162(1):53. [DOI] [PubMed] [Google Scholar]
Bobokhodzhaev 1984 {published data only}
- Bobokhodzhaev II, Rudoi DG, Bobkhodzhaev OI, Kovaleva IF, Vlasova IF. Effectiveness of the early inclusion of physical methods in the combined therapy of bronchial asthma [Russian]. Voprosy Kurortologii, Fizioterapii i Lechebnoi Fizicheskoi Kultury 1984;1:58‐9. [PubMed] [Google Scholar]
Bowler 1998 {published data only}
- Bowler SD, Green A, Mitchell CA. Buteyko breathing techniques in asthma: a blinded randomised controlled trial. Medical Journal of Australia 1998;169(11‐12):575‐8. [DOI] [PubMed] [Google Scholar]
Cambach 1997 {published data only}
- Cambach W, Chadwick‐Straver RV, Wagenaar RC, Keimpema AR, Kemper HC. The effects of a community‐based pulmonary rehabilitation programme on exercise tolerance and quality of life: a randomized controlled trial. European Respiratory Journal 1997;10:104‐13. [DOI] [PubMed] [Google Scholar]
Cambach 1999 {published data only}
- Cambach W, Wagenaar RC, Koelman TW, Keimpema T, Kemper HC. The long‐term effects of pulmonary rehabilitation in patients with asthma and chronic obstructive pulmonary disease: a research synthesis. Archives of Physical Medicine Rehabilitation 1999;80:103‐11. [DOI] [PubMed] [Google Scholar]
Coll 1994 {published data only}
- Coll R, Tello A. Yoga in bronchial asthma letter [Yoga en el asma bronquial]. Archivos de Bronconeumologia 1994;30(7):369. [DOI] [PubMed] [Google Scholar]
Cooper 2003 {published data only}
- Cooper S, Oborne J, Newton S, Harrison V, Thompson Coon J, Lewis S, et al. Effect of two breathing exercises (Buteyko and pranayama) in asthma: a randomised controlled trial. Thorax 2003;58(8):674‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cowie 2008 {published data only}
- Cowie RL, Conley DP, Underwood MF, Reader PG. A randomised controlled trial of the Buteyko technique as an adjunct to conventional management of asthma. Respiratory Medicine 2008;102(5):726‐32. [DOI] [PubMed] [Google Scholar]
Emtner 1998 {unpublished data only}
- Emtner M. Rehabilitation of adults with asthma: a quantitative and qualitative Study (comprehensive summaries of Uppsala dissertations from the Faculty of Medicine). Acta Universitatis Upsaliensis (1998), 1998. [Google Scholar]
Erskine 1979 {published data only}
- Erskine J, Schonell M. Relaxation therapy in bronchial asthma. Journal of Psychosomatic Research 1979;23:131‐9. [DOI] [PubMed] [Google Scholar]
Falkenbach 1993 {published data only}
- Falkenbach A, Kirchner P, Richter R, Kaiser C, Schultze‐Werninghaus G, Meier‐Sydow J. The influence of a comprehensive respiratory therapy and educational program on the symptoms during metacholine‐induced acute airway obstruction in asthmatic adults. European Journal of Physical Medicine and Rehabilitation 1993;3(3):95‐100. [Google Scholar]
Gallefoss 1999 {published data only}
- Gallefoss F, Bakke PS, Kjaersgaard P. Quality of life assessment after patient education in a randomised controlled study on asthma and chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1999;159:812‐7. [DOI] [PubMed] [Google Scholar]
Gosselink 1993 {published data only}
- Gosselink HA, Wagenaar RC. Efficacy of breathing exercises in chronic obstructive pulmonary disease and asthma. Journal of Rehabilitation Sciences 1993;6:66‐79. [Google Scholar]
Hoang 2015 {published data only}
- Hoang KA, Nguyen HM. The effectiveness of practicing pranayama yoga on some respiratory indicators in patients suffering from bronchial disease. International Journal of Science Culture and Sport 2015;3(2):6‐12. [Google Scholar]
Holmes 1990 {published data only}
- Holmes P. An air of hope. Nursing Times 1990;86(33):21. [PubMed] [Google Scholar]
Johansson 2017 {published data only}
- Johansson EL, Ternestén‐Hasseus E, Olsén MF, Millgvist E. Physical therapy treatment impaired chest mobility in patients with airway sensory hyperreactivity. Physiotherapy Research International 2017;22(2):e1658. [DOI] [PubMed] [Google Scholar]
Joseph 1999 {published data only}
- Joseph CL, Foxman B, Leickly FE, Peterson E, Ownby D. Sensitivity and specificity of asthma definitions and symptoms used in a survey of childhood asthma. Journal of Asthma 1999;36(7):565‐73. [DOI] [PubMed] [Google Scholar]
Karam 2017 {published data only}
- Karam M, Kaur BP, Baptist AP. A modified breathing exercise program for asthma is easy to perform and effective. Journal of Asthma 2017;54(2):217‐22. [DOI] [PubMed] [Google Scholar]
Khanam 1996 {published data only}
- Khanam AA, Sachdevaq U, Guleria R, Deepak KK. Study of pulmonary and autonomic functions of asthma patients after yoga training. Indian Journal of Physiology and Pharmacology 1996;40(4):318‐24. [PubMed] [Google Scholar]
Kotses 1978 {published data only}
- Kotses H, Glaus KD, Bricel SK, Edwards JE, Crawford PL. Operant muscular relaxation and peak expiratory flow rate in asthmatic children. Journal of Psychosomatic Research 1978;22:17‐23. [DOI] [PubMed] [Google Scholar]
Kurabayashi 1998 {published data only}
- Kurabayashi H, Machida I, Handa H, Akiba T, Kubota K. Comparison of three protocols for breathing exercises during immersion in 38C water for chronic obstructive pulmonary disease. American Journal of Physical Medicine Rehabilitation 1998;77(2):145‐8. [PubMed] [Google Scholar]
Lacasse 1997 {published data only}
- Lacasse Y, Guyatt GH, Goldstein RS. The components of a respiratory rehabilitation program. Chest 1997;111:1077‐88. [DOI] [PubMed] [Google Scholar]
Loew 1996 {published data only}
- Loew TH, Siegfried W, Martus P, Tritt K, Hahn EG. 'Functional relaxation' reduces acute airway obstruction in asthmatics as effectively as inhaled terbutaline. Psychotherapy and Psychosomatics 1996;65:124‐8. [DOI] [PubMed] [Google Scholar]
Manocha 2002 {published data only}
- Manocha R, Marks GB, Kenchington P, Peters D, Salome CM. Sahaja yoga in the management of moderate to severe asthma: a randomised controlled trial. Thorax 2002;57:110‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mass 1991 {published data only}
- Mass R, Harden H, Leplow B, Wessel M, Richter R, Dahme B. A device for functional residual capacity controlled biofeedback of respiratory resistance [Ein messaufbau zur ruckmeldung des atemwiderstandes unter kontrolle der funktionellen residualkapazitat]. Biomedizinische Technik 1991;36(4):78‐85. [DOI] [PubMed] [Google Scholar]
McFadden 1986 {published data only}
- McFadden ER Jr, Lenner KA, Strohl KP. Postexertional airway rewarming and thermally induced asthma. Journal of Clinical Investigation 1986;78:18‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
McHugh 2003 {published data only}
- McHugh P, Aitcheson F, Duncan B, Houghton F. Buteyko breathing technique for asthma: an effective intervention. New Zealand Medical Journal 2003;116(1187):U710. [PubMed] [Google Scholar]
Mussell 1986 {published data only}
- Mussell MJ. Trachea noise biofeedback device to help reduce bronchospasm in asthmatics. Journal of Biomedical Engineering 1986;8:341‐4. [DOI] [PubMed] [Google Scholar]
Opat 2000 {published data only}
- Opat AJ, Cohen MM, Bailey MJ, Abramson MJ. A clinical trial of the Buteyko breathing technique in asthma as taught by a video. Journal of Asthma 2000;37(7):557‐64. [DOI] [PubMed] [Google Scholar]
Paleev 1988 {published data only}
- Paleev NR, Tsar Kova LN, Chenko VA. Anti‐recurrence treatment of bronchial asthma and chronic bronchitis [Russian]. Klinicheskaia Meditsina 1988;66(12):15‐21. [PubMed] [Google Scholar]
Pryor 1979 {published data only}
- Pryor J, Webber BA. An evaluation of the forced expiration technique as an adjunct to postural drainage. Physiotherapy 1979;65(10):304‐7. [PubMed] [Google Scholar]
Raghavendra 2016 {published data only}
- Raghavendra P, Shetty P, Shetty S, Manjunath NK, Saoji AA. Effect of high‐frequency yoga breathing on pulmonary functions in patients with asthma. Annals of Allergy, Asthma and Immunology 2016;117(5):550–1. [DOI] [PubMed] [Google Scholar]
Redchits 1986 {published data only}
- Redchits IV, Treumova SI. Effectiveness of the differential treatment of bronchial asthma patients on the southern coast of the Crimea. Voprosy Kurortologii, Fizioterapii, i Lechebnoi Fizicheskoi Kultury 1986;4:54‐6. [PubMed] [Google Scholar]
Sabina 2005 {published data only}
- Sabina AB, Williams A‐L, Wall HK, Bansal S, Chupp G, Katz DL. Yoga intervention for adults with mild‐to‐moderate asthma: a pilot study. Annals of Allergy, Asthma and Immunology 2005;94(5):543‐8. [DOI] [PubMed] [Google Scholar]
Saxena 2009 {published data only}
- Saxena T, Saxena M. The effect of various breathing exercises (pranayama) in patients with bronchial asthma of mild to moderate severity. International Journal of Yoga 2009;2(1):22‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulze 2000 {published data only}
- Schulze J, Riel B, Fischer S, Lecheler J, Hofmann D. Improvement of the quality of life by asthma training [Verbesserung der Lebensqualität durch Asthmaschulung]. Pravent‐Rehabil 2000;12(3):91‐8. [Google Scholar]
Shaw 2011 {published data only}
- Shaw BS, Shaw I. Static standing posture and pulmonary function in moderate‐persistent asthmatics following aerobic and diaphragmatic breathing training. Pakistan Journal of Medical Sciences 2011;27(3):549‐52. [Google Scholar]
Singh 1987 {published data only}
- Singh V. Effect of respiratory exercises on asthma. Journal of Asthma 1987;24(6):355‐9. [DOI] [PubMed] [Google Scholar]
Singh 1990 {published data only}
- Singh V, Wisniewski A, Britton J, Tattersfield A. Effect of yoga breathing exercises (pranayama) on airway reactivity in subjects with asthma. Lancet 1990;335:1381‐3. [DOI] [PubMed] [Google Scholar]
Slader 2006 {published data only}
- Slader CA, Reddel HK, Spencer LM, Belousova EG, Armour CL, Bosnic‐Anticevich SZ, et al. Double blind randomised controlled trial of two different breathing techniques in the management of asthma. Thorax 2006;61(2):651‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smyth 1999 {published data only}
- Smyth JM, Soeffer MH, Hurewitz A, Stone AA. The effect of tape recorded relaxation training on well being symptoms, and peak expiratory flow rate in adult asthmatics: a pilot study. Psychology and Health 1999;14:487‐501. [Google Scholar]
Tandon 1978 {published data only}
- Tandon MK. Adjunct treatment with yoga in chronic severe airways obstruction. Thorax 1978;33:514‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van der Schans 1997 {published data only}
- Schans CP, Jong W, Vries G, Postma DS, Koeter GH, Mark TW. Respiratory muscle activity and pulmonary function during acutely induced airways obstruction. Physiotherapy Research International 1997;2(3):167‐94. [DOI] [PubMed] [Google Scholar]
Weiner 1992 {published data only}
- Weiner MD, Azgad Y, Ganem R, Weiner M. Inspiratory muscle training in patients with bronchial asthma. Chest 1992;102(5):1357‐61. [DOI] [PubMed] [Google Scholar]
Wilson 1975 {published data only}
- Wilson AF, Honsberger R, Chiu JT, Novey HS. Transcendental meditation and asthma. Respiration 1975;32:74‐80. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Agnihotri 2013 {published data only}
- Agnihotri S. Significance of yoga in asthma management to improve the status of quality of life. European Journal of Allergy and Clinical Immunology. Conference: European Academy of Allergy and Clinical Immunology and World Allergy Organization World Allergy and Asthma Congress 2013. Milan, Italy, 2013; Vol. 68:378.
Divya 2013 {published data only}
- Divya, Kusumadevi MS, Veeriah S. Effect of yoga training on airway resistance and specific airway conductance in patients of bronchial asthma. Indian Journal of Physiology and Pharmacology. Conference: 59th Annual Conference of Association of Physiologists and Pharmacologists of India, APPI 2013. Bengaluru, India, 2013; Vol. 57 (5 SUPPL. 1):126‐127.
Kant 2013 {published data only}
- Kant K, Agnihotri S. Asthma diagnosis and treatment – 1029. Yoga as an adjuvant therapy in asthma management. World Allergy Organization Journal 2013;6(1):P28. [Google Scholar]
References to ongoing studies
Andreasson 2017 {unpublished data only}
- Andreasson KH, Bødtger U, Skou ST, et al. Breathing Exercises in Asthma Targeting Dysfunctional Breathing. Clinical Trials 2017.
Elahi 2016 {unpublished data only}
- Elahi N. Effect of breathing exercises on indicators of clinical,spirometric parameters and quality of life of patientswith asthma reffered to Allergic Asthma clinic ofImam Khomeini in Ahwaz.. Iranian Registry of Clinical Trials 2016.
Murthy 2010 {unpublished data only}
- Murthy. Effect of naturopathy interventions in bronchial asthma. 2010.
Vagedes 2017 {unpublished data only}
- Vagedes J. Effectiveness of a Buteyko‐based Breathing Technique for Asthma Patients. Clinical Trials 2017.
Additional references
Arden‐Close 2017
- Arden‐Close E, Yardley L, Kirby S, Thomas M, Bruton A. Patients' experiences of breathing retraining for asthma: a qualitative process analysis of participants in the intervention arms of the BREATHE trial. NPJ Primary Care Respiratory Medicine 2017;27(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Arun 2012
- Arun JJ, Lodha R, Kabra SK. Bronchodilatory effect of inhaled budesonide/formoterol and budesonide/salbutamol in acute asthma: a double‐blind, randomized controlled trial. BMC Pediatrics 2012;12:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Austin 2013
- Austin G. Buteyko technique use to control asthma symptoms. Nursing Times 2013;109(16):16‐7. [PubMed] [Google Scholar]
Bailey 2016
- Bailey NW, Bridgman TK, Marx W, Fitzgerald PB. Asthma and mindfulness: an increase in mindfulness as the mechanism of action behind breathing retraining techniques?. Mindfulness 2016;7(6):1249–55. [Google Scholar]
Bandy 2012
- Bandy WD, Sanders B. Therapeutic exercise for physical therapy assistants: techniques for intervention. 3. Philadelphia: Wolters Kluwer: Lippincott Williams & Wilkins, 2012. [Google Scholar]
Barreiro 2004
- Barreiro TJ, Perillo I. An approach to interpreting spirometry. American Family Physician 2004;69(5):1107‐14. [PubMed] [Google Scholar]
Bateman 2008
- Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M. Global strategy for asthma management and prevention: GINA executive summary. European Respiratory Journal 2008;31(1):143‐78. [DOI] [PubMed] [Google Scholar]
Beasley 2016
- Beasley R, Hancox RJ, Harwood M, Perrin K, Poot B, Pilcher J, et al. Asthma and respiratory foundation NZ adult asthma guidelines: a quick reference guide. New Zealand Medical Journal 2016;129(1445):83‐102. [PubMed] [Google Scholar]
Bernardi 2000
- Bernardi L, Wdowczyk‐Szulc J, Valenti C, Castoldi S, Passino C, Spadacini G, et al. Effects of controlled breathing, mental activity and mental stress with or without verbalization on heart rate variability. Journal of the American College of Cardiology 2000;35(6):1462‐9. [DOI] [PubMed] [Google Scholar]
Blanc 2001
- Blanc PD, Trupin L, Earnest G, Katz PP, Yelin EH, Eisner MD. Alternative therapies among adults with a reported diagnosis of asthma or rhinosinusitis: data from a population‐based survey. Chest 2001;120(5):1461‐7. [DOI] [PubMed] [Google Scholar]
Boulding 2016
- Boulding R, Stacey R, Niven R, Fowler SJ. Dysfunctional breathing: a review of the literature and proposal for classification. European Respiratory Review 2016;25(141):287‐94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Braido 2013
- Braido F. Failure in asthma control: reasons and consequences. Hindawi Scientifica 2013;2013:549252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brightling 2012
- Brightling CE, Gupta S, Gonem S, Siddiqui S. Lung damage and airway remodelling in severe asthma. Clinical and Experimental Allergy 2012;42(5):638‐49. [DOI] [PubMed] [Google Scholar]
Bruton 2005
- Bruton A, Lewith GT. The Buteyko breathing technique for asthma: a review. Complementary Therapies in Medicine 2005;13(1):41‐6. [DOI] [PubMed] [Google Scholar]
Bruurs 2013
- Bruurs ML, Giessen LJ, Moed H. The effectiveness of physiotherapy in patients with asthma: a systematic review of the literature. Respiratory Medicine 2013;107(4):483‐94. [DOI] [PubMed] [Google Scholar]
Burgess 2011
- Burgess J, Ekanayake B, Lowe A, Dunt D, Thien F, Dharmage SC. Systematic review of the effectiveness of breathing retraining in asthma management. Expert Review of Respiratory Medicine 2011;5(6):789‐807. [DOI] [PubMed] [Google Scholar]
Chaitow 2014
- Chaitow L, Gilbert C, Morrison D. Recognizing and treating breathing disorders: a multidisciplinary approach. 2nd Edition. Elsevier Health Sciences, 2014. [Google Scholar]
Courtney 2008
- Courtney R. Strengths, weaknesses, and possibilities of the Buteyko breathing method. Biofeedback 2008;36(2):59‐63. [Google Scholar]
Courtney 2017
- Courtney R. Breathing training for dysfunctional breathing in asthma: taking a multidimensional approach. ERJ Open Research 2017;3(4):00065‐2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Courtney 2019
- Courtney R, Biland G, Ryan A, Grace S, Gordge R. Improvements in multi‐dimensional measures of dysfunctional breathing in asthma patients after a combined manual therapy and breathing retraining protocol: a case series report. International Journal of Osteopathic Medicine 2019;31:36‐43. [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors), on behalf ofthe Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Denehy 2016
- Denehy L, Main E. Cardiorespiratory physiotherapy: adults and paediatrics. 5th Edition. Elsevier, 2016. [Google Scholar]
Dennis 2000
- Dennis JA, Cates CJ. Alexander technique for chronic asthma. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000995] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dennis 2012
- Dennis JA, Cates CJ. Alexander technique for chronic asthma. Cochrane Database of Systematic Reviews 2013, Issue 9. [DOI: 10.1002/14651858.CD000995.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Eisner 2012
- Eisner MD, Yegin A, Trzaskoma B. Severity of asthma score predicts clinical outcomes in patients with moderate to severe persistent asthma. Chest 2012;141(1):58‐65. [DOI] [PubMed] [Google Scholar]
Enright 2004
- Enright PL, Beck K, Sherrill DL. Repeatability of spirometry in 18,000 adult patients. American Journal of Respiratory and Critical Care Medicine 2004;169(2):235‐8. [DOI] [PubMed] [Google Scholar]
Evans 1993
- Evans D. To help patients control asthma the clinician must be a good listener and teacher. Thorax 1993;48(7):685‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giavina‐Bianchi 2010
- Giavina‐Bianchi P, Aun MV, Bisaccioni C, Agondi R, Kalil J. Difficult‐to‐control asthma management through the use of a specific protocol. Clinics 2010;65(9):905‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gillis 2017
- Gillis RM, Van Litsenburg, Balkom WR, Muris JW, Smeenk FW. The contribution of an asthma diagnostic consultation service in obtaining an accurate asthma diagnosis for primary care patients: results of a real‐life study. NPJ Primary Care Respiratory Medicine 2017;27(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
GINA 2018
- Global Initiative for Asthma. Global Strategy for Asthma Management and Prevention 2018. ginasthma.org/ (accessed 2 April 2018).
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 01 August 2018. Hamilton (ON): McMaster University (developed by Evidence Prime).
Graham 2019
- Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, et al. Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. American Journal of Respiratory and Critical Care Medicine 2019;200(8):e70‐e88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Grammatopoulou 2014
- Grammatopoulou EP, Skordilis EK, Georgoudis G, Haniotou A, Evangelodimou A, Fildissis G, et al. Hyperventilation in asthma: a validation study of the Nijmegen Questionnaire‐‐NQ. Journal of Asthma 2014;51(8):839‐46. [DOI] [PubMed] [Google Scholar]
Hakked 2017
- Hakked CS, Balakrishnan R, Krishnamurthy MN. Yogic breathing practices improve lung functions of competitive young swimmers. Journal of Ayurveda and Integrative Medicine 2017;8(2):99‐104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Deeks JJ. Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
James 2016
- James DR, Lyttle MD. British guideline on the management of asthma: SIGN Clinical Guideline. Archives of Disease in Childhood: Education & Practice 2016;101(6):319‐22. [DOI] [PubMed] [Google Scholar]
Jones 2002
- Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. European Respiratory Journal 2002;19(3):398‐404. [DOI] [PubMed] [Google Scholar]
Juniper 2004
- Juniper EF, Wisniewski ME, Cox FM, Emmett AH, Nielsen KE, O'Byrne PM. Relationship between quality of life and clinical status in asthma: a factor analysis. European Respiratory Journal 2004;23(2):287‐91. [DOI] [PubMed] [Google Scholar]
Juniper 2005
- Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shorted versions of the asthma control questionnaire. Respiratory Medicine 2005;99(5):553‐8. [DOI] [PubMed] [Google Scholar]
Kew 2016
- Kew KM, Cates CJ. Home telemonitoring and remote feedback between clinic visits for asthma. Cochrane Database of Systematic Reviews 2016, Issue 8. [DOI: 10.1002/14651858.CD011714.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lavoie 2005
- Lavoie KL, Cartier A, Labrecque M, Bacon SL, Lemière C, Malo JL, et al. Are psychiatric disorders associated with worse asthma control and quality of life in asthma patients?. Respiratory Medicine 2005;99(10):1249‐57. [DOI] [PubMed] [Google Scholar]
Lopes 2018
- Lopes CP, Danzmann LC, Moraes RS, Vieira PJ, Meurer FF, Soares DS. Yoga and breathing technique training in patients with heart failure and preserved ejection fraction: study protocol for a randomized clinical trial. Trials 2018;19(1):405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2017
- Ma X, Yue ZQ, Gong ZQ, Zhang H, Duan NY, Shi YT, et al. The effect of diaphragmatic breathing on attention, negative affect and stress in healthy adults. Frontiers in Psychology 2017;8:874. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mark 2015
- Mark JD, Chung Y. Complementary and alternative medicine in pulmonology. Current Opinion in Pediatrics 2015;27(3):334‐40. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA Statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2012
- Moore VC. Spirometry: step by step. Breathe 2012;8(3):232‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morgan 2002
- Morgan MD. Dysfunctional breathing in asthma: is it common, identifiable and correctable?. Thorax 2002;57(Suppl II):ii31‐ii35. [PMC free article] [PubMed] [Google Scholar]
Muktibodhananda 2002
- Muktibodhananda S. Hatha yoga pradipika: light on hatha yoga. 2. Bihar: Yoga Publication Trust, 2002. [Google Scholar]
National Asthma Council Australia 2016
- National Asthma Council Australia. Australian Asthma Handbook. www.asthmahandbook.org.au/diagnosis/adults (accessed 21 August 2018).
Nunes 2017
- Nunes C, Pereira AM, Morais‐Almeida M. Asthma costs and social impact. Asthma Research and Practice 2017;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ram 2003
- Ram FS, Holloway EA, Jones PW. Breathing retraining for asthma. Respiratory Medicine 2003;97(5):501‐7. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2004
- Riley D. Hatha yoga and the treatment of illness. Alternative Therapies in Health and Medicine 2004;10(2):20‐1. [PubMed] [Google Scholar]
Rimington 2001
- Rimington LD, Davies DH, Lowe D, Pearson MG. Relationship between anxiety, depression, and morbidity in adult asthma patients. Thorax 2001;56(4):266‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sankar 2018
- Sankar J, Das RR. Asthma ‐ a disease of how we breathe: role of breathing exercises and pranayama. Indian Journal of Pediatrics 2018;85(10):905‐10. [DOI] [PubMed] [Google Scholar]
Sterne 2017
- Sterne JA, Egger M, Moher D, Boutron I (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Sveum 2012
- Sveum R, Bergstrom J, Brottman G, Hanson M, Heiman M, Johns K, et al. Institute for Clinical Systems Improvement. Diagnosis and management of asthma. Updated July 2012. www.icsi.org/_asset/rsjvnd/Asthma‐Interactive0712.pdf (accessed 23 September 2013).
Tepper 2012
- Tepper RS, Wise RS, Covar R, Irvin CG, Kercsmar CM, Kraft M, et al. Asthma outcomes: pulmonary physiology. Journal of Allergy Clinical Immunology 2012;129(30):S65–S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2001
- Thomas M, McKinley RK, Freeman E, Foy C. Prevalence of dysfunctional breathing in patients treated for asthma in primary care: cross sectional survey. BMJ 2001;322(7294):1098‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
To 2012
- To T, Stanojevic S, Moores G, Gershon AS, Bateman ED, Cruz AA, et al. Global asthma prevalence in adults: findings from the cross‐sectional world health survey. BMC Public Health 2012;12:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Torres‐Llenza 2010
- Torres‐Llenza V, Bhogal S, Davis M, Ducharme F. Use of complementary and alternative medicine in children with asthma. Canadian Respiratory Journal 2010;17(4):183–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
van Dixhoorn 2015
- Dixhoorn J, Folgering H. The Nijmegen Questionnaire and dysfunctional breathing. ERJ Open Research 2015;1(1):00001‐2015. [DOI: 10.1183/23120541.00001-2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Varvogli 2011
- Varvogli L, Darviri C. Stress management techniques: evidence‐based procedures that reduce stress and promote health. Health Science Journal 2011;5(2):74‐89. [Google Scholar]
Veidal 2017
- Veidal S, Jeppegaard M, Sverrild A, Backer V, Porsbjerg C. The impact of dysfunctional breathing on the assessment of asthma control. Respiratory Medicine 2017;123:42‐7. [DOI] [PubMed] [Google Scholar]
Vidotto 2019
- Vidotto LS, Carvalho CR, Harvey A, Jones M. Dysfunctional breathing: what do we know?. Jornal Brasileiro de Pneumologia 2019;45(1):e20170347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Watchie 2010
- Watchie J. Cardiovascular and pulmonary physical therapy: a clinical manual. 2. Saunders Elsevier, 2010. [Google Scholar]
Welsh 2011
- Welsh EJ, Hasan M, Li P. Home‐based educational interventions for children with asthma. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD008469.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2018
- World Health Organization. Asthma fact sheets. www.who.int/en/news‐room/fact‐sheets/detail/asthma (accessed 2 April 2018).
Yang 2016
- Yang ZY, Zhong HB, Mao C, Yuan JQ, Huang YF, Wu XY, et al. Yoga for asthma. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD010346] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2010
- Zhang X, Köhl J. A complex role for complement in allergic asthma. Expert Review of Clinical Immunology 2010;6(2):269‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Freitas 2013
- Freitas DA, Holloway EA, Bruno SS, Chaves GS, Fregonezi GA, Mendonça KM. Breathing exercises for adults with asthma. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD001277.pub3] [DOI] [PubMed] [Google Scholar]
Holloway 2004
- Holloway E, Ram FS. Breathing exercises for asthma. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD001277.pub2] [DOI] [PubMed] [Google Scholar]


