Abstract
Objective:
To characterize behavior of 2-year-old children based on the severity of bronchopulmonary dysplasia (BPD).
Study design:
We studied children born at 22–26 weeks’ gestation and assessed at 22–26 months’ corrected age with the Child Behavior Checklist (CBCL). BPD was classified by level of respiratory support at 36 weeks’ postmenstrual age. CBCL syndrome scales were the primary outcomes. The relationship between BPD grade and behavior was evaluated, adjusting for perinatal confounders. Mediation analysis was performed to evaluate whether cognitive, language, or motor skills mediated the effect of BPD grade on behavior.
Results:
Of 2310 children, 1208 (52%) had no BPD, 806 (35%) had grade 1 BPD, 177 (8%) had grade 2 BPD, and 119 (5%) had grade 3 BPD. Withdrawn behavior (P<.001) and pervasive developmental problems (P<.001) increased with worsening BPD grade. Sleep problems (P=.008) and aggressive behavior (P=.023) decreased with worsening BPD grade. Children with grade 3 BPD scored 2 points worse for withdrawn behavior and pervasive developmental problems and 2 points better for externalizing problems, sleep problems, and aggressive behavior than children without BPD. Cognitive, language, and motor skills mediated the effect of BPD grade on the attention problems, emotionally reactive, somatic complaints, and withdrawn CBCL syndrome scales (Ps<.05).
Conclusions:
BPD grade was associated with increased risk of withdrawn behavior and pervasive developmental problems but with decreased risk of sleep problems and aggressive behavior. The relationship between BPD and behavior is complex. Cognitive, language, and motor skills mediate the effects of BPD grade on some problem behaviors.
Keywords: BPD, premature infants, Child Behavior Checklist, Bayley Scales of Infant and Toddler Development, cognitive, language, motor
Behavioral challenges are among the developmental sequelae of extremely preterm birth. Early childhood behavioral outcomes may be determinants of functional attainment, independence, and mental health later in life. Extremely preterm children have more difficulties with internalizing behaviors (i.e., anxiety and affective disorders) and attention problems than full term peers.1,2 Inattention, anxiety, and social problems occur in more than 20% of extremely babies and were recently characterized as a ‘preterm behavioral phenotype’.3,4
Bronchopulmonary dysplasia (BPD) is a major pulmonary morbidity affecting nearly half of extremely preterm infants.5 The diagnosis of BPD is associated with adverse neurodevelopmental outcomes above and beyond those anticipated with extreme prematurity.6–9 Yet we do not fully understand how BPD severity impacts behavioral outcomes. Protracted duration of mechanical ventilation is associated with increased risk of neurodevelopmental impairment.10 Short et al demonstrated that children with severe BPD performed more poorly on mental and psychomotor developmental indices as well as language measures at 3 years compared with children with mild BPD.11 In the Extremely Low Gestational Age Newborns (ELGAN) study, children with the most severe BPD performed the worst on cognitive and executive function assessments at 10 years.12
Our purpose was to better understand how BPD, across the severity spectrum, is related to behavior. We used a recent classification of BPD severity more predictive of late death or serious respiratory morbidity13 than the NIH Consensus definition.14 We aimed to describe how BPD grade relates to behavior at 2 years’ corrected age and to evaluate whether language, motor, or cognitive skills mediate the effect of BPD grade on problem behaviors.
Methods
We performed a secondary analysis of a prospective cohort from the NICHD Neonatal Research Network (NRN) premature infant registry and follow-up database. The sample includes children born before 27 weeks’ gestation and cared for at NRN centers between July 2012 and February 2016 for whom a primary caregiver completed the Child Behavior Checklist (CBCL)15 at 22–26 months’ corrected age. Children with major congenital anomalies or syndromes known to affect development were excluded. Children with birth weight less than the 10th centile for gestational age were considered small for gestational age (SGA).16 Intracranial hemorrhage (ICH) was determined for children who had cranial sonography performed within 28 days of birth, with findings classified by Papile criteria.17 Severe retinopathy of prematurity (ROP) was defined as having undergone ophthalmologic intervention for ROP or having retinal detachment. BPD status was categorized according to level of respiratory support at 36 weeks’ postmenstrual age (PMA), irrespective of oxygen therapy: no BPD (no respiratory support including supplemental oxygen), grade 1 (nasal cannula ≤2 L/min), grade 2 (nasal cannula >2 L/min or noninvasive positive airway pressure), and grade 3 (invasive mechanical ventilation).13 Children participated in a comprehensive follow-up evaluation at 22–26 months’ corrected age, which included administration of the Bayley Scales of Infant and Toddler Development, 3rd Edition (Bayley-III)18 and the CBCL. The CBCL consists of 100 behavior-related questions for which the primary caregiver rates each problem behavior on a three-point scale that produces a T-score as its standard score. It is broken down into seven syndrome scales. The CBCL produces an internalizing problems score (composed of four syndrome scales: emotionally reactive, anxious/depressed, somatic complaints, withdrawn behavior), an externalizing problems score (composed of two syndrome scales: attention problems, aggressive behavior), a total problems score, and five DSM-oriented scales (scales oriented to the classifications of the Diagnostic and Statistical Manual of Mental Disorders of the American Psychiatric Association). The CBCL syndrome scales were the primary outcomes of interest; the CBCL problem scores, DSM-oriented scales, and Bayley-III composite scores were among the secondary outcomes.
Statistical Analyses
To characterize the study sample, bivariate comparisons were made by BPD status for maternal and neonatal characteristics, neonatal therapies and morbidities, and 2-year neurosensory outcomes, including the Bayley-III composite scores (cognitive, language, and motor). Comparisons were made using chi-square tests for categorical variables and ANOVA for continuous variables. To control for type 1 error, an overall effect across all BPD grades was first tested, and if significant, pairwise comparisons between individual BPD grades were conducted after adjusting for multiple comparisons using the Holm correction for categorical variables and the Tukey method for continuous variables
To evaluate whether children with more severe BPD have more behavioral difficulties, bivariate analyses using ANOVA tests were first conducted to compare mean scores on the CBCL syndrome scales and other CBCL scores by BPD grade, followed by pairwise comparisons among individual BPD grades using Tukey adjustment for multiple comparisons. To determine if BPD grade differences remained after controlling for other factors, linear mixed effect regression models were fit using SAS PROC MIXED to compare CBCL scores by BPD grade, including center as random factor to account for clustering of participants by center and controlling for sex, gestational age, SGA, race, Hispanic ethnicity, maternal education, insurance type, grade III/IV ICH, severe ROP, and postnatal steroids.
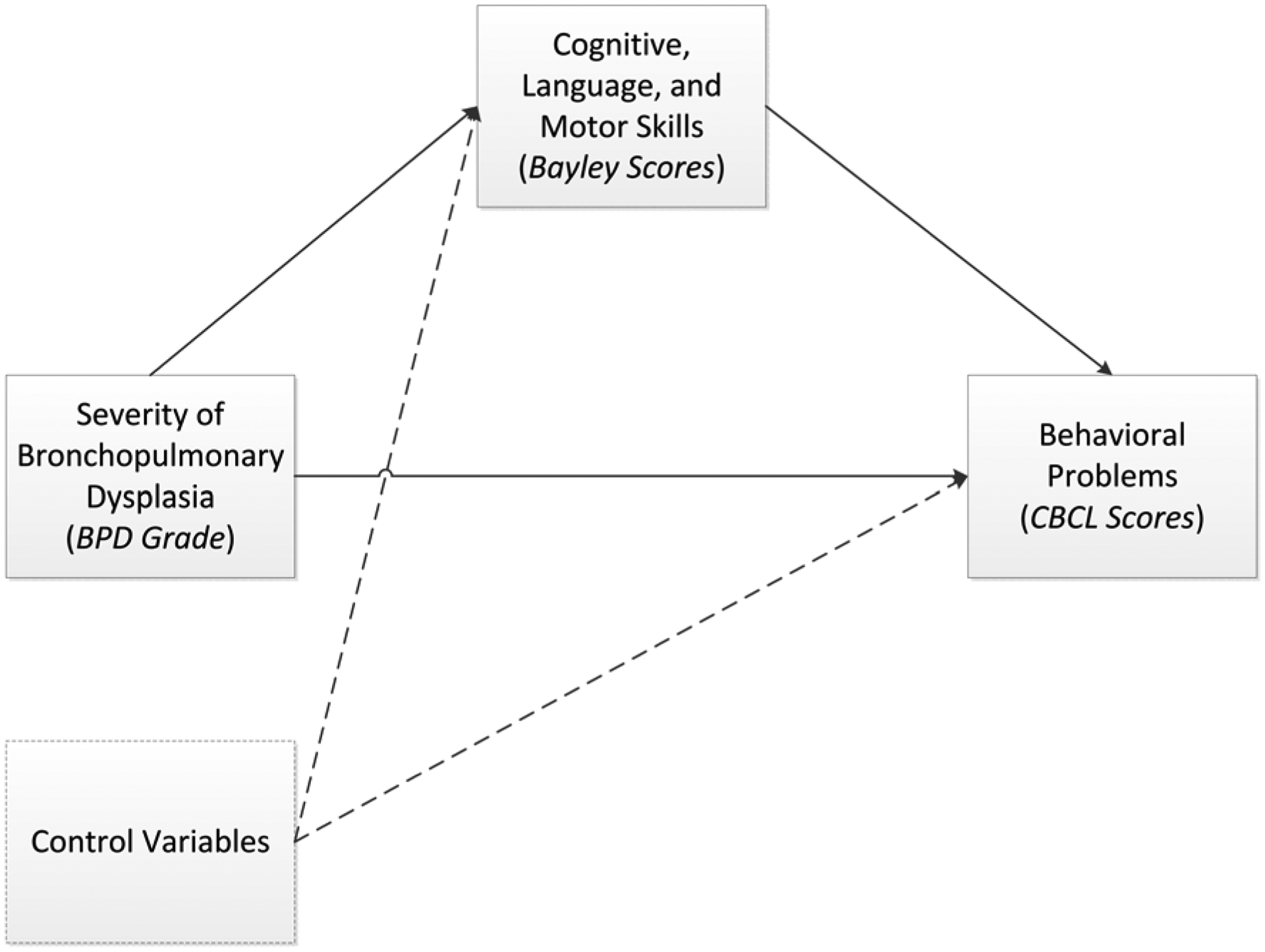
The mediation analyses investigated whether a child’s cognitive, language, or motor skills mediate the relationship between BPD grade and problem behaviors (Figure 1; available at www.jpeds.com). Because BPD grade is a multi-category nominal variable, we used the mediation analysis approach for multi-categorical independent variables presented by Hayes and Preacher.19 For these analyses, BPD status was classified as each grade compared with no BPD. The mediation analyses were conducted in the structural equation modeling framework with Mplus software version 8.3, using bootstrapping to determine confidence intervals. The mediation models controlled for the same potential confounders identified above. Each possible mediator (cognitive, language, or motor skills) was first analyzed separately. To enhance usability of the results, after identifying the significant mediators for each CBCL scale, we then combined the results into one overall path model, including the significant paths for the mediators of the relationship between BPD grades and the CBCL syndrome scales and controlling for potential confounding factors. Due to high correlations between the Bayley cognitive, language, and motor scales and to improve model parsimony, we removed paths between Bayley scores and CBCL scores that were no longer significant and did not affect overall model fit when all three Bayley scores were combined into the same model. Model fit was based on several indices, including the comparative fit index (CFI), Tucker-Lewis index (TLI), and root mean square error of approximation (RMSEA).
Figure 1.
Mediation model.
Results
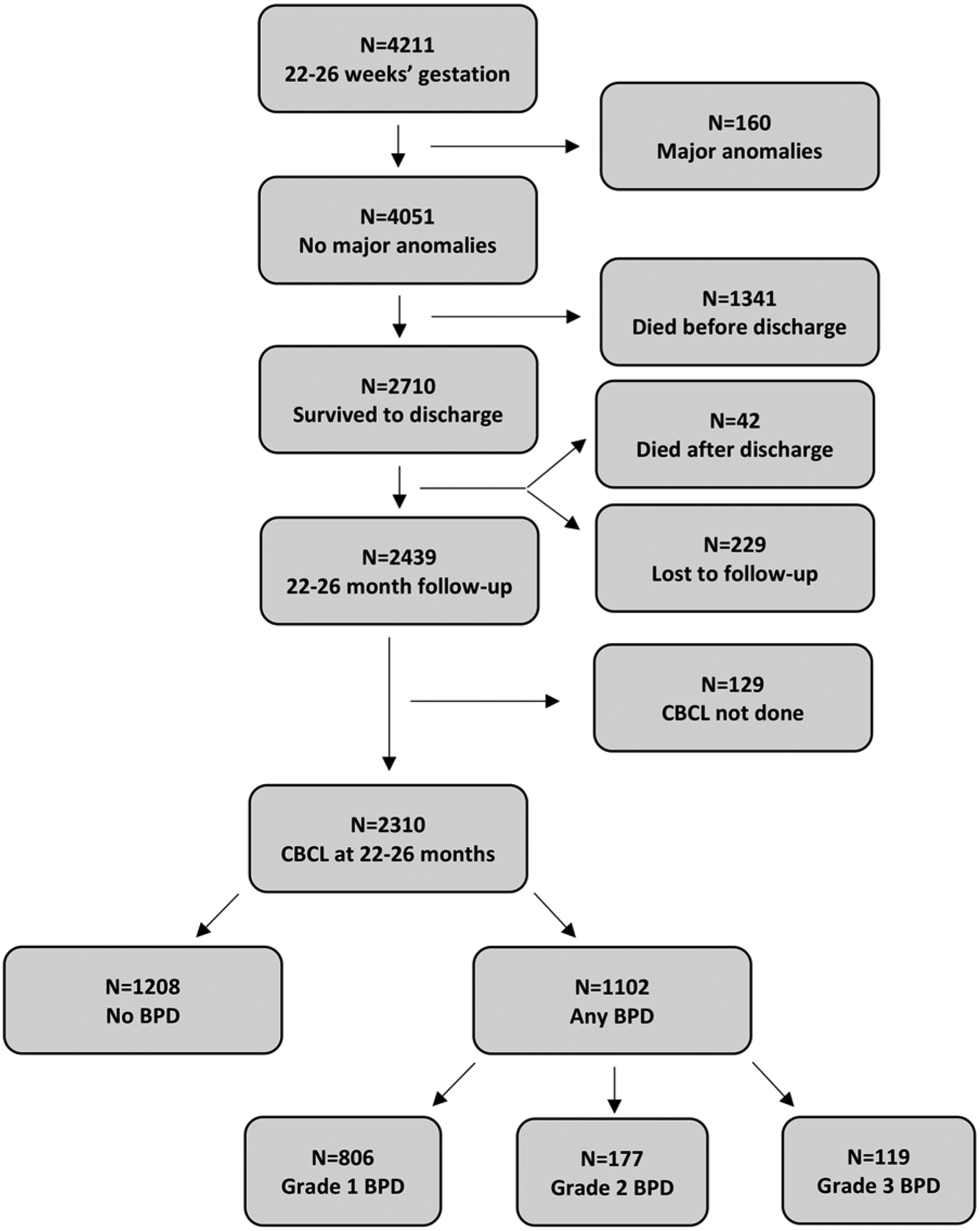
Between July 2012 and February 2016, 4211 extremely preterm infants were cared for in NRN centers with 2710 surviving to discharge (Figure 2; available at www.jpeds.com). At 22–26 months’ corrected age, 2439/2710 children were seen (90% follow-up rate), of which 2310 had the CBCL completed during their comprehensive follow-up assessment. History of BPD was present in 1102 (47.7%) children and 1208 (52.3%) children had no history of BPD. Among those with a history of BPD, 806 children (73.1%) had grade 1 BPD, 177 (16.1%) had grade 2 BPD, and 119 (10.8%) had grade 3 BPD.
Figure 2.
Flow chart of children.
Mothers of children with BPD were more likely to have private insurance (37.6% vs. 35.2%, P=.005) and be of non-black race (59.8% vs. 51.1%, P<.001) than mothers of children without BPD (Table I). Children with BPD were more likely to be outborn (5.2% vs. 3.3%, P=.026), male (54.1% vs. 47.5%, P=0.002), and SGA (8.4% vs. 3.2%, P <.001) than children without BPD. Mothers of children for whom CBCL data were not available, including those who died or were lost to follow-up, were younger (P<.001) and were less likely to have received antenatal corticosteroids (P<.001) than mothers of children for whom the CBCL was completed. The children for whom the CBCL was not completed (including those who died or were lost to follow-up) had a younger gestational age (P<.001) and lower birth weight (P<.001), were less likely to be exposed to antenatal steroids (P<.001), and were more likely to be SGA (P<.001) and to be male (P=.012) than children for whom the CBCL was completed.
Table I.
Characteristics, morbidities, and therapies by BPD grade.
| Variable, mean (SD) or N (%) | BPD Grade | ||||
|---|---|---|---|---|---|
| No BPD | Grade 1 | Grade 2 | Grade 3 | P-value | |
| Maternal | |||||
| Age (years) | 28.3 (6.3) | 28.3 (6.2) | 28.6 (6.6) | 28.3 (6.0) | .977 |
| Marital status | |||||
| Married | 435/1075 (40.5) | 333/728 (45.7) | 80/165 (48.5) | 37/106 (34.9) | .020 |
| Education | .898 | ||||
| Less than high school | 175/884 (19.8) | 112/575 (19.5) | 25/132 (18.9) | 14/73 (19.2) | |
| High school diploma | 289/884 (32.7) | 170/575 (29.6) | 36/132 (27.3) | 26/73 (35.6) | |
| Partial college or trade school | 218/884 (24.7) | 154/575 (26.8) | 38/132 (28.8) | 16/73 (21.9) | |
| College degree or higher | 202/884 (22.9) | 139/575 (24.2) | 33/132 (25.0) | 17/73 (23.3) | |
| Medical insurance | .021 | ||||
| Public | 632/1075 (58.8) | 412/728 (56.6) | 91/165 (55.2) | 67/106 (63.2) | |
| Private | 378/1075 (35.2) | 271/728 (37.2) | 68/165 (41.2) | 37/106 (34.9) | |
| Self-pay/uninsured | 56/1075 (5.2) | 28/728 (3.9) | 2/165 (1.2) | 1/106 (0.9) | |
| Other | 9/1075 (0.8) | 17/728 (2.3) | 4/165 (2.4) | 1/106 (0.9) | |
| Race | <.001*†¶ǂ | ||||
| Black | 508/1038 (48.9) | 275/706 (39.0) | 55/162 (34.0) | 61/105 (58.1) | |
| White | 479/1038 (46.2) | 381/706 (54.0) | 98/162 (60.5) | 42/105 (40.0) | |
| Other | 51/1038 (4.9) | 50/706 (7.1) | 9/162 (5.6) | 2/105 (1.9) | |
| Hispanic ethnicity | 174/1063 (16.4) | 105/719 (14.6) | 24/163 (14.7) | 11/106 (10.4) | .358 |
| Multiple birth | 185/1075 (17.2) | 132/728 (18.1) | 26/165 (15.8) | 11/106 (10.4) | .246 |
| Chorioamnionitis (clinical) | 182/1071 (17.0) | 120/723 (16.6) | 24/164 (14.6) | 23/106 (21.7) | .496 |
| Chorioamnionitis (histological) | 618/992 (62.3) | 378/654 (57.8) | 82/150 (54.7) | 54/93 (58.1) | .144 |
| Antenatal steroids | 959/1074 (89.3) | 655/726 (90.2) | 153/165 (92.7) | 95/106 (89.6) | .576 |
| Infant | |||||
| Gestational age (weeks) | 25.1 (0.9) | 24.7 (1.1) | 24.4 (1.2) | 24.4 (1.1) | <.001*†‡§¶ |
| Birth weight (grams) | 796.7 (157.5) | 724.3 (153.7) | 688.6 (166.1) | 657.5 (136.8) | <.001*†‡§¶ |
| Small for gestational age | 39/1208 (3.2) | 56/806 (6.9) | 20/177 (11.3) | 16/119 (13.4) | <.001*†‡ |
| Outborn | 40/1208 (3.3) | 36/806 (4.5) | 10/177 (5.6) | 11/119 (9.2) | .012‡ |
| Female sex | 634/1208 (52.5) | 375/806 (46.5) | 79/177 (44.6) | 52/119 (43.7) | .015* |
| PDA | 570/1208 (47.2) | 513/806 (63.6) | 112/177 (63.3) | 75/119 (63.0) | <.001*†‡ |
| Grade III/IV ICH | 154/1203 (12.8) | 130/801 (16.2) | 27/174 (15.5) | 26/119 (21.8) | .019‡ |
| Early-onset sepsis | 29/1208 (2.4) | 14/806 (1.7) | 4/176 (2.3) | 1/119 (0.8) | .571 |
| Late-onset sepsis | 256/1208 (21.2) | 208/806 (25.8) | 60/176 (34.1) | 57/119 (47.9) | <.001†‡¶ |
| Meningitis | 19/1208 (1.6) | 8/806 (1.0) | 3/176 (1.7) | 2/119 (1.7) | .699 |
| Severe ROP | 91/1173 (7.8) | 119/783 (15.2) | 35/163 (21.5) | 21/115 (18.3) | <.001*†‡ |
| Surgeries | 215/1208 (17.8) | 167/806 (20.7) | 29/177 (16.4) | 68/119 (57.1) | <.001‡¶ǂ |
| Surfactant use | 1035/1208 (85.7) | 772/806 (95.8) | 167/176 (94.9) | 116/119 (97.5) | <.001*†‡ |
| Invasive ventilation (days) | 15.6 (34.9) | 34.9 (21.7) | 56.9 (32.1) | 87.0 (31.7) | <.001*‡§†¶ǂ |
| Postnatal steroids for BPD | 125/1136 (11.0) | 224/695 (32.2) | 81/152 (53.3) | 75/105 (71.4) | <.001*†‡§¶ǂ |
Statistically significant differences at p<0.05 after adjustment for multiple comparisons:
No BPD vs. Grade 1;
No BPD vs. Grade 2;
No BPD vs. Grade 3;
Grade 1 vs. Grade 2;
Grade 1 vs. Grade 3;
Grade 2 vs. Grade 3.
PDA: patent ductus arteriosus. ICH: intracranial hemorrhage. ROP: retinopathy of prematurity. BPD: bronchopulmonary dysplasia.
Children with BPD had higher rates of patent ductus arteriosus (63.5% vs. 47.2%, P<.001), late-onset sepsis (29.7% vs. 21.2%, P<.001), surfactant use (95.8% vs. 85.7%, P<.001), and postnatal corticosteroid use for BPD (39.9% vs. 11.0%, P<.001) than children without BPD (Table I). With regard to neurosensory morbidities, children with BPD experienced higher rates of severe ICH (16.7% vs. 12.8%, P=.008) and severe ROP (16.5% vs. 7.8%, P<.001) compared with those without BPD.
At 2 years’ corrected age, the majority of children were within normal limits by parent report for all domains assessed by the CBCL (Table II; available at www.jpeds.com). Children with grade 3 BPD were more likely to score in the borderline or clinically significant range for withdrawn behavior (P=.007) and pervasive developmental problems (P=.007) compared with children without BPD.
Table II.
CBCL score classification by BPD grade.
| BPD Grade | |||||
|---|---|---|---|---|---|
| CBCL Score ClassificationA | No BDP | Grade 1 | Grade 2 | Grade 3 | P-valueB |
| Syndrome Scales | |||||
| Emotionally Reactive | .939 | ||||
| Within normal limits | 1079/1208 (89.2%) | 716/805 (88.9%) | 158/177 (89.3%) | 105/119 (88.2%) | |
| Borderline | 92/1208 (7.6%) | 58/805 (7.2%) | 14/177 (7.9%) | 11/119 (9.2%) | |
| Clinically significant | 38/1208 (3.1%) | 31/805 (3.9%) | 5/177 (2.8%) | 3/119 (2.5%) | |
| Anxious/Depressed | .535 | ||||
| Within normal limits | 1129/1208 (93.4%) | 755/807 (93.6%) | 165/177 (93.2%) | 114/119 (95.8%) | |
| Borderline | 54/1208 (4.5%) | 33/807 (4.1%) | 11/177 (6.2%) | 4/119 (3.4%) | |
| Clinically significant | 26/1208 (2.2%) | 19/807 (2.4%) | 1/177 (0.6%) | 1/119 (0.8%) | |
| Somatic Complaints | .456 | ||||
| Within normal limits | 1110/1207 (92.0%) | 722/806 (89.6%) | 159/177 (89.8%) | 105/119 (88.2%) | |
| Borderline | 64/1207 (5.3%) | 59/806 (7.3%) | 14/177 (7.9%) | 10/119 (8.4%) | |
| Clinically significant | 33/1207 (2.7%) | 25/806 (3.1%) | 4/177 (2.3%) | 4/119 (3.4%) | |
| Attention Problems | .450 | ||||
| Within normal limits | 966/1206 (80.1%) | 629/803 (78.3%) | 145/177 (81.9%) | 93/118 (78.8%) | |
| Borderline | 91/1206 (7.5%) | 63/803 (7.8%) | 14/177 (7.9%) | 14/118 (11.9%) | |
| Clinically significant | 149/1206 (12.4%) | 111/803 (13.8%) | 18/177 (10.2%) | 11/118 (9.3%) | |
| Aggressive Behavior | .302 | ||||
| Within normal limits | 1072/1208 (88.7%) | 722/807 (89.5%) | 166/177 (93.8%) | 109/119 (91.6%) | |
| Borderline | 61/1208 (5.0%) | 47/807 (5.8%) | 5/177 (2.8%) | 5/119 (4.2%) | |
| Clinically significant | 76/1208 (6.3%) | 38/807 (4.7%) | 6/177 (3.4%) | 5/119 (4.2%) | |
| Sleep Problems | .134 | ||||
| Within normal limits | 1104/1204 (91.7%) | 737/805 (91.6%) | 169/176 (96%) | 110/118 (93.2%) | |
| Borderline | 28/1204 (2.3%) | 30/805 (3.7%) | 2/176 (1.1%) | 2/118 (1.7%) | |
| Clinically significant | 72/1204 (6.0%) | 38/805 (4.7%) | 5/176 (2.8%) | 6/118 (5.1%) | |
| Withdrawn | .007‡ | ||||
| Within normal limits | 1058/1207 (87.7%) | 681/806 (84.5%) | 145/177 (81.9%) | 91/119 (76.5%) | |
| Borderline | 48/1207 (4.0%) | 43/806 (5.3%) | 15/177 (8.5%) | 10/119 (8.4%) | |
| Clinically significant | 101/1207 (8.4%) | 82/806 (10.2%) | 17/177 (9.6%) | 18/119 (15.1%) | |
| Problem Scores | |||||
| Externalizing Problems | .524 | ||||
| Within normal limits | 1061/1208 (87.8%) | 708/807 (87.7%) | 161/177 (91.0%) | 110/119 (92.4%) | |
| Borderline | 69/1208 (5.7%) | 51/807 (6.3%) | 10/177 (5.6%) | 4/119 (3.4%) | |
| Clinically significant | 79/1208 (6.5%) | 48/807 (5.9%) | 6/177 (3.4%) | 5/119 (4.2%) | |
| Internalizing Problems | .397 | ||||
| Within normal limits | 1102/1208 (91.1%) | 717/807 (88.8%) | 165/177 (93.2%) | 111/119 (93.3%) | |
| Borderline | 68/1208 (5.6%) | 58/807 (7.2%) | 7/177 (4.0%) | 6/119 (5.0%) | |
| Clinically significant | 39/1208 (3.2%) | 32/807 (4.0%) | 5/177 (2.8%) | 2/119 (1.7%) | |
| Total Problems | |||||
| Within normal limits | 1047/1208 (86.7%) | 700/806 (86.9%) | 157/177 (88.7%) | 110/119 (92.4%) | .212 |
| Borderline | 87/1208 (7.2%) | 51/806 (6.3%) | 15/177 (8.5%) | 4/119 (3.4%) | |
| Clinically significant | 74/1208 (6.1%) | 55/806 (6.8%) | 5/177 (2.8%) | 5/119 (4.2%) | |
| DSM-Oriented Scales | |||||
| Oppositional Defiant Problems | .669 | ||||
| Within normal limits | 1069/1208 (88.4%) | 723/807 (89.6%) | 162/177 (91.5%) | 110/119 (92.4%) | |
| Borderline | 49/1208 (4.1%) | 29/807 (3.6%) | 7/177 (4.0%) | 3/119 (2.5%) | |
| Clinically significant | 91/1208 (7.5%) | 55/807 (6.8%) | 8/177 (4.5%) | 6/119 (5.1%) | |
| Anxiety Problems | .570 | ||||
| Within normal limits | 1120/1208 (92.7%) | 745/807 (92.3%) | 167/177 (94.4%) | 112/119 (94.1%) | |
| Borderline | 26/1208 (2.2%) | 24/807 (3.0%) | 5/177 (2.8%) | 4/119 (3.4%) | |
| Clinically significant | 62/1208 (5.1%) | 38/807 (4.7%) | 5/177 (2.8%) | 3/119 (2.5%) | |
| Affective Problems | .561 | ||||
| Within normal limits | 1098/1208 (90.9%) | 727/806 (90.2%) | 163/177 (92.1%) | 114/119 (95.8%) | |
| Borderline | 42/1208 (3.5%) | 26/806 (3.2%) | 5/177 (2.8%) | 2/119 (1.7%) | |
| Clinically significant | 68/1208 (5.6%) | 53/806 (6.6%) | 9/177 (5.1%) | 3/119 (2.5%) | |
| AD/Hyperactivity Problems | .117 | ||||
| Within normal limits | 1031/1208 (85.3%) | 688/806 (85.4%) | 160/177 (90.4%) | 111/119 (93.3%) | |
| Borderline | 83/1208 (6.9%) | 48/806 (6.0%) | 7/177 (4.0%) | 4/119 (3.4%) | |
| Clinically significant | 95/1208 (7.9%) | 70/806 (8.7%) | 10/177 (5.6%) | 4/119 (3.4%) | |
| Pervasive Developmental Problems | .007*‡ | ||||
| Within normal limits | 1007/1208 (83.4%) | 634/806 (78.7%) | 148/177 (83.6%) | 84/118 (71.2%) | |
| Borderline | 91/1208 (7.5%) | 65/806 (8.1%) | 12/177 (6.8%) | 15/118 (12.7%) | |
| Clinically significant | 110/1208 (9.1%) | 107/806 (13.3%) | 17/177 (9.6%) | 19/118 (16.1%) | |
CBCL standard scores are scaled with a mean of 50 and standard deviation of 10. Scores <65 are within normal limits. Scores 65–69 are borderline, and scores ≥70 (i.e. 2 standard deviations or more above the mean) are considered clinically significant.
Statistically significant differences at p<0.05 after abdjustment for multiple comparisons:
No BPD vs. Grade 1;
No BPD vs. Grade 2;
No BPD vs. Grade 3;
Grade 1 vs. Grade 2;
Grade 1 vs. Grade 3;
Grade 2 vs. Grade 3.
For the unadjusted bivariate comparisons on the CBCL scores, children with BPD scored higher (worse) for somatic complaints (mean 54.2 ± SD 6.3 vs. 53.6±5.7, P=.025), withdrawn behavior (56.9±8.4 vs. 55.8±7.5, P<.001), and pervasive developmental problems (57.5±8.5 vs. 56.2±7.9, P<.001) compared with those without BPD (Table III). The sleep problems scale revealed statistically lower (better) scores among children with BPD compared with those without BPD (54.1±7.5 vs. 54.8±7.8, P=.025) as did the aggressive behavior scale (54.4±7.3 vs. 55.1±8.1, P=.0496). When examined by BPD grade, withdrawn behavior and pervasive developmental problems increased with worsening BPD grade (P<.001 for both), whereas sleep problems and aggressive behavior decreased with worsening BPD (P=.008 and P=.023, respectively).
Table III.
Outcomes at 22–26 months’ corrected age by BPD grade.
| Variable | |||||||
|---|---|---|---|---|---|---|---|
| No BPD (N=1,208) | Any BPD (N=1,102) | P-valueA | Grade 1 (N=806) | Grade 2 (N=177) | Grade 3 (N=119) | P-valueA | |
| Corrected age (months), mean (SD) | 24.4 (2.8) | 24.6 (2.7) | 0.128 | 24.5 (2.6) | 24.8 (3.3) | 24.4 (2.2) | .176 |
| Bayley-III composite, mean (SD)B | |||||||
| Cognitive | 89.1 (14.2) | 83.0 (16.2) | <0.001^ | 85.5 (15.4) | 79.5 (16.3) | 71.0 (15.5) | <.001*†‡§¶ǂ |
| Language | 85.5 (16.4) | 78.7 (17.5) | <0.001^ | 81.2 (17.0) | 74.6 (17.1) | 67.1 (16.6) | <.001*†‡§¶ǂ |
| Motor | 89.4 (15.1) | 80.8 (17.2) | <0.001^ | 83.6 (15.6) | 76.9 (18.2) | 68.0 (19.0) | <.001*†‡§¶ǂ |
| CBCL syndrome scales, mean (SD) | |||||||
| Aggressive Behavior | 55.1 (8.1) | 54.4 (7.3) | 0.050^ | 54.8 (7.6) | 53.6 (5.8) | 53.5 (7.2) | .023 |
| Anxious/Depressed | 53.5 (5.6) | 53.5 (5.5) | 0.896 | 53.7 (5.7) | 53.1 (5.1) | 52.8 (4.7) | .332 |
| Attention Problems | 56.9 (8.0) | 57.5 (8.1) | 0.067 | 57.6 (8.3) | 56.8 (7.7) | 57.3 (7.1) | .189 |
| Emotionally Reactive | 54.1 (6.5) | 54.2 (6.7) | 0.747 | 54.4 (6.9) | 53.7 (5.9) | 53.7 (6.5) | .454 |
| Sleep Problems | 54.8 (7.8) | 54.1 (7.5) | 0.024^ | 54.5 (7.6) | 53.1 (6.4) | 52.9 (6.9) | .008† |
| Somatic Complaints | 53.6 (5.7) | 54.2 (6.3) | 0.025^ | 54.0 (6.4) | 54.3 (5.8) | 55.0 (6.0) | .055 |
| Withdrawn | 55.8 (7.5) | 56.9 (8.4) | <0.001^ | 56.6 (8.4) | 57.5 (8.4) | 58.5 (8.8) | <.001†‡ |
| CBCL DSM-oriented scales, mean (SD) | |||||||
| Affective Problems | 54.6 (6.4) | 54.7 (6.4) | 0.619 | 54.8 (6.7) | 54.7 (5.8) | 54.1 (5.2) | .656 |
| Anxiety Problems | 54.1 (6.4) | 53.9 (6.5) | 0.583 | 54.2 (6.7) | 53.4 (6.1) | 53.3 (5.5) | .320 |
| Attention Deficit/Hyperactivity Problems | 56.2 (7.3) | 56.1 (7.2) | 0.752 | 56.4 (7.4) | 55.5 (6.8) | 55.4 (5.8) | .289 |
| Oppositional Defiant Problems | 54.7 (7.1) | 54.4 (6.8) | 0.374 | 54.7 (6.9) | 53.7 (6.2) | 53.7 (6.2) | .150 |
| Pervasive Developmental Problems | 56.2 (7.9) | 57.5 (8.5) | <0.001^ | 57.3 (8.7) | 57.4 (7.9) | 59.0 (8.2) | <.001*‡ |
| CBCL problem scores, mean (SD) | |||||||
| Externalizing Problems | 51.1 (11.9) | 50.6 (11.5) | 0.247 | 51.1 (11.7) | 49.1 (10.8) | 49.1 (10.7) | .051 |
| Internalizing Problems | 49.2 (11.0) | 49.9 (11.3) | 0.094 | 49.8 (11.5) | 49.9 (10.8) | 51.3 (9.8) | .179 |
| Total Problems | 51.0 (11.7) | 51.1 (11.5) | 0.882 | 51.4 (11.9) | 50.1 (10.9) | 50.8 (10.0) | .598 |
| Neurosensory outcomes, N (%) | |||||||
| Cerebral palsy | 149 (12.4) | 232 (21.2) | <0.001^ | 139 (17.3) | 43 (24.9) | 50 (42.0) | <.001*†‡§¶ǂ |
| Hearing impairment | 24 (2.0) | 38 (3.5) | 0.028^ | 24 (3.0) | 8 (4.7) | 6 (5.1) | .059 |
| Bilateral blindness | 9 (0.7) | 21 (1.9) | 0.014^ | 9 (1.1) | 5 (2.9) | 7 (5.9) | <.001ঠ|
| Pulmonary outcomes, N (%) | |||||||
| Current supplemental oxygen use | 12 (1.0) | 115 (10.5) | <0.001^ | 41 (5.1) | 36 (20.5) | 38 (31.9) | <.001*†‡§¶ǂ |
| Current ventilator or CPAP use | 4 (0.3) | 34 (3.1) | <0.001^ | 6 (0.7) | 8 (4.6) | 20 (16.8) | <.001†‡§¶ǂ |
| Current tracheostomy | 1 (0.1) | 75 (7) | <0.001^ | 7 (0.9) | 15 (8.5) | 53 (44.5) | <.001*†‡§¶ǂ |
Statistically significant differences at p<0.05 after adjustment for multiple comparisons:
No BPD vs. Any BPD;
No BPD vs. Grade 1;
No BPD vs. Grade 2;
No BPD vs. Grade 3;
Grade 1 vs. Grade 2;
Grade 1 vs. Grade 3;
Grade 2 vs. Grade 3.
Bayley-III composite scores were available for a subset of children for whom the CBCL was completed: N=1151 children with no BPD, N=773 children with grade 1 BPD, N=169 children with grade 2 BPD, and N=110 children with grade 3 BPD.
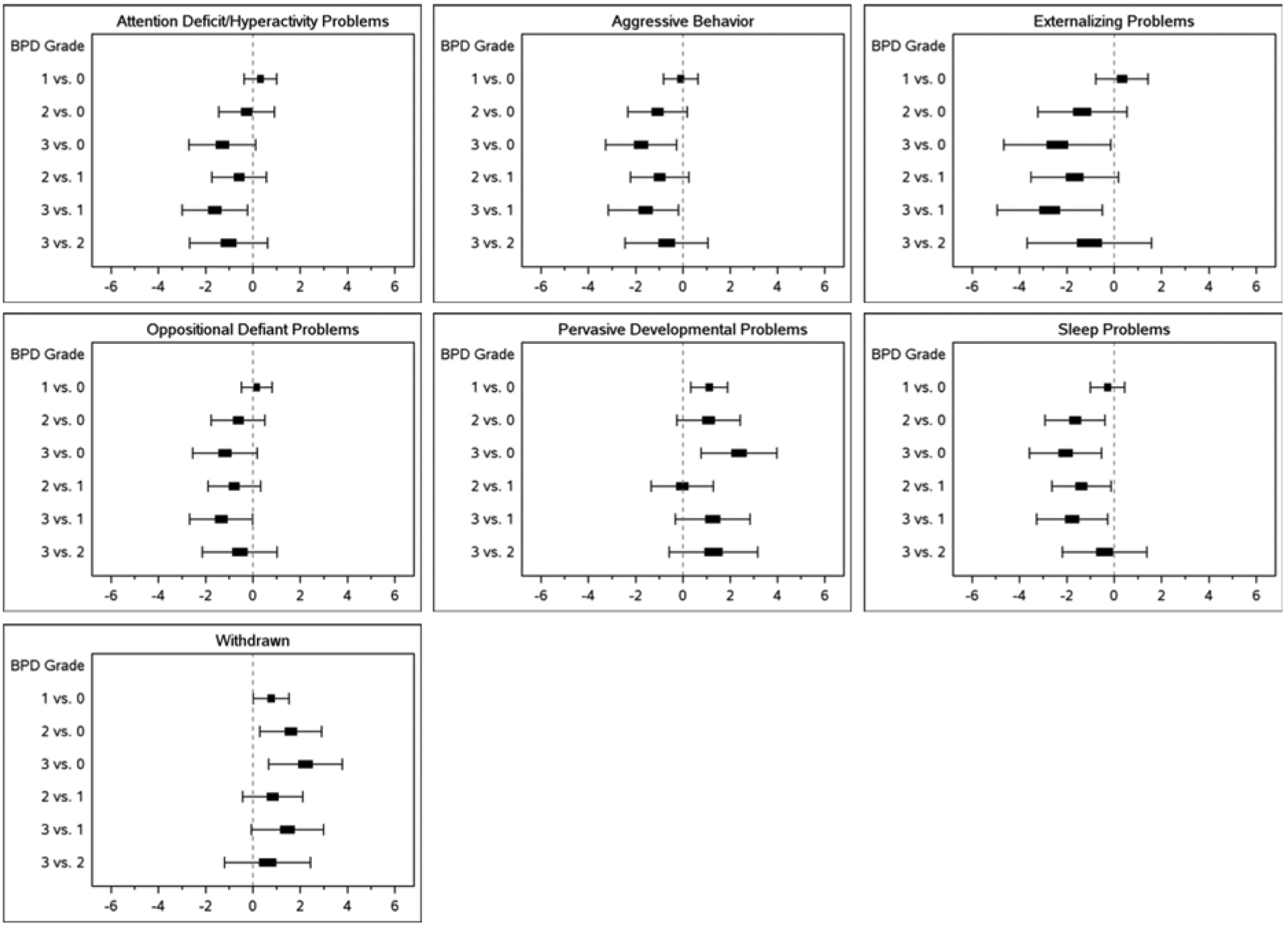
After controlling for potential confounding factors, children with grade 3 BPD scored 2.4 points higher (worse) on pervasive developmental problems (95% CI 0.76, 3.98) and 2.2 points higher (worse) on withdrawn behavior (95% CI 0.67, 3.78) than those with no BPD (Figure 3). In contrast, children with grade 3 BPD scored 2.4 points lower (better) on externalizing problems (95% CI −4.66, −0.14), 2.1 points lower (better) on sleep problems (95% CI −3.58, −0.53), and 1.8 points lower (better) on aggressive behavior (95% CI −3.27, −0.27) than children with no BPD. The adjusted R2 values were small (0.023–0.067), suggesting that although statistically significant, BPD grade and other variables in the model did not explain a large portion of the variability observed in problem behaviors.
Figure 3.
Adjusted mean differences in CBCL scores by bronchopulmonary dysplasia grade.
Mean differences are adjusted for sex, gestational age, SGA, race, Hispanic ethnicity, maternal education, insurance type, center, grade III/IV ICH, severe ROP, and postnatal steroids.
Mediation analysis revealed that cognitive skills assessed by the Bayley-III were significant mediators for all of the CBCL scales except for aggressive behavior, sleep problems, and oppositional defiant problems (Table IV; available at www.jpeds.com). In addition, both language and motor skills were significant mediators for attention problems, emotionally reactive, somatic complaints, withdrawn, affective problems, pervasive developmental problems, internalizing problems, and total problems. In some cases, motor skills also were a significant mediator for anxious/depressed behavior. The size of the mediation effect increased with BPD severity. For example, there was a 0.14 standard deviation (SD) difference in mean CBCL attention problems scores due to cognitive skills among those with BPD grade 1 versus no BPD; however, this value increased to 0.46 SD for grade 2 and 0.85 SD for grade 3 versus no BPD.
Table IV.
Cognitive, language, and motor skills as mediators of the relationship between BPD grade and problem behaviors.
| Indirect Effect of BPD Grade on Problem Behavior through a Mediator | |||
|---|---|---|---|
| Cognitive Skills | Language Skills | Motor Skills | |
| Problem Behavior | Adj. SMD (95% CI) | Adj. SMD (95% CI) | Adj. SMD (95% CI) |
| CBCL Syndrome Scales | |||
| Aggressive Behavior | |||
| BPD Grade 1 (vs. 0) | 0.03 (−0.01, 0.10) | 0.00 (−0.08, 0.08) | −0.04 (−0.13, 0.02) |
| BPD Grade 2 (vs. 0) | 0.10 (−0.05, 0.29) | 0.00 (−0.21, 0.20) | −0.12 (−0.33, 0.06) |
| BPD Grade 3 (vs. 0) | 0.19 (−0.09, 0.52) | −0.01 (−0.32, 0.31) | −0.21 (−0.57, 0.12) |
| Anxious/Depressed | |||
| BPD Grade 1 (vs. 0) | 0.05 (0.01, 0.11)A | 0.05 (0.00, 0.13) | 0.05 (0.00, 0.13)A |
| BPD Grade 2 (vs. 0) | 0.17 (0.05, 0.33)A | 0.14 (−0.01, 0.31) | 0.15 (0.00, 0.33)A |
| BPD Grade 3 (vs. 0) | 0.32 (0.09, 0.59)A | 0.21 (−0.02, 0.47) | 0.26 (−0.01, 0.57) |
| Attention Problems | |||
| BPD Grade 1 (vs. 0) | 0.12 (0.04, 0.23)A | 0.14 (0.05, 0.27)A | 0.09 (0.02, 0.20)A |
| BPD Grade 2 (vs. 0) | 0.41 (0.22, 0.67)A | 0.37 (0.15, 0.65)A | 0.24 (0.04, 0.49)A |
| BPD Grade 3 (vs. 0) | 0.77 (0.42, 1.20)A | 0.57 (0.24, 0.98)A | 0.44 (0.06, 0.86)A |
| Emotionally Reactive | |||
| BPD Grade 1 (vs. 0) | 0.11 (0.04, 0.21)A | 0.14 (0.06, 0.26)A | 0.13 (0.06, 0.25)A |
| BPD Grade 2 (vs. 0) | 0.37 (0.20, 0.61)A | 0.38 (0.19, 0.62)A | 0.36 (0.18, 0.61)A |
| BPD Grade 3 (vs. 0) | 0.69 (0.38, 1.08)A | 0.58 (0.29, 0.95)A | 0.65 (0.32, 1.07)A |
| Sleep Problems | |||
| BPD Grade 1 (vs. 0) | 0.00 (−0.05, 0.07) | −0.03 (−0.12, 0.05) | 0.01 (−0.07, 0.08) |
| BPD Grade 2 (vs. 0) | 0.01 (−0.18, 0.21) | −0.07 (−0.30, 0.15) | 0.02 (−0.19, 0.22) |
| BPD Grade 3 (vs. 0) | 0.02 (−0.32, 0.39) | −0.11 (−0.45, 0.23) | 0.04 (−0.34, 0.40) |
| Somatic Complaints | |||
| BPD Grade 1 (vs. 0) | 0.06 (0.02, 0.13)A | 0.09 (0.03, 0.17)A | 0.10 (0.04, 0.20)A |
| BPD Grade 2 (vs. 0) | 0.19 (0.06, 0.37)A | 0.23 (0.08, 0.42)A | 0.27 (0.11, 0.49)A |
| BPD Grade 3 (vs. 0) | 0.36 (0.11, 0.66)A | 0.36 (0.11, 0.64)A | 0.49 (0.19, 0.83)A |
| Withdrawn | |||
| BPD Grade 1 (vs. 0) | 0.35 (0.12, 0.59)A | 0.58 (0.33, 0.87)A | 0.49 (0.26, 0.75)A |
| BPD Grade 2 (vs. 0) | 1.22 (0.81, 1.70)A | 1.57 (1.11, 2.06)A | 1.33 (0.90, 1.83)A |
| BPD Grade 3 (vs. 0) | 2.30 (1.72, 2.98)A | 2.37 (1.73, 3.07)A | 2.43 (1.82, 3.17)A |
| DSM-Oriented Scales | |||
| Affective Problems | |||
| BPD Grade 1 (vs. 0) | 0.13 (0.05, 0.24)A | 0.20 (0.11, 0.33)A | 0.19 (0.10, 0.31)A |
| BPD Grade 2 (vs. 0) | 0.45 (0.27, 0.68)A | 0.55 (0.34, 0.80)A | 0.50 (0.31, 0.77)A |
| BPD Grade 3 (vs. 0) | 0.84 (0.53, 1.22)A | 0.83 (0.53, 1.22)A | 0.91 (0.57, 1.32)A |
| Anxiety Problems | |||
| BPD Grade 1 (vs. 0) | 0.04 (0.00, 0.11)A | −0.01 (−0.08, 0.06) | 0.03 (−0.04, 0.10) |
| BPD Grade 2 (vs. 0) | 0.14 (0.00, 0.32) | −0.03 (−0.21, 0.15) | 0.07 (−0.10, 0.26) |
| BPD Grade 3 (vs. 0) | 0.26 (−0.02, 0.57) | −0.04 (−0.32, 0.24) | 0.12 (−0.17, 0.46) |
| Attention Deficit/Hyperactivity | |||
| BPD Grade 1 (vs. 0) | 0.05 (0.01, 0.13)A | 0.04 (−0.03, 0.13) | −0.01 (−0.08, 0.06) |
| BPD Grade 2 (vs. 0) | 0.18 (0.02, 0.37)A | 0.11 (−0.09, 0.31) | −0.02 (−0.20, 0.16) |
| BPD Grade 3 (vs. 0) | 0.33 (0.04, 0.66)A | 0.16 (−0.13, 0.48) | −0.04 (−0.37, 0.29) |
| Oppositional Defiant Problems | |||
| BPD Grade 1 (vs. 0) | 0.03 (−0.01, 0.10) | 0.03 (−0.04, 0.11) | −0.03 (−0.10, 0.03) |
| BPD Grade 2 (vs. 0) | 0.10 (−0.04, 0.28) | 0.08 (−0.11, 0.27) | −0.08 (−0.27, 0.09) |
| BPD Grade 3 (vs. 0) | 0.19 (−0.09, 0.49) | 0.12 (−0.17, 0.42) | −0.14 (−0.47, 0.17) |
| Pervasive Developmental Problems | |||
| BPD Grade 1 (vs. 0) | 0.30 (0.10, 0.51)A | 0.53 (0.29, 0.80)A | 0.41 (0.22, 0.64)A |
| BPD Grade 2 (vs. 0) | 1.04 (0.68, 1.46)A | 1.43 (1.02, 1.90)A | 1.11 (0.74, 1.55)A |
| BPD Grade 3 (vs. 0) | 1.97 (1.44, 2.58)A | 2.19 (1.63, 2.86)A | 2.01 (1.47, 2.66)A |
| Problem Scores | |||
| Externalizing Problems | |||
| BPD Grade 1 (vs. 0) | 0.07 (0.00, 0.19)A | 0.09 (−0.02, 0.25) | −0.02 (−0.14, 0.10) |
| BPD Grade 2 (vs. 0) | 0.24 (−0.02, 0.54) | 0.25 (−0.07, 0.61) | −0.05 (−0.37, 0.25) |
| BPD Grade 3 (vs. 0) | 0.44 (−0.04, 0.98) | 0.39 (−0.10, 0.94) | −0.09 (−0.66, 0.46) |
| Internalizing Problems | |||
| BPD Grade 1 (vs. 0) | 0.33 (0.12, 0.57)A | 0.54 (0.31, 0.83)A | 0.44 (0.24, 0.71)A |
| BPD Grade 2 (vs. 0) | 1.14 (0.74, 1.63)A | 1.45 (1.00, 1.98)A | 1.19 (0.79, 1.71)A |
| BPD Grade 3 (vs. 0) | 2.14 (1.52, 2.86)A | 2.20 (1.55, 2.96)A | 2.16 (1.52, 2.93)A |
| Total Problems | |||
| BPD Grade 1 (vs. 0) | 0.21 (0.07, 0.39)A | 0.35 (0.19, 0.59)A | 0.24 (0.11, 0.44)A |
| BPD Grade 2 (vs. 0) | 0.72 (0.42, 1.12)A | 0.95 (0.59, 1.40)A | 0.65 (0.33, 1.05)A |
| BPD Grade 3 (vs. 0) | 1.35 (0.82, 1.98)A | 1.46 (0.91, 2.14)A | 1.18 (0.61, 1.85)A |
p<0.05.
Note: Adj. SMD: adjusted standardized mean difference
Values shown are the indirect effect of BPD grade on CBCL scores through the mediator and are expressed as the standardized mean difference in CBCL scores for each BPD grade versus no BPD. Analyses were adjusted for sex, gestational age, small-for-gestational age, race, Hispanic ethnicity, maternal education, insurance type, center, grade III/IV ICH, severe ROP, and postnatal steroids.
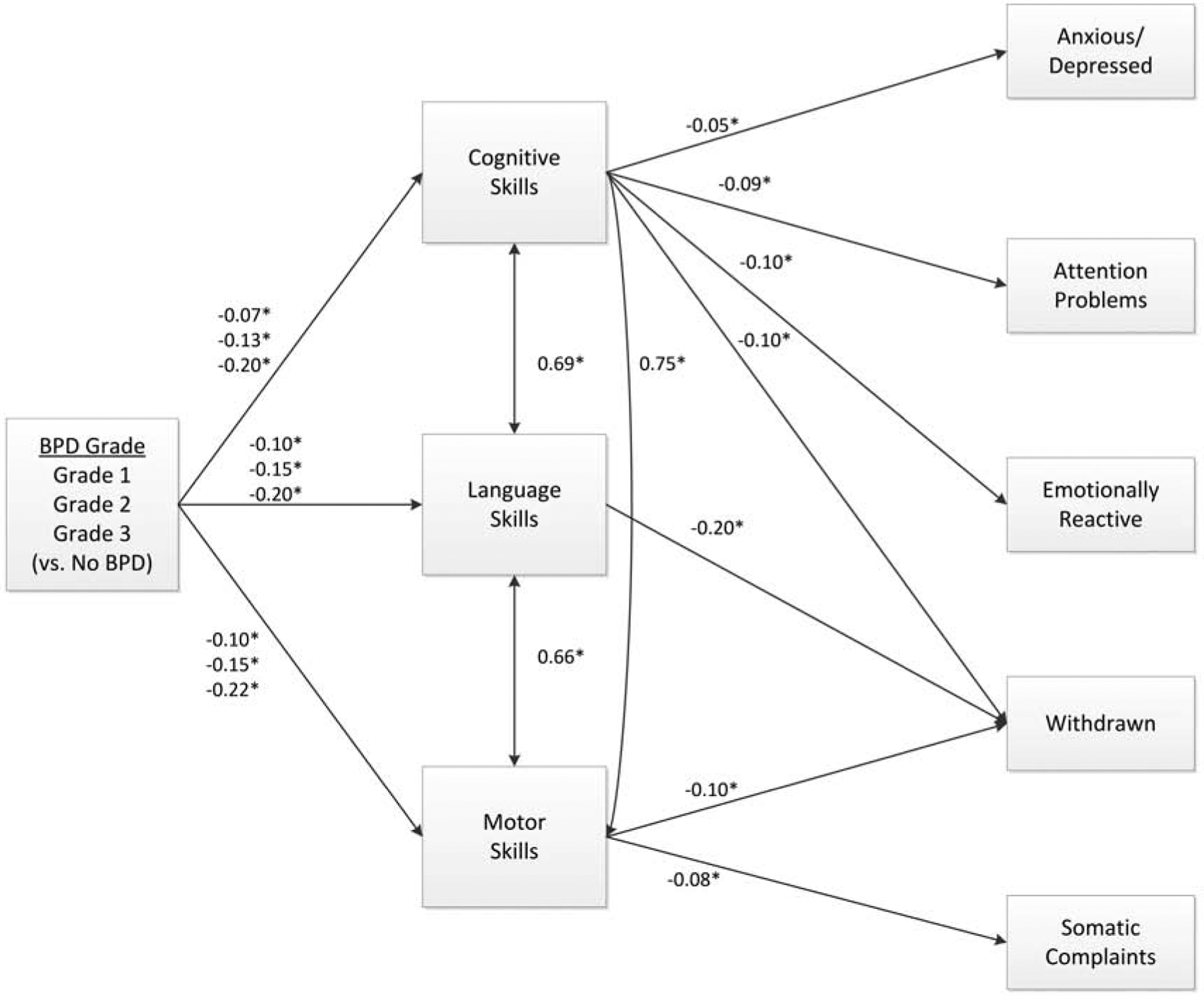
Figure 4 shows the path diagram integrating the mediation results into an overall model for the CBCL syndrome scales. The model fit very well (CFI=1.000, TLI=0.998, RMSEA=0.005). This model indicates that higher BPD grades are associated with poorer cognitive, language, and motor skills, which are in turn associated with worse problem behaviors. Cognitive skills were significant mediators of BPD grade on anxious/depressed, attention problems, emotionally reactive, and withdrawn scales. When combining all 3 Bayley scores into a single model, language skills remained a significant mediator only for the withdrawn scale and motor skills remained a significant mediator of both the withdrawn and somatic complaints scales.
Figure 4.
Path diagram of Bayley cognitive, language, and motor scores as mediators of bronchopulmonary dysplasia grade’s effect on CBCL syndrome scales.
*p <0.05. Values shown are standardized path coefficients. Path coefficients are adjusted for sex, gestational age, SGA, race, Hispanic ethnicity, maternal education, insurance type, center, grade III/IV ICH, severe ROP, and postnatal steroids.
Discussion
Although children with BPD had some increased behavioral difficulties (withdrawn behavior and pervasive developmental problems) compared with those without BPD, children with BPD had decreased problems in other behavior domains (sleep problems and aggressive behavior). The magnitude of the effects of BPD on behavior was small. Although statistically significant, these differences may not be clinically significant at less than half a standard deviation (ie, <5 points with standard deviation of 10 for the CBCL).20 What is a clinically important difference may vary for pediatric populations and may vary between children. In the current sample, difficulties on the withdrawn and pervasive developmental problems scales were more common in children with BPD and increased with increasing BPD grade. This may signal a role for screening children with BPD for autism spectrum disorder and for monitoring children with BPD for anxiety, because higher scores on withdrawn and pervasive developmental problems scales may correlate with autism spectrum disorder or anxiety diagnoses in childhood.21,22
Behavioral outcomes are complex given the endogenous and exogenous factors at play. Cognitive, language, and motor skills, markers of neurodevelopment, all mediated the effect of BPD grade on attention problems, emotional reactivity, somatic complaints, withdrawn, affective problems, pervasive developmental problems, internalizing problems, and total problems. None of the Bayley-III measures of neurodevelopment mediated the effect of BPD grade on aggressive behavior, oppositional defiant problems, or sleep problems. In an earlier NICHD NRN cohort, both language and cognitive skills mediated the relationship between sociodemographic risk factors and problem behaviors.23 To address the home environment, we included maternal education and insurance type as confounders. Other work by the NRN found language and cognitive skills to be associated with problem behaviors.24 In contrast, in a previous single center cohort, there was no independent association of BPD severity with cognition.25
Significant differences in problem behaviors were most commonly found between those without BPD and those with the most severe grade of BPD. Mechanisms by which BPD may have an effect on behavior include chronic hypercarbia,26 hypoxemia,27 and postnatal steroid exposure.28 The impact of BPD alone on CBCL scores was small, changing the CBCL scores by at most three points. In an Australian cohort, preterm children with BPD displayed more internalizing behaviors (derived from withdrawn behavior, somatic complaints, and anxious behavior) than children without BPD at 8 years; again, the magnitude of difference was small.2 This is not to minimize the importance of understanding behavioral outcomes of children born prematurely. The EXPRESS investigators found the incidence of clinical range internalizing and externalizing behaviors on the CBCL to be 20.9% and 19.5% in extremely preterm children at 2 years, which was higher than our cohort (Table II).29 Although behavioral challenges are not included in definitions of neurodevelopmental impairment, they have functional significance for children, their families, and society, particularly as children grow older. The ELGAN investigators evaluated behavioral outcomes at 10 years for children whose BPD status was known; autism spectrum disorder and communication impairment were more common among children with more severe BPD relative to children with milder or no BPD.6
The preterm behavioral phenotype is not a temporary problem limited to toddlerhood. As more extremely preterm children survive to adulthood, we are beginning to better characterize the enduring effects of prematurity. The preterm behavioral phenotype is unusual in the co-occurrence of externalizing behaviors, such as attention difficulties, and internalizing behaviors, such as anxiety. Deficits in executive function, which includes higher order cognitive processes engaged in behavioral control, are more common in adults born preterm with histories of BPD relative to adults born preterm without BPD.30 In a systematic review and meta-analysis, parents of extremely low birth weight adolescents reported more ADHD symptoms and internalizing behaviors than normal birth weight peers.31 In adulthood, those born preterm reported more internalizing problems and fewer externalizing problems than adults born at term.32 Because the effect of prematurity on neurobehavioral outcome measures may not depend on the age of assessment,10 behavioral difficulties in toddlerhood may hint at adult mental health outcomes. If behavioral difficulties do not improve with age, characterization of risk factors for problem behaviors and identification of behavioral difficulties in toddlerhood raise the potential for early intervention.
Strengths of this study include the application of a novel classification system for BPD that reflects current respiratory support modalities.13 A second strength is the large size of the cohort (n=2310), which allowed for analyses based on BPD by grade rather than as a binary outcome, and low attrition. Another strength of the study is its nuanced assessment of neurodevelopmental indicators as possible mediators of the relationship between medical illness, specifically BPD grade, and behavior.
This work also has limitations. Prematurity and sociodemographic risk factors are intertwined. Although our analyses adjusted for multiple potential confounders including baseline maternal sociodemographic characteristics, there may be additional confounding factors pertaining to the home environment or neonatal morbidities that we did not consider. Although the analyses were adjusted for multiple comparisons across BPD grades, given the large number of CBCL scales, it is possible that up to 5% of the tests could be significant by chance. The CBCL is a parent report measure, and no standardized direct child assessment of behavior was performed. An additional limitation is the age at follow-up. Two-year follow-up hinders the ability to evaluate behavior in multiple environments by different caretakers. Children may be identified as having behavioral problems beyond toddlerhood, with new challenges identified after immersion into the classroom setting.
This research informs postnatal counseling for families of children with BPD and comprehensive follow-up for children with BPD. It also contributes to our understanding of the complex relationship between BPD, behavior, and cognitive, language, and motor skills. The effects of BPD grade on behavior were subtle, and the directionality varied for different problem behaviors with some of the differences in behavioral outcomes mediated by cognitive, language, and motor development. Behavioral and mental health services may be a valued addition to the medical home for children born extremely preterm, including those affected by BPD. Given the high demands for pediatric behavioral health services, risk stratification based on neonatal morbidities and disease severity for comprehensive behavioral health screening may be one approach to optimize resource utilization for the highest risk population of children born prematurely, including those with grade 3 BPD.
Supplementary Material
Acknowledgements
We thank our medical and nursing colleagues and the infants and their parents who agreed to take part in this study.
Funded by awards to the participating institutions from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. Detailed funding information is available in the Appendix (available at www.jpeds.com). The authors declare no conflicts of interest.
Appendix
List of additional investigators and participating hospitals of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network.
NRN Steering Committee Chairs: Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006–2011); Richard A. Polin, MD, Division of Neonatology, College of Physicians and Surgeons, Columbia University, (2011-present).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904) – Abbot R. Laptook, MD; Martin Keszler, MD; Angelita M. Hensman, PhD RNC-NIC; Elisa Vieira, RN BSN; Emilee Little, RN BSN; Robert T. Burke, MD MPH; Bonnie E. Stephens, MD; Barbara Alksninis, RNC PNP; Carmena Bishop; Mary L. Keszler, MD; Teresa M. Leach, MEd CAES; Victoria E. Watson, MS CAS; Andrea M. Knoll.
Case Western Reserve University, Rainbow Babies & Children’s Hospital (U10 HD21364) – Michele C. Walsh, MD MS; Avroy A. Fanaroff, MD; Nancy S. Newman, RN; Deanne E. Wilson-Costello, MD; Allison Payne, MD MSCR; Monika Bhola, MD; Gulgun Yalcinkaya, MD; Bonnie S. Siner, RN; Harriet G. Friedman, MA; Elizabeth Roth, MS.
Children’s Mercy Hospital (U10 HD68284) – William E. Truog, MD; Eugenia K. Pallotto, MD MSCE; Howard W. Kilbride MD; Cheri Gauldin, RN BS CCRC; Anne Holmes RN MSN MBA-HCM CCRC; Kathy Johnson RN, CCRC; Allison Knutson, RN BSNC-NIC.
Cincinnati Children’s Hospital Medical Center, University Hospital, and Good Samaritan Hospital (U10 HD27853, UL1 TR77) – Kurt Schibler, MD; Brenda B. Poindexter, MD MS; Stephanie Merhar, MD MS; Kimberly Yolton, PhD; Teresa L. Gratton, PA; Cathy Grisby, BSN CCRC; Kristin Kirker, CRC; Sandra Wuertz, RN BSN CLC.
Duke University School of Medicine, University Hospital, University of North Carolina, and Duke Regional Hospital (U10 HD40492, UL1 TR1117) – C. Michael Cotten, MD MHS; Ronald N. Goldberg, MD; Ricki F. Goldstein, MD; William F. Malcolm, MD; Patricia L. Ashley, MD; Joanne Finkle, RN JD; Kimberley A. Fisher, PhD FNP-BC IBCLC; Sandra Grimes, RN BSN; Kathryn E. Gustafson, PhD; Matthew M. Laughon, MD MPH; Carl L. Bose, MD; Janice Bernhardt, MS RN; Gennie Bose, RN; Diane Warner, MD MPH; Janice Wereszczak, CPNP-AC/PC; Stephen D. Kicklighter, MD; Ginger Rhodes-Ryan, ARNP MSN NNP-BC.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (U10 HD27851, UL1 TR454) – David P. Carlton, MD; Ira Adams-Chapman, MD; Ellen C. Hale, RN BS CCRC; Yvonne C. Loggins, RN BSN; Diane I. Bottcher, RN MSN; Colleen Mackie, BS RRT; Sheena L. Carter, PhD; Maureen Mulligan LaRossa, RN; Lynn C. Wineski, RN MS; Gloria V. Smikle, PNP MSN; Angela Leon-Hernandez, MD; Salathiel Kendrick-Allwood, MD.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Rosemary D. Higgins, MD; Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, UL1 TR6) – Brenda B. Poindexter, MD MS; Gregory M. Sokol, MD; Lu Ann Papile, MD; Abbey C. Hines, PsyD; Dianne E. Herron, RN; Susan Gunn, NNP CCRC; Lucy Smiley, CCRC.
McGovern Medical School at The University of Texas Health Science Center at Houston, Children’s Memorial Hermann Hospital, and Memorial Hermann Southwest Hospital (U10 HD21373) – Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Julie Arldt-McAlister, RN BSN; Katrina Burson, RN BSN; Allison G. Dempsey, PhD; Patricia W. Evans, MD; Carmen Garcia, RN CCRP; Margarita Jiminez, MD MPH; Janice John, CPNP; Patrick M. Jones, MD MA; M. Layne Lillie, RN BSN; Karen Martin, RN; Sara C. Martin, RN BSN; Georgia E. McDavid, RN; Shawna Rodgers, RN BSN; Saba Khan Siddiki, MD; Daniel Sperry, RN; Patti L. Pierce Tate, RCP; Sharon L. Wright, MT (ASCP).
Nationwide Children’s Hospital and the Ohio State University Medical Center (U10 HD68278) – Pablo J. Sánchez, MD; Leif D. Nelin, MD; Sudarshan R. Jadcherla, MD; Patricia Luzader, RN; Christine A. Fortney, PhD RN; Gail E. Besner; Nehal A. Parikh, MD.
RTI International (U10 HD36790) – Dennis Wallace, PhD; Marie G. Gantz, PhD; Jamie E. Newman, PhD MPH; Jeanette O’Donnell Auman, BS; Margaret Crawford, BS; Jenna Gabrio, BS CCRP; David Leblond, BS; Carolyn M. Petrie Huitema, MS; Kristin M. Zaterka-Baxter, RN BSN.
Stanford University, Dominican Hospital, El Camino Hospital, and Lucile Packard Children’s Hospital (U10 HD27880, UL1 TR93) – Krisa P. Van Meurs, MD; Valerie Y. Chock, MD MS Epi; David K. Stevenson, MD; Marian M. Adams, MD; M. Bethany Ball, BS CCRC; Barbara Bentley, PsychD, MS Ed; Maria Elena DeAnda, PhD; Anne M. Debattista, RN PNP PhD; Beth Earhart, PhD; Lynne C. Huffman, MD; Magdy Ismael, MD MPH; Casey E. Krueger, PhD; Andrew W. Palmquist, RN BSN; Melinda S. Proud, RCP; Elizabeth N. Reichert, MA CCRC; Meera N. Sankar, MD; Nicholas H. St. John, PhD; Heather L. Taylor, PhD; Hali E. Weiss, MD.
Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54) – Ivan D. Frantz III, MD; John M. Fiascone, MD; Brenda L. MacKinnon, RNC; Ellen Nylen, RN BSN; Anne Furey, MPH; Cecelia E Sibley PT MHA; Ana K. Brussa, MS OTR/L.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10HD34216) – Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Kirstin J. Bailey, PhD; Fred J. Biasini, PhD; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN; Vivien A. Phillips, RN BSN; Richard V. Rector, PhD; Sally Whitley, MA OTR-L FAOTA.
University of California - Los Angeles, Mattel Children’s Hospital, Santa Monica Hospital, Los Robles Hospital and Medical Center, and Olive View Medical Center (U10 HD68270) – Uday Devaskar, MD; Meena Garg, MD; Isabell B. Purdy, PhD CPNP; Teresa Chanlaw, MPH; Rachel Geller, RN BSN.
University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns (U10 HD40461) – Neil N. Finer, MD; Yvonne E. Vaucher, MD MPH; David Kaegi, MD; Maynard R. Rasmussen, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Martha G. Fuller, RN MSN; Wade Rich, BSHS RRT; Radmila West PhD.
University of Iowa, Mercy Medical Center, and Sanford Health (U10 HD53109, UL1 TR442) – Michelle L. Baack, MD; Dan L. Ellsbury, MD; Laurie A. Hogden, MD; Jonathan M. Klein, MD; John M. Dagle, MD PhD; Karen J. Johnson, RN BSN; Tracy L. Tud, RN; Chelsey Elenkiwich, RN BSN; Megan M. Henning, RN; Megan Broadbent, RN BSN; Mendi L. Schmelzel, MSN RN; Jacky R. Walker, RN; Claire A. Goeke, RN.
University of New Mexico Health Sciences Center (U10 HD53089, UL1 TR41) – Kristi L. Watterberg, MD; Robin K. Ohls, MD; Conra Backstrom Lacy, RN; Sandra Brown, BSN; Janell Fuller, MD; Carol Hartenberger, MPH BSN; Jean R. Lowe, PhD; Sandra Sundquist Beauman, MSN RNC-NIC; Mary Ruffner Hanson, RN BSN; Tara Dupont, MD; Elizabeth Kuan, RN BSN.
University of Pennsylvania, Hospital of the University of Pennsylvania, Pennsylvania Hospital, and Children’s Hospital of Philadelphia (U10 HD68244) – Barbara Schmidt, MD MSc; Haresh Kirpalani, MB MSc; Aasma S. Chaudhary, BS RRT; Soraya Abbasi, MD; Toni Mancini, RN BSN CCRC; Dara M. Cucinotta, RN; Judy C. Bernbaum, MD; Marsha Gerdes, PhD; Hallam Hurt, MD.
University of Rochester Medical Center, Golisano Children’s Hospital, and the University of Buffalo Women’s and Children’s Hospital of Buffalo (U10 HD68263, UL1 TR42) – Carl T. D’Angio, MD; Ronnie Guillet, MD PhD; Gary J. Myers, MD; Satyan Lakshminrusimha, MD; Anne Marie Reynolds, MD; Michelle E. Hartley-McAndrew, MD; Holly I.M. Wadkins, MA; Michael G. Sacilowski, BS; Linda J. Reubens, RN CCRC; Rosemary L. Jensen; Joan Merzbach, LMSW; William Zorn, PhD; Osman Farooq, MD; Deanna Maffett, RN; Ashley Williams, MS Ed; Julianne Hunn, BS; Stephanie Guilford, BS; Kelley Yost, PhD; Mary Rowan, RN; Diane M. Prinzing, AAS; Karen Wynn, NNP RN; Cait Fallone, MA; Ann Marie Scorsone, MS CCRC.
University of Texas Southwestern Medical Center, Parkland Health & Hospital System, and Children’s Medical Center Dallas (U10 HD40689) – Myra H. Wyckoff, MD; Pablo J. Sánchez, MD; Luc P. Brion, MD; Roy J. Heyne, MD; Diana M. Vasil, MSN RN BSNC-NIC; Sally S. Adams, MS RN CPNP; Lijun Chen, RN PhD; Maria M. De Leon, RN BSN; Frances Eubanks, RN BSN; Alicia Guzman; Elizabeth T. Heyne, MS MA PA-C PsyD; Linda A. Madden, RN BSN CPNP; Nancy A. Miller, RN; Lizette E. Lee, RN; Lara Pavageau, MD; Pollieanna Sepulveda, RN; Cathy Twell Boatman, MS CIMI.
University of Utah University Hospital, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (U10 HD53124, M01 RR64, UL1 TR105) – Roger G. Faix, MD; Bradley A. Yoder, MD; Mariana Baserga, MD MSCI; Karen A. Osborne, RN BSN CCRC; Shawna Baker, RN; Karie Bird, RN BSN; Jill Burnett, RNC BSN; Susan Christensen, RN; Brandy Davis, RN; Jennifer O. Elmont, RN BSN; Jennifer J. Jensen, RN BSN; Manndi C. Loertscher, BS CCRP; Trisha Marchant, RNC; Earl Maxson, RN CCRN; Stephen D. Minton, MD; D. Melody Parry, RN; Carrie A. Rau, RN BSN CCRC; Susan T. Schaefer, RN BSN RRT; Mark J. Sheffield, MD; Cynthia Spencer, RNC BSN; Mike Steffen, PhD; Kimberlee Weaver-Lewis, RN MS; Sarah Winter, MD; Kathryn D. Woodbury, RN BSN; Karen Zanetti, RN.
Wayne State University, Hutzel Women’s Hospital, Children’s Hospital of Michigan, and University of Michigan (U10 HD21385) – Seetha Shankaran, MD; Sanjay Chawla, MD; Beena G. Sood, MD MS; Athina Pappas, MD; Girija Natarajan, MD; Monika Bajaj, MD; Rebecca Bara, RN BSN; Mary E. Johnson, RN BSN; Laura Goldston, MA, Stephanie A. Wiggins, MS; Mary K. Christensen, BA RRT; Martha Carlson, MD; John Barks, MD; Diane F. White, RRT CCRP.
Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital (U10 HD27871, UL1-RR024139, UL1 TR142) – Richard A. Ehrenkranz, MD; Harris Jacobs, MD; Christine G. Butler, MD; Patricia Cervone, RN; Sheila Greisman, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Janet Taft, RN BSN; Elaine Romano, MSN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Sharing: Data reported in this paper may be requested through a data use agreement. Further details are available at https://neonatal.rti.org/index.cfm?fuseaction=DataRequest.Home.
References
- 1.Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics 2009;124:717–28. [DOI] [PubMed] [Google Scholar]
- 2.Samuelsson M, Holsti A, Adamsson M, Serenius F, Hägglöf B, Farooqi A. Behavioral patterns in adolescents born at 23 to 25 weeks of gestation. Pediatrics 2017;140:e20170199. [DOI] [PubMed] [Google Scholar]
- 3.Johnson S, Marlow N. Preterm birth and childhood psychiatric disorders. Pediatr Res 2011;69:11R–8R. [DOI] [PubMed] [Google Scholar]
- 4.Burnett AC, Youssef G, Anderson PJ, Duff J, Doyle LW, Cheong JLY, et al. Exploring the “preterm behavioral phenotype” in children born extremely preterm. J Dev Behav Pediatr 2019;40:200–7. [DOI] [PubMed] [Google Scholar]
- 5.Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993–2012. JAMA 2015;314:1039–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson PJ, Doyle LW. Neurodevelopmental outcome of bronchopulmonary dysplasia. Semin Perinatol 2006;30:227–32. [DOI] [PubMed] [Google Scholar]
- 7.Gray PH, O’Callaghan MJ, Poulsen L. Behaviour and quality of life at school age of children who had bronchopulmonary dysplasia. Early Hum Dev 2008;84:1–8. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt B, Asztalos EV, Roberts RS, Robertson CM, Sauve RS, Whitfield MF, et al. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA 2003;289:1124–9. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt B, Roberts RS, Davis PG, Doyle LW, Asztalos EV, Opie G, et al. Prediction of late death or disability at age 5 years using a count of 3 neonatal morbidities in very low birth weight infants. J Pediatr 2015;167:982–6.e2. [DOI] [PubMed] [Google Scholar]
- 10.Walsh MC, Morris BH, Wrage LA, Vohr BR, Poole WK, Tyson JE, et al. Extremely low birthweight neonates with protected ventilation; mortality and 18-month neurodevelopmental outcomes. J Pediatr 2005;146:798–804. [DOI] [PubMed] [Google Scholar]
- 11.Short EJ, Kirchner HL, Asaad GR, Fulton SE, Lewis BA, Klein N, et al. Developmental sequelae in preterm infants having a diagnosis of bronchopulmonary dysplasia: analysis using a severity-based classification system. Arch Pediatr Adolesc Med 2007;161:1082–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sriram S, Schreiber MD, Msall ME, Kuban KCK, Joseph RM, O’Shea TM, et al. Cognitive development and quality of life associated with BPD in 10-year-olds born preterm. Pediatrics 2018;141:e20172719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jensen EA, Dysart K, Gantz MG, McDonald S, Bamat NA, Keszler M, et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am J Resp Crit Care Med. 2019;200:751–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med 2001;163:1723–9. [DOI] [PubMed] [Google Scholar]
- 15.Achenbach TM, Rescorla LA. Manual for the ASEBA School-age Forms & Profiles. Burlington, VT: University of Vermont Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- 16.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol 1996;87:163–8. [DOI] [PubMed] [Google Scholar]
- 17.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr 1978;92:529–34. [DOI] [PubMed] [Google Scholar]
- 18.Albers CA, Grieve AJ. Bayley scales of infant and toddler development, 3rd edition J Psychoeduc Assess; 2007;25:180–90. [Google Scholar]
- 19.Hayes AF, Preacher KJ. Statistical mediation analysis with a multicategorical independent variable. Br J Math Stat Psychol. 2014;67:451–70. [DOI] [PubMed] [Google Scholar]
- 20.Norman GR, Sloan JA, Wyrwich KW. Interpretation of changes in health-related quality of life: the remarkable universality of half a standard deviation. Med Care 2003;41:582–92. [DOI] [PubMed] [Google Scholar]
- 21.Rescorla LA, Winder-Patel BM, Paterson SJ, Pandey J, Wolff JJ, Schultz RT, et al. Autsim spectrum disorder screening with the CBCL/1½-5: findings for young children at high risk for autism spectrum disorder. Autism 2019;23:29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Read KL, Settipani CA, Peterman J, Kendall PC, Compton S, Piacentini J, et al. Predicting anxiety diagnoses and severity with the CBCL-A: improvement relative to other CBCL scales? J Pscyhopathol Behav Assess 2015;37:100–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peralta-Carcelen M, Carlo WA, Pappas A, Vaucher YE, Yeates KO, Phillips VA, et al. Behavioral problems and socioemotional competence at 18 to 22 months of extremely premature children. Pediatrics 2017;139:e20161043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowe JR, Fuller JF, Do BT, Vohr BR, Das A, Hintz SR, et al. Behavioral problems are associated with cognitive and language scores in toddlers born extremely preterm. Early Hum Dev 2019;128:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brumbaugh JE, Colaizy TT, Patel NM, Klein JM. The changing relationship between bronchopulmonary dysplasia and cognition in very preterm infants. Acta Paediatr 2018;107:1339–44. [DOI] [PubMed] [Google Scholar]
- 26.Thome UH, Carroll W, Wu TJ, Johnson RB, Roane C, Young D, et al. Outcomes of extremely preterm infants randomized at birth to different PaCO2 targets during the first seven days of life. Biol Neonate 2006;90:218–25. [DOI] [PubMed] [Google Scholar]
- 27.Poets CF, Roberts RS, Schmidt B, Whyte RK, Asztalos EV, Bader D, et al. Association between intermitten hypoxemia or bradycardia and late death or disability in extremely preterm infants. JAMA 2015;314:595–603. [DOI] [PubMed] [Google Scholar]
- 28.Qin G, Lo JW, Marlow N, Calvert SA, Greenough A, Peacock JL. Postnatal dexamethasone, respiratory and neurodevelopmental outcomes at two years in babies born extremely preterm. PLoS One 2017;12:e0181176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Månnson J, Stjernqvist K, Bäckström M. Behavioral outcomes at corrected age 2.5 years in children born extremely preterm. J Dev Behav Pediatr 2014;35:435–42. [DOI] [PubMed] [Google Scholar]
- 30.Gough A, Linden MA, Spence D, Halliday HL, Patterson CC, McGarvey L. Executive functioning deficits in young adult survivors of bronchopulmonary dysplasia. Disabil Rehabil 2015;37:1940–5. [DOI] [PubMed] [Google Scholar]
- 31.Mathewson KJ, Chow CH, Dobson KG, Pope EI, Schmidt LA, Van Lieshout RJ. Mental health of extremely low birth weight survivors: a systematic review and meta-analysis. Psychol Bull 2017;143:347–83. [DOI] [PubMed] [Google Scholar]
- 32.Pyhälä R, Wolford E, Kautiainen H, Andersson S, Bartmann P, Baumann N, et al. Self-reported mental health problems among adults born preterm: a meta-analysis. Pediatrics 2017;139:e20162690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


