Abstract
Objective.
To test whether the composite outcome of death or neurodevelopmental impairment (NDI) at 18-22 months corrected age for infants ≤1000 grams at birth is reduced by continuous monitoring of heart rate characteristics during neonatal intensive care.
Study design.
We studied a subset of participants enrolled in a multi-center randomized trial of HRC monitoring. Survivors were evaluated at 18-22 months corrected age with a standardized neurological examination and the Bayley Scales of Infant Development-III (BSID-III). NDI was defined as Gross Motor Function Classification System >2 (moderate or severe cerebral palsy), BSID-III language or cognitive scores <70, severe bilateral hearing impairment, and/or bilateral blindness.
Results.
The composite outcome, death or NDI, was obtained for 628/884 (72%) study infants. The prevalence of this outcome was 44.4% (136/306) among controls (infants randomized to HRC monitored but not displayed) and 38.9% (125/322) among infants randomized to HRC monitoring displayed (RR 0.87, 95% CI 0.73 to 1.05, p=0.17). Mortality was reduced from 32.0% (99/307) among controls to 24.8% (81/326) among monitoring displayed infants (RR 0.75, 95% CI 0.59 to 0.97, p=0.028). The composite outcomes of death or severe CP and death or mildly low Bayley Cognitive score occurred less frequently in the displayed group (p < 0.05).
Conclusions.
We found no difference in the composite outcome of death or NDI for extremely preterm infants whose HRC were and were not displayed during neonatal intensive care. Two outcomes that included mortality or a specific neurodevelopmental impairment were less frequent in the displayed group.
Advances in neonatal intensive care have increased survival of extremely low-birth-weight infants (ELBW, weighing ≤1000 g at birth) but about one third of survivors have moderate to severe neurodevelopmental impairment when assessed at 18-22 months.1, 2 By school age approximately 50% of ELBW infants show cognitive deficits and 17% have cerebral palsy (CP).3, 4 Systemic infections, a frequent complication among ELBW preterm infants, are associated with short-term sequelae and an increased risk of death.5, 6 There is mounting evidence that nosocomial infections and the systemic inflammatory response damage cerebral white matter7, 8 and contribute to long-term disability in ELBW infants.9–11
The diagnosis of sepsis in the newborn period is challenging. The clinical signs of nosocomial sepsis and/or meningitis in ELBW infants are often non-specific. Blood and body fluid cultures are reported sterile in more than one third of ELBW infants with a clinical sepsis syndrome.10 There are numerous adjunctive, nonspecific diagnostic and screening tests used to aid in the diagnosis of neonatal sepsis and/or meningitis. Although abnormalities of the complete blood count and neutrophil indices, acute phase reactants, and cytokines may suggest serious infection, few of these adjunctive tests have a high specificity and only rarely have a positive predictive value of more than 40% for proven neonatal sepsis.12
A recent advance in the diagnosis of neonatal sepsis is continuous heart rate characteristics (HRC) monitoring to detect the presence of reduced variability and transient decelerations, which occur with increased frequency in the preclinical phases of septicemia.13, 14 The result is a monitoring system that has allowed diagnosis and treatment of bacterial sepsis in infants prior to showing overt signs of illness. HRC monitoring underwent rigorous testing as part of a NICHD-funded multi-center, randomized, controlled trial of 3003 very low birth weight infants (<1500 g at birth), including 1513 ELBW infants.15 The current study was motivated by the need to assess the impact of HRC technology on the prevention or amelioration of disability in survivors of extreme prematurity. We hypothesized that HRC monitoring of ELBW infants leads to a reduction of moderate to severe NDI or death at 18-22 months for these infants.
METHODS
The HRC monitoring trial was a multi-center, randomized controlled trial in which 3003 very low birth weight infants (≤1500 g) were enrolled at 9 hospitals (ClinicalTrials.gov: NCT00307333) The methods and results of the trial have been reported.15, 16 Briefly, in the HRC trial, infants were randomly assigned to one of two groups after written, informed parental consent. One group of infants had the HRC index displayed to the care team for the infants (Display group). Providers used the HRC index to aid in early detection and treatment of nosocomial infection in the Display group. Infants in the Control group had the HRC index recorded by the data collection devices, but this information was not displayed locally to the team caring for the infants. Infants had blood, urine, and cerebrospinal fluid cultures obtained and antibiotics started based on clinical suspicion of infection. For the Control group infants, this management continued as usual practice. For the infants in the Display group, the use of the HRC index may have influenced the frequency and timing of obtaining cultures and starting antibiotics. When the HRC index was considered abnormal by the clinician, then a complete blood count, blood culture, and other laboratory tests to assist with the assessment of the infant may have been obtained. In addition, the clinician may have obtained cultures and started antibiotics at his/her discretion if there was a clinical suspicion of infection, but the HRC index had not reached a concerning level or was not displayed. Antibiotics were administered at the discretion of the attending neonatologist. Thus, infants were not denied antibiotics if the clinicians felt that antibiotics were indicated.
Centers were selected for the follow up component of the HRC trial a priori based on the merits of their neurodevelopmental follow up programs and consistency of the 18-22 months follow up protocol. Investigators at the University of Alabama at Birmingham, Wake Forest University, and the University of Miami enrolled over 50% of the patients in the HRC trial. All extremely low birth weight infants (≤1000 grams) in the HRC trial enrolled at these three sites were eligible to participate in the current study. Follow up data were added to the HRC trial in hospital database. Infant characteristics during the first 120 days from randomization were collected for the HRC trial. These included birth weight, gestational age, pregnancy, labor, delivery complications, and Apgar scores. The research ethics board at each of the clinical centers approved the protocol.
Survivors underwent a comprehensive neurodevelopmental and neurological assessment at 18 to 22 months of corrected age by examiners unaware of the treatment group. Cognitive function was assessed with the Bayley Scales of Infant and Toddler Development, third edition (BSID-III); scores are assessed relative to a standardized mean (±SD) of 100±15 in the normative data, with higher scores indicating better performance. 17 The modified Gross Motor Function Classification System (GMFCS) was used to classify gross-motor performance, with levels ranging from 0 (normal) to 5 (most impaired).18 Moderate cerebral palsy was defined as a GMFCS level of 2-3 and severe cerebral palsy was defined as a GMFCS level of 4-5.19, 20 Permanent hearing loss was defined as a requirement for bilateral hearing aids and blindness was defined as no functional vision in either eye.
The pre-specified primary composite outcome for this study was death before 18 to 22 months or neurodevelopmental impairment (NDI) at 18 to 22 months of corrected age. Neurodevelopmental impairment was defined as one or more of the following: a cognitive or language composite score on the BSID-III of <70, GMFCS of 2 or higher (moderate or severe cerebral palsy), hearing impairment, or bilateral visual impairment. The other pre-specified secondary outcomes were individual components of the primary outcome, and mild NDI (GMFCS 1 or BSID-III 70-84).
The sample size was based on the number of infants with birth weight < 1000 grams enrolled at the three study sites and was sufficient to detect a reduction in the proportion with death/NDI of 10 percentage points (ie, 20% in the intervention group vs. 30% for the control group) with 0.80 power and 2-sided significance level of 0.05 which would require 313 infants per group, for a total of 626.
Data were entered on a database maintained at the University of Virginia. Baseline data and treatment group differences for infants who have known and unknown primary outcome (death or NDI) were compared by Student t tests for continuous data and χ2 tests for categorical data. Fisher exact test was used for categorical outcomes with frequency counts <6. Secondary outcomes include the components of NDI in survivors and death or moderate to severe CP, death or BSID-III Cognitive and Language scale <70, death or hearing impairment, and death or blindness in all participants.
RESULTS
Death or NDI, the primary composite outcome, was known in 322 (73%) infants in the monitor Display group and 306 (69%) in the Control Group. Of the 884 infants enrolled in the follow up component of the original trial, 636 infants were available for follow-up at 18-22 months corrected age. A total of 458 infants was seen (248 in the Display group, 210 in the Control group), which was 74.7% and 69.2% of surviving infants, respectively (Figure).
Figure 1.
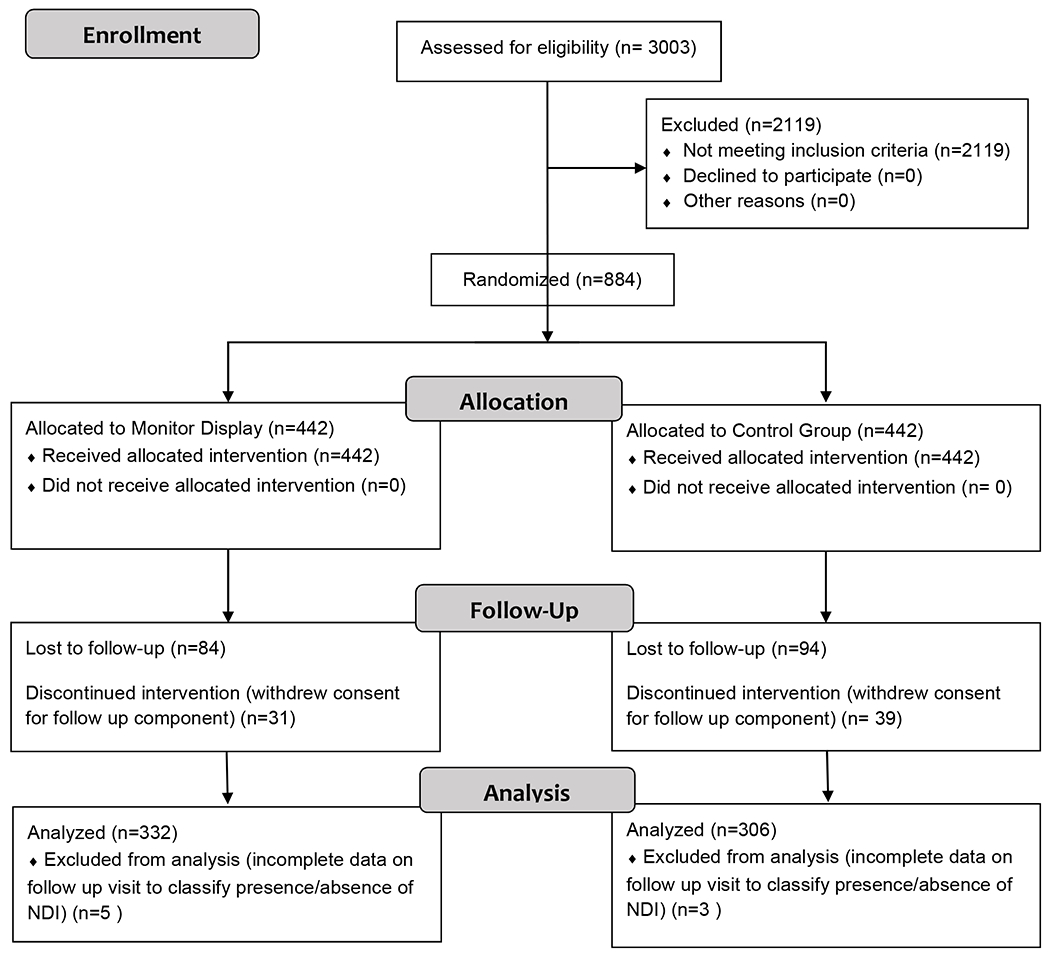
Consolidated Standards of Reporting Trials diagram
Baseline perinatal variables for the infants, including, gestational age, male sex, maternal chorioamnionitis, antenatal steroids and Apgar score <7 at 5 minutes did not differ between treatment groups. Infants in the monitor Display Group were on average 23 grams heavier at birth than the Control group (746 grams ± 154 grams versus 723 ± 152, p = 0.05). The mean corrected age at the time of follow-up evaluation was 19.6 ± 2 (SD) months for both the monitor Display and Control groups. Infants lost to follow up were heavier at birth (794 versus 735 grams), P < .001; more mature (27 versus 26 weeks); and less likely to have maternal antenatal steroids administered (71% versus 79%), p =0.01 when compared with the infants whose primary outcome was known. There were no detectable differences in the loss to follow up group with respect to sex, maternal chorioamnionitis and Apgar scores <7 at five minutes of age (Table I).
Table 1.
Characteristics of the infants with outcome known and not known according to monitoring assignment
| Variable | Outcome Known Display |
Control | P1 | Outcome not known Display |
Control | P1 | Comparison P-value2 |
|---|---|---|---|---|---|---|---|
| n = 322 | n = 306 | n = 120 | n = 136 | ||||
| Baseline variables | |||||||
| Birth weight – g.* | 746±154 | 723±152 | 0.05 | 811±140 | 779±148 | 0.07 | 0.0001 |
| Gestational age – wk.* | 26±2.1 | 26±1.9 | 0.3 | 27±2.1 | 27±2.1 | 0.11 | 0.0001 |
| Male – no. (%) | 172(53.4) | 153(50.0) | 0.42 | 59(49.2) | 57(41.9) | 0.26 | 0.08 |
| Maternal Chorioamnionitis - no. (%) | 37(11.5) | 37(12.1) | 0.9 | 10(8.3) | 17(12.5) | 0.31 | 0.64 |
| Antenatal steroids - no. (%) | 256(79.5) | 239(78.1) | 0.69 | 83(69.2) | 98(72.1) | 0.68 | 0.01 |
| Neonatal Outcomes** | |||||||
| Apgar 5 min <7 - no. (%) | 112/319(37.1) | 118/302(37.0) | 1.0 | 38(32.2) | 46(34.9) | 0.69 | 0.35 |
| Culture proven sepsis | 130/322(40.4) | 131/306(42.8) | 0.57 | 42/120(35.0) | 49/136(36.0) | 0.89 | 0.11 |
Plus-minus values are means ± SD.
Total number refers to the number of infants assessed for the outcome if different from the number in that group.
Among the infants with known primary outcome, the proportion with death or NDI did not differ between groups (RR = 0.89 [95% CI = 0.74-1.07]). Death was reduced from 32.0% (99/309) for infants in the Control group to 24.2% (79/327) in monitor Display group (RR 0.75 95% CI 0.59 to 0.97, p=0.028). Regarding death or individual components of NDI, all relative risk estimates were < 1 and the 95% CI crossed over 1. (Table II). Among survivors seen at follow up, the proportion of infants who were blind and who had abnormal neurological examination did not significantly differ. More infants in the Display group than the Control group were deaf 11/248 (4.4%) versus 1/210 (0.5%), p=0.008.
Table 2.
Mortality and neurodevelopmental findings at 18 to 22 months according to treatment assignment
| Variable | Display No. / total No. (%) |
Control | RR (95% CI) | P-value |
|---|---|---|---|---|
| Primary outcome | ||||
| Death or NDI | 127/321(39.6) | 136/306(44.4) | 0.89(0.74,1.07) | 0.223 |
| Death | 79/327(24.2) | 99/309(32.0) | 0.75(0.59,0.97) | 0.028 |
| Death or the following specific components of NDI | ||||
| GMFCS Level 2-5 -- moderate/severe CP | 127/321(39.6) | 136/306(44.4) | 0.89(0.74,1.07) | 0.223 |
| Bilateral blindness | 83/326(25.5) | 99/309(32.0) | 0.79(0.62-1.02) | 0.079 |
| Deafness | 90/327(27.5) | 100/309(32.4) | 0.85(0.67,1.08) | 0.194 |
| Bayley Cognitive <70 | 102/322(31.7) | 113/306(36.9) | 0.86(0.69,1.07) | 0.179 |
| Bayley Language <70 | 115/320(35.9) | 127/305(41.6) | 0.86(0.71,1.05) | 0.163 |
| NDI and specific components of NDI, survivors only | ||||
| NDI | 53/247(21.5) | 40/210(19.1) | 1.12(0.78-1.63) | 0.561 |
| GMFCS Level 2-5 -- moderate/severe CP | 23/246(9.4) | 13/210(6.2) | 1.51(0.78,2.9) | 0.223 |
| Bilateral blindness | 4/247(1.6) | 0/210(0) | 0(0-0) | 0.128 |
| Deafness | 11/248(4.4) | 1/210(0.5) | 9.31(1.21,71.55) | 0.008 |
| Bayley Cognitive <70 | 23/243(9.5) | 15/207(7.3) | 1.31(0.70,2.43) | 0.497 |
| Bayley Language <70 | 36/241(14.9) | 28/206(13.6) | 1.01(0.69,1.74) | 0.787 |
NDI is neurodevelopmental impairment (GMFCS Level 2-5 -- moderate/severe cerebral palsy, blind, deaf, language or cognitive Bayley score <70). CP is cerebral palsy. GMFCS is the Gross Motor Function Classification System. RR is the relative risk of the outcome in the Display group as compared with the Control group. CI denotes confidence interval.
To further delineate the impact of HRC monitoring on specific neurodevelopmental outcomes, while controlling for death, we examined mild, moderate and severe CP individually as well as intermediate Bayley III cognitive and language levels (70 – 84). Fewer infants in the Display group than those in the Control group had death or mild CP (114/325, 35.4% versus 134/309, 43.4% p = 0.035), severe CP (85/325, 26.2% versus 104/309, 33.7% p = 0.046), and Bayley Cognitive scores 70 – 84 (106/321, 33.0% versus 125/306, 40.8% p = 0.047) Table III.
Table 3.
Mortality and additional neurodevelopmental findings at 18 to 22 months according to treatment assignment
| Variable | Display No. / total No. (%) |
Control | RR (95% CI) | P-value |
|---|---|---|---|---|
| Death or the following neurodevelopmental outcomes: | ||||
| GMFCS Level 1 -- mild CP | 114/325(35.1) | 134/309(43.4) | 0.81(0.67,0.98) | 0.035 |
| GMFCS Level 2-3 -- moderate CP | 96/325(29.5) | 107/309(34.6) | 0.85(0.68,1.07) | 0.174 |
| GMFCS Level 4-5 -- severe CP | 85/325(26.2) | 104/309(33.7) | 0.78(0.61,0.99) | 0.046 |
| Bayley Cognitive 70 - 84 | 106/321(33.0) | 125/306(40.8) | 0.81(0.66-0.99) | 0.047 |
| Bayley Language 70 - 84 | 148/320(46.3) | 164/306(53.6) | 0.86(0.74,1.01) | 0.079 |
GMFCS is the Gross Motor Function Classification System. CP is cerebral palsy. RR is the relative risk of the outcome in the Display group as compared with the Control Group. CI denotes confidence interval.
DISCUSSION
For the ELBW infants enrolled in the 18-22 month follow up component of the original HRC trial, there was a significant reduction in mortality for those infants whose heart rate characteristics were displayed to the clinical care team. Although no specific evaluation or treatment for infection was dictated by the original study 15 for increases in the HRC predicted infection risk score, providing the risk information resulted in significantly lower mortality. This mortality reduction may have been due to earlier recognition and empirical treatment for nosocomial infection.16 We hypothesized that earlier recognition and treatment for infection would ameliorate disability in survivors of extreme prematurity. Contrary to our hypothesis, survivors in the HRC Displayed group did not have discernable reductions in the predetermined components of NDI, nor was there excess morbidity.
Increased hearing loss noted among survivors in the HRC Display group was an unanticipated finding. Improved survival in the HRC Display group seems a likely explanation for this group’s higher rate of deafness, as an intervention that improves survival of infants most vulnerable to deafness could increase the rate of deafness among survivors. Consistent with this possibility is that infants with hearing loss in the HRC follow up study were smaller (695 ± 129 grams versus 776 ± 137 grams, p=0.04) and more immature at birth (25 ± 2 weeks versus 26 ± 2 weeks, p=0.01). A less likely explanation for the higher rate of hearing loss among survivors in the HRC-display group is ototoxicity due to the 3-day increase in antibiotic exposure in the HRC Display group, as previously reported. 16 However, treatment with ototoxic aminoglycoside antibiotics alone has not been definitively linked to excess hearing loss.21 The higher prevalence of increased deafness among survivors in the HRC-display group should be considered in light of the finding that death or deafness occurred less frequently in the HRC-display group, although the difference was not statistically significant, and other factors potentially associated with deafness.
In an exploratory analysis, we questioned the influence of HRC monitoring for ELBW infants who develop less severe cognitive impairment and varying severity of cerebral palsy. In the HRC Display group, there was an 8.3% absolute reduction of death or mild CP, a 7.5% absolute reduction of death or severe CP, and a 7.8% absolute reduction of death or moderate cognitive impairment (Bayley III Cognitive level 70-84) when compared with the Control group (p = 0.035, 0.046 and 0.047 respectively). An intervention that decreases mortality and reduces moderate cognitive delays, without increasing severe disability, could have important implications for ELBW survivors. Early intervention tends to have a greater impact on long-term outcome for infants with moderate delays than for those with severe delays.22, 23 However, such sub group analyses should be interpreted with caution.
There are limitations to our study. Although the rate of loss to follow up was similar in the HRC Display and Control groups (27.1% and 30.8%), this is a potential source of bias and loss of statistical power. Infants whose outcome could not be determined due to loss to follow up were on average 59 grams heavier at birth and one week more mature, 27 weeks versus 26 weeks. Because of the known influence of birth weight and gestational age on prevalence of neurodevelopmental disability, we might have over-estimated the proportion of infants with the composite primary outcome and individual components of NDI. We identified neurodevelopmental impairments at 18-22 months; often impairments observed at that age do not persist at school age.24 There are also strengths to our study. We prospectively followed a large cohort of ELBW infants who participated in a randomized controlled trial with pre-planned analysis and pre-specified definitions for outcomes. Trained examiners used standardized neurological assessments and psychometric evaluations. The patient population demographics and neurodevelopmental outcomes of patients in the HRC trial are comparable with large observational studies of ELBW infants from the Eunice Kennedy Shriver National Institute of Child Health and Human Developmental Neonatal Research Network.6, 10, 11,25, 26
ACKNOWLEDGMENTS
We thank M. Pamela Griffin, MD (Global Clinical Lead, BioPharmaceuticals AstraZeneca), and William E. King MS, (Chief Executive Officer of Medical Predictive Science Corporation) for contributions in the clinical application of heart rate characteristics monitoring in neonates and data warehousing for the original clinical trial.
Supported by the National Institutes of Health grant (R01-HD48562 [to J.M.], R01 HD048562 [to T.O.]), and Florida-Children’s Medical Services (COQZH [to C.B.]). J.M. and D.L. have consulting agreements and equity shares in Medical Predictive Science Corporation, Charlottesville, VA. The other authors declare no conflicts of interest.
ABBREVIATIONS
- NDI
neurodevelopmental impairment
- BSID-III
Bayley Scales of Infant and Toddler Development, third edition
- CP
cerebral palsy
- GMFCS
Gross Motor Function Classification System
- ELBW
extremely low-birth-weight.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Rysavy MA, Li L, Bell EF, Das A, Hintz SR, Stoll BJ, et al. Between-hospital variation in treatment and outcomes in extremely preterm infants. The New England journal of medicine. 2015;372:1801–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Fanaroff AA, Stoll BJ, Wright LL, Carlo WA, Ehrenkranz RA, Stark AR, et al. Trends in neonatal morbidity and mortality for very low birthweight infants. American journal of obstetrics and gynecology. 2007;196:147.e1–8. [DOI] [PubMed] [Google Scholar]
- [3].Hirschberger RG, Kuban KCK, O’Shea TM, Joseph RM, Heeren T, Douglass LM, et al. Co-occurrence and Severity of Neurodevelopmental Burden (Cognitive Impairment, Cerebral Palsy, Autism Spectrum Disorder, and Epilepsy) at Age Ten Years in Children Born Extremely Preterm. Pediatric neurology. 2018;79:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Joseph RM, O’Shea TM, Allred EN, Heeren T, Hirtz D, Jara H, et al. Neurocognitive and Academic Outcomes at Age 10 Years of Extremely Preterm Newborns. Pediatrics. 2016;137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Stoll BJ, Hansen N. Infections in VLBW infants: studies from the NICHD Neonatal Research Network. Seminars in perinatology. 2003;27:293–301. [DOI] [PubMed] [Google Scholar]
- [6].Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–91. [DOI] [PubMed] [Google Scholar]
- [7].Dammann O, Kuban KC, Leviton A. Perinatal infection, fetal inflammatory response, white matter damage, and cognitive limitations in children born preterm. Mental retardation and developmental disabilities research reviews. 2002;8:46–50. [DOI] [PubMed] [Google Scholar]
- [8].Hagberg H, Mallard C. Effect of inflammation on central nervous system development and vulnerability. Current opinion in neurology. 2005;18:117–23. [DOI] [PubMed] [Google Scholar]
- [9].O’Shea TM, Klinepeter KL, Meis PJ, Dillard RG. Intrauterine infection and the risk of cerebral palsy in very low-birthweight infants. Paediatric and perinatal epidemiology. 1998;12:72–83. [PubMed] [Google Scholar]
- [10].Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, et al. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. Jama. 2004;292:2357–65. [DOI] [PubMed] [Google Scholar]
- [11].Pappas A, Kendrick DE, Shankaran S, Stoll BJ, Bell EF, Laptook AR, et al. Chorioamnionitis and early childhood outcomes among extremely low-gestational-age neonates. JAMA pediatrics. 2014;168:137–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Polin RA. The “ins and outs” of neonatal sepsis. The Journal of pediatrics. 2003;143:3–4. [DOI] [PubMed] [Google Scholar]
- [13].Griffin MP, Lake DE, O’Shea TM, Moorman JR. Heart rate characteristics and clinical signs in neonatal sepsis. Pediatric research. 2007;61:222–7. [DOI] [PubMed] [Google Scholar]
- [14].Griffin MP, Lake DE, Bissonette EA, Harrell FE Jr., O’Shea TM, Moorman JR. Heart rate characteristics: novel physiomarkers to predict neonatal infection and death. Pediatrics. 2005;116:1070–4. [DOI] [PubMed] [Google Scholar]
- [15].Moorman JR, Carlo WA, Kattwinkel J, Schelonka RL, Porcelli PJ, Navarrete CT, et al. Mortality reduction by heart rate characteristic monitoring in very low birth weight neonates: a randomized trial. The Journal of pediatrics. 2011;159:900–6.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fairchild KD, Schelonka RL, Kaufman DA, Carlo WA, Kattwinkel J, Porcelli PJ, et al. Septicemia mortality reduction in neonates in a heart rate characteristics monitoring trial. Pediatric research. 2013;74:570–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bayley N, Bayley N, PsychCorp. Bayley scales of infant and toddler development, third edition : technical manual; 2006. [Google Scholar]
- [18].Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Developmental medicine and child neurology. 1997;39:214–23. [DOI] [PubMed] [Google Scholar]
- [19].Rosenbaum P, Paneth N, Leviton A, Goldstein M, Bax M, Damiano D, et al. A report: the definition and classification of cerebral palsy April 2006. Developmental medicine and child neurology Supplement. 2007;109:8–14. [PubMed] [Google Scholar]
- [20].Amiel-Tison C, Gosselin J. Neurological development from birth to six years : guide for examination and evaluation. Baltimore: Johns Hopkins University Press; 2001. [Google Scholar]
- [21].Finitzo-Hieber T, McCracken GH Jr., Roeser RJ, Allen DA, Chrane DF, Morrow J. Ototoxicity in neonates treated with gentamicin and kanamycin: results of a four-year controlled follow-up study. Pediatrics. 1979;63:443–50. [PubMed] [Google Scholar]
- [22].Guralnick MJ. The next decade of research on the effectiveness of early intervention. Exceptional children. 1991;58:174–83. [DOI] [PubMed] [Google Scholar]
- [23].McCormick MC, Brooks-Gunn J, Buka SL, Goldman J, Yu J, Salganik M, et al. Early intervention in low birth weight premature infants: results at 18 years of age for the Infant Health and Development Program. Pediatrics. 2006;117:771–80. [DOI] [PubMed] [Google Scholar]
- [24].Hack M, Taylor HG, Drotar D, Schluchter M, Cartar L, Wilson-Costello D, et al. Poor predictive validity of the Bayley Scales of Infant Development for cognitive function of extremely low birth weight children at school age. Pediatrics. 2005;116:333–41. [DOI] [PubMed] [Google Scholar]
- [25].Boghossian NS, McDonald SA, Bell EF, Carlo WA, Brumbaugh JE, Stoll BJ, et al. Association of Antenatal Corticosteroids With Mortality, Morbidity, and Neurodevelopmental Outcomes in Extremely Preterm Multiple Gestation Infants. JAMA pediatrics. 2016;170:593–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Vohr BR, Stephens BE, Higgins RD, Bann CM, Hintz SR, Das A, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. The Journal of pediatrics. 2012;161:222–8.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]


