Abstract
Objective
To describe the epidemiology of and risk factors associated with acute kidney injury (AKI) during acyclovir treatment in neonates and infants.
Study design
We conducted a multicenter (n = 4), retrospective cohort study of all hospitalized infants age <60 days treated with intravenous acyclovir (≥1 dose) for suspected or confirmed neonatal herpes simplex virus disease from January 2011 to December 2015. Infants with serum creatinine measured both before acyclovir (baseline) and during treatment were included. We classified AKI based on changes in creatinine according to published neonatal AKI criteria and performed Cox regression analysis to evaluate risk factors for AKI during acyclovir treatment.
Results
We included 1017 infants. The majority received short courses of acyclovir (median, 5 doses). Fifty-seven infants (5.6%) developed AKI during acyclovir treatment, with an incidence rate of AKI at 11.6 per 1000 acyclovir days. Cox regression analysis identified having confirmed herpes simplex virus disease (OR, 4.35; P = .002), receipt of ≥2 concomitant nephrotoxic medications (OR, 3.07; P = .004), receipt of mechanical ventilation (OR, 5.97; P = .001), and admission to an intensive care unit (OR, 6.02; P = .006) as risk factors for AKI during acyclovir treatment.
Conclusions
Among our cohort of infants exposed to acyclovir, the rate of AKI was low. Sicker infants and those exposed to additional nephrotoxic medications seem to be at greater risk for acyclovir-induced toxicity and warrant closer monitoring.
Neonatal herpes simplex virus (HSV) infections cause significant morbidity and mortality even with adequate treatment. More than 70% of surviving infants with HSV-related central nervous system (CNS) disease develop long-term impairment, and mortality in infants with disseminated HSV exceeds 50%.1 Owing to concerns about the devastating effects of neonatal HSV, intravenous (IV) acyclovir is often administered empirically to young infants with suspected serious infections while diagnostic studies are pending. However, neonatal HSV infections are uncommon, with an estimated incidence in the US being approximately 10–60 cases per 100 000 births.2–4 The signs and symptoms of neonatal HSV infection are nonspecific, leading to significant variability in HSV testing and empiric treatment in at-risk infants.5,6 Although delayed initiation of treatment is associated with poor outcomes in infants with HSV disease,6 the vast majority of acyclovir-exposed infants do not have HSV infections. Therefore, clinicians must balance the risk of disease and potential adverse effects related to acyclovir exposure, such as possible prolonged hospitalization awaiting test results and nephrotoxicity during each evaluation.7
Acute kidney injury (AKI) is a known side effect of IV acyclovir that occurs in more than one-third of older children who receive the drug.8 However, the incidence of AKI in infants exposed to acyclovir is unknown. In a study by Ericson et al, only 2 of 89 infants who received treatment with acyclovir for >14 days developed elevated creatinine.9 However, the definition of nephrotoxicity used in this study (creatinine >1.7 mg/dL) was based on a priori criteria set forth to identify adverse events in clinical trials involving premature infants, which is not congruent with current consensus definitions of neonatal AKI.10 The mechanism of acyclovir-associated nephrotoxicity is crystal nephropathy, which can develop following as little as a single dose of medication.11 Because the bulk of acyclovir exposure is among infants without HSV disease, it is important to understand the toxicity risk among all infants exposed to acyclovir, including those who have received empiric therapy for suspected neonatal HSV disease.
The primary objective of this study was to evaluate the nephrotoxicity of acyclovir in infants. We assessed the incidence rate of AKI among infants exposed to acyclovir and describe the severity and timing of AKI relative to acyclovir initiation when present. A secondary goal was to identify important risk factors for AKI during therapy, which could help to inform clinicians about the risks of toxicity during HSV treatment.
Methods
We performed a retrospective cohort study at 4 US hospitals: Children’s Hospital of Philadelphia (CHOP), Children’s Hospital Los Angeles, Inova Children’s Hospital, and Monroe Carell Jr. Children’s Hospital at Vanderbilt. Our study protocol was approved by the institutional review board at each hospital as well as at the US Food and Drug Administration. CHOP served as the data coordination site for this study. De-identified data from each center were shared with CHOP and the US Food and Drug Administration, who performed data analysis as described herein.
Patients
We identified all hospitalized infants age <60 days old treated with ≥1 dose of IV acyclovir for suspected or confirmed neonatal HSV disease from January 2011 to December 2015. Infants were eligible for inclusion if they had serum creatinine measurements both before acyclovir initiation and during or ≤48 hours after last dose of acyclovir. Children were excluded if they had congenital kidney disease, treatment with IV acyclovir for an indication other than HSV, or AKI detected within 72 hours before initiation of acyclovir.
Data Sources
The electronic medical record (EMR) served as the source of data at each hospital. Investigators at each site used a combination of automated and manual data abstraction methods based on the availability of resources at their institution. A standard set of definitions and data collection instructions were applied to ensure consistency across hospitals. The following data elements were collected: demographics; birth history; biometrics; concurrent illnesses/diagnoses; acyclovir dosages; other medication exposures including antibiotics, IV fluids, and nephrotoxic medications (Table I; available at www.jpeds.com); laboratory measurements including serum creatinine, HSV polymerase chain reaction testing, and microbiology cultures; admission location; and disposition. Study data were managed using REDCap electronic data capture tools hosted at CHOP.12 Quality control using a de-identified dataset was performed by investigators at CHOP, with all discrepancies resolved before final data analysis.
Table I.
Nephrotoxic medications
| Antimicrobials |
| Antifungals: Amphotericin b products |
| Antivirals: Cidofovir, foscarnet, ganciclovir, valacyclovir, valganciclovir |
| Antibacterials: Aminoglycosides (amikacin, gentamicin, tobramycin), colistin, dapsone, nafcillin, vancomycin |
| ACE inhibitors |
| Benazapril, captopril, enalapril, fosinopril, lisinopril, perindopril, quinapril, ramipril, trandolapril |
| Loop diuretics |
| Furosemide and bumetanide |
| Vasopressor medications |
| Dopamine, dobutamine, norepinephrine, epinephrine, norepinephrine, vasopressin |
| Immunosuppressives and chemotherapeutics |
| Carboplatin, cisplatin, cyclosporine, ifosfamide, mesalamine, methotrexate, sirolimus, sulfasalazine, tacrolimus |
| Nonsteroidal anti-inflammatory drugs |
| Ibuprofen, indomethacin, ketorolac, naproxen |
Outcome Measures
The primary outcome was detection of AKI at any time after the first dose of acyclovir through 48 hours after the last dose. AKI was defined as either (a) a ≥50% increase in creatinine value from baseline or (b) an absolute increase of ≥0.3 mg/dL within a 48-hour period. These definitions are consistent with neonatal AKI criteria put forth by Selewski et al.10 Because an infant’s creatinine decreases over the first few weeks of life owing to elimination of maternal creatinine, the lowest creatinine value documented at any time before acyclovir initiation was considered the baseline.13 Urine output AKI criteria were not used because urine volume measurement was not routinely performed in all infants and oliguria is an insensitive marker of AKI in neonates.14 The severity of AKI was defined based on the maximum creatinine measurement obtained after the first dose through 48 hours after the last dose.10 The timing of AKI onset was determined based on the earliest day of AKI detection, with day 0 being the first calendar day of acyclovir administration.
Statistical Analyses
We compared the characteristics of infants with and without AKI using χ2 tests or Fisher exact tests for proportions and Wilcoxon rank-sum tests for medians. Summary statistics were reported using frequencies with proportions and medians with IQRs. The incidence rate of AKI was calculated per 1000 acyclovir treatment days.
Cox regression analysis stratified by study site was performed to evaluate factors associated with AKI during IV acyclovir treatment. Data were censored at day of AKI onset, hospital discharge, death, or 2 days after acyclovir completion, whichever occurred first. The following factors were considered for inclusion in the model: sex; race; preterm or full-term gestational age; age at acyclovir start; weight at acyclovir start; baseline creatinine level; receipt of IV fluids; presence of a positive blood, urine, or cerebrospinal fluid (CSF) bacterial culture; admission location; receipt of mechanical ventilation during acyclovir course; and HSV disease detection. The number of concomitant nephrotoxic medications was included in the model a priori as a time-varying covariate based on the number of nephrotoxic medications administered the previous hospital day. HSV disease was defined based on the results of HSV polymerase chain reaction testing: (a) disseminated disease if positive from blood with or without positive testing from any other site, (b) CNS disease if positive from CSF sample, or (c) skin/eye/mouth disease if positive from a skin or mucosal surface sample only. Because polymerase chain reaction testing alone may be insufficient to categorize the extent of HSV infection, the classification of HSV disease was confirmed through clinical documentation in the EMR. Acyclovir daily dosage depends on gestational age and weight. To avoid collinearity, acyclovir dosage was not considered for the model. Separately, backward elimination was used to remove any covariates with P values of >.2 from the final model.
To better understand the representativeness of our study population among all infants exposed to acyclovir, we compared the characteristics of infants eligible for inclusion and those who were otherwise eligible but lacked baseline creatinine measurement to determine AKI status. Complete data collection was not performed for infants with no creatinine measurements during acyclovir treatment. Therefore, this analysis was limited to the subset of infants with ≥1 creatinine measurements after acyclovir initiation, but no baseline creatinine measurement. The χ2 or Wilcoxon rank-sum tests were used to compare the following characteristics of the 2 groups: age at acyclovir start, hospital, gestational age (dichotomized as <37 weeks [preterm] vs ≥37 weeks or [full term]), admission location (floor/neonatal intensive care unit/pediatric intensive care unit), weight at acyclovir start, duration of acyclovir therapy, HSV testing, and HSV disease detection.
Because changes in creatinine values owing to nephrotoxicity may take time to develop after the initiation of a drug, we performed a sensitivity analysis using Cox regression whereby infants with AKI on day 0 of acyclovir treatment were excluded. In this multivariate analysis, only infants who had AKI detected on day 1 or later after initiation of acyclovir were included in the AKI group. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, North Carolina) or Stata 13 (StataCorp, College Station, Texas).
Results
We identified 2686 infants exposed to acyclovir at the 4 participating hospitals. Of these, 72 had congenital kidney disease, 88 had AKI before acyclovir initiation, and 1191 did not have creatinine measured during or within 48 hours after acyclovir treatment; an additional 318 infants were missing baseline creatinine measurements. Thus, a total of 1017 infants met all eligibility criteria including having sufficient creatinine measurements available to assess AKI (Table II). In this cohort, >75% of patients (n = 806) started acyclovir treatment during the first 3 weeks of life and >25% (n = 274) were preterm infants. Acyclovir was most often prescribed at a dosage of 60 mg/kg/day, which is consistent with recommendations for treatment of neonatal HSV disease.15 Almost three-quarters of infants (71%) received ≤2 days of acyclovir treatment.
Table II.
Cohort characteristics (n = 1017)
| Characteristics | |
|---|---|
| Demographics | |
| Age in days at start of acyclovir | 6 (2–18) |
| Male sex | 563 (55.4) |
| Race | |
| Black | 185 (18.2) |
| Asian | 46 (4.5) |
| White | 507 (49.9) |
| Other race/multirace | 9 (0.9) |
| Unknown | 270 (26.6) |
| Hispanic ethnicity | 158 (15.5) |
| Gestational age | |
| Preterm gestation (<37 wk) | 274 (26.9) |
| Preterm (33–36 wk) | 217 (79.2) |
| Very preterm (28–32 wk) | 38 (13.9) |
| Extremely preterm (<28 wk) | 19 (6.9) |
| Full-term gestation (≥37 wk) | 726 (71.4) |
| Unknown | 17 (1.7) |
| Clinical characteristics | |
| Hospital | |
| 1 | 182 (17.9) |
| 2 | 330 (32.5) |
| 3 | 43 (4.2) |
| 4 | 462 (45.4) |
| Acyclovir administration location | |
| Floor/other | 239 (23.5) |
| NICU | 594 (58.4) |
| PICU | 184 (18.1) |
| Initial acyclovir dose in mg/kg/dose | 20.0 (19.4–20.4) |
| Maximum acyclovir daily dose in mg/kg/day | 60.0 (57.7–61.1) |
| No. of acyclovir doses | 5 (3–7) |
| Maximum concomitant nephrotoxic medications received on a single day | |
| 0 | 316 (31.1) |
| 1 | 511 (50.3) |
| 2–4 | 190 (18.7) |
| Maximum number of nephrotoxic medications received on a single day during acyclovir | |
| None | 302 (29.7) |
| 1 | 517 (50.8) |
| 2 | 172 (16.9) |
| ≥3 | 26 (2.6) |
| Receipt of specific nephrotoxic medications during acyclovir | |
| Gentamicin | 517 (50.8) |
| Vancomycin | 226 (22.2) |
| Baseline creatinine value | 0.50 (0.36–0.70) |
| Mechanical ventilation during acyclovir course* | |
| Started mechanical ventilation after acyclovir initiation | 93 (9.2) |
| Receiving mechanical ventilation at the time of acyclovir initiation | 298 (29.3) |
| No mechanical ventilation | 625 (61.5) |
| Receipt of any IV fluids during acyclovir administration | 866 (85.2) |
| Bolus infusion around doses | 116 (13.4) |
| Maintenance (>2 mL/kg/h) | 493 (56.9) |
| IV fluids for full acyclovir course | 649 (75.5) |
| HSV status | |
| Not tested | 75 (7.4) |
| HSV− | 911 (89.6) |
| HSV+ | 31 (3.0) |
NICU, neonatal intensive care unit; PICU, pediatric intensive care unit.
Values are number (%) or median (IQR).
One participant with unknown mechanical ventilation status.
Among our final study population, 93% (n = 942) had HSV testing performed. Thirty-one infants (3.0%) had confirmed HSV disease including 15 (48%) with disseminated disease, 9 (29%) with CNS disease, and 7 (23%) with skin/eye/mouth disease. The median age at HSV diagnosis was 10 days (IQR, 6–16) and 13% (n = 4) were premature. The median duration of acyclovir treatment in infants with HSV was 18 days (IQR, 7–22). Seven infants (23%) died during hospitalization.
In total, 57 infants (6%) developed AKI during acyclovir treatment or within 48 hours of acyclovir treatment completion (Table III). The median first day of AKI detection was day 1 (IQR, 1–2). Although the majority of AKI was stage I (defined as 50%–99% or ≥0.3 mg/dL increase in creatinine from baseline), 16 infants (28%) who developed AKI had stage ≥II disease (≥100% increase in creatinine). Seven infants with confirmed HSV (23%) had AKI detected during acyclovir treatment, including 4 of 15 with disseminated disease, 2 of 9 with CNS disease, and 1 of 7 with skin/eye/mouth disease. Of these 7 infants, 4 had AKI detected on day 1.
Table III.
AKI during acyclovir
| Variables | No. (%) |
|---|---|
| AKI from start of acyclovir through 48 h after last acyclovir dose | 57 (5.6) |
| Day of first detection | |
| 0 | 13 (22.8) |
| 1 | 21 (36.8) |
| 2 | 11 (19.3) |
| ≥3 | 12 (21.1) |
| Maximal severity of AKI | |
| Stage I | 41 (71.9) |
| Stage II | 12 (21.1) |
| Stage III | 4 (7.0) |
| Incidence rate of AKI per 1000 acyclovir days | 11.6 |
There were no significant differences in the proportion of infants with AKI across the 4 hospitals (Table IV). Infants with AKI were more often female (P = .04) and more often received ≥2 concurrent nephrotoxic medications during acyclovir treatment (P = .02). The proportion of infants with AKI who received gentamicin, the most commonly administered nephrotoxin, was similar to those without AKI (P = .17). Infants co-administered vancomycin more often developed AKI (P = .006).
Table IV.
Characteristics of infants with and without AKI
| Variables | With AKI (n = 57) | Without AKI (n = 960) | P value* |
|---|---|---|---|
| Demographics | |||
| Age (days) at start of acyclovir | 9 (5–25) | 6 (2–17) | .07 |
| Weight (g) at start of acyclovir | 3200 (2500–3600) | 3180 (2700–3700) | .39 |
| Male sex | 24 (42.1) | 538 (56.2) | .04 |
| Race | .07 | ||
| Black | 10 (17.5) | 175 (18.2) | |
| Asian | 2 (3.5) | 44 (4.6) | |
| White | 21 (36.8) | 486 (50.6) | |
| Other race/multirace/unknown | 24 (42.1) | 255 (26.6) | |
| Hispanic ethnicity | 5 (8.8) | 153 (15.9) | .15 |
| Preterm gestation (<37 wk) | 14 (24.6) | 260 (27.1) | .68 |
| Clinical characteristics | |||
| Hospital | .08 | ||
| 1 | 14 (24.6) | 168 (17.5) | |
| 2 | 24 (42.1) | 306 (31.9) | |
| 3 | 1 (1.8) | 42 (4.4) | |
| 4 | 18 (31.6) | 444 (46.3) | |
| Acyclovir administration location | .003 | ||
| Floor/other | 4 (7.0) | 235 (24.5) | |
| PICU | 17 (29.8) | 167 (17.4) | |
| NICU | 36 (63.2) | 558 (58.1) | |
| Initial acyclovir dose, mg/kg/dose | 20.0 (19.5–20.5) | 20.0 (19.4–20.4) | .43 |
| Maximum acyclovir daily dose, mg/kg/day | 60.0 (56.8–60.9) | 60.0 (57.7–61.1) | .70 |
| No. of acyclovir doses | 4 (2–8) | 5 (3–7) | .38 |
| Maximum concomitant nephrotoxic medications received on a single day | .02 | ||
| 0 | 21 (36.8) | 295 (30.7) | |
| 1 | 19 (33.3) | 492 (51.3) | |
| 2–4 | 17 (29.8) | 173 (18.0) | |
| Receipt of specific nephrotoxic medications during acyclovir | |||
| Gentamicin | 24 (42.1) | 493 (51.4) | .17 |
| Vancomycin | 21 (36.8) | 205 (21.4) | .006 |
| Baseline creatinine value | 0.35 (0.21–0.66) | 0.50 (0.37–0.70) | .0003 |
| Mechanical ventilation during acyclovir course† | <.0001 | ||
| Started mechanical ventilation after acyclovir initiation | 14 (24.6) | 79 (8.2) | |
| Receiving mechanical ventilation at the time of acyclovir initiation | 25 (43.9) | 273 (28.5) | |
| No mechanical ventilation | 18 (31.6) | 607 (63.3) | |
| Receipt of any IV fluids during acyclovir administration | 52 (91.2) | 814 (84.8) | .18 |
| HSV status | .0002 | ||
| Not tested | 4 (7.0) | 71 (7.4) | |
| HSV− | 46 (80.7) | 865 (90.1) | |
| HSV+ | 7 (12.3) | 24 (2.5) |
Values are number (%) or median (IQR).
The χ2 tests were used to compare proportions of categorical or binary variables. Wilcoxon rank-sum tests used to compare the distribution of continuous variables.
One subject without AKI missing mechanical ventilation status.
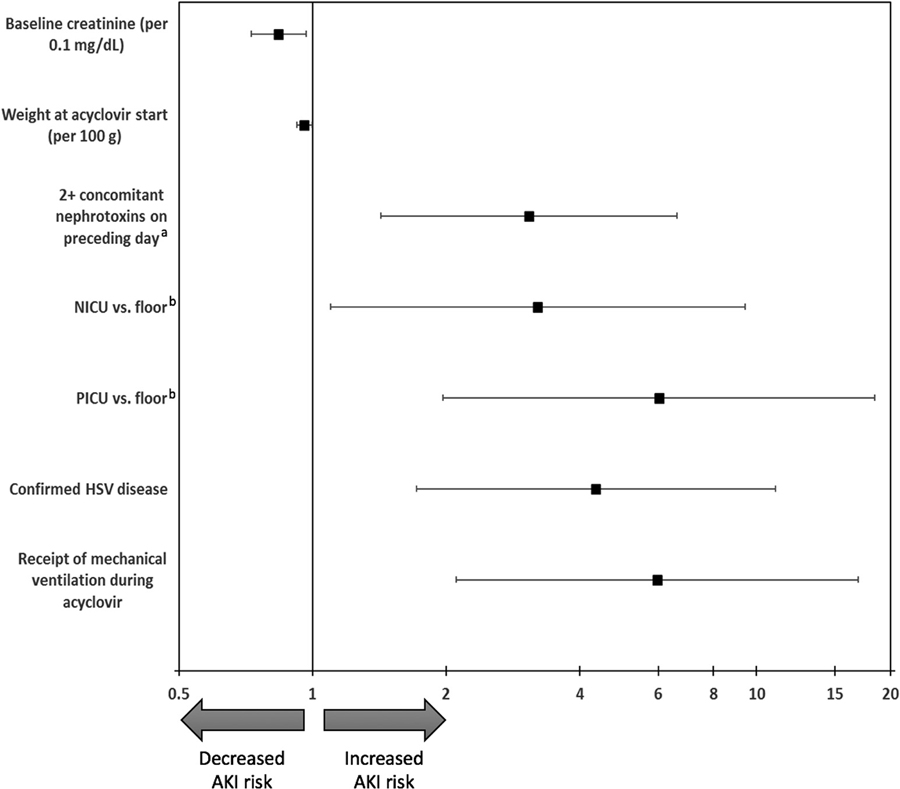
The results of the Cox regression analysis evaluating factors associated with AKI during acyclovir treatment are shown in the Figure and (Table V; available at www. jpeds.com). After adjustment for other covariates, increased hazard of AKI was associated with confirmed HSV disease (OR, 4.35; P = .002), receipt of mechanical ventilation during acyclovir course (OR, 5.97; P = .001), admission to the pediatric intensive care unit or neonatal intensive care unit (OR, 6.02 and 3.21, respectively; P = .006), and receipt of ≥2 concomitant nephrotoxic medications on the preceding day (OR, 3.07; P = .004). Increased weight (OR, 0.96 per 100 grams; P = .03) and higher baseline creatinine values (OR, 0.84 per 0.1 mg/dL; P = .01) were associated with a decreased hazard of AKI. Receipt of IV fluids, admission year, bacterial infection (positive blood, CSF, or urine culture), sex, race, prematurity, and age were not significantly associated with AKI.
Figure.
Cox regression evaluating factors associated with the detection of AKI during IV acyclovir. The model was stratified by study site. Backward elimination was used to remove covariates with a P value of >.2 from final model (admission year, receipt of IV fluids, positive urine culture, positive CSF culture). Only covariates with a P value of <.05 are displayed; covariates with a P value of >.05 and ≤.2 that were included in model but not displayed in figure include sex, race, age at start of acyclovir, premature gestation, receipt of 1 concomitant nephrotoxin on preceding day, and positive blood culture. a Incorporated as a time-varying covariate in model. Reference is no concomitant nephrotoxic medications. b Floor designation includes 10 subjects whose location of first dose is other.
Table V.
Cox regression evaluating factors associated with detection of AKI during IV acyclovir in infants*
| Variables | Hazard ratio | 95% Confidence Limits | P value | |
|---|---|---|---|---|
| Male sex | 0.66 | 0.38 | 1.15 | .14 |
| Age (days) at the start of acyclovir (per day) | 1.02 | 1.00 | 1.04 | .08 |
| Baseline creatinine value (per 0.1 mg/dL) | 0.84 | 0.73 | 0.97 | .01 |
| Race | .10 | |||
| African American or Black | 0.39 | 0.05 | 3.35 | |
| Asian/Pacific Islander | 0.38 | 0.03 | 4.43 | |
| Hispanic | 0.37 | 0.05 | 3.00 | |
| Unknown | 0.88 | 0.11 | 7.06 | |
| Weight at acyclovir start (per 100 g) | 0.96 | 0.92 | 1.00 | .03 |
| Gestational age <37 wk | 0.46 | 0.20 | 1.03 | .06 |
| 1 concomitant nephrotoxic medications on preceding day† | 1.73 | 0.89 | 3.38 | .11 |
| ≥2 concomitant nephrotoxic medications on preceding day† | 3.07 | 1.43 | 6.60 | .004 |
| Admission location at acyclovir start | .006 | |||
| Floor‡ | REF | |||
| PICU | 6.02 | 1.96 | 18.47 | |
| NICU | 3.21 | 1.10 | 9.42 | |
| Confirmed HSV disease | 4.35 | 1.71 | 11.01 | .002 |
| Receipt of mechanical ventilation during acyclovir | 5.97 | 2.11 | 16.92 | .001 |
| Positive blood culture | 0.23 | 0.03 | 1.70 | .15 |
NICU, neonatal intensive care unit; PICU, pediatric intensive care unit.
Model stratified by study site. Backward elimination used to remove covariates with P value > .2 from final model (admission year, receipt of IV fluids, positive urine culture, positive CSF culture).
Incorporated as a time-varying covariate in model. Reference is no concomitant nephrotoxins on the preceding day.
Includes 11 subjects whose admission location/location of first dose is other.
There were differences between infants with and without baseline creatinine measurements available (Table VI; available at www.jpeds.com). Infants without baseline creatinine measurements were significantly older and heavier at acyclovir initiation, less often tested for HSV, and more often admitted to the floor vs an intensive care unit setting. There were also significant differences in the availability of baseline creatinine measurements by hospital. The 2 groups were similar in terms of preterm gestation, HSV positivity, and duration of acyclovir therapy. The sensitivity analysis evaluating factors associated with AKI on day 1 or later of acyclovir treatment identified similar risk factors (Table VII; available at www.jpeds.com), although weight at acyclovir initiation was no longer significant in the model.
Table VI.
Characteristics of infants missing baseline creatinine excluded from the study vs final study population
| Characteristics | Missing baseline creatinine (n = 318) | Study population (n = 1017) | P value* |
|---|---|---|---|
| Age (days) at start of acyclovir | 9 (4–20) | 6 (2–18) | .03 |
| Weight (g) at start of acyclovir | 3400 (2860–3890)† | 3180 (2700–3700) | <.01 |
| Preterm gestation (<37 wk) | 59 (27.1)‡ | 274 (26.9) | .92 |
| Hospital | <.01 | ||
| 1 | 23 (7.2) | 182 (17.9) | |
| 2 | 167 (52.5) | 330 (32.5) | |
| 3 | 4 (1.3) | 43 (4.2) | |
| 4 | 124 (39.0) | 462 (45.4) | |
| Tested for HSV | 260 (81.8) | 942 (92.6) | <.01 |
| HSV+ PCR | 13 (4.1) | 31 (3.0) | .48 |
| Days of acyclovir administration | 2 (2–4) | 2 (2–3) | .65 |
| Location of first acyclovir dose | <.01 | ||
| Floor/other | 139 (44.1)§ | 239 (23.5) | |
| PICU | 32 (10.2) | 184 (18.1) | |
| NICU | 144 (45.7) | 594 (58.4) |
PCR, polymerase chain reaction.
Values are number (%) or median (IQR).
The χ2 tests or Fisher exact tests (if cells <5) were used to compare proportions of categorical or binary variables. Wilcoxon rank-sum tests were used to compare distribution of continuous covariates.
Data available for 316 of 318 infants with missing baseline.
Data available for 218 of 318 infants with missing baseline.
Data available for 315 of 318 infants with missing baseline.
Table VII.
Cox regression evaluating factors associated with detection of AKI on day 1 or later during IV acyclovir in infants*
| Variables | Hazard ratio | 95% Confidence Limits | P value | |
|---|---|---|---|---|
| Male sex | 0.63 | 0.34 | 1.14 | .13 |
| Baseline creatinine value (per 0.1 mg/dL) | 0.73 | 0.61 | 0.87 | <.001 |
| 1 concomitant nephrotoxic medication on preceding day† | 1.91 | 0.91 | 4.02 | .09 |
| ≥2 concomitant nephrotoxic medications on preceding day† | 2.90 | 1.23 | 6.84 | .02 |
| Admission location at acyclovir start | ||||
| Floor/other | REF | |||
| PICU | 4.37 | 1.37 | 13.90 | .01 |
| NICU | 2.97 | 0.99 | 8.89 | .05 |
| Confirmed HSV disease | 5.34 | 2.03 | 14.07 | <.001 |
| Receipt of mechanical ventilation during acyclovir | 5.10 | 1.75 | 14.89 | .003 |
Model stratified by study site. Backward elimination used to remove covariates with a P value of >.2 from final model (admission year, receipt of IV fluids, positive urine culture, positive CSF culture, positive blood culture, age, race, weight, gestational age, gestational age <37 weeks).
Incorporated as a time-varying covariate in model. Reference is no concomitant nephrotoxins on the preceding day.
Discussion
In our multicenter retrospective cohort study of infants exposed to acyclovir, the rate of AKI was low; only 6% of infants developed AKI during treatment. AKI developed in roughly 1 of every 100 days of acyclovir administration and was more common in sicker infants, for example, those admitted to an intensive care unit, with confirmed HSV disease, requiring mechanical ventilation, or those receiving multiple concurrent nephrotoxic medications. Given the frequency of empiric acyclovir use in infants with suspected serious infections, clinicians should be cognizant of the toxicity risk, recognize infants who are at higher risk for AKI, and monitor at-risk infants at the start of and throughout treatment.
The incidence of AKI in our study was lower than some previous reports of toxicity in older children and adults treated with IV acyclovir where toxicity developed in 18%–30% of recipients.8,16 However, the majority of acyclovir administration in our study population was empiric and, thus, of short duration.
The bulk of AKI in our study occurred early during acyclovir treatment with 79% of AKI cases detected in the first 3 days of treatment. This may be reflective of the generally short duration of acyclovir exposure; however, this finding is also consistent with the mechanism of acyclovir toxicity. Acyclovir has low urine solubility and can precipitate in the lumen of renal tubules causing obstruction (eg, crystal nephropathy) after as little as a single dose of the drug.8,11 Although changes in creatinine take time to develop after a renal insult,17 we felt it was important to report all AKI that occurred after the first dose because acyclovir toxicity is plausible at any time during treatment. However, our study was not designed to implicate acyclovir as the cause of renal injury. Instead, we believe that our findings highlight the importance of close monitoring of acyclovir-exposed infants, even when treatment courses are short. This monitoring is particularly imperative in sicker infants and those receiving additional nephrotoxic medications, in whom the risk of AKI is high regardless of acyclovir exposure. The implementation of approaches to identify infants with exposure to multiple concomitant nephrotoxic medications, such as use of EMR-based alerts,18 may be a prudent strategy.
Administration of acyclovir in the setting of low urine output or dehydration can contribute to the development of toxicity.11 Although co-administration of IV fluids was not associated with decreased AKI in our study, the vast majority of infants received IV fluids. We were unable to fully assess hydration status owing to the retrospective design of our study and the limitations of available clinical documentation. For example, urine specific gravity results were not available for abstraction at all sites. IV hydration has been protective against AKI in previous reports, but its benefit in neonates warrants further study.19
Nearly one-quarter of infants with HSV disease developed AKI during treatment with more than one-half of these infants having AKI detected on day 1. This finding differs significantly from the incidence of nephrotoxicity in infants with HSV reported by Ericson et al, where only 2% of 89 infants with HSV disease developed renal dysfunction.9 This discrepancy is likely due to the different AKI definitions used in the 2 studies. We applied the Neonatal AKI Classification criteria put forth by Selewski et al, whereas the Ericson study applied a specific creatinine value cut-point for toxicity (>1.7 mg/dL).10 Defining baseline creatinine values in infants is challenging and there is no universally accepted approach. In our study, we used the lowest creatinine measurement available before the first dose of acyclovir. Additional studies are needed to establish accurate baseline renal function measurements in newborn infants, which will facilitate future medication safety studies in this population. Because creatinine decreases over the first few weeks of life because of elimination of maternal creatinine from serum, and the median age of infants in our study was 6 days, we may have underestimated AKI by using a higher creatinine value (eg, influenced by maternal creatinine).13 Nevertheless, we advise clinicians to obtain creatinine measurements upon initiation of acyclovir so that changes in creatinine can be recognized early. As has been previously described, systematic evaluation of children exposed to nephrotoxic medications can detect AKI sooner and potentially mitigate subsequent AKI by identifying modifiable risk factors in patients exposed to nephrotoxin.20 Based on the risk factors identified for AKI in our study, we believe that early and frequent monitoring of kidney function is warranted during acyclovir treatment for all infants who (a) have HSV disease, (b) are receiving ≥2 concomitant nephrotoxic medications, or (c) are admitted to an intensive care unit. Daily creatinine measurement could even be considered in the highest risk infants.
It is notable that fewer than one-half of all infants treated with acyclovir at our institutions had adequate creatinine measurements to assess AKI status. This factor may affect the generalizability of our findings. Although the incidence of AKI was low, the timing of AKI onset demonstrates that it can occur even during short courses of treatment. Infants without baseline creatinine values available were older, less often tested for HSV, and more often admitted to the floor. Because the factors associated with AKI can be considered signals of sicker patient status, it is possible that the population without baseline creatinine values represents infants less likely to develop AKI during therapy.
There are important additional limitations to our study. First, because this was a retrospective evaluation, data collection was restricted to that which could be accurately collected. Infants without creatinine measured during acyclovir were excluded because we could not assess AKI, which could bias results. Additionally, we focused the collection of data in the EMR that were universally available across all 4 hospitals, such as laboratory results and medication records, rather than clinical assessments. Information such as fluid balance, severity of illness indicators, and concurrent illnesses may have contributed to the risk of AKI, but could not be captured adequately in all patients. Moreover, it is also possible that some infants received acyclovir at another institution before admission at our 4 study hospitals. This factor could explain why a small subset of patients in our cohort were treated with acyclovir but did not have HSV testing performed. Alternatively, some infants may not have had testing performed because of an inability to obtain adequate samples for testing. Regardless, to avoid misclassification of their HSV status, we categorized these infants separately; notably, their AKI rate was similar to infants who were HSV negative (5%). Second, variability in creatinine monitoring could have contributed to misclassification. Because changes in creatinine values can be delayed, it is possible that toxicity was not captured if a subject did not have creatinine measured after cessation of the drug. Additionally, monitoring practices may have differed across institutions and hospital units. Finally, there were differences in how creatinine results were reported across the 4 hospitals. At CHOP, creatinine values are reported to the nearest 0.1 mg/dL, whereas they were reported to the nearest 0.01 mg/dL at the other institutions. Although the rate of AKI was similar across the 4 hospitals, it is possible that this difference could have contributed to misclassification for infants at CHOP with change in creatinine close to 1.5-fold of baseline.
Among our large cohort of infants exposed to acyclovir, the incidence of AKI was low. However, most acyclovir treatment courses were short and, when AKI was detected, it was soon after initiation of therapy. AKI was more common among sicker infants and in those receiving multiple other nephrotoxic medications. Close monitoring of renal function should be considered throughout acyclovir treatment courses in neonates and young infants, even during the empiric treatment phase, especially in sicker patients.
Acknowledgments
K.D. has received research support from Merck, Inc and Pfizer unrelated to the current work and is supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health (K23 HD091365). B.H. has received support from Pfizer, Inc, unrelated to the current work. S.V. is funded by the Burroughs Wellcome Innovation in Regulatory Science Award. S.C. has received support from Merck for participation on an unrelated DSMB. The views expressed in the article are the personal views of the authors and may not be understood, quoted or stated on behalf of or reflecting the views of their respective employers. No specific funding was received for the performance of this work.
Glossary
- AKI
Acute kidney injury
- CHOP
Children’s Hospital of Philadelphia
- CNS
Central Nervous System
- CSF
Cerebrospinal fluid
- EMR
Electronic medical record
- HSV
Herpes simplex virus
- IV
Intravenous
References
- 1.Kimberlin DW, Lin CY, Jacobs RF, Powell DA, Frenkel LM, Gruber WC, et al. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics 2001;108:223–9. [DOI] [PubMed] [Google Scholar]
- 2.Flagg EW, Weinstock H. Incidence of neonatal herpes simplex virus infections in the United States, 2006. Pediatrics 2011;127:e1–8. [DOI] [PubMed] [Google Scholar]
- 3.Handel S, Klingler EJ, Washburn K, Blank S, Schillinger JA. Population-based surveillance for neonatal herpes in New York City, April 2006-September 2010. Sex Transm Dis 2011;38:705–11. [DOI] [PubMed] [Google Scholar]
- 4.Whitley R, Davis EA, Suppapanya N. Incidence of neonatal herpes simplex virus infections in a managed-care population. Sex Transm Dis 2007;34:704–8. [DOI] [PubMed] [Google Scholar]
- 5.Cruz AT, Freedman SB, Kulik DM, Okada PJ, Fleming AH, Mistry RD, et al. Herpes simplex virus infection in infants undergoing meningitis evaluation. Pediatrics 2018;141(2). in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shah SS, Aronson PL, Mohamad Z, Lorch SA. Delayed acyclovir therapy and death among neonates with herpes simplex virus infection. Pediatrics 2011;128:1153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long SS. Delayed acyclovir therapy in neonates with herpes simplex virus infection is associated with an increased odds of death compared with early therapy. Evid Based Med 2013;18:e20. [DOI] [PubMed] [Google Scholar]
- 8.Rao S, Abzug MJ, Carosone-Link P, Peterson T, Child J, Siparksy G, et al. Intravenous acyclovir and renal dysfunction in children: a matched case control study. J Pediatr 2015;166:1462–8.e1–4. [DOI] [PubMed] [Google Scholar]
- 9.Ericson JE, Gostelow M, Autmizguine J, Hornik CP, Clark RH, Benjamin DK Jr, et al. Safety of high-dose acyclovir in infants with suspected and confirmed neonatal herpes simplex virus infections. Pediatr Infect Dis J 2017;36:369–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Selewski DT, Charlton JR, Jetton JG, Guillet R, Mhanna MJ, Askenazi DJ, et al. Neonatal acute kidney injury. Pediatrics 2015;136:e463–73. [DOI] [PubMed] [Google Scholar]
- 11.Izzedine H, Launay-Vacher V, Deray G. Antiviral drug-induced nephrotoxicity. Am J Kidney Dis 2005;45:804–17. [DOI] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoseini R, Otukesh H, Rahimzadeh N, Hoseini S. Glomerular function in neonates. Iran J Kidney Dis 2012;6:166–72. [PubMed] [Google Scholar]
- 14.Jetton JG, Askenazi DJ. Update on acute kidney injury in the neonate. Curr Opin Pediatr 2012;24:191–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poole CL, Kimberlin DW. Antiviral approaches for the treatment of herpes simplex virus infections in newborn infants. Ann Rev Virol 2018;5: 407–25. [DOI] [PubMed] [Google Scholar]
- 16.Lee EJ, Jang HN, Cho HS, Bae E, Lee TW, Chang SH, et al. The incidence, risk factors, and clinical outcomes of acute kidney injury (staged using the RIFLE classification) associated with intravenous acyclovir administration. Ren Fail 2018;40:687–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta RL, Awdishu L, Davenport A, Murray PT, Macedo E, Cerda J, et al. Phenotype standardization for drug-induced kidney disease. Kidney Int 2015;88:226–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldstein SL, Mottes T, Simpson K, Barclay C, Muething S, Haslam DB, et al. A sustained quality improvement program reduces nephrotoxic medication-associated acute kidney injury. Kidney Int 2016;90:212–21. [DOI] [PubMed] [Google Scholar]
- 19.Kim S, Byun Y. Comparison of renal function indicators according to hydration volume in patients receiving intravenous acyclovir with CNS infection. Biol Res Nurs 2015;17:55–61. [DOI] [PubMed] [Google Scholar]
- 20.Goldstein SL, Kirkendall E, Nguyen H, Schaffzin JK, Bucuvalas J, Bracke T, et al. Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics 2013;132: e756–67. [DOI] [PubMed] [Google Scholar]