Abstract
Introduction
Tripterygium glycosides (TGs) have been widely used in China to treat diabetic nephropathy (DN); however, proof of their use is scarce. The present study aimed to evaluate the effectiveness and safety of adding TGs to angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs).
Methods
By searching Embase, MEDLINE, Cochrane Library, SINOMED, China National Knowledge Infrastructure, VIP Information/Chinese Scientific Journals, and WANFANG databases, we identified previous studies that met the specific selection criteria and included them in the meta-analysis. Analyses were performed using Review Manager (version 5.3).
Results
Nine randomized controlled trials were included in the final meta-analysis. Patients were compared before and after treatment with ACE inhibitors or ARBs plus TGs, or ACE inhibitors or ARBs alone. The results revealed that treatment with ACE inhibitors or ARBs plus TGs resulted in significantly greater reductions in 24-h urinary total protein (UTP) levels (trial duration <2 months, mean difference [MD]: −0.25; 95% confidence interval [CI]: −0.32, −0.18; trial duration between 2 and 6 months, MD: −0.39; 95% CI: −0.44, −0.33; trial duration >6 months, MD: −2.09; 95% CI: −2.89, −1.29) compared with treatment using ACE inhibitors or ARBs alone. Additionally, ACE inhibitors or ARBs plus TGs showed better results after long-term administration. Treatment with ACE inhibitors or ARBs plus TGs resulted in significantly greater reductions in serum creatinine (SCr) compared with ACE inhibitors or ARBs alone (MD: −9.87; 95% CI: −13.76, −5.97).
Conclusion
In patients with DN, adding TGs to ACE inhibitors or ARBs significantly lowered both the 24-h UTP and SCr levels. Therefore, ACE inhibitors or ARBs plus TGs might improve the treatment of DN in patients.
Keywords: Tripterygium glycosides, Diabetic nephropathy, Angiotensin-converting enzyme inhibitor, Angiotensin receptor blockers, Meta-analysis
Introduction
Diabetic nephropathy (DN) is a severe microvascular complication of diabetes. DN presents as albuminuria, hypertension, renal injury, and finally, renal failure. Approximately 30–40% of patients with diabetes mellitus develop DN,1,2 that can severely damage patients’ physical and mental health, ultimately increasing the economic burden on society.3 In developed countries, such as the USA and Norway, DN was the major cause of end-stage renal diseases (ESRDs) until a decade ago.4 In China, with economic development and the change of lifestyles, DN has become the most common cause of ESRDs.1 Thus, increased attention has been paid to traditional Chinese medicine in the treatment of DN in recent years. Tripterygium glycosides (TGs) were the earliest traditional Chinese medicines that were used to treat DN; therefore, Chinese physicians have the most clinical experience of their use.
Tripterygium wilfordii Hook. F (TwHF) is a medicinal plant from the genera Tripterygium and the family Celastraceae. In addition, TwHF is used to treat chronic nephritis,5 active rheumatoid arthritis,6, 7, 8 and systemic lupus erythematosus,9 among others. TGs are extracted from TwHF, and can be used to regulate immunity, reduce blood sugar, or as anti-inflammatories.10,11 TGs have also been used to treat proteinuria in patients with DN.12,13 Angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) are common treatments for DN.14 In recent years, TGs have been used widely in China. However, randomized controlled trials (RCTs) are lacking, particularly those comparing treatment using ACE inhibitors or ARBs plus TGs with treatment using ACE inhibitors or ARBs alone. This meta-analysis only includes RCTs that examined the effectiveness and safety of adding TGs to ACE inhibitors or ARBs to treat patients with DN. The results will provide a basis for clinical use of TGs.
Methods
The meta-analysis was performed according to the recommendations of the Cochrane handbook for systematic reviews of interventions.15,16 It also was reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines.17
Study selection
The inclusion criteria for this meta-analysis were: (1) Patients with DN with a urine protein filtration rate > 20 μg/min or a quantitative 24-h urinary total protein (UTP) > 0.15 g/d (stages 3–5 of DN); (2) one study group treated with ACE inhibitors or ARBs plus TGs; (3) another study group treated with ACE inhibitors or ARBs alone, regardless of dosage, type, or duration of treatment; (4) RCTs with a parallel or crossover design, in both English and Chinese languages, regardless of the use of a blinding method; and (5) studies including 24-h UTP levels as an observed indicator.
The exclusion criteria for this meta-analysis were: (1) Patients with other kidney diseases, such as IgA Nephropathy, focal segmental glomerulosclerosis (FSGS), lupus nephritis, or membranous nephropathy; (2) patients with other severe diseases that could influence the outcomes, such as severe heart failure, cancer, disseminated intravascular coagulation (DIC), or severe infection; or (3) literature with repetitive content.
Data Sources and Searches
This study used the Embase, MEDLINE, Cochrane Library, SINOMED, China National Knowledge Infrastructure, VIP Information/Chinese Scientific Journals, and WANFANG databases to search for relevant studies. The literature search included studies that were published between the establishment of the databases and July 31, 2018. We conducted electronic searches using expanded Medical Subject Headings (MeSH) terms and corresponding key words.
The search terms used were (MeSH expanded term “Diabetic Nephropathy” and key words “diabetic nephropathy”) (MeSH expanded term “Angiotensin Receptor Antagonists” and key words “receptor antagonist*”) (MeSH expanded term “Angiotensin Converting Enzyme Inhibitors”), and (MeSH expanded term “tripterygium glycosides”). At the same time, the reference lists of included textbooks, all retrieved studies, review articles, and reports of academic congresses were checked manually. The comprehensive search strategy is shown in Appendix A.
Data extraction and quality assessment
Two investigators (Fang JY and Yang Y) independently researched studies from the retrieved literature, based on the inclusion criteria, and extracted their analytical results and data. If the two investigators had differing opinions regarding the quality of a study, differences were resolved by a third investigator (Yu TY). Data were only included for consideration if a consensus was achieved among all three investigators.
Two investigators (Fang JY and Yang Y) independently assessed the risk of bias using the Cochrane risk-of-bias tool. Each trial was reviewed and scored as high risk of bias (if the answer was yes), low risk of bias (if the answer was no), or unclear (if there were insufficient details to allow a definite judgment), based on the following criteria: (1) Random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinded assessment of the outcome, (5) incomplete outcome data assessments, (6) selective outcome reporting, and (7) other bias.
Statistical analysis
In this meta-analysis, the data and analytical results were extracted to compare the effects of ACE inhibitors or ARBs with the effects of ACE inhibitors or ARBs plus TGs on 24-h UTP and serum creatinine (SCr) levels in patients with DN. Analyses were performed using Review Manager software (version 5.3; The Cochrane Collaboration, Denmark). The weighted mean difference ((WMD = √SD12 + SD22 – SD1 × SD2); SD1 = baseline endpoint of control group; SD2 = baseline endpoint of experimental group) was used to evaluate the measured data. Tests for heterogeneity were performed using the I2 statistic and the χ2 test, in which I2 > 50% indicated significant heterogeneity and I2 < 50% indicated minor heterogeneity.18 If the data showed greater significant heterogeneity, subgroup (meta-regression) or sensitivity analysis were used. A P value < 0.05 was considered statistically significant. Both fixed and random-effect models were used in the meta-analysis; publication bias was assessed using funnel plots and evaluated using Begg's or Egger's tests.
Trial sequential analysis
To evaluate whether the present meta-analysis had a sufficient sample size to reach firm conclusions about the effects of the interventions, we used trial sequential analysis (TSA) for the outcomes. Traditionally, interim analysis of a single trial evaluates whether the monitoring boundaries for a predefined estimated effect are reached before the optimal sample size has been accrued. TSA performs a cumulative meta-analysis, which creates a Z-curve of the cumulative number of included patients and events, and the monitoring boundaries for benefit and harm, and estimates the optimal sample size. A sufficient level of evidence for the anticipated intervention effect might have been reached when the cumulative 8 Z-curve crosses the trial sequential monitoring boundary. If the Z-curve does not reach any of the boundaries and the required information size has not been reached, there is insufficient evidence to reach a conclusion. We used TSA software, version 0.9 Beta (Copenhagen Trial Unit, Denmark) for these analyses.
Results
Background information on the included studies
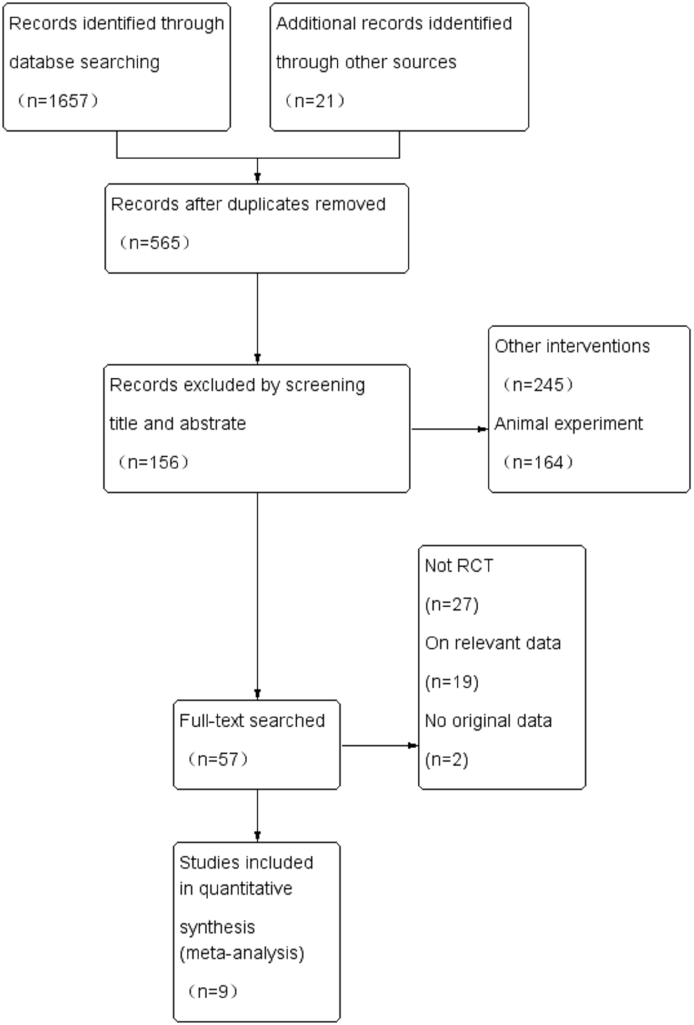
Electronic and manual searches yielded 1678 possibly relevant papers: 2 from Embase, 4 from MEDLINE®, 6 from the Cochrane Library, 347 from SINOMED, 556 from China National Knowledge Infrastructure, 196 from VIP Information/Chinese Scientific Journals, 546 from WANFANG, and 21 from manual searching. After removing duplicated publications, 565 papers remained. Of those, 156 papers were selected after review of their titles and abstracts. After reviewing the full text of each publication, 57 papers were selected. Based on the exclusion criteria, nine papers,19, 20, 21, 22, 23, 24, 25, 26, 27 with 851 patients, were included in the final meta-analysis. This selection process is shown in Fig. 1 Detailed information on the included studies is provided in Table 1, and an overview of the baseline characteristics of the study participants is shown in Table 2.
Fig. 1.
Flow diagram of study identification process, and the inclusion and exclusion criteria.
Table 1.
Characteristics of randomized controlled trials included in the meta-analysis.
| References | Province | ACEI/ARBs | ACEI/ARBs + TG | Trial duration | Sample size | Study design | Primary outcome | Dropout |
|---|---|---|---|---|---|---|---|---|
| Chen 201219 | Xizang | Erbesartan 150 mg qd | Erbesartan 150 mg qd; TG 40 mg qd | 3 months | 50 | Random number table | 24-h UTP SCr | 0 |
| Song 200520 | Shandong | Benazepril 5–20 mg qd | Benazepril 5–20 mg qd; TG 1–2 mg/kg qd | 6 months | 67 | Lottery | 24-h UTP SCr | 0 |
| He 201621 | Guizhou | Benazepril 5 mg bid | Benazepril 5 mg bid; TG 0.3–0.5 mg/kg bid/tid | 2 months | 70 | Random number table | 24-h UTP | 0 |
| Zhang 201222 | Guangzhou | Erbesartan 75 mg bid | Erbesartan 75 mg bid; TG 10–20 mg tid | 6 months | 100 | Envelope method | 24-h UTP SCr | 0 |
| Tu 201723 | Zhejiang | Telmisartan 40 mg qd | Telmisartan 40 mg qd; TG 1.5 mg/kg tid | 1 months | 216 | Random number table | 24-h UTP SCr | 0 |
| Wu 201824 | Zhejiang | Valsartan 80–160 mg qd | Valsartan 40–80 mg qd; TG 10–20 mg tid | 6 months | 68 | Random number table | 24-h UTP SCr | 0 |
| Zhang 201625 | Hebei | Valsartan 80 mg qd | Valsartan 80 mg qd; TG 30 mg bid | 12 months | 140 | Random number table | 24-h UTP SCr | 9a |
| Li 201526 | Beijing | Erbesartan 150 mg qd | Erbesartan 150 mg qd; TG 40 mg qd | 6 months | 60 | Random number table | 24-h UTP SCr | 3b |
| Song 201427 | Henan | Valsartan 40–80 mg qd | Valsartan 40–80 mg qd; TG 20 mg tid | 6 months | 80 | Random number table | 24-h UTP SCr | 0 |
TG: Tripterygium glycoside; ACEI: angiotensin-converting enzyme inhibitor; ARBs: angiotensin receptor blockers; ACEI/ARBs: angiotensin-converting enzyme inhibitors/angiotensin receptor blockers; tid: 3 times daily; bid: 2 times daily; qd: once a day; 24-h UTP: 24-h urine total protein quantitation; SCr: serum creatinine.
In the ACEI/ARBs + TG group, four patients dropped out. Two were excluded because they were not strictly prescribed according to the doctor's orders and two were rejected because of missing visits; in the ACEI/ARBs group, five patients dropped out. One was excluded because they were not strictly prescribed according to the doctor's orders, one was rejected because of missing visits and three dropped out without reason.
Two patients dropped out in the ACEI/ARB group because of repeated hyperkalemia; one patient dropped out in the ACEI/ARBs + TG group because of increased alanine aminotransferase.
Table 2.
Baseline characteristics of participants included in the meta-analysis.
| References | Group | Gender (M/F) | Mean age | UTP (g/24 h) |
SCr (μmol/L) |
||
|---|---|---|---|---|---|---|---|
| Baseline | End point | Baseline | End point | ||||
| Chen 201219 | ACEI/ARBs | 11/14 | 56.90 ± 12.10 | 8.57 ± 0.53 | 7.10 ± 0.79 | 125.60 ± 29.42 | 127.60 ± 31.3 |
| ACEI/ARBs + TG | 12/13 | 57.30 ± 11.60 | 8.34 ± 1.29 | 6.42 ± 0.95 | 131.12 ± 27.21 | 129.6 ± 26.13 | |
| Song 200520 | ACEI/ARBs | 22/10 | 53.10 ± 11.30 | 1.65 ± 0.62 | 1.23 ± 0.53 | 85.77 ± 19.10 | 88.15 ± 17.13 |
| ACEI/ARBs + TG | 21/14 | 50.10 ± 10.50 | 1.63 ± 0.51 | 0.88 ± 0.31 | 83.15 ± 20.56 | 70.56 ± 17.32 | |
| He 201621 | ACEI/ARBs | 25/10 | 58.50 ± 6.80 | 2.56 ± 0.57 | 1.97 ± 0.39 | – | – |
| ACEI/ARBs + TG | 24/11 | 57.80 ± 6.90 | 2.55 ± 0.57 | 1.38 ± 0.20 | – | – | |
| Zhang 201222 | ACEI/ARBs | 35/15 | 54.23 ± 8.23 | 1.71 ± 0.15 | 1.25 ± 0.14 | 125.90 ± 27.50 | 118.30 ± 26.40 |
| ACEI/ARBs + TG | 33/17 | 53.66 ± 7.80 | 1.73 ± 0.16 | 0.89 ± 0.12 | 128.40 ± 27.60 | 105.70 ± 22.70 | |
| Tu 201723 | ACEI/ARBs | 62/46 | 52.30 ± 6.30 | 1.44 ± 0.28 | 0.73 ± 0.24 | 127.99 ± 25.44 | 110.62 ± 20.35 |
| ACEI/ARBs + TG | 66/42 | 51.20 ± 5.90 | 1.43 ± 0.29 | 0.47 ± 0.24 | 126.96 ± 24.40 | 101.31 ± 20.29 | |
| Wu 201824 | ACEI/ARBs | 18/16 | 55.00 ± 8.00 | 4.00 ± 1.70 | 3.00 ± 1.60 | 137.00 ± 50.00 | 125.00 ± 46.00 |
| ACEI/ARBs + TG | 19/15 | 55.00 ± 9.00 | 4.00 ± 2.10 | 1.70 ± 1.20 | 133.00 ± 51.00 | 104.00 ± 42.00 | |
| Zhang 201625 | ACEI/ARBs | 40/30 | 59.82 ± 6.79 | 4.65 ± 2.93 | 3.50 ± 1.95 | 199.70 ± 65.83 | 235.96 ± 73.45 |
| ACEI/ARBs + TG | 46/24 | 59.94 ± 6.53 | 4.68 ± 2.57 | 1.44 ± 1.03 | 198.35 ± 63.94 | 210.35 ± 68.50 | |
| Li 201526 | ACEI/ARBs | 17/13 | 56.12 ± 10.34 | 2.65 ± 1.32 | 2.29 ± 0.10 | 147.31 ± 28.62 | 145.25 ± 29.10 |
| ACEI/ARBs + TG | 16/14 | 55.37 ± 9.97 | 2.74 ± 1.43 | 1.47 ± 0.69 | 145.78 ± 24.79 | 140.12 ± 23.16 | |
| Song 201427 | ACEI/ARBs | 24/16 | 50.33 ± 5.32 | 2.43 ± 1.04 | 1.98 ± 0.93 | 101.42 ± 31.35 | 105.32 ± 38.32 |
| ACEI/ARBs + TG | 22/18 | 51.24 ± 5.77 | 2.54 ± 1.21 | 1.82 ± 0.89 | 102.48 ± 29.45 | 109.87 ± 41.43 | |
M/F: male/female; UTP: urine total protein; SCr: serum creatinine; ACEI: angiotensin-converting enzyme inhibitor; ARBs: angiotensin receptor blockers; TG: Tripterygium glycoside.
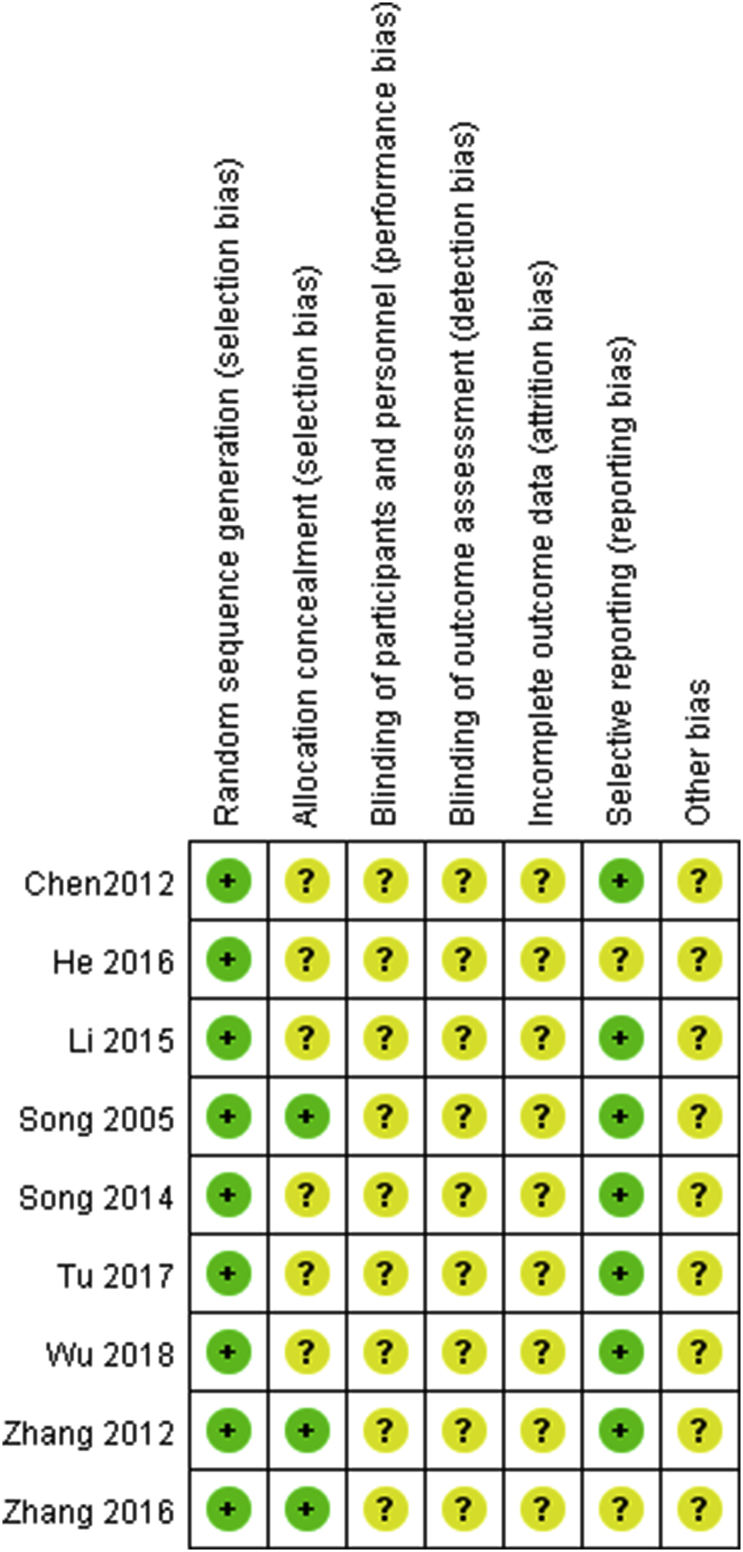
Risk of bias
The overall quality of the studies included in this investigation was not satisfactory; details on the risk-of-bias assessment are shown in Fig. 2. All nine RCTs used the random number acquisition method, but without a detailed description. Of the nine papers included, seven used a random number table, one used a lottery table, and one used the envelope method. None of the papers referred to the use of a blinded method (Fig. 2).
Fig. 2.
Risk of bias graph: Each risk of bias item was included for each study.
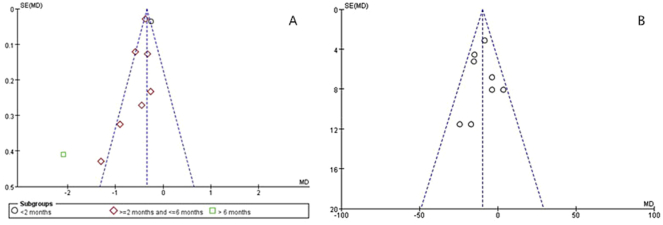
Publication bias
Using the Begg's tests, no meta-analysis had a significant publication bias (24-h UTP: P > 0.802, SCr: P > 0.736) among all meta-analyses in the present study, as shown in Fig. 3.
Fig. 3.
Publication bias analysis with funnel plots. a: Change in 24-h urinary total protein (24-h UTP); b: Change in serum creatinine (SCr) levels.
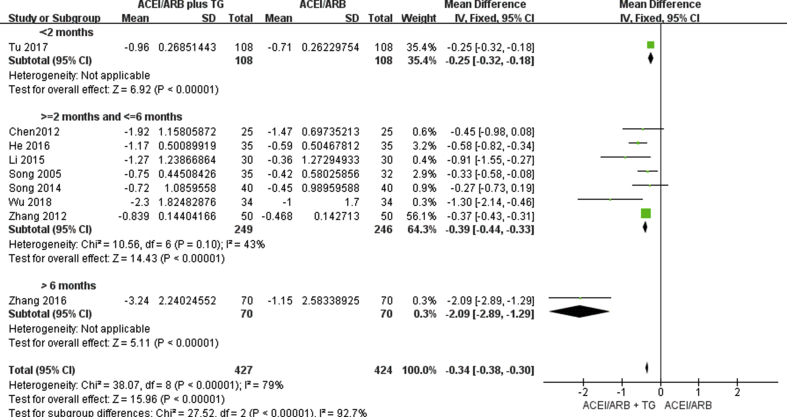
Effects on 24-h UTP levels
All the included studies reported the efficacy of ACE inhibitors or ARBs alone versus ACE inhibitors or ARBs plus TGs on 24-h UTP levels. We established three subgroups to distinguish between the effects of the length of treatment: Treatment >6 months; treatment between 2 and 6 months; and treatment <2 months. A comparison of the changes in 24-h UTP levels before and after treatment showed that the addition of TGs to the ACE inhibitor or ARB regimen produced significantly greater reductions in 24-h UTP levels in all three subgroups. The reduction in 24-h UTP levels in patients treated with ACE inhibitors or ARBs plus TGs might improve with increased treatment time. However, only one study used a treatment time >6 months (Fig. 4).
Fig. 4.
Forest plot for the change in 24-h urinary total protein (24-h UTP).
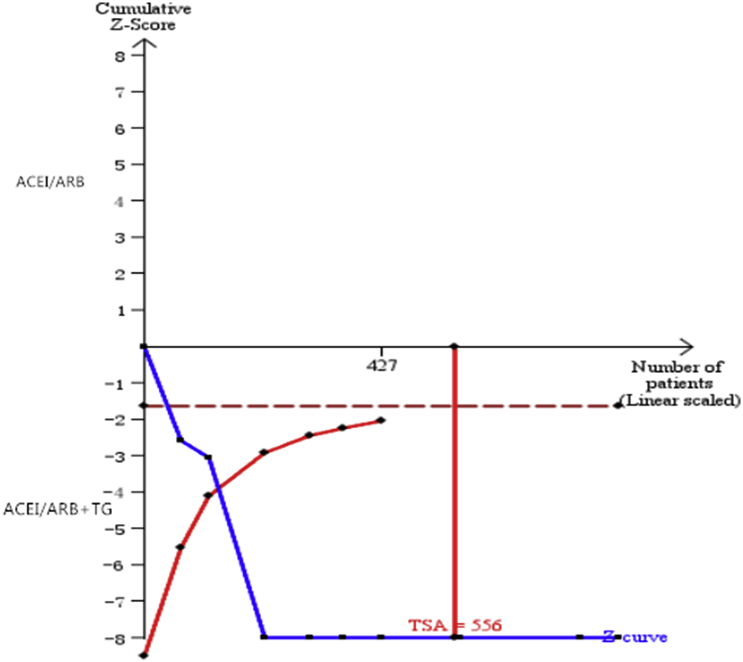
TSA of the nine comparisons illustrated that the cumulative Z curve crossed both the conventional boundary for benefit and the trial sequential monitoring boundary for benefit (Fig. 5).
Fig. 5.
Trial sequential analysis (TSA) of 24-h urinary total protein (24-h UTP).
Effects on SCr levels
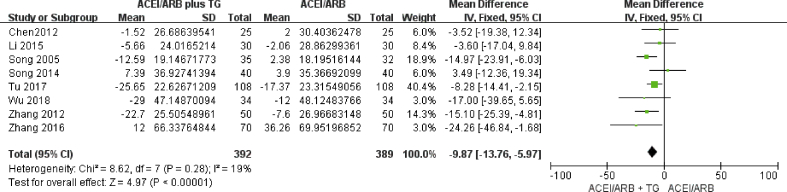
Eight of the included studies reported the efficacy of ACE inhibitors or ARBs alone versus ACE inhibitors or ARBs plus TGs on SCr levels. A comparison of the changes in SCr levels before and after treatment showed that the addition of TGs to the ACE inhibitor or ARB regimen produced significantly greater reductions in SCr levels (Fig. 6).
Fig. 6.
Forest plot for the change in serum creatinine (SCr).
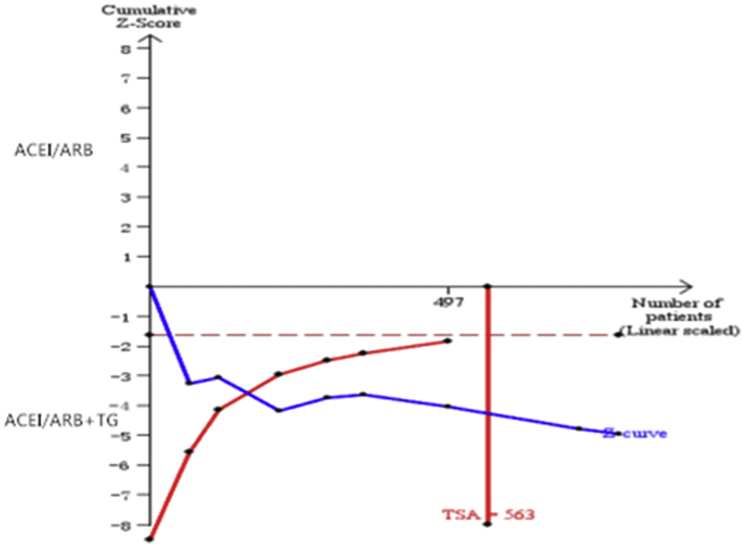
TSA of the eight comparisons illustrated that the cumulative Z curve crossed both the conventional boundary for benefit and the trial sequential monitoring boundary for benefit (Fig. 7).
Fig. 7.
Trial sequential analysis (TSA) of SCr.
Tolerability and safety
The major adverse effects of TGs include nausea, vomiting, liver injury, rash, and reproductive toxicity.28, 29, 30 Three of the included studies reported on the side effects of TGs during the treatment process. One article reported that, among those treated with ACE inhibitors or ARBs plus TGs, one patient had a headache and one had vasculitis. Among those treated with ACE inhibitors or ARBs alone, one patient reported a headache, one had vasculitis, and one had dry cough. Another paper reported that, among those treated with ACE inhibitors or ARBs plus TGs, two patients experienced liver injury, one had leukocyte reduction, and one had irregular menses. Among those treated with ACE inhibitors or ARBs alone, two patients reported hyperkalemia. In the studies by Wu et al24 and Zhang et al,22 two patients and three patients, respectively, appeared to have elevated levels of transaminase after using TGs. After stopping the use of TGs, their transaminase levels returned to normal. Another study reported that, among those treated with ACE inhibitors or ARBs plus TGs, one patient had a gastrointestinal reaction, three had liver injury, and one had leukocyte reduction. In terms of side effects, no statistical differences were observed between treatment with ACE inhibitors or ARBs plus TGs and ACE inhibitors or ARBs alone. All treatment drugs were well tolerated by nearly all the patients.
Discussion
The results of this meta-analysis showed that the addition of TGs to the ACE inhibitor or ARB regimen provided better renal protective effects on albuminuria in patients with DN than treatment with ACE inhibitors or ARBs alone. Moreover, the addition of TGs to the ACE inhibitor or ARB regimen showed better results in terms of the reduction in SCr levels compared with that induced by ACE inhibitors or ARBs alone. There were no significant differences in the reported side effects between the two groups.
The pathogenesis of DN is multifactorial, and is often linked to hemodynamics, oxidative stress, inflammation, and, especially, the immune inflammatory response.10,11,31,32 TGs are extracted from the traditional Chinese medicine, TwHF, which has been used to dispel wind and dampness, relieve swelling and pain, and promoting blood circulation to dredge collaterals. The active ingredients in TGs are diterpenoid alkaloids, as well as three terpenes.33 Some cell and animal experiments have shown that TGs can inhibit the expression of hypoxia-inducible factor 1-α, endothelin-1, and vascular endothelial growth factor in rats with DN to reduce inflammation, the number of mesangial cells, and mesangial matrix proliferation, thus delaying glomerulosclerosis.34,35 Some studies have reported that TGs can intervene in nuclear factor-κB and toll-like receptor signaling pathways to reduce the production of tumor necrosis factor-α, interleukin-5, and immunoglobulin E, thus exerting immunosuppressive effects.13,36 In addition, TGs can inhibit the proliferation of antigen-specific lymphocytes, reduce the deposition of antigen–antibody immune complexes, reduce the production of collagen fibers, and protect podocytes to reduce the degree of renal inflammation and delay damage to renal function.13,37,38 ACE inhibitors or ARBs are used commonly to treat DN, and can reduce urinary protein levels and improve renal function. However, we still lack a specific medication to treat DN, and the effects of treatment need to be improved. Thus, adding TGs to the ACE inhibitor or ARB regimen might represent an advance in DN treatment, especially in patients requiring long-term treatment. Although this meta-analysis did not show any differences in side effects between ACE inhibitors or ARBs plus TGs and ACE inhibitors or ARBs alone, some studies reported the side effects of TGs.28,29 Thus, attention should be paid to this aspect during long-term treatment.
A meta-analysis in 2014 showed that treatment with ACE inhibitors or ARBs plus TGs had an better effect on patients with stage 4 DN compared to treatment with ACE inhibitors or ARBs alone.39 However, that study only included patients with stage 4 DN. Additionally, the meta-analysis included a quasi-RCT, which increased statistical differences. Unfortunately, the data were not sufficient to draw solid conclusions.
Our meta-analysis had some limitations. First, during the literature evaluation, we found that certain studies did not describe the randomization process or the procedure of allocation concealment in detail; therefore, we could not completely exclude selection bias. Second, the sample size was small in some of the studies and the term of treatment was short. Third, TGs were extracted from the traditional Chinese medicine, TwHF, which is not commonly prescribed in other countries. Therefore, all the selected studies in this meta-analysis were Chinese-based, which may have caused regional, language, and racial biases. Finally, some studies paid little attention to side effects, which may have led to unreliable conclusions. The data were not sufficient to draw solid conclusions. We await future RCTs to further provide reliable data.
Conclusions
In conclusion, ACE inhibitors or ARBs plus TGs produce greater reductions in 24-h UTP and SCr levels in patients with DN than ACE inhibitors or ARBs alone, and even better effects might be achieved after long-term administration. There were no differences in side effects between ACE inhibitors or ARBs plus TGs and ACE inhibitors or ARBs alone. Thus, ACE inhibitors or ARBs administered together with TGs might improve the treatment of patients with impaired renal function.
Data Availability
All data are fully available without restriction.
Funding
This study was supported by grants from the Science and Technology Project of Beijing (D171100002817003; D171100002817002), National Key R&D Program of China (2016YFC1305500), China Health Promotion Foundation (DKD-MBD project, 2018–2022).
Declaration of Competing Interest
None.
Edited by Yan-Gang Ren and Yi Cui
Footnotes
Peer review under responsibility of Chinese Medical Association.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.cdtm.2019.12.008.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- 1.Tuttle K.R., Bakris G.L., Bilous R.W. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37:2864–2883. doi: 10.2337/dc14-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amemiya N., Takei T., Kojima C., Nokiba H., Itabashi M., Nitta K. Induction of remission following a single dose of rituximab alone in a patient with minimal change nephrotic syndrome. Clin Exp Nephrol. 2011;15:933–936. doi: 10.1007/s10157-011-0510-5. [DOI] [PubMed] [Google Scholar]
- 3.Zhang L., Wang F., Wang L. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379:815–822. doi: 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 4.Eckardt K.U., Coresh J., Devuyst O. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158–169. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 5.Liu L., Zhao H., Sun X. Efficacy and safety of Tripterygium wilfordii hook F for chronic urticaria: a systematic review and meta-analysis. BMC Complement Altern Med. 2018;18:243. doi: 10.1186/s12906-018-2305-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lv Q.W., Zhang W., Shi Q. Comparison of Tripterygium wilfordii Hook F with methotrexate in the treatment of active rheumatoid arthritis (TRIFRA): a randomised, controlled clinical trial. Ann Rheum Dis. 2015;74:1078–1086. doi: 10.1136/annrheumdis-2013-204807. [DOI] [PubMed] [Google Scholar]
- 7.Jiang M., Zha Q., Zhang C. Predicting and verifying outcome of Tripterygium wilfordii Hook F. based therapy in rheumatoid arthritis: from open to double-blinded randomized trial. Sci Rep. 2015;5:9700. doi: 10.1038/srep09700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paoliello-Paschoalato A.B., Marchi L.F., de Andrade M.F., Kabeya L.M., Donadi E.A., Lucisano-Valim Y.M. Fcγ and complement receptors and complement proteins in neutrophil activation in rheumatoid arthritis: contribution to pathogenesis and progression and modulation by natural products. Evid Based Complement Alternat Med. 2015;2015:429878. doi: 10.1155/2015/429878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang L., Feng S., Wang H. Decreased bone mineral density in female patients with systemic lupus erythematosus after long-term administration of Tripterygium Wilfordii Hook. F Chin Med J (Engl) 2000;113:159–161. [PubMed] [Google Scholar]
- 10.Wu W., Yang J.J., Yang H.M. Multi-glycoside of Tripterygium wilfordii Hook. f. attenuates glomerulosclerosis in a rat model of diabetic nephropathy by exerting anti-microinflammatory effects without affecting hyperglycemia. Int J Mol Med. 2017;40:721–730. doi: 10.3892/ijmm.2017.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li T., Xie J., Li Y. Tripterygium wilfordii Hook F extract in cART-treated HIV patients with poor immune response: a pilot study to assess its immunomodulatory effects and safety. HIV Clin Trials. 2015;16:49–56. doi: 10.1179/1528433614Z.0000000005. [DOI] [PubMed] [Google Scholar]
- 12.Wan Y., Gu L., Suzuki K. Multi-glycoside of Tripterygium wilfordii Hook f. ameliorates proteinuria and acute mesangial injury induced by anti-Thy 1.1 monoclonal antibody. Nephron Exp Nephrol. 2005;99:e121–e129. doi: 10.1159/000083980. [DOI] [PubMed] [Google Scholar]
- 13.Ma Z.J., Zhang X.N., Li L. Tripterygium glycosides tablet ameliorates renal tubulointerstitial fibrosis via the toll-like receptor 4/nuclear factor kappa B signaling pathway in high-fat diet fed and streptozotocin-induced diabetic rats. J Diabetes Res. 2015;2015:390428. doi: 10.1155/2015/390428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kincaid-Smith P., Fairley K., Packham D. Randomized controlled crossover study of the effect on proteinuria and blood pressure of adding an angiotensin II receptor antagonist to an angiotensin converting enzyme inhibitor in normotensive patients with chronic renal disease and proteinuria. Nephrol Dial Transplant. 2002;17:597–601. doi: 10.1093/ndt/17.4.597. [DOI] [PubMed] [Google Scholar]
- 15.Cumpston M., Li T., Page M.J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.JPTGS H. 2011. Cochrane Handbook for Systematic Reviews of Interventions.http://www.cochraneorg/handbookCochraneHandbook version 5.1.0. [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ (Clinical research ed) 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Higgins J.P., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H., Zhuang L.P., Liu J.F., Ci R.L.B., Bian W. Effects of irbesartan combined with tripterygium wilfordii on urinary protein in patients with diabetic nephropathy in highland areas (in Chinese) Clin Med Chin. 2012;28:1149–1151. [Google Scholar]
- 20.Song H.X., Gong J., Chen W. Effect of triptolide on urinary monocyte chemottractant protein-1 in patients with diabetic nephropathy (in Chinese) Chin J Integr Tradit West Med. 2005;25:416–418. [PubMed] [Google Scholar]
- 21.HeJM The effect of tripterygium wilfordii combined with benazeprilon proteinuria in diabetic nephropathy (in Chinese) Guangdong Med J. 2016;37:212–213. [Google Scholar]
- 22.Zhang Y.J., Sun Y.P., Liu D. Effect of tripterygium glycosides and irbesartan on type 2 diabetic nephropathy (in Chinese) Chin Med Herald. 2012;9:73–74. [Google Scholar]
- 23.Tu C.F., Wang L.J., Gu L.J., Tao H.Y. Effects of tripterygium glycosides combined with telmisartan on renal function and hemorheology in patients with diabetic nephropathy (in Chinese) Chin J Gen Pract. 2017;15:1527–1528. [Google Scholar]
- 24.Wu Y.P., Shi N.C. Effect of tripterygium glycosides combined with valsartan on proteinuria in stage IV diabetic nephropathy (in Chinese) Chin Remedies Clin. 2018;18:753–754. [Google Scholar]
- 25.Zhang H.C. Tripterygium glycosides tablets in the treatment of 66 cases of diabetic nephropathy in stage IV (in Chinese) Chin Pharm. 2015;2:248–249. [Google Scholar]
- 26.Li Z.X., Ma L.J., Li Y.C. Clinical efficacy of tripterygium wilfordii combined with irbesartan in the treatment of early and middle stage diabetic nephropathy (in Chinese) J Clin Res. 2015:1048–1051. [Google Scholar]
- 27.Song M.A. Effect of valsartan dispersible tablets and tripterygium wilfordii on proteinuria in diabetic nephropathy (in Chinese) China Med Eng. 2014;22:154–156. [Google Scholar]
- 28.Li X.J., Jiang Z.Z., Zhang L.Y. Triptolide: progress on research in pharmacodynamics and toxicology. J Ethnopharmacol. 2014;155:67–79. doi: 10.1016/j.jep.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Xu X.L., Yang L.J., Jiang J.G. Renal toxic ingredients and their toxicology from traditional Chinese medicine. Expert Opin Drug Metabol Toxicol. 2016;12:149–159. doi: 10.1517/17425255.2016.1132306. [DOI] [PubMed] [Google Scholar]
- 30.Long H., Li R. Clinical observation 48 cases of elderly-onset rheumatoid arthritis treating with Tripterygium glycoside combined with leflunomide (in Chinese) Contemp Med. 2014;20:152–153. [Google Scholar]
- 31.Mora C., Navarro J.F. Inflammation and diabetic nephropathy. Curr Diabetes Rep. 2006;6:463–468. doi: 10.1007/s11892-006-0080-1. [DOI] [PubMed] [Google Scholar]
- 32.Tuttle K.R. Linking metabolism and immunology: diabetic nephropathy is an inflammatory disease. J Am Soc Nephrol. 2005;16:1537–1538. doi: 10.1681/ASN.2005040393. [DOI] [PubMed] [Google Scholar]
- 33.Giuffrida D., Paola D., Francesco C. Comprehensive two-dimensional liquid chromatography coupled to triple quadrupole mass spectrometry: application to a challenging food case study. LC GC Eur. 2014;32:S42–S47. [Google Scholar]
- 34.Chen W.D., Chang B.C., Zhang Y., Yang P., Liu L. Effect of Tripterygium glycosides on expression of hypoxia inducible factor-1α and endothelin-1 in kidney of diabetic rats (in Chinese) J South Med Univ. 2015;35:499–505. [PubMed] [Google Scholar]
- 35.Wan Y.G., Sun W., Zhen Y.J. Preventive effect of multi-glycoside of tripterygium Wilfordii Hook. f. on proteinuria and mesangial injury in experimental mesangial proliferative glomerulonephritis (in Chinese) Chin J Integr Tradit West Med. 2005;25:817–821. [PubMed] [Google Scholar]
- 36.Wan Y.G., Che X.Y., Sun W. Low-dose of multi-glycoside of Tripterygium wilfordii Hook. f., a natural regulator of TGF-β1/Smad signaling activity improves adriamycin-induced glomerulosclerosis in vivo. J Ethnopharmacol. 2014;151:1079–1089. doi: 10.1016/j.jep.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 37.Zheng C.X., Chen Z.H., Zeng C.H., Qin W.S., Li L.S., Liu Z.H. Triptolide protects podocytes from puromycin aminonucleoside induced injury in vivo and in vitro. Kidney Int. 2008;74:596–612. doi: 10.1038/ki.2008.203. [DOI] [PubMed] [Google Scholar]
- 38.Yang Y.Q., Liang J., Han X.D. Dual-function of triptriolide in podocytes injury: inhibiting of apoptosis and restoring of survival. Biomed Pharmacother. 2019;109:1932–1939. doi: 10.1016/j.biopha.2018.11.031. [DOI] [PubMed] [Google Scholar]
- 39.Huang J., Zhang J.Q., Chen Z., Zhang Y., Chen W.D., Wu X.P. Systematic evaluation for efficacy of tripterygium glycosides in treating diabetic nephropathy stage IV (in Chinese) China J Chin Mater Med. 2015;40:3100–3109. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are fully available without restriction.