Abstract
Allostatic load is an indicator of multisystem physiologic dysregulation that may arise from prolonged or accumulated exposure to stress, including adverse childhood experiences (ACEs) and chronic stressors persisting into adulthood. People living with HIV (PLWH) may be particularly vulnerable given their high burdens of adversity across the life course. Using data from a cohort of middle aged PLWH, we examined associations between ACEs and two measures of allostatic load. In order to determine whether the negative impact of ACEs on allostatic load operates through increasing the adoption of adverse coping behaviors, we tested for mediation by smoking and alcohol use. PLWH who had experienced 4 or more ACEs had on average higher allostatic load in adulthood compared to those who experienced fewer. Neither smoking nor alcohol use mediated this relationship, however, suggesting alternative mechanisms may be at play.
Keywords: allostatic load, adverse childhood experiences, health behaviors
INTRODUCTION
The environment in which children are born, grow and develop has the ability to shape trajectories of health and well-being for the rest of their lives. Early childhood adversity, including experiences of physical abuse, sexual abuse, emotional abuse, neglect, and other traumas can lead to life-long physical and mental health consequences.[1, 2] Unfortunately, such experiences are not uncommon in the US where an estimated 1 in 4 children will experience some form of childhood maltreatment in their lifetime.[3]
The ACE (Adverse Childhood Experiences) Study begun by Kaiser Permanente in the mid-1990s established an index of exposure to abuse and household dysfunction including such items as substance abuse, mental illness, violence and incarceration.[4] In collaboration with the Centers for Disease Control and Prevention, the ACE study has documented robust associations between ACEs and early mortality, adulthood morbidities comprising the leading causes of death, and poor quality of life in the US.[5] Moreover, additional research on broad national samples suggests a dose-response relationship between the cumulative number of ACEs experienced and increasing risk for chronic health conditions.[6, 7]
As the negative impacts of ACEs on adult health are increasingly documented, explorations of potential mechanisms underlying these associations are beginning to emerge. Accelerated biological aging as a result of prolonged and cumulative exposure to psychosocial stress may explain earlier onset of age-related chronic diseases.[8] One biologic measure that has been linked to stress across the life course is allostatic load, a measure of multisystemic physiologic dysregulation as a result of wear and tear brought on the body by prolonged or cumulative exposure to stress. Chronic activation of physiological stress response can lead to dysregulation of parameters involved in maintaining homeostasis, including metabolic, inflammatory, cardiovascular, and neuroendocrine systems.[9]
Allostatic load has been identified as a promising tool for understanding how harmful social contexts get “under the skin” to disrupt physiological functioning, placing individuals at earlier and increased risk for adverse clinical outcomes and engendering social inequities in health at a population level.[10, 11] Measures of allostatic load in adulthood may reflect the accumulation of physical consequences directly resulting from stressful experiences themselves, but also changes in lifestyle that result from living under chronic stress, including adoption of unhealthy coping behaviors such as smoking and alcohol use.[12] While documentation of the adverse population health impacts of ACEs continues to grow, empirical tests of such potential mechanisms linking ACEs to adult health remain a critical gap in the literature. Such information is necessary for the development of interventions aiming to interrupt the harmful trajectory set by adverse childhood events. A large number of studies have examined risk of adverse health behaviors after ACEs, and their collective results – summarized in a recent meta-analysis[13] – suggest a two- to three-fold increase in odds of smoking and heavy alcohol use. Less is known about the relationship between smoking and alcohol use and allostatic load, but preliminary evidence suggests their role as potential mediators.[14–16]
Persons living with HIV (PLWH) may be uniquely vulnerable to the negative health consequences of accumulated stressors. Exposure to chronic and lifetime social stressors is a pervasive experience of PLWH,[17–20] with more than one-third often reporting sexual and /or physical abuse before age 18.[21] Early life trauma resulting from ACEs may increase risk of contracting HIV.[22] PLWH, in turn, may continue to experience an accumulation of physical and social stressors directly or indirectly related to living with HIV, manifesting in higher allostatic load than adults in the general population.[23] The purpose of this study was to explore the relation between ACEs and allostatic load in later life among a cohort of PLWH. We hypothesized that adverse experiences in childhood would be associated with greater accumulation of physiologic stress (allostatic load) as PLWH age into older adulthood, and that adoption of harmful coping behaviors across the life course (smoking and alcohol use) may partially explain this relationship.
METHODS
Study Population
In-care PLWH were screened and invited to participate in a translational longitudinal study, the New Orleans Alcohol Use in HIV [NOAH] Study. PLWH were recruited from an HIV outpatient clinic and a local federally qualified health center (FQHC) from October 2015 to October 2017. The overall goal of the parent study is to examine the impact of early life and adult stress on biological and clinical outcomes of PLWH over 2.5 years. We provide a brief description of study recruitment and data collection here, greater detail are provided in an additional publication.[24]
Study eligibility included non-pregnant PLWH age 18 and older who were without acute illness or intoxication at the baseline study visit. There were no further exclusion criteria. Consenting individuals attended a baseline visit at which point data on residential address, alcohol use, physical and mental health measures, and other health-related factors were obtained. In addition to survey data, participants provided blood samples for analysis of HIV clinical outcomes and allostatic load biomarkers, blood pressure and anthropometric measures. Three hundred and sixty-five individuals completed a baseline visit, and those data were used in the present analysis.
Allostatic load
Biomarkers available for the allostatic load index included systolic and diastolic blood pressure; body mass index and waist-to-hip ratio, total cholesterol, high density lipoproteins, low density lipoproteins, and triglycerides; fasting glucose and glycosylated hemoglobin; albumin and C-reactive protein; and cortisol. These indicators capture functioning across the following five physiologic systems, respectively: cardiovascular, metabolic-lipids, glucose metabolism, inflammation, and hypothalamic pituitary-adrenal axis. Height, weight, hip and waist circumference were measured with the participant in a standing position. Blood pressure was measured twice at the right arm with the participant seated, feet flat on the floor, arm resting at heart level. Total cholesterol, lipoproteins, triglycerides, fasting glucose, C-reactive protein, and albumin were assayed from blood collected in vacutainer lithium heparin tubes and processed on the Beckman Coulter AU5822 Automated Clinical Chemistry Analyzer. Glycosylated hemoglobin was assayed from blood collected in vacutainer EDTA tubes and processed on the AU5822 machine. Cortisol was assayed from blood drawn at the study visit (occurring between 7:30–11AM) using the Unicel Dxl 600 Access Immunoassay System.
As is most common in published literature on allostatic load, an allostatic load index was operationalized as the count of biomarkers measured in the highest risk quartile (<25th percentile for high density lipoprotein, >75th percentile for all others) based on the distribution in this sample. Additionally, there is growing evidence of bi-directionality of risk associated with neuroendocrine biomarkers being too high or too low particularly among individuals with high levels of perceived stress, posttraumatic stress, or depression.[23, 25, 26] Given our sample of low income PLHW (91% had annual household incomes <$20,000), many of whom reported high levels of stress and trauma, we operationalized a second measure that included a bidirectional measure of cortisol risk (either <25th or >75th percentile), and conducted a sensitivity analysis using this alternative measure.
Adverse Childhood Experiences
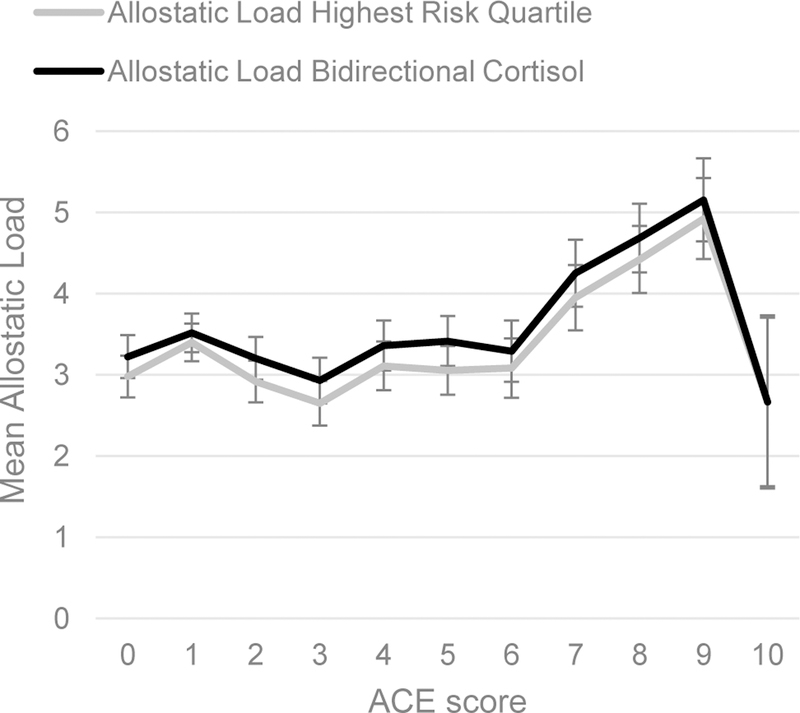
The Adverse Childhood Experiences (ACE) instrument is a 10-item inventory of childhood hardships including physical, sexual, and emotional abuse, neglect, and household dysfunction.[4] Interviewers asked participants to report on events that took place in childhood, before age 18. We examined associations with each ACE individually, as well as the association between allostatic load and a total ACE score. We derived the total ACE score by summing the number of affirmative responses ranging from a possible 0 to 10. We plotted allostatic load means and standard errors (SE) by total ACE score (Figure 1) and patterns were consistent with previous reports of a threshold at 4 or more ACEs increasing physiological impact.[4] Therefore, we dichotomized the total ACE score with values of 4 or more indicating high exposure to ACEs and less than 4 indicating lower exposure.
Figure 1.
Mean allostatic load by number of ACEs.
Smoking and Alcohol Use
Participants completed a lifetime drinking history questionnaire to report the quantity and frequency of various alcohol use by decade since age 10. Lifetime intensity of alcohol use was operationalized as grams per year and dichotomized at ≥75th percentile to identify high lifetime intensity drinkers (≥124 grams per year). We operationalized smoking as lifetime history of ever having smoked or used tobacco products for as long as one year (yes/no). Participants who reported having smoked or used tobacco products for at least a year of their life were identified as ever smokers.
Covariates
Participant demographics (age, race, sex), and educational attainment were collected during the study visit and included in adjusted models. We additionally controlled for adherence to prescribed antiretroviral medication doses given the potential for medication-related physiological alterations that may be independent of allostatic load. HIV medication adherence in the past 3 months was categorically defined as high (took 100% of the prescribed doses), medium (took 90–99% of prescribed doses) or low (took <90% of prescribed doses, or not currently taking medication).
Statistical Analyses
Eleven participants with missing data on allostatic load biomarkers and two with missing data on ACEs and were excluded, leaving an analytic total of n=352. Descriptive statistics characterized the study population. We fit a crude linear regression model to estimate the mean difference (and 95% confidence interval) in allostatic load comparing individuals with high exposure to ACEs to those with low exposure. We then adjusted the model for medication status and sociodemographic characteristics only (age, sex, race, education) in order to avoid over-adjusting for factors that may be along the causal pathway from ACEs to allostatic load. We confirmed the appropriateness of our threshold operationalization of total ACE score (<4 vs. ≥4) by additionally modeling dose response scores from 0 up to 4 or more and testing for liner trend in allostatic load. We fit the same adjusted model in order to identify associations between individual ACE items and allostatic load score. Finally, we tested for heterogeneity in the relationship between high ACE exposure and allostatic load by sex, given previous evidence of important sex differences in neurological responses to stressors[27] and the physical consequences of exposure to ACEs.[28] Based on a priori hypotheses that ACEs may lead to greater alcohol use or smoking – adverse health behaviors which may in turn increase allostatic load – we applied a counterfactual approach to mediation analysis in order to estimate the natural direct and natural indirect effects of ACEs on allostatic load mediated through these factors.[29]
All modeling described above was repeated using the bidirectional cortisol measure of allostatic load as a sensitivity analyses to identify any differential impacts of this alternative operationalization of allostatic load in this highly stressed population.
RESULTS
The study population was predominantly African American (83%) male (68%) and age 50 or older (59%) (Table I). The vast majority of participants reported taking their HIV medication more than 90% of the time, and only twelve participants reported not taking HIV medication. These individuals were grouped with the lowest reported adherence group in order to retain their data for the multivariable modeling. Almost 40% had less than a high school education. On average participants reported 3.4 ACEs and 42% had experienced 4 or more ACEs, classified as high exposure. ACEs ranged in prevalence from 56% (parents separated or divorced) to 14% (parental neglect). Allostatic load ranged from 0 (no biomarkers measured in the highest risk quartile) to 9 with a mean of 3.2 in this sample. Mean allostatic load based on the bidirectional cortisol measure was slightly higher at 3.5 (STD=1.9). Crude mean allostatic load highest risk quartile score was 3.5 among individuals with four or more ACEs and 3.0 among those with fewer than four (p<0.05). This was consistent after adjustments with higher allostatic load among individuals who had experienced four or more ACEs (adjusted mean difference in allostatic load highest risk quartile score = 0.46, 95% CI=0.06, 0.86; Table II). Estimates based on the bidirectional cortisol measure mirrored these findings with scores averaging 0.52 points higher among individuals with a high number of ACEs compared to those with fewer (95% CI=0.11, 0.93).
Table I.
Characteristics of the Study Population (n=352).
| N (%) | |
|---|---|
| Race | |
| Black/African American | 291(82.7) |
| Other | 61 (17.3) |
| Gender | |
| Female | 114 (32.4) |
| Male | 238 (67.6) |
| Age | |
| <40 | 58 (16.5) |
| 40–<50 | 86 (24.4) |
| 50–<60 | 157 (44.6) |
| >=60 | 51 (14.5) |
| Education | |
| Less than High School | 135 (38.4) |
| High School Graduate/GED | 112 (31.9) |
| At least some college | 105 (29.8) |
| Antiretroviral medication | |
| High (100% of prescribed doses) | 161 (45.7) |
| Medium (90–99% of prescribed doses | 134 (38.1) |
| Low (<90% of prescribed doses, or not taking medication) | 57 (16.2) |
| Lifetime alcohol intensity | |
| Low | 251 (74.9) |
| High | 84 (25.1) |
| Ever smoker (yes) | 261 (74.2) |
| Adverse Childhood Experiences (ACEs) | |
| Parents separated or divorced | 196 (55.7) |
| Household member alcoholic, problem drinker, or street drugs user | 166 (47.2) |
| Physical abuse | 148 (42.1) |
| Emotional abuse | 127 (36.1) |
| Never feeling loved or important or supported | 121 (34.5) |
| Household member in prison | 109 (31.0) |
| Witnessed domestic violence towards mother or stepmother | 108 (30.9) |
| Sexual abuse | 87 (24.9) |
| Household member depressed, mentally ill, or attempted suicide | 82 (23.3) |
| Not enough to eat, had to wear dirty clothes, parents too drunk or too high to care take | 50 (14.2) |
| High ACE threshold | |
| <4 | 201 (57.1) |
| ≥4 | 151 (42.9) |
| Mean (SD) | |
| Ace score | 3.4 (2.7) |
| Allostatic load highest risk quartile measure | 3.2 (1.9) |
| Allostatic load highest risk quartile with bidirectional cortisol measure | 3.5 (1.9) |
Table II.
Adjusted mean difference (beta) and 95% confidence interval (CI) for associations with allostatic load scores.
| AL highest risk quartile | AL highest risk quartile with bidirectional cortisol | |||||||
|---|---|---|---|---|---|---|---|---|
| Beta | 95% CI | P | Beta | 95% CI | P | |||
| ACE scorea | ||||||||
| Low | Ref | |||||||
| High | 0.46 | 0.06 | 0.86 | 0.02 | 0.52 | 0.11 | 0.93 | 0.01 |
| Sex | ||||||||
| Men | Ref | |||||||
| Women | −0.12 | −0.54 | 0.30 | 0.58 | −0.30 | −0.72 | 0.13 | 0.17 |
| Age | ||||||||
| <40 | Ref | |||||||
| 40–49 | 0.23 | −0.39 | 0.85 | 0.47 | 0.17 | −0.46 | 0.81 | 0.59 |
| 50–59 | −0.17 | −0.74 | 0.39 | 0.55 | −0.15 | −0.73 | 0.43 | 0.61 |
| 60 and older | 0.11 | −0.60 | 0.81 | 0.76 | −0.03 | −0.75 | 0.69 | 0.94 |
| Race | ||||||||
| Non-black | Ref | |||||||
| Black | −0.09 | −0.55 | 0.53 | 0.96 | −0.03 | −0.52 | 0.58 | 0.92 |
| Education | ||||||||
| Less than high school | Ref | |||||||
| High school graduate/GED | 0.07 | −0.40 | 0.54 | 0.77 | 0.17 | −0.30 | 0.65 | 0.47 |
| Some college or more | 0.24 | −0.25 | 0.72 | 0.34 | 0.37 | −0.13 | 0.87 | 0.15 |
| Medication adherence | ||||||||
| High (100% of prescribed doses) | Ref | |||||||
| Medium (90–99% of prescribed doses | −0.14 | −0.58 | 0.29 | 0.52 | −0.16 | −0.61 | 0.29 | 0.49 |
| Low (<90% of prescribed doses, or not taking medication) | 0.06 | −0.51 | 0.62 | 0.84 | −0.01 | −0.59 | 0.57 | 0.97 |
Statistically significant, p < 0.05
We did not find evidence of a dose-response relationship between ACE scores from 0 up to 4 or more (test for linear trend p-value>0.10), confirming a threshold effect of ACEs on allostatic load as suggested by the plot shown in Figure 1 and as operationalized in our primary analyses described above (<4 vs. ≥4). Non-significant tests for effect modification by gender in both allostatic load models indicated that the association between high exposure to ACEs and allostatic load did not differ between men and women (both interaction term p-values>0.10).
Among individual ACEs, experiences of physical abuse, never feeling loved or important or supported, or living with a household member who was depressed, mentally ill, or attempted suicide were associated with significantly increased allostatic load scores of similar magnitude to the dichotomized ACE total score across both measures of allostatic load (Table III).
Table III.
Adjusted mean difference (beta) and 95% confidence interval (CI) for associations between individual ACEs and allostatic load scores.
| AL highest risk quartile | AL highest risk quartile with bidirectional cortisol | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Beta | 95% CI | P | Beta | 95% CI | P | ||||
| ACEa | |||||||||
| Parents separated or divorced | |||||||||
| No | Ref | Ref | |||||||
| Yes | 0.39 | −0.01 | 0.79 | 0.06 | 0.53 | 0.12 | 0.94 | 0.01 | |
| Household member alcoholic, problem drinker, or street drugs user | |||||||||
| No | Ref | Ref | |||||||
| Yes | 0.31 | −0.08 | 0.70 | 0.12 | 0.36 | −0.46 | 0.76 | 0.04 | |
| Physical abuse | |||||||||
| No | Ref | Ref | |||||||
| Yes | 0.54 | 0.14 | 0.94 | 0.01 | 0.54 | 0.13 | 0.95 | 0.01 | |
| Emotional abuse | |||||||||
| No | Ref | Ref | |||||||
| Yes | 0.29 | −0.12 | 0.70 | 0.16 | 0.26 | −0.16 | 0.68 | 0.23 | |
| Never feeling loved or important or supported | |||||||||
| No | Ref | Ref | |||||||
| Yes | 0.46 | 0.04 | 0.88 | 0.03 | 0.50 | 0.07 | 0.93 | 0.02 | |
| Household member in prison | |||||||||
| No | Ref | Ref | |||||||
| Yes | 0.41 | −0.01 | 0.84 | 0.06 | 0.38 | −0.06 | 0.81 | 0.09 | |
| Witnessed domestic violence towards mother or stepmother | |||||||||
| No | Ref | Ref | |||||||
| Yes | 0.42 | −0.01 | 0.85 | 0.05 | 0.46 | 0.03 | 0.90 | 0.04 | |
| Sexual abuse | |||||||||
| No | Ref | Ref | |||||||
| Yes | 0.16 | −0.32 | 0.63 | 0.52 | 0.19 | −0.30 | 0.67 | 0.45 | |
| Household member depressed, mentally ill, or attempted suicide | |||||||||
| No | Ref | Ref | |||||||
| Yes | 0.51 | 0.04 | 0.98 | 0.03 | 0.58 | 0.10 | 1.06 | 0.02 | |
| Not enough to eat, had to wear dirty clothes, parents too drunk or too high to care take | |||||||||
| No | Ref | Ref | |||||||
| Yes | 0.27 | −0.30 | 0.84 | 0.36 | 0.20 | −0.38 | 0.78 | 0.50 | |
Models adjusted for race, sex, educational attainment, age, and HIV medication adherence
In this sample, lifetime intensity of alcohol use or history of smoking did not appear to mediate the relationship between ACEs and allostatic load after controlling for sociodemographic confounders (Table IV). Non-significant indirect effects were estimated for both potential mediators for both our primary and alternative measures of allostatic load, indicating that the effect of ACEs on allostatic load was not explained by greater smoking or alcohol use over the lifecourse. The natural direct effect in the smoking mediation model indicated that if lifetime prevalence of smoking was the same between participants with a high number of ACEs and those with a low number, those with a high number of ACEs would still have on average approximately 0.50 points higher allostatic load compared with those with fewer ACEs (0.48 [95% CI=0.08, 0.89] points higher on the allostatic load highest risk quartile measure , 0.54 [95% CI=0.13, 0.95] points higher on the allostatic load bidirectional cortisol measure). The natural direct effect of ACEs on the bidirectional cortisol allostatic load measure in the alcohol use mediation mode indicated that even if lifetime alcohol use was equally high among participants with high ACEs and those with fewer, those with high ACEs would still have on average 0.42 points higher allostatic load (95% CI=0.01, 0.84).
Table IV.
Estimates of the natural direct, natural indirect and total effect of the association between ACEs and allostatic load mediated through alcohol use or smoking.a
| Outcome measure | Mediator | Natural direct effect Beta (95% CIb) | P | Natural indirect effect Beta (95% CI) | P | Total effect Beta (95% CI) | P |
|---|---|---|---|---|---|---|---|
| AL highest risk quartile | |||||||
| Alcohol use | 0.37 (−0.04, 0.77) | 0.07 | 0.01 (−0.03, 0.06) | 0.59 | 0.38 (−0.03, 0.80) | 0.06 | |
| Smoking | 0.48 (0.08, 0.89)c | 0.02 | −0.02 (−0.08, 0.04) | 0.50 | 0.46 (0.05, 0.87) c | 0.03 | |
| AL highest risk quartile with bidirectional cortisol | |||||||
| Alcohol use | 0.42 (0.01, 0.84) c | 0.05 | 0.01 (−0.03, 0.06) | 0.54 | 0.44 (0.02, 0.85) c | 0.04 | |
| Smoking | 0.54 (0.13, 0.95) c | 0.01 | −0.02 (−0.08, 0.04) | 0.50 | 0.52 (0.10, 0.94) c | 0.01 |
Models adjusted for race, sex, educational attainment, age, and HIV medication adherence
CI confidence interval
Statistically significant, p < 0.05
DISCUSSION
Accumulating evidence suggests an important role of biologically-mediated pathways through which stress exposure across the lifespan “gets under the skin” and results in gradients of health and clinical outcomes among seronegative [11] and seropositive [30] individuals, and across socioeconomic and racial lines.[31–33] In this cohort of predominantly older and in care PLWH, we find evidence of increased multisystemic physiologic dysregulation following early life adversities. This finding is in line with a growing body of research that suggests adverse childhood experiences become biologically embedded by inducing changes to nervous, endocrine, and immune systems that endure into adulthood.[34] Prevalence of ACEs among our participants was vastly higher than national averages, with respondents experiencing an average of 3.5 adverse experiences in childhood compared to 1.6, the mean number of ACEs reported in the largest and most diverse nationally-representative sample of adults in the US. Furthermore, 43% of our participants reported having experienced 4 or more ACES compared with only 16% nationally.[35] Still, we detected physiologic impact (higher allostatic load) at the threshold of 4 or more ACEs, consistent with previous reports despite ours being a highly stressed cohort.
Ours is among the first to measure allostatic load among PLWH in particular, a population with higher burdens not only of early adversities, but of HIV-related physical and emotional challenges, and chronic stressors arising from sociocontextual disadvantages associated with being low income and non-white. Glover et al.[23] reported higher allostatic load (a composite biomarker index) among Latina and African American women living with HIV compared to HIV-control women involved in their study (means of 2.54 vs 1.75, respectively). We are unable to directly compare levels of allostatic load among PLWH between their study and ours due to the differing number and type of biomarkers collected for analysis (9 in Glover et al., 13 in ours). This is an ongoing challenge in the allostatic load literature. Immense variation in the methodologies and biomarkers used to calculate allostatic load scores prohibits comparison of score values across studies (of the 21 allostatic load publications based on data from the National Health and Nutrition Examination Survey alone, authors employed 18 different ways to calculate allostatic load using 26 different biomarkers).[36]
Heavy alcohol consumption and other substance use has been linked to early life adversity and chronic psychosocial stress,[37, 38] but the role of substance use in the link between adverse experiences in childhood and biologic and clinical outcomes is not well-understood. We theorized that higher allostatic load in adulthood was a reflection of an adverse stress response trajectory set by exposure to multiple ACEs during childhood as well as the physiological harms caused by coping behaviors – smoking and alcohol use – accumulated over the life course. However, we did not find evidence that either behavior mediated the association between ACEs and allostatic load in this sample. This corroborates previous findings in seronegative adults which also found no mediation by health behaviors in the relation between concurrent stress and allostatic load.[39]
Previous evidence documents the deleterious effect of substance use on physiologic stress response pathways, in particular among those experiencing a high level of perceived stress.[40] The number of empirical studies examining the relation between tobacco and alcohol use on allostatic load and not just individual biomarkers of allostatic load are limited, [41–43] but the two have been linked to allostatic load. The relationship, however, has not always been in the hypothesized direction with greater engagement in unhealthy behaviors linked to higher allostatic load, and may differ by sociodemographic groups.[43] Engagement in heavy alcohol use and tobacco use may be a result of exposure to lifetime or current chronic stressors, reflecting coping mechanisms aimed at alleviating stress.[44, 45] While this and other studies have not found evidence of a mediated effect of substance use between stress and allostatic load, this may be due to limitations in study design (all cross-sectional) as well as measurement issues discussed below. Future work should continue to examine these stress pathways longitudinally.
The direct effect of adverse childhood experiences on allostatic load as well as other health outcomes must also be considered and addressed, irrespective of mechanisms. Life course adversity has been associated with adult health, including obesity, hypertension, heart failure and mortality.[46–49] A feature common to those experiencing early life adversity is exposure to greater amounts of psychosocial stress, both within the household and neighborhood,[50] and these stressors may be heightened among PLWH.[17–19] It is also increasingly clear that the impact of early life adversity may persist throughout the lifespan and is potentially transmitted across generations.[51–54] Our results suggest that early life adversity may impact physiological changes directly, rather than through negative health practices associated with stress (e.g., increase substance use).[55]
Our findings should be considered in light of their limitations. These include a limited geographic representation, cross-sectional nature of the baseline study design, and reliance on self-reported exposure data. Furthermore, our measure of allostatic load was limited, particularly for neuroendocrine markers but not unlike many published studies on allostatic load. The sample of PLWH in this study were recruited from an HIV clinic, and therefore findings may not represent PLWH less engaged in care. Additionally, given that our cohort was predominantly older (majority over age 50), it may be that our sample reflects only individuals able to survive to this age and less healthy individuals – those who may have adopted fatally hazardous levels of smoking and alcohol use in response to adverse childhood experiences, for example – were not included. Nonetheless, our results add to the limited literature on the impact of early life adversity among PLWH.
PLWH have an extremely high prevalence of early life adversity and pattern of repeated trauma and stress across the lifespan.[21, 30, 56] A better understanding of the role that both early life and current adversity play in clinical and behavioral outcomes, as well as in overall well-being, would aid in improving the continuum of care in this population. Interventions that identify individuals in HIV clinical settings with histories of traumatic experiences are warranted.
Acknowledgements
We thank the research subjects for their willingness to participate. We acknowledge the hard work and time devoted by study staff, and referring clinicians. They are key to the success of the study. The authors recognize the contributions of study personnel Mary Meyaski-Schluter, RN, and Virginia Garrison, RN. The study was supported by grants from the National Institutes of Health (NIH, P60AA009803). This study was approved by the Tulane University and Louisiana State University Health Sciences Center Institutional Review Boards. The data were collected in compliance with ethical standards regarding treatment of human participants. All authors have contributed significantly to the manuscript, approved the submission of this version, and consent to having their names on the manuscript. No form of payment was given to anyone to produce the manuscript.
Funding. This study was funded by NIH grant P60AA009803.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest. Maeve Wallace declares that she has no conflicts of interest. Erica Felker-Kantor declares that she has no conflicts of interest. Aubrey Madkour declares that she has no conflicts of interest. Tekeda Ferguson declares that she has no conflicts of interest. David Welsh declares that he has no conflicts of interest. Patricia Molina declares that she has no conflicts of interest. Katherine P. Theall declares that she has no conflicts of interest.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all individual participants included in the study.
References
- 1.Lindert J, von Ehrenstein OS, Grashow R, Gal G, Braehler E, Weisskopf MG. Sexual and physical abuse in childhood is associated with depression and anxiety over the life course: systematic review and meta-analysis. Int J Public Health. 2014;59(2):359–72. [DOI] [PubMed] [Google Scholar]
- 2.Buckingham ET, Daniolos P. Longitudinal outcomes for victims of child abuse. Curr Psychiatry Rep. 2013;15(2):342. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Child Maltreatment: Facts at a Glance. Centers for Disease Control and Prevention; 2014. [Available from https://www.cdc.gov/violenceprevention/pdf/childmaltreatment-facts-at-a-glance.pdf] [Google Scholar]
- 4.Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–58. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. Injury Prevention and Control: Division of Violence Prevention: ACE Study 2015. [Available from: http://www.cdc.gov/violenceprevention/acestudy/.
- 6.Gilbert LK, Breiding MJ, Merrick MT, Thompson WW, Ford DC, Dhingra SS, et al. Childhood adversity and adult chronic disease: an update from ten states and the District of Columbia, 2010. Am J Prev Med. 2015;48(3):345–9. [DOI] [PubMed] [Google Scholar]
- 7.Campbell JA, Walker RJ, Egede LE. Associations Between Adverse Childhood Experiences, High-Risk Behaviors, and Morbidity in Adulthood. Am J Prev Med. 2015. [DOI] [PMC free article] [PubMed]
- 8.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338(3):171–9. [DOI] [PubMed] [Google Scholar]
- 9.Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev. 2010;35(1):2–16. [DOI] [PubMed] [Google Scholar]
- 10.Seeman TE, Singer BH, Rowe JW, Horwitz RI, McEwen BS. Price of adaptation--allostatic load and its health consequences. MacArthur studies of successful aging. Arch Intern Med. 1997;157(19):2259–68. [PubMed] [Google Scholar]
- 11.Geronimus AT, Hicken M, Keene D, Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96(5):826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrovic D, Pivin E, Ponte B, Dhayat N, Pruijm M, Ehret G, et al. Sociodemographic, behavioral and genetic determinants of allostatic load in a Swiss population-based study. Psychoneuroendocrinology. 2016;67:76–85. [DOI] [PubMed] [Google Scholar]
- 13.Hughes K, Bellis MA, Hardcastle KA, Sethi D, Butchart A, Mikton C, et al. The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. Lancet Public Health. 2017;2(8):e356–e66. [DOI] [PubMed] [Google Scholar]
- 14.Crimmins EM, Kim JK, Seeman TE. Poverty and biological risk: the earlier “aging” of the poor. J Gerontol A Biol Sci Med Sci. 2009;64(2):286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomfohr LM, Pung MA, Dimsdale JE. Mediators of the Relationship Between Race and Allostatic Load in African and White Americans. Health Psychol. 2016;35(4):322–32. [DOI] [PubMed] [Google Scholar]
- 16.Doan SN, Dich N, Evans GW. Childhood Cumulative Risk and Later Allostatic Load: Mediating Role of Substance Use. Health Psychol. 2014;33(11):1402–9. [DOI] [PubMed] [Google Scholar]
- 17.Arnold M, Hsu L, Pipkin S, McFarland W, Rutherford GW. Race, place and AIDS: the role of socioeconomic context on racial disparities in treatment and survival in San Francisco. Soc Sci Med. 2009;69(1):121–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joy R, Druyts EF, Brandson EK, Lima VD, Rustad CA, Zhang W, et al. Impact of neighborhood-level socioeconomic status on HIV disease progression in a universal health care setting. J Acquir Immune Defic Syndr. 2008;47(4):500–5. [DOI] [PubMed] [Google Scholar]
- 19.Kelly JA, Murphy DA, Bahr GR, Koob JJ, Morgan MG, Kalichman SC, et al. Factors associated with severity of depression and high-risk sexual behavior among persons diagnosed with human immunodeficiency virus (HIV) infection. Health Psychol. 1993;12(3):215. [DOI] [PubMed] [Google Scholar]
- 20.Latkin CA, German D, Vlahov D, Galea S. Neighborhoods and HIV: a social ecological approach to prevention and care. Am Psychol. 2013;68(4):210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whetten K, Leserman J, Lowe K, Stangl D, Thielman N, Swartz M, et al. Prevalence of childhood sexual abuse and physical trauma in an HIV-positive sample from the deep south. Am J of Public Health. 2006;96(6):1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fang L, Chuang DM, Lee Y. Adverse childhood experiences, gender, and HIV risk behaviors: Results from a population-based sample. Prev Med Rep. 2016;4:113–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glover DA, Garcia-Aracena EF, Lester P, Rice E, Rothram-Borus MJ. Stress biomarkers as outcomes for HIV+ prevention: participation, feasibility and findings among HIV+ Latina and African American mothers. AIDS Behav. 2010;14(2):339–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Welsh DA, Ferguson T, Theall KP, Simon L, Amedee A, Siggins RW, et al. The New Orleans Alcohol Use in HIV Study: Launching a Translational Investigation of the Interaction of Alcohol Use with Biological and Socioenvironmental Risk Factors for Multimorbidity in People Living with HIV. Alcohol Clin Exp Res. 2019;43(4):704–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glover DA, Stuber M, Poland RE. Allostatic load in women with and without PTSD symptoms. Psychiatry. 2006;69(3):191–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hellhammer J, Schlotz W, Stone AA, Pirke KM, Hellhammer D. Allostatic load, perceived stress, and health - A prospective study in two age groups. Biobehavioral Stress Response: Protective and Damaging Effects. 2004;1032:8–13. [DOI] [PubMed] [Google Scholar]
- 27.McEwen BS. Neurobiological and Systemic Effects of Chronic Stress. Chronic Stress (Thousand Oaks). 2017;1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alcala HE, Tomiyama AJ, von Ehrenstein OS. Gender Differences in the Association between Adverse Childhood Experiences and Cancer. Women Health Iss. 2017;27(6):625–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013;18(2):137–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pence BW, Mugavero MJ, Carter TJ, Leserman J, Thielman NM, Raper JL, et al. Childhood Trauma and Health Outcomes in HIV-Infected Patients: An Exploration of Causal Pathways. J Acquir Immune Defic Syndr. 2012;59(4):409–16 10.1097/QAI.0b013e31824150bb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adler NE, Boyce W. Thomas, chesney, Margaret A, Folkman, Susan, Syme S. Leonard. Socioeconomic Inequalities in Health: No Easy Solution. JAMA. 1993;Vol. 269(No. 24):3140–5. [PubMed] [Google Scholar]
- 32.Lupien S, King S, Meaney M, McEwen B. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev Psychopathol. 2001;13(03):653–76. [DOI] [PubMed] [Google Scholar]
- 33.Felitti M, Vincent J, Anda M, Robert F, Nordenberg M, Williamson M, et al. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the Adverse Childhood Experiences (ACE) Study. Am J Prev Med. 1998;14(4):245–58. [DOI] [PubMed] [Google Scholar]
- 34.Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106(1):29–39. [DOI] [PubMed] [Google Scholar]
- 35.Merrick MT, Ford DC, Ports KA, Guinn AS. Prevalence of Adverse Childhood Experiences From the 2011–2014 Behavioral Risk Factor Surveillance System in 23 States. JAMA Pediatr. 2018;172(11):1038–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duong MT, Bingham BA, Aldana PC, Chung ST, Sumner AE. Variation in the Calculation of Allostatic Load Score: 21 Examples from NHANES. J Racial Ethn Health. 2017;4(3):455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pence BW. The impact of mental health and traumatic life experiences on antiretroviral treatment outcomes for people living with HIV/AIDS. J Antimicrob Chemother. 2009;63(4):636–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brief DJ, Bollinger A, Vielhauer M, Berger-Greenstein J, Morgan E, Brady S, et al. Understanding the interface of HIV, trauma, post-traumatic stress disorder, and substance use and its implications for health outcomes. AIDS Care. 2004;16(sup1):97–120. [DOI] [PubMed] [Google Scholar]
- 39.Schulz AJ, Mentz G, Lachance L, Johnson J, Gaines C, Israel BA. Associations between socioeconomic status and allostatic load: effects of neighborhood poverty and tests of mediating pathways. Am J Public Health. 2012;102(9):1706–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Obasi EM, Shirtcliff EA, Brody GH, MacKillop J, Pittman DM, Cavanagh L, et al. The relationship between alcohol consumption, perceived stress, and CRHR1 genotype on the hypothalamic-pituitary-adrenal axis in rural African Americans. Front Psychol. 2015;6:832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crimmins EM, Kim JK, Seeman TE. Poverty and biological risk: the earlier “aging” of the poor. Journals of Gerontology Series A: Biomedical Sciences and Medical Sciences. 2009;64(2):286–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hu P, Wagle N, Goldman N, Weinstein M, Seeman TE. The associations between socioeconomic status, allostatic load and measures of health in older Taiwanese persons: Taiwan social environment and biomarkers of aging study. J Biosoc Sci. 2007;39(4):545–56. [DOI] [PubMed] [Google Scholar]
- 43.Rodriquez EJ, Livaudais-Toman J, Gregorich SE, Jackson JS, Nápoles AM, Pérez-Stable EJ. Relationships between allostatic load, unhealthy behaviors, and depressive disorder in US adults, 2005–2012 NHANES. Prev Medicine. 2018;110:9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peele S, Brodsky A. Exploring psychological benefits associated with moderate alcohol use: a necessary corrective to assessments of drinking outcomes? Drug Alcohol Depen. 2000;60(3):221–47. [DOI] [PubMed] [Google Scholar]
- 45.Lipton RI. The effect of moderate alcohol use on the relationship between stress and depression. Am J Public Health. 1994;84(12):1913–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Baltrus PT, Lynch JW, Everson-Rose S, Raghunathan TE, Kaplan GA. Race/ethnicity, life-course socioeconomic position, and body weight trajectories over 34 years: the Alameda County Study. Am J Public Health. 2005;95(9):1595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roberts CB, Couper DJ, Chang PP, James SA, Rosamond WD, Heiss G. Influence of life-course socioeconomic position on incident heart failure in blacks and whites: the Atherosclerosis Risk in Communities Study. Am J Epidemiol. 2010;172(6):717–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.James SA, Van Hoewyk J, Belli RF, Strogatz DS, Williams DR, Raghunathan TE. Life-course socioeconomic position and hypertension in African American men: the Pitt County Study. Am J Public Health. 2006;96(5):812–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mishra GD, Chiesa F, Goodman A, De Stavola B, Koupil I. Socio-economic position over the life course and all-cause, and circulatory diseases mortality at age 50–87 years: results from a Swedish birth cohort. Eur J Epidemiol. 2013;28(2):139–47. [DOI] [PubMed] [Google Scholar]
- 50.Evans GW, Marcynyszyn LA. Environmental Justice, Cumulative Environmental Risk, and Health Among Low- and Middle-Income Children in Upstate New York. Am J Public Health. 2004;94(11):1942–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drury S, Theall K, Gleason M, Smyke A, De Vivo I, Wong J, et al. Telomere length and early severe social deprivation: linking early adversity and cellular aging. Mol Psychiatry. 2011;1:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Theall KP, Brett Z, Shirtcliff EA, Dunn E, Drury S. Neighborhood disorder and telomeres: Connecting children’s exposure to community level stress and cellular response. Soc Sci Med. 2013;85 50–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fonagy P. The transgenerational transmission of holocaust trauma. Hum Dev. 1999;1(1):92–114. [DOI] [PubMed] [Google Scholar]
- 54.Yehuda R, Bierer LM. Transgenerational transmission of cortisol and PTSD risk In: Ronald De Kloet MSO E, Eric V, editors. Prog Brain Res. Volume 167: Elsevier; 2007. p. 121–35. [DOI] [PubMed] [Google Scholar]
- 55.Loucks EB, Juster RP, Pruessner JC. Neuroendocrine biomarkers, allostatic load, and the challenge of measurement: A commentary on Gersten. Soc Sci Med. 2008;66(3):525–30. [Google Scholar]
- 56.Machtinger E, Wilson T, Haberer J. Psychological trauma and PTSD in HIV-positive women: a meta analysis. AIDS Behav. 2012;16(8):2091–100. [DOI] [PubMed] [Google Scholar]