Abstract
Background and Aims:
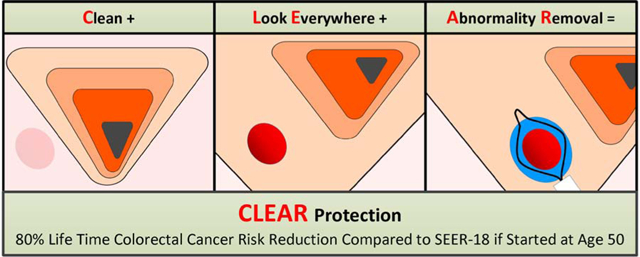
Colorectal cancer (CRC) prevention by colonoscopy has been lower than expected. We studied CRC prevention outcomes of a colonoscopy protocol based on CLEAR principles, an acronym for: (1) Clean the colon, (2) Look Everywhere, and (3) complete Abnormality Removal.
Methods:
Observational follow-up study of patients provided screening colonoscopy at a free-standing private ambulatory surgery center in South Carolina by 80 endoscopists from October 2001 to December 2014, followed through December 2015. The colonoscopy protocol, optimized for polyp clearance, featured (1) in-person bowel preparation instructions reinforced by phone; (2) polyp search and removal throughout insertion and gradual withdrawal with circumferential tip movements, and (3) team approach using all personnel present to maximize polyp detection, patient safety, and clear-margin polypectomy including requesting repeat inspection or additional tissue removal. Outcome measures were postscreening lifetime CRC risk relative to SEER-18 and interval cancer rate (postcolonoscopy CRCs among cancer-free patients at screening)
Results:
Of 25,862 patients (mean age 58.1 years, 52% black, 205,522 person-years, PYO), 159 had CRC at screening and 67 patients developed interval CRC. Interval CRC rate was 3.34/10,000 PYO, 5.79 and 2.24 among patients with and without adenomas, respectively. The rate was similar among older patients (mean 68.5 years at screening) and with prolonged follow-up. Postscreening lifetime CRC risk was 1.6% (bootstrap 95% confidence interval, 1.3% - 1.8%), versus 4.7% in SEER-18, 67% lower. Subgroups with mean screening age 50 and 68.5 years showed risk reductions of 80% and 72%, respectively. Adverse event rate was less than usually reported rates: perforation 2.6/10,000, bleeding with hospitalization 2.4/10,000, and no deaths.
Conclusions:
A colonoscopy protocol optimized for polyp clearance prevented 67% of CRC compared with SEER-18 population given ongoing population screening.
Keywords: colonoscopy screening, colorectal cancer prevention, lifetime colorectal cancer risk, interval cancer
Graphical Abstract
INTRODUCTION
Colorectal cancer (CRC) affects about 4% to 6% of the Western population.1,2 In theory, most CRCs can be prevented by screening with colonoscopy to remove precancerous polyps. Experts agree that colonoscopy is the most accurate screening method to prevent CRC. However, community-based clinical trials have reported modest CRC reductions compared with 76% prevention documented in the National Polyp Study (NPS).3–6 In 2013, 58% of age-eligible Americans had completed colonoscopy screening; yet CRC deaths (~51,000) and lifetime CRC risk (~4.3%) remain almost similar to 1997 rates.1,7 Simulation modeling studies project optimistic population-wide reductions in the future, based, however, on certain assumptions that are contradicted by empirical studies of community-based, usual-practice colonoscopy.2,8–10
BACKGROUND
Clinical trials have relied on usual-practice colonoscopy provided by community-based physicians without specifying a colonoscopy protocol, and reported outcomes similar to population screening programs, 26% to 43% prevention.11,12 A key weakness of most trials is viewing colonoscopy as a commodity, a binary treatment, either “done” or “not done” within the trial protocol timeline. The “treatment” (colonoscopy procedure) is not protocolized to ensure the goal of colonoscopy, complete polyp clearance, which requires adherence to the CLEAR criteria, an acronym for (1) Clean the colon (adequate bowel preparation), (2) Look Everywhere (maximum adenoma detection), and (3) Abnormality Removal (complete polypectomy of all detected polyps).13 Thus, usual-practice “colonoscopy done” does not translate to complete polyp clearance, the true test of “done.” Current quality indicators, withdrawal time and adenoma detection rate (ADR) are imperfect measures of colonoscopy quality and do not reflect completeness of polyp clearance.
The most challenging and weakest links for achieving polyp clearance are endoscopist-dependent variations in the adenoma detection rate (ADR) and completeness of polypectomy.14–19 ADR is adversely affected by time-constrained colonoscopy.20 Tandem colonoscopies show that 22% to 27% of polyps are missed.14,15 To prevent CRC, all abnormal tissue must be completely removed (akin to R0 tumor resection). Incomplete polypectomy (R1 resection) leaves behind residual neoplastic tissue that may evolve into interval cancer. Polyp clearance requires time and optimum technique, described in the United Kingdom’s Joint Advisory Group (JAG) criteria applied during direct observation of procedure skills (DOPS) and direct observation of polypectomy skills (DOPyS) for credentialing National Health Service physicians for colonoscopy.16,21–24
We present here a colonoscopy protocol and the long-term outcomes of an endoscopy group who―preceding publication of CLEAR principles and the JAG guidelines―created and consistently implemented across 80 endoscopists, a standardized colonoscopy protocol optimized to overcome all weak links in the CLEAR chain of events. Similar to the JAG the study center credentialed all endoscopists based on direct observation of procedure and polypectomy skills as well as ADR.16,21,22 We present their screening cohort’s interval CRC rates and cumulative lifetime CRC risk over the remaining life expectancy compared with the prevailing risk of the Surveillance, Epidemiology, and End Results program (SEER) population given ongoing population screening activities.
METHODS
Study design and data sources
An independent observational study was conducted to assess CRC incidence prevention after screening colonoscopies performed by 80 endoscopists at a licensed ambulatory surgery center for endoscopy in South Carolina (SC) from October 2001 to December 2014. Patient data were linked to the SC Central Cancer Registry’s (SCCCR) incidence and mortality databases from January 1, 1996 to December 31, 2015 using patient identifying variables (Appendix Note 1).25 The study was approved by the University of South Carolina Institutional Review Board.
The SCCCR, a state population-based registry was gold-certified by the North American Association of Central Cancer Registries (NAACCR) in most study years (criteria: >95% cancer data complete within 2 years, ~100% error-free data, <3% of cancers based on death certificates), and silver-rated in 4 years (>90% data completeness).26 The Registry’s final data completeness was >96.5% (99.7% in 2012), achieved by continued addition of new data indefinitely, based on cancer care/cancer death abstracts on SC residents received from 43 states under reciprocal data exchange agreements.
Colonoscopy protocol
All endoscopists adhered to the colonoscopy protocol featuring (1) bowel preparation with phospho-soda until 2009 and magnesium citrate-bisacodyl since 2007, both split doses, and importantly, bowel preparation instructions explained in person and reinforced by a phone call when preparation was due to begin; (2) midazolam-meperidine or propofol sedation; (3) team approach using all personnel present to achieve efficient cecal intubation with minimal endoscope shaft insertion, optimum patient/endoscope/snare positioning, maximal polyp detection and R0 resection with clear margins, and minimal adverse events including bleeding and perforation; (4) maximizing mucosal exposure by gradual shaft withdrawal while cleaning the mucosa and use of circumferential tip movements; (5) polyp search and polypectomy during both insertion and withdrawal except for flat polyps larger than 20 mm (removed only during withdrawal); (6) all team members observe the monitor and can request repeat inspection or additional tissue removal as needed (the Jidoka principle); (7) hot snare/hot biopsy forceps, as appropriate for polyp size, used for all adenomatous-appearing polyps, all right-sided colon polyps, and hyperplastic-appearing left-sided colon polyps ≥5 mm (cold snare for anticoagulated patients and diminutive hyperplastic-appearing polyps in the rectum); (8) submucosal lift used for flat/depressed polyps and tattooing polyps >20 mm, unsure of complete removal or appearance suggesting high-grade dysplasia; (9) retroflexion in the rectum during insertion; (10) real-time documentation of procedure and polyp details by note-taker assistant and photo-documentation of cecal landmarks, polyps, polypectomy sites, and rectal retroflexion field; (11) an expert in difficult polyp removal available onsite if needed; (12) quarterly in-house quality reviews and identification-protected display of providers’ cumulative ADRs and procedure time averages on a bulletin board in the physicians’ breakroom to encourage self-driven benchmarking.16,27–34
All polyps were removed except ≤2 mm hyperplastic polyps in the rectum (identified by narrow-band imaging), and invasive, large, or vascular polyps (referred for removal at a hospital). All polyps were sent for histopathology examination, except clusters of similar polyps <2 mm or a carpet-like patch (sample collected and the remaining destroyed). Nonvillous appearing polyps from a colonic segment were placed in a single container.25 Surveillance colonoscopy schedules complied with prevailing professional consensus/U.S. Multi-Society Task Force guidelines.28 Patient compliance was not systematically monitored; however, the initial letter conveying the histopathology results mentioned the due date of next colonoscopy. When surveillance became due (tracked by an informal chart marking system), a letter was sent. No additional follow-up was done to pursue compliance.
Patients and data
The study population consisted of average-risk patients from primary care practices provided screening colonoscopy from October 2001 to December 2014. By policy the center does not accept patients with known bowel pathology or CRC-predisposing genetic syndromes. Accuracy of procedure and polyp data was verified by a 2% sample chart review (error rate 0.6%). Missing/discrepant data were extracted from patient records.28 Study exclusion criteria were age below 40 or over 89 years, other bowel pathology, and prior colon resection or CRC diagnosis.35–37 Patients with CRC at screening (SCCCR diagnosis date within 6 months of colonoscopy) were included in the lifetime risk analyses. Interval CRC was SCCCR-documented incident CRC or CRC death more than 6 months after colonoscopy. CRC family history, smoking, and lifestyle data were not available.
Measures of CRC prevention
Two measures of CRC prevention were used (1) interval CRC rate per 10,000 person-years of observation (PYO) compared with the NPS and community-based colonoscopy studies using chi-square tests, and (2) postscreening lifetime CRC risk ratio relative to SEER-18 population. Patient PYO were calculated from the colonoscopy date up to death, CRC diagnosis or end of study period, December 31, 2015 whichever occurred first. Postscreening lifetime CRC risk ratio relative to SEER-18 population (usual-care, comparison group) was calculated using published age-conditional SEER-18 lifetime risk, the cumulative probability of incident CRC after a given age over the remaining life expectancy estimated from historic incidence and mortality data.38 For the main analysis, we estimated the SEER lifetime risk at age 58, (cohort mean age at screening) by prorating SEER lifetime risks at age 50 and 60 in 2007 to 2009 (cohort accrual mid-point, 2008).3,38,39 This was the cohort’s expected CRC risk. SEER-18 comparison was deemed appropriate because the SC CRC incidence has closely tracked SEER-18 rates (122.8/100,000 population aged over 50 in 2009–2013 vs 119.3/100,000 in SEER-18 in 2016, the difference consistent with the annual rate of CRC decline).1
Postscreening lifetime CRC risk
The cohort’s postscreening lifetime CRC risk was calculated based on follow-up CRCs, the sum of all CRCs found at screening and interval CRCs. This assumes that CRCs detected at screening are equivalent to clinically silent, undiagnosed CRCs in the SEER “CRC-free” population (dwell-time CRC).37 Postscreening lifetime CRC risk was the sum of the follow-up CRC rate and projected CRC risk during post–follow-up life expectancy. Postscreening life expectancy was life expectancy at the cohort mean age at screening, sourced from United States life tables.40 Follow-up CRC rate was follow-up CRCs converted into a per-person per-year rate multiplied by mean years of follow-up per patient. Projected CRC risk during post–follow-up life-expectancy was the observed annual interval cancer rate during follow-up multiplied by years of post-follow-up life-expectancy. The lifetime risk ratio was the calculated cohort lifetime risk divided by SEER lifetime risk at the corresponding age. Its complement is the cohort’s risk reduction relative to SEER-18. Detailed calculations are shown in Appendix Exhibit 1. To assess accuracy of the lifetime CRC risk and interval cancer rate estimates, we used bootstrapping (1000 random samples with replacement) to estimate 95% confidence intervals (95% CI).
Sensitivity analyses were done as follows: (1) lifetime CRC prevention estimated after adjustment for racial composition of the cohort (the expected SEER_18 lifetime risk for the cohort weighted to reflect cohort racial composition), and (2) calculating the cohort lifetime risk after prorating the cohort baseline CRC rate to SEER using the annual CRC incidence rate (instead of including all CRCs found at screening). The resulting annual rate of follow-up CRC was applied to all life expectancy years at age 58.
Subgroup analyses were conducted to (1) assess the risk reduction among patients screened around the U.S. Preventive Services Taskforce-recommended age (up to 55 years, mean 50 years) and compare it with the full cohort using chi-square test, and (2) detect potential attrition of prevention at advanced age or over prolonged follow-up. Nationally, CRC incidence among the 65+ age group is thrice the rate of the 50 to 65 age group.41
To test attrition of CRC prevention with time since screening and at advanced ages, we (1) compared the full cohort interval CRC rate and lifetime risk ratio with patients screened between 2001 and 2008 who had longer follow-up using chi-square tests, (2) plotted the 2 groups’ trends of calendar year-wise interval CRC rates, and (3) plotted the trends among patients aged 70 to 90 years at the end of follow-up. These analyses also verify the validity of assuming stable interval CRC rates over subjects’ remaining life-expectancy.
Control cancer
A control cancer, primary brain cancer was used to verify the completeness of follow-up CRC data. Brain cancer incidence and mortality has been stable for decades, is unrelated to lifestyle or CRC risk, and unlikely to be misclassified in death certificates. Expected brain cancer deaths were estimated using SC mortality rates within age-sex-race strata during 2001 to 2015 (Appendix Note 2). The standardized mortality ratio (SMR) was observed divided by expected deaths. Statistical analyses were performed using SAS (v.9.4, Cary, NC), except for bootstrapping performed in R.
RESULTS
Study Population
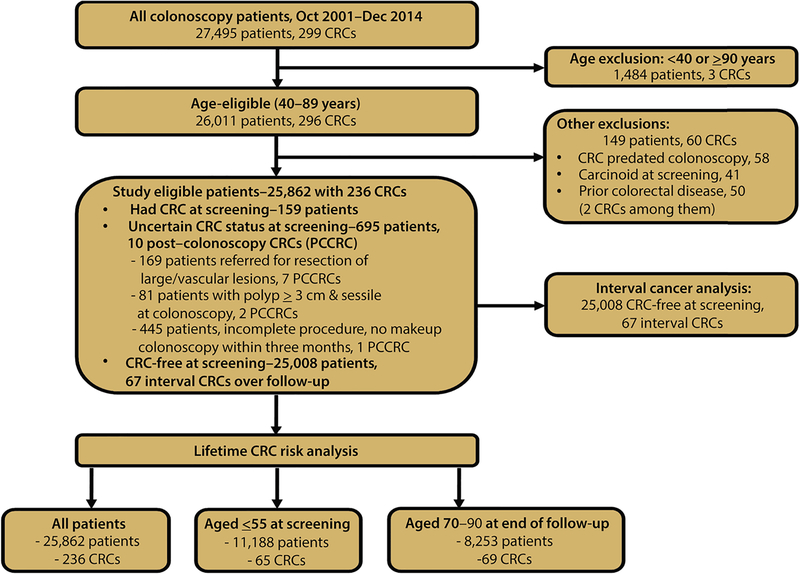
Figure 1 presents the exclusion criteria and patients studied in the main and subgroup analyses. Of 25,862 study-eligible patients, 159 had CRC at screening, a rate of 611/100,000, 5 times the SC incidence (122.8/100,000 population aged over 50 years).1 Table 1 presents the characteristics of 25,008 CRC-free patients at screening, 54% female, 52% black, mean age 58.1 years (SD 9.2), cecum intubation rate 97.6%, total procedure time, median 22 minutes, (25th and 75th percentiles, interquartile (I-Q) range, 16, 30), and withdrawal time, median 10, I-Q range 7, 14). Polypectomy occurred when a polyp was found including during insertion except for flat polyps larger than 20 mm. Mean ADR was 30.1%. ADR range for endoscopists with at least 200 procedures was 14.1% to 42.3%; 24.2% to 42.3% after excluding 2 outliers who declined to follow protocol and left the practice. Advanced adenomas or ≥3 nonadvanced adenomas were present in 8.3%, and 67 patients developed interval cancer.
Figure 1:
Study inclusion criteria and patient selection for each analysis.
Table 1.
Demographic characteristics of CRC-free patients at screening by adenoma status
| Characteristics | CRC-free patients at screening | Patients without adenoma | Patients with adenoma | ||||
|---|---|---|---|---|---|---|---|
| Number of CRC-free patients at screening (%) | 25,008 | (69.9) | (30.1) | ||||
| (15.2) | (77.2) | (22.8) | |||||
| (47.2) | (72.8) | (27.2) | |||||
| (25.8) | (65.1) | (34.9) | |||||
| (118) | (59.2) | (40.8) | |||||
| Mean age (SD) | (9.2) | (9.0) | (9.4) | ||||
| (45.7) | (64.8) | (35.2) | |||||
| (53.9) | (74.1) | (25.9) | |||||
| Missing | (0.4) | (84.6) | (15.4) | ||||
| (43.7) | (67.0) | (33.0) | |||||
| (51.9) | (72.1) | (27.9) | |||||
| (3.9) | (72.0) | (28.0) | |||||
| Missing | (0.5) | (81.0) | (19.0) | ||||
| (30.1) | |||||||
| (5.7) | |||||||
| ≥3 nonadvanced adenomas* | (2.6) | ||||||
| Cecum intubation rate | 97.6% | 97.6% | 97.5% | ||||
| (68.7) | (77.7) | (22.3) | |||||
| (21.4) | (60.0) | (40.0) | |||||
| +2 or more procedures | (9.9) | (36.7) | (63.3) | ||||
| Total procedure time, minutesα Median (25th, 75th percentile) | (16,30) | (14,25) | (18,32) | ||||
| Withdrawal time, minutesα Median (25th, 75th percentile) | (7,14) | (6,10) | (8,16) | ||||
| Interval CRCs (% of total) | 67 | (46.3) | (53.7) | ||||
| 2001–2014 screened cohort - PYO | 200,834 | 138,608 | 62,226 | ||||
| Mean years of follow-up (median) | (8.5) | (8.5) | (8.5) | ||||
| Mean years of follow up (median) | (10.5) | (10.5) | (10.5) | ||||
PYO = Person-years of observation; ADR = adenoma detection rate
p<0.001.
Includes 55 persons with missing polyp histopathology reports.
In a previous study the 2001–2008 screened cohort followed through 2009 was documented using the standardized incidence ratio method.25 The current study uses this cohort followed through 2015 to compare the lifetime risk ratio with that of the full cohort to evaluate persistence of CRC risk reduction with cohort aging and time since first screening.
Notes: Advanced adenoma is defined as adenoma with villous/tubulo-villous features, size ≥1 cm, or high-grade dysplasia. IQ range: interquartile range. Follow-up duration: From screening date to the first of 3 events: diagnosed with CRC, died from any cause, or end of follow-up period (December 31, 2015). Student t-test used for continuous variables, chi-square test for count variables.
Satisfactory bowel preparation was achieved for 93.7% of procedures; among patients with unsatisfactory preparation, 90.7% complete colonoscopy was achieved by lavage, and the remaining were subjected to make-up colonoscopy (data not shown). ADR among patients with satisfactory bowel preparation was 31.4% versus 27.9% in procedures completed despite unsatisfactory preparation. Adverse event rates were perforation 2.6/10,000, bleeding 2.4/10,000, aspiration 1.2/10,000, and no deaths.
Of total patients, 11,188 patients (43.3%) were aged ≤55 years at screening, (mean 50.0 years), and 8,253 patients (33%) were aged 70 to 90 years at the end of follow-up (mean screening age, 68.5 years), who contributed 88,793 and 77,404 person-years of observation (PYO), respectively (Supplementary Table 1). Additional colonoscopies (surveillance or rescreening) during the study period were completed by 31.3% of patients, average 1 colonoscopy per patient every 5.6 years including the screening procedure (1 every 6.6 years for 2001 to 2008 screened patients).
Interval CRC rate
The interval cancer rate was 3.34/10,000 PYO (bootstrap 95% CI, 2.54 – 4.13), being 5.79 and 2.24 among the adenoma and no-adenoma groups, respectively (Table 2). Similar interval cancer rates were observed among the subgroup aged 70 to 90 at the end of follow-up (3.10/1,000; p=0.76), and the 2001 to 2008 screened subgroup (3.46/10,000 PYO, p=0.87). Patients aged ≤55 years at screening (mean 50.0 years) had a significantly lower rate, 1.81/10,000 (p=0.03). Rates were similar for right-sided versus left-sided colon cancers (p=0.35).
Table 2:
Interval colorectal cancer rate among CRC-free patients
| No. of patients | Person-years of obs. (PYO) | Interval CRCs | Interval CRCs/10,000 PYO | P value* | |
|---|---|---|---|---|---|
| Full cohort | |||||
| All patients, all ages | 25,008 | 200,834 | 67 | 3.34 | - |
| Adenoma at screening | 7,530 | 62,226 | 36 | 5.79 | |
| No adenoma | 17,478 | 138,608 | 31 | 2.24 | |
| Age ≤55 at screening | 11,139 | 88,404 | 16 | 1.81 | 0.03 |
| Age 70–90 years at end of follow-up | 8,253 | 77,404 | 24 | 3.10 | 0.76 |
| Anatomic location* | |||||
| Right-colon segment | 25,008 | 200,834 | 31 | 1.54 | |
| Left-colon segment | 25,008 | 200,834 | 24 | 1.2 | 0.35 |
| Subgroup | |||||
| screened 2001–2008 | 15,808 | 165,984 | 57 | 3.46 | 0.87 |
P value compared with the full cohort all ages, except anatomic location where P is for difference between right-and left-sided CRC.
Right-colon segment CRC excludes 4 appendix cancers (considered as other location in current literature).45 For 7 CRCs laterality data were missing. P value shown is upon comparing right- vs left-interval CRCs.
Note: Expected right- and left-sided colon cancers out of 67 are 28 and 34, respectively, (per 42% and 51% of CRCs among SEER-18 incidence cases).45 Observed right colon segment CRCs vs expected are similar, 31 vs 28 (p=0.70); left colon segment CRCs, 24 vs 34 (p=0.19).
Control cancer standardized mortality ratio
The standardized mortality ratio for brain cancer was 1.0 (19 deaths observed vs 19.0 expected, p=0.42).
Postscreening lifetime CRC risk relative to SEER-18
Table 3 shows that the study cohort had a postscreening lifetime CRC risk of 1.57% (bootstrap 95% CI, 1.3% - 1.8%) after counting all 159 CRCs at screening as follow-up CRCs. SEER-18 lifetime risk at age 58 was 4.71%, cohort CRC risk ratio 0.33, and CRC reduction, 66.7% (95% CI, 61.8% - 71.9%). The subgroup aged ≤55 years at screening showed substantially greater risk reduction than the full cohort: 79.5% less CRC than the corresponding SEER-18 population. Patients aged 70 to 90 years at the end of follow-up (mean 68.5 years at screening) experienced CRC reduction similar to the full cohort, 71.7% (risk calculations and P values shown in Appendix 1 Exhibit 1). Sensitivity analyses showed a race-adjusted lifetime CRC reduction of 67.2%. The rate was 69.2% when the cohort baseline CRCs were standardized to the SEER incidence rate.
Table 3:
Postscreening lifetime CRC risk reduction relative to SEER-18 population*
| Full cohort (screened 2001 to 2014) | Screened 2001 to 2008 | |
|---|---|---|
| Patients | 25,862 | 16,306 |
| Person-years of observation | 205,522 | 168,643 |
| Mean age at screening (year) | 58.1 | 58.6 |
| Mean age at end of follow-up (year) | 66.1 | 69.1 |
| Follow-up CRCs (interval CRCs + CRCs at screening) | 236 | 176 |
| Life expectancy at mean screening age (year) | 27.4 | 27.4 |
| Postscreening lifetime CRC risk (%)* | 1.57 | 1.77 |
| Expected SEER-18 lifetime CRC risk at 58 years (%) | 4.71 | 4.71 |
| Lifetime CRC risk ratio relative to SEER** | 0.333 | 0.376 |
| Lifetime CRC risk reduction | 66.7% | 62.4% |
| Patients | 11,188 | 6,731 |
| Mean age at screening (year) | 50.0 | 50.0 |
| Lifetime CRC risk reduction relative to SEER at age 50 | 79.5%α | 80.7%α |
| Patients | 8,253 | 6,669 |
| Mean age at screening (year) | 68.5 | 67.8 |
| Lifetime CRC risk reduction relative to SEER at age 68 | 71.7% | 75.1% |
See Appendix Exhibit 1 for detailed calculations and sources of reference population (SEER-18) data.
Estimated risk ÷ expected risk per SEER-18
Statistically significant difference relative to the full cohort-all ages, all p<0.05.
CRC risk reductions of all other subgroups are statistically similar to that of the full cohort-all ages (p>0.05).
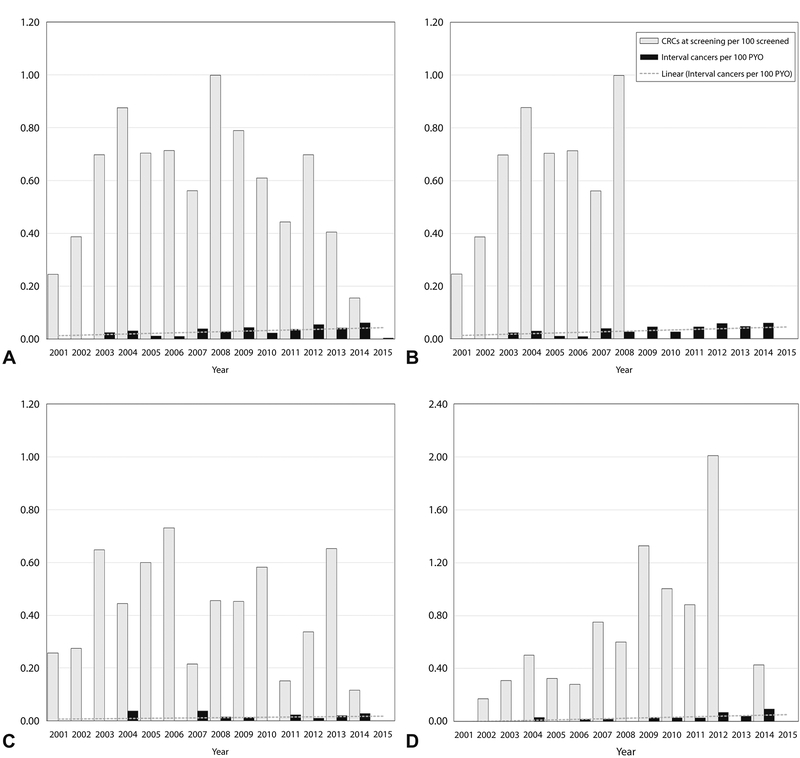
Figure 2 presents calendar year-wise interval CRC rate per 100 person-years, and the CRC rate at screening per 100 screened subjects. Figure 2A (full cohort) and 2B (2001–2008 screened cohort) show stable interval CRC rates over follow-up (and by implication, stable CRC prevention effect). Figure 2C and D presenting patients aged ≤55 years at screening, and 70 to 90 years at the end of follow-up, respectively, illustrate stable CRC prevention effects over prolonged follow-up regardless of age. The data also show homogeneity of subjects’ baseline CRC rates over the long study period.
Figure 2:
CRCs detected at screening per 100 persons, and interval CRCs per 100 PYO over follow-up, 2001 to 2015. A, Cohort screened 2001 to 2014 (mean age at screening 58.1 years, mean age at end of follow-up 66.1 years). B, Cohort screened 2001–2008 (mean age at screening 58.6 years, mean age at end of follow-up 69.1 years). C, Cohort screened at or below 55 years (mean age 50.01 years, mean age at end of follow-up 58.01 years). D, Cohort aged 70 to 90 years at end of follow-up (mean age at screening, 68.5 years). In A, interval CRC rate of 0.06/100 PYO in 2014 is random; in 2015 it was 0.0, 2-year average, 0.03/10,000. A similar anomaly is seen in part D.
Interval cancer rate compared with documented studies
Table 4 shows that the cohort’s interval CRC rate among the adenoma subgroup was similar to the NPS rate of 5.9/10,000, a study that used protocolized colonoscopy personally performed by the study investigators (p=0.95).3,36 All other documented studies of community-based, usual-practice colonoscopy service showed much higher interval CRC rates than the study cohort, all p<0.001. The Polyp Prevention Trial and Kaiser Permanente 2014 studies (cohort age comparable with the study cohort) showed 3 times and twice the study cohort rate, respectively. The PLCO cohort, 10 years older, had 3 times the study cohort rate.4–6,11,35 The Kaiser Permanente 2019 study of adenoma-free patients at screening showed twice the study cohort rate (p<0.001) with half the follow-up duration of the study cohort.42 Supplementary Table 2 presents the exclusions applied by each study.
Table 4:
Interval CRC rate among CRC-free study patients at screening compared with published studies
| Current study | National Polyp Study3,36 | Polyp Prevention Trial4,5,11 | PLCO study6 | KP 201435 | KP 201942 | |
|---|---|---|---|---|---|---|
| Procedure used | CLEAR colonoscopy | All performed by study investigators | Usual-care, community-based colonoscopy | |||
| Total patients (age, year) | 26,011 (>40) | 9,112 (NA) | 37,175 (>35 years) | 15,395 (50–75 years) | 273,742 (≥50 years) | 99,166 (50–75 years) |
| Indication | Screening | Diagnostic | Screen/diagnostic | Diagnostic | 57% diagnostic | Screening |
| Exclusions@ | 1,003 (3.9%) | 7694 (84.4%) | 35,096 (94.4%) | NA | 49,900 (18.2%) | - |
| CRC-free patients (adenoma status at screening) | 25,008 (7,530 adenoma, 17,478 no-adenoma) | 1,418 (adenoma patients) | 2,079 (adenoma patients) | 15,935 (7950 adenoma, 7985 no-adenoma) | 223,842 (all patients) | 99,166 (No-adenoma) |
| Mean age at colonoscopy (year, (SD)) | 58.1 (±9.2) | 61 (±10) | 61.0 (±0.3) | 64 (median) | 64 (median) | 55.6 |
| Interval CRCs | 67 | 5 | 14 | 196 | 712 | 184 |
| Person-Years of Observation (PYO) (mean follow-up years) | 200,834 (8.0) | 8,401 (5.9) | 7,620 (4.0) | 189,891 (119) | 927,523 (4.1) | 417,987 |
| Interval CRC/10,000 PYO a) All patients | 3.34 | - | - | 10.32 | 7.68 | - |
| b) Adenoma patients | 5.79 | 5.9 | 18.3 | 13.07 | NA | - |
| c) No adenoma | 2.24 | - | - | NA | NA | 4.4 |
| P value, Current vs Comparison study* | - | 0.95 | <0.0001 | <0.0001 | <0.0001 | 0.0004 |
| Interval CRC rate ratio | 1.0 | 1.0 | 3.2 | 3.1 | 2.3 | 2.0 |
See Supplementary Table 2 for the exclusion criteria applied in each study. NA: Not available for the category.
KP: Kaiser Permanente
DISCUSSION
We report here that a group of endoscopists adhering to a rigorous colonoscopy protocol reduced the postscreening lifetime CRC risk by 67% compared with SEER-18 population given its prevailing screening activity with usual-practice colonoscopy. This is the estimated prevention after counting all CRCs detected at screening as follow-up CRCs. To clarify in simple terms, cohort patients provided screening colonoscopy have 1/3 of the lifetime risk of getting CRC compared with SEER-18 population, which already had a colonoscopy screening rate of 48% in 2008, the SEER reference year (Table 3).43 Accuracy of the lifetime CRC risk estimates is supported by narrow 95% bootstrap CIs. Bootstrapping generates the sampling distribution of lifetime risk estimates for the bootstrapped random samples, and the 95% CI shows the range within which the true lifetime risk lies. The narrow confidence intervals, both for lifetime risk and interval cancer rate validate accuracy of the calculated rates. The cohort’s low interval cancer rate is validated by comparisons with published cohorts of usual-practice colonoscopy, which show multifold higher rates than those of the cohort, all differences being statistically significant. Finally, the magnitude of CRC risk reduction is supported by the colonoscopy procedure quality indicators shown in Table 1. Our estimated lifetime risk reduction assumes and requires continuing compliance with surveillance and receipt of CLEAR colonoscopy over patients’ remaining life expectancy. Notably, the total colonoscopy burden was only 1 procedure per patient every 6.6 years including screening and surveillance procedures.
A recent study showed declining CRC prevention in adenoma-free subjects’ later years of follow-up, zero prevention after 12 years.42,44 The interval CRC rate was 6.29/10,000 PYO in patients’ first year of follow-up, increasing to 22.48/10,000 after the 12th year.42 In our study, the rate among adenoma-free patients was 2.24/10,000 with no change over time or with advanced age. The consistent multifold higher rates of interval cancer in every other study that used usual-practice colonoscopy implies that the standardized CLEAR protocol implemented by the study endoscopists merits consideration for widespread use. Incomplete polypectomy affects up to 36% of polyps (50% if polyps are >5 mm) under direct observation by peers.16 Missed adenoma rates are 22% to 26% despite the endoscopists anticipating in tandem colonoscopy by a peer.15,20 Both rates under usual-practice conditions are likely higher.
A simulation study of lifetime CRC risk reduction under Germany’s colonoscopy screening program projected very high prevention rates after 15 and 25 years, assuming near-100% adenoma detection, all complete polypectomies, and unvarying CRC risk regardless of adenoma status, assumptions that are contradicted by empirical studies of usual-practice colonoscopy.2,3,15,16,44
Previous observational studies without internal comparison groups excluded CRCs detected at screening to calculate standardized incidence ratios relative to the general population, potentially overestimating the prevention rate.3,11,25 When this method was used, our earlier study of 4.8 year follow-up reported 83% CRC prevention.25 In the current study, we included all CRCs detected at screening as follow-up CRCs, attributing the excess CRCs to dwell time CRC. Dwell time is the lag between preclinical CRC and clinical diagnosis, estimated at 4.5 to 5.8 years.37 Including all CRCs at screening in estimating the postscreening lifetime risk strongly assures that our cohort baseline CRC status is not biased toward showing greater-than-actual CRC prevention (overestimation bias). The number of CRCs detected at screening testifies to near-100% detection of all cancers including small lesions by the CLEAR protocol.
However, racial composition did impact our CRC rate at screening, 52% blacks with 46% higher rate than white/other race (SEER-18 has 11% black population). To assess the impact of cohort racial composition on the estimated lifetime CRC prevention, a sensitivity analysis was done adjusting the analysis for race, which showed that the prevention outcome (67.2% CRC risk reduction, Appendix Exhibit 1) is robust to race-associated CRC risk (66.3% without race adjustment). This is because CLEAR colonoscopy confers high postscreening CRC prevention (ie, a low interval cancer rate) over the remaining lifetime regardless of race.
Our study may underestimate the full preventive effect of CLEAR colonoscopy on 2 counts. We compare our cohort with SEER-18, which already reflects CRC reductions achieved by the prevailing usual-practice colonoscopy screenings (48% nationally in 2008 per National Health Interview Survey data, and 57.8% in South Carolina per 2008 Behavioral Risk Factor Surveillance System data analysis).43 Second, colonoscopy-seeking populations may contain a greater-than-average proportion of higher-risk individuals (eg, CRC family history).
Our calculated CRC risk reduction should be robust to potential estimation errors due to certain approximations used: using the midpoint of cohort accrual as the SEER reference year,3 prorating the available age-conditional risks at 50 and 60 years to estimate SEER risk at age 58 (the cohort mean age at screening), and applying life expectancy at the cohort’s mean screening age to the full cohort. The observed stable interval cancer rates throughout follow-up and among advanced age groups mitigate this concern.
Single-center study remains a limitation; however, it may be mitigated by the number of participating endoscopists. Another study limitation is the use of SEER-18 as the comparison population (instead of SC population for which lifetime risk estimates were not available). This issue is mitigated by the similarity of SC and SEER incidence rates and the sensitivity analysis that adjusted for cohort racial composition. Another potential limitation is the absence of data on CRC family history, lifestyle factors, and bowel symptoms. Despite potential inclusion of such higher-risk patients in the cohort, the observed prevention rate testifies to the strengths of the CLEAR colonoscopy protocol.
Why are these results exceptional? First, the group adhered to a rigorous colonoscopy protocol which addressed all weak links in the CLEAR chain of events for 13 years. Colon preparation is personally instructed by phone at the right time, when the patient starts colon cleansing.34 Significant expertise and shaft manipulation skill/effort/mucosal inspection are contributed by team members, facilitating endoscopist’s focus on key instrument manipulations for error-free and safe performance in every case. During insertion the cecum is reached with minimum shaft length inserted without looping and straightening the entire colon into the shape of a “?” allowing precise tip control during withdrawal. All people present observe the endoscopy monitor for abnormalities. Studies show increasing polyp detection with more observers.32
During withdrawal, mucosal inspection with circumferential tip movements maximizes lesion detection in folds and flexures. Polypectomy also is a team effort. Team members contribute to patient/shaft/snare repositioning as needed and support safe application of hot snare/forceps for most polyps, ensuring destruction of all abnormal tissue. When difficulties arise, an expert in difficult polypectomies (performing their own cases) is available onsite to advise or assist. Any team member can request repeat inspection, resection, or an alternative technique method to achieve clear-margin resection―the Jidoka principle.33 Procedure time-points, landmarks, polyps, and resection details are documented concurrently by a note-taker assistant, sparing endoscopist distraction (to mentally note these details), and enabling appropriate surveillance scheduling and periodic quality review.
Second, the group has proactively used published studies to set its own quality targets ahead of the gastroenterology societies’ quality guidelines. Individual endoscopist ADRs are tracked and ADR maintenance in the group’s ADR range is required to retain endoscopy privileges. Self-driven benchmarking, encouraged by displaying privacy-protected individual ADRs may also contribute to generally high ADRs. Third, the team approach reinforces mutual accountability for patient outcomes and ensures diligent adherence to protocol in every procedure. Fourth, adequate procedure time slots―45 minutes per procedure for endoscopists―are set to maximize procedure quality and patient outcome; patients are allocated to procedure rooms for 30 minutes giving endoscopists 15 minutes for administrative tasks and patient interaction.
Are these results replicable in the general population? Potential healthy subject bias is mitigated by the high rate of CRC at screening (consistent with the cohort racial composition and CRC dwell time), adenoma detection rate (30.1%), and similar lung cancer incidence as the SC general population computed over 4.8 years follow-up documented earlier.25 Completeness of follow-up cancer data is robustly validated by the control cancer, identical observed versus expected primary brain cancer deaths. Completeness is also validated by SC’s NAACCR-certified Cancer Registry data completeness of 97% to 100%, achieved by active data collection from diagnostic and treatment facilities within the state and supplemented by new cases identified from out-of-state cancer care abstracts on SC residents received continually from 43 states under data exchange agreements. A potential challenge to replicate the prevention rate in the general population is the study center’s policy of accepting primarily average-risk patients. High-risk patients contribute 5% to 10% of CRCs (genetic syndromes and prior bowel pathology). However, high-risk patients develop CRC at an early age. The cohort mean age was 58 years and the comparison benchmark, SEER lifetime risk at 58 years excludes younger-onset CRC. Therefore, the estimated CRC prevention ratio should be robust to this concern. Finally, the feasibility and financial sustainability of this protocol is self-evident, being sustained over 17 years to date at a free-standing private endoscopy center under prevailing reimbursement rates.
In conclusion, this study demonstrates that a strict colonoscopy protocol produced CRC prevention comparable with the NPS, and multifold higher prevention than published studies of usual-practice colonoscopy cohorts. With an average of just one colonoscopy per patient every 6.6 years, a low interval cancer rate was maintained throughout follow-up and at advanced ages. Despite being a single-arm, unblinded observational study, 4 study characteristics are critical to evaluate the strength of evidence contributed by this study. First, consistent, uniform delivery of a protocolized intervention, CLEAR principles-based colonoscopy that is verifiable by documented procedure details and photo-images for every patient (in these respects similar to clinical trials of experimental interventions). Second, completeness of follow-up CRC data verified by an independent control cancer. Third, postscreening lifetime CRC risk incorporating interval cancers and CRCs at screening used to study prevention outcomes. Fourth, multifold lower interval cancer rates than other published studies. The observed 80% CRC reduction after screening colonoscopy by age 55 makes a compelling case for pursuing rapid CRC reductions to 30,000 new cases and 10,000 deaths annually (currently 150,000 and 50,000, respectively), by delivering on average, one CLEAR colonoscopy per person every 6.6 years.
Supplementary Material
Financial support and acknowledgements
US National Institutes of Health: 1R21CA202477 and 3R21CA202477-01S1, PI: Sudha Xirasagar; Co-I: Piet de Groen)], and R01DK106130, PI: Piet de Groen] and the University of Minnesota. The funding organization had no role in study planning, interpretation of findings, manuscript preparation or journal submission.
The authors are grateful to the Carolina Colonoscopy Center (formerly the South Carolina Medical Endoscopy Center) in Columbia, South Carolina for providing access to their colonoscopy data, facilitating data quality audit and missing data extraction by the study team using medical records, and facilitating data linkage with the South Carolina Central Cancer Registry. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health or of the authors’ affiliated organizations.
Acronyms/abbreviations used:
- CRC
Colorectal cancer
- CLEAR
Clean the colon, Look Everywhere, complete Abnormality Removal
- SC
South Carolina
- SCCCR
South Carolina Central Cancer Registry
- SEER
Surveillance Epidemiology and End Results Program
- ADR
Adenoma Detection Rate
- DOPS
Direct observation of procedure skills
- DOPyS
Direct observation of polypectomy skills
- JAG
Joint Advisory Group
- R0 resection
Clear margin resection
- NPS
National Polyp Study
- PYO
Person-years of observation
- CI
Confidence interval
- PLCO study
Prostate Lung Colorectal and Ovarian Cancer study
Appendix Exhibit 1: Post-screening lifetime CRC risk analyses to estimate % CRC reduction achieved by CLEAR colonoscopy relative to SEER-18 population3,36,38
Data used for estimations:
Midpoint of current study cohort accrual3 = 2008 (reference year for SEER-18 lifetime CRC risk)
-
Applicable age conditional lifetime CRC risk is 2007–2009:36,37
At age 50 years (given alive and no CRC diagnosis at 50 years) = 4.97 (all races, both sexes)
At age 60 years = 4.64
Study cohort mean age at screening = 58.1 years.
Reference population and year: We estimate the expected SEER-18 cumulative lifetime CRC risk at age 58 years by prorating the risk change between 50 and 60 years (0.33%), to annual change (0.033 per year of age), multiplied by 8 years, 0.033 × 8 = 0.264%. Lifetime risk is 0.264% less at 58 years than at 50 years.37
Expected lifetime risk of CRC-free individuals at age 58 in the absence of study intervention = 4.71% (if cohort members had similar screening experience as the SEER-18 population). (Interpretation: In 2007–2009 Americans without a CRC diagnosis aged 58 years had a 4.71% risk of being ever-diagnosed with CRC in their remaining lifetime. This is the expected risk of study subjects in the absence of intervention, CLEAR colonoscopies.)
Although the mostly average-risk screening study cohort does not contain known high-risk sub-groups of the population that contributes up to 10% of all CRCs, it is not necessary to adjust the expected lifetime CRC risk of the cohort (4.71%) because most high-risk CRCs likely occur before 58 years of age.
Published life expectancy for the 55–60 years age group (midpoint 58 years, also the cohort mean age) = 27.4 years (applied to the study cohort).39
% CRC reduction relative to SEER-18 using lifetime risk analysis (full cohort, n = 25,862 patients)
Post-screening lifetime risk was calculated by adding all CRCs found at screening colonoscopy to interval CRCs and counting them as “follow-up CRCs” during the follow-up period. This approach assumes that all the cohort CRCs found at screening represent the true prevalence of CRC in the population, which includes clinically diagnosed CRCs (represented in the incidence rate number) and dwell-time or preclinical CRCs. CRCs found at screening amount to 5 times the population incidence rate (cohort rate 611/100,000 vs. SC incidence 122·8/100,000 population aged over 50 years). The documented CRC dwell time from pre-clinical CRC to clinically diagnosed CRC is 4.5 – 5.8 years. The observed 5-fold CRC incidence in the cohort is consistent with the CRC dwell time.35 All CRCs found at screening are counted towards “follow-up” CRCs to calculate the post-colonoscopy CRC risk during observed follow-up. For the lifetime after observed follow-up, we multiply the observed interval cancer rate during follow-up by the remaining life expectancy. This is supported by the unchanging CRC incidence with time since colonoscopy and with advancing age shown in Figure 2, which supports the assumption of constant interval CRC rate over all years of post-follow-up life expectancy.
Analysis-eligible cohort = 25,862 patients including patients with CRC found at screening (25,008 CRC-free at screening + 159 patients with CRC at screening + 695 unclear CRC status at screening – see Figure 1)
PYO = 205,522 after censoring at CRC diagnosis, death or end of follow-up.
Follow-up CRCs = 236 (159 at screening + 10 among unclear baseline status + 67 interval CRCs)
CRC risk per person per year = 236/205,522 = 0.001148
Cohort CRC risk over 8.0 years of observed follow-up = 0.001148 × 8.0 = 0.00918
CRC risk over unobserved 19.4 years’ life expectancy = Interval CRC risk per-person per-year × 19.4 = (67/200,834) × 19.4 = 0.00647
Total lifetime CRC risk = 0.0092 + 0.0065 = 0.0157 or 1.57% lifetime risk
Expected CRC risk per SEER-18 = 4.71%
Lifetime CRC risk ratio for the cohort = 1.57/4.71 = 0.333
CRC reduction relative to SEER-18 = 66.7%
Sub-group analysis: Age ≤55 years at screening (mean 50.01 years) – estimated CRC reduction after including all CRCs found at screening in the analysis
Analysis-eligible cohort = 11,188 patients including patients with CRC found at screening (11,139 CRC-free at screening + 49 patients with CRC at screening)
PYO =88,793
Life expectancy at age 50 years (mean age at screening) = 31.7 years39
CRCs = 65 (49 at screening + 16 interval CRCs)
CRC risk per person per year = 65/88,793 = 0.000732
Cohort CRC risk over 8.0 years of observed follow-up = 0.000732 × 8.0 = 0.00586
CRC risk over unobserved life expectancy = Interval CRC risk per-person per-year × 23.7 = (16/88,793) × 23.7 = 0.00427
Total lifetime CRC risk of cohort = 0.0059 + 0.0043 = 0.0102 or 1.02% (using Approach 2 above)
Expected CRC risk at age 50 years in SEER-18 population = 4.97%
Lifetime CRC risk ratio for the cohort = 1.02/4.97 = 0.205
CRC reduction rate = 79.5% relative to SEER-18 (greater than the reduction in the full cohort, p=0.0012)
Subgroup analysis: Mean age 68.5 years at screening (aged 70–90 at end of follow-up) – Estimated CRC reduction after including all CRCs found at screening in the analysis
Analysis-eligible cohort = 8,253 patients including all patients with CRC found at screening (7,972 CRC-free at screening + 39 patients with CRC at screening + 242 unclear CRC status at screening)
PYO = 77,404
Life expectancy at age 68 years (mean age at screening) = 19.4 years39
CRCs = 69 (39 at screening + 6 among unclear status at screening + 24 interval CRCs)
CRC risk per person per year = 69/ 77,404 = 0.00089
Cohort CRC risk over 9.38 years of observed follow-up = 0.00089 × 9.38 = 0.0084
CRC risk over unobserved 10.02 years’ life expectancy = Interval CRC risk per-person per-year × 10.02 = (24/75,394) × 10.02 = 0.00319
Total lifetime CRC risk = 0.0084 + 0.00319 = 0.01159 or 1.159% lifetime risk
Expected CRC risk at age 68 years in SEER-18 population = 4.10%
Lifetime CRC risk ratio for the cohort = 1.159/4.10 = 0.2827
CRC reduction rate = 71.73% relative to SEER-18 (similar to CRC reduction in the full cohort, p=0.06)
Conclusion:
CLEAR colonoscopy screening at a mean age of 58 years with subsequent surveillance colonoscopy produced an estimated 67% lifetime CRC incidence reduction relative to SEER-18 with its ongoing population screening activity.
Colonoscopy screening performed at a mean age of 50 years with subsequent surveillance colonoscopy produced lifetime CRC incidence reduction of 79.5%, and among patients aged 68.5 years at screening, 71.7% reduction (both estimated after including all CRCs at screening among follow-up CRCs).
SENSITIVITY ANALYSES
Race-adjusted CRC incidence reduction (SEER-18 lifetime risk weighted to adjust for cohort race composition)
% CRC incidence reduction estimated by standardizing cohort baseline risk to SEER-18 based on annual CRC incidence
A. Race-adjusted CRC incidence reduction (SEER-18 lifetime risk weighted to adjust for cohort race composition) – Full cohort 25,862 patients including patients with CRC at screening
Notes: According to SEER18 published rates, Other races are close to White LT risk. White LT risk are lower than Black. Because of few other race patients, they are combined with Whites.
White and Other CRC lifetime risk at age 50 years = 4.89 (2007–2009)
At age 60 years = 4.58
White and Other mean age = 58.9 years, ~59 years
Expected SEER-18 cumulative lifetime CRC risk at age 59 years = 4.61 (White and Other race)
Black race: CRC lifetime risk at age 50 years = 5.23 (2007–2009)
At age 60 years = 4.86
Black mean age = 57.5 ~58 years
Expected SEER-18 cumulative lifetime CRC risk at age 58 years = 4.93 (Black)
Proportion of White/Other and Black in cohort = 48% and 52% (for race-weighted expected lifetime risk of cohort)
Race adjusted lifetime CRC risk of cohort = (0.48 × 4.61) + (0.52 × 4.93) = 2.213 + 2.564 = 4.78
Total lifetime CRC risk = 1.57% (calculated earlier)
(Published life expectancy at age 58 race-wise are not available. Because of nearly equal Blacks and White/Other race in the cohort, the higher LE of White/Other at 55 and 60 years of age is expected to make up for lower LE of Blacks resulting in overall cohort LE being unaffected by the slight variation in LE among the races. Hence lifetime CRC risk calculation remains unchanged.)
Race-adjusted cohort lifetime CRC risk ratio relative to SEER-18 = 1.57/4.78 = 0.328
Race adjusted CRC risk reduction relative to SEER-18 = 67.2%
B. % CRC incidence reduction estimated by standardizing cohort baseline risk to SEER-18 based on annual CRC incidence
The principle in this approach was to “equalize” the follow-up CRC experience of the study cohort with that of SEER-18 “CRC-free” population, except for the intervention effect. Because our patients are all from South Carolina, we used the South Carolina CRC incidence rate of 122.8/100,000 in the population aged over 50 years to prorate CRCs found at screening and added these CRCs to interval CRCs to standardize the CRC risk of the two groups.1 (SEER-18 incidence is similar to SC incidence.) The analytic sample for this analysis consisted of CRC-free patients at colonoscopy, patients with uncertain CRC status at screening, and patients with CRC detected at screening prorated to the population incidence rate (Figure 1).3 The annual CRC rate based on total “follow-up” CRCs was used to calculate their post-colonoscopy lifetime CRC risk per Figure 1.
Calculations:
Step 1: Standardize study cohort CRC risk profile at baseline to SEER-18 CRC-free population, establish analytic sample and follow-up CRCs
To standardize the CRC risk profile of the study cohort at start of follow-up to the comparison population, we attributed follow-up CRCs in the cohort as the sum of the following: a) interval CRCs during follow-up among those who were CRC-free at screening, b) CRCs diagnosed among patients who had uncertain CRC status at screening and did not comply with referral instructions (see Figure 1), and, c) CRCs found at screening in the cohort prorated to the population incidence rate (122/100,000 population aged over 50 years).
The denominator patients for the above CRCs are added to generate the SEER-comparable “CRC-free cohort”. Analysis-eligible cohort = 25,735 patients (25,008 CRC-free at screening + 695 patients of uncertain CRC status at screening, + 32 patients with CRC at screening (after prorating 159 CRCs to the general population incidence rate) –
Follow-up CRCs consisted of: 67 interval cancers among the CRC-free + 10 CRCs among uncertain status patients + 32 prorated CRCs = 109
PYO of 25,735 patients = 205,776
Step 2: Estimate the incidence-standardized lifetime CRC risk ratio for the cohort and % CRC prevention
Expected lifetime CRC risk at 58 years of age per SEER 18 = 4.71%
Baseline risk-standardized follow-up CRC rate of the study cohort over observed follow-up = (109/205,776) = 0.000529 per PYO or 0.0529% (annual risk per person per year)
CRC rate applied to calculate cohort’s estimated lifetime CRC risk: Annual risk multiplied by life expectancy at cohort mean age) = 0.0529 × 27.4 = 1.45%
Study subjects exchanged the SEER population’s lifetime risk of 4.71% for 1.45% by getting CLEAR colonoscopy screening.
Ratio of observed lifetime CRC risk to expected lifetime risk = 1.45/4.71 = 0.308 (standardized lifetime risk ratio for study subjects).
Reduction in lifetime CRC risk achieved = (4.71–1.45) = 3.26
CRC prevention rate = 3.26/4.71 = 69.2% prevention achieved by CLEAR colonoscopy screening relative to SEER-18 given ongoing US population-wide screening activities in 2007–09.
Appendix Note 1: Data linkage procedures to identify colorectal cancer cases and primary brain cancer deaths in the SC Central Cancer Registry databases
Incident CRC cases in the cohort were identified by linking the study cohort data with the South Carolina Central Cancer Registry cancer data for1996–2015 (to ensure exclusion of those with a pre-dating CRC diagnosis). We used LinkPlus®, a probabilistic record linkage program developed by the Centers for Diseases Control and Prevention to support data extraction from the National Program of Cancer Registries.23
Cases were matched on first and last name, social security number (SSN) and date of birth (DOB) as blocking variables, and race and sex as matching variables. LinkPlus® uses the Soundex phonetic coding of last and first names to generate a comprehensive list of perfect, approximate, partial, and remotely plausible matches. It uses a weighted algorithm (accommodating typographical errors and transposition of digits in SSNs and DOBs, and matching patients on race and sex) and calculates a probabilistic record linkage score, higher the score, better is the match. LinkPlus® recommends a cut-off score of 7.0–10.0, suggesting manual review of cases above the cut-off score to finalize cancer matches.23 For this study, however, we used a much more conservative cut-off score of 1.0, resulting in manual review of a large number of pairs with remote plausibility of being true matches. The score range for the study patients was 0.2 to 48 (48 being the perfect match). All pairs with scores of 30–48 were reviewed to confirm that they were indeed true matches. Pairs with linkage scores between 1 and 29.9 were subjected to in-depth manual review by the study PI and a research associate. The goal was to ensure capture of potential cancer cases despite typographical errors or differences in name or address (e.g. upon marriage). To determine match status, we used additional variables: middle name, sex, race, address, zip code. Manual review yielded no additional CRC match. The same process was used for deaths and primary brain cancer.
Appendix Note 2: Person-years of observation calculation for brain cancer standardized mortality ratio estimation:33
Person-years of observation (PYO) were calculated using brain cancer death as the primary censoring event, secondary censoring events being death from other cause and end of study period. Each patient’s PYO were aggregated into age-sex-race strata, and the corresponding brain cancer mortality rates applied to calculate total expected brain cancer deaths in the cohort. PYO were prorated to appropriate age strata when cusp birthdays occurred (e.g. 60th birthday).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Sudha Xirasagar, University of South Carolina.
Yuqi Wu, University of South Carolina.
Meng-han Tsai, California State University-Monterey Bay.
Jiajia Zhang, University of South Carolina.
Stephanie Chiodini, South Carolina Central Cancer Registry, SC Department of Health and Environmental Control.
Piet C. de Groen, University of Minnesota.
References
- 1.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA: a cancer journal for clinicians 2017;67:177–93. [DOI] [PubMed] [Google Scholar]
- 2.Brenner H, Kretschmann J, Stock C, Hoffmeister M. Expected long-term impact of screening endoscopy on colorectal cancer incidence: a modelling study. Oncotarget 2016;7:48168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. New England Journal of Medicine 1993;329:1977–81. [DOI] [PubMed] [Google Scholar]
- 4.Schatzkin A, Lanza E, Corle D, et al. Lack of effect of a low-fat, high-fiber diet on the recurrence of colorectal adenomas. New England Journal of Medicine 2000;342:1149–55. [DOI] [PubMed] [Google Scholar]
- 5.Schatzkin A, Lanza E, Freedman LS, et al. The polyp prevention trial I: rationale, design, recruitment, and baseline participant characteristics. Cancer Epidemiology and Prevention Biomarkers 1996;5:375–83. [PubMed] [Google Scholar]
- 6.Click B, Pinsky PF, Hickey T, Doroudi M, Schoen RE. Association of colonoscopy adenoma findings with long-term colorectal cancer incidence. Jama 2018;319:2021–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1997. CA: A cancer journal for clinicians 1997;47:5–27. [DOI] [PubMed] [Google Scholar]
- 8.Zauber AG, Lansdorp-Vogelaar I, Knudsen AB, Wilschut J, van Ballegooijen M, Kuntz KM. Evaluating test strategies for colorectal cancer screening—age to begin, age to stop, and timing of screening intervals. Evidence Synthesis, No. 65.2 2009 report No: 08–05124-EF-2. Agency for Healthcare Research and Quality, Rockville, MD. [PubMed] [Google Scholar]
- 9.Meester RG, Peterse EF, Knudsen AB, et al. Optimizing colorectal cancer screening by race and sex: microsimulation analysis II to inform the American Cancer Society colorectal cancer screening guideline. Cancer 2018;124:2974–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ladabaum UMA, Meester RGS, GUpta S, Schoen RE. Cost-effectiveness and National Effects of Initiating Colorectal Cancer Screening for Average-risk Persons at Age 45 Years Instead of 50 Years. Gastroenterology 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leung K, Pinsky P, Laiyemo AO, Lanza E, Schatzkin A, Schoen RE. Ongoing colorectal cancer risk despite surveillance colonoscopy: the Polyp Prevention Trial Continued Follow-up Study. Gastrointestinal endoscopy 2010;71:111–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishihara R, Wu K, Lochhead P, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. New England Journal of Medicine 2013;369:1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Groen PC. Advanced systems to assess colonoscopy. Gastrointestinal Endoscopy Clinics 2010;20:699–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rex DK, Cutler CS, Lemmel GT, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 1997;112:24–8. [DOI] [PubMed] [Google Scholar]
- 15.Van Rijn JC, Reitsma JB, Stoker J, Bossuyt PM, Van Deventer SJ, Dekker E. Polyp miss rate determined by tandem colonoscopy: a systematic review. The American journal of gastroenterology 2006;101:343. [DOI] [PubMed] [Google Scholar]
- 16.Duloy AM, Kaltenbach TR, Keswani RN. Assessing colon polypectomy competency and its association with established quality metrics. Gastrointestinal endoscopy 2018;87:635–44. [DOI] [PubMed] [Google Scholar]
- 17.Ferlitsch MMA, Hassan C et al. Colorectal polypectomy and endoscopic mucosal resection (EMR): European Society of Gastrointestinal Endoscopy (ESGE) Clinical Guideline. Endoscopy 2017;49:270–97. [DOI] [PubMed] [Google Scholar]
- 18.Pohl HSA, Bensen SP, Anderson P, Rothstein RI,, Gordon SR ea. Incomplete Polyp Resection During Colonoscopy—Results of the Complete Adenoma Resection (CARE) Study. Gastroenterology 2013;144:74–80. [DOI] [PubMed] [Google Scholar]
- 19.Moss ANK. Standardisation of polypectomy technique. Best Practice & Research Clinical Gastroenterology 2017;31:447–53. [DOI] [PubMed] [Google Scholar]
- 20.Barclay RL, Vicari JJ, Greenlaw RL. Effect of a time-dependent colonoscopic withdrawal protocol on adenoma detection during screening colonoscopy. Clinical Gastroenterology and Hepatology 2008;6:1091–8. [DOI] [PubMed] [Google Scholar]
- 21.Gupta S, Anderson J, Bhandari P, et al. Development and validation of a novel method for assessing competency in polypectomy: direct observation of polypectomy skills. Gastrointestinal endoscopy 2011;73:1232–9. e2. [DOI] [PubMed] [Google Scholar]
- 22.Patel K, Faiz O, Rutter M, Dunckley P, Thomas-Gibson S. The impact of the introduction of formalised polypectomy assessment on training in the UK. Frontline gastroenterology 2017;8:104–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walsh CM, Ling SC, Khanna N, et al. Gastrointestinal Endoscopy Competency Assessment Tool: development of a procedure-specific assessment tool for colonoscopy. Gastrointestinal endoscopy 2014;79:798–807. e5. [DOI] [PubMed] [Google Scholar]
- 24.Barton JR, Corbett S, van der Vleuten CP, Programme EBCS. The validity and reliability of a Direct Observation of Procedural Skills assessment tool: assessing colonoscopic skills of senior endoscopists. Gastrointestinal endoscopy 2012;75:591–7. [DOI] [PubMed] [Google Scholar]
- 25.Xirasagar S, Li YJ, Hurley TG, et al. Colorectal cancer prevention by an optimized colonoscopy protocol in routine practice. International journal of cancer 2015;136:E731–E42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.North American Association of Central Cancer Registries: NAACCR certification by registry and year, and Certification criteria. June 28, 2018. at https://www.naaccr.org/certified-registries/.)
- 27.Rex DK. Colonoscopic withdrawal technique is associated with adenoma miss rates. Gastrointestinal endoscopy 2000;51:33–6. [DOI] [PubMed] [Google Scholar]
- 28.Xirasagar S, Hurley TG, Sros L, Hebert JR. Quality and safety of screening colonoscopies performed by primary care physicians with standby specialist support. Medical care 2010;48:703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA: a cancer journal for clinicians 2008;58:130–60. [DOI] [PubMed] [Google Scholar]
- 30.Loeve AJ, Fockens P, Breedveld P. Mechanical analysis of insertion problems and pain during colonoscopy: why highly skill-dependent colonoscopy routines are necessary in the first place… and how they may be avoided. Canadian Journal of Gastroenterology and Hepatology 2013;27:293–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderson J. Colonoscopy: do operator motions and posture count? Endoscopy international open 2015;3:E627–E8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aslanian HR, Shieh FK, Chan FW, et al. Nurse observation during colonoscopy increases polyp detection: a randomized prospective study. The American journal of gastroenterology 2013;108:166. [DOI] [PubMed] [Google Scholar]
- 33.Liker JK. The 14 principles of the Toyota way: an executive summary of the culture behind TPS. The Toyota Way 2004;14:35–41. [Google Scholar]
- 34.Johnson DA, Barkun AN, Cohen LB, et al. Optimizing adequacy of bowel cleansing for colonoscopy: recommendations from the US multi-society task force on colorectal cancer. The American journal of gastroenterology 2014;109:1528. [DOI] [PubMed] [Google Scholar]
- 35.Corley DA, Jensen CD, Marks AR, et al. Adenoma detection rate and risk of colorectal cancer and death. New england journal of medicine 2014;370:1298–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zauber AG, Winawer SJ, O’brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. New England Journal of Medicine 2012;366:687–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rutter MD, Beintaris I, Valori R, et al. World Endoscopy Organization Consensus Statements on Post-Colonoscopy and Post-Imaging Colorectal Cancer. Gastroenterology 2018. [DOI] [PubMed] [Google Scholar]
- 38.Fay MP. Estimating age conditional probability of developing disease from surveillance data. Population Health Metrics 2004;2:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.SEER. Surveillance Research Program. SEER Cancer Statistics Review 1975–2009: Table 6.17: Lifetime Risk of Being Diagnosed with Cancer Given Alive and Cancer-free at Current age, 2007–2009. August 2011. https://seer.cancer.gov/csr/previous.html.
- 40.United States Life Tables, 2014. National Vital Statistics Reports; 66(4). August 14, 2017. (Accessed August 27, 2018, at https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_04.pdf.) [PubMed]
- 41.Siegel R, DeSantis C, Jemal A. Colorectal cancer statistics, 2014. CA: a cancer journal for clinicians 2014;64:104–17. [DOI] [PubMed] [Google Scholar]
- 42.Lee JK, Jensen CD, Levin TR, et al. Long-term Risk of Colorectal Cancer and Related Deaths After a Colonoscopy With Normal Findings. JAMA internal medicine 2019;179:153–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klabunde CN CK, Breen N, Waldron WR, Ambs AH, Nadel MR. Trends of colorectal cancer test use among vulnerable populations in the US.. Cancer Epidemiol Biomarkers Prev 2011;20:1611–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imperiale TF, Glowinski EA, Lin-Cooper C, Larkin GN, Rogge JD, Ransohoff DF. Five-year risk of colorectal neoplasia after negative screening colonoscopy. New England Journal of Medicine 2008;359:1218–24. [DOI] [PubMed] [Google Scholar]
- 45.Siegel RL. Colorectal Cancer Statistics, 2014. CA Cancer J Clin 2014;64:104–17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.