Abstract
Background
Brain metastasis from breast cancer (bca) in young women is doubly devastating because both quality of life and life expectancy are significantly reduced. With new radiation technology and drugs that have emerged, survival is expected to increase for these young women.
Methods
Using the oacis and sardo patient databases, we identified 121 patients diagnosed with bca and brain metastasis between 2006 and 2016 at the University of Montreal Hospital Centre. Those patients were divided into Group A, patients who developed brain metastasis during the evolution of metastatic bca, and Group B, patients whose first metastasis was to the brain. For each group, we compared young patients (<40 years of age) with older patients (≥40 years of age).
Results
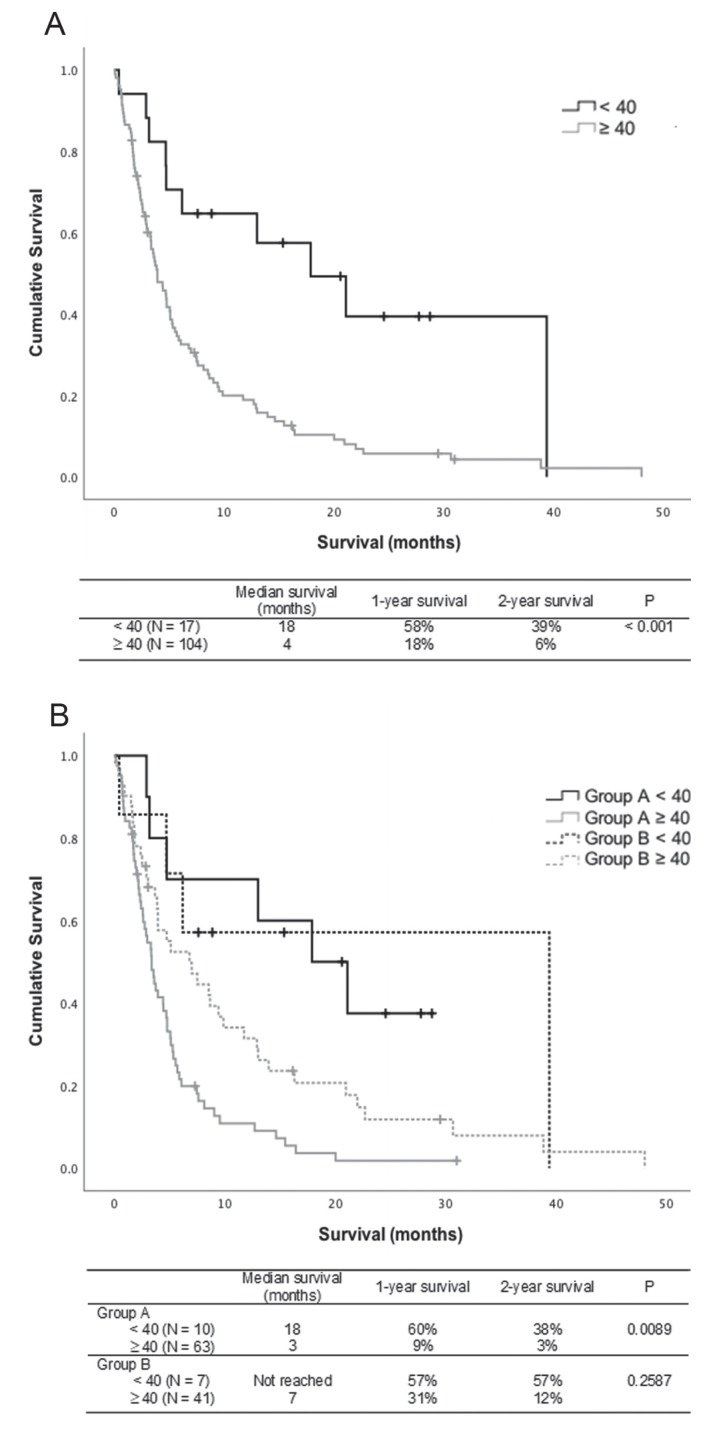
Among the 121 patients with brain metastasis, median overall survival (mos) was significantly longer for those less than 40 years of age than for those 40 or more years of age (18 months vs. 4 months, p < 0.001). With respect to the timing of brain metastasis, survival was significantly longer in Group B than in Group A (7 months vs. 4 months, p = 0.032). In Group A, mos was significantly longer for patients less than 40 years of age than for patients 40 or more years of age (18 months vs. 3 months, p = 0.0089). In Group B, the 2-year overall survival rate was 57% for patients less than 40 years of age and 12% for those 40 or more years of age (mos: not reached vs. 7 months; p = 0.259).
Conclusions
In our single-centre retrospective cohort of women with brain metastasis from bca, prognosis was better for young women (<40 years) than for older women (≥40 years). Survival was also longer for patients whose initial metastasis was to the brain than for patients whose brain metastasis developed later in the disease course. In patients who received systemic treatment, median survival remained significantly higher in women less than 40 years of age. Further studies are needed to validate those results.
Keywords: Breast cancer, brain metastasis, age, prognosis
INTRODUCTION
Breast cancer (bca) is the cancer most frequently diagnosed in women in Canada and worldwide1,2. Although many advances in treatments have been made, which have successfully increased survival, no cure for metastatic bca has yet been achieved.
Breast cancer is the 2nd most frequent source of brain metastases3–6. An estimated 10%–16% of patients with metastatic bca develop symptomatic brain metastasis6. After brain metastasis, prognosis is grim, with greatly reduced quality of life and life expectancy in affected patients. The 1-year survival for those patients has been estimated at 20%7. The impact of such a diagnosis is even more devastating in young women.
Younger age has been associated with more aggressive forms of cancer, and thus survival is expected to decrease for younger compared with older women8. However, studies conducted in women less than 40 years of age with bca have been limited in number, and even fewer have been conducted in women with brain metastasis. A population-based cohort study from Australia (n = 1166) found no significant difference in survival based on age in women with metastatic bca (18 months for those <40 years vs. 14 months for those ≥40 years, p = 0.21)9.
The goals of the present study were to determine the effect of age on prognosis after brain metastasis from bca and the effects of systemic compared with local treatment on prognosis.
METHODS
Patient Selection
We conducted a retrospective study using the sardo and oacis patient databases. The sardo patient database for the oncology department at the Centre hospitalier de l’Université de Montréal (chum) actively collects data for patients seen in clinic. At the chum, oacis is the electronic patient database. Using sardo, we identified all patients with bca metastatic to the brain who were followed by the oncology team between 2006 and 2016. Patients with leptomeningeal carcinomatosis were included with patients having brain metastasis. All bca histology types—ductal, lobular, small-cell carcinoma, malignant phyllodes tumour, and metaplastic carcinoma—were included in the study. Of 122 patients located in sardo, only 1 was excluded from the analysis (she was lost to follow-up).
Before data collection, the study protocol was submitted for approval by the Research Ethics Committee of the chum. Data processing was carried out under complete anonymity.
Patient Distribution
We allocated the patients to two groups based on the site of their initial metastasis. In Group A patients, the initial metastasis was to a site other than the brain, and brain metastasis developed later during progression of their cancer. In Group B patients, the initial metastasis was to the brain. Group B also included patients who had concurrent distant metastasis elsewhere in the body at the time of their brain metastasis diagnosis. We then compared young patients (<40 years of age) with older patients (≥40 years of age) in each group. Patient age was defined as age at the time of the initial bca diagnosis. The cut-off of 40 years for young compared with older women was used because that cut-point is conventional in the literature9–13. In our preliminary analysis, women 40–59 years of age and women 60 or more years of age showed no difference in median overall survival—a finding that also supported the merging of those two groups.
Data Sources
The primary source for data extraction was the sardo database. Necessary additional information was obtained from oacis.
Age at bca diagnosis, sex, family history of bca, tumour histology, and bca details such as hormone receptor (hr) and her2 (human epidermal growth factor receptor 2) status, date of diagnosis with brain metastasis, progression dates, date of death, date of last contact, inpatient or outpatient status at the time of the brain metastasis diagnosis, and treatments were extracted from the sardo database and completed with information from oacis.
The date of the cerebral metastasis diagnosis was determined by neuroimaging using computed tomography and magnetic resonance imaging. We defined inpatients as patients who presented to the emergency room or who were admitted to the wards at the time of their brain metastasis diagnosis. Patients who were diagnosed with brain metastasis outside the hospital setting were defined as outpatients.
Outcomes
The primary study outcome was the overall survival of patients with brain metastasis from bca based on age (<40 years and ≥40 years). Survival was defined as the time from the diagnosis of brain metastasis to the date of death or last contact.
Secondary outcomes were time to first metastasis in patients with eventual brain metastasis, time to brain metastasis, progression-free survival, and the effect on survival of inpatient or outpatient status at the time of brain metastasis diagnosis. Time to first metastasis was defined as the time from the bca diagnosis to diagnosis of the first metastasis. For patients whose metastasis first occurred at a site other than brain (Group A), we also calculated the time from the diagnosis of first metastasis to the diagnosis of brain metastasis.
Based on age, we examined local cerebral treatment and systemic treatment independently of the site of initial metastasis. Local cerebral treatment was defined as brain radiotherapy or brain surgery with or without radiotherapy. Systemic treatment was defined as chemotherapy or anti-her2 therapy with or without hormonal therapy, or hormonal therapy alone (without chemotherapy).
Statistical Analysis
Chi-square tests were used to test non-continuous variables. Kaplan–Meier analysis with log-rank test was used to determine survival in groups A and B, and in the subgroups based on age. Those values were also calculated based solely on age, independent of the site of initial metastasis.
Additional Kaplan–Meier analyses were used to determine the effect on survival of treatment received at the time of cerebral metastasis.
The IBM SPSS Statistics software application (version 25: IBM, Armonk, NY, U.S.A.) was used for all statistical analyses. A significance level of α < 0.05 was used for all analyses.
RESULTS
Patient Characteristics
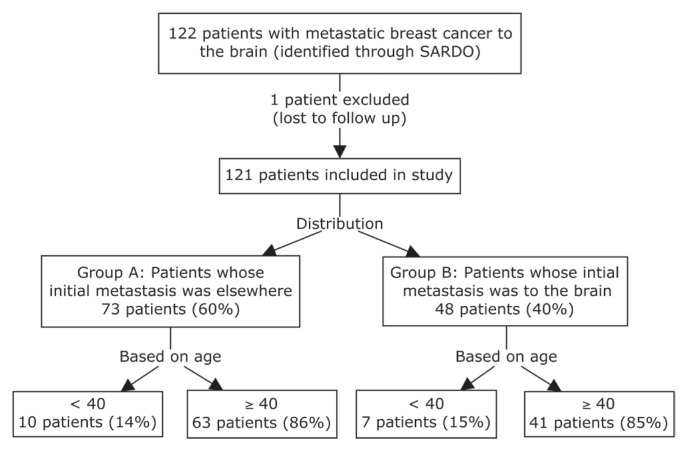
Our study included 121 patients with bca metastatic to the brain. Of initial metastases in those patients, 73 (60%) occurred at other sites before the brain metastasis, and 48 (40%) occurred in the brain (Figure 1). Of the 121 patients, 17 (14%) were less than 40 years of age, and 104 (86%) were 40 or more years of age. With respect to the site of initial metastasis, no statistically significant difference in age distribution was observed in the two groups. Women less than 40 years of age represented 14% of Group A and 15% of Group B.
FIGURE 1.
Flowchart detailing patient selection for the study. SARDO = patient database for the oncology department at the Centre hospitalier de l’Université de Montréal.
Patient and Tumour Characteristics
BRCA1/2 status was available for 19% of patients. It was unknown for 65% of women less than 40 years of age and for 84% of women 40 or more years of age. No patient in the study received a parp inhibitor.
Ductal carcinoma was the most frequent histologic type in both Group A and Group B (90% and 75% respectively, p = 0.023); however, site of initial metastasis was significantly different depending on the histologic type. Lobular tumours were more frequent in patients whose initial metastasis was to the brain than in patients whose initial metastasis was elsewhere (23% Group B vs. 4% Group A, p = 0.002). Also, ductal carcinoma was found to be the most frequent histologic type in women whether they were less than 40 years of age or 40 or more years of age (94% vs. 83%, p = 0.230).
TABLE I.
Demographics and tumour characteristics of patients with breast cancer (BCa) based on site of initial metastasis and age
| Variable | Group A: Initial metastasis to a non-brain site | Group B: Initial metastasis to the brain | p Value | ||
|---|---|---|---|---|---|
|
| |||||
| <40 Years | ≥40 Years | <40 Years | ≥40 Years | ||
| Patients (n) | 10 | 63 | 7 | 41 | — |
|
| |||||
| Median age (years) | 36 | 55 | 35 | 56 | <40: 0.755 |
| ≥40: 0.389 | |||||
|
| |||||
| Sex [n (%)] | |||||
| Women | 9 (90) | 63 (100) | 7 (100) | 41 (100) | 0.011 |
| Men | 1 (10) | 0 (0) | 0 (0) | 0 (0) | |
|
| |||||
| Positive family history of BCa (first degree)[ n (%)] | |||||
| Unavailable | 3 (30) | 11 (17) | 1 (14) | 7 (17) | 0.248 |
| Yes | 1 (14) | 18 (35) | 0 (0) | 9 (26) | |
| No | 6 (86) | 34 (65) | 6 (100) | 25 (74) | |
|
| |||||
| BRCA1/2 status [n (%)] | |||||
| Unavailable | 6 (60) | 53 (84) | 5 (71) | 34 (83) | 0.442 |
| Yes | 1 (25) | 5 (50) | 1 (50) | 1 (14) | |
| No | 3 (75) | 5 (50) | 1 (50) | 6 (86) | |
|
| |||||
| Histology [n (%)] | |||||
| Ductal | 10 (100) | 56 (89) | 6 (86) | 30 (73) | 0.005 |
| Lobular | 0 (0) | 3 (5) | 0 (0) | 11 (27) | |
| Othera | 0 (0) | 4 (6) | 1 (14) | 0 (0) | |
|
| |||||
| BCa subtype [n (%)] | |||||
| HR-negative, HER2-negative | 1 (10) | 12 (19) | 2 (28,5) | 15 (36.5) | 0.644 |
| HR-negative, HER2-positive | 1 (10) | 4 (6) | 0 (0) | 3 (7) | |
| HR-positive, HER2-negative | 4 (40) | 30 (48) | 3 (43) | 15 (36.5) | |
| HR-positive, HER2-positive | 4 (40) | 17 (27) | 2 (28.5) | 8 (20) | |
Includes 1 malignant phyllodes tumour (≥40 years, initial metastasis at site other than brain), 2 ductal and lobular tumours (≥40 years, initial metastasis at site other than brain), 1 small-cell carcinoma (≥40 years, initial metastasis at site other than brain), 1 metaplastic carcinoma (<40 years, initial metastasis to brain).
HR = hormone receptor; HER2 = human epidermal growth factor receptor 2.
Compared with women 40 or more years of age, those less than 40 years of age tended to be less likely to have triple-negative tumours (18% vs. 26%, p = 0.462) and more likely to have hr-positive, her2-positive disease (35% vs. 24%, p = 0.324); however, those results were not statistically significant. A first metastasis of bca to brain was significantly more likely in patients with triple-negative tumours (35% vs. 18%, p = 0.028). The most frequent bca tumour subtype in both age groups was hr-positive, her2-negative (41% vs. 43%).
We observed no significant difference of age with respect to histopathology, her2 status, or triple-negative tumours. Compared with patients having initial metastasis at other sites, patients with brain metastasis as first bca metastasis more frequently had lobular or triple-negative tumours.
Leptomeningeal Carcinomatosis
Leptomeningeal carcinomatosis occurred in 21% of the study cohort overall (n = 25), but twice as frequently in women less than 40 years of age than in women 40 or more years of age (35% vs. 18%, p < 0.001, Table II). However, younger women were half as likely to be treated specifically for their meningeal carcinomatosis with intrathecal methotrexate (17% vs. 37%, p < 0.001). That difference in treatment did not affect survival, because compared with their older counterparts, younger women with leptomeningeal carcinomatosis tended to experience longer survival (6 months vs. 3 months, p = 0.056).
TABLE II.
Treatment and survival by inpatient or outpatient status at the time of brain metastasis diagnosis
| Variable | Age group | p Value | |
|---|---|---|---|
| <40 Years | ≥ 40 Years | ||
| Patients (n) | 17 | 104 | 0.059a |
|
| |||
| Outpatient or inpatient statusb | |||
| Outpatient [n (%)] | 13 (76) | 54 (52) | |
| Mean survival (months) | 24 | 7 | 0.001c |
| Inpatient [n (%)] | 4 (24) | 50 (48) | |
| Mean survival (months) | 13 | 8 | 0.248c |
|
| |||
| Local treatment status [n (%)] | 17 | 104 | <0.001 |
| Supportive care | 0 (0) | 14 (13.5) | |
| No local treatment; any systemic treatment | 2 (12) | 12 (11.5) | |
| Brain surgery ± RT | 1 (6) | 13 (12.5) | |
| Brain RT, no brain surgery | 14 (82) | 65 (62.5) | |
|
| |||
| Systemic treatment status [n (%)] | 17 | 104 | <0.001 |
| CTx, anti-HER2 therapyd, ± hormonal therapy | 12 (71) | 37 (36) | |
| Hormonal therapy (without CTx) | 2 (12) | 16 (15) | |
| None | 3 (18) | 51 (49) | |
|
| |||
| Cohort receiving systemic treatment (n) | 14 | 53 | 0.014 |
| Mean survival (months) | 21 | 6 | |
| 1-Year survival (%) | 63 | 26 | |
| 2-Year survival (%) | 42 | 10 | |
Relationship between inpatient or outpatient status and age.
At the time of brain metastasis diagnosis.
Relationship between mean survival in months and age.
When appropriate.
RT = radiation therapy; CTx = chemotherapy.
Survival
In a preliminary analysis, we compared median overall survival for women less than 40 years of age, women 40–59 years of age, and women 60 or more years of age (18 months vs. 4 months vs. 4 months). Because no difference in median survival was evident between women 40–59 years of age and women 60 or more years of age, we established a cut-off of 40 years for all further analyses.
Regardless of age, patients whose first metastasis was to the brain experienced longer survival than did patients who developed metastasis at another site before brain metastasis (7 months vs. 4 months, p = 0.032).
Regardless of the site of initial metastasis, women less than 40 years of age experienced significantly longer survival than did women 40 or more years of age (18 months vs. 4 months, p < 0.001). Young women with initial metastasis to the brain or initial metastasis to another site both experienced longer survival than did older women in the same group (Group A: 18 months vs. 3 months, p = 0.009; Group B: not reached vs. 7 months, p = 0.26; Figure 2).
FIGURE 2.
Kaplan–Meier survival analyses. (A) Survival of women with metastatic breast cancer based on age only (<40 years vs. ≥40 years). (B) Survival of women with metastatic breast cancer based on age (<40 years vs. ≥40 years) and site of initial metastasis. Tick marks represent censored individuals, as previously defined. Group A = patients whose initial metastasis was to a site other than brain and who later developed brain metastasis during the progression of their metastatic cancer; Group B = patients whose initial metastasis was to the brain.
Follow-Up
Median follow-up for the overall study cohort was 4 months (range: 2 days to 48 months). Median follow-up for patients less than 40 years of age was 13 months (range: 13 days to 39 months), and for patients 40 or more years of age, it was 4 months (range: 2 days to 48 months; p = 0.0014).
Inpatient or Outpatient Status at the Time of Brain Metastasis Diagnosis
We observed no significant difference based on patient age with respect to inpatient or outpatient status at the time of the brain metastasis diagnosis (76% vs. 54% for outpatients and 24% vs. 48% for inpatients, p = 0.059). When the cohort was stratified by inpatient or outpatient status, survival was significantly longer for outpatients less than 40 years of age than for outpatients 40 or more years of age (24 months vs. 7 months, p = 0.001). No significant difference was observed for inpatients less than 40 years of age and those 40 or more years of age (13 months vs. 8 months, p = 0.248; Table II).
Treatment
Local treatment to the brain—surgical resection with or without radiotherapy, or radiotherapy only—was given in 77% of patients. Most women received local treatment to the brain regardless of age: 88% of women less than 40 years of age and 75% of women 40 or more years of age (p < 0.001, Table II). However, women less than 40 years of age received significantly more local treatment than did women 40 or more years of age. Radiotherapy alone was the most frequent form of local treatment to the brain in both age groups (82% vs. 63%, p < 0.001). No women less than 40 years of age received supportive care only.
Of the 121 patients in the present study, 67 (55%) received systemic treatment at the time of brain metastasis: either chemotherapy (with anti-her2 therapy when indicated) with or without hormonal therapy, or hormonal therapy alone. Of those 67 patients, 14 (21%) were less than 40 years of age and 53 (79%) were 40 or more years of age (Table II).
More systemic treatment was given in women less than 40 years of age than in those 40 or more years of age (82% vs. 51%, p < 0.001). Chemotherapy (with anti-her2 therapy when indicated) was the most frequent form of systemic treatment in both age groups. However, twice as many women less than 40 years of age than women 40 or more years of age were treated with chemotherapy (71% vs. 36%, p < 0.001).
Survival in Patients Who Received Systemic Treatment
When controlled for systemic treatment, median survival remained significantly higher in women less than 40 years of age than in women 40 or more years of age (21 months vs. 6 months, p = 0.014; Table II).
DISCUSSION
Multiple studies have set out to clarify the effect of age on survival for patients with bca. The consensus is that complex biologic factors explain the significant difference in survival based on age in those women. Several studies identified young age as a negative prognostic factor14–17. That conclusion has been attributed to a higher prevalence of aggressive cancers and differences in biomarker expression in younger patients18,19. Other studies have shown young age to be favourable20,21. A study conducted in Singapore found that patients with bca of all stages in the 40- to 44-year age group had the best relative survival rate at both 2 and 5 years21. Our study similarly showed young age to be a favourable prognostic factor for women with bca metastatic to the brain. A longer time to first metastasis from the initial diagnosis of bca in younger women, as found in our study, reflects slower progression and more indolent disease. That observation correlates with their longer survival (compared with that for older women) from the time of brain metastasis. One hypothesis that might potentially explain those findings is an increase in comorbidities in older women as they age, which can lead to a decrease in survival. Our study did not include comorbidities in its analyses, however.
Very few studies have set out to determine the effect of age on survival specifically for patients with brain metastasis. A study similar to ours was performed in Australia using data from 2001 to 2007 for patients with metastatic bca (n = 1166). They found no significant difference in survival based on age9. However, their study measured survival in patients with metastatic bca overall rather than specifically in patients with brain metastasis. Also, their study did not include hr and her2 status, nor treatments received by patients. Thus, their population could not easily be compared with ours, which could potentially explain the differing results in our study.
In the present study, younger age was not found to be a risk factor for initial metastasis to the brain compared with initial metastasis to other sites because women less than 40 years of age constituted 14% and 15% of Group A and Group B respectively. Therefore, in our study, young women and women 40 or more years of age presented the same prevalence of brain metastasis. However, leptomeningeal carcinomatosis was more prevalent in young women. The young women were also less likely to receive treatment with intrathecal methotrexate. However, that difference in treatment did not affect prognosis.
Compared with women 40 or more years of age, younger women in our study received significantly more systemic treatment at the time of brain metastasis. A strong trend toward inpatient status in women 40 or more years of age could explain why less systemic treatment was received by older women than by younger women, because the older women were more ill at the time of brain metastasis. Among inpatients, prognosis was better for women less than 40 years of age than for women 40 or more years of age (24 months vs. 7 months, p = 0.001). Nonetheless, the more favourable outcome in younger women remained for patients who received systemic treatment at the time of brain metastasis (21 months vs. 6 months, p = 0.014). However, a larger proportion of patients less than 40 years of age received chemotherapy as a systemic treatment, which might have an effect on survival when controlled for systemic treatment.
When women in our study were stratified by age group, no significant differences in the distributions of histopathology, her2 status, or triple-negative status were observed in the groups less than 40 years of age and 40 or more years of age. However, regardless of age, women with triple-negative tumours more often presented with initial metastasis to brain (35% vs. 18%). Also, brain metastasis as initial metastasis from bca compared with initial metastasis to another site occurred significantly more frequently with lobular tumours (23% vs. 4%, p = 0.002). Survival was longer for women with initial metastasis to the brain than for women with initial metastasis to another site (7 months vs. 4 months, p = 0.032). A higher metastatic burden at the time of brain metastasis can potentially explain shorter survival in patients with initial metastasis to another site. Women who have already received treatment for other metastatic progressions are also more limited in subsequent treatment options at the time of brain metastasis, which might further explain their shorter survival.
The retrospective nature of our study and its small sample size are the major limitations of our analysis. Also, we included only patients followed at our oncology clinics rather than all patients who received treatment for brain metastasis at our hospital centre, because detailed information for the analysis was lacking for the latter patient group. Further research into this subject therefore has to be conducted to clarify the effect of age on survival, particularly for bca metastatic to the brain. The results obtained in our study have to be validated in further multicentric studies with a larger sample size.
The strength of our study is the precise granular data for women with brain metastases from bca, which are valuable compared with the more general data from other studies14–17. Our cohort represents 10 years of patient data at one of the largest tertiary hospital centres in Canada. Given that our study was exploratory in nature, it would therefore be interesting, in the future, to pool data with other hospital centres for further analysis.
CONCLUSIONS
In our retrospective single-centre study, we found that, regardless of the site of initial metastasis, median survival from the time of brain metastasis diagnosis is significantly longer for young women less than 40 years of age than for women 40 or more years of age. In analyses stratified by age, that more favourable outcome persists in younger patients who receive systemic treatment. If corroborated by larger studies, the prolonged survival seen in younger women with initial brain metastasis from bca potentially suggests that more aggressive treatments might be indicated in young women. However, further studies with larger sample sizes are required to validate our results, because reasons for our findings cannot be discerned in the present work.
ACKNOWLEDGMENTS
The authors thank Caroline Cherbal for providing the sardo patient database. They also thank Dr. Philip Wong and the medical oncology and radiation oncology teams of the chum for helpful discussions.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: This study was supported by a grant from the chum’s Centre intégré de cancérologie and Boehringer Ingelheim. Those funding sources had no involvement in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. MF has consulted for AstraZeneca, Boehringer Ingelheim, Bristol–Myers Squibb, Merck, and Roche. DC has participated as an expert on the advisory board for Merck immunotherapy and head-and-neck cancer. JPA has consulted for Eisai, NanoString Technologies, Novartis, and Pfizer, and has received research funding from AbbVie, Celgene, Novartis, Puma Biotechnology, and Tesaro. LY has received research funding from Boehringer Ingelheim, Celgene, Eisai, Genentech, GlaxoSmithKline, MedImmune, Nektar Therapeutics, Puma Biotechnology, and Roche. All remaining authors have no conflicts of interest to disclose.
REFERENCES
- 1.Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2018. Toronto, ON: Canadian Cancer Society; 2018. [Google Scholar]
- 2.Stewart BW, Wild CP, editors. World Cancer Report 2014. Geneva, Switzerland: World Health Organization; 2014. [Google Scholar]
- 3.Chang EL, Lo S. Diagnosis and management of central nervous system metastases from breast cancer. Oncologist. 2003;8:398–410. doi: 10.1634/theoncologist.8-5-398. [DOI] [PubMed] [Google Scholar]
- 4.Zimm S, Wampler GL, Stablein D, Hazra T, Young HF. Intracerebral metastases in solid-tumor patients: natural history and results of treatment. Cancer. 1981;48:384–94. doi: 10.1002/1097-0142(19810715)48:2<384::AID-CNCR2820480227>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 5.Lee YT. Breast carcinoma: pattern of metastasis at autopsy. J Surg Oncol. 1983;23:175–80. doi: 10.1002/jso.2930230311. [DOI] [PubMed] [Google Scholar]
- 6.Lin NU, Bellon JR, Winer EP. cns metastases in breast cancer. J Clin Oncol. 2004;22:3608–17. doi: 10.1200/JCO.2004.01.175. [DOI] [PubMed] [Google Scholar]
- 7.Engel J, Eckel R, Aydemir U, et al. Determinants and prognoses of locoregional and distant progression in breast cancer. Int J Radiat Oncol Biol Phys. 2003;55:1186–95. doi: 10.1016/S0360-3016(02)04476-0. [DOI] [PubMed] [Google Scholar]
- 8.Shannon C, Smith IE. Breast cancer in adolescents and young women. Eur J Cancer. 2003;39:2632–42. doi: 10.1016/S0959-8049(03)00669-5. [DOI] [PubMed] [Google Scholar]
- 9.Tjokrowidjaja A, Lee CK, Houssami N, Lord S. Metastatic breast cancer in young women: a population-based cohort study to describe risk and prognosis. Intern Med J. 2014;44:764–70. doi: 10.1111/imj.12481. [DOI] [PubMed] [Google Scholar]
- 10.Buckley JM, Coopey S, Samphao S, et al. Recurrence rates and long-term survival in women diagnosed with breast cancer at age 40 and younger [abstract 70] J Clin Oncol. 2011;29 doi: 10.1200/jco.2011.29.27_suppl.70. [Available online at: https://ascopubs.org/doi/abs/10.1200/jco.2011.29.27_suppl.70; cited 7 January 2020] [DOI] [Google Scholar]
- 11.Ruddy KJ, Gelber S, Tamimi R, et al. Presentation of breast cancer in young women [abstract 6608] J Clin Oncol. 2009;27 [Available online at: https://ascopubs.org/doi/abs/10.1200/jco.2009.27.15_suppl.6608; cited 7 January 2020] [Google Scholar]
- 12.Assi HA, Khoury KE, Dbouk H, Khalil LE, Mouhieddine TH, El Saghir NS. Epidemiology and prognosis of breast cancer in young women. J Thorac Dis. 2013;5(suppl 1):S2–8. doi: 10.3978/j.issn.2072-1439.2013.05.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brenner DR, Brockton NT, Kotsopoulos J, et al. Breast cancer survival among young women: a review of the role of modifiable lifestyle factors. Cancer Causes Control. 2016;27:459–72. doi: 10.1007/s10552-016-0726-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gil-Gil MJ, Martinez-Garcia M, Sierra A, et al. Breast cancer brain metastases: a review of the literature and a current multidisciplinary management guideline. Clin Transl Oncol. 2014;16:436–46. doi: 10.1007/s12094-013-1110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anders CK, Hsu DS, Broadwater G, et al. Young age at diagnosis correlates with worse prognosis and defines a subset of breast cancers with shared patterns of gene expression. J Clin Oncol. 2008;26:3324–30. doi: 10.1200/JCO.2007.14.2471. [DOI] [PubMed] [Google Scholar]
- 16.Chung M, Chang HR, Bland KI, Wanebo HJ. Younger women with breast carcinoma have a poorer prognosis than older women. Cancer. 1996;77:97–103. doi: 10.1002/(SICI)1097-0142(19960101)77:1<97::AID-CNCR16>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 17.El Saghir NS, Seoud M, Khalil MK, et al. Effects of young age at presentation on survival in breast cancer. BMC Cancer. 2006;6:194. doi: 10.1186/1471-2407-6-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azim HA, Jr, Partridge AH. Biology of breast cancer in young women. Breast Cancer Res. 2014;16:427. doi: 10.1186/s13058-014-0427-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azim HA, Jr, Michiels S, Bedard PL, et al. Elucidating prognosis and biology of breast cancer arising in young women using gene expression profiling. Clin Cancer Res. 2012;18:1341–51. doi: 10.1158/1078-0432.CCR-11-2599. [DOI] [PubMed] [Google Scholar]
- 20.Adami HO, Malker B, Holmberg L, Persson I, Stone B. The relation between survival and age at diagnosis in breast cancer. N Engl J Med. 1986;315:559–63. doi: 10.1056/NEJM198608283150906. [DOI] [PubMed] [Google Scholar]
- 21.Chia KS, Du WB, Sankaranarayanan R, et al. Do younger female breast cancer patients have a poorer prognosis? Results from a population-based survival analysis. Int J Cancer. 2004;108:761–5. doi: 10.1002/ijc.11632. [DOI] [PubMed] [Google Scholar]