Abstract
BACKGROUND:
Microvascular decompression (MVD) is highly effective in managing the neuropathic facial pain of trigeminal neuralgia (TN). Its utility in patients with TN and concurrent multiple sclerosis (MS) has been a subject of debate. The goal of this study was to identify demographic and perioperative variables associated with favorable outcome after MVD over the past 20 years in patients from our institution.
METHODS:
A retrospective analysis of our cohort of 33 patients diagnosed with MS and TN who underwent MVD between 1997 and 2017 to treat neuropathic facial pain was performed. Perioperative variables included MS disease burden, findings on preoperative magnetic resonance imaging (MRI), TN pain severity, and the presence of intraoperative neurovascular compression. MS disease burden was quantified using the Expanded Disability Status Scale. Preoperative and postoperative pain severity was quantified using the Barrow Neurological Institute (BNI) pain severity scale.
RESULTS:
A total of 33 patients with TN and MS were treated with MVD at our institution (out of the 632 total MVDs performed) between 1997 and 2017. Twenty-two patients (67%) maintained a reduction in pain at a mean follow-up of 53.5 months. Higher preoperative BNI pain intensity score was associated with unfavorable outcome after MVD (P = 0.006). No associations were identified between MS disease burden, presence of neurovascular compression or pontine demyelinating plaques on MRI, or intraoperative findings of neurovascular compression and treatment outcomes.
CONCLUSIONS:
MVD is a reasonable treatment option for patients with TN and MS, although the rate of freedom from pain is lower than that for the general TN population. Preoperative pain severity may be a predictor of treatment success.
Keywords: Microvascular decompression, Multiple sclerosis, Trigeminal neuralgia
INTRODUCTION
Microvascular decompression (MVD) has had a profound impact on our management of patients with trigeminal neuralgia (TN).1,2 Typical TN (Burchiel classification type I or II) is characterized by sharp, shooting pain in the dermatomal distributions of the trigeminal nerve. TN is widely considered a result of vascular compression of the trigeminal nerve in the prepontine cistern, resulting in demyelination and pathological nerve conduction.3,4 Consistent with this proposed pathophysiological mechanism, MVD, which entails separation of the trigeminal nerve from the offending vascular structure through a small craniotomy, results in immediate and permanent relief of TN pain in roughly 90% of patients who undergo the procedure.5–8
A controversial clinical dilemma, however, has been the management of TN in patients with concurrent multiple sclerosis (MS), a relapsing and remitting autoimmune disease associated with the formation of demyelinating plaques along the white matter tracts of the brain and spinal cord.9–11 In patients with MS experiencing neuropathic facial pain, it may be unclear whether the pain is a sequela of MS plaques in the pons, which may affect trigeminal pathways but would not benefit from MVD, or of vascular compression in the prepontine cistern, which may benefit from surgical decompression.9,12,13 Reported rates of pain freedom in MS patients with TN range from 20% to 74% in patients treated with MVD, lower than those reported for the general TN population.9–11 Given these concerns, some neuro-surgeons will not offer MVD to patients with both TN and MS.9–11 However, withholding surgical treatment precludes offering potentially significant lasting relief to this vulnerable patient population.
Although rhizotomy through a variety of techniques is usually offered to patients with trigeminal neuralgia and MS, it is possible that the 2 diseases are independent and may not be directly related. Because MVD offers longer-lasting benefit (with 74% of patients remaining pain-free at 10 years), is less injurious to the nerve, and addresses the potential pathology, it is important to understand the predictors of successful MVD treatment in patients with MS.17 Fortunately, modern diagnostic tools, including high-resolution magnetic resonance imaging (MRI) and TN pain rating scales, improve the quality of preoperative information available in these patients and facilitate population studies.6,18–20 Our experience supports the notion that MVD in patients with MS in fact may be unrelated to the presence or severity of the MS in these patients and may respond to MVD and padding of the nerve. Therefore, the goal of this study was to perform a quantitative analysis of demographic and preoperative variables, with emphasis on MS disease burden, MRI findings, and pain severity, in an attempt to identify predictors of treatment success in patients with MS and TN treated with MVD at our institution. Moreover, given the paucity of the literature on these concurrent diseases, this article aims to guide further treatment options by reporting MVD outcomes to date in a 33-patient cohort with TN and MS to date.10
METHODS
Patient Selection
For this retrospective longitudinal cohort study, we reviewed the medical records of 542 consecutive MVD procedures at Vanderbilt University Medical Center between 1999 and 2017. Individuals with a diagnosis of MS who underwent MVD to treat TN were included in the study, a cohort comprising 33 patients. The perioperative period was defined as the first 30 days after surgery. The study duration for each patient ended with his or her last follow-up with a neurosurgeon or neurologist (no fixed study duration). The Vanderbilt University Medical Center Institutional Review Board approved all components of this study.
Operative Technique
Our MVD technique for TN has been described in detail previously.21,22 In brief, a 3-cm retrosigmoid craniotomy was performed with the patient in a park bench position. Using a surgical microscope, the trigeminal nerve was identified, circumferentially isolated from surrounding vessels and pia, and padded by 1 or more Teflon pads. A cranioplasty with methyl methacrylate was then performed. Brainstem auditory evoked responses were monitored throughout all cases to reduce the likelihood of hearing deficits following surgery. Following surgery, patients were instructed to maintain head elevation of at least 60° for 6 days to avoid cerebrospinal fluid leak. Patients were typically discharged 2–3 days following surgery on a 7- to 10-day tapering dose of steroids and returned to the clinic by 1 month for follow-up.
Data Collection
Preoperative data were obtained from a review of the electronic medical record and preoperative imaging. Demographic data collected included patient age, sex, and weight. Details of each patient’s MS history included disease subtype, duration since diagnosis, and symptom severity on the Expanded Disability Status Scale (EDSS). The EDSS is a widely used method to assess MS symptomatology, functioning, and quality of life that quantifies the level of disability in individuals with MS by integrating 8 functional systems: pyramidal, cerebellar, brainstem, sensory, bowel and bladder, visual, cerebral function, and other. The EDSS ranges from 0 to 10, with a score of 0 indicating no disability and 10 indicates death due to MS.23 TN disease variables included degree of pain intensity measured using the Barrow Neurological Institute (BNI) rating scale, pain frequency, perceived impact on quality of life, number of TN medications previously tried, TN medication regimen at the time of surgery, duration of TN symptoms, and previous TN procedures. The BNI pain scale is a composite of subjective pain rating and medication use, with scores ranging from no medication and no pain (I) to severe pain not responsive to medications (V). Generally, scores of I–III are considered a favorable pain outcome and scores of IV and V are considered unfavorable.18,24 Preoperative MRI images were reviewed for evidence of plaques near the trigeminal nucleus or compressive vessels along the trigeminal nerve. Fast imaging employing steady-state acquisition (FIESTA) sequence MRIs were obtained for 30 of the 33 patients. Intraoperative data recorded included whether a vessel was observed in contact with cranial nerve (CN) V, which vessel (presumed by the surgeon), whether a Teflon pad was placed, and the occurrence of operative complications.
Postoperative data included hospital length of stay, presence of neurologic deficits, pain level, pain medication use after the procedure, late complications, subsequent TN procedures, and pain level after subsequent TN procedures. Pain outcome was determined at the most recent follow-up visit by a neurologist or neurosurgeon using the BNI pain rating scale.18,24
Statistical Analysis
To analyze factors associated with favorable or unfavorable pain outcome after MVD, we used Fisher’s exact test for categorical variables and the Student t test for continuous variables. Before parametric testing, normality of the data was verified using the Levene test for equality of variance. Statistical significance was assessed at P < 0.05. The false discovery rate was set at 0.1. All statistical analyses were performed using SPSS version 25 (IBM, Armonk, New York, USA).
RESULTS
Demographics
Of the 33 patients with MS who received MVD to treat TN at our institution, 81.8% were female, with a mean ± SEM age of 53.2 ± 2.1 years. The mean duration of postoperative follow-up was 53.5 ± 9.2 months. The mean duration of diagnosis with MS and TN before surgery was 11.8 ± 1.6 years and 6.6 ± 1.0 years, respectively. At the time of surgery, patients were taking a median of 1 medication for their MS and had trialed a median of 3 medications for their TN before surgery. Five patients (15%) had undergone a previous TN procedure, including previous MVD in 2 patients, radiofrequency rhizotomy in 2 patients, and balloon rhizotomy in 1 patient (Table 1).
Table 1.
Demographic and Perioperative Patient Characteristics
| Characteristic | Value |
|---|---|
| Age at surgery (years), mean ± SEM | 53.1 ± 2.1 |
| Female sex (%) | 81.8 |
| First TN surgery (%) | 84.8 |
| Disease duration of MS (years), mean ± SEM | 11.8 ±1.6 |
| Disease duration of TN (years), mean ± SEM | 6.6 ± 1.04 |
| Teflon pad placement (%) | 97 |
| Operative findings (%) | |
| Compressive artery | 75.8 |
| Compressive vein | 21.2 |
| Other | 3.0 |
| Perioperative complications (%) | 21.2 |
TN, trigeminal neuralgia; MS, multiple sclerosis.
MS Clinical and Imaging Features
The most common subtype of MS in our cohort was secondary progressive MS, seen in 11 patients (33%), followed by relapsing-remitting MS in 10 patients (30%), and 7 (21%) with primary progressive MS. Five patients (15%) did not have a listed MS subtype. The daily impact of MS on patients’ daily lives varied. Our cohort had a mean score of a 4.3 ± 2.7 on the EDSS (range, 1.0–8.5), indicating that patients had significant disability but were able to complete their activities of daily living without limitations and could walk without aid for 300–500 meters.
Of the 30 FIESTA sequence MRI scans performed, 26 (78.8%) showed MS plaques. No scans showed plaques in the vicinity of the trigeminal nerve root or the trigeminal pathways in the pons.
Preoperative Pain Severity and Imaging Features
TN pain was most commonly located in the V2 and V3 distributions (n = 12). Other pain distributions were V3 (n = 9), V2 (n = 6), V1 and V2 (n = 2), and V1–V3 (n = 4). Patients most commonly reported multiple pain episodes daily (II patients; 33%). Eight patients (24%) reported 1 episode daily. Five (15%) had continuous pain. Five (15%) reported weekly episodes, and 4 (12%) had fewer than 1 episode per week. The mean preoperative BNI pain intensity score was 3.9 ± 0.6. The findings on MRI read by an independent attending neuroradiologist indicated the presence of a suspicious vessel or mass in 13 scans (39%).
Intraoperative Findings and Postoperative Complications
A compressive artery was in contact with the trigeminal nerve in 25 patients (75.8%). The most commonly associated artery was the superior cerebellar artery (66.6%). A compressive vein was found in 8 patients (24.2%). Prominent arachnoid adhesions with no offending vessel were found in 1 patient. Teflon pads were placed between the vessel and the nerve in all but 1 of the patients, in whom a vein compressing the trigeminal nerve was coagulated and divided.
During the perioperative period, 24 patients progressed without complications. Complications in the remaining 9 patients included aseptic meningitis in 2 (6%), cerebrospinal fluid leak from the incision in 2 (6%), and pseudomeningocele in 5 (15%). Two patients (6%) ultimately required a lumbar drain followed by ventriculoperitoneal shunt. There were no infections, new neurologic deficits, or perioperative mortality.
Postoperative Pain Severity and Predictors of Treatment Success
As of the latest post-operative follow-up after MVD, 8 patients (24%) were completely pain free and did not require medication (BNI I), 1 patient (3%) had occasional TN pain, but did not require medication (BNI II), and 13 patients (39%) had residual pain that was adequately managed with medication (BNI III). Otherwise, 4 patients (12.1%) still had TN pain that was not controlled with medications (BNI IV), and 7 (21.2%) received no relief from surgery (BNI V). Overall, 67% of the patients with MS (n = 22) reported some degree of pain relief (BNI I–III outcome) from MVD (Table 2, Figure 1).
Table 2.
Qualitative Subcategorization of Pain Outcomes
| Postsurgical Pain Outcome | Number of Patients (%) |
|---|---|
| No improvement | 11 (33) |
| Slight improvement in pain | 3 (9) |
| Dramatic improvement in pain | 6 (18) |
| Pain free | 13 (40) |
Twenty-two patients (67%) experienced some degree of pain relief from MVD.
Figure 1.
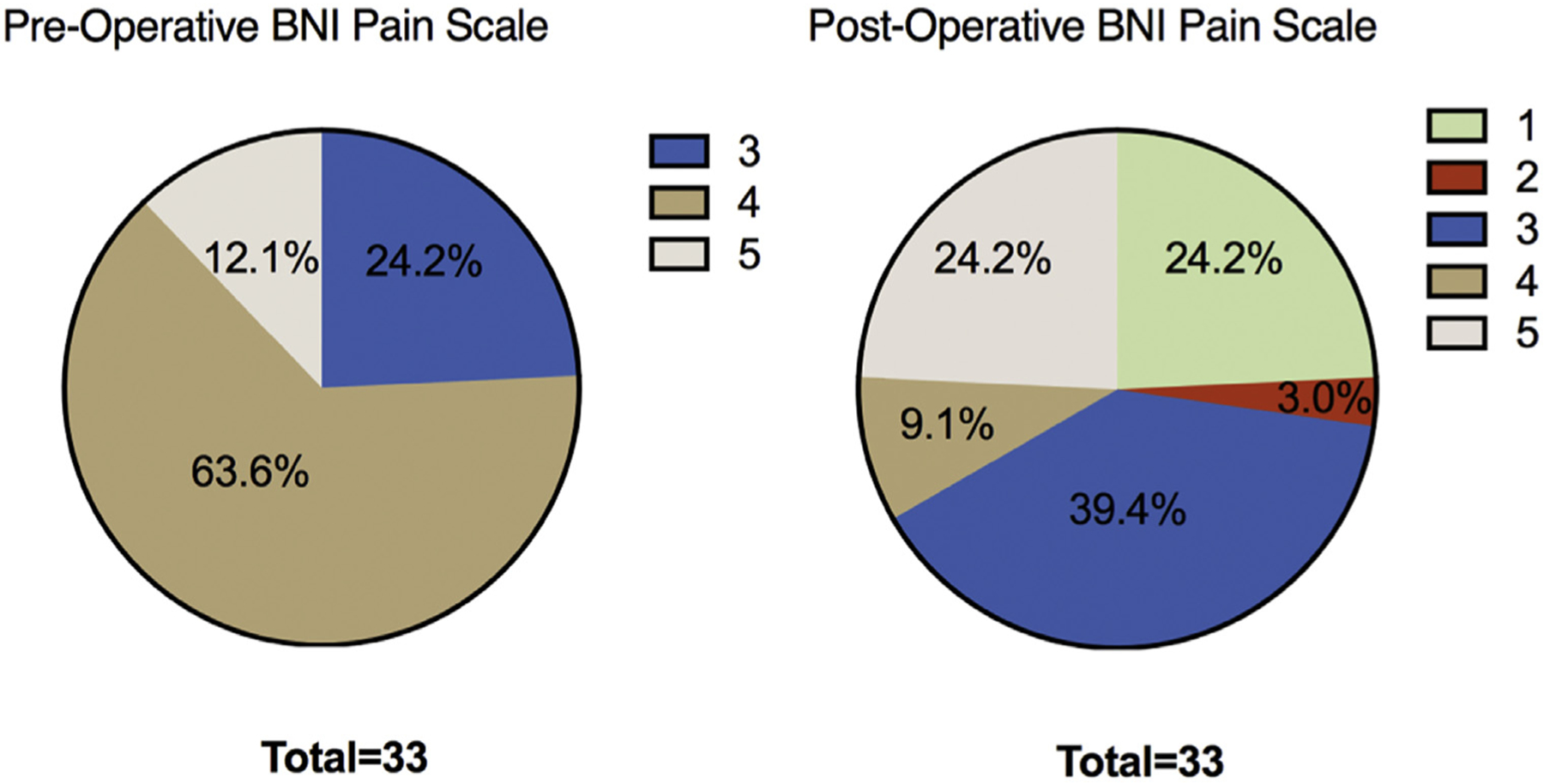
Pie charts comparing preoperative and postoperative BNI pain scores (I–V), depicted as percentage of the total cohort (n = 33) for each score.
When preoperative variables were compared between patients with favorable pain outcome (improvement in pain and reduction in TN medication; BNI I–III) and those with unfavorable pain outcome (no improvement in pain; BNI IV or V), univariate analysis showed an association between preoperative BNI pain scores and treatment success. Specifically, higher preoperative BNI score was associated with lower postoperative improvement in BNI score (P = 0.006). Each patient in the cohort that rated their pain at the highest severity level of V had a poor surgical outcome with little to no benefit from surgery. To rule out the possibility that follow-up time was a confounding variable contributing to this statistically significant association, we next examined whether follow-up time was different for patients with favorable versus less favorable outcome, however, no statistically significant association was observed between the groups (t = 0.96; P = 0.16). In addition, sex, MS subtype, the presence of MS plaques or vessel compression on MRI, and nerve segment pain distribution were not associated with postoperative pain outcome (Table 3).
Table 3.
Fisher Exact Analysis of Perioperative Predictors of Treatment Success
| Variables | Favorable Pain Outcome | Unfavorable Pain Outcome | P Value* |
|---|---|---|---|
| Continuous variables, mean ± SEM | |||
| Age at surgery (years) | 54.6 ± 2.5 | 50.27 ± 4.1 | 0.38 |
| Duration of TN (years) | 7.5 ± 1.5 | 4.9 ± 0.9 | 0.25 |
| Duration of MS (years) | 13.81 ± 1.9 | 8.1 ± 2.6 | 0.16 |
| MS medications at surgery (number) | 2.1 ± 0.2 | 2.27 ± 0.30 | 0.64 |
| Categorical variables, number (%) | |||
| Sex | |||
| Male | 5 (83.3) | 1 (16.7) | 0.64 |
| Female | 17 (63.0) | 10 (37.0) | |
| MS subtype | |||
| RRMS | 6 (60.0) | 4 (40.0) | 0.42 |
| SPMS | 9 (81.8) | 2 (18.2) | |
| PPMS | 5 (71.4) | 2 (28.6) | |
| Not listed | 2 (40.0) | 3 (60.0) | |
| MS MRI findings | |||
| Normal | 1 (33.3) | 2 (66.7) | 0.69 |
| Plaques, but not near CN V nucleus | 19 (69.2) | 8 (30.8) | |
| Plaques near CN V nucleus | 0 (0.0) | 0 (0.0) | |
| MRI unobtainable | 2 (66.7) | 1 (33.3) | |
| MRI vessel findings | |||
| No suspicious vessel | 9 (52.9) | 8 (47.1) | 0.56 |
| Yes suspicious vessel | 9 (75.0) | 3 (25.0) | |
| Possible suspicious vessel | 1 (100.0) | 0 (0.0) | |
| Missing | 0 (100.0) | 0 (0.0) | |
| Preoperative BNI score | |||
| I | 0 (0.0) | 0 (0.0) | 0.006† |
| II | 0 (0.0) | 0 (0.0) | |
| III | 5 (62.5) | 3 (37.5) | |
| IV | 17 (91.0) | 4 (19.0) | |
| V | 0 (0.0) | 4 (100.0) | |
| Nerve segment distribution | |||
| V2 | 4 (66.7) | 2 (33.3) | 0.95 |
| V3 | 6 (66.7) | 3 (33.3) | |
| V1 and V2 | 2 (100.0) | 0 (0.0) | |
| V2 and V3 | 8 (66.7) | 4 (33.3) | |
| V1, V2, and V3 | 2 (50.0) | 2 (50.0) |
Continuous and categorical variables analyzed for association with successful pain reduction. A lower preoperative pain score was significantly associated with greater pain reduction from MVD.
EDSS, Expanded Disability Status Scale; MS, multiple sclerosis; TN, trigeminal neuralgia; PPMS, primary progressive multiple sclerosis; RRMS, relapsing remitting multiple sclerosis; SPMS, secondary progressive multiple sclerosis; CN, cranial nerve; MRI, magnetic resonance imaging; BNI, barrow neurological institute.
Due to multiple comparisons, P values were corrected at a false discovery rate of 0.1.
Statistically significant at P < 0.01.
DISCUSSION
The controversy over whether to offer MVD in MS patients with TN stems largely from uncertainty of the pathophysiological mechanisms involved and limitations of current diagnostic methods. Nevertheless, here we show that a majority of these patients could benefit from MVD, although the rate of favorable outcome may be somewhat lower than that in patients with TN only. We suggest that the severity of facial pain on the BNI scale may be a prognostic indicator of treatment success, although validation of this in larger trials is ultimately required.
Previous reports of MVD in patients with TN and MS show varying degrees of treatment efficacy. Ariai et al.25 reported favorable initial outcomes in 80% of patients, which decreased to 15% at 2 years. Broggi et al.10 found favorable outcomes in 61% of patients at 44 months, and Sandell and Eide26 reported demonstrated favorable outcomes in 74% of patients at 55 months. The discrepancies among these studies likely stem from differences in patient selection; for example, whereas the present study did not include patients with ipsilateral MS plaques involving the pons, all patients in the study of Arial et al.25 did. Furthermore, more palliative surgical treatments for TN, such as percutaneous rhizotomy or radiosurgical approaches, can be considered in this patient population as well, either as an initial treatment or in cases of MVD failure.25,27,28 Arial et al.25 reported that 4 of 5 MS patients who failed MVD were pain-free following glycerol rhizotomy, radio-surgery, or balloon compression.
Collectively, the literature on treatment of TN in patients with MS suggests that perhaps the pathophysiological mechanisms leading to their neuropathic facial pain cannot be simply dichotomized into patients with vascular compression and those with pontine plaques. Another possibility is that the pathology at the trigeminal nerve or pontine trigeminal pathways caused by MS results in a vulnerable trigeminal system susceptible to peripheral insult. A likely cause of this vulnerability is inflammation leading to demyelination in the proximal trigeminal nerve or pontine trigeminal pathways, which has been shown in histological specimens obtained during surgery and at autopsy.25,29,30 Consistent with this, TN symptoms are reportedly 20-fold greater in patients with MS. In addition, although not observed in the present study, previous reports have found a higher incidence of trigeminal nerve compression by veins (which likely causes less compressive trauma than compression by arteries) than is typically reported for in patients without MS with TN.6,7,26 A double-insult mechanism for TN in patients with MS was proposed by Truini et al.31 after identifying the co-occurrence of neurovascular compression and demyelinating plaques, to greater degree ipsilateral versus contralateral to facial pain, in patients with MS and TN.
When selecting patients with MS and TN that may be amenable to MVD, careful consideration of both clinical and diagnostic factors is required. In general, TN pain from microvascular compression is unilateral, episodic, shock-like pain that may be triggered by external stimulation, whereas facial pain associated with MS plaques is often atypical and constant, associated with burning, numbness, and paresthesias. However, frequently these pain phenotypes are difficult to distinguish, and a favorable response to antiepileptic medications may be seen irrespective of etiology of facial pain. The presence of ipsilateral plaques in the pons is likely associated with worse outcome, although freedom from pain can be achieved in these patients as well.10,25 Here we show that the most severe pain phenotypes are less responsive to MVD. Whether this is because these patients have a significant component of neuropathic facial pain may be difficult to quantify when patients have multiple pain components (e.g., burning and lancinating, triggering events on top of background pain state) before surgery. Nevertheless, patients with severe, unremitting pain should be approached with caution during preoperative evaluation for MVD.
The utility of MRI in the diagnosis of microvascular compression and its relevance to outcomes after MVD is controversial, owing to the limited spatial resolution of MRI; however, an association between microvascular compression and TN has been identified in recent studies.20,22,26,31 It is likely that as MRI techniques evolve, our ability to identify clinically significant microvascular compression will improve. Diffusion tensor imaging, an MRI technique that permits in vivo visualization of white matter tracts, could be of particular benefit to MS patients with TN. A recent study in patients with idiopathic TN demonstrated that preoperative diffusion tensor imaging is highly predictive of response to MVD by distinguishing central and peripheral sources of pain.32
Limitations of the present study include most notably its small cohort size and retrospective nature. In addition, our analysis was limited to patients who underwent MVD at our institution and did not examine patients with MS experiencing facial pain who either underwent percutaneous surgery or did not undergo surgery at all. Therefore, our data do not reflect the majority of patients with MS with TN symptoms who present for neurosurgical evaluation.
CONCLUSIONS
Careful patient selection is critical when considering MVD for the treatment of TN in the setting of MS. In patients who do not have radiographic evidence of plaque formation near the trigeminal nucleus, trigeminal pain can be a result of vascular compression, mutually exclusive of demyelination, which would benefit from decompression. A majority of patients in this study were pain free following MVD, suggesting a peripheral component to their facial pain. However, outcomes appear less favorable than with MVD for TN reported across the literature, suggesting that this subset of patients should be approached cautiously, with careful evaluation of symptomatology and MRI results, and counseled appropriately regarding expected outcomes. As our diagnostic capabilities evolve, we expect patient selection to improve, maximizing the chance of achieving freedom from pain in this severely debilitated patient population.
Abbreviations and Acronyms
- BNI
Barrow Neurological Institute
- EDSS
Expanded Disability Status Scale
- FIESTA
Fast imaging employing steady-state acquisition
- MRI
Magnetic resonance imaging
- MS
Multiple sclerosis
- MVD
Microvascular decompression
- TN
Trigeminal neuralgia
Footnotes
Conflict of interest statement: The authors declare that the article content was composed in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
REFERENCES
- 1.Yadav YR, Nishtha Y, Sonjjay P, Vijay P, Shailendra R, Yatin K. Trigeminal neuralgia. Asian J Neurosurg. 2017;12:585–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maarbjerg S, Di Stefano G, Bendtsen L, Cruccu G. Trigeminal neuralgia - diagnosis and treatment. Cephalalgia. 2017;37:648–657. [DOI] [PubMed] [Google Scholar]
- 3.Burchiel KJ. A new classification for facial pain. Neurosurgery. 2003;53:1164–1166 [discussion: 1166–1167]. [DOI] [PubMed] [Google Scholar]
- 4.Devor M, Amir R, Rappaport ZH. Pathophysiology of trigeminal neuralgia: the ignition hypothesis. Clin J Pain. 2002;18:4–13. [DOI] [PubMed] [Google Scholar]
- 5.Jannetta PJ, McLaughlin MR, Casey KF. Technique of microvascular decompression. Technical note. Neurosurg Focus. 2005;18:E5. [PubMed] [Google Scholar]
- 6.Holste K, Chan AY, Rolston JD, Englot DJ. Pain outcomes following microvascular decompression for drug-resistant trigeminal neuralgia: a systematic review and meta-analysis [e-pub ahead of print]. Neurosurgery. 2019. 10.1093/neuros/nyz075. Accessed XXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tronnier VM, Rasche D, Hamer J, Kienle AL, Kunze S. Treatment of idiopathic trigeminal neuralgia: comparison of long-term outcome after radiofrequency rhizotomy and microvascular decompression. Neurosurgery. 2001;48:1261–1267 [discussion: 1267–1268]. [PubMed] [Google Scholar]
- 8.Love S, Hilton DA, Coakham HB. Central demyelination of the Vth nerve root in trigeminal neuralgia associated with vascular compression. Brain Pathol. 1998;8:1–11 [discussion: 11–12]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zakrzewska JM, Wu J, Brathwaite TS. A systematic review of the management of trigeminal neuralgia in patients with multiple sclerosis. World Neurosurg. 2018;111:291–306. [DOI] [PubMed] [Google Scholar]
- 10.Broggi G, Ferroli P, Franzini A, et al. Operative findings and outcomes of microvascular decompression for trigeminal neuralgia in 35 patients affected by multiple sclerosis. Neurosurgery. 2004; 55:830–838 [discussion: 838–839]. [PubMed] [Google Scholar]
- 11.Crooks DA, Miles JB. Trigeminal neuralgia due to vascular compression in multiple sclerosis–postmortem findings. Br J Neurosurg. 1996;10:85–88. [DOI] [PubMed] [Google Scholar]
- 12.Gass A, Kitchen N, MacManus DG, Moseley IF, Hennerici MG, Miller DH. Trigeminal neuralgia in patients with multiple sclerosis: lesion localization with magnetic resonance imaging. Neurology. 1997; 49:1142–1144. [DOI] [PubMed] [Google Scholar]
- 13.Brisman R Trigeminal neuralgia and multiple sclerosis. Arch Neurol. 1987;44:379–381. [DOI] [PubMed] [Google Scholar]
- 14.Liang X, Dong X, Zhao S, Ying X, Du Y, Yu W. A retrospective study of neurocombing for the treatment of trigeminal neuralgia without neurovascular compression. Ir J Med Sci. 2017;186: 1033–1039. [DOI] [PubMed] [Google Scholar]
- 15.Zhou X, Liu Y, Yue Z, Luan D, Zhang H, Han J. Comparison of nerve combing and percutaneous radiofrequency thermocoagulation in the treatment for idiopathic trigeminal neuralgia. Braz J Otorhinolaryngol. 2016;82:574–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo H, Song G, Wang X, Bao Y. Surgical treatment of trigeminal neuralgia with no neurovascular compression: a retrospective study and literature review. J Clin Neurosci. 2018;58:42–48. [DOI] [PubMed] [Google Scholar]
- 17.Barker FG 2nd, Jannetta PJ, Bissonette DJ, Larkins MV, Jho HD. The long-term outcome of microvascular decompression for trigeminal neuralgia. N Engl J Med. 1996;334:1077–1083. [DOI] [PubMed] [Google Scholar]
- 18.Chen HI, Lee JY. The measurement of pain in patients with trigeminal neuralgia. Clin Neurosurg. 2010;57:129–133. [PubMed] [Google Scholar]
- 19.Noble DJ, Scoffings D, Ajithkumar T, Williams MV, Jefferies SJ. Fast imaging employing steady-state acquisition (FIESTA) MRI to investigate cerebrospinal fluid (CSF) within dural reflections of posterior fossa cranial nerves. Br J Radiol. 2016;89:20160392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leal PR, Hermier M, Souza MA, Cristino-Filho G, Froment JC, Sindou M. Visualization of vascular compression of the trigeminal nerve with high-resolution 3T MRI: a prospective study comparing preoperative imaging analysis to surgical findings in 40 consecutive patients who underwent microvascular decompression for trigeminal neuralgia. Neurosurgery. 2011;69:15–25 [discussion: 26]. [DOI] [PubMed] [Google Scholar]
- 21.Forbes J, Cooper C, Jermakowicz W, Neimat J, Konrad P. Microvascular decompression: salient surgical principles and technical nuances. J Vis Exp. 2011:e2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mistry AM, Niesner KJ, Lake WB, et al. Neurovascular compression at the root entry zone correlates with trigeminal neuralgia and early microvascular decompression outcome. World Neurosurg. 2016;95:208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology. 1983;33:1444–1452. [DOI] [PubMed] [Google Scholar]
- 24.Kumar S, Rastogi S, Kumar S, Mahendra P, Bansal M, Chandra L. Pain in trigeminal neuralgia: neurophysiology and measurement: a comprehensive review. J Med Life. 2013;6:383–388. [PMC free article] [PubMed] [Google Scholar]
- 25.Ariai MS, Mallory GW, Pollock BE. Outcomes after microvascular decompression for patients with trigeminal neuralgia and suspected multiple sclerosis. World Neurosurg. 2014;81:599–603. [DOI] [PubMed] [Google Scholar]
- 26.Sandell T, Eide PK. The effect of microvascular decompression in patients with multiple sclerosis and trigeminal neuralgia. Neurosurgery. 2010;67: 749–753 [discussion: 753–754]. [DOI] [PubMed] [Google Scholar]
- 27.Alvarez-Pinzon AM, Wolf AL, Swedberg HN, et al. Comparison of percutaneous retrogasserian balloon compression and gamma knife radio-surgery for the treatment of trigeminal neuralgia in multiple sclerosis. World Neurosurg. 2017;97: 590–594. [DOI] [PubMed] [Google Scholar]
- 28.Przybylowski CJ, Cole TS, Baranoski JF, Little AS, Smith KA, Shetter AG. Radiosurgery for multiple sclerosis-related trigeminal neuralgia: retrospective review of long-term outcomes. J Neurosurg. 2018:1–8. [DOI] [PubMed] [Google Scholar]
- 29.Olafson RA, Rushton JG, Sayre GP. Trigeminal neuralgia in a patient with multiple sclerosis. An autopsy report. J Neurosurg. 1966;24:755–759. [DOI] [PubMed] [Google Scholar]
- 30.Lazar ML, Kirkpatrick JB. Trigeminal neuralgia and multiple sclerosis: demonstration of the plaque in an operative case. Neurosurgery. 1979;5: 711–717. [DOI] [PubMed] [Google Scholar]
- 31.Truini A, Prosperini L, Calistri V, et al. A dual concurrent mechanism explains trigeminal neuralgia in patients with multiple sclerosis. Neurology. 2016;86:2094–2099. [DOI] [PubMed] [Google Scholar]
- 32.Hung PS, Chen DQ, Davis KD, Zhong J, Hodaie M. Predicting pain relief: use of presurgical trigeminal nerve diffusion metrics in trigeminal neuralgia. Neuroimage Clin. 2017;15: 710–718. [DOI] [PMC free article] [PubMed] [Google Scholar]


