Abstract
Plants are routinely utilized as efficient production platforms for the development of anti-cancer biologics leading to novel anti-cancer vaccines, immunotherapies, and drug-delivery modalities. Various biosimilar/biobetter antibodies and immunogens based on tumor-associated antigens have been produced and optimized for plant expression. Plant virus nanoparticles, including those derived from cowpea mosaic virus or tobacco mosaic virus in particular have shown promise as immunotherapies stimulating tumor-associated immune cells and as drug carriers delivering conjugated chemotherapeutics effectively to tumors. Advancements have also been made towards the development of lectins that can selectively recognize cancer cells. The ease at which plant systems can be utilized for the production of these products presents an opportunity to further develop novel and exciting anti-cancer biologics.
Keywords: Plant-made pharmaceutical, cancer biologic, biopharmaceutical, plant virus, plant virus nanoparticle, therapeutic vaccine, lectin, lectibody, biosimilar, biobetter
Graphical abstract
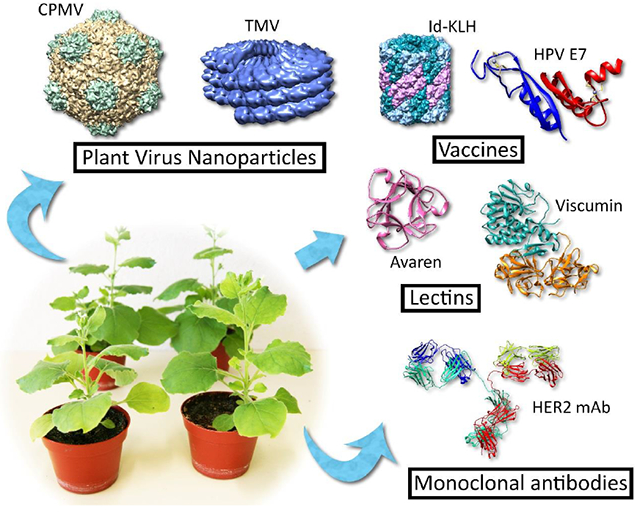
INTRODUCTION
The 1997 approval of rituximab, which was the first anti-cancer monoclonal antibody (mAb) approved for use in the U.S., began a biologic explosion that has transformed the landscape of cancer therapy and dramatically altered and improved patient survival and quality of life. According to the U.S. National Cancer Institute (URL: https://www.cancer.gov/about-cancer/treatment/types/immunotherapy/bio-therapies-fact-sheet), this broad category of pharmaceuticals includes immune checkpoint inhibitors, immune cell therapy, therapeutic mAbs and other immune system molecules, therapeutic vaccines and immune system modulators, which now combined make up the majority of total pharmaceutical sales globally (with a market value of approximately 1 trillion dollars in 2016) [1]. Since 1997 hundreds of biologic drugs have been approved or clinically evaluated, and the development of mAbs targeting immune checkpoints like PD-1 and CTLA-4 was even the subject of the 2018 Nobel Prize in Medicine or Physiology, a testament to the impact that biologics have had on medicine. Despite their promise, biologic drugs remain expensive due to manufacturing costs and the lack of significant generic competition from biosimilars (the first was only approved in 2015) [2]. Cell-culture based manufacturing systems are also slow to implement for initial screening and proof-of-concept (POC) studies, prolonging preclinical development of novel drugs, though alternative methods have some important advantages.
Cancer biologic production in plants has a long history, beginning with the early production of mAbs against tumor-associated antigens (TAAs) like CO17-A [3]. In contrast to transgenic plants, the recent advent of transient overexpression vectors allow relatively short time for novel biologic drugs to be produced at scale and tested, making plants an ideal platform for preclinical biologic development [4]. A large number of recent advances in the field have come from the area of plant virus nanoparticles (PVNs), particularly those derived from cowpea mosaic virus (CPMV), tobacco mosaic virus (TMV), and potato virus X (PVX), which have shown efficacy as both immunostimulatory agents/therapeutic vaccines and as drug delivery modalities capable of delivering chemotherapy payloads to tumor sites in vivo. While much of the literature is dominated by these advancements, steps have also been made towards the development of recombinant cancer vaccines based on tumor antigens and anti-cancer lectins. This review sets out to catalog recent advancements in plant-made cancer biologics and their future.
CANCER VACCINES AND IMMUNOTHERAPY
The goal of cancer vaccination is to induce tumor-specific immunity and activate immune cells in the tumor microenvironment to elicit anti-cancer activity. Cancer vaccines are immunostimulatory agents that often make use of TAAs, which are antigens capable of distinguishing cancer and non-cancer tissue or antigens that are overexpressed in cancer tissues compared to normal tissue, such as epidermal growth factor receptor (EGFR) and its family in some cancers. One such example is human prostatic acid phosphatase, or PAP, which is a secreted glycoprotein used historically as a marker for prostate cancer. A recombinant PAP fused to granulocyte-macrophage colony-stimulation factor (GM-CSF) is used as a part of spuleucel-T (Provenge®) vaccine, an FDA-approved autologous cellular immunotherapy for prostate cancer [5]. To enhance immunogenicity and expression, Kang et al. has successfully expressed PAP-fused to the Fc region of human IgM in transgenic tobacco (Nicotiana tabacum) [6]. Other tumor antigens that have been expressed in plants include the colorectal cancer antigen GA733-2-Fc fusion with an additional KDEL receptor, which was reported to have increased immunotherapeutic effects [7], and idiotypic antibody-keyhole limpet hemocyanin (Id-KLH) conjugate vaccines for Non-Hodgkin’s Lymphoma [8–10]. More recently, a phase I safety and immunogenicity trial of Id-KLH conjugate vaccines in 11 patients showed that immunization resulted in a vaccine-induced, idiotype-specific cellular and humoral immune response without any serious adverse events reported [8].
Some chronic infections are known to be risk factors for cancer. A recent report estimated that approximately 15% (2.2 million) of 14 million worldwide new cancer cases in 2012 were attributable to infectious agents, including Helicobacter pylori, human papillomavirus (HPV), hepatitis B virus, hepatitis C virus and Epstein-Barr virus [11]. Thus, vaccines against these infections have significant implications for cancer prevention, and a number of efforts have been made for the development of plant-made vaccines against cancer-causing pathogens. However, these vaccines are beyond the scope of this review as they are not strictly categorized as “cancer biologics” with the exception of therapeutic vaccines against HPV E6 and E7 oncoproteins. HPV infection is the cause of approximately 5% of all human cancers, in particular, malignancies of the genitalia (penile, vulval, anal, and cervical cancers) and oral cavity [11]. The E6 and E7 proteins are ideal targets because they are constitutively expressed in HPV-associated malignant cells and thus may be more effective at generating an immune response to infected cells than L1-based vaccines [12]. One interesting vaccine candidate that has been produced by transient expression in N. benthamiana is LALF32-51-E7, which is a fusion of the HPV E7 protein to the bacterial cell-penetrating peptide LALF [13]. LALF, or Limulus polyphemus anti-lipopolysaccharide factor, can penetrate mammalian cell membranes and has immunomodulatory properties. While plant-made LALF32-51-E7 has not been evaluated in animals, it does form the appropriate protein body-like structures in leaf tissue and can be purified to a high degree, and may be a cost-effective therapeutic vaccine candidate [14].
Extensive work in the plant-made pharmaceutical (PMP) research field has been dedicated to the use of PVNs as in situ vaccination/immunostimulatory agents with or without the delivery of tumor antigen epitopes, beginning with the first POC study in 2006 with TMV-peptide fusion vaccines [15]. This strategy ultimately aims at the reactivation of tumor-suppressed immune cells in the tumor microenvironment and the induction of systemic anti-cancer immunity. The most well-studied of these PVNs are derived from CPMV, which have demonstrated efficacy in murine 4T1 breast, CT-26, colon, B16F10 melanoma, GL261 glioma, and ID8 ovarian cancer models [16–21]. The icosahedral structure of CPMV appears to be more efficiently taken up by antigen presenting cells (APCs), resulting in higher APC activation and better transport of PVNs to and retention in lymph nodes than high-aspect-ratio viruses like PVX [17,22]. Additionally, as has been recently demonstrated, in situ vaccination can result in the conversion of immunosuppressive cells like M2 macrophages and N2 neutrophils to their M1 and N1 counterparts, helping to break tumor immunotolerance [19]. What remains to be seen is the potential efficacy of these particles in humans, as mouse models in these studies used immunodeficient mice for human cancer xenograft, which may have a limited predictive value for immunotherapeutic effects in humans. Nevertheless, the results obtained in recent years hold much promise for their development.
Plants have long been used as production platforms for cancer-targeting immunotherapeutics, including mAbs and cytokines. One such example is the production of CCL21 in tomato, which may potentially be used as an anti-metastatic agent for many cancer types [23]. Recently, several groups have published the production of anti-cancer mAbs including the anti-HER2 mAb trastuzumab [24], the anti-GA733 mAb C017-1A [25], and the anti-CD20 mAbs ofatumumab and rituximab [26,27], as well as an anti-HER2 single chain variable fragment-Fc (scFv-Fc) fusion [28]. Importantly, trastuzumab and rituximab were produced in glycoengineered plants and showed greater anti-cancer activity owing to the lack of core fucose on the single N-glycan in the Fc region, which increases the affinity for FcγRIIIa and potently elicits antibody-dependent cell-mediated cytotoxicity [29]. Similar increases in Fc effector functions were also seen for a plant-produced anti-CD20-hIL-2 immunocytokine, made by the fusion of an anti-CD20 mAb and human IL-2 [30]. The resulting immunocytokine was also highly efficient at activating T cells, potentially resulting in greater cytotoxic T cell responses against malignant cells. The relative ease of plant glycoengineering compared to mammalian or insect cell culture systems make them a useful alternative for mAb production [31].
DRUG DELIVERY AND IMAGING
Considerable research has been conducted into novel drug delivery systems, with the goal of improving the pharmacokinetics and pharmacodynamics of small molecule and biologic drugs by affecting their absorption and distribution in the body. PVNs have been particularly attractive owing their ability to deliver larger payloads than antibody-drug conjugates, the relative ease at which they can be decorated with targeting ligands for tissue-specific delivery of drugs, the wide array of possible chemistries, and the ease of manufacturing them in planta. So-called high-aspect-ratio viruses, like TMV and PVX, are particularly useful as they are not taken up as easily by phagocytosis and may have a prolonged serum half-life compared to icosahedral viruses. Several recent reports have described PVNs for chemotherapeutic and imaging reagent delivery, including those derived from TMV [32–37], PVX [38–40], red clover necrotic mosaic virus (RCNMV) [41], Pepino mosaic virus (PeMV) [40], and Johnsongrass chlorotic stripe mosaic virus (JgCSMV) [42,43].
Much of the recent work regarding PVNs as drug delivery modalities has focused on the use of high-aspect ratio, or filamentous, viruses. Among these, TMV PVNs have been extensively researched and used in medical imaging and animal models of cancer. Notably, Franke et al. demonstrated that the conjugation of cisplatin to tobacco mosaic virus PVNs restored the efficacy of the chemotherapeutic to OVCAR3 cells, which are typically cisplatin resistant [36]. Because resistance to cisplatin is common among patients with recurrent ovarian cancer [44], the results justify further preclinical validation. Similarly, PVX particles loaded with doxorubicin or presenting tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) have also shown to be efficacious in mice xenografted with the human breast cancer cell line MDA-MB-231 [38,39]. PVX however may have less desirable pharmacokinetic and tumor homing properties compared to another filamentous virus, PeMV [40]. Overall, much work remains to demonstrate how these particles end up at the tumor site and how that can be improved, in addition to further demonstration of efficacy in animal models.
A number of icosahedral viruses have also been used as drug delivery systems, including RCNMV and JgCSMV. While icosahedral virus particles have short serum half-life as efficiently taken up by the immune system, these PVNs appear to have excellent tumor penetration and drug carrying capacity. For example, RCNMV particles loaded with doxorubicin showed more efficacy at a lower dose in an SKOV3 human ovarian cancer xenograft model than the pegylated liposomal form of the drug, indicating a greater degree of tumor targeting by the PVNs [41]. Alemzadeh et al. also recently demonstrated the loading of JgCSMV particles with doxorubicin and their efficacy in the MCF-7 human xenograft breast cancer model in mice [42,43]. Interestingly, encapsulation of drug in JgCSMV particles led to increased uptake of doxorubicin in the breast cancer tissue and actually led to decreased cardiotoxicity [42].
Many recent advances have come in the area of medical imaging. TMV PVNs have, for example, been successfully conjugated to the contrast agent dysprosium and used to image prostate cancer cells in mice using both ultra-high-field magnetic resonance and near-infrared fluorescence imaging [34]. Serum-albumin-coated particles, which may reduce the potential immune response to the TMV, have also been characterized and used to deliver doxorubicin and the contrast agent gadolinium in mouse models of human breast cancer. Dubbed a “theranostic”, this approach successfully combined treatment and MRI imaging, demonstrating the dual capacity of PVNs and their superiority over conventional drug-delivery modalities.
ANTI-CANCER LECTINS
Lectins are a diverse group of carbohydrate-binding proteins that have garnered much interest for their potential immunomodulating and cancer-targeting abilities. In recent years, a great number of fungal and plant lectins with anti-cancer activity have been isolated, characterized, and described in the literature [45,46]. Plant lectins in particular have been historically important as alternative or adjuvant therapies for cancer especially in Europe, where arguably the most well-known is a lectin-containing extract from European mistletoe (Viscum album). One of the active ingredients in the extract, viscumin (also called mistletoe lectin or ML), is a holotoxin consisting of a single ribosome-inactivating A chain and a single sialic acid-specific lectin B chain covalently linked with a disulfide bond [47,48]. Though a comprehensive systematic review of the use of mistletoe extract in addition to chemotherapy concluded that it offered no additional benefit in terms of survival or quality of life, isolated viscumin may still have useful anti-cancer activity in vivo [49–51]. To this end, Gengenbach et al. recently published the expression and purification of recombinant viscumin in N. benthamiana, with a yield of ≈ 7 mg/kg fresh weight. The plant-derived lectin exhibited greater cytotoxicity to THP-1 cells than E. coli-made viscumin and was significantly more cost effective to produce [52]. While promising, further in vitro and in vivo studies are warranted to demonstrate efficacy in multiple models and to demonstrate the selectivity of viscumin for cancer over healthy tissue. Additionally, improvements in yield are necessary to facilitate preclinical development.
Cholera toxin B subunit (CTB) is a non-toxic lectin component of the holotoxin that recognizes the Galβ1–3GalNAc moiety of GM1 ganglioside found on the surface of intestinal epithelial cells (where the toxin normally exerts its activity). We have recently demonstrated that oral administration of a CTB variant (containing a KDEL endoplasmic reticulum retention motif) produced in N. benthamiana can facilitate mucosal healing and reduce tumorigenesis in a colitis-associated colorectal cancer mouse model [53]. Epidemiological evidence has pointed to an increase in colorectal cancer incidence in inflammatory bowel disease patients [54,55]. Thus, the plant-made CTB variant as a treatment for chronic intestinal inflammation may also have anti-cancer properties that should be investigated further.
Lastly, aberrant glycosylation has been recognized a hallmark of cancer, and in particular high-mannose glycans have been demonstrated to be over-represented in the glycocalyx of many human cancers [56,57], making them a potentially useful biomarker or druggable target [58]. Our lab has recently developed Avaren-Fc, a plant-produced “lectibody” targeting a cluster of high-mannose glycans that are widely found on the surface of enveloped viruses and malignant cells [59,60]. While originally developed as an anti-HIV agent, we observe that Avaren-Fc also has strong anti-cancer activity in vitro and in human cancer cell xenograft and syngeneic mouse models (Dent and Matoba, unpublished), highlighting the druggability of HIV- and tumor-associated high-mannose glycans.
CONCLUSIONS
Transient expression of proteins in plants is a powerful method for the rapid, robust production of recombinant proteins, which will significantly facilitate the preclinical development of biosimilar, biobetter and innovator anti-cancer proteins as well as vaccines. PVNs show promise as immunostimulatory agents, drug delivery platforms and imaging probes. Since aberrant protein glycosylation is a hallmark of cancer [61,62], plant-derived lectins and their derivatives such as “lectibodies” may have unique potential as cancer biologics.
As regulatory frameworks for plant-based biomanufacturing system are becoming well established [8,63–66], the technology has finally come of age. In addition to transient expression, other technologies based on transgenic plants and plant-cell culture offer some potential advantages that may facilitate the commercialization of plant-made biologics. Transgenic plants, for instance, offer greater scalability and simpler upstream processing, while plant-cell culture is similar to existing platforms that are well-established in the pharmaceutical industry, allowing for the adaptation of conventional chemical engineering and regulatory approaches. Though there are still challenges to be addressed in regard to plant growth conditions, transgene expression regulation, post-translational modifications, and product isolation and recovery, we will soon witness some plant-made cancer biologic products being tested for their clinical efficacy – the most important step that will further cement plants as a viable alternative to other more established production methods.
Table 1 –
Recent developments in plant-made cancer biologics. CPMV: cowpea mosaic virus; ΔXF: with plant xylose/fucose deleted glycans; Fc: crystallizable fragment of human antibody; HPV: human papillomavirus; JgCSMV: Johnson grass chlorotic stripe mosaic virus; mAb: monoclonal antibody; PAP: prostatic acid phosphatase; PVX: potato virus X; RCNMV: red clover necrotic mosaic virus; TAA: tumor-associated antigen; TMV: tobacco mosaic virus; TRAIL: tumor necrosis factor-related apoptosis-inducing ligand
| Protein | Application | Produced in | Reference |
|---|---|---|---|
| CPMV particles | Immunotherapy, drug delivery | Vigna unguiculata (viral propagation) or N. benthamiana (transient expression) | [16–21] |
| TMV particles | Immunotherapy, drug delivery, imaging | N. benthamiana (propagation or transient expression) | [16,33–36,40] |
| PVX particles | Immunotherapy, drug delivery | N. benthamiana (viral propagation) | [17,22,38] |
| PeMV particles | Immunotherapy | N. benthamiana (viral propagation) | [40] |
| TMV-Albumin particles | Immunotherapy, drug delivery | N. benthamiana (viral propagation), | [32] |
| RCNMV particles | Drug delivery | - | [41] |
| TMV-coated mesoporous silica nanoparticles | Drug delivery | N. benthamiana (viral propagation) | [37] |
| TRAIL-coated PVX | Drug delivery, therapeutic | N.benthamiana (viral propagation) | [39] |
| JgCSMV particles | Drug delivery, therapeutic | Sorghum halepense (viral propagation) | [42,43] |
| PAP-IgM Fc | Immunotherapy | Nicotiana tabacum (transgenic, in vitro cultured) | [6] |
| GA733–2-Fc-KDEL | Immunotherapy | N. benthamiana (transient expression) | [7] |
| Oligomannose rituximab | Immunotherapy | N. benthamiana (transient expression) | [27] |
| Ofatumumab | Immunotherapy | N. benthamiana (transient expression) | [26] |
| Trastuzumab | Immunotherapy | N. benthamiana (transient expression) | [24] |
| CO17-1A-KDEL | Immunotherapy | N. benthamiana (transient expression) | [25] |
| Anti-CD20 scFv-Fc-hIL2 immunocytokine (ΔXF) | Therapeutic | N. benthamiana (transient expression) | [30] |
| Anti-HER2 scFv-Fc | Therapeutic | Arabidopsis thaliana | [28] |
| Viscumin (mistletoe lectin) | Therapeutic | N. benthamiana (transient expression) | [52] |
| Avaren-Fc | Therapeutic | N. benthamiana (transient expression) | [59] |
| CTB-KDEL | Immunotherapy | N. benthamiana (transient expression) | [53] |
| LALF32-51-E7 HPV vaccine | Therapeutic vaccine | N. benthamiana (transient expression) | [13,14] |
| CCL21 | Immunotherapy | Solanum lycopersicum (transient expression) | [23] |
HIGHLIGHTS.
Plant overexpression systems facilitate preclinical biologics development
Plants have been used to produce many protein vaccines, mAbs and immunotherapeutics
Plant virus nanoparticles are effective cancer-targeting agents
Plant-derived lectins and lectibodies may have unique anti-cancer potential
ACKNOWLEDGEMENTS
This work was in part funded by the National Institute of Health (R21CA216447).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTERESTS
Nothing declared.
REFERENCES
- 1.Lindsley CW: New 2016 data and statistics for global pharmaceutical products and projections through 2017. ACS Publications; 2017. [DOI] [PubMed] [Google Scholar]
- 2.Raedler LA: Zarxio (filgrastim-sndz): first biosimilar approved in the United States. American health & drug benefits 2016, 9:150. [PMC free article] [PubMed] [Google Scholar]
- 3.Verch T, Yusibov V, Koprowski H: Expression and assembly of a full-length monoclonal antibody in plants using a plant virus vector. Journal of Immunological Methods 1998, 220:69–75. [DOI] [PubMed] [Google Scholar]
- 4.Pujol M, Gavilondo J, Ayala M, Rodríguez M, González EM, Pérez L: Fighting cancer with plant-expressed pharmaceuticals. Trends in Biotechnology 2007, 25:455–459. [DOI] [PubMed] [Google Scholar]
- 5.Sims RB: Development of sipuleucel-T: autologous cellular immunotherapy for the treatment of metastatic castrate resistant prostate cancer. Vaccine 2012, 30:4394–4397. [DOI] [PubMed] [Google Scholar]
- 6.Kang YJ, Kim D-S, Myung S-C, Ko K: Expression of a human prostatic acid phosphatase (PAP)-IgM Fc fusion protein in plants using in vitro tissue subculture. Frontiers in plant science 2017, 8:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *7.Fu YY, Zhao J, Park JH, Choi GW, Park KY, Lee YH, Chung IS: Human colorectal cancer antigen GA733-2-Fc fused to endoplasmic reticulum retention motif KDEL enhances its immunotherapeutic effects. J Cancer Res Ther 2018, 14:S748–S757. [DOI] [PubMed] [Google Scholar]; By attaching the ER-retention signal KDEL to GA733-Fc, a colon cancer antigen, the authors found improved immunogenicity in mice. These results corroborate other studies showing that an increase in oligomannose glycans caused by ER retention can result in greater immunogenicity.
- **8.Tuse D, Ku N, Bendandi M, Becerra C, Collins R Jr., Langford N, Sancho SI, Lopez-Diaz de Cerio A, Pastor F, Kandzia R, et al. : Clinical Safety and Immunogenicity of Tumor-Targeted, Plant-Made Id-KLH Conjugate Vaccines for Follicular Lymphoma. Biomed Res Int 2015, 2015:648143. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this landmark study of a plant-made cancer vaccine, 11 NHL patients received an individualized idiotype antibody-KLH fusion vaccine, and the majority of them developed specific anti-tumor immune responses without serious adverse events.
- 9.McCormick A, Reddy S, Reinl S, Cameron T, Czerwinkski D, Vojdani F, Hanley K, Garger S, White E, Novak J: Plant-produced idiotype vaccines for the treatment of non-Hodgkin’s lymphoma: safety and immunogenicity in a phase I clinical study. Proceedings of the National Academy of Sciences 2008, 105:10131–10136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendandi M, Marillonnet S, Kandzia R, Thieme F, Nickstadt A, Herz S, Fröde R, Inoges S, Lòpez-Díaz de Cerio A, Soria E: Rapid, high-yield production in plants of individualized idiotype vaccines for non-Hodgkin’s lymphoma. Annals of Oncology 2010, 21:2420–2427. [DOI] [PubMed] [Google Scholar]
- 11.Plummer M, de Martel C, Vignat J, Ferlay J, Bray F, Franceschi S: Global burden of cancers attributable to infections in 2012: a synthetic analysis. Lancet Glob Health 2016, 4:e609–616. [DOI] [PubMed] [Google Scholar]
- 12.Chabeda A, Yanez RJ, Lamprecht R, Meyers AE, Rybicki EP, Hitzeroth II: Therapeutic vaccines for high-risk HPV-associated diseases. Papillomavirus Research 2018, 5:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *13.Yanez RJR, Lamprecht R, Granadillo M, Weber B, Torrens I, Rybicki EP, Hitzeroth II: Expression optimization of a cell membrane-penetrating human papillomavirus type 16 therapeutic vaccine candidate in Nicotiana benthamiana. PLoS One 2017,12:e0183177. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, the authors described the production of LALF(32-51)-E7, an experimental therapeutic vaccine for high risk human papillomaviruses (in particular, HPV-16). Such a therapeutic vaccine could allow for the effective treatment of people with HPV-related cancers and lesions who did not or could not vaccinate before exposure.
- 14.Yanez RJR, Lamprecht R, Granadillo M, Torrens I, Arcalis E, Stoger E, Rybicki EP, Hitzeroth II: LALF32-51-E7, a HPV-16 therapeutic vaccine candidate, forms protein body-like structures when expressed in Nicotiana benthamiana leaves. Plant Biotechnol J 2018,16:628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McCormick AA, Corbo TA, Wykoff-Clary S, Nguyen LV, Smith ML, Palmer KE, Pogue GP: TMV-peptide fusion vaccines induce cell-mediated immune responses and tumor protection in two murine models. Vaccine 2006, 24:6414–6423. [DOI] [PubMed] [Google Scholar]
- 16.Murray AA, Wang C, Fiering S, Steinmetz NF: In Situ Vaccination with Cowpea vs Tobacco Mosaic Virus against Melanoma. Mol Pharm 2018,15:3700–3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shukla S, Myers JT, Woods SE, Gong X, Czapar AE, Commandeur U, Huang AY, Levine AD, Steinmetz NF: Plant viral nanoparticles-based HER2 vaccine: Immune response influenced by differential transport, localization and cellular interactions of particulate carriers. Biomaterials 2017,121:15–27. [DOI] [PubMed] [Google Scholar]
- 18.Czapar AE, Tiu BDB, Veliz FA, Pokorski JK, Steinmetz NF: Slow-Release Formulation of Cowpea Mosaic Virus for In Situ Vaccine Delivery to Treat Ovarian Cancer. Adv Sci (Weinh) 2018, 5:1700991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **19.Wang C, Fiering SN, Steinmetz NF: Cowpea Mosaic Virus Promotes Anti - Tumor Activity and Immune Memory in a Mouse Ovarian Tumor Model. Advanced Therapeutics 2019, 2:1900003. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using CPMV in an aggressive murine model of ovarian cancer (ID8-Defb29-VEGFA), the authors demonstrated that the antitumor activity of CPMV is due to activation of normally immunosuppressive myeloid and lymphoid cells, boosting the activity of the mouse immune system by coordinating both innate and adaptive immune responses.
- 20.Kerstetter-Fogle A, Shukla S, Wang C, Beiss V, Harris PLR, Sloan AE, Steinmetz NF: Plant Virus-Like Particle In Situ Vaccine for Intracranial Glioma Immunotherapy. Cancers (Basel) 2019,11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang C, Steinmetz NF: CD47 Blockade and Cowpea Mosaic Virus Nanoparticle In Situ Vaccination Triggers Phagocytosis and Tumor Killing. Adv Healthc Mater 2019, 8:el801288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee KL, Murray AA, Le DH, Sheen MR, Shukla S, Commandeur U, Fiering S, Steinmetz NF: Combination of plant virus nanoparticle-based in situ vaccination with chemotherapy potentiates antitumor response. Nano letters 2017, 17:4019–4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beihaghi M, Marashi H, Bagheri A, Sankian M: Transient expression of CCL21as recombinant protein in tomato. Biotechnology reports 2018, 17:10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLean MD: Trastuzumab Made in Plants Using vivoXPRESS® Platform Technology. 2017.
- 25.Song I, Kang Y, Lee YK, Myung S-C, Ko K: Endoplasmic reticulum retention motif fused to recombinant anti-cancer monoclonal antibody (mAb) C017–1A affects mAb expression and plant stress response. PloS one 2018,13:e0198978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin N, Lee JW, Heo W, Ryu MY, So MK, Ko BJ, Kim H-Y, Yoon SM, Lee J, Kim JY : Low binding affinity and reduced complement-dependent cell death efficacy of ofatumumab produced using a plant system (Nicotiana benthamiana L.). Protein expression and purification 2019. [DOI] [PubMed] [Google Scholar]
- *27.Kommineni V, Markert M, Ren Z, Palle S, Carrillo B, Deng J, Tejeda A, Nandi S, McDonald KA, Marcel S, et al. : In Vivo Glycan Engineering via the Mannosidase I Inhibitor (Kifunensine) Improves Efficacy of Rituximab Manufactured in Nicotiana benthamiana Plants. Int J Mol Sci 2019, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrated that an oligomannose form of a rituximab biosimilar produced transiently in Nicotiana benthamiana, generated through the use of the mannosidase inhibitor kifunensine, had a 14-fold increase in ADCC activity compared to the wild type rituximab.
- 28.Dong Y, Li J, Yao N, Wang D, Liu X, Wang N, Li X, Wang F, Li H, Jiang C: Seed-specific expression and analysis of recombinant anti-HER2 single-chain variable fragment (scFv-Fc) in Arabidopsis thaliana. Protein Expr Purif 2017, 133:187–192. [DOI] [PubMed] [Google Scholar]
- 29.Liu SD, Chalouni C, Young JC, Junttila TT, Sliwkowski MX, Lowe JB: Afucosylated antibodies increase activation of FcyRIIIa-dependent signaling components to intensify processes promoting ADCC. Cancer immunology research 2015, 3:173–183. [DOI] [PubMed] [Google Scholar]
- 30.Marusic C, Pioli C, Stelter S, Novelli F, Lonoce C, Morrocchi E, Benvenuto E, Salzano AM, Scaloni A, Donini M: N-glycan engineering of a plant-produced anti-CD20-hIL-2 immunocytokine significantly enhances its effector functions. Biotechnol Bioeng 2018,115:565–576. [DOI] [PubMed] [Google Scholar]
- 31.Strasser R, Stadlmann J, Schähs M, Stiegler G, Quendler H, Mach L, Glössl J, Weterings K, Pabst M, Steinkellner H: Generation of glyco - engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human - like N - glycan structure. Plant biotechnology journal 2008, 6:392–402. [DOI] [PubMed] [Google Scholar]
- *32.Pitek A, Hu H, Shukla S, Steinmetz N: Cancer Theranostic Applications of Albumin-Coated Tobacco Mosaic Virus Nanoparticles. ACS applied materials & interfaces 2018, 10:39468–39477. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using TMV PVNs, the authors combined chemotherapy and contrast imaging reagents to create a “theranostic”, uniquely capable of acting as a drug delivery modality as well as an imaging reagent. These could potentially see applications in both diagnostics and therapy.
- 33.Gulati NM, Pitek AS, Steinmetz NF, Stewart PL: Cryo-electron tomography investigation of serum albumin-camouflaged tobacco mosaic virus nanoparticles. Nanoscale 2017, 9:3408–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu H, Zhang Y, Shukla S, Gu Y, Yu X, Steinmetz NF: Dysprosium-modified tobacco mosaic virus nanoparticles for ultra-high-field magnetic resonance and near-infrared fluorescence imaging of prostate cancer. ACS nano 2017, 11:9249–9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kernan DL, Wen AM, Pitek AS, Steinmetz NF: Featured Article: Delivery of chemotherapeutic vcMMAE using tobacco mosaic virus nanoparticles. Experimental Biology and Medicine 2017, 242:1405–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *36.Franke CE, Czapar AE, Patel RB, Steinmetz NF: Tobacco Mosaic Virus-Delivered Cisplatin Restores Efficacy in Platinum-Resistant Ovarian Cancer Cells. Molecular pharmaceutics 2017, 15:2922–2931. [DOI] [PubMed] [Google Scholar]; This work demonstrates how the use of an immunotherapy agent like TMV can restore sensitivity to chemotherapeutics like cisplatin in cell lines where resistance occured previously. This is important, as cisplatin resistance is a common barrier to the treatment of recurring ovarian cancer.
- 37.Marin-Caba L, Chariou PL, Pesquera C, Correa-Duarte MA, Steinmetz NF: Tobacco Mosaic Virus-Functionalized Mesoporous Silica Nanoparticles, a Wool-Ball-like Nanostructure for Drug Delivery. Langmuir 2019, 35:203–211. [DOI] [PubMed] [Google Scholar]
- 38.Le DH, Lee KL, Shukla S, Commandeur U, Steinmetz NF: Potato virus X, a filamentous plant viral nanoparticle for doxorubicin delivery in cancer therapy. Nanoscale 2017, 9:2348–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Le DHT, Commandeur U, Steinmetz NF: Presentation and Delivery of Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand via Elongated Plant Viral Nanoparticle Enhances Antitumor Efficacy. ACS Nano 2019, 13:2501–2510. [DOI] [PubMed] [Google Scholar]
- *40.Le DH, Méndez-López E, Wang C, Commandeur U, Aranda MA, Steinmetz NF: Biodistribution of Filamentous Plant Virus Nanoparticles: Pepino Mosaic Virus versus Potato Virus X. Biomacromolecules 2018, 20:469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors evaluated the pharmacokinetics and dynamics of two distinctly-shaped PVNs: one an icosahedral virus and one a filamentous virus. The conclusion that icosahedral viruses have greater tissue distribution and cellular uptake compared to filamentous viruses is important to consider when designing nanoparticle-based treatments or diagnostics.
- 41.Madden AJ, Oberhardt B, Lockney D, Santos C, Vennam P, Arney D, Franzen S, Lommel SA, Miller CR, Gehrig P: Pharmacokinetics and efficacy of doxorubicin-loaded plant virus nanoparticles in preclinical models of cancer. Nanomedicine 2017, 12:2519–2532. [DOI] [PubMed] [Google Scholar]
- *42.Alemzadeh E, Dehshahri A, Dehghanian AR, Afsharifar A, Behjatnia AA, Izadpanah K, Ahmadi F: Enhanced anti-tumor efficacy and reduced cardiotoxicity of doxorubicin delivered in a novel plant virus nanoparticle. Colloids Surf B Biointerfaces 2019, 174:80–86. [DOI] [PubMed] [Google Scholar]; Here, the authors reported that the delivery of Johnson grass chlorotic stripe mosaic virus nanoparticles encapsulating the chemotherapeutic doxorubicin showed marked decrease in cardiotoxicity while decreasing tumor growth in athymic nude mice.
- 43.Alemzadeh E, Izadpanah K, Ahmadi F: Generation of recombinant protein shells of Johnson grass chlorotic stripe mosaic virus in tobacco plants and their use as drug carrier. Journal of virological methods 2017, 248:148–153. [DOI] [PubMed] [Google Scholar]
- 44.Oronsky B, Ray CM, Spira AI, Trepel JB, Carter CA, Cottrill HM: A brief review of the management of platinum-resistant-platinum-refractory ovarian cancer. Med Oncol 2017, 34:103. [DOI] [PubMed] [Google Scholar]
- 45.Varrot A, Basheer SM, Imberty A: Fungal lectins: structure, function and potential applications. Current Opinion in Structural Biology 2013, 23:678–685. [DOI] [PubMed] [Google Scholar]
- 46.Bhutia SK, Panda PK, Sinha N, Praharaj PP, Bhol CS, Panigrahi DP, Mahapatra KK, Saha S, Patra S, Mishra SR, et al. : Plant lectins in cancer therapeutics: Targeting apoptosis and autophagy-dependent cell death. Pharmacol Res 2019, 144:8–18. [DOI] [PubMed] [Google Scholar]
- 47.Pfüller U: Chemical Constituents of European Mistletoe (Viscum album L.) Isolation and Characterisation of the Main Relevant Ingredients: Lectins, Viscotoxins, Oligo-/polysaccharides, Flavonoides, Alkaloids In Mistletoe. CRC Press; 2000:117–138. [Google Scholar]
- 48.Muthing J, Meisen I, Bulau P, Langer M, Witthohn K, Lentzen H, Neumann U, Peter-Katalinic J: Mistletoe lectin I is a sialic acid-specific lectin with strict preference to gangliosides and glycoproteins with terminal Neu5Ac alpha 2-6Gal beta 1-4GlcNAc residues. Biochemistry 2004, 43:2996–3007. [DOI] [PubMed] [Google Scholar]
- 49.Freuding M, Keinki C, Kutschan S, Micke O, Buentzel J, Huebner J: Mistletoe in oncological treatment: a systematic review : Part 2: quality of life and toxicity of cancer treatment. J Cancer Res Clin Oncol 2019, 145:927–939. [DOI] [PubMed] [Google Scholar]
- 50.Freuding M, Keinki C, Micke O, Buentzel J, Huebner J: Mistletoe in oncological treatment: a systematic review : Part 1: survival and safety. J Cancer Res Clin Oncol 2019,145:695–707. [DOI] [PubMed] [Google Scholar]
- 51.Thies A, Dautel P, Meyer A, Pfüller U, Schumacher U: Low-dose mistletoe lectin-I reduces melanoma growth and spread in a scid mouse xenograft model. British Journal Of Cancer 2007, 98:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *52.Gengenbach BB, Keil LL, Opdensteinen P, Müschen CR, Melmer G, Lentzen H, Bührmann J, Buyel JF: Comparison of microbial and transient expression (tobacco plants and plant - cell packs) for the production and purification of the anti - cancer mistletoe lectin viscumin. Biotechnology and bioengineering 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this work the authors compared the expression of viscumin, the active ingredient in mistletoe extract (a common alternative therapy for cancer in Europe), between a plant system and a bacterial system. They concluded that the plant system was superior and that the complex lectin produced was equally cytotoxic.
- **53.Baldauf K, Royal J, Kouokam J, Haribabu B, Jala V, Yaddanapudi K, Hamorsky K, Dryden G, Matoba N: Oral administration of a recombinant cholera toxin B subunit promotes mucosal healing in the colon. Mucosal immunology 2017, 10:887. [DOI] [PubMed] [Google Scholar]; In this study, the authors present evidence that a plant-produced cholera toxin B subunit variant can faciliate mucosal wound healing and reduce the rate of colon cancer formation in a mouse model of colitis-associated colorectal cancer, an important finding given that great increase in risk of colorectal cancer for patients with ulcerative colitis.
- 54.Axelrad JE, Lichtiger S, Yajnik V: Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol 2016, 22:4794–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang M, Chang L, Chang HM, Chang F: Intestinal and Extraintestinal Cancers Associated With Inflammatory Bowel Disease. Clin Colorectal Cancer 2018, 17:e29–e37. [DOI] [PubMed] [Google Scholar]
- 56.Oliveira-Ferrer L, Legler K, Milde-Langosch K: Role of protein glycosylation in cancer metastasis. Semin Cancer Biol 2017. [DOI] [PubMed] [Google Scholar]
- 57.Loke I, Kolarich D, Packer NH, Thaysen-Andersen M: Emerging roles of protein mannosylation in inflammation and infection. Mol Aspects Med 2016, 51:31–55. [DOI] [PubMed] [Google Scholar]
- 58.Ruhaak LR, Miyamoto S, Lebrilla CB: Developments in the identification of glycan biomarkers for the detection of cancer. Molecular & Cellular Proteomics 2013, 12:846–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **59.Hamorsky KT, Kouokam JC, Dent MW, Grooms TN, Husk AS, Hume SD, Rogers KA, Villinger F, Morris MK, Hanson CV, et al. : Engineering of a lectibody targeting high-mannose-type glycans of the HIV envelope. Mol Ther In press [DOI] [PMC free article] [PubMed] [Google Scholar]; In this study, the authors developed Avaren-Fc, a novel “lectibody” consisting of a variant of the oligomannose glycan-binding lectin actinohivin fused to the Fc region of human IgG1. The plant-made lectibody potently inhibited primary HIV isolates and killed HIV-infected cells, while not exhibiting any siginificant toxicity to normal human blood cells or upon systemic administration in mice and rhesus macaques.
- 60.Seber Kasinger LE, Dent MW, Mahajan G, Hamorsky KT, Matoba N: A novel anti-HIV-1 bispecific bNAb-lectin fusion protein engineered in a plant-based transient expression system. Plant Biotechnol J 2019, 17:1646–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reily C, Stewart TJ, Renfrow MB, Novak J: Glycosylation in health and disease. Nat Rev Nephrol 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oliveira-Ferrer L, Legler K, Milde-Langosch K: Role of protein glycosylation in cancer metastasis. Semin Cancer Biol 2017, 44:141–152. [DOI] [PubMed] [Google Scholar]
- 63.Tuse D: Safety of plant-made pharmaceuticals: product development and regulatory considerations based on case studies of two autologous human cancer vaccines. Hum Vaccin 2011, 7:322–330. [DOI] [PubMed] [Google Scholar]
- 64.Ma JK, Drossard J, Lewis D, Altmann F, Boyle J, Christou P, Cole T, Dale P, van Dolleweerd CJ, Isitt V, et al. : Regulatory approval and a first-in-human phase I clinical trial of a monoclonal antibody produced in transgenic tobacco plants. Plant Biotechnol J 2015, 13:1106–1120. [DOI] [PubMed] [Google Scholar]
- 65.Pillet S, Aubin E, Trepanier S, Bussiere D, Dargis M, Poulin JF, Yassine-Diab B, Ward BJ, Landry N: A plant-derived quadrivalent virus like particle influenza vaccine induces cross-reactive antibody and T cell response in healthy adults. Clin Immunol 2016, 168:72–87. [DOI] [PubMed] [Google Scholar]
- 66.Chichester JA, Green BJ, Jones RM, Shoji Y, Miura K, Long CA, Lee CK, Ockenhouse CF, Morin MJ, Streatfield SJ, et al. : Safety and immunogenicity of a plant-produced Pfs25 virus-like particle as a transmission blocking vaccine against malaria: A Phase 1 dose-escalation study in healthy adults. Vaccine 2018, 36:5865–5871. [DOI] [PMC free article] [PubMed] [Google Scholar]


