Abstract
The attitudes of individuals who receive, provide, or influence opioid use disorder (OUD) medication services, also called stakeholders, may enhance or hinder their dissemination and adoption. Individuals who have resolved a significant alcohol or other drug (AOD) problem are a group of key stakeholders whose OUD medication attitudes are not well understood empirically. This group subsumes, but is not limited to, individuals who identify as being “in recovery.” Analyses leveraged the National Recovery Study, a geo-demographically representative survey of U.S. adults who resolved a significant AOD problem (N = 1,946). We examined the prevalence of positive, neutral, and negative attitudes toward agonists, such as buprenorphine/naloxone and methadone, and antagonists, such as oral and extended-release depot injection naltrexone. Single-predictor logistic regression models tested for demographic, clinical, and recovery-related correlates of these attitudes and, for those significant at the .1 level, multivariable-predictor logistic regression models tested unique associations between these correlates and attitudes. Results showed that participants were equally likely to hold positive (21.4 [18.9–24.0]%) and negative agonist (23.8 [21.2–26.7]%) attitudes but significantly more likely to hold negative (30.3 [27.4–33.3]%) than positive antagonist attitudes (18.0 [15.9–20.4]%). Neutral attitudes were most commonly endorsed for both agonists (54.8 [51.6–57.9]%) and antagonists (51.7 [48.5–54.8]%). For agonists, more recent AOD problem resolution was a unique predictor of positive attitude, whereas Black and Hispanic races/ethnicities, compared with White, were unique predictors of negative attitude. For antagonists, older age group (45–59 and 60+ vs. 18–29 years), lifetime opioid antagonist medication prescription, and past 90-day non–12-step mutual-help attendance were unique predictors of positive attitude, whereas greater spirituality was a unique predictor of negative attitude. This population-level study of U.S. adults who resolved an AOD problem showed that agonist attitudes may be more positive than anecdotal evidence suggests. Certain characteristics and experiences, however, highlight a greater likelihood of negative attitudes, suggesting these factors may be potential barriers to OUD medication adoption.
Keywords: opioid use disorder, medications, buprenorphine, implementation, addiction recovery
The precipitous increase in opioid-involved overdose deaths in the United States (Centers for Disease Control and Prevention, 2018) necessitates scrutiny of interventions that can help protect against opioid-related mortality. Opioid use disorder (OUD) agonist medications, such as methadone, and partial agonist medications, such as buprenorphine (typically prescribed in a formulation with naloxone that can be taken sublingually, marketed in the United States as Suboxone), reduce the likelihood of illicit opioid use and opioid-involved overdose (Marsden et al., 2017; Mattick, Breen, Kimber, & Davoli, 2014; Sordo et al., 2017). Antagonists, specifically the once-per-month extended-release depot injection formulation of naltrexone (marketed in the United States as Vivitrol), have similar clinical benefits as buprenorphine/naloxone but can be more difficult to initiate because their induction requires an initial period of opioid abstinence (Lee et al., 2018; Tanum et al., 2017).
The overall public health impact of these OUD medications vis-à-vis reduced opioid use and overdose depends on several factors, including not only clinical efficacy but also the degree to which they are disseminated and adopted (Glasgow, Klesges, Dzewaltowski, Estabrooks, & Vogt, 2006). OUD medication attitudes among relevant stakeholders—individuals who engage with and provide OUD medication services, as well as those who are positioned to influence service delivery—are likely to affect these adoption and dissemination efforts. Indeed, OUD policymakers, scientists, and health-care providers and administrators have called for broad dissemination of these potentially life-saving medications, with an emphasis on opioid agonists (Congressional Research Service, 2018; Green et al., 2014; Molfenter et al., 2015; Saloner et al., 2018; Samet & Kertesz, 2018; Volkow & Collins, 2017; Wakeman & Rich, 2018).
The large population of individuals who have resolved a significant alcohol or other drug (AOD) problem in the United States (Kelly, Bergman, Hoeppner, Vilsaint, & White, 2017), half of whom identify as individuals “in recovery” (Kelly, Abry, Milligan, Bergman, & Hoeppner, 2018), are also important and potentially unique stakeholders in the dissemination of OUD medications. Many fill key gatekeeper roles, such as treatment providers and administrators (Roman & Johnson, 2004a, 2004b), peer recovery support specialists or “recovery coaches” (Bassuk, Hanson, Greene, Richard, & Laudet, 2016), policymakers and scientists, and peers who interact with individuals taking or considering OUD medication.
Clinical commentaries (Wakeman & Rich, 2018) and journalism geared toward lay individuals (e.g., Siegel, 2016) suggest that individuals in AOD recovery hold negative or otherwise skeptical views of OUD medication. For agonists, recovering individuals, who typically adhere to a “program of complete abstinence” (Ginter, 2012; White, 2011), these views may center on continued use of other substances among individuals in methadone maintenance treatment program (Kleber, 2008) and misuse of buprenorphine “to get high or change [one’s] mood” (Cicero, Ellis, & Chilcoat, 2018). Antagonists, like naltrexone, however, block opioid receptors and thus are unable to produce rewarding or withdrawal-relieving effects, posing no risk for misuse irrespective of dose. Given the different mechanisms of therapeutic action and the potential to evoke different perceptions as a result, agonists and antagonists may well evoke different attitudes.
Despite widely held assumptions about recovering individuals’ OUD medication attitudes, there has been very little empirical scrutiny of this phenomenon. To help address this gap, we examined both opioid agonist and antagonist medication attitudes using data from the National Recovery Study, a survey of U.S. adults who resolved a significant AOD problem (Kelly et al., 2017).
Perspectives of Individuals With AOD Disorders
A review of OUD medication attitudes for those with current AOD disorder provides additional context. Among those admitted to inpatient opioid withdrawal management programs, 60–80% express interest in taking medication (Bailey, Herman, & Stein, 2013; Uebelacker, Bailey, Herman, Anderson, & Stein, 2016). Whereas individuals attending outpatient methadone maintenance programs and other patients with OUD report modestly positive views of methadone’s helpfulness, purpose, and physical effects (Kelly et al., 2012; Schwartz et al., 2008), those with OUD typically see buprenorphine as more useful, safer, and consistent with a drug-free lifestyle than methadone (Schwartz et al., 2008; Uebelacker et al., 2016; Yarborough et al., 2016). This may be related to buprenorphine’s partial agonism, associated with a lower likelihood of physical dependence, misuse, and overdose, compared to methadone’s full agonism (Lutfy & Cowan, 2004). Individuals with OUD may also view buprenorphine more positively because they can access the medication via prescription in office-based settings such as primary care (American Society of Addiction Medicine, 2004) rather than opioid treatment programs, which initially require daily administration for methadone. Furthermore, patients may see the monthly depot formulation of naltrexone as more consistent with a drug-free lifestyle than buprenorphine-naloxone (Uebelacker et al., 2016).
Thus, given the predominance of buprenorphine-naloxone among OUD medication interventions (Alderks, 2017; Morgan, Schackman, Leff, Linas, & Walley, 2018) and more positive attitudes toward buprenorphine relative to methadone, it is reasonable to hypothesize that agonist attitudes are becoming more positive over time. In addition, individuals who resolved their AOD problem during the current opioid overdose crisis may have greater acceptance of “multiple pathways to recovery” (White & Kurtz, 2005). Finally, individuals may have formed more positive agonist attitudes given the easier access to online media that highlight scientific evidence for the clinical and public health utilities of buprenorphine-naloxone.
In the current study, we examined both opioid agonist and antagonist medication attitudes in a nationally representative sample of U.S. adults who resolved a significant AOD problem. Specifically, in Aim 1, we characterized opioid agonist and antagonist medication attitudes; in Aim 2, we examined associations between demographic, clinical, and recovery-related variables with agonist and antagonist attitudes; and, in Aim 3, we tested whether those variables associated with attitudes in Aim 2 were uniquely associated with agonist and antagonist attitudes. We hypothesized that more recent problem resolution would be uniquely associated with a greater likelihood of positive opioid agonist attitude but made no other a priori hypotheses given the lack of prior data in the area to inform predictions.
Method
Procedure
National Recovery Study (NRS) participants were members of the GfK (recently acquired by Ipsos) Knowledge Panel, which employs address-based sampling to randomly select individuals from 97% of all U.S. households, including those with unlisted phone numbers or without a landline. GfK provides individuals with a web-enabled computer and free Internet service if needed. For the NRS, GfK conducted web-based screening with a representative subset of the GfK Knowledge Panel (N = 39,809) in July and August 2016, targeting individuals who responded yes to the question: “Did you used to have a problem with drugs or alcohol, but no longer do?” These individuals completed a brief assessment battery related to their AOD problem resolution experiences (see Measures section), with a median time to completion of 24 min (interquartile range [IQR] = 18–36 min; Kelly et al., 2017). The current study focused on NRS participants who completed five items assessing attitudes toward the use of medication in the treatment of AOD problems and emotional difficulties, as detailed in the Measures section (N = 1,946; 97.2% of the full NRS sample).
NRS data were weighted using iterative proportional fitting (Battaglia, Hoaglin, & Frankel, 2009), which first adjusts weights for survey response rate biases and then accounts for participants’ over- or underrepresentation in the data set along eight geodemographic dimensions according to benchmarks from the March 2015 Current Population Survey (U.S. Census Bureau, 2015; e.g., gender, age, race/ethnicity, education, geographic location, household income, homeownership status, and metropolitan area). When these participant weights are applied statistically, analyses yield unbiased, representative estimates of the U.S. adult population. In the current study, we applied these weights for all analyses. This process resulted in a geodemographically representative sample of U.S. adults who resolved a significant AOD problem (Kelly et al., 2017). It is important to note that in addition to the series of studies our group has published from these data (e.g., Bergman, Greene, Hoeppner, & Kelly, 2018; Kelly, Greene, & Bergman, 2018a, 2018b), GfK’s Knowledge Panel has also been used to conduct other rigorous, epidemiological studies in the substance use field (e.g., Heeren et al., 2008).
Measures
Demographic characteristics
We used the demographic data participants reported as part of their initial GfK Knowledge Panel recruitment, including the following: age (subsequently categorized into 18–29, 30–44, 45–59, and 60+ years), level of education (highest grade completed), race/ethnicity (White, Black—non-Hispanic, other race—non-Hispanic, Hispanic, 2+ races), gender (male or female), household income (ordinal variable with 19 levels ranging from less than $5,000 to $175,000 or more), and U.S. region (Northeast, Midwest, South, or West).
Substance use
Participants answered a series of questions about 15 substances/classes of substances (hereafter simply referred to as substances) used 10 or more times in their lifetime from the Global Appraisal of Individual Needs (Dennis, Titus, White, Unsicker, & Hodgkins, 2002). Of these, they indicated which was ever a problem, from which we computed the total number of lifetime problem substances (with a maximum of 16, including the 15 substances and an “other” option) and their primary problem substance. For all lifetime substances used, participants also reported whether they were currently using that substance, from which we determined current AOD abstinence (i.e., abstinence from all substances, including alcohol and other drugs, and no misuse of medications such as opioids and benzodiazepines). We categorized these problem/primary substances into the groups of alcohol, cannabis, cocaine, methamphetamine, opioids, or other (e.g., benzodiazepines).
Medication history
Participants reported whether they had ever been prescribed a medication “to prevent you from drinking alcohol” (i.e., AUD medication; acamprosate, naltrexone, nalmefene, topiramate, disulfiram, baclofen) or “to prevent you from using opioids” (OUD medication; methadone, levomethadyl acetate, buprenorphine/naloxone, buprenorphine, oral naltrexone, long-acting injectable naltrexone; Miller, 1996). Given the current study’s conceptual focus on the comparison of agonist to antagonist attitudes, we further categorized history of OUD medication prescription into opioid agonists (e.g., buprenorphine, methadone) and antagonists (e.g., naltrexone).
OUD medication attitudes
OUD medication attitude items were based on Rychtarik, Connors, Dermen, and Stasiewicz (2000) and operationalized by participant agreement (1 = strongly disagree, 2 = disagree, 3 = somewhat disagree, 4 = somewhat agree, 5 = agree, and 6 = strongly agree) with two statements: (a) “It is a good idea for someone with an opioid problem to take a substitute medication like Suboxone or methadone to help them stop using” for opioid agonist attitude and(b) “It is a good idea for someone with an opioid problem to take an opioid blocking medication like naltrexone/Vivitrol to help them stop using” for opioid antagonist attitude. We defined “positive” attitude as agree or strongly agree, “neutral” attitude as somewhat agree or somewhat disagree, and “negative” attitude as disagree or strongly disagree.
We also conducted sensitivity analyses (see Analysis Plan section) in which positive attitude was operationalized by any agreement with the item (somewhat agree, agree, or strongly agree), and negative attitude was operationalized by any disagreement with the item (somewhat disagree, disagree, and strongly disagree).
Clinical history
Participants indicated lifetime engagement with professional treatment (e.g., outpatient or inpatient/residential treatment; Institute of Behavioral Research, 2002). Co-occurring psychiatric disorder was determined by an affirmative response to one or more of 16 non-AOD psychiatric disorders (Dennis et al., 2002), including anxiety disorders, mood disorders, eating disorders, psychotic disorders, and personality disorders.
Psychosocial functioning
The questionnaire included the Kessler-6 (Kessler et al., 2003), a six-item scale assessing psychiatric symptoms (also referred to as psychological distress) that asks participants to rate how often they experienced mental health difficulties (e.g., nervousness and depression), from 0 = none of the time to 4 = all of the time during the past 30 days (current sample Cronbach’s alpha = .92). It also included the EUROHIS-QOL (Schmidt, Mühlan, & Power, 2006), a quality-of-life measure that asks participants to rate life satisfaction along eight dimensions from 1 = very dissatisfied to 5 = very satisfied (current sample Cronbach’s alpha = .89).
Recovery history
Participants reported on attendance at mutual-help organizations (MHOs), including Alcoholics Anonymous (AA), Narcotics Anonymous (NA), and SMART Recovery (Kelly, Urbanoski, Hoeppner, & Slaymaker, 2011), as well as other community-based recovery supports (e.g., recovery community centers, collegiate recovery programs, etc.; Institute of Behavioral Research, 2002). For each MHO attended during the lifetime, attendees reported whether they ever engaged in regular attendance (i.e., once per week or more) as well as the number of meetings attended during the past 90 days, which we dichotomized into any attendance (yes/no) in the past 90 days. We separated 12-step MHOs (e.g., AA and NA) from non–12-step MHOs (e.g., SMART Recovery). Participants also indicated whether they considered themselves to be “in recovery” (yes/no).
Criminal justice history
Participants indicated whether they had been arrested (yes/no) and, if yes, whether they participated in a drug court (yes/no).
Religiosity/spirituality
The survey assessed the extent to which individuals considered themselves religious or spiritual (in separate items) on a Likert scale from 1 = not religious/spiritual at all to 4 = very religious/spiritual (Idler et al., 2003).
Analysis Plan
To characterize and compare agonist and antagonist attitudes, we used frequencies to describe the percentages with positive (strongly agree or agree), neutral (somewhat agree or somewhat disagree), and negative (strongly disagree or disagree) attitudes, including 95% confidence intervals. Nonoverlapping confidence intervals are conservative methods for indicating significant differences between frequency estimates (Schenker & Gentleman, 2001). We also computed the correlation between agonist and antagonist attitudes modeling attitude continuously from 1 (strongly disagree) to 6 (strongly agree).
Given how little is known empirically regarding OUD medication attitudes, we also examined correlations between participant attitudes and several demographic, clinical, recovery-related, and criminal justice history variables. To test for correlates of agonist attitudes, we first conducted single-predictor, multinomial logistic regressions where opioid agonist medication attitude was the outcome (2 = positive; 1 = neutral; and 0 = negative). For these models, we analyzed whether variables were associated with agonist attitude chosen one at a time from a series of demographic (age, gender, race/ethnicity, geographic region, level of education, household income, religiosity, and spirituality), clinical (AUD medication, OUD medication separated by agonists and antagonists, formal treatment history, co-occurring psychiatric disorder, and psychiatric symptoms), recovery-related (recovery identity, primary substance, current abstinence from all substances, any regular 12-step attendance lifetime, any regular non–12-step MHO attendance lifetime, any 12-step attendance past 90 days, any non–12-step MHO attendance past 90 days, lifetime use of non-MHO recovery support services, and quality of life), and criminal justice history variables.
Then, in order to examine which variables accounted for significant, unique variance in the prediction of agonist attitude, we conducted a multivariable-predictor logistic regression model with the set of variables significant at the .10 level in the single-predictor models as predictors in the multivariable model. For variables with three or more categories, we used the omnibus significance level to determine whether the association met the .10 threshold. We then replicated this analytic framework for antagonist attitudes. In order to test the hypothesis that more recent problem resolution was a unique predictor of agonist attitude, we first examined whether it was associated with the outcome at the .10 level. Then, if meeting this threshold, we examined its adjusted odds ratio and the statistical significance (p < .05) of its semipartial coefficient in the multivariable-predictor model (i.e., controlling for other predictors in the model).
Regarding model interpretation, we chose neutral as the reference outcome. Thus, for each effect, we present the omnibus p-value (i.e., Does the likelihood of having a positive, neutral, or negative medication attitude depend on the level of the predictor variable?). We also present odds ratios comparing the likelihood of a positive attitude to a neutral attitude, as well as a negative attitude to a neutral attitude. In multivariable models, these are adjusted odds ratios because they control for the other predictors in the model. If an odds ratio is greater than 1, it suggests that the attitude in question, positive or negative, is more likely than neutral, and if less than 1, it suggests that the attitude in question is less likely than neutral. In cases where a variable is significantly associated with both positive and negative attitude in the same direction compared to neutral (e.g., both positive and negative attitude are more likely than neutral), the direction of the association would be unclear. Thus, in these cases, we conducted post hoc analyses, changing the reference group to negative so that we could determine if there was a difference between the likelihood of positive and negative attitudes. Supplementary Table 1 shows the associations between all variables and the likelihood of positive versus negative attitude in the single-predictor and multivariable-predictor multinomial logistic regression models, including odds ratios and significance testing, for both agonist and antagonist attitudes. This shift in reference group from neutral to negative attitude to facilitate positive versus negative attitude comparisons simply offers a different perspective on the same set of analyses (e.g., omnibus p-values are identical).
Finally, we conducted sensitivity analyses to examine whether our findings would be different if the medication attitude variable were dichotomous (positive and negative) rather than trichotomous (positive, neutral, and negative). For these analyses, we operationalized positive attitude as strongly agree, agree, or somewhat agree and negative attitude as strongly disagree, disagree, or somewhat disagree. Odds ratios greater than 1 reflect a greater likelihood of a positive attitude, and odds ratios less than 1 reflect a greater likelihood of negative attitude. Although we conducted sensitivity analyses in parallel with the primary analyses (e.g., first examined correlates significant at the .1 level and then tested those in a multivariable model to examine whether each variable is uniquely associated with attitude), we compare only the multivariable model findings.
All analyses were survey-weighted and conducted using the Complex Samples module in IBM SPSS Statistics 24, with a significance level of .05 unless specified otherwise. All study procedures were approved by the Partners HealthCare Institutional Review Board.
Results
Participant Characteristics and OUD Medication Attitudes
We present participant demographic, clinical, recovery-related, and criminal justice history characteristics in Table 1. Of note, compared with excluded participants who did not complete the medication attitude items (n = 34), included participants were significantly more likely to be White (compared to other—non-Hispanic), to have higher levels of education, to identify as a person in recovery, to have a history of a co-occurring psychiatric disorder, and to have engaged with a range of AOD services (e.g., inpatient or outpatient treatment) but were significantly less likely to be abstinent from alcohol and other drugs (p < .05). Included and excluded individuals were similar on all other measured characteristics.
Table 1.
Descriptive Statistics for Demographic, Clinical, and Recovery-Related Characteristics (N = 1,946)
| Characteristics | % or M | SE |
|---|---|---|
| Age | ||
| 18–29 | 17.2% | 1.5% |
| 30–44 | 27.6% | 1.5% |
| 45–59 | 34.5% | 1.5% |
| 60+ | 20.7% | 1.0% |
| Female | 40.2% | 1.5% |
| Race/ethnicity | ||
| 2+ races | 1.7% | .3% |
| Black—non-Hispanic | 13.9% | 1.2% |
| Other—non-Hispanic | 5.9% | .9% |
| Hispanic | 17.0% | 1.4% |
| White | 61.5% | 1.7% |
| Geographical region | ||
| Northeast | 16.5% | 1.2% |
| Midwest | 21.1% | 1.3% |
| South | 34.2% | 1.6% |
| West | 28.2% | 1.5% |
| Education level (1–14)a | 9.79 | .07 |
| Household income (1–19)b | 10.43 | .16 |
| Religiosity (1–4) | 2.38 | .03 |
| Spirituality (1– 4) | 2.75 | .03 |
| Criminal justice history | ||
| Arrested, drug court | 7.9% | 1.0% |
| Arrested, no drug court | 43.0% | 1.6% |
| None | 49.1% | 1.6% |
| Psychiatric symptoms (0–24) | 4.91 | .18 |
| Quality of life (8–40) | 29.18 | .22 |
| Lifetime AUD medication prescription | 5.4% | .7% |
| Lifetime OUD agonist medication prescription | 4.2% | .7% |
| Lifetime OUD antagonist medication prescription | .5% | .3% |
| Inpatient or outpatient treatment | 25.3% | 1.4% |
| History of a co-occurring psychiatric disorder | 33.6% | 1.5% |
| Alcohol problemc | 63.1% | 1.6% |
| Cannabis problem | 18.1% | 1.3% |
| Cocaine problem | 20.5% | 1.2% |
| Methamphetamine problem | 12.8% | 1.1% |
| Opioid problem | 11.1% | 1.0% |
| Number lifetime problem substances | 3.41 | .09 |
| Primary substance | ||
| None | 12.6% | 1.1% |
| Alcohol | 51.2% | 1.6% |
| Cannabis | 10.8% | 1.1% |
| Cocaine | 10.1% | .9% |
| Methamphetamine | 7.4% | .9% |
| Opioids | 5.4% | .8% |
| Other | 2.5% | .5% |
| Years since AOD problem resolution | 11.83 | .31 |
| Total abstinence | 51.0% | 1.6% |
| Recovery identity | 45.6% | 1.6% |
| Any regular 12-step attendance | 32.3% | 1.5% |
| Any regular non–12-step MHO attendance | 5.1% | .7% |
| Past 90-day 12-step MHO attendance | 10.9% | 1.0% |
| Past 90-day non–12-step attendance | 2.2% | .5% |
| Any non-MHO recovery support service | 22.3% | 1.4% |
Note. AUD = alcohol use disorder; OUD = opioid use disorder; AOD = alcohol and other drug; MHO = mutual-help organization.
Education is an ordinal variable from 1 (no formal education) to 14 (professional or doctoral degree).
Household income is an ordinal variable from 1 (less than $5,000) to 19 ($175,000 or more).
Substance problems (alcohol, cannabis, cocaine, methamphetamine, and opioid) were dichotomous yes/no variables and not mutually exclusive, such that participants could report more than one problem substance. Of those that participants reported as a problem, they were asked to choose just one primary substance; thus, these responses were mutually exclusive.
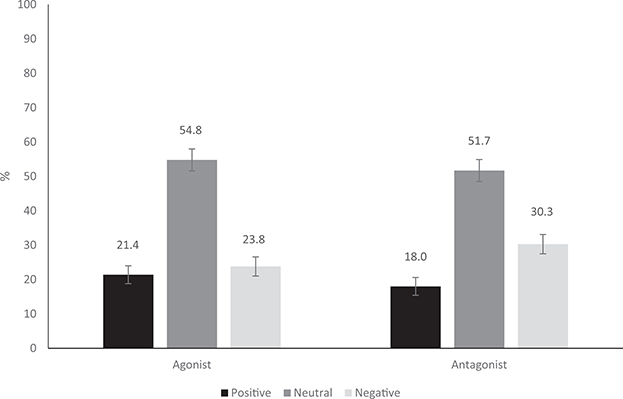
For agonist attitudes, participants had greater rates of neutral (54.8%; 95% confidence interval [CI: 51.6, 57.9%]) than both positive (21.4%; 95% CI [18.9, 24.0%]) and negative (23.8%; 95% CI [21.2, 26.7%]) attitudes, whereas positive and negative agonist attitude rates were similar (see Figure 1). For antagonists, participants were significantly more likely to have a neutral (51.7%; 95% CI [48.5, 54.8%]) than a negative attitude (30.3%; 95% CI [27.4, 33.3%]) and more likely to have a negative than a positive attitude (18.0%; 95% CI [15.9, 20.4%]; see Figure 1). Comparing attitudes by type of OUD medication, participants were equally likely to hold a positive agonist and positive antagonist attitude, as well as a neutral agonist and neutral antagonist attitude. On the other hand, participants were more likely to hold a negative antagonist than a negative agonist attitude. As continuous ordinal variables, agonist and antagonist attitudes were strongly correlated, r = .760, p < .001.
Figure 1.
Proportion with positive (strongly agree or agree), neutral (somewhat agree or somewhat disagree), and negative (disagree or strongly disagree) agonist attitudes and with positive (strongly agree or agree), neutral (somewhat agree or somewhat disagree), and negative (disagree or strongly disagree) antagonist attitudes (error bars represent 95% CI upper and lower bounds). Participants had a similar likelihood of positive and negative agonist attitudes but a greater likelihood of a negative antagonist attitude compared with a positive antagonist attitude.
Correlates of Medication Attitudes
Agonists
Significant correlates of agonist attitude at the .1 level in single-predictor models were race/ethnicity (p < .001), lifetime AUD medication prescription (p = .025), lifetime OUD agonist medication prescription (p = .067), inpatient or outpatient treatment (p = .026), history of a co-occurring psychiatric disorder (p = .006), and fewer years since AOD problem resolution (p = .008; see Table 2).
Table 2.
Odds Ratios (ORs) and Significance Testing for Single-Predictor Logistic Regressions (Single; Ns = 1,932–1,946) and Adjusted Odds Ratios (aORs) and Significance Testing for Multivariable-Predictor Logistic Regression (Multi; N = 1,912) Examining Correlates of Opioid Agonist Medication Attitudes (Positive, Neutral, or Negative)a by Positive Versus Neutral and Negative Versus Neutral Pairwise Comparisons
|
p |
Positive versus neutral |
Negative versus neutral |
||||
|---|---|---|---|---|---|---|
| Variable | Singleb | Multib | OR | aOR | OR | aOR |
| Age | .102 | |||||
| 18–29 (ref)c | — | — | ||||
| 30–44 | 1.26 | .69 | .67 | |||
| 45–59 | 1.36 | .66 | .66 | |||
| 60+ | .92 | .59* | .66 | |||
| Gender (female = ref) | .758 | 1.06 | .92 | |||
| Race/Ethnicity | .001 | <.001 | ||||
| 2 + Races | 3.38** | 3.61* | 2.20* | 1.78 | ||
| Black—non-Hispanic | .75 | .76 | 1.67* | 1.71* | ||
| Other—non-Hispanic | 1.08 | 1.00 | .46 | .41 | ||
| Hispanic | 1.08 | 1.06 | 1.94** | 1.84* | ||
| White (ref) | — | — | — | — | ||
| Geographical region | .577 | |||||
| Northeast | 1.17 | 1.20 | ||||
| Midwest | .98 | 1.14 | ||||
| South | .76 | 1.10 | ||||
| West | — | — | ||||
| Education level | .219 | 1.02 | .93 | |||
| Household income | .352 | 1.00 | .98 | |||
| Religiosity | .599 | .93 | 1.01 | |||
| Spirituality | .190 | .95 | 1.11 | |||
| Criminal justice history | .104 | |||||
| Arrested, drug court | 1.03 | 1.91 | ||||
| Arrested, no drug court | 1.33 | 1.02 | ||||
| None (ref) | — | — | — | |||
| Psychiatric symptoms | .238 | 1.02 | .99 | |||
| Quality of life | .193 | .99 | 1.01 | |||
| Lifetime AUD medication prescription (no = ref) | .025 | .104 | 2.18* | 1.65 | .87 | .70 |
| Lifetime opioid agonist medication prescription (no = ref) | .067 | .115 | 2.25* | 1.62 | 2.23 | 2.57* |
| Lifetime opioid antagonist medication prescription (no = ref) | .995 | .90 | d | |||
| Inpatient or outpatient treatment (no = ref) | .026 | .131 | 1.57** | 1.43 | .99 | 1.01 |
| History of a co-occurring psychiatric disorder (no = ref) | .006 | .112 | 1.67** | 1.43* | 1.01 | .98 |
| Alcohol problem (no = ref) | .469 | .89 | .95 | |||
| Cannabis problem (no = ref) | .246 | .78 | 1.20 | |||
| Cocaine problem (no = ref) | .583 | 1.20 | 1.13 | |||
| Methamphetamine problem (no = ref) | .814 | .86 | .95 | |||
| Opioid problem (no = ref) | .673 | 1.26 | 1.11 | |||
| Number problem substances | .708 | .99 | 1.04 | |||
| Primary substance | .661 | |||||
| None | .76 | 1.52 | ||||
| Alcohol | .80 | .96 | ||||
| Cannabis | .89 | 1.42 | ||||
| Cocaine | 1.05 | 1.19 | ||||
| Methamphetamine | .78 | .70 | ||||
| Opioids (ref) | — | — | ||||
| Other | 1.32 | 1.70 | ||||
| Years since AOD problem resolution | .008 | .021 | .98** | .98** | .99 | .99 |
| Total abstinence (no = ref) | .239 | 1.00 | 1.31 | |||
| Recovery identity (no = ref) | .580 | 1.18 | 1.04 | |||
| Any regular 12-step attendance (no = ref) | .433 | 1.24 | 1.13 | |||
| Any regular non–12-step MHO attendance (no = ref) | .698 | 1.13 | .76 | |||
| Past 90-day 12-step MHO attendance (no = ref) | .641 | 1.10 | 1.29 | |||
| Past 90-day non–12-Step Attendance (no = ref) | .809 | .96 | 1.41 | |||
| Any non-MHO recovery support service (no = ref) | .842 | 1.10 | 1.09 | |||
Note. AUD = alcohol use disorder; OUD = opioid use disorder; AOD = alcohol and other drug; MHO = mutual-help organization.
Attitude was determined by response to the statement, “It is a good idea for someone with an opioid problem to take a substitute opioid medication like Suboxone or methadone to help them stop using.” Positive attitude = strongly agree or agree; neutral attitude = somewhat agree or somewhat disagree; negative attitude = disagree or strongly disagree.
Multivariable-predictor logistic regression models included only the predictors significant at the .10 level in single-predictor models.
ref = reference group in logistic regression; if reference group is not specified (e.g., education level), the variable is continuous/ordinal, and the odds ratio represents the increase in log-odds for each 1-unit increase.
None of those with lifetime opioid antagonist prescription had a negative opioid agonist attitude. Odds ratio could not be computed.
p < .05.
p < .01.
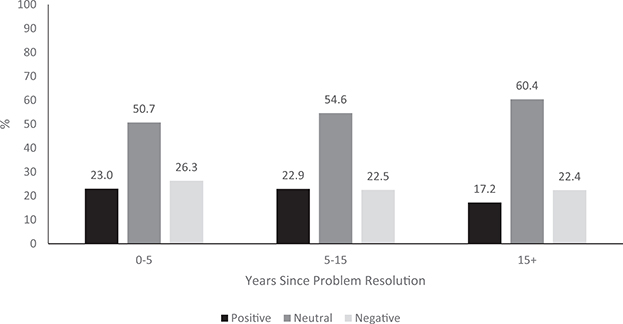
In the multivariable predictor model (see Table 2), only race/ethnicity (p < .001) and fewer years since AOD problem resolution (p = .021) remained statistically significant (p < .05). As illustrated by adjusted odds ratios (aOR) in Table 2, compared with identifying as White, identifying as two or more races was uniquely associated with a greater likelihood of a positive attitude, but identifying as Black (non-Hispanic) or Hispanic was uniquely associated with a greater likelihood of a negative attitude. Fewer years since problem resolution was uniquely associated with a greater likelihood of a positive attitude. Figure 2 depicts the proportions holding positive, neutral, and negative agonist attitudes by years since AOD problem resolution, with the continuous variable trichotomized for illustrative purposes into (a) 0–4 years 11 months, (b) 5–14 years 11 months, and (c) 15 years or more. All variables taken together accounted for 6.8% of the variance in agonist attitude (Nagelkerke’s R2 = .068).
Figure 2.
Proportion with positive (strongly agree or agree), neutral (somewhat agree or somewhat disagree), and negative (disagree or strongly disagree) agonist attitude as a function of years since problem resolution. Years since problem resolution, modeled continuously, was a significant, unique predictor of positive agonist attitude, controlling for the other significant correlates: race/ethnicity, lifetime AUD medication prescription, lifetime OUD agonist medication prescription, history of inpatient or outpatient AOD treatment, and having been diagnosed with a co-occurring psychiatric disorder.
Antagonists
Correlates of antagonist attitudes at the .1 level in the single-predictor models were age (p = .063), household income (p = .039), spirituality (p = .075), criminal justice history (p = .019), quality of life (p = .016), lifetime AUD medication prescription (p = .039), lifetime opioid agonist medication prescription (p < .001), outpatient or inpatient treatment (p = .003), history of a co-occurring psychiatric disorder (p = .001), identifying as being “in recovery” (p = .004), lifetime regular 12-step MHO attendance (p = .030), past 90-day 12-step MHO attendance (p = .064), and past 90-day non–12-step MHO attendance (p = .003; see Table 3).
Table 3.
Odds Ratios (ORs) and Significance Testing for Single-Predictor Logistic Regressions (Single; Ns = 1,932–1,946) and Adjusted Odds Ratios (aORs) and Significance Testing for Multivariable Predictor Logistic Regression (Multi; N = 1,912) Examining Correlates of Opioid Antagonist Medication Attitudes (Positive, Neutral, or Negative)a by Positive Versus Neutral and Negative Versus Neutral Pairwise Comparisons
|
p |
Positive versus neutral |
Negative versus neutral |
||||
|---|---|---|---|---|---|---|
| Variable | Single | Multi | OR | aORb | OR | aORb |
| Age | .063 | .027 | ||||
| 18–29 (ref)c | — | — | ||||
| 30–44 | 2.52* | 2.21 | .82 | .96 | ||
| 45–59 | 2.90** | 3.16** | .82 | .92 | ||
| 60+ | 2.49* | 3.21** | .70 | .83 | ||
| Gender (female = ref) | .669 | .91 | .88 | |||
| Race/ethnicity | .104 | |||||
| 2+ races | 1.76 | 1.50 | ||||
| Black—non-Hispanic | 1.09 | 1.73* | ||||
| Other—non-Hispanic | 1.00 | .71 | ||||
| Hispanic | .89 | 1.59* | ||||
| White (ref) | — | — | — | |||
| Geographical region | .505 | |||||
| Northeast | 1.38 | 1.16 | ||||
| Midwest | 1.02 | 1.14 | ||||
| South | .86 | 1.17 | ||||
| West | — | — | ||||
| Education level | .377 | .96 | .94 | |||
| Household income | .039 | .172 | .96* | .98 | .97* | .97 |
| Religiosity | .268 | 1.02 | 1.12 | |||
| Spirituality | .075 | .025 | 1.00 | .89 | 1.17* | 1.15* |
| Criminal justice history | .019 | .179 | ||||
| Arrested, drug court | 1.14 | .62 | 2.23** | 1.70 | ||
| Arrested, no drug court | 1.31 | 1.02 | .94 | .98 | ||
| None (ref) | — | — | — | |||
| Psychiatric symptoms | .288 | 1.02 | 1.00 | |||
| Quality of life | .016 | .446 | .97** | .99 | 1.00 | 1.00 |
| Lifetime AUD medication prescription (no = ref) | .039 | .777 | 2.29* | 1.13 | 1.31 | .85 |
| Lifetime OUD agonist medication prescription (no = ref) | <.001 | <.001 | 15.5*** | 13.05*** | 5.86*** | 3.91** |
| Lifetime OUD antagonist medication prescription (no = ref) | .031 | .454 | 25.82** | 7.47 | 9.11 | 4.58 |
| Inpatient or outpatient treatment (no = ref) | .003 | .060 | 1.66** | 1.27 | .86 | .68 |
| History of a co-occurring psychiatric disorder (no = ref) | .001 | .079 | 1.91*** | 1.44 | 1.11 | .93 |
| Alcohol problem (no = ref) | .862 | 1.03 | .93 | |||
| Cannabis problem (no = ref) | .224 | .80 | 1.24 | |||
| Cocaine problem (no = ref) | .689 | 1.05 | 1.16 | |||
| Methamphetamine problem (no = ref) | .711 | 1.06 | .85 | |||
| Opioid problem (no = ref) | .116 | 1.72 | 1.16 | |||
| Number problem substances | .351 | 1.06 | 1.07 | |||
| Primary substance | .350 | |||||
| None | .55 | 1.54 | ||||
| Alcohol | .75 | 1.05 | ||||
| Cannabis | .80 | 1.43 | ||||
| Cocaine | .90 | 1.30 | ||||
| Methamphetamine | .82 | .80 | ||||
| Opioids (ref) | — | — | ||||
| Other | 1.30 | 1.79 | ||||
| Years since AOD problem resolution | .149 | .99 | .99 | |||
| Total abstinence (no = ref) | .164 | 1.01 | 1.32 | |||
| Recovery identity (no = ref) | .004 | .387 | 1.74*** | 1.26 | 1.16 | .96 |
| Any regular 12-step attendance (no = ref) | .030 | .543 | 1.53* | 1.15 | 1.33 | 1.25 |
| Any regular non–12-step MHO attendance (no = ref) | .304 | 1.62 | 1.03 | |||
| Past 90-day 12-step MHO attendance (no = ref) | .064 | .259 | 1.33 | .89 | 1.76* | 1.47 |
| Past 90-day non–12-Step attendance (no = ref) | .003 | .105 | 5.04*** | 2.86* | 2.48 | 1.83 |
| Any non-MHO recovery support service (no = ref) | .158 | 1.32 | 1.40 | |||
Note. AUD = alcohol use disorder; OUD = opioid use disorder; AOD = alcohol and other drug; MHO = mutual-help organization.
Attitude was determined by response to the statement, “It is a good idea for someone with an opioid problem to take an opioid blocking medication like naltrexone/Vivitrol to help them stop using.” Positive attitude = strongly agree or agree; neutral attitude = somewhat agree or somewhat disagree; negative attitude = disagree or strongly disagree.
Multivariable-predictor logistic regression models included only the predictors significant at the .10 level in single-predictor models.
ref = reference group in logistic regression; if reference group is not specified (e.g., education level), the variable is continuous/ordinal, and the odds ratio represents the increase in log-odds for each 1-unit increase.
p < .05.
p < .01.
p < .001.
In the multivariable predictor model, age (p = .027), spirituality (p = .025), and lifetime opioid agonist medication prescription (p < .001) remained significant (p < .05; see Table 3). Specifically, compared to ages 18–29, ages 45–59 and 60+ were uniquely associated with a greater likelihood of a positive attitude. More spirituality was uniquely associated with a greater likelihood of a negative attitude. Lifetime opioid agonist medication prescription was uniquely associated with both a greater likelihood of a positive and a greater likelihood of a negative attitude compared with a neutral attitude, but the association with a positive attitude was significantly greater than that with a negative attitude (aOR = 3.34; Supplementary Table 1). All variables taken together accounted for 12.3% of the variance in antagonist attitude (Nagelkerke’s R2 = .123).
Sensitivity Analyses
Agonists
The results of the sensitivity analyses, where positive attitude was operationalized as any agreement and negative attitude as any disagreement, were different from the primary analyses in several respects for agonist attitudes. There were no significant unique effects of race/ethnicity or fewer years since AOD problem resolution. Three effects emerged in the sensitivity analyses that were not present in the primary analyses. Participation in drug court compared to no arrest history (aOR = 0.47), current AOD abstinence (aOR = 0.75), and number of problem substances (aOR = 0.90) were uniquely associated with a negative agonist attitude.
Antagonists
For antagonist attitudes, similar to the primary analyses, younger age was uniquely associated with a negative attitude but with stronger effects. Compared to ages 18–29, ages 30–44 (aOR = 1.77), 45–59 (aOR = 2.67), and 60+ (aOR = 2.57) were significantly associated with a greater likelihood of a positive attitude. There were several differences, however. Like agonists, drug court participation compared with no arrest history (aOR = 0.51), current AOD abstinence (aOR = 0.60), and number of problem substances emerged as significant, unique predictors of a negative antagonist attitude. On the other hand, lifetime inpatient/outpatient treatment (aOR = 1.40) and co-occurring psychiatric disorder (aOR = 1.34) emerged as significant predictors of a positive antagonist attitude. Whereas spirituality and lifetime opioid agonist medication prescription were uniquely associated with antagonist attitudes in the primary analyses, these were not significant in the sensitivity analyses.
Discussion
This study of OUD medication attitudes in a representative sample of U.S. adults who resolved a significant AOD problem showed that for opioid agonist medications, individuals were equally as likely to endorse positive and negative attitudes. Our hypothesis was supported, as more recent AOD problem resolution was uniquely associated with a greater likelihood of a positive attitude. Of note, post hoc exploratory analyses showed that this effect of fewer years since problem resolution on a more positive agonist attitude held even when controlling for age in addition to the existing set of predictors. Thus, the association between fewer years since problem resolution and positive agonist attitude is not explained by younger age among those who resolved their problem more recently. Although statistically significant, context suggests the relationship between recent problem resolution and positive agonist attitude may be relatively modest. Compared with those who resolved their AOD problem 15 or more years ago (before 2002), those who resolved their problem in the past 15 years (2002–2017) held only slightly more positive agonist attitudes in terms of absolute difference (23% positive for the 2002–2017 cohort vs. 17% positive for those who resolved their problem before 2002).
The finding that participants were more likely to endorse negative attitudes toward antagonists than agonists was somewhat unexpected. Anecdotal evidence suggests that recovering individuals, and the policies of the recovery-related organizations and programs in which they often participate or reside (e.g., residential treatment programs), may focus on the potential for agonist misuse (Cicero et al., 2018; White, 2011). One possible explanation for this counterintuitive finding is that because buprenorphine-naloxone is the most widely prescribed OUD medication (Hadland et al., 2017; Kelly et al., 2017; Morgan et al., 2018), participants may have more absolute exposure to agonist “medication success stories” (White, 2014), helping to dispel myths and decrease negative attitudes.
Taken together, these data suggest that although negative agonist attitudes persist, positive attitudes are equally as prevalent, and these more positive attitudes may be on the rise. This positive shift in agonist attitudes coincides with the U.S. Food and Drug Administration’s approval of buprenorphine-naloxone in 2002 (U.S. Food and Drug Administration, 2002). Thus, the positive shift in agonist attitudes may be due to the proliferation of buprenorphine-naloxone compared with the more negatively perceived methadone (Schwartz et al., 2008; Uebelacker et al., 2016; Yarborough et al., 2016). Our results also highlight several attitudinal correlates that could facilitate or hinder OUD medication adoption and dissemination.
Indicators of OUD Medication Attitudes
Age
Young adults (18–29) had more negative antagonist attitudes compared with older adults. Despite magnified harms in their age cohort as a result of the opioid overdose crisis (Gomes, Tadrous, Mamdani, Paterson, & Juurlink, 2018), young adults who have resolved AOD problems may have broadly negative attitudes toward addiction and mental health treatment, not just OUD medications (Gonzalez, Alegria, & Prihoda, 2005). In exploratory post hoc analyses (not shown), we found that young adults had more negative attitudes toward all AOD and psychiatric medications. It may also be that these young adults are simply less agreeable than older adults (Costa & McCrae, 1994). Irrespective of underlying explanatory factors, our findings suggest that attitudinal barriers may partially explain the lower rates of OUD medication prescription among youth (Hadland et al., 2017).
Racial/ethnic minority status
Compared with White/non-Hispanic identification, identification as a Black or Hispanic minority was related to more negative agonist attitudes. Compared with their White counterparts, racial/ethnic minorities may have greater access to methadone than buprenorphine and are less likely to have experienced an increase in buprenorphine access (Hansen, Siegel, Wanderling, & DiRocco, 2016; Stein et al., 2018). Thus, ethnic minorities’ negative perceptions of OUD medications may be driven primarily by their perceptions of methadone more specifically. In addition, they may have negative opinions of opioid agonist medications due to greater systemic responsiveness to the opioid overdose crisis in communities with more individuals who are White and have higher socioeconomic status (Stein et al., 2018).
Service utilization and clinical severity
There was a general pattern in which greater clinical and substance use severity indicators (e.g., history of outpatient or inpatient treatment and AUD/OUD medication) were associated with more positive medication attitudes in single-predictor models as well as sensitivity analyses, and lifetime opioid agonist medication prescription was associated with positive antagonist attitudes. The health beliefs model (Finney & Moos, 1995; Humphreys, Finney, & Moos, 1994; Rosenstock, 1990) predicts that greater perceived severity is associated with a greater likelihood of seeking treatment. This willingness to seek treatment for AOD and psychiatric disorders more generally, and positive experiences therein, may yield more positive attitudes toward a range of recovery pathways (Kelly et al., 2017; White & Kurtz, 2005), including but not limited to OUD medication.
Also striking was the absence of association between 12-step MHO participation (including exploratory post hoc analyses examining only NA) and negative agonist medication attitudes given preliminary data to this effect (Monico et al., 2015), historical context (White, 2014), negative attitudes toward agonists in NA’s organizational literature (Narcotics Anonymous World Services, 2016), and anecdotal reports of 12-step participants’ negative agonist attitudes in lay media (Brico, 2017; Siegel, 2016). To be sure, 12-step recovery communities contain historical threads of suspiciousness about the use of medications and other formal health-care interventions to address substance use problems. Folk wisdom regarding potential iatrogenic effects of ostensibly well-intentioned medical interventions (e.g., relapse subsequent to tranquilizer prescriptions for individuals with alcohol use disorder) has persisted over time in some circles despite seismic changes in the medical AOD treatment landscape (White, 2014). Thus, the generalization of these suspicious and perhaps negative attitudes to OUD medications may be motivated, at least in part, by self-protection. At the same time, however, such attitudes might also lead individuals to conclude that taking opioid agonist medication is a prima facie negative outcome and to stigmatize those taking such medications. Indeed, NA literature states that the organization “may be compatible for addicts [sic] on medically assisted protocols if they have a desire to become clean one day,” differentiating abstinence for individuals taking medication versus those who are “drug-free” (Narcotics Anonymous World Services, 2016).
The divergence of our findings from these historical and anecdotal contexts may be explained by the “vocal minority” phenomenon, whereby broad perceptions are driven by a small, but active and influential, group of stakeholders (Tonigan & Kelly, 2004). Our data showing no relationship between 12-step MHO attendance and agonist attitudes adds a scientific perspective to this important issue. Despite the absence of association between 12-step MHO attendance and attitudes, current AOD abstinence, the recovery pathway endorsed most strongly in 12-step MHOs, was associated with both negative agonist and antagonist attitudes in the sensitivity analyses. In addition, greater spirituality was uniquely associated with more negative antagonist attitudes in the primary analyses, and a national survey of NA members showed that nearly all (94%) identify as spiritual (Galanter, Dermatis, Post, & Santucci, 2013). It may be that more active 12-step MHO participants do, in fact, have negative OUD medication attitudes, although more fine-grained questions regarding current MHO participation were not included in the survey. Given the central role of medications in the treatment of OUD and the traditional predominance of 12-step MHOs among individuals who seek help for drug use disorders (Grant et al., 2016), further study is needed on the intersection between OUD medications and 12-step MHO participation.
Finally, drug court participation was associated with both negative agonist and antagonist attitudes in the sensitivity analyses. Only half of drug courts offer OUD medications, and administrators and in-depth qualitative analyses cite several barriers to integration (Matusow et al., 2013; Physicians for Human Rights, 2017). Irrespective of whether drug courts are now more likely to integrate OUD medications into participant treatment plans (Substance Abuse and Mental Health Services Administration, 2014), our data suggest that individuals who have participated in drug court may have negative OUD medication attitudes.
Future Directions
There are many advantages to using a nationally representative data set as was done in the current study. This type of broad-scale approach, however, makes it difficult to fully explain complex phenomena such as attitudes given the heterogeneity of participants’ experiences (Kelly et al., 2017). In fact, the multivariable predictor models explained only 7–12% of the variance in medication attitudes. Future studies may focus on subsets of recovering individuals to inform OUD medication adoption. For example, consistent with epidemiological data showing that alcohol use disorder is far more prevalent than other drug use disorders (Grant et al., 2015, 2016), the majority of this nationally representative sample of individuals who resolved an AOD problem identified alcohol as their primary substance. Importantly, OUD medication attitudes did not differ as a function of primary substance. That said, given that the role of opioids in the current overdose crisis (Gomes et al., 2018) highlights an urgent need to focus on individuals with OUD, future work might focus on medication attitudes specifically among individuals in OUD recovery. Other potential target subpopulations based on our findings include racial/ethnic minorities, younger individuals, and drug court participants. Studies may also examine OUD medication attitudes in the general population because lay community members’ attitudes may influence the decisions of policymakers and other relevant stakeholders.
In addition, 20–40% of individuals receiving opioid withdrawal management do not want medication as part of their treatment plan (Bailey et al., 2013; Uebelacker et al., 2016). Taken together with our finding that 20–30% of U.S. adults who resolved an AOD problem have negative OUD medication attitudes, with no difference by opioid versus other primary substances, these data suggest an important, but understudied, subset of individuals with OUD who may be reluctant to take OUD medications. Thus, in order to mount an immediate response to the sharp increase in opioid-involved overdoses, and a sustained approach to OUD treatment and recovery over the long term, future studies might investigate psychosocial approaches that (a) increase opioid abstinence, reduce overdose, and enhance functioning (Bergman, Fallah-Sohy, Hoffman, & Kelly, 2019) and (b) enhance positive OUD medication attitudes and adherence, such as those shown to be effective for HIV medications (Safren et al., 2012).
Limitations
The study’s findings and implications should be contextualized within its limitations. First, items referred to opioid agonists as opioid “substitution” medications and opioid antagonists as opioid “blocking” medications. We used this terminology to increase the likelihood that participants, with a range of educational backgrounds, would know to which medications the items referred. As some have noted, although “substitution” is commonly used to refer to agonist treatments in Europe and Australia (e.g., Sordo et al., 2017), the term could enhance stigma (National Alliance of Advocates for Buprenorphine Treatment, 2008) and thus may have enhanced the likelihood of endorsing a negative attitude. Also, agonists like buprenorphine act as opioid receptor “blockers” in addition to their activating effects (Lutfy & Cowan, 2004). It is possible that use of different medication terminology may evoke different attitudinal responses (Ashford, Brown, & Curtis, 2018), an area ripe for future investigation. Second, the developmental implications gleaned from this cross-sectional design—for example, those who resolved their AOD problem longer ago had less positive agonist attitudes—require further study with truly longitudinal data. Third, NRS participants who completed the medication attitude items and were included in this study had a history of greater AOD service engagement than excluded participants who did not complete these items; thus, caution should be taken when generalizing to individuals with no history of AOD service engagement.
Summary and Conclusion
Individuals who have resolved a significant AOD problem are key stakeholders in the dissemination, adoption, and implementation of medication interventions for OUD. The current study leveraged a nationally representative data set to examine OUD medication attitudes in this seldom-studied stakeholder group. Overall, the results showed that these individuals were equally likely to endorse positive and negative opioid agonist attitudes, although they had a greater likelihood of a positive attitude if they had resolved their AOD problem more recently. Racial/ethnic minority identification and younger age may be indicators of more negative OUD medication attitudes. Although 12-step MHO attendance was not associated with OUD medication attitudes, current AOD abstinence and greater spiritual identification, characteristics related to the 12-step MHO philosophy, may be markers of more negative attitudes. Continued elucidation of factors that may influence OUD medication adoption could help enhance the overall impact of these life-saving interventions. Greater attention to OUD medication attitudes may also clarify potential implementation barriers, thereby informing the nature and effectiveness of strategies intended to help overcome these obstacles.
Public Health Significance.
This study of opioid use disorder (OUD) medication attitudes among individuals who resolved a significant alcohol or other drug problem suggest more recent problem resolution is associated with a greater likelihood of positive attitude. Younger age and racial/ethnic minority status are associated with a greater likelihood of negative attitudes. The findings may help identify facilitators of, and obstacles to, OUD medication dissemination efforts.
Acknowledgments
Brandon G. Bergman (K23AA025707) and John F. Kelly (K24AA022136) are supported by the National Institute on Alcohol Abuse and Alcoholism. Funding sources had no roles other than financial support.
We thank Alexandra Abry, BA, for her assistance in data management and preparation.
Footnotes
All authors contributed to the design, analyses, and interpretation of findings.
The authors have no conflicts of interest to report.
None of the original material in this article has been published or disseminated elsewhere. Ideas from the discussion overlap with ideas outlined in the book chapter “Psychosocial Approaches in the Treatment of Opioid Use Disorders” published by Springer (Bergman, Fallah-Sohy, Hoffman, & Kelly, 2019).
Supplemental materials: http://dx.doi.org/10.1037/pha0000325.supp
Contributor Information
Brandon G. Bergman, Massachusetts General Hospital, Boston, Massachusetts, and Harvard Medical School.
Robert D. Ashford, University of the Sciences
John F. Kelly, Massachusetts General Hospital, Boston, Massachusetts, and Harvard Medical School
References
- Alderks CE (2017). Trends in the use of methadone, buprenorphine, and extended-release naltrexone at substance abuse treatment facilities: 2003–2015 (Update). Rockville, MD: Substance Abuse and Mental Health Services Administration. [PubMed] [Google Scholar]
- American Society of Addiction Medicine. (2004). Public policy statement on office-based opioid agonist treatment (OBOT). Retrieved from https://www.asam.org/docs/default-source/public-policy-statements/1obot-treatment-7-04.pdf?sfvrsn6ecf77e3_0 [DOI] [PubMed]
- Ashford RD, Brown AM, & Curtis B (2018). Substance use, recovery, and linguistics: The impact of word choice on explicit and implicit bias. Drug and Alcohol Dependence, 189, 131–138. 10.1016/j.drugalcdep.2018.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey GL, Herman DS, & Stein MD (2013). Perceived relapse risk and desire for medication assisted treatment among persons seeking inpatient opiate detoxification. Journal of Substance Abuse Treatment, 45, 302–305. 10.1016/j.jsat.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassuk EL, Hanson J, Greene RN, Richard M, & Laudet A (2016). Peer-delivered recovery support services for addictions in the United States: A systematic review. Journal of Substance Abuse Treatment, 63, 1–9. 10.1016/j.jsat.2016.01.003 [DOI] [PubMed] [Google Scholar]
- Battaglia MP, Hoaglin DC, & Frankel MR (2009). Practical considerations in raking survey data. Survey Practice, 2, 1–10. 10.29115/SP-2009-0019 [DOI] [Google Scholar]
- Bergman BG, Fallah-Sohy N, Hoffman LA, & Kelly JF (2019). Psychosocial approaches in the treatment of opioid use disorders In Kelly JF & Wakeman SE (Eds.), Treating opioid addiction (pp. 109–138). New York, NY: Springer; 10.1007/978-3-030-16257-3_6 [DOI] [Google Scholar]
- Bergman BG, Greene MC, Hoeppner BB, & Kelly JF (2018). Expanding the reach of alcohol and other drug services: Prevalence and correlates of U.S. adult engagement with online technology to address substance problems. Addictive Behaviors, 87, 74–81. 10.1016/j.addbeh.2018.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brico E (2017). By shunning medication-assisted therapy, 12-step meetings are making the opioid crisis worse. Retrieved from https://www.statnews.com/2017/10/04/medication-assisted-therapy-12-step/
- Centers for Disease Control and Prevention. (2018). 2018 annual surveillance report of drug-related risks and outcomes—United States. Surveillance special report. Retrieved from https://www.cdc.gov/drugoverdose/pdf/pubs/2018-cdc-drug-surveillance-report.pdf
- Cicero TJ, Ellis MS, & Chilcoat HD (2018). Understanding the use of diverted buprenorphine. Drug and Alcohol Dependence, 193, 117–123. 10.1016/j.drugalcdep.2018.09.007 [DOI] [PubMed] [Google Scholar]
- Congressional Research Service. (2018). H.R. 6: Support for Patients and Communities Act. Retrieved from https://www.congress.gov/bill/115th-congress/house-bill/6.
- Costa PT, & McCrae RR (1994). Stability and change in personality from adolescence through adulthood In Halverson JCF, Kohnstamm GA, & Martin RP (Eds.), The developing structure of temperament and personality from infancy to adulthood (pp. 139–150). Hillsdale, NJ: Erlbaum. [Google Scholar]
- Dennis ML, Titus JC, White MK, Unsicker J, & Hodgkins D (2002). Global Appraisal of Individual Needs: Administration guide for the GAIN and related measures. Bloomington, IL: Chestnut Health Systems. [Google Scholar]
- Finney JW, & Moos RH (1995). Entering treatment for alcohol abuse: A stress and coping model. Addiction, 90, 1223–1240. 10.1111/j.1360-0443.1995.tb01092.x [DOI] [PubMed] [Google Scholar]
- Galanter M, Dermatis H, Post S, & Santucci C (2013). Abstinence from drugs of abuse in community-based members of Narcotics Anonymous. Journal of Studies on Alcohol and Drugs, 74, 349–352. 10.15288/jsad.2013.74.349 [DOI] [PubMed] [Google Scholar]
- Ginter W (2012). Methadone Anonymous and mutual support for medication-assisted recovery. Journal of Groups in Addiction & Recovery, 7, 189–201. 10.1080/1556035X.2012.705699 [DOI] [Google Scholar]
- Glasgow RE, Klesges LM, Dzewaltowski DA, Estabrooks PA, & Vogt TM (2006). Evaluating the impact of health promotion programs: Using the RE-AIM framework to form summary measures for decision making involving complex issues. Health Education Research, 21, 688–694. 10.1093/her/cyl081 [DOI] [PubMed] [Google Scholar]
- Gomes T, Tadrous M, Mamdani MM, Paterson JM, & Juurlink DN (2018). The burden of opioid-related mortality in the United States. Journal of the American Medical Association Network Open, 1, e180217–e180217. 10.1001/jamanetworkopen.2018.0217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Alegria M, & Prihoda TJ (2005). How do attitudes toward mental health treatment vary by age, gender, and ethnicity/race in young adults? Journal of Community Psychology, 33, 611–629. 10.1002/jcop.20071 [DOI] [Google Scholar]
- Grant BF, Goldstein RB, Saha TD, Chou SP, Jung J, Zhang H, … Hasin DS (2015). Epidemiology of DSM-5 alcohol use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. Journal of the American Medical Association Psychiatry, 72, 757–766. 10.1001/jamapsychiatry.2015.0584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Saha TD, Ruan WJ, Goldstein RB, Chou SP, Jung J, … Hasin DS (2016). Epidemiology of DSM-5 drug use disorder: Results from the National Epidemiologic Survey on Alcohol and Related Conditions III. Journal of the American Medical Association Psychiatry, 73, 39–47. 10.1001/jamapsychiatry.2015.2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CA, McCarty D, Mertens J, Lynch FL, Hilde A, Firemark A, … Anderson BM (2014). A qualitative study of the adoption of buprenorphine for opioid addiction treatment. Journal of Substance Abuse Treatment, 46, 390–401. 10.1016/j.jsat.2013.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland SE, Wharam JF, Schuster MA, Zhang F, Samet JH, & Larochelle MR (2017). Trends in receipt of buprenorphine and naltrexone for opioid use disorder among adolescents and young adults, 2001–2014. Journal of the American Medical Association Pediatrics, 171, 747–755. 10.1001/jamapediatrics.2017.0745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen H, Siegel C, Wanderling J, & DiRocco D (2016). Buprenorphine and methadone treatment for opioid dependence by income, ethnicity and race of neighborhoods in New York City. Drug and Alcohol Dependence, 164, 14–21. 10.1016/j.drugalcdep.2016.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren T, Edwards EM, Dennis JM, Rodkin S, Hingson RW, & Rosenbloom DL (2008). A comparison of results from an alcohol survey of a prerecruited Internet panel and the National Epidemiologic Survey on Alcohol and Related Conditions. Alcoholism: Clinical and Experimental Research, 32, 222–229. 10.1111/j.1530-0277.2007.00571.x [DOI] [PubMed] [Google Scholar]
- Humphreys K, Finney JW, & Moos RH (1994). Applying a stress and coping framework to research on mutual help organizations. Journal of Community Psychology, 22, 312–327. [DOI] [Google Scholar]
- Idler EL, Musick MA, Ellison CG, George LK, Krause N, Ory MG, … Williams DR (2003). Measuring multiple dimensions of religion and spirituality for health research. Research on Aging, 25, 327–365. 10.1177/0164027503025004001 [DOI] [Google Scholar]
- Institute of Behavioral Research. (2002). TCU Comprehensive Intake (TCU CI). Fort Worth: Institute of Behavioral Research, Texas Christian University. [Google Scholar]
- Kelly JF, Abry AW, Milligan CM, Bergman BG, & Hoeppner BB (2018). On being “in recovery”: A national study of prevalence and correlates of adopting or not adopting a recovery identity among individuals resolving drug and alcohol problems. Psychology of Addictive Behaviors, 32, 595–604. 10.1037/adb0000386 [DOI] [PubMed] [Google Scholar]
- Kelly JF, Bergman B, Hoeppner BB, Vilsaint C, & White WL (2017). Prevalence and pathways of recovery from drug and alcohol problems in the United States population: Implications for practice, research, and policy. Drug and Alcohol Dependence, 181(Suppl. C), 162–169. 10.1016/j.drugalcdep.2017.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JF, Greene MC, & Bergman BG (2018a). Beyond abstinence: Changes in indices of quality of life with time in recovery in a nationally representative sample of U.S. adults. Alcoholism: Clinical and Experimental Research, 42, 770–780. 10.1111/acer.13604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JF, Greene MC, & Bergman BG (2018b). Is recovery from cannabis use problems different from alcohol and other drugs? Results from a national probability-based sample of the United States adult population. International Journal of Drug Policy, 53, 55–64. 10.1016/j.drugpo.2017.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JF, Urbanoski KA, Hoeppner BB, & Slaymaker V (2011). Facilitating comprehensive assessment of 12-step experiences: A multidimensional measure of mutual-help activity. Alcoholism Treatment Quarterly, 29, 181–203. 10.1080/07347324.2011.586280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SM, Brown BS, Katz EC, O’Grady KE, Mitchell SG, King S, & Schwartz RP (2012). A comparison of attitudes toward opioid agonist treatment among short-term buprenorphine patients. The American Journal of Drug and Alcohol Abuse, 38, 233–238. 10.3109/00952990.2011.643983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, … Zaslavsky AM (2003). Screening for serious mental illness in the general population. Archives of General Psychiatry, 60, 184–189. 10.1001/archpsyc.60.2.184 [DOI] [PubMed] [Google Scholar]
- Kleber HD (2008). Methadone maintenance 4 decades later: Thousands of lives saved but still controversial. Journal of the American Medical Association, 300, 2303–2305. 10.1001/jama.2008.648 [DOI] [PubMed] [Google Scholar]
- Lee JD, Nunes EV Jr., Novo P, Bachrach K, Bailey GL, Bhatt S, … Rotrosen J (2018). Comparative effectiveness of extended-release naltrexone versus buprenorphine-naloxone for opioid relapse prevention (X:BOT): A multicentre, open-label, randomised controlled trial. Lancet, 391, 309–318. 10.1016/S0140-6736(17)32812-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutfy K, & Cowan A (2004). Buprenorphine: A unique drug with complex pharmacology. Current Neuropharmacology, 2, 395–402. 10.2174/1570159043359477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden J, Stillwell G, Jones H, Cooper A, Eastwood B, Farrell M, … Hickman M (2017). Does exposure to opioid substitution treatment in prison reduce the risk of death after release? A national prospective observational study in England. Addiction, 112, 1408–1418. 10.1111/add.13779 [DOI] [PubMed] [Google Scholar]
- Mattick RP, Breen C, Kimber J, & Davoli M (2014). Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database of Systematic Reviews, 2, CD002207 10.1002/14651858.CD002207.pub4 [DOI] [PubMed] [Google Scholar]
- Matusow H, Dickman SL, Rich JD, Fong C, Dumont DM, Hardin C, … Rosenblum A (2013). Medication assisted treatment in U.S. drug courts: Results from a nationwide survey of availability, barriers and attitudes. Journal of Substance Abuse Treatment, 44, 473–480. 10.1016/j.jsat.2012.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WR (1996). Form 90: A structured assessment interview for drinking and related behaviors. Project MATCH Monograph Series (Vol. 5). Rockville, MD: National Institute on Alcohol Abuse and Alcoholism. [Google Scholar]
- Molfenter T, Sherbeck C, Zehner M, Quanbeck A, McCarty D, Kim JS, & Starr S (2015). Implementing buprenorphine in addiction treatment: Payer and provider perspectives in Ohio. Substance Abuse Treatment, Prevention, and Policy, 10, 13 10.1186/s13011-015-0009-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monico LB, Gryczynski J, Mitchell SG, Schwartz RP, O’Grady KE, & Jaffe JH (2015). Buprenorphine treatment and 12-step meeting attendance: Conflicts, compatibilities, and patient outcomes. Journal of Substance Abuse Treatment, 57, 89–95. 10.1016/j.jsat.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan JR, Schackman BR, Leff JA, Linas BP, & Walley AY (2018). Injectable naltrexone, oral naltrexone, and buprenorphine utilization and discontinuation among individuals treated for opioid use disorder in a United States commercially insured population. Journal of Substance Abuse Treatment, 85, 90–96. 10.1016/j.jsat.2017.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narcotics Anonymous World Services. (2016). Narcotics anonymous and persons receiving medication-assisted treatment. Retrieved from https://www.na.org/admin/include/spaw2/uploads/pdf/pr/2306_NA_PRMAT_1021.pdf
- National Alliance of Advocates for Buprenorphine Treatment. (2008). The words we use matter. Reducing stigma through language. Retrieved from https://www.naabt.org/documents/NAABT_Language.pdf
- Physicians for Human Rights. (2017). Neither justice nor treatment: Drug courts in the United States. Retrieved from https://phr.org/wp-content/uploads/2017/06/phr_drugcourts_report_singlepages.pdf
- Roman PM, & Johnson JA (2004a). National treatment center study report: Public treatment centers. Athens: Institute for Behavioral Research, University of Georgia. [Google Scholar]
- Roman PM, & Johnson JA (2004b). National treatment center study summary report: Private treatment centers. Athens: Institute for Behavioral Research, University of Georgia. [Google Scholar]
- Rosenstock IM (1990). The health belief model: Explaining health behavior through expectancies In Glanz K, Lewis FM, & Rimer BK (Eds.), Health behavior and health education: Theory, research, and practice (pp. 39–62). San Francisco, CA: Jossey-Bass. [Google Scholar]
- Rychtarik RG, Connors GJ, Dermen KH, & Stasiewicz PR (2000). Alcoholics Anonymous and the use of medications to prevent relapse: An anonymous survey of member attitudes. Journal of Studies on Alcohol, 61, 134–138. 10.15288/jsa.2000.61.134 [DOI] [PubMed] [Google Scholar]
- Safren SA, O’Cleirigh CM, Bullis JR, Otto MW, Stein MD, & Pollack MH (2012). Cognitive behavioral therapy for adherence and depression (CBT-AD) in HIV-infected injection drug users: A randomized controlled trial. Journal of Consulting and Clinical Psychology, 80, 404–415. 10.1037/a0028208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saloner B, McGinty EE, Beletsky L, Bluthenthal R, Beyrer C, Botticelli M, & Sherman SG (2018). A public health strategy for the opioid crisis. Public Health Reports, 133(Suppl. 1), 24S–34S. 10.1177/0033354918793627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JH, & Kertesz SG (2018). Suggested paths to fixing the opioid crisis: Directions and misdirections. Journal of the American Medical Association Network Open, 1, e180218 10.1001/jamanetworkopen.2018.0218 [DOI] [PubMed] [Google Scholar]
- Schenker N, & Gentleman JF (2001). On judging the significance of differences by examining the overlap between confidence intervals. The American Statistician, 55, 182–186. 10.1198/000313001317097960 [DOI] [Google Scholar]
- Schmidt S, Mühlan H, & Power M (2006). The EUROHIS-QOL 8-item index: Psychometric results of a cross-cultural field study. European Journal of Public Health, 16, 420–428. 10.1093/eurpub/cki155 [DOI] [PubMed] [Google Scholar]
- Schwartz RP, Kelly SM, O’Grady KE, Mitchell SG, Peterson JA, Reisinger HS, … Brown BS (2008). Attitudes toward buprenorphine and methadone among opioid-dependent individuals. The American Journal on Addictions, 17, 396–401. 10.1080/10550490802268835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel Z (2016). We know how to treat opioid addiction, but our antiquated conceptions of addicts prevent us from doing so. Retrieved from https://slate.com/technology/2016/11/we-do-not-use-an-evidence-backed-method-for-treating-heroin-addiction.html
- Sordo L, Barrio G, Bravo MJ, Indave BI, Degenhardt L, Wiessing L, … Pastor-Barriuso R (2017). Mortality risk during and after opioid substitution treatment: Systematic review and meta-analysis of cohort studies. British Medical Journal, 357, j1550 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein BD, Dick AW, Sorbero M, Gordon AJ, Burns RM, Leslie DL, & Pacula RL (2018). A population-based examination of trends and disparities in medication treatment for opioid use disorders among Medicaid enrollees. Substance Abuse, 39, 419–425. 10.1080/08897077.2018.1449166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2014). Adult drug courts and medication-assisted treatment for opioid dependence. In Brief, 8(1), 1–8. Retrieved from https://store.samhsa.gov/system/files/sma14-4852.pdf [Google Scholar]
- Tanum L, Solli KK, Latif ZE, Benth JS, Opheim A, Sharma-Haase K, … Kunøe N (2017). Effectiveness of injectable extended-release naltrexone vs daily buprenorphine-naloxone for opioid dependence: A randomized clinical noninferiority trial. Journal of the American Medical Association Psychiatry, 74, 1197–1205. 10.1001/jamapsychiatry.2017.3206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonigan JS, & Kelly JF (2004). Beliefs about AA and the use of medications: A comparison of three groups of AA-exposed alcohol dependent persons. Alcoholism Treatment Quarterly, 22, 67–78. 10.1300/J020v22n02_06 [DOI] [Google Scholar]
- Uebelacker LA, Bailey G, Herman D, Anderson B, & Stein M (2016). Patients’ beliefs about medications are associated with stated preference for methadone, buprenorphine, naltrexone, or no medication-assisted therapy following inpatient opioid detoxification. Journal of Substance Abuse Treatment, 66, 48–53. 10.1016/j.jsat.2016.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Census Bureau. (2015). Current Population Survey (CPS). Retrieved from https://www.census.gov/programs-surveys/cps/about.html
- U.S. Food and Drug Administration. (2002). Subutex and suboxone approval letter. Retrieved from https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2002/20732,20733ltr.pdf
- Volkow ND, & Collins FS (2017). The role of science in addressing the opioid crisis. The New England Journal of Medicine, 377, 391–394. 10.1056/NEJMsr1706626 [DOI] [PubMed] [Google Scholar]
- Wakeman SE, & Rich JD (2018). Barriers to medications for addiction treatment: How stigma kills. Substance Use & Misuse, 53, 330–333. 10.1080/10826084.2017.1363238 [DOI] [PubMed] [Google Scholar]
- White W, & Kurtz E (2005). The varieties of recovery experience: A primer for addiction treatment professionals and recovery advocates. International Journal of Self Help & Self Care, 3, 21–61. 10.2190/911R-MTQ5-VJ1H-75CU [DOI] [Google Scholar]
- White WL (2011). Narcotics Anonymous and the pharmacotherapeutic treatment of opioid addiction in the United States. Philadelphia Department of Behavioral Health and Intellectual Disability Services and the Great Lakes Addiction Technology Transfer Center. Retrieved from http://atforum.com/documents/2011NAandMedication-assistedTreatment.pdf
- White WL (2014). Slaying the dragon: The history of addiction treatment and recovery in America (2nd ed.). Bloomington, IL: Chestnut Health Systems/Lighthouse Institute. [Google Scholar]
- Yarborough BJ, Stumbo SP, McCarty D, Mertens J, Weisner C, & Green CA (2016). Methadone, buprenorphine and preferences for opioid agonist treatment: A qualitative analysis. Drug and Alcohol Dependence, 160, 112–118. 10.1016/j.drugalcdep.2015.12.031 [DOI] [PMC free article] [PubMed] [Google Scholar]