Abstract
The Mediterranean Diet, characterized by higher intakes of plant foods including plant proteins, monounsaturated fat, fish, and lower consumption of animal products and saturated fat, has long been associated with reduced cardiovascular risk, but the molecular mechanisms underlying these associations have not been fully elucidated. We conducted a pilot study to evaluate associations of an Alternate Mediterranean Diet Score, reflective of adherence to this diet pattern and adapted for US populations, and its components, with markers of endothelial inflammation directly measured in endothelial cells harvested from a diverse sample of women (n=25, mean±SD age 33±10.5y, 68% racial/ethnic minorities). Cardiovascular risk markers including nuclear factor kappa B (NF-κB)- a marker of inflammation, as well as oxidative stress and endothelial nitric oxide synthase (eNOS) gene expression-markers of endothelial function, were evaluated in harvested endothelial cells. We hypothesized that the Mediterranean diet pattern would be associated with lower inflammation and oxidative stress and higher eNOS expression in endothelial cells. Results showed that lower oxidative stress was associated with higher plant-based protein (Exp(β)=0.96; p=0.007), overall protein (Exp(β)=0.99; p=0.007), and red and processed meat intake (Exp(β)=0.93; p=0.012). Lower NFκB was associated with higher legume (Exp(β)=0.79; p=0.045) intake, and higher eNOS was associated with higher red and processed meat intake (Exp(β)=1.13; p=0.005). Our findings suggest potential novel mechanisms through which certain Mediterranean dietary components may influence pre-clinical vascular alterations that may be associated with cardiovascular risk through lower endothelial oxidative stress, lower inflammation, and greater endothelial functioning. These findings warrant confirmation prospectively in a larger sample.
Keywords: Endothelial Function, Mediterranean Diet, Inflammation, Cardiovascular Risk Markers, Women
1. INTRODUCTION
Healthful lifestyle behaviors including consuming a high-quality diet are associated with ~80% reduction in risk for obesity, type 2 diabetes and cardiovascular disease (CVD) [1]. Poor diet quality alone accounts for ~ 45% deaths from these cardiometabolic diseases [2]. Among women in the United States, it is estimated that poor diet quality accounts for 22.3% of CVD mortality, highlighting the importance of targeting diet for lowering the CVD burden [3,4]. Poor quality diets, characterized by high intakes of refined carbohydrate and processed food products rich in added sugar and salt, contribute to CVD risk [5] through their influence of cardiometabolic risk factors including elevated adiposity, blood pressure, blood glucose, cholesterol and other biomarkers [6,7], but there may be additional mechanisms at play.
The Mediterranean diet and its components in particular have long been associated with reduced cardiovascular risk [8]. It has been proposed that various components of this diet pattern, including polyphenols, antioxidants, and micro and macro nutrients may reduce cardiovascular risk through biological pathways related to inflammation, oxidative stress, and endothelial functioning [9]. However, the biological mechanisms through which the Mediterranean diet pattern decreases risk for CVD have not yet been fully elucidated. Compared to commonly used biochemical plasmatic inflammation markers assays, measures of inflammation and oxidative stress from harvested endothelial cells serve as direct measures of cardiovascular risk and may provide greater insight into the role of diet in promoting the health of the endothelium, a highly metabolically active organ that is involved in many physiopathological processes related to CVD [10]. Evaluation of the associations between the Mediterranean diet and direct measures of cardiovascular risk factors in harvested endothelial cells would allow us to gain insight into possible early and subtle changes in endothelial function that may affect the systemic vascular endothelium. Therefore, we conducted a pilot study to examine associations of the Mediterranean diet and its components with these direct measures of inflammation and oxidative stress from endothelial cells in women. We tested the hypothesis that an Alternate Mediterranean Diet (aMed) score, reflective of this diet pattern and adapted for the United States population, and its components will be associated with lower inflammation and oxidative stress and higher endothelial nitric oxide synthase (eNOS) expression in endothelial cells.
2. METHODS AND MATERIALS
2.1. Study Population:
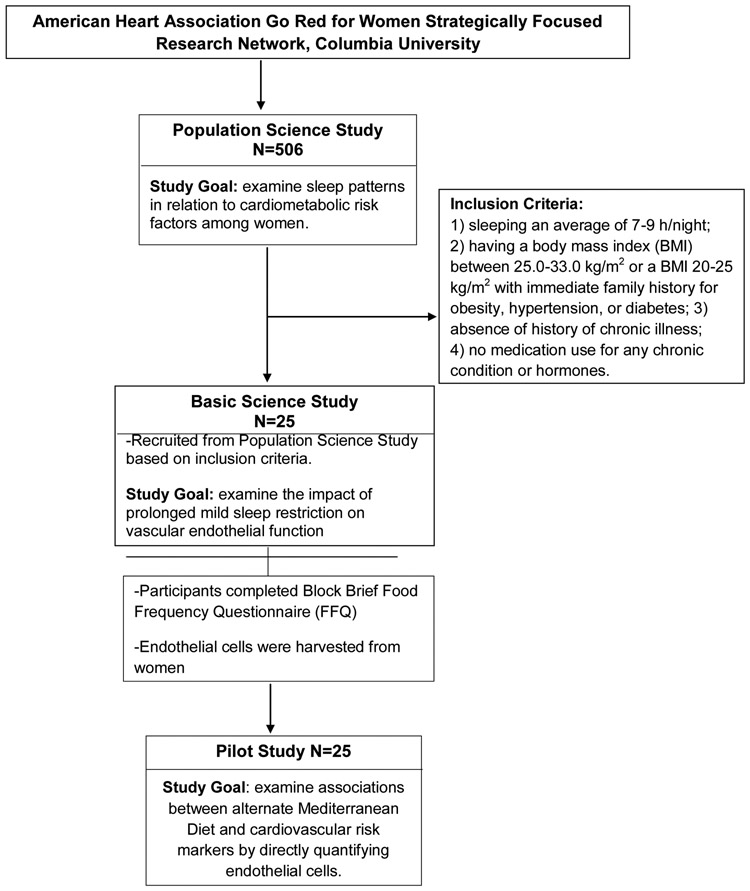
Baseline data from a subset of 25 healthy women enrolled in the American Heart Association Go Red for Women (AHA GRFW) Strategically Focused Research Network (SFRN) at Columbia University Irving Medical Center (CUIMC) for whom harvested endothelial cell samples were available, were analyzed. Women in the analytic sample participated in both the AHA GRFW SFRN Population Science and Basic Science studies. Study data collected in the years 2016-2018 were included in the study. During the baseline visit of the Population Science study, which aims to examine sleep patterns and other lifestyle behaviors in relation to cardiometabolic risk, women were asked to complete the validated Block Brief Food Frequency Questionnaire (FFQ) to assess their habitual dietary intakes. A subset of women from Population Science study were also enrolled in the Basic Science study based on inclusion criteria: (1) sleeping an average of 7 to 9 hours/ night; (2) having a body mass index (BMI) between 25.0 and33.0 kg/m2 or a BMI 20 to 25 kg/m2 without immediate family history of obesity, hypertension, or diabetes mellitus; (3) absence of history of chronic illness; and (4) no medication use for any chronic condition or hormones. The overall purpose of basic science study is to examine the impact of prolonged mild sleep restriction on vascular endothelial function. As part of their participation in the CUIMC AHA GRFW Basic Science study, they provided endothelial cell samples for Nuclear Factor kappa B (NF-κB) activation, a marker of inflammation, as well as oxidative stress assessments, and endothelial nitric oxide synthase (eNOS) gene expression measurements. Data from the screening visit (prior to any study intervention), which coincided with the baseline visit of the Population Science study, were analyzed (Figure 1). This study was approved by the CUIMC Institutional Review Board. All participants provided written informed consent. The study has been preregistered at ClinicalTrials.gov ( NCT02835261).
Figure 1:
Study Participant Recruitment
2.2. Dietary Assessment:
The validated Block Brief FFQ, [11] which queries participants regarding their habitual intake over the preceding year, was used to assess diet. The food list for this questionnaire was developed from the National Health and Nutrition Examination Survey (NHANES) III dietary recall data. The nutrient database was developed from the United States Department of Agriculture (USDA) Nutrient Database for Standard Reference. The FFQ contains approximately 70 food items. Participants reported a standard portion size and nine possible frequencies of consumption responses, ranging from “never” to “every day” for each food item on the FFQ. The FFQ was designed to be administered with portion-size photos as an aid to estimating usual portion size for each food. The Block FFQ has demonstrated validity and reliability in previous studies among women [12]. Questionnaires were made available in English and Spanish.
An Alternate Mediterranean Diet (aMed) score based on the Mediterranean diet scoring scale initially developed for Greek populations by Trichopoulou et al [13] and adapted for US populations by Fung et al [14] was computed. The aMed score focuses on higher consumption of plant foods including plant proteins, monounsaturated fat, fish, and lower consumption of animal products and saturated fat. The aMed score components in this analysis were vegetables, fruits, nuts, whole grains, legumes, fish, monounsaturated-to-saturated fatty acid (MUFA/SFA) ratio, red and processed meats, and alcohol. Total vegetable, fruit, nut, fish, MUFA and SFA intakes were already available from the FFQ data. Because total whole grain intake was not available from the FFQ, we used intake of dark bread as a proxy for whole grain intake. Legume intake was computed as the sum of refried bean, green bean and other bean intake. Participants with intake above the median intake for vegetables, fruits, nuts, whole grains, legumes, fish and MUFA/SFA ratio received 1 point for these categories; otherwise they received 0 points. In contrast, participants with red and processed meat consumption below the median received 1 point, and those with intakes above the median received 0 points for this category. We assigned 1 point for alcohol intake between 5-15 grams/day, which represents one 12-oz can of regular beer, 5 oz of wine, or 1.5 oz of liquor. The aMed scores ranged from 0–9 such that higher scores represented closer resemblance to the Mediterranean diet pattern.
2.3. Assessment of Markers of Inflammation and Endothelial Function:
Endothelial cells were collected from women in the forearm vein using three guidewires sequentially inserted through a 20-gauge angiocatheter. ECs were retrieved from wire tips by washing with an EC dissociation buffer. Harvesting yielded ~2000-5000 ECs [15]. For immunofluorescence, ECs were recovered by centrifugation at 4°C, 150G for 6 minutes, the cell pellet was re-suspended in red blood cell lysis buffer and incubated at 4°C for 5 min, then centrifuged at 150G for 6 minutes, fixed with 4% paraformaldehyde (Santa Cruz, Dallas, TX) in PBS for 10 minutes, washed twice with PBS, transferred to poly-L-lysine coated slides (Sigma, St. Louis, MO), and air dried at 37°C. The slides were stored at −80°C until analyzed. For mRNA extraction, the cell pellet was re-suspended with isolation buffer, incubated with biotinylated mouse anti-human monoclonal antibody directed against CD146 (Millipore, Temecula, CA,1:200) at 4°C for 15 min, then incubated at 4°C with Streptavidin FlowComp Dynabeads (Invitrogen, Solo, Norway; 1:100) for 45 min, followed by EC isolation by magnet [16].
Harvested ECs were identified by positive staining with goat anti-human polyclonal antibodies directed against CD144 (VE-Cadherin) (R&D Systems, Minneapolis, MN; 1:20) followed by Texas Red-conjugated donkey anti-goat secondary antibodies (Jackson ImmunoResearch, 1:50) and stained with DAPI (Molecular Probes by Life Technology, Carlsbad, CA). At least 25 consecutive ECs were analyzed from each slide. For oxidative stress, Cell ROX Green reagent (5μM, Life Technologies), a fluorogenic probe activated by reactive oxygen species and subsequent binding to DNA, was used. Oxidative stress was quantified using florescence microscopy (Nikon Eclipse E600) and ImageJ. Nuclear fluorescence of NF-κB was quantified using confocal microscopy (Nikon A1, Nikon Instruments Inc., Melville, NY) and ImageJ after staining with rabbit anti-human antibodies directed against p65 subunit of NF-κB (Novus, Littleton, CO,1:50) followed by Texas Red-conjugated donkey anti-rabbit secondary antibodies (Jackson ImmunoResearch, 1:50) [16].
We used TaqMan (Life Technology) method on Applied Biosystems StepOne Plus Real-Time PCR System. Messenger RNA (mRNA) was extracted using RNAqueous Micro Kit (Invitrogen by Thermo Fisher Scientific) according to manufacturer’s protocol. The range of 10 pg – 2 μg of mRNA was used for first-strand cDNA synthesis with qScriptXTL cDNA SuperMix (Quantabio) according to the manufacturer’s protocol. The first strand cDNA was further diluted 2.5 times with Tris-EDTA buffer and stored in −80°C. For 20 μl real-time PCR reaction the following reagents were added: 10μl TaqMan Gene Expression Master Mix (2X) (Life Technology), 1 μl TaqMan Gene Expression Assay for eNOS (NOS3- Hs01574665_m1) (20X), 5 μl Nuclease-fee water and 4μl cDNA. All the real-time PCR primers and probes span an exon junction to eliminate the contamination of genomic DNA. TaqMan primers and probes were used for eNOS and β-actin. Results were expressed by Ct values normalized to the housekeeping gene β-actin [15-16].
2.4. Assessment of Other Covariates:
A standardized health questionnaire was used to evaluate sociodemographic factors including age, race/ethnicity, and medical history including menopausal status. Anthropometric measures including height, weight, and waist circumference were obtained by trained personnel. Body mass index (BMI), in kg/m2, was calculated from weight and height. Fasting blood samples were collected along with endothelial cell harvesting. Serum and plasma were stored at −80°C until further analysis.
2.5. Statistical Analyses:
Descriptive demographic and clinical characteristics for the overall study population were generated as means and standard deviations (SD) for continuous variables and as frequencies (N) and percentages (%) for categorical variables. Means and SDs were computed for markers of vascular health and for the aMed score and its components. Univariate linear regression models were used to examine the associations of the total aMed score and its components with NF-κB, oxidative stress, eNOS gene expression. Age- and energy-adjusted linear regression models were also used to evaluate associations between the aMed score and its components in relation to NF-κB, oxidative stress and eNOS. Outcome variables were log-transformed. Because the Mediterranean diet focuses on higher overall consumption of plant-based foods, we also examined associations of percent energy intake of plant-based protein and overall protein intake with the outcomes of interest in age- and energy-adjusted linear regression models. Analyses were conducted using SAS version 9.4 (SAS Inc. Cary, North Carolina). A p-value<0.05 was considered significant for all analyses. Power analyses were not conducted as this was a pilot hypothesis-generating study with a sample size of 25 women.
3. RESULTS
Descriptive characteristics of the 25 women included in the analysis are shown in Table 1. The mean age was 33 ± 10.5 y, and the mean BMI was 24.62 ± 2.35 kg/m2. The majority of the women (68%) were racial/ethnic minorities, and the racial/ethnic distribution was as follows: 12% white, 6% African American, 7% Asian, 20% Hispanic. The mean aMed score was 4.52±1.66 (range: 0-9), Table 2.
Table 1:
Descriptive Characteristics of the Study Population
| (N = 25) Means ± SD/ N (%) |
|
|---|---|
| A. Demographic Characteristics | |
| Age (Years) | 33 ± 10.5 |
| Race | |
| White | 12 (48%) |
| African American | 6 (24%) |
| Asian | 7 (28%) |
| Ethnicity | |
| Hispanic | 5 (20%) |
| Non-Hispanic | 20 (80%) |
| Race/ Ethnicity Combined | |
| White/Non-Hispanic | 8 (32%) |
| Minority/Hispanic | 17 (68%) |
| Married | 9 (36%) |
| B. Socio-economic Status | |
| Health Insurance | 20(80%) |
| Education (Post Graduation/Professional Degree) | 11(44%) |
| Employment | 22(88%) |
| Smoking | 0(0%) |
| C. Clinical Characteristics | |
| Premenopausal | 22 (88%) |
| Systolic BP (mmHg) | 111.60 ± 14.27 |
| Diastolic BP (mmHg) | 70.32 ± 9.45 |
| Waist Circumference (Inches) | 34.23 ± 2.99 |
| BMI (kg/m2) | 24.62 ± 2.35 |
| Glucose (mg/L) | 86.83 ± 8.06 |
| Cholesterol (mg/L) | 192.75 ± 44.33 |
| Triglycerides (mg/L) | 79.17 ± 40.05 |
| HDL-Cholesterol (mg/L) | 65.17 ± 17.32 |
| LDL-Cholesterol (mg/L) | 111.80 ± 40.63 |
| C-Reactive Protein (mg/L) | 1.80 ± 2.49 |
| Cardiovascular Risk Markers | |
| Nuclear Florescence Intensity of NF-κB | 359.49 ± 139.22 |
| Oxidative Stress (Reactive Oxygen Species Florescence Intensity) | 16.71 ± 9.11 |
| Endothelial Nitric Oxide Synthase (eNOS) gene expression (n=22) | 0.93 ± 1.04 |
BMI, Body Mass Index; HDL, high-density lipoprotein; LDL, low-density lipoprotein. All values are shown as Means ± SD for continuous variable and percentages for categorical variables.
Table 2:
Mediterranean Diet Score and Individual Components
| Mediterranean Diet Score and Components | (N = 25) Means ± SD |
|---|---|
| Total Mediterranean Diet Score (Range 0-9) | 4.52 ± 1.66 |
| Individual Components (grams/day) | |
| a. Vegetable intake (excluding potatoes and French fries) | 3.21 ± 1.85 |
| b. Fruit intake | 1.25 ± 0.85 |
| c. Nuts intake | 0.17 ± 0.18 |
| d. Fish intake | 0.54 ± 0.64 |
| e. Red and Processed Meat intake | 2.48 ± 4.05 |
| f. Whole Grain intake (includes dark bread, whole wheat and rye) | 0.33 ± 0.43 |
| g. Legume intake | 0.66 ± 0.64 |
| h. Ratio of monounsaturated to saturated fats | 1.35 ± 0.34 |
| i. Alcohol | 6.09 ± 6.72 |
| Plant-based Protein (Percentage of protein as plant-based verses animal-based per day) | 19.32 ± 8.32 |
| Overall Protein | 57.27 ± 32.85 |
All values are shown as Means ± SD for continuous variable.
The Mediterranean Diet Score was calculated based on 9 individual components (fruit, nuts, fish, red and processed meat, whole grains, legumes, ratio of monosaturated to saturated fats, alcohol). Each component contributes 0 –9 points to the total score.
In univariate linear regression models (Table 3), lower NF-κB was associated with higher legume intake (Exp(β)=0.805; p=0.054) and lower oxidative stress was associated with higher red and processed meat intake (Exp(β)=0.94; p=0.012), plant-based protein (Exp(β)=0.97; p=0.007) and total protein intake (Exp(β)=0.99; p=0.006). Higher eNOS gene expression was associated with higher nut intake (Exp(β)=5.60; p=0.028).
Table 3:
Unadjusted Associations between Mediterranean Diet and Cardiovascular Risk Markers in Harvested Human Endothelial Cells
| Diet Components/Outcomes | Exponential Beta (β) | p Value |
|---|---|---|
| NF-κB | ||
| Total Mediterranean Diet Score | 0.933 | 0.110 |
| a. Vegetable intake (excluding potatoes and French fries) | 0.949 | 0.182 |
| b. Fruit intake | 0.954 | 0.591 |
| c. Nuts intake | 0.832 | 0.232 |
| d. Fish intake | 0.963 | 0.751 |
| e. Red and Processed Meat intake | 0.969 | 0.084† |
| f. Whole Grain intake (includes dark bread, whole wheat and rye) | 1.111 | 0.541 |
| g. Legume intake | 0.805 | 0.054* |
| h. Ratio of monounsaturated to saturated fats | 0.819 | 0.361 |
| i.Alcohol | 0.997 | 0.772 |
| Plant-based Protein (Percentage of protein as plant-based verses animal-based per day) | 0.986 | 0.092 |
| Total Protein | 0.996 | 0.063 |
| Oxidative Stress | ||
| Total Mediterranean Diet Score | 0.932 | 0.265 |
| a. Vegetable intake (excluding potatoes and French fries) | 0.959 | 0.474 |
| b. Fruit intake | 0.879 | 0.302 |
| c. Nuts intake | 0.719 | 0.571 |
| d. Fish intake | 0.894 | 0.455 |
| e. Red and Processed Meat intake | 0.939 | 0.012* |
| f. Whole Grain intake (includes dark bread, whole wheat and rye) | 0.864 | 0.551 |
| g. Legume intake | 1.015 | 0.932 |
| h. Ratio of monounsaturated to saturated fats | 0.826 | 0.541 |
| i. Alcohol | 0.997 | 0.839 |
| Plant-based Protein (Percentage of protein as plant-based verses animal-based per day) | 0.968 | 0.007* |
| Total Protein | 0.992 | 0.006* |
| eNOS | ||
| Total Mediterranean Diet Score | 0.945 | 0.535 |
| a. Vegetable intake (excluding potatoes and French fries) | 0.980 | 0.812 |
| b. Fruit intake | 0.837 | 0.308 |
| c. Nuts intake | 5.597 | 0.028* |
| d. Fish intake | 0.955 | 0.844 |
| e. Red and Processed Meat intake | 1.062 | 0.078† |
| f. Whole Grain intake (includes dark bread, whole wheat and rye) | 0.902 | 0.758 |
| g. Legume intake | 1.093 | 0.741 |
| h. Ratio of monounsaturated to saturated fats | 1.053 | 0.414 |
| i. Alcohol | 0.994 | 0.768 |
| Plant-based Protein (Percentage of protein as plant-based verses animal-based per day) | 1.003 | 0.826 |
| Total Protein | 1.001 | 0.776 |
Values expressed in Exponential Beta (β) generated using univariate linear regression models. Significant and borderline P-values between Diet Score and Components vs Outcomes are indicated as * or †
p-value significant at <.05
p-value borderline significance at >0.05 to 0.085. NF-κB, nuclear factor kappa B; eNOS, endothelial nitric oxide synthase.
In age-adjusted models (Table 4), lower NF-κB was associated with higher legume intake (Exp(β)=0.79; p=0.045);a borderline significant association with overall protein intake was also observed (Exp(β)=0.99; p=0.058). Lower oxidative stress was associated with higher red and processed meat intake (Exp(β)=0.93; p=0.012), plant-based protein intake (Exp(β)=0.96; p=0.007) and overall protein intake (Exp(β)=0.99; p=0.007). There was also a borderline significant association between higher eNOS gene expression and higher nut intake (Exp(β)=4.76; p=0.062) and red and processed meat intake (Exp(β)=1.06; p=0.075). The overall aMed score was not associated with outcomes.
Table 4:
Multivariate Associations between Mediterranean Diet and Cardiovascular Risk Markers in Harvested Human Endothelial Cells
| Diet Components/Outcomes | Exponential Beta (β) – Adjusted by Age |
p Value | Exponential Beta (β) – Adjusted by Age and Energy |
p Value |
|---|---|---|---|---|
| NF-κB | ||||
| Total Mediterranean Diet Score | 0.934 | 0.146 | 0.944 | 0.148 |
| a. Vegetable intake (excluding potatoes and French fries) | 0.944 | 0.161 | 0.973 | 0.549 |
| b. Fruit intake | 0.948 | 0.551 | 0.990 | 0.907 |
| c. Nuts intake | 0.631 | 0.291 | 0.696 | 0.362 |
| d. Fish intake | 0.934 | 0.590 | 1.000 | 0.998 |
| e. Red and Processed Meat intake | 0.969 | 0.087 | 0.997 | 0.916 |
| f. Whole Grain intake (includes dark bread, whole wheat and rye) | 3.803 | 0.455 | 1.228 | 0.203 |
| g. Legume intake | 0.794 | 0.045* | 0.819 | 0.057† |
| h. Ratio of monounsaturated to saturated fats | 0.830 | 0.409 | 0.695 | 0.079 |
| i. Alcohol | 0.886 | 0.906 | 0.997 | 0.808 |
| Plant-based Protein (Percentage of protein as plant-based verses animal-based per day) | 0.984 | 0.086 | 1.010 | 0.557 |
| Total Protein | 0.995 | 0.058† | 1.002 | 0.586 |
| Oxidative Stress | ||||
| Total Mediterranean Diet Score | 0.923 | 0.238 | 0.934 | 0.231 |
| a. Vegetable intake (excluding potatoes and French fries) | 0.959 | 0.478 | 1.015 | 0.771 |
| b. Fruit intake | 0.878 | 0.311 | 0.943 | 0.607 |
| c. Nuts intake | 0.682 | 0.544 | 0.807 | 0.694 |
| d. Fish intake | 0.883 | 0.492 | 0.990 | 0.950 |
| e. Red and Processed Meat intake | 0.939 | 0.012* | 0.973 | 0.411 |
| f. Whole Grain intake (includes dark bread, whole wheat and rye) | 0.854 | 0.540 | 0.953 | 0.832 |
| g. Legume intake | 1.016 | 0.923 | 1.078 | 0.609 |
| h. Ratio of monounsaturated to saturated fats | 0.821 | 0.544 | 0.616 | 0.085 |
| i. Alcohol | 0.996 | 0.821 | 0.994 | 0.685 |
| Plant-based Protein (Percentage of protein as plant-based verses animal-based per day) | 0.967 | 0.007* | 0.985 | 0.542 |
| Total Protein | 0.991 | 0.007* | 0.997 | 0.722 |
| eNOS Gene Expression | ||||
| Total Mediterranean Diet Score | 0.370 | 0.293 | 0.912 | 0.248 |
| a. Vegetable intake (excluding potatoes and French fries) | 0.998 | 0.985 | 0.887 | 0.881 |
| b. Fruit intake | 0.863 | 0.396 | 0.846 | 0.309 |
| c. Nuts intake | 4.769 | 0.062† | 3.592 | 0.096 |
| d. Fish intake | 1.061 | 0.796 | 1.033 | 0.889 |
| e. Red and Processed Meat intake | 1.062 | 0.075† | 1.133 | 0.005* |
| f. Whole Grain intake (includes dark bread, whole wheat and rye) | 0.116 | 0.531 | 0.781 | 0.4495 |
| g. Legume intake | 1.166 | 0.561 | 1.137 | 0.552 |
| h. Ratio of monounsaturated to saturated fats | 1.317 | 0.521 | 1.302 | 0.533 |
| i. Alcohol | 0.854 | 0.481 | 0.983 | 0.445 |
| Plant-based Protein (Percentage of protein as plant-based verses animal-based per day) | 1.007 | 0.682 | 1.024 | 0.474 |
| Total Protein | 1.002 | 0.680 | 1.014 | 0.152 |
Values expressed in Exponential Beta (β) generated using multivariate linear regression models adjusted to age and energy. Significant and borderline P-values between Diet Score and Components vs Outcomes are indicated as * or †
p-value significant at <.05
p-value borderline significance at >0.05 to 0.08. NF-κB, nuclear factor kappa B; eNOS, endothelial nitric oxide synthase.
In age- and energy-adjusted models (Table 4), higher eNOS gene expression remained significantly associated with higher red and processed meat intake (Exp(β)=1.13; p=0.005), and a borderline significant inverse association between NF-κB and legume intake was observed (Exp(β)=0.81; p=0.057).
4. DISCUSSION
This study uniquely demonstrates significant associations between specific aMed components and cardiovascular risk markers directly assessed in the harvested endothelial cells of women. In particular, intakes of legumes, red and processed meat, plant-based protein and overall protein, were significantly associated with reduced inflammation and oxidative stress, and greater eNOS expression, a marker of healthier vascular function, among women. No associations were observed between the overall aMed score and the study outcomes. Although we reject our initial hypothesis that the overall aMed score is related to the cardiovascular risk markers measured in this study, we can accept our second hypothesis that certain individual components of the Mediterranean diet pattern are associated with greater endothelial function, and lower inflammation and oxidative stress. Previous studies have examined the association of Mediterranean diet and markers of inflammation and endothelial dysfunction in plasma, but our pilot study is the first attempt to show an association between Mediterranean diet components and the biomarkers assessed in endothelial cells, which provides direct insight to cardiovascular risk [10,17]. Oxidative stress, NF-κB and eNOS expression are measured in the endothelium to deduce the homeostatic state of the cells, as endothelium dysfunction can lead towards the pathogenesis of CVD [18].
Surprisingly, we also observed that higher intakes of red and processed meat were associated with lower oxidative stress and higher eNOS expression. To explain this observed inverse association, we performed t-tests to determine if intakes of food groups and micronutrients known to be associated with lower cardiovascular risk were higher among women with red and processed meat intake above and below the median in this cohort. Women with higher red and processed meat intake in this cohort also had higher intakes of antioxidant nutrients. For instance, the energy-adjusted mean intake of dietary selenium was significantly greater in women with red and processed meat intake above versus below the median intake (58.95±7.95 vs 44.88±11.16mcg/1000kcal/day, p=0.001). This finding suggests that higher intakes of antioxidant nutrients among those with greater consumption of red and processed meat may help explain, at least in part, the observed protective association of red meat intake with oxidative stress and eNOS expression.
A previous report showed that higher selenium intake is associated with lower CVD risk [19], and the inclusion of low saturated fatty acid containing lean beef in the diet has been shown to help in lowering LDL-cholesterol and further reducing CVD risk [20]. Evidence related to red meat consumption and its role in the development of CVD are mixed, and further research is needed to better understand this relationship [21,22].
There are limitations of our study which should be considered, including the cross-sectional design, and the small sample size. Dietary data collected via FFQ, in general, is prone to recall bias and measurement error. Underreporting is also an issue, which may have biased associations towards the null.
In conclusion, our findings highlight additional mechanisms through which a Mediterranean diet pattern may lower CVD risk in women. Results indicate that several aspects of a Mediterranean diet may work to lower oxidative stress and inflammation and improve endothelial dysfunction. To our knowledge, this represents one of the earliest reports on the Mediterranean diet in relation to measures of inflammation and oxidative stress from endothelial cells, as direct measures of cardiovascular risk, in women. However, our results warrant confirmation prospectively in a larger sample and among men.
ACKNOWLEDGMENT
The authors have no conflicts of interest to disclose. This research was supported by an American Heart Association Go Red for Women Strategically Focused Research Network Award: Grant # 16SFRN27960011 to Aggarwal, Grant # 16SFRN29050000 and NIH NHLBI R01HL106041 to Dr. Jelic. The work was also supported in part by Columbia University’s CTSA Grant # UL1TR001873 from NCATS, NIH. The funding sources had no involvement in study design, in the collection, analysis and interpretation of data, in writing of the report and in the discussion to submit the article for publication.
ABBREVIATIONS
- CVD
Cardiovascular Disease
- aMed
Alternate Mediterranean Diet
- eNOS
endothelial Nitric Oxide Synthase
- CUIMC
Columbia University Irving Medical Center
- AHA GRFW
American Heart Association Go Red for Women
- FFQ
Food Frequency Questionnaire
- NHANES
National Health and Nutrition Examination Survey
- USDA
United States Department of Agriculture
- NF-κB
Nuclear Factor kappa B
- MUFA/SFA
ratio of Mono-saturated to Saturated Fatty Acids
- SD
Standard Deviation
- N
Frequency
- %
Percentage
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Whatnall M, Collins C, Callister R, Hutchesson M. Associations between Unhealthy Diet and Lifestyle Behaviours and Increased Cardiovascular Disease Risk in Young Overweight and Obese Women. Healthcare 2016;4:57 10.3390/healthcare4030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Willett WC, Koplan JP, Nugent R, Dusenbury C, Puska P, Gaziano TA. Prevention of Chronic Disease by Means of Diet and Lifestyle Changes In: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, et al. , editors. Disease Control Priorities in Developing Countries. 2nd edition. Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2006. Chapter 44. [PubMed] [Google Scholar]
- [3].George SM, Ballard-Barbash R, Manson JAE, Reedy J, Shikany JM, Subar AF, et al. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the women’s health initiative observational study: Evidence to inform national dietary guidance. Am J Epidemiol. 2014;180(6):616–25. 10.1093/aje/kwu173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Centers for Disease Control and Prevention. Leading Causes of Death in Females United States, 2013. 3025;2015:2–4. Available from: http://www.cdc.gov/women/lcod/2013/index.htm [Google Scholar]
- [5].Anand SS, Hawkes C, De Souza RJ, Mente A, Dehghan M, Nugent R, et al. Food Consumption and its impact on Cardiovascular Disease. J Am Coll Cardiol. 2016;66:1590–1614. 10.1016/j.jacc.2015.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ros E, Martínez-gonzález MA, Estruch R, Salas-salvadó J, Martínez JA, Corella D. Mediterranean Diet and Cardiovascular Health : Teachings of the PREDIMED Study. Adv Nutr. 2014;5:330S–6S. 10.3945/an.113.005389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Whatnall M, Collins C, Callister R, Hutchesson M. Associations between Unhealthy Diet and Lifestyle Behaviours and Increased Cardiovascular Disease Risk in Young Overweight and Obese Women. Healthcare 2016;4:pii:E57. 10.3390/healthcare4030057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Grosso G, Marventano S, Yang J, Micek A, Pajak A, Scalfi L, et al. A comprehensive meta-analysis on evidence of Mediterranean diet and cardiovascular disease: Are individual components equal? Crit Rev Food Sci Nutr. 2017;57:3218–3232. 10.1080/10408398.2015.1107021. [DOI] [PubMed] [Google Scholar]
- [9].Castiglione D, Platania A, Conti A, Falla M, D’Urso M, Marranzano M. Dietary Micronutrient and Mineral Intake in the Mediterranean Healthy Eating, Ageing, and Lifestyle (MEAL) Study. Antioxidants 2018; 7:pii:E79. doi: 10.3390/antiox7070079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sureda A, Bibiloni MDM, Julibert A, Bouzas C, Argelich E, Llompart I, et al. Adherence to the Mediterranean Diet and Inflammatory Markers. Nutrients 2018; 10:pii:E62. 10.3390/nu10010062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Block G, Hartman AM, Naughton D. A reduced dietary questionnaire: development and validation. Epidemiology 1990;1:58–64. 10.1097/00001648-199001000-0013. [DOI] [PubMed] [Google Scholar]
- [12].Boucher BA, Cotterchio M, Kreiger N, Nadalin V, Block TJ, Block G. Validity and reliability of the Block98 food-frequency questionnaire in a sample of Canadian women. Public Health Nutrition 2006;1:84–93. 10.1079/PHN2005763. [DOI] [PubMed] [Google Scholar]
- [13].Trichopoulou A, Costacou T, Bamia C, Trichopoulous D. Adherence to a Mediterranean diet and survival in a Greek population. New England Journal of Medicine 2003;348:2599–2608. 10.1056/NEJMMoa025039. [DOI] [PubMed] [Google Scholar]
- [14].Fung TT, McCullough ML, Newby PK, Manson JE, Meigs JB, Rifai N, et al. Diet-quality scores and plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr. 2005;82:163–173. 10.1093/ajcn.82.1.163. [DOI] [PubMed] [Google Scholar]
- [15].Jelic S, Lederer DJ, Adams T, Padeletti M, Colombo PC, Factor PH, et al. Vascular inflammation in obesity and sleep apnea. Circulation 2010;121:1014–21. 10.1161/CIRCULATIONAHA.109.900357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Emin M, Wang G, Castagna F, Rodriguez-Lopez J, Wahab R, Wang J, et al. Increased internalization of complement inhibitor CD59 may contribute to endothelial inflammation in obstructive sleep apnea. Sci Transl Med. 2016;8:1–14. 10.1126/scitranslmed.aad0634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Casas R, Sacanella E, Urpí-Sardà M, Chiva-Blanch G, Ros E, Martínez-González M-A, et al. The Effects of the Mediterranean Diet on Biomarkers of Vascular Wall Inflammation and Plaque Vulnerability in Subjects with High Risk for Cardiovascular Disease. A Randomized Trial. PLoS ONE 2014;9:e100084 10.1371/journal.pone.0100084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Defagó MD, Elorriaga N, Irazola VE, Rubinstein AL. Influence of food patterns on endothelial biomarkers: a systematic review. J Clin Hypertens. (Greenwich) 2014; 16:907–913. 10.1111/jch.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, Guallar E. Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr. 2006;84:762–73. 10.1093/ajcn/84.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Roussell MA, Hill AM, Gaugler TL, West SG, Heuvel JPV, Alaupovic P, et al. Beef in an Optimal Lean Diet study: effects on lipids, lipoproteins, and apolipoproteins. Am J Clin Nutr. 2012;95:9–16. 10.3945/ajcn.111.016261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Meta-analysis of prospective cohort studies evaluating the association of saturated fat with cardiovascular disease. Am J Clin Nutr. 2010;91:535–46. 10.3945/ajcn.2009.27725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Guasch-Ferré M, Babio N, Martinez-González MA, Corella D, Ros E, Martin-Peláez S et al. Dietary fat intake and risk of cardiovascular disease and all-cause mortality in a population at high risk of cardiovascular disease. Am J Clin Nutr. 2015; 102:1563–73. 10.3945/ajcn.115.116046. [DOI] [PubMed] [Google Scholar]