Abstract
Context
Menstrual cycle function is determined by a complex endocrine axis that controls the ovaries and endometrium. While the late luteal phase is characterized by declining progesterone and estrogen, how these hormonal profiles relate to menstrual bleeding patterns is not well understood.
Objective
Characterize associations between luteal phase hormonal profiles and subsequent menstrual bleeding patterns, specifically spotting before bleeding.
Design, Setting, and Participants
We examined creatinine-adjusted urinary estrone 3-glucuronide (E13G) and pregnanediol 3-glucuronide (Pd3G) levels in relation to spotting in 116 premenopausal women (ages 20–47) who kept daily menstrual diaries and collected first morning urine samples for ≥ 2 consecutive cycles or 1 luteal-follicular transition (n = 283 transitions). We used linear mixed models to estimate associations between luteal phase hormone levels and spotting before bleeding.
Main Outcome Measure(s) and Results
Transitions with ≥ 1 days of spotting before menstrual bleeding (n = 118) had greater luteal phase Pd3G levels vs nonspotting transitions (n = 165). Differences in Pd3G between spotting and nonspotting transitions were largest at menses onset (34.8%, 95% confidence interval, 18.9%, 52.7%). Pd3G levels for spotting transitions dropped to similar levels as nonspotting transitions an average of 1 day later, which aligned with the first day of bleeding for transitions with contiguous spotting. Spotting transitions were preceded by slower rates of Pd3G decline than nonspotting transitions, whereas E13G declines were similar.
Conclusions
Self-reported bleeding patterns may provide insight into luteal phase Pd3G levels. First bleed appears to be the best choice for defining the end of the luteal phase and achieving hormonal consistency across transitions.
Keywords: menstruation, luteal phase, menses, progesterone, estrogen
Menstrual cycle function and menstruation are important markers of female reproductive health and have also been found to be related to chronic disease risk (1). However, measuring menstrual cycle function is complex because hormonal dynamics and ovarian processes that characterize the menstrual cycle are internal and difficult to observe. By convention, menstruation marks the end of one cycle and the start of the next. Thus, defining individual menstrual cycles requires identifying menses onset. Although menses is overt, defining its onset can be complicated by heterogeneous menstrual bleeding patterns that include irregular bleeding and spotting (ie, very light bleeding) (2). Algorithms for defining menses onset and length have been developed using self-reported menstrual diary data (2, 3). Although such algorithms have been used (4–6), none have been informed by the underlying hormonal dynamics that govern the menstrual cycle.
The late luteal phase is characterized by declining levels of progesterone and estrogen (7), but little is known about how these hormone levels may affect subsequent menstrual bleeding patterns. In general, while the withdrawal of progesterone due to corpora luteal regression leads to menses, it is unclear what hormonal profile stimulates the onset of menses to occur and/or the type of menstrual flow (eg, the absolute levels of steroid hormones or their rates of decline). Understanding this process would expand our knowledge about the endocrine basis for menstrual bleeding and, conversely, provide a noninvasive index of the sex steroid hormonal milieu.
The purpose of this study was to characterize the relationship between hormonal profiles during the luteal phase and subsequent menstrual bleeding patterns; in particular, bleeding with antecedent day(s) of spotting. Using longitudinally collected urine samples across multiple consecutive menstrual cycles, we evaluated daily estrogen and progesterone metabolite concentrations and rates of decline during the luteal-follicular transition in relation to the presence of spotting before bleeding.
Materials and Methods
Study population
The Michigan Polybrominated Biphenyl (PBB) Registry is a cohort of individuals who were exposed to persistent organic pollutants in the early 1970s through a contamination of the food supply (8, 9). This cohort has been followed for over forty years and now includes multiple generations (10). This report describes a subset of women in this cohort who participated in a longitudinal study on menstrual cycle function (11).
Participants for the Menstrual Cycle Function Study were recruited from 2004 to 2006 (11). Eligible women were 18 to 45 years of age at the time of initial questionnaire and were premenopausal with a menstrual cycle within the past 3 months. Women who were pregnant, breastfeeding, taking hormonal medications or had existing implanted hormonal medications, developmentally disabled, ever diagnosed with or treated for cancer, and those who had a hysterectomy were ineligible. Participation in the Menstrual Cycle Function Study included a blood draw; a health questionnaire with details on medical history, current medications, behaviors, and demographics; and menstrual function monitoring which included daily urine collections and daily diaries (see Menstrual function protocol). This study was approved by the Institutional Review Boards at Emory University and the State Health Department in Michigan.
Menstrual cycle function protocol
Women were asked to keep daily menstrual diaries and collect first morning urine every day for at least 3 menstrual cycles (defined as 4 bleeding episodes). Diaries included daily information on bleeding, menstrual cramping, stress, smoking and alcohol consumption, and exercise. Bleeding was reported on the following scale: 0 = no bleeding, 1 = spotting, 2 = light, 3 = moderate, 4 = heavy. Spotting was described as bloody discharge that required no more than a thin panty liner. Estrogen and progesterone metabolites, estrone 3-glucuronide (E13G) and pregnanediol 3-glucuronide (Pd3G), respectively, were measured in urine samples collected 21 days before through 4 days after the day of menses onset. The timing of ovulation was based on the day of luteal transition (DLT), which was determined by an algorithm based on changes in the E13G:Pd3G ratio (12). The DLT has been shown to be an accurate and precise estimator of ovulation and to correlate well with the plasma luteinizing hormone surge (13–16).
Urinary E13G and Pd3G were measured in triplicate using competitive double-antibody time-resolved fluoroimmunoassays, as has been previously described (11, 17). Urinary creatinine (Cr) was measured in duplicate using a Vitros 250 Chemistry Analyzer (Ortho-Clinical Diagnostics, Raritan, NJ, USA) (18,19). Urinary endocrine concentrations were divided by Cr concentrations to adjust for sample dilution.
Statistical analysis
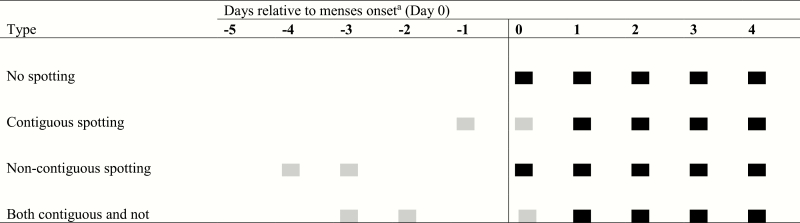
An algorithm described by Hornsby (3) to define menses onset and duration (3–6) defines the day of menses onset as the first of 2 consecutive days of spotting or bleeding in which only 1 day is spotting. Menses is preceded and followed by 3 consecutive days of spotting and/or nonbleeding. This algorithm was developed to distinguish mid-cycle spotting from menstrual bleeding and to minimize variation in cycle and bleed length. Fig. 1 shows several possible presentations of menses onset in relation to defined menses start using this algorithm.
Figure 1.
Bleeding patterns and corresponding terminology used in the Menstrual Cycle Function Study (2004–2006). Gray rectangles denote spotting days; black rectangles denote bleeding days. aAs defined by the Hornsby algorithm (3)
In our analyses, daily E13G and Pd3G concentrations and rates of change were examined with respect to the day of menses onset, which was defined by the Hornsby algorithm (which may be a day of spotting) (3), with an alternative approach where menses onset was based on first bleed (ie, excluding spotting days) (2). This proposed “first bleed” algorithm defines the day of menses onset as the first day of bleeding (ie, not spotting) followed by at least 1 more day of either bleeding or spotting.
The menstrual bleeding pattern and outcome of interest in this study was spotting before menstrual bleeding. Spotting before menstrual bleeding was defined as at least 1 reported day of spotting (ie, a diary bleeding report of 1) within 5 days before the menstrual period (ie, onset as defined by the Hornsby algorithm; Fig. 1). Mid-cycle spotting outside of this window was rare (n = 9 cycles) and was not examined in primary analyses.
In statistical analyses, we examined the associations between day-specific E13G and Pd3G concentrations and spotting before menstrual bleeding (yes/no) by fitting linear mixed models with random effects for woman and cycle to account for the nesting of days within cycles and cycles within women. In these models, spotting before menstrual bleeding was treated as the independent variable, and E13G and Pd3G concentrations were treated as the dependent variables, in order to leverage the mechanics of linear mixed models that accommodate repeated measures and nested observations. E13G and Pd3G levels were adjusted for Cr and natural log-transformed. Two sets of models were fit: first, models were aligned by the day of menses onset (calculated using both algorithms); and second, by the day after ovulation (ie, start of the luteal phase). Because hormone concentrations are more proximal to menstrual cycle function than characteristics such as age (ie, age is likely to exert its effect on menstrual cycle function by influencing hormone concentrations), models were not adjusted for age or other covariates.
Lastly, rates of E13G and Pd3G decline in the luteal phase were examined in relation to spotting before menstrual bleeding. Rates of decline were calculated in 2 ways. First, they were calculated as the change in hormone levels from 4 days before menses onset to the day before menses onset (calculated using both algorithms). Days −4 and −1 relative to menses onset were selected based on visual inspection of plots which showed declining hormone levels during this time for most transitions. Second, rates of hormone decline were calculated as the difference between the luteal phase peak Pd3G or E13G levels and the corresponding concentration on the day before menses onset (calculated using both algorithms) divided by the number of days between the peak and the last luteal phase day (ie, ΔY/ΔX; slope). Finally, the associations between slopes of hormone decline and spotting were estimated by fitting log binomial models with generalized estimating equations with exchangeable correlation structures to account for multiple transitions per woman.
In secondary analyses, we compared hormonal profiles of those with mid-cycle spotting with those with spotting before (ie, within 5 days of) menses.
Results
One-hundred sixteen women contributed daily urine samples and corresponding diaries during a total of 283 luteal-follicular transitions. Women were between 20 and 47 years of age at the start of their menstrual cycle function study collections, with an average age of 36 years (Table 1). At least 1 day of spotting before menstrual bleeding was common (41.7%, n = 118 transitions). Among these transitions with spotting, most had spotting contiguous with bleeding (86.4%, n = 102); where the spotting day directly preceded the first bleed day (Fig. 1). The remaining transitions with spotting had spotting within 5 days of the first bleed (ie, noncontiguous spotting). Women over 30 years of age, with a college degree, who had previously been pregnant, or who had never smoked were more likely to report spotting before or at menses onset (Table 1).
Table 1.
Characteristics of 116 Premenopausal Women (283 transitions) in the Menstrual Cycle Function Study (2004–2006)
| N (%) women | N (%) transitions | Spotting transitionsa N (%)b | P e | |
|---|---|---|---|---|
| Age c | ||||
| 20–29 | 17 (14.7) | 39 (13.8) | 10 (25.6) | 0.08 |
| 30–39 | 61 (52.6) | 152 (53.7) | 69 (45.4) | |
| 40–47 | 38 (32.8) | 92 (32.5) | 39 (42.4) | |
| Education | ||||
| ≤High school | 31 (26.7) | 73 (25.8) | 18 (24.7) | <0.001 |
| Some college | 44 (37.9) | 109 (38.5) | 45 (41.3) | |
| ≥College graduate | 41 (35.3) | 101 (35.7) | 55 (54.5) | |
| Income | ||||
| <$50 000/year | 45 (40.5) | 110 (40.6) | 45 (40.9) | 0.67 |
| ≥$50 000/year | 66 (59.5) | 161 (59.4) | 70 (43.5) | |
| Missing | 5 | 12 | 3 | |
| Gravidity | ||||
| Nulligravid | 21 (18.1) | 50 (17.7) | 15 (30.0) | 0.18 |
| 1–2 pregnancies | 47 (40.5) | 113 (39.9) | 50 (44.2) | |
| ≥3 pregnancies | 48 (41.4) | 120 (42.4) | 53 (44.2) | |
| Body mass index (BMI) | ||||
| ≤Normal (BMI: <25 kg/m2) | 52 (44.8) | 124 (43.8) | 51 (41.1) | 0.68 |
| Overweight (BMI: 25 to <30 kg/m2) | 43 (37.1) | 108 (38.2) | 48 (44.4) | |
| Obese (BMI: ≥30 kg/m2) | 21 (18.1) | 51 (18.0) | 19 (37.3) | |
| Smoking status d | ||||
| Never smoker | 85 (73.3) | 212 (74.9) | 104 (49.1) | <0.001 |
| Former smoker | 13 (11.2) | 28 (9.9) | 9 (32.1) | |
| Current smoker | 18 (15.5) | 43 (15.2) | 5 (11.6) |
a118 transitions from 72 women
bPercents calculated as the number of spotting transitions divided by the number of total transitions in each category
cAge when started menstrual cycle function study
dBased on questionnaire responses and daily diaries during the study
e P value from chi-square test
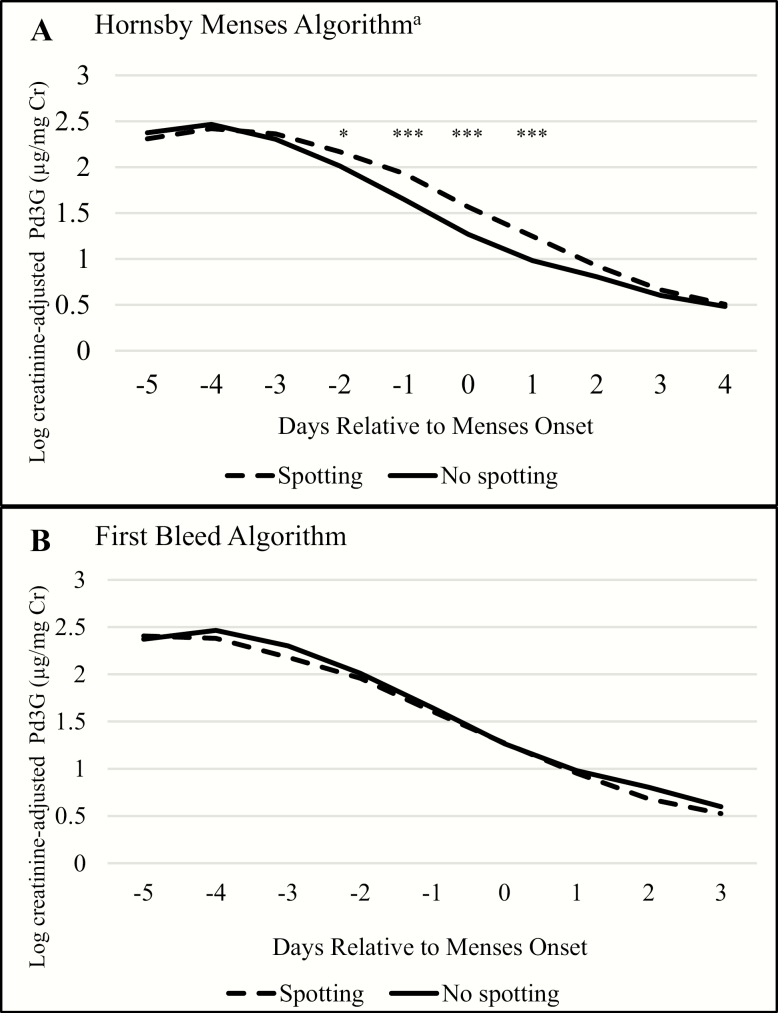
When defining menses onset using the Hornsby algorithm, late luteal phase Pd3G levels were higher for transitions with spotting compared to those without spotting. This difference was most pronounced on the day of menses onset (34.8%; 95% confidence interval [CI]: 18.9%, 52.7%; P < 0.0001) (Fig. 2A). The trajectory of Pd3G decline for transitions with spotting appeared shifted to the right by 1 day compared with that for transitions without spotting. Pd3G levels for transitions with spotting dropped to similar levels as transitions without spotting an average of 1 day later (Fig. 2). This aligned with the first day of bleeding for contiguous spotting transitions. Correspondingly, on the first day of bleeding, spotting and nonspotting transitions had similar Pd3G levels (geometric mean (GM) = 3.8 vs 3.3 µg/mg Cr, respectively).
Figure 2.
Daily predicted log Cr-adjusted Pd3G during the luteal-follicular transition by day relative to menses onset using two definitions of menses onset. Abbreviations: Pd3G, pregnanediol 3-glucuronide; Cr, creatinine. aAccording to the Hornsby algorithm, the day of menses onset may be a day of spotting. *P < 0.05; ** P < 0.01; *** P < 0.001
Accordingly, when menses onset was defined as the first day of bleeding, Pd3G trajectories of spotting and nonspotting transitions were nearly identical throughout the luteal-follicular transition (Fig. 2B).
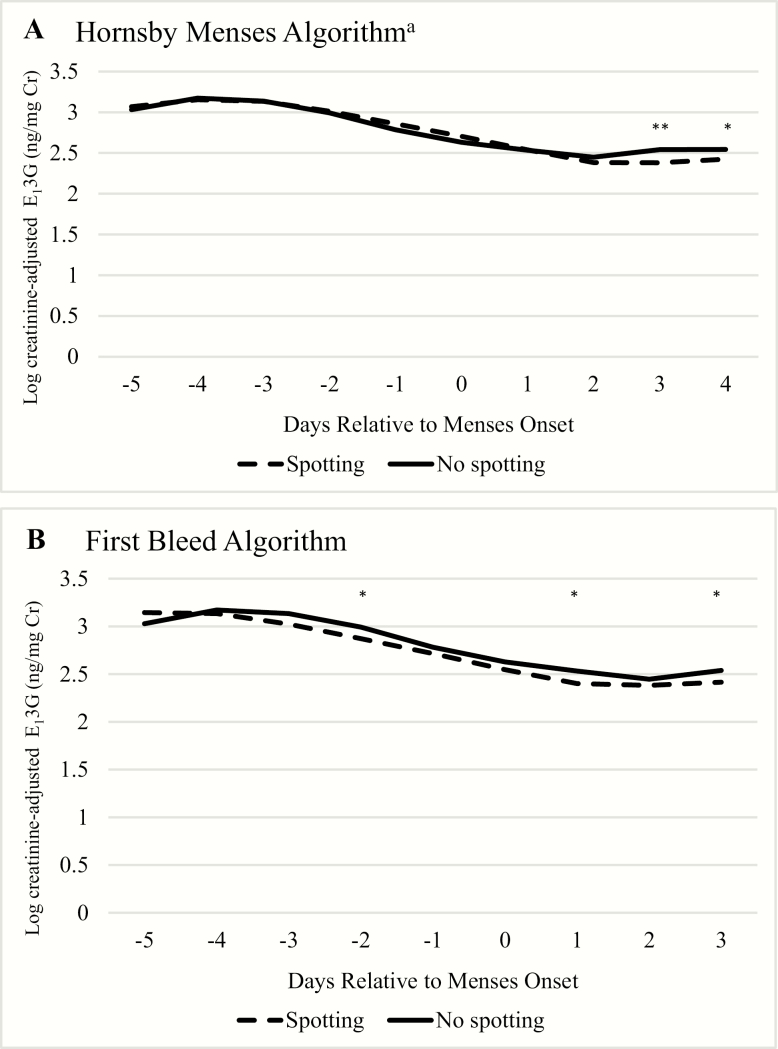
Overall, E13G levels and trajectories were similar for spotting and nonspotting transitions using either definition of menses onset (Fig. 3). When using the Hornsby algorithm for defining menses onset, E13G levels were virtually identical throughout the luteal-follicular transition, but spotting transitions had slightly lower levels on days 3 and 4 of the cycle (Fig. 3A). However, when using first bleed as the day of menses onset, spotting transitions had slightly lower E13G levels throughout, with significantly lower E13G 2 days before, and 1 and 3 days after menses onset (Fig. 3B); although the trajectory was similar.
Figure 3.
Daily predicted log Cr-adjusted E13G during the luteal-follicular transition by day relative to menses onset using two definitions of menses onset. Abbreviations: E13G, estrone 3-glucuronide; Cr, creatinine. aAccording to the Hornsby algorithm, the day of menses onset may be a day of spotting. * P < 0.05; ** P < 0.01; *** P < 0.001
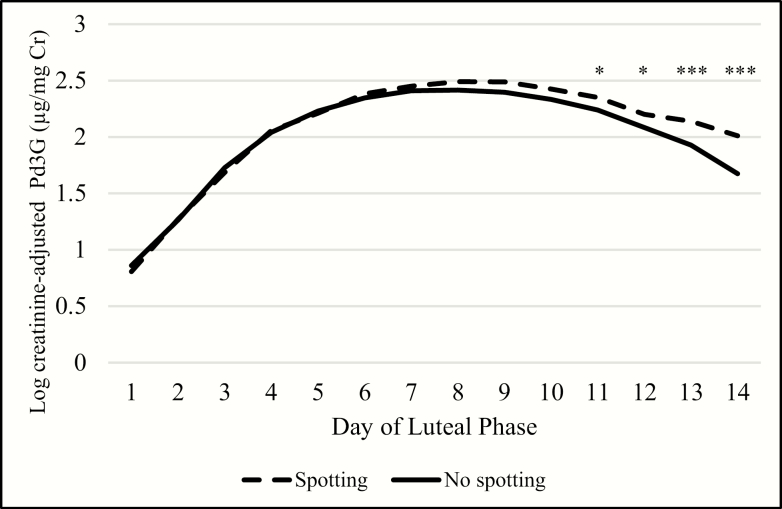
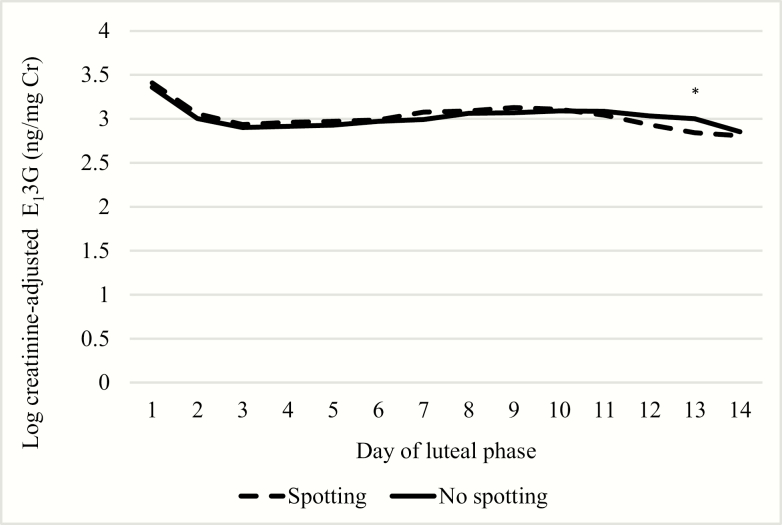
When aligning data on day 1 of the luteal phase, transitions with spotting were preceded by luteal phases with higher peak Pd3G (3-day maximum GM = 14.6 µg/mg Cr) compared with nonspotting transitions (12.1 µg/mg Cr) (Fig. 4). This difference in Pd3G became increasingly apparent as the luteal phase progressed. Again, luteal phase E13G concentrations and trajectories were similar for spotting and nonspotting transitions (Fig. 5). Luteal phases preceding transitions with spotting were shorter (median = 11, interquartile range [IQR] = 10, 12) than those that had no spotting (median = 12, IQR = 11, 13) only when the Hornsby algorithm for menses onset was used. However, when menses onset was defined by the first day of bleeding, luteal phase lengths were more similar (for both: median = 12, IQR = 11, 13).
Figure 4.
Daily predicted log Cr-adjusted Pd3G concentrations over the luteal phase by luteal phase day. Abbreviations: Pd3G, pregnanediol 3-glucuronide; Cr, creatinine. * P < 0.05; ** P < 0.01; *** P < 0.001
Figure 5.
Daily predicted log Cr-adjusted E13G concentrations over the luteal phase by luteal phase day. Abbreviations: E13G, estrone 3-glucuronide; Cr, creatinine; * P < 0.05; ** P < 0.01; *** P < 0.001
The rate of Pd3G decline during the late luteal phase was associated with spotting before menstrual bleeding. Faster rates of Pd3G decline between −4 and −1 days before menses onset (defined by the Hornsby algorithm) were associated with a decreased likelihood of spotting (for a 1-unit decrease in slope [further away from zero in the negative direction, thus a steeper slope], risk ratio (RR) = 0.92; 95% CI: 0.86, 0.98). In other words, transitions with spotting had slightly slower rates of Pd3G decline compared with those without spotting. When transitions with spotting were redefined as those with more than ≥ 2 days of spotting before bleeding, the estimate became stronger (RR = 0.79; 95% CI: 0.75, 0.82). However, when menses onset was defined as the first day of bleeding, rates of Pd3G decline were more similar for transitions with and without spotting. Still, there was some evidence of this pattern: transitions with spotting had slower Pd3G decline during the interval of −4 to −2 days before the first day of bleeding (for a 1-unit decrease in slope, RR = 0.81; 95% CI: 0.69, 0.96). Finally, when considering the entire interval from the luteal phase Pd3G peak to the day before menses onset, as defined by either algorithm, the rate of Pd3G decline was not related to presence or absence of spotting.
Rates of E13G decline (calculated using both approaches) were not associated with spotting when menses onset was defined by either algorithm (using Hornsby definition of menses onset: RR = 0.97, 95% CI: 0.92, 1.03; using first bleed as menses onset: RR = 1.00, 95% CI: 0.95, 1.05).
Secondary analyses of cycles with spotting in the mid-cycle (n = 9) showed that most (n = 7, 77.8%) also had spotting before menses. Hormonal profiles were similar for those with both mid-cycle spotting and spotting directly preceding menses (data not shown).
Discussion
In a cohort of premenopausal women with longitudinal menstrual cycle function measurements, urinary progesterone metabolite concentrations were related to the occurrence of spotting before menstrual bleeding. Transitions with spotting prior to or at the onset of menses were preceded by luteal phases with higher peak Pd3G concentrations compared with those with no spotting. However, by the day of first bleed, Pd3G concentrations were similar for transitions that included spotting days and those that did not. Lastly, slower rates of Pd3G decline in the late luteal phase were associated with an increased likelihood of spotting prior to menses. Urinary E13G concentrations were more similar for transitions with and without spotting, and there were no associations between rates of E13G decline and spotting before menstrual bleeding.
Our results are consistent with previous observations that older women are more likely to experience spotting (20) and have slower rates of progesterone decline in the luteal phase compared with younger women (21). In our study, women in their thirties and forties were more likely to spot before bleeding than younger women, and transitions with spotting were found to have slightly slower rates of Pd3G decline. Another study assessed hormone concentrations in relation to subsequent bleeding (22) and found that serum progesterone concentrations were positively associated with the volume of menstrual blood loss, but did not consider menstrual patterns. We observed consistency in hormonal profiles across the luteal-follicular transition when menses was defined as the first day of bleeding. This may suggest that the phenomenon of spotting may be due to factors other than hormonal support, such as endometrial responsiveness and sensitivity. Studies among women using intrauterine devices have found that insertion and initial use increases inflammation through up-regulation of endometrial cytokines and prostaglandins (23), which is thought to elicit vascular changes in the endometrium and subsequent irregular bleeding (24,25). Although not applicable in this study, there is also a documented inflammatory response to progesterone withdrawal by decidualized endometrial stromal cells in the perimenstrual phase (26) that increases leukocyte formation and influx (27). This process precipitates changes to endometrial vascularization (27) and affects observed bleeding patterns. It is therefore possible that heterogeneity in proinflammatory responses, specifically levels of prostaglandins (28), may have manifested in spotting in some women.
This study benefited from prospective collection of menstrual cycle function data over multiple consecutive cycles per woman, including daily urine samples and corresponding daily diaries. This allowed endocrine assessment during the luteal-follicular transition. However, our study relied on self-reporting of menstrual bleeding and spotting, which is subjective and can vary from person to person (29, 30). For example, women with greater levels of education were more likely to report spotting, which may signify that differential patterns in reporting based on demographic characteristics. In addition, we did not use the International Federation of Gynecology and Obstetrics definition for spotting (“Any bloody vaginal discharge that is not large enough to require sanitary protection”) (31) because it has been shown that many women feel that any discharge requires at least some degree of protection (24). Accordingly, in this study, spotting was defined as bloody discharge that required no more than a thin panty liner. Still, interpretation and application of this definition may be subjective and we had no way to evaluate this in this study. Other studies have utilized pictograms to guide participants’ reporting of menstrual bleeding based on visual appearance of blood loss and/or feminine sanitary product use (13), which may result in less heterogeneity across individuals (29, 30). Despite this, when we restricted the spotting group to those who reported spotting for at least 2 days before bleeding, the association with the rate of Pd3G decline was similar and perhaps even stronger. Another limitation was that we measured progesterone and estrogen metabolites in urine, which may introduce error, owing to differential excretion of hormones. For example, it is theoretically possible that progesterone could be metabolized differently (ie, into different forms and/or at different rates) in women whose menstruation is preceded by spotting versus those without initial spotting. However, we are unaware of any studies that have shown this. In spite of this, an advantage to using the urinary biomarkers Pd3G and E13G is that they have been shown to be strong correlates of the serum sex hormone levels (17, 32-35), and have proven useful in evaluating menstrual function in epidemiological (11, 36, 37), medical (38–41), and athletic populations (42). Furthermore, urine integrates the secretory episodes which complicate interpretation of blood sample measurements (33, 43); altogether making urine a feasible option for conducting epidemiologic, population-level research on menstrual cycle function.
This study has implications for both clinical and epidemiologic research. The relationship between luteal phase Pd3G levels and spotting in the transition suggests that for studies without daily hormone levels, diary records of bleeding may lend insight into endocrine function. In addition, since spotting and nonspotting groups had similar Pd3G levels on the first day of bleeding and similar Pd3G trajectories during the late luteal phase when menses onset was defined by the first day of bleeding (ie, ignoring spotting days), these relationships may be able to be exploited for algorithms to assign onset of menses and the cycle. In conclusion, by defining menses onset as first bleed and excluding contiguous spotting days in the definition of menses onset, this may yield more consistency and more comparable hormonal profiles across women. For this reason, first bleed appears to be the best choice for defining the end of the luteal phase and the beginning of menses and the follicular phase.
Acknowledgments
Financial Support: This work was supported by the National Institute of Environmental Health Sciences/National Institutes of Health (NIEHS/NIH) (grants R01ES012014, P30ES019776, R21ES023927, R01ES024790, R01ES08341, and R01ES025775), the U.S. Environmental Protection Agency (EPA) (agreement number R825300), the Eunice Kennedy Shriver National Institute of Child Health and Human Development Reproductive, Perinatal, & Pediatric Training Grant (T32HD052460)
Glossary
Abbreviations
- Cr
creatinine
- E13G
estrone 3-glucuronide
- Pd3G
pregnanediol 3-glucuronide
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
Disclaimers: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health (NIOSH). Mention of any company or product does not constitute endorsement by NIOSH.
References
- 1. Harlow SD, Ephross SA. Epidemiology of menstruation and its relevance to women’s health. Epidemiol Rev. 1995;17(2):265–286. [DOI] [PubMed] [Google Scholar]
- 2. Belsey EM, Farley TM. The analysis of menstrual bleeding patterns: a review. Contraception. 1988;38(2):129–156. [DOI] [PubMed] [Google Scholar]
- 3. Hornsby PP. The Effects of in Utero Exposure to Diethylstilbestrol (DES) on the Menstrual Cycle. Chapel Hill, NC: Department of Epidemiology, University of North Carolina at Chapel Hill; 1993. [Google Scholar]
- 4. Reutman SR, LeMasters GK, Knecht EA, et al. Evidence of reproductive endocrine effects in women with occupational fuel and solvent exposures. Environ Health Perspect. 2002;110(8):805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cragin LA, Kesner JS, Bachand AM, et al. Menstrual cycle characteristics and reproductive hormone levels in women exposed to atrazine in drinking water. Environ Res. 2011;111(8):1293–1301. [DOI] [PubMed] [Google Scholar]
- 6. Wainman BC, Kesner JS, Martin ID, et al. Menstrual cycle perturbation by organohalogens and elements in the Cree of James Bay, Canada. Chemosphere. 2016;149:190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mais V, Cetel NS, Muse KN, Quigley ME, Reid RL, Yen SS. Hormonal dynamics during luteal-follicular transition. J Clin Endocrinol Metab. 1987;64(6):1109–1114. [DOI] [PubMed] [Google Scholar]
- 8. Wolff MS, Anderson HA, Selikoff IJ. Human tissue burdens of halogenated aromatic chemicals in Michigan. Jama. 1982;247(15):2112–2116. [PubMed] [Google Scholar]
- 9. Fries GF. The PBB episode in Michigan: an overall appraisal. Crit Rev Toxicol. 1985;16(2):105–156. [DOI] [PubMed] [Google Scholar]
- 10. Small CM, DeCaro JJ, Terrell ML, et al. Maternal exposure to a brominated flame retardant and genitourinary conditions in male offspring. Environ Health Perspect. 2009;117(7):1175–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Howards PP, Terrell ML, Jacobson MH, et al. Polybrominated Biphenyl Exposure and Menstrual Cycle Function. Epidemiology. 2019;30(5):687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baird DD, Weinberg CR, Wilcox AJ, McConnaughey DR, Musey PI. Using the ratio of urinary oestrogen and progesterone metabolites to estimate day of ovulation. Stat Med. 1991;10(2):255–266. [DOI] [PubMed] [Google Scholar]
- 13. Baird DD, McConnaughey DR, Weinberg CR, et al. Application of a method for estimating day of ovulation using urinary estrogen and progesterone metabolites. Epidemiology. 1995;6(5):547–550. [DOI] [PubMed] [Google Scholar]
- 14. Baird DD, Weinberg CR, Zhou H, et al. Preimplantation urinary hormone profiles and the probability of conception in healthy women. Fertil Steril. 1999;71(1):40–49. [DOI] [PubMed] [Google Scholar]
- 15. Kesner JS, Knecht EA, Krieg EF Jr, Wilcox AJ, O’Connor JF. Detecting pre-ovulatory luteinizing hormone surges in urine. Hum Reprod. 1998;13(1):15–21. [DOI] [PubMed] [Google Scholar]
- 16. Baird DD, Wilcox AJ, Weinberg CR, et al. Preimplantation hormonal differences between the conception and non-conception menstrual cycles of 32 normal women. Hum Reprod. 1997;12(12):2607–2613. [DOI] [PubMed] [Google Scholar]
- 17. Kesner JS, Knecht EA, Krieg EF Jr, et al. Validations of time-resolved fluoroimmunoassays for urinary estrone 3-glucuronide and pregnanediol 3-glucuronide. Steroids. 1994;59(3):205–211. [DOI] [PubMed] [Google Scholar]
- 18. Findlay J, Wu A, Knott V, Mauck L, Frickey P, Norton G. Development of a Kodak Ektachem® clinical chemistry slide for CK-B activity. Clin Chem. 1985;31(6):1000. [Google Scholar]
- 19. Mauck J, Mauck L, Novros J, Norton G, Toffaletti J. Development of a single slide Kodak Ektachem thin-film assay for serum and urine creatinine. Clin Chem. 1986;32(6):1197–1198. [Google Scholar]
- 20. Belsey EM, d’Arcangues C, Carlson N. Determinants of menstrual bleeding patterns among women using natural and hormonal methods of contraception. II. The influence of individual characteristics. Contraception. 1988;38(2):243–257. [DOI] [PubMed] [Google Scholar]
- 21. Santoro N. The menopausal transition. Am J Med. 2005;118(Suppl 12B):8–13. [DOI] [PubMed] [Google Scholar]
- 22. Dasharathy SS, Mumford SL, Pollack AZ, et al. Menstrual bleeding patterns among regularly menstruating women. Am J Epidemiol. 2012;175(6):536–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones RL, Critchley HO. Morphological and functional changes in human endometrium following intrauterine levonorgestrel delivery. Hum Reprod. 2000;15(Suppl 3):162–172. [DOI] [PubMed] [Google Scholar]
- 24. Madden T, Proehl S, Allsworth JE, Secura GM, Peipert JF. Naproxen or estradiol for bleeding and spotting with the levonorgestrel intrauterine system: a randomized controlled trial. Am J Obstet Gynecol. 2012;206(2):129.e1–129.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Guttinger A, Critchley HO. Endometrial effects of intrauterine levonorgestrel. Contraception. 2007;75(6 Suppl):S93–S98. [DOI] [PubMed] [Google Scholar]
- 26. Evans J, Salamonsen LA. Decidualized human endometrial stromal cells are sensors of hormone withdrawal in the menstrual inflammatory cascade. Biol Reprod. 2014;90(1):14. [DOI] [PubMed] [Google Scholar]
- 27. Maybin JA, Critchley HO. Menstrual physiology: implications for endometrial pathology and beyond. Hum Reprod Update. 2015;21(6):748–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Smith OP, Jabbour HN, Critchley HO. Cyclooxygenase enzyme expression and E series prostaglandin receptor signalling are enhanced in heavy menstruation. Hum Reprod. 2007;22(5):1450–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hald K, Lieng M. Assessment of periodic blood loss: interindividual and intraindividual variations of pictorial blood loss assessment chart registrations. J Minim Invasive Gynecol. 2014;21(4):662–668. [DOI] [PubMed] [Google Scholar]
- 30. Janssen CA, Scholten PC, Heintz AP. A simple visual assessment technique to discriminate between menorrhagia and normal menstrual blood loss. Obstet Gynecol. 1995;85(6):977–982. [DOI] [PubMed] [Google Scholar]
- 31. Fraser IS, Critchley HO, Broder M, Munro MG. The FIGO recommendations on terminologies and definitions for normal and abnormal uterine bleeding. Semin Reprod Med. 2011;29(5):383–390. [DOI] [PubMed] [Google Scholar]
- 32. Kesner JS, Krieg EF Jr, Knecht EA, Wright DM. Power analyses and immunoassays for measuring reproductive hormones in urine to assess female reproductive potential in field studies. Scand J Work Environ Health. 1992;18(Suppl 2):33–36. [PubMed] [Google Scholar]
- 33. Kesner JS, Wright DM, Schrader SM, Chin NW, Krieg EF Jr. Methods of monitoring menstrual function in field studies: efficacy of methods. Reprod Toxicol. 1992;6(5):385–400. [DOI] [PubMed] [Google Scholar]
- 34. Adlercreutz H, Brown J, Collins W, et al. The measurement of urinary steroid glucuronides as indices of the fertile period in women. World Health Organization, task force on methods for the determination of the fertile period, special programme of research, development and research training in human reproduction. J Steroid Biochem. 1982;17(6):695–702. [DOI] [PubMed] [Google Scholar]
- 35. Gray RH, Campbell OM, Zacur HA, Labbok MH, MacRae SL. Postpartum return of ovarian activity in nonbreastfeeding women monitored by urinary assays. J Clin Endocrinol Metab. 1987;64(4):645–650. [DOI] [PubMed] [Google Scholar]
- 36. Chin HB, Jukic AM, Wilcox AJ, et al. Association of urinary concentrations of phthalate metabolites and bisphenol A with early pregnancy endpoints. Environ Res. 2019;168:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Z. Jukic AM, Weinberg CR, Wilcox AJ, Baird DD. Effects of early pregnancy loss on hormone levels in the subsequent menstrual cycle. Gynecol Endocrinol. 2010;26(12):897–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stanczyk FZ, Miyakawa I, Goebelsmann U. Direct radioimmunoassay of urinary estrogen and pregnanediol glucuronides during the menstrual cycle. Am J Obstet Gynecol. 1980;137(4):443–450. [DOI] [PubMed] [Google Scholar]
- 39. Lasley BL, Stabenfeldt GH, Overstreet JW, Hanson FW, Czekala N, Munro C. Urinary hormone levels at the time of ovulation and implantation. Fertil Steril. 1985;43(6):861–867. [DOI] [PubMed] [Google Scholar]
- 40. Miller MM, Hoffman DI, Creinin M, et al. Comparison of endometrial biopsy and urinary pregnanediol glucuronide concentration in the diagnosis of luteal phase defect. Fertil Steril. 1990;54(6):1008–1011. [DOI] [PubMed] [Google Scholar]
- 41. Sauer MV, Paulson RJ. Utility and predictive value of a rapid measurement of urinary pregnanediol glucuronide by enzyme immunoassay in an infertility practice. Fertil Steril. 1991;56(5):823–826. [DOI] [PubMed] [Google Scholar]
- 42. Loucks AB, Mortola JF, Girton L, Yen SS. Alterations in the hypothalamic-pituitary-ovarian and the hypothalamic-pituitary-adrenal axes in athletic women. J Clin Endocrinol Metab. 1989;68(2):402–411. [DOI] [PubMed] [Google Scholar]
- 43. Munro CJ, Stabenfeldt GH, Cragun JR, Addiego LA, Overstreet JW, Lasley BL. Relationship of serum estradiol and progesterone concentrations to the excretion profiles of their major urinary metabolites as measured by enzyme immunoassay and radioimmunoassay. Clin Chem. 1991;37(6):838–844. [PubMed] [Google Scholar]