Abstract
Background
Epidermal growth factor receptor (egfr) tyrosine kinase inhibitors (tkis) are standard therapy for patients with advanced or metastatic non-small-cell lung cancer harbouring an EGFR mutation. Upon progression, 50%–60% develop a secondary T790M mutation. Recent trials demonstrated outcome improvement with osimertinib compared with standard platinum-based chemotherapy as second-line therapy for patients with secondary T790M mutation. To identify T790M, a biopsy of the tumour or, more recently, plasma is necessary. This retrospective study aimed to evaluate biopsy procedures and mutational analysis at 2 Canadian cancer centres.
Methods
In a retrospective review of patients who were approached to enrol in the aura2, aura3, or astris studies, demographics, eligibility for rebiopsy upon progression after an egfr tki, rebiopsy methods and complications, number of rebiopsies, and incidence of the T790M mutation were collected.
Results
Of 84 patients considered for trial enrolment, 80 signed a consent. In 78 patients who underwent rebiopsy, computed tomography or ultrasonography guidance were the most common methods used. The most common biopsy sites were lung and lymph nodes. The median number of rebiopsies performed to find a T790M mutation was 2. Only 9% of patients experienced complications. Of samples obtained, 74% were adequate for testing after initial rebiopsy. A T790M mutation was found in 47 patients, of whom 44 were enrolled on a trial. After multiple rebiopsies, only 5% of samples were inadequate for molecular analysis.
Conclusions
In the Canadian setting, the acceptance of rebiopsy on progression was high. Multiple rebiopsies were clinically feasible and could increase the yield for T790M mutation. The incidence of complications was low despite the most common site for rebiopsy being lung.
Keywords: Biopsy, non-small-cell lung cancer, EGFR mutation
INTRODUCTION
An activating mutation in exon 19 or 21 of the kinase domain of the EGFR gene, first reported in 20041,2, confers sensitivity to epidermal growth factor receptor (egfr) tyrosine kinase inhibitors (tkis), including first-generation (gefitinib, erlotinib) and second-generation types (afatinib and, more recently, dacomitinib). Subsequently, randomized phase iii trials showed superior median progression-free survival, objective response rate, safety and tolerability, and in some instances, median overall survival for egfr tkis compared with platinum-based chemotherapy3–12. As a result, egfr tkis have been widely adopted into clinical practice throughout the world for patients with recurrent and metastatic nonsquamous non-small-cell lung cancer (nsclc) that harbours activating EGFR mutations: either an exon 19 deletion or an exon 21 L858R point mutation.
Despite an initial rapid response to egfr tki treatment, patients will inevitably experience disease progression after a median duration of 10–20 months3–8,11,13. As summarized by Gainor and Shaw14, multiple resistance mechanisms found in post-progression biopsies have been documented in the literature. The most common secondary resistance mechanism is a T790M mutat ion in exon 20 of EGFR, which leads to steric hindrance for binding of both the first- and second-generation egfr tkis at the kinase domain. Osimertinib was found to yield improvements in the overall response rate, progression-free survival, and tolerability in phase i/ii studies (aura and aura2)15–17 involving patients with that mutat ion. Later, osimertinib was associated with superior clinical outcomes (progression-free survival, overall response rate, and tolerability) when compared with both standard platinum–pemetrexed palliative chemotherapy in patients pre-treated with an egfr tki (aura3)18 and with first-generation egfr tkis in patients with treatment-naïve EGFR mutation–positive (EGFRm+) recurrent and metastatic nsclc (flaura)19.
To tailor subsequent therapy to resistant molecular aberrations, including T790M, a new tissue or plasma biopsy (“rebiopsy”) is necessary, and up to 7% of patients refused to undergo biopsy for various reasons, including perceived risk, occurrence of complications during an earlier biopsy, and poor health status, among others. Approximately 20%–50% of patients did not undergo a rebiopsy upon progression because of the absence of a lesion that was amenable to rebiopsy, physician decision, age of the patient, comorbidity that was deemed unsafe to permit rebiopsy, and declining performance status20–26. However, little research is available about how frequently post-progression rebiopsies of recurrent and metastatic EGFRm+ nsclc are used in clinical practice in the Canadian setting. In addition, little is known about the clinical utility of post-progression rebiopsy procedures in terms of acceptance by patients and of sufficiency and quality to effectively guide treatment. Furthermore, the rate of rebiopsy-related complications in Canada is not well documented. We therefore conducted a retrospective analysis of biopsy-on-progression procedures and mutational analysis in patients who were approached to enrol in the aura2, aura3, and astris trials at 2 Canadian centres.
METHODS
Study Design
This retrospective cohort study of patients who were being considered for potential enrolment in the aura2 (NCT02094261 at https://ClinicalTrials.gov/), aura3 (NCT02151981), or astris (NCT02474355) trials had the primary objective to describe and characterize the acceptance rate by patients and the feasibility rate for rebiopsy on progression of EGFRm+ nonsquamous nsclc at 2 Canadian academic cancer centres. Data from June 2014 to February 2017 (based on date of progression) were included.
Secondary objectives included
■ describing the demographic and clinical characteristics of patients with nonsquamous nsclc harbouring activating EGFR mutations who were considered for rebiopsy on progression.
■ describing the reasons for exclusion of, and barriers to testing for, patients judged to be ineligible for rebiopsy on progression.
■ quantifying the timelines associated with rebiopsy on progression.
■ characterizing the procedure used for rebiopsy on progression (for example, needle type, anatomic localization).
■ describing complications associated with rebiopsy on progression and their clinical management.
Study Population
The study included patients with EGFRm+ nonsquamous recurrent or metastatic nsclc from 2 Canadian institutions (BC Cancer–Vancouver in British Columbia, and the Cross Cancer Institute in Edmonton, Alberta) who were evaluated to undergo post-progression rebiopsy for potential enrolment on the aura2, aura3, and astris studies. Research ethics approval was obtained from the respective institutional research ethics boards before study initiation. Patients who were T790M-positive, -negative, or -unknown (that is, test results were inconclusive) and those who did not undergo rebiopsy on progression were included in the analysis.
Variables and Outcomes
Demographic and clinical characteristics were abstracted from patient charts and electronic medical records. Study measures, tied directly to each objective, included a full description of the patient with respect to initial diagnosis, treatment, biopsy history and T790M testing, summary of acceptance or refusal to undergo rebiopsy on progression, timelines for the rebiopsy procedure, eligibility or ineligibility for T790M-targeted therapy, and an overview of complications for those who underwent rebiopsy.
Statistical Analysis
Descriptive analyses on the study measures were conducted.
RESULTS
Baseline Characteristics
The screening logs from aura2 (June–September 2014), aura3 (November 2014–August 2015), and astris (April 2015–February 2017) were reviewed, and 84 patients with an activating EGFR mutation who had progressed on an egfr tki and who had been approached for trial enrolment were identified.
Median age of the 84 patients was 63 years (range: 36–86 years), with 79% being women, 75% having an Eastern Cooperative Oncology Group performance status of 0–1, and 73% being never-smokers (Table I). Exon 19 was the more common EGFR mutation (60%). Monotherapy with an egfr tki was given as first-line treatment in 61 patients. In 20 patients, prior platinum-based chemotherapy with or without pemetrexed maintenance had been given for their recurrent or metastatic EGFRm+ nsclc. Only 3 patients received an egfr tki plus 2 or more lines of palliative systemic therapy. Furthermore, 68 patients had received an egfr tki as their most recent line of therapy, and 14 had received an egfr tki followed by platinum-based chemotherapy. Only 2 patients had received an egfr tki followed by more than 1 line of prior therapy before their rebiopsy.
TABLE I.
Baseline patient characteristics
| Characteristic | Value |
|---|---|
| Participants (n) | 84 |
|
| |
| Age (years) | |
| Median | 63 |
| Range | 36–86 |
|
| |
| Sex [n (%)] | |
| Men | 27 (31) |
| Women | 57 (79) |
|
| |
| ECOG PS [n (%)] | |
| 0 | 9 (11) |
| 1 | 54 (64) |
| 2 | 19 (23) |
| Missing or not reported | 2 (2) |
|
| |
| Smoking status [n (%)] | |
| Former smoker | 18 (21) |
| Current smoker | 5 (6) |
| Never-smoker | 61 (73) |
|
| |
| EGFR mutation [n (%)] | |
| Exon 19 deletion | 50 (60) |
| Exon 21 L858R | 30 (36) |
| L861Q | 1 (1) |
| Not reported | 3 (4) |
|
| |
| Prior curative therapy [n (%)] | |
| Yes | 15 (18) |
| No | 69 (82) |
|
| |
| Prior systemic therapy [n (%)] | |
| EGFR TKI | |
| Alone | 61 (73) |
| Plus platinum-based chemotherapy ± maintenance pemetrexed | 20 (24) |
| Plus platinum-based chemotherapy ± maintenance pemetrexed + other systemic therapy | 3 (3) |
|
| |
| Prior response to EGFR TKI [n (%)] | |
| Complete response | 1 (1) |
| Partial response | 59 (70) |
| Stable disease | 18 (21) |
| Progressive disease | 5 (6) |
| Unknown or missing data | 1 (1) |
|
| |
| Prior palliative radiation [n (%)] | |
| Yes | 56 (67) |
| No | 28 (34) |
|
| |
| Managing site [n (%)] | |
| BC Cancer–Vancouver | 60 (71) |
| Cross Cancer Institute | 24 (29) |
|
| |
| Trial participation [n (%)] | |
| ASTRIS | |
| Assessed | 49 (58) |
| Enrolled | 27 (32) |
| AURA2 | |
| Assessed | 14 (17) |
| Enrolled | 6 (7) |
| AURA3 | |
| Assessed | 21 (25) |
| Enrolled | 11 (13) |
ECOG PS = Eastern Cooperative Oncology Group performance status.
Characteristics of Rebiopsy
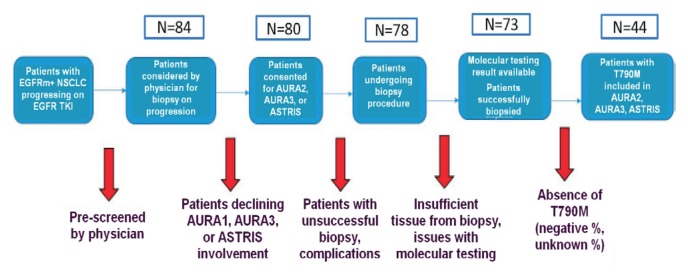
Of 80 patients who signed consent for trial enrolment, 78 underwent rebiopsy, and of those 78, 58 (74%) had adequate dna for EGFR mutational analysis for both activating and T790M mutations, with 33 patients being found to be T790M-positive. Interestingly, T790M positivity was more likely to be detected in samples from patients who had undergone image-guided rebiopsy by either computed tomography (cr) or ultrasonography (48%–57% vs. 11%–36% in patients whose biopsy was not image-guided). In 7 samples, insufficient tumour cells were obtained for molecular analysis. An additional 11 rebiopsy samples failed to yield adequate dna for molecular analysis. A 2nd rebiopsy in 25 patients produced 15 samples deemed to be adequate for testing, with 94% of patients having adequate tissue after 2 attempts. Of 25 patients, 8 tested positive for T790M mutation. A 3rd rebiopsy in 13 patients led to 9 specimens being deemed adequate for testing, with 5 additional patients being found to harbour the T790M mutation. A 4th rebiopsy by endoscopic bronchial ultrasonography (ebus) in 1 patient was positive for T790M mutation. In total, 47 patients were found to have the T790M mutation after a median of 2 rebiopsy attempts during the screening period for the 3 osimertinib studies. Of the patients found to be T790M-positive, 44 were successfully enrolled onto one of the clinical studies (Figure 1).
FIGURE 1.
CONSORT diagram of patient deposition in this analysis. EGFRm = EGFR mutation; NSCLC = non-small-cell lung cancer; TKI = tyrosine kinase inhibitor.
Lung and mediastinal nodes (64%) were the most common biopsy sites, followed by thoracentesis (14%), liver (8%), bone (4%), and others (Table II). The 3 most common methods of rebiopsy were ct-guided lung or liver biopsy (32%), followed by bronchoscopy or ebus (32%) and ultrasonography-guided biopsy of liver, lymph nodes, or a chest wall lesion (18%). Core biopsy was the most common pathology sample (48% of patients), followed by fine-needle aspiration (18%), and transbronchial samples (14%). Plasma T790M testing was performed for only 4% of patients.
TABLE II.
Operational characteristics of biopsy
| Characteristic | Value |
|---|---|
| Patients (n) | 78 |
|
| |
| Sample type [n (%)] | |
| Core needle | 38 (48) |
| Fine-needle aspiration | 14 (18) |
| Transbronchial biopsy | 11 (14) |
| Pleural fluid cytology or cell block | 6 (7.7) |
| Plasma | 3 (3.8) |
| Brain resection | 2 (2.6) |
| Lung resection | 1 (1.3) |
| Othera | 2 (2.6) |
|
| |
| Biopsy method [n (%)] | |
| Overall | |
| Computed tomography–guided | 25 (32) |
| Bronchoscope or EBUS | 25 (32) |
| Ultrasonography-guided | 14 (18) |
| Plasma | 3 (3.8) |
| Other | 9 (12) |
| Unknown/Not reported | 2 (2.6) |
| Sample showing T790M positivity | |
| Computed tomography–guided | 12 (15) |
| Bronchoscope or EBUS | 9 (12) |
| Ultrasonography-guided | 8 (10) |
| Plasma | 1 (1) |
| Other | 1 (1) |
| Unknown/Not reported | 1(1) |
|
| |
| Biopsy location [n (%)] | |
| Lung or lymph nodesb | 50 (64) |
| Pleural fluid | 11 (14) |
| Liver | 6 (7.7) |
| Bone | 3 (3.8) |
| Plasma | 3 (3.8) |
| Brain | 2 (2.6) |
| Chest wall | 1 (1.3) |
| Peritoneal mass | 1 (1.3) |
| Unknown or missing data | 1 (1.3) |
Endobronchial brushing or pericardial fluid cytology.
Mediastinal or supraclavicular.
CT = computed tomography; EBUS = endoscopic bronchial ultrasonography
Median time from documented radiologic progression to first rebiopsy was 73.9 ± 171.3 days); median time from rebiopsy to receipt of the sample at the central or local molecular laboratory was 35 ± 161.7 days; and median time from receipt at the molecular laboratory to result availability was 19.8 ± 69.3 days.
Complications from Biopsy and Rebiopsy
Of the 84 patients, 9 (11%) experienced complications when they underwent biopsy at the initial diagnosis of their nsclc. The most common complications were pneumothorax with or without bleeding [4% (2 patients undergoing ct-guided lung biopsy, and 1 patient undergoing bronchoscopy or ebus)], followed by desaturation post-bronchoscope (1 patient), pain from puncture of the femoral cortex (1 patient), and bleeding and pain at the biopsy site (1 patient). None of the complications required invasive measures except in the patient who developed pneumothorax and bleeding after the bronchoscopy or ebus (treated with balloon tamponade and lavage).
Of the 78 patients (9%) who underwent rebiopsy for T790M mutation analysis, 7 experienced complications; all except 1 experienced the complication during the 1st rebiopsy (Table III). Pneumothorax occurred in 4 patients after ct-guided biopsy of a lung lesion. All cases of pneumothorax resolved spontaneously without aggressive interventions. After ct-guided lung biopsy, 1 patient each experienced fever requiring antibiotics, minor bleeding in the lung, and chest pain and cough requiring no further therapy. No biopsy-related death was observed.
TABLE III.
Outcome of biopsy samples
| Outcome | Value |
|---|---|
| Patients (n) | 78 |
|
| |
| Complications of rebiopsy [n (%)] | |
| Yes | 7 (9) |
| No | 67 (86) |
| Not reported | 4 (5.1) |
|
| |
| Adequacy of tissue for molecular analysis after initial rebiopsy [n (%)] | |
| Yes | 58 (74) |
| No | 18 (23) |
| Inadequate tumour tissue | 7 (9) |
| Inadequate DNA in tumour tissue | 11 (14) |
| Missing or not reported | 2 (3) |
|
| |
| Molecular testing facility used for first adequate rebiopsy [ n (%)] | |
| Local | 27 (35) |
| Central | 18 (23) |
| Third-party | 9 (12) |
| Local and central | 1 (1.3) |
| Unknown or not reported | 3 (4) |
|
| |
| Sample type [n (%)] | |
| Tissue | 58 (74) |
| Plasma | 3 (3.8) |
| Pleural or pericardial fluid | 3 (3.8) |
| Missing or not reported | 14 (18) |
Reasons for Ineligibility for a Study
Of the 40 patients who were ineligible for the osimertinib studies, reasons included T790M negativity (72.5%), decline in performance status or death (15%), inadequate tissue (5%), biopsy refusal by patient (2.4%), inability to biopsy because of the absence of accessible metastatic lesions (2.5%), and symptomatic brain metastases requiring radiation (2.5%).
DISCUSSION
Our study of rebiopsy for characterization of molecular signatures in patients with advanced EGFRm+ nsclc progressing on first- or second-generation egfr tkis demonstrated a refusal rate of 5%, a successful biopsy rate of 74% on 1st attempt and of 94% on 2nd attempt, and a complication rate less than 10%. Those real-world data suggest that rebiopsy is feasible and acceptable to patients when associated with a need for clinical decision-making.
EGFR activating mutations occur in 15%–40% of patients with nonsquamous nsclc, with the highest incidence being seen in patients of Asian ethnicity, women, and never-smokers27, which aligns with our retrospective study population (79% women and 73% never-smokers). Of our patients, 56% (47 of 84) were found to harbour the T790M mutation after progression on gefitinib, afatinib, or erlotinib—an incidence comparable to that reported in aura318 and other contemporary series (33%–70%)20,21,23–25,28–30. In addition, patients with an EGFR exon 19 deletion (60%) were more commonly represented in our series—an observation possibly related to their more prolonged response to an egfr tki31. Of the 44 patients with a secondary T790M mutation who were enrolled onto a clinical trial, 32 initially had an exon 19 deletion mutation, which was similar to observations in some series20,21,25, but not others24,26,29. Prolonged response to an egfr tki, especially in patients initially harbouring an exon 19 deletion, might predict the occurrence of a secondary T790M mutation31. Interestingly, no small-cell transformation was detected in the present series21,22.
At the time that the osimertinib studies in this series were being conducted, tumour biopsy was the only validated method for detecting the T790M mutation. Only 2 of the 84 patients who were approached for enrolment (2.4%) refused to undergo a tumour biopsy. The refusal rate for biopsy ranged from 1.2% to 7.1% in other studies22,23,30,32. Mirroring other reports, further reasons that rebiopsy was infeasible included absence of an accessible lesion, worsening performance status, physician decision, old age, comorbidity, and the presence of brain metastasis alone upon progression from prior therapy20,22,23,26,30,32. Overall, patient input did not seem to be the major barrier to tumour rebiopsy for the studies in question, nor was it a barrier in our analysis. Patients are likely to be receptive to invasive procedures if additional pathology information can gain them access to novel therapies and significantly affect their clinical outcomes.
In some series, a barrier to rebiopsy more commonly arose from the risk of complications perceived by the physician, based on patient comorbidity, age, and performance status22,32. In our study, rates of complications at initial diagnosis and at the time of rebiopsy for T790M analysis were low. The most common methods of tissue acquisition were ct- or ultrasonography-guided biopsy, and 30% of the patients (25 of 84) underwent more than 1 rebiopsy upon progression. Pneumothorax was the most common complication. Most of the affected patients did not require aggressive measures, as per prior studies22,24,25. To minimize risk, collaboration with radiologists, invasive pulmonary physicians, and thoracic surgeons is important so that the rebiopsy is performed on the most accessible lesions, with the lowest risk of complications, and with the highest chance of adequate sample acquisition and detection of resistance mutations.
The likelihood of detecting T790M positivity can be further improved if the lesion biopsied has documented progression on imaging. In our study, image-guided biopsy was the most common method used for tissue acquisition, as in some other series20,21,30,32; still other series reported use of bronchoscopy or ebus22–25. The predominant method of tissue acquisition at an institution is probably related to accessibility, local expertise, and wait times.
Even with a well-devised biopsy plan, the tissue acquired might not have sufficient dna for a mutational analysis—a situation that occurred in 23% of the initial rebiopsies in our series (18 of 78) and that aligns with the expected 10%–40% previously reported20,22–24,29. After repeated rebiopsies, only in 5 patients could sufficient tumour tissue for mutational analysis not be acquired. Repeated rebiopsy was feasible and safe.
Although circulating tumour dna for EGFR and T790M mutational analysis is being adopted into the clinic, with sensitivity ranging from 50% to 80% depending on the method used33,34, a substantial number of patients will still require tissue biopsy to determine if they are candidates for egfr tki therapy targeting both activating EGFR and T790M mutations (for example, if circulating tumour dna tests are negative or inconclusive). Thus, the findings of the present report remain relevant for the foreseeable future.
The median time from detection of progression to rebiopsy comprised imagining (7–10 days before the clinic appointment), time to organize a biopsy (7–21 days after the appointment, depending on the modality required), and the practice of treatment beyond radiologic progression for asymptomatic patients22,35,36. Median times from biopsy to receipt by the laboratory and to result for EGFR T790M mutation were longer than expected. In the present study, biopsies were performed at the 2 Canadian centres, and testing was performed at either a local or a central laboratory. The recommended time from receipt of the sample to result is 10 business days according to evidence-based best practice in Canada37. Ongoing communication between the medical oncologists and the Molecular Pathology Laboratory has to be in place to ensure adherence to that guideline, because substantial delay might compromise access to osimertinib and thus outcomes in this patient population.
Because the present analysis is retrospective, some of the data were incomplete, including the duration of benefit, details of the response to egfr tki treatment, and the sites of metastatic disease at the time of biopsy for T790M mutation. Those data might provide further insight into the characteristics of the patients who were more likely to harbour T790M mutation upon progression on an egfr tki. In addition, the actual number of patients with EGFRm+ recurrent or metastatic nsclc who progressed during the enrolment period for aura2, aura3, and astris might not have been fully captured. Patients approached for trial enrolment might represent a subset who were willing to undergo biopsy or who had been determined to have more indolent and biopsy-feasible metastatic disease. Thus, the rates of biopsy refusal, complication, and tissue inadequacy might be underestimated.
CONCLUSIONS
In this contemporary retrospective analysis, the incidence of T790M mutation in patients with EGFRm+ nsclc who had received prior egfr tki therapy was comparable to incidences reported in the literature. The consent rate for rebiopsy was high, and complication rates were clinically acceptable, which supports the practice of precision medicine in nsclc treatment in Canada in addition to—or in advance of—widespread use of testing based on circulating tumour dna.
ACKNOWLEDGMENTS
Partial results of this retrospective study were presented at the European Society for Medical Oncology Congress; Munich, Germany; 19–23 October 2018.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: QC has received honoraria or fees, or both, for participation on advisory boards from AbbVie, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol–Myers Squibb, Eisai, Eli Lilly, Merck, Novartis, Pfizer, and Roche, and research funding from AstraZeneca and Bayer. QC’a institution receives funding from AbbVie, Astellas, AstraZeneca, AurKa Pharma, Boehringer Ingelheim, Bristol–Myers Squibb, Celgene, Debiopharm, Eisai, Eli Lilly, Esperas Pharma, Exactis, GlaxoSmithKline, Merck, Pfizer, Roche, Sanofi Genzyme, Spectrum, Turning Point Therapeutics, and Xcovery. ND and RNW are employees of AstraZeneca. CH has received honoraria and fees for participation on advisory boards from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol–Myers Squibb, Eisai, Eli Lilly, Merck, and Roche; grants from AstraZeneca and Eisai; and a travel grant from Roche.
REFERENCES
- 1.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 2.Paez JG, Jänne PA, Lee JC, et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 3.Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361:947–57. doi: 10.1056/NEJMoa0810699. [DOI] [PubMed] [Google Scholar]
- 4.Han JY, Park K, Kim SW, et al. First-signal: first-line single-agent Iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30:1122–8. doi: 10.1200/JCO.2011.36.8456. [DOI] [PubMed] [Google Scholar]
- 5.Maemondo M, Inoue A, Kobayashi K, et al. on behalf of the North-East Japan Study Group. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–8. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 6.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation–positive non-small-cell lung cancer (optimal, ctong-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–42. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 7.Rosell R, Carcereny E, Gervais R, et al. on behalf of the Spanish Lung Cancer Group in collaboration with the Groupe français de pneumo-cancérologie and Associazione Italiana Oncologia Toracica. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation–positive non-small-cell lung cancer (eurtac): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–46. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 8.Sequist LV, Yang JC, Yamamoto N, et al. Phase iii study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327–34. doi: 10.1200/JCO.2012.44.2806. [DOI] [PubMed] [Google Scholar]
- 9.Yang JC, Hirsh V, Schuler M, et al. Symptom control and quality of life in lux-Lung 3: a phase iii study of afatinib or cisplatin/pemetrexed in patients with advanced lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3342–50. doi: 10.1200/JCO.2012.46.1764. [DOI] [PubMed] [Google Scholar]
- 10.Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (lux-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15:213–22. doi: 10.1016/S1470-2045(13)70604-1. [DOI] [PubMed] [Google Scholar]
- 11.Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (archer 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18:1454–66. doi: 10.1016/S1470-2045(17)30608-3. [DOI] [PubMed] [Google Scholar]
- 12.Mok TS, Cheng Y, Zhou X, et al. Improvement in overall survival in a randomized study that compared dacomitinib with gefitinib in patients with advanced non-small-cell lung cancer and EGFR-activating mutations. J Clin Oncol. 2018;36:2244–50. doi: 10.1200/JCO.2018.78.7994. [DOI] [PubMed] [Google Scholar]
- 13.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (wjtog3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–8. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]
- 14.Gainor JF, Shaw AT. Emerging paradigms in the development of resistance to tyrosine kinase inhibitors in lung cancer. J Clin Oncol. 2013;31:3987–96. doi: 10.1200/JCO.2012.45.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jänne PA, Yang JC, Kim DW, et al. AZD9291 in egfr inhibitor-resistant non-small-cell lung cancer. N Engl J Med. 2015;372:1689–99. doi: 10.1056/NEJMoa1411817. [DOI] [PubMed] [Google Scholar]
- 16.Yang JC, Ahn MJ, Kim DW, et al. Osimertinib in pretreated T790M-positive advanced non-small-cell lung cancer: aura study phase ii extension component. J Clin Oncol. 2017;35:1288–96. doi: 10.1200/JCO.2016.70.3223. [DOI] [PubMed] [Google Scholar]
- 17.Goss G, Tsai CM, Shepherd FA, et al. Osimertinib for pretreated EGFR Thr790Met-positive advanced non-small-cell lung cancer (aura2): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2016;17:1643–52. doi: 10.1016/S1470-2045(16)30508-3. [DOI] [PubMed] [Google Scholar]
- 18.Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. N Engl J Med. 2017;376:629–40. doi: 10.1056/NEJMoa1612674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soria JC, Ohe Y, Vansteenkiste J, et al. on behalf of the flaura investigators. Osimert inib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378:113–25. doi: 10.1056/NEJMoa1713137. [DOI] [PubMed] [Google Scholar]
- 20.Arcila ME, Oxnard GR, Nafa K, et al. Rebiopsy of lung cancer patients with acquired resistance to egfr inhibitors and enhanced detection of the T790M mutation using a locked nucleic acid–based assay. Clin Cancer Res. 2011;17:1169–80. doi: 10.1158/1078-0432.CCR-10-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandrasekharan A, Patil V, Norohna V, et al. Rebiopsy post progression in EGFR mutated lung cancer. J Thorac Oncol. 2017;12(suppl):S1248–9. doi: 10.1016/j.jtho.2016.11.1761. [DOI] [Google Scholar]
- 22.Chouaid C, Dujon C, Do P, et al. Feasibility and clinical impact of re-biopsy in advanced non-small-cell lung cancer: a prospective multicenter study in a real-world setting (gfpc study 12-01) Lung Cancer. 2014;86:170–3. doi: 10.1016/j.lungcan.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura T, Kenmotsu H, Taira T, et al. Rebiopsy for patients with non-small-cell lung cancer after epidermal growth factor receptor–tyrosine kinase inhibitor failure. Cancer Sci. 2016;107:1001–5. doi: 10.1111/cas.12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim TO, Oh IJ, Kho BG, et al. Feasibility of re-biopsy and EGFR mutation analysis in patients with non–small cell lung cancer. Thorac Cancer. 2018;9:856–64. doi: 10.1111/1759-7714.12762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nosaki K, Satouchi M, Kurata T, et al. Re-biopsy status among non–small cell lung cancer patients in Japan: a retrospective study. Lung Cancer. 2016;101:1–8. doi: 10.1016/j.lungcan.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Uozu S, Imaizumi K, Yamaguchi T, et al. Feasibility of tissue re-biopsy in non–small cell lung cancers resistant to previous epidermal growth factor receptor tyrosine kinase inhibitor therapies. BMC Pulm Med. 2017;17:175. doi: 10.1186/s12890-017-0514-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for egfr and alk tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Arch Pathol Lab Med. 2013;137:828–60. doi: 10.5858/arpa.2012-0720-OA. [Erratum in: Arch Pathol Lab Med 2013;137:1174] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kakihana M, Maeda J, Matsubayashi J, et al. Role of re-biopsy during disease progression non–small cell lung cancer for acquired resistance analysis and directing oncology treatments [abstract P1.01-041] J Thorac Oncol. 2017;12(suppl 2):S1909. doi: 10.1016/j.jtho.2017.09.695. [DOI] [Google Scholar]
- 29.Oguri T, Ohyanagi F, Takano N, et al. Current situation of re-biopsy in non-small-cell lung cancer treated with egfr tki [abstract e20563] J Clin Oncol. 2016;34 doi: 10.1200/JCO.2016.34.15_suppl.e20563. [Available online at: https://ascopubs.org/doi/abs/10.1200/JCO.2016.34.15_suppl.e20563; cited 3 January 2020] [DOI] [Google Scholar]
- 30.Wang H, Zhang L, Si X, Zhang X, Wang M. Rebiopsy status among non–small cell lung cancer patients after icotinib therapy in China [abstract e21146] J Clin Oncol. 2018;36 [Available online at: https://ascopubs.org/doi/abs/10.1200/JCO.2018.36.15_suppl.e21146; cited 3 January 2020] [Google Scholar]
- 31.Yang JC, Wu YL, Schuler M, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation–positive lung adenocarcinoma (lux-Lung 3 and lux-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–51. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 32.Oguri T, Horiike A, Ariyasu R, et al. The reasons why rebiopsies were not performed after failure with egfr tki [abstract e20540] J Clin Oncol. 2017;36 [Available online at: https://ascopubs.org/doi/abs/10.1200/JCO.2017.35.15_suppl.e20540; cited 3 January 2020] [Google Scholar]
- 33.Thompson JC, Yee SS, Troxel AB, et al. Detection of therapeutically targetable driver and resistance mutations in lung cancer patients by next-generation sequencing of cell-free circulating tumor dna. Clin Cancer Res. 2016;22:5772–82. doi: 10.1158/1078-0432.CCR-16-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu T, Kang X, You X, et al. Cross-platform comparison of four leading technologies for detecting EGFR mutations in circulating tumor dna from non–small cell lung carcinoma patient plasma. Theranostics. 2017;7:1437–46. doi: 10.7150/thno.16558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goto Y, Tanai C, Yoh K, et al. Continuing egfr-tki beyond radiological progression in patients with advanced or recurrent, EGFR mutation–positive non-small-cell lung cancer: an observational study. ESMO Open. 2017;2:e000214. doi: 10.1136/esmoopen-2017-000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Auliac JB, Fournier C, Audigier Valette C, et al. Impact of continuing first-line egfr tyrosine kinase inhibitor therapy beyond recist disease progression in patients with advanced EGFR-mutated non-small-cell lung cancer (nsclc): retrospective gfpc 04-13 study. Target Oncol. 2016;11:167–74. doi: 10.1007/s11523-015-0387-4. [DOI] [PubMed] [Google Scholar]
- 37.Stockley T, Souza CA, Cheema PK, et al. Evidence-based best practices for EGFR T790M testing in lung cancer in Canada. Curr Oncol. 2018;25:163–9. doi: 10.3747/co.25.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]