Abstract
Paclitaxel is the top-selling chemotherapeutic drug used for the treatment of lung, ovarian and breast cancer as well as Kaposi’s sarcoma. Cell suspension culture (CSC) of Corylus avellana has been addressed as a promising alternative for producing paclitaxel. In this study, endophytic fungus strain YEF33 was isolated from Taxus baccata and identified as Coniothyrium palmarum. The effects of the elicitors derived from this fungus including cell extract, culture filtrate and cell wall (CW) and also chitin, alone or in combination with Methyl-β-Cyclodextrin (MBCD), on paclitaxel biosynthesis in C. avellana CSC were assayed for the first time. CW of C. palmarum was the most efficient fungal elicitor for paclitaxel biosynthesis in C. avellana CSC. The results revealed that MBCD affected paclitaxel biosynthesis differently depending on fungal elicitor type and vice versa. MBCD, either alone or in combination with fungal elicitors, induced a high secretion of paclitaxel, suggesting the decrement of toxicity and retro-inhibition processes of paclitaxel for cells. The joint effects of C. palmarum CW (2.5% (v/v) on 17th day) and 50 mM MBCD synergistically enhanced paclitaxel biosynthesis (402.4 µg l−1; 5.8-fold), 78.6% of which (316.5 µg l−1) were secreted into culture medium, a level 146% higher than that in control.
Subject terms: Plant biotechnology, Plant biotechnology, Secondary metabolism, Secondary metabolism
Introduction
Paclitaxel, the most effective chemotherapy agent against lung, ovarian and breast cancer, and also Kaposi’s sarcoma1, was originally extracted from Taxus brevifolia bark in 19672 and its structure was published in 19713, and then it was joined the drug development program of National Cancer Institute (NCI)4. Since the bark harvest is mortal for the trees, T. brevifolia was set on the endangered species list4,5. Plant cell suspension culture (CSC) is a hopeful and nature-friendly system to mass production of paclitaxel6–8. The worldwide demand for paclitaxel is rising at a high speed and its biosynthesis via Taxus cell factories is inadequate to handle the growing need of this medicine, mostly because of Taxus recalcitrant manner under in vitro conditions6,7,9,10. Thus, finding the alternative sources of this valuable secondary metabolite is prompted.
Corylus avellana, common hazel, has likewise been reported as a paclitaxel-producing species among angiosperms6,7,10–15. The major superiority of producing paclitaxel by C. acellana cell culture is that in vitro culture of C. avellana is more facile than that of Taxus6,7,9,12,16,17. In vitro culture of C. avellana has been reported as a hopeful and inexpensive method for producing paclitaxel6,7,10,12,18. High-yielding in vitro culture setup is essential for producing secondary metabolites through plant cell culture19. Bioactive compounds are usually fluctuated quantitatively/qualitatively under different conditions either in vivo or in vitro6,7,10,12,20–23. Even engineered plant cells for overexpressing key genes still need using the elicitors for mass- biosynthesis of relevant secondary metabolite. Thus, screening the efficient elicitors for stimulating the biosynthesis of secondary metabolite in a plant cell culture system is vital24. Amongst the various elicitors, fungal elicitors because of their high effectiveness and low cytotoxicity are mainly used for inducing the biosynthesis of secondary metabolites in plant cell cultures25.
The first defense line of plants is the recognition of specific conserved molecules of the microbes known as microbe-associated molecular patterns (MAMPs). The receptors localized on plant cell surface recognize MAMPs; this is the first defense induction phase which is known MAMP-triggered immunity26–28. Chitin is one of fungal MAMPs29 and induces the biosynthesis of different secondary metabolites in plant cell cultures30–33. Chitin forms a small percentage of fungal cell wall while function as a strong elicitor of plant defense system34. Previous research35 suggested that fungal cell wall stimulated the biosynthesis of phenylpropanoid derivatives in hairy root culture of Linum album. It is noteworthy that the informational fragments released from fungal cell wall through enzymatic degradation function as the signals for activating the genes involved in defensive chemical production36. Chitin, an important MAMP in plants, is hydrolyzed via plant chitinases and then short oligomers act as the signaling component for triggering plant defense response34.
Our previous studies7,10 showed that cell extract and culture filtrate of endophytic fungi enhanced paclitaxel biosynthesis in C. avellana. Nevertheless, no data is available respecting the effects of chitin, fungal cell wall and also comparing the efficiency of different fungal elicitors on paclitaxel content enhancement in C. avellana CSC. Fungal elicitor type, its concentration and adding-time, and also the exposure time of cell culture with it affected the paclitaxel biosynthesis in C. avellana CSC7,10. The optimal selection of these factors would set the scene for significant biosynthesis of paclitaxel by C. avellana cell culture.
The combined use of biotic and abiotic elicitors in Taxus37 and Corylus avellana38 CSCs has been shown to highly enhance the biosynthesis of paclitaxel.
Cyclodextrin has recently absorbed remarkable attention not only as an agent inducing the biosynthesis of secondary metabolites in plant cell cultures, the consequence of defense response induction, but also for its capability to constitute the inclusion complexes with poorly water-soluble apolar compounds and facilitate the secretion of metabolites from cell to culture medium, thus act as a genuine elicitor39–41. Some studies40,42,43 have been indicated that Methyl-β-Cyclodextrin (MBCD) enhanced paclitaxel biosynthesis, and also its secretion from cells to culture medium in Taxus cell culture. Therefore, the exploration of the combined effect of fungal elicitors with the elicitor/secretion activator MBCD on the biosynthesis and secretion of paclitaxel in C. avellana CSC is considered as crucial.
The main objective of this study was to enhance paclitaxel biosynthesis and also its secretion from cells to culture medium in a promising new biotechnological platform founded on C. avellana cell culture by optimizing elicitors. For achieving this purpose, potent new fungal elicitors such as cell wall and also MBCD were assayed for the first time in C. avellana CSC, either individually or as a combined treatment.
Results and Discussion
Identification of endophytic fungus
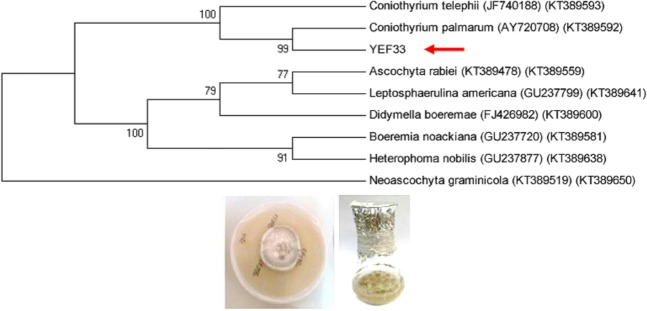
Strain YEF33 was isolated from the inner bark of T. baccata and identified as Coniothyrium palmarum by analysis of the sequences of ITS1-5.8S-ITS2 region and RPB2 gene (Fig. 1). Coniothyrium species contain very few helpful morphological features of taxonomic relationship44. This is the first report of this endophytic fungus on T. baccata tree. The partial sequences of ITS rDNA and RPB2 obtained from C. palmarum strain YEF33 was deposited in GenBank (NCBI) under accession numbers MK530082 and MT113119, respectively.
Figure 1.
Molecular identification of strain YEF33 based on the analysis of the sequences of ITS1-5.8S-ITS2 region and RPB2 gene.
Effects of elicitors on C. avellana cell growth
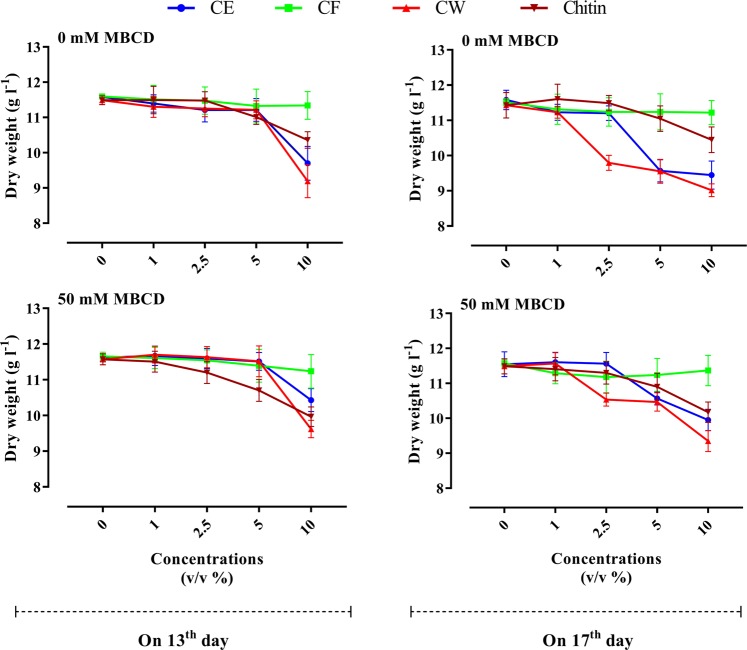
Analysis of variance (ANOVA) displayed that the main effects of the examined factors (MBCD, fungal elicitor type, concentration level and elicitor-adding day) and reciprocal interactions of MBCD × fungal elicitor type; fungal elicitor type × concentration level and also fungal elicitor type × elicitor-adding day on DW were significant (Table S1). The significant interaction effect of fungal elicitor type (CE, CF, CW and chitin) and concentration level showed that the effect of elicitors on cell growth was concentration level-dependent. Meanwhile, the significant interaction effect of fungal elicitor (CE, CF, CW and chitin) and elicitor-adding time (mid and late log phase) indicated that fungal elicitor type affected cell growth differently depending on elicitor-adding time. By reason of these significant interactions, the effects of fungal elicitor type were surveyed on each adding time and concentration level of elicitors. Means comparison showed that adding 1 and 2.5% (v/v) CE of C. palmarum on 13th and 17th days of cell culture cycle to C. avellana CSC did not significantly affect the cell growth, whereas adding 10% (v/v) of this elicitor in mid (day 13) and late (day 17) log phase significantly reduced DW as compared with control (Fig. 2). Cell culture treated with 10% (v/v) CE displayed an average growth rate of 0.456 g l−1 day−1, i.e. 16.4% lower than that of control (0.545 g L−1 day−1). Cell growth inhibition in cell culture exposed with 10% (v/v) CE seems to be as a result of CE toxicity at high concentration. As shown by Fig. 2, adding 5% (v/v) CE on 13th day of culture cycle did not affect cell growth. However, adding 5% (v/v) of this elicitor at day 17 significantly repressed cell growth (Fig. 2). Average growth rate in CSC treated with 5% (v/v) CE of C. palmarum on 17th of cell culture cycle was 0.456 g l−1 day−1, about 16.7% lower than that in control culture. It is noteworthy that C. avellana CSC exposed with 5% (v/v) CE on 17th day exhibited higher paclitaxel biosynthesis than that on 13th day (Fig. 3). The negative relation between paclitaxel accumulation and cell growth has been reported previously12,45. Also, the studies reported that high paclitaxel producing cell cultures can display cell growth inhibition46,47.
Figure 2.
Effects of adding cell extract (CE), culture filtrate (CF) and cell wall (CW) of Coniothyrium palmarum and also chitin on 13th (a) and 17th (b) days of culture cycle, either individually or as a combined treatment with 50 mM of Methyl- β –Cyclodextrin (MBCD), on cell growth of Corylus avellana L. Average values are given, standard error are represented by vertical lines.
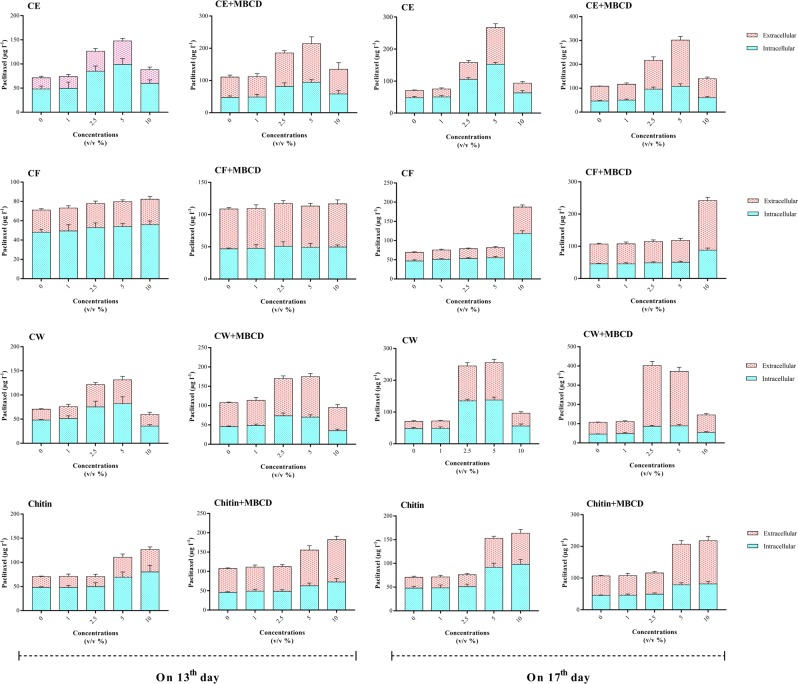
Figure 3.
Effects of adding cell extract (CE), culture filtrate (CF) and cell wall (CW) of Coniothyrium palmarum and also chitin on 13th and 17th days of culture cycle, either individually or as a combined treatment with 50 mM of Methyl- β –Cyclodextrin (MBCD), on paclitaxel production in Coryllus avellana cell suspension culture. Average values are given, standard error are represented by vertical lines.
As shown by Fig. 2, adding the different concentrations of C. palmarum CF, and also 1, 2.5 and 5% (v/v) chitin to C. avellana CSC in mid and late log phase did not significantly affect cell growth, whiles adding 10% (v/v) chitin to CSC significantly reduced cell growth as compared with control. Cell culture exposed with 10% (v/v) chitin (0.495 g l−1 day−1) displayed a decrement of 8.9% in average growth rate as compared with control (0.544) (Fig. 2). Also, cell cultures exposed with 10% (v/v) CW on 13th day, and also 2.5, 5 and 10% (v/v) CW of C. palmarum on day 17 displayed an average growth rate of 0.447 g l−1 day−1, i.e. 17.8% lower than that of control (0.544 g l−1 day−1) (Fig. 2). Cell growth inhibition in cell culture exposed with 10% (v/v) CW in mid and late log phase can be as a result of CW toxicity at high concentration. Given that C. avellana CSC exposed with 2.5 and 5% (v/v) CW on 17th day exhibited significantly higher paclitaxel biosynthesis than that on 13th day (Fig. 3), cell growth decrement in CSC subjected to mentioned treatment at day 17 can be attributed to reverse relation between paclitaxel accumulation and cell growth. Significant interaction effect of MBCD × fungal elicitor type (Table S1) showed that MBCD affected cell growth differently depending on fungal elicitor type and vice versa (i.e. fungal elicitors affected cell growth differently depending on presence or absence of MBCD) (Fig. 2). Average growth rate was not significantly influenced by MBCD alone, as has likewise been reported in Vitis vinifera48 and Taxus × media42. As mentioned above, adding 5% (v/v) CE, and also 2.5 and 5% (v/v) CW on 17th day significantly repressed cell growth (Fig. 2). However, the presence of MBCD in culture medium significantly reduced the negative effect of adding 5% (v/v) CE, and also 2.5 and 5% (v/v) CW on 17th day (Fig. 2), as it was also observed that pre-treatment of Taxus CSCs with MBCD decreased the negative effect of methyl jasmonate42 and coronatine40 on cell growth. Adding 5% (v/v) CE, and also 2.5 and 5% (v/v) CW on 17th day to cell culture previously treated with MBCD resulted in cell growth increment of 9.2% as compared with that not treated with MBCD (Fig. 2). Indeed, MBCD constitute inclusion complexes with paclitaxel and other taxanes, thus boosting their secretion from cells to culture medium, also reducing cellular toxicity42. The effect of MBCD on decreasing the negative effect of mentioned treatment (adding 5% (v/v) CE, and also 2.5 and 5% (v/v) CW on 17th day) can be attributed to secretion increment of taxanes to culture medium, and decreasing cellular toxicity.
Effect of exposure period of fungal elicitors on paclitaxel content
To figure out the relevance between paclitaxel content and exposure period of fungal elicitors, the contents of paclitaxel in C. avellana CSCs treated with four concentrations (1, 2.5, 5 and 10% (v/v)) of CE, CF, CW and chitin in mid and late log phase were determined in 2-day periods after elicitation (Fig. S1). Generally, the increment of paclitaxel biosynthesis was observed throughout the period of cell growth and its maximum significant level was determined at day 21 (Fig. S1). Decreasing paclitaxel biosynthesis after 21st day in non-treated cell culture with MBCD could be ascribed to enzymatic degradation of paclitaxel. However, C. avellana CSCs treated with MBCD exhibited no significant differences in paclitaxel produced on 21st and 23rd days (Fig. S1). It is noteworthy that MBCD forms the inclusion complexes with paclitaxel and inhibit its possible enzymatic degradation42. This could explain why paclitaxel content in CSCs treated with MBCD displayed no significant difference at days 21 and 23. Since maximum significant contents of paclitaxel were measured on day 21, this time was considered as the benchmark of paclitaxel biosynthesis in CSCs.
Paclitaxel biosynthesis in elicited cell suspension cultures
The effects of CE, CF and CW of C. palmarum, as well as chitin on paclitaxel content were studied in a concentration level-, elicitor-adding time-dependent way, either individually or as a combined treatment with MBCD. The results of eliciting paclitaxel biosynthesis in C. avellana CSC using elicitors disclosed that intracellular, extracellular and total yield of paclitaxel were significantly affected by the mentioned elicitors (Table S1). The main effects of measured factors (MBCD, fungal elicitor type, concentration level and adding time of fungal elicitors) and their interactions (reciprocal and trilateral effects) except MBCD × concentration level, MBCD × fungal elicitor-adding time, MBCD × fungal elicitor type × concentration level, and MBCD × concentration level × fungal elicitor-adding time on total yield of paclitaxel were highly significant (p < 0.01) (Table S1). The significant interactions of fungal elicitor type and concentration level and adding time of fungal elicitors (Table S1) displayed that the concentration level and adding time of fungal elicitors impressed paclitaxel content differently at each fungal elicitor type. Also, the significant interaction of MBCD × fungal elicitor type revealed that the fungal elicitors affected the content of paclitaxel differently depending on the presence of MBCD and vice versa (i.e. MBCD affected paclitaxel biosynthesis differently depending on fungal elicitor type). To carefully analyze these significant interactions, the fungal elicitors were further examined on each concentration level and adding time of fungal elicitors as well as the presence or absence of MBCD.
Effects of concentration level and adding time of fungal elicitors on the biosynthesis of paclitaxel
Means comparison revealed that cell cultures exposed with 2.5 and 5% (v/v) CE and CW of C. palmarum on 13th day of culture cycle displayed a slight increase in paclitaxel biosynthesis (Fig. 3). As indicated in Fig. 3, adding 2.5 and 5% (v/v) CE and also CW at day 17 led to significantly higher paclitaxel contents (1.2-, 1.8-, 2.0 and 1.9-fold, respectively) than that on day 13. The most total yield of paclitaxel in cell cultures exposed to C. palmarum CE (266.9 μg L−1) was obtained by using 5% (v/v) of this elicitor on 17th day of cell culture cycle, about 3.9- fold that detected in control culture (Fig. 3). The contents of extracellular and intracellular paclitaxel in CSC exposed to 5% (v/v) CE on 17th day were 114.5 μg L−1 (5.2-fold) and 152.4 μg L−1 (3.2-fold), respectively (Fig. 3). It is noteworthy that cell cultures exposed with 5% (v/v) CE and also 2.5 and 5% (v/v) CW of C. palmarum displayed no significant difference in paclitaxel content (Fig. 3).
The results displayed that adding the different concentrations of C. palmarum CF on 13th day of cell culture cycle and also 1, 2.5 and 5% (v/v) of it on 17th day did not significantly affect paclitaxel biosynthesis (Fig. 3). However, cell cultures treated with 10% (v/v) CF of C. palmarum on 17th day exhibited a pronounced increment in paclitaxel biosynthesis (2.8-fold) than control, measured 187.1 μg L−1 (Fig. 3).
As illustrated in Fig. 3, adding chitin to cell culture in mid and late log phase only at concentration levels of 5 and 10% (v/v) significantly enhanced paclitaxel biosynthesis. No significant difference was observed between paclitaxel biosynthesis in CSCs exposed to the concentration levels of 5 and 10% (v/v) chitin (Fig. 3) and the optimal concentration of it was 5% (v/v). Cell cultures subjected to 5 and 10% (v/v) chitin at day 17 had paclitaxel productivity of 7.54 μg l−1 day−1, about 33.7% higher than that at day 13 (5.64 μg l−1 day−1) (Fig. 3).
Out of CSCs exposed to four concentrations of 1, 2.5, 5 and 10% (v/v) of fungal elicitors in mid (day 13) and late (day 17) log phase of cell culture cycle, the highest yield of paclitaxel was measured in cell cultures treated with 5% (v/v) CE and also 2.5 and 5% (v/v) CW of C. palmarum added at day 17 (Fig. 3). Out of these treatments, 2.5% (v/v) CW is preferable as less volume of fungal elicitor was added to cell culture.
The results clearly showed that fungal elicitors had remarkable effects on improving paclitaxel biosynthesis in C. avellana cell culture. Several fungal elicitors applied in this study led to different responses regarding the enhancement of paclitaxel biosynthesis. CW of C. palmarum strain YEF33, isolated from the inner bark of T. baccata, has been demonstrated to be the most impressive fungal elicitor for inducing paclitaxel biosynthesis in in vitro cell culture of C. avellana. The varied responses of plant cells to fungal elicitors in enhancing the biosynthesis of secondary metabolite as observed in our research for paclitaxel biosynthesis can be associated with specific interactions of fungi and plant cells7,49. The receptors localized on plant cell surface recognize fungal elicitors and transfer the information for motivating cell defense system50. The specific structure of receptors leads to specially distinguish the specific elicitors7,51. Accordingly, all fungal elicitors are unable to induce a cell culture, and the selection of an efficient elicitor for the most biosynthesis of a favorite product in a special cell culture is essential.
Taken together, our results show that the influences of fungal elicitors on paclitaxel biosynthesis are affected by fungal elicitor concentration levels and its adding time to cell culture. So, optimizing these factors is required for the maximum biosynthesis of paclitaxel. The influences of the mentioned factors on paclitaxel biosynthesis in C. avellana cell culture were also reported in the previous studies using the elicitors derived from another endophytic fungi7,10.
Effects of cyclodextrin and fungal elicitors on paclitaxel biosynthesis
As shown in Fig. 3, C. avellana CSCs treated with MBCD, alone or in combination with fungal elicitors, significantly enhanced paclitaxel biosynthesis. Significant interaction effect of MBCD × fungal elicitor type (Table S1) showed that MBCD affected paclitaxel biosynthesis differently depending on fungal elicitor type and vice versa (i.e. fungal elicitors affected paclitaxel biosynthesis differently depending on the presence or absence of MCBD). C. avellana CSCs treated with MBCD alone or in combination with fungal elicitors displayed a considerable variation in improving paclitaxel biosynthesis, ranging from 13% to 64% (Fig. 3). The most efficient treatment for increment of paclitaxel biosynthesis in C. avellana cell culture showed to be the combined one of MBCD and 2.5% (v/v) CW added at day 17 (Fig. 3), 5.8-fold higher than in control. By comparison, the individual use of MBCD and CW induced paclitaxel biosynthesis only 1.5- and 3.5-fold higher than control, respectively. These results show a synergistic effect of MBCD and CW on paclitaxel biosynthesis in C. avellana cell culture. The previous studies reported the synergistic effect of MBCD and methyl jasmonate42 or coronatine40 on paclitaxel biosynthesis in Taxus cell culture, but this is the first report on the synergistic effect of MBCD and fungal elicitor (CW) on paclitaxel biosynthesis. Intra- and extracellular paclitaxel of MBCD-pretreated CSC exposed with 2.5% (v/v) of C. palmarum CW on 17th day of cell culture cycle were 86.0 μg L−1 (1.8-fold) and 316.5 μg l−1 (14-fold), respectively. It is noteworthy that paclitaxel content in cell culture treated with 2.5 and 5% (v/v) CW displayed no statistically significant difference.
When examining cell capacity to secrete paclitaxel to the medium (Fig. 4), MBCD, either alone or in combination with fungal elicitors, induced a high secretion, whereas control and fungal elicitor-treated CSC maintained more than 50% of produced paclitaxel inside C. avellana cells. Facilitating paclitaxel secretion from cells into the medium in MBCD-pretreated CSCs was reported in previous studies40,42. MBCD, through the chemical structure, facilitates paclitaxel secretion from cells into the medium and mitigates feedback inhibition. Decreasing toxicity and retro-inhibition processes of paclitaxel could explain the high biosynthesis of paclitaxel found in CSC treated with MBCD and CW. Aforementioned synergistic effect on paclitaxel biosynthesis was not observed between MBCD and other fungal elicitors. It can be concluded that out of different fungal elicitors used in this study, only C. palmarum CW had high potential to induce paclitaxel biosynthesis, but the high accumulation of paclitaxel in cells led to feedback inhibition which is a drawback for its high biosynthesis. MBCD, due to paclitaxel secretion increment, declined the retro-inhibition processes and toxicity caused by paclitaxel accumulation in the cytoplasm and thus improved paclitaxel biosynthesis. Cell cultures treated with MBCD and fungal elicitors, individually and combined treatment, exhibited a remarkable variation in paclitaxel secretion, ranging from 31.8% to 78.6% (Fig. 4). Overall, MBCD-pretreated CSCs displayed a statistically significant increase in paclitaxel secretion as compared to control and also CSCs exposed with different fungal elicitors (Fig. 4). Out of different treatments, MBCD-pretreated cell culture exposed with 2.5 and also 5% (v/v) CW on 17th day exhibited the best results (78.6 and 76.1, respectively) regarding extracellular paclitaxel portion, i.e.,137.4 and 70.7% higher than that in control and CSCs individually treated with 2.5 or 5% (v/v) CW. Cell capacity to secrete paclitaxel to the medium is essential for the commercial production because it enables continuous production with no destroying the cells and causes the extraction and purification processes to be easier and more economic.
Figure 4.
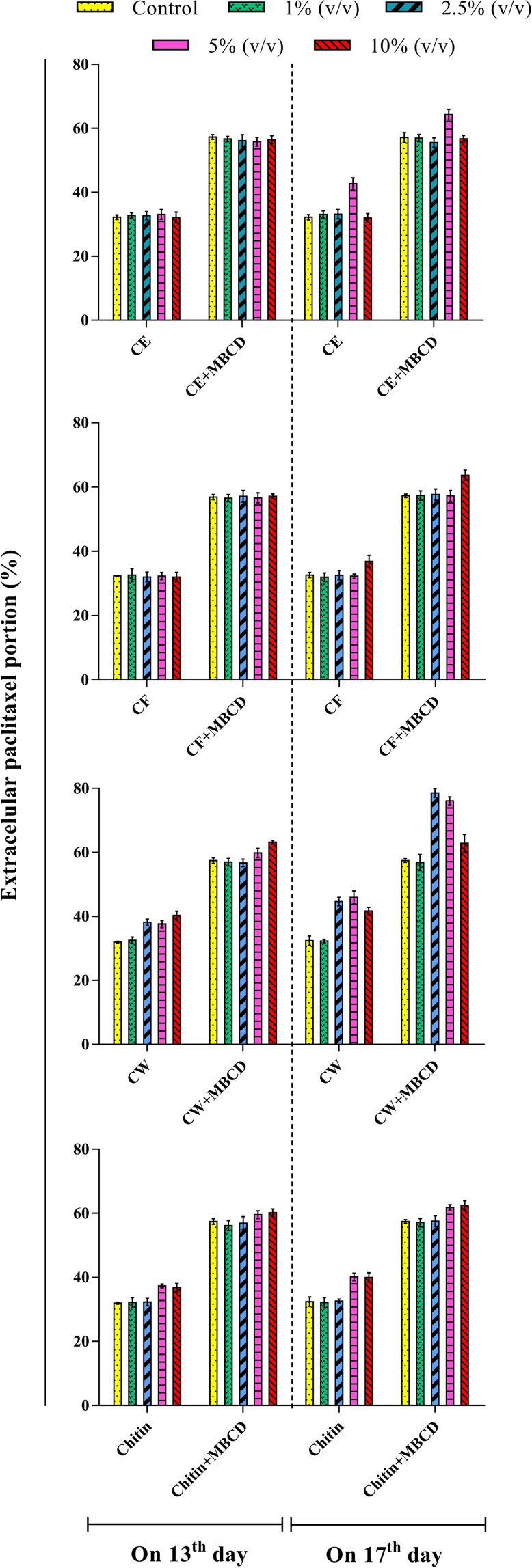
Extracellular paclitaxel portion in Corylus avellana cell suspension culture exposed with 1, 2.5, 5 and 10% (v/v) of cell extract (CE), culture filtrate (CF), cell wall (CW) and also chitin on 13th and 17th days of cell culture cycle, either individually or as a combined treatment with 50 mM of Methyl- β –Cyclodextrin (MBCD).
The various treatments have been applied in C. avellana CSCs to enhance paclitaxel productivity. C. avellana CSC treated with the combined treatment of ultrasound (40 kHz for 3 min at days 10 and 12) and 50 mg l−1 salicylic acid displayed a 14-folds increment in paclitaxel biosynthesis, while a significant decrement in cell growth was observed by salicylic acid38. Also, C. avellana CSC subjected with ultrasound (29 KHz for 20 min) produced 6.07 mg kg−1 paclitaxel52. In another report, a slight increase in paclitaxel biosynthesis displayed in C. avellana cell culture affected by silver nano particles53. Gallego et al.54 also reported that coronatine highly induced paclitaxel biosynthesis, but strongly reduced cell growth in C. avellana CSC. In another attempt to find an efficient treatment, a combined treatment of salicylic acid and dibutyl phthalate highly enhanced paclitaxel biosynthesis in C. avellana CSC with displaying a synergistic effect, but these treatments decrease cell viability55. Also, the addition of benzoic acid to C. avellana CSC resulted in a 4-fold in paclitaxel biosynthesis56. In another research, Rahpeyma et al.15 displayed that the joint effects of phenylalanine (3 μM) and vanadyl sulfate (0.05 and 0.1 mM) in culture medium completed with fructose (3% (v/v)) led to a 2.3-fold increment in paclitaxel biosynthesis.
In the light of remarkable positive effect of ultrasound on paclitaxel biosynthesis without the negative effect on cell growth38, it can be suggested to evaluate the effects of ultrasound, CW and MBCD, either individually or in a combined treatment with each other using the factorial arrangement.
Conclusion
Out of the elicitors evaluated in this study, the joint effect of C. palmarum CW (2.5% (v/v) on 17th day) and 50 mM MBCD resulted in the highest stimulation of paclitaxel biosynthesis in C. avellana CSC. Although cell growth was decreased by about 8%, the total yield of paclitaxel was improved by 480% as compared with control. Indeed, C. palmarum CW is an efficient elicitor for paclitaxel biosynthesis in C. avellana CSC, although the presence of MBCD synergistically enhanced paclitaxel biosynthesis. Also, among the various elicitors, adding 2.5% (v/v) CW of C. palmarum on 17th day to cell culture pre-treated with MBCD displayed the best results regarding extracellular paclitaxel portion (78.6%). The secretion of paclitaxel from cells into culture medium indubitably facilitates its extraction and the purification for paclitaxel production at the commercial level. Overall, the results show the potential of C. avellana CSC as a promising alternative for paclitaxel production, though this eco-friendly system yet needs the optimization.
Material and Methods
Isolation of endophytic fungi
Healthy samples of the stem, bud, bark pieces, and leaves were collected from T. baccata grown in Iran, in July, August, and September 2014. The surface sterilization of the samples was performed as described by Salehi et al.7,12. The surface sterilized pieces of stem, bud, bark, and leaves segments were excised and placed on PDAC (Potato Dextrose Agar (PDA); supplemented with 250 mg l−1 Chloramphenicol) in unique Petri dishes (100 × 15 mm), incubated at 25 °C to growth endophytic fungi. The isolates were purified by hyphal tip culture57. All fungal isolates were numbered as YEF# series and maintained on PDA at 4 °C.
Molecular identification of endophytic fungus
Our group recently evaluated the effects of Cell extract (CE) and culture filtrate (CF) of a number of fungal endophytes isolated from T. baccata and C. avellana on the biosynthesis of paclitaxel in C. avellana cell culture7,10. CE of Chaetomium globosum7 and strain YEF33 were selected as the most impressive elicitors for stimulating paclitaxel biosynthesis in C. avellana CSC. C. avellana CSCs exposed with 10% (v/v) C. globosum CE and 5% (v/v) CE of strain YEF33 displayed no significant difference in paclitaxel production. Given that the elicitation effect of CE of strain YEF33 was stronger than that of C. globosum, the strain YEF33 was used in this study.
Strain YEF33 was cultured in potato dextrose broth (PDB) and maintained in a shaker incubator at 110 rpm and 25 °C for 7 days. The mycelia were harvested; freeze-dried and then genomic DNA extraction was performed as described by Salehi et al.7,12. ITS fragments were amplified using universal primers ITS1 and ITS4 (White et al.)58, RPB2 using fRPB2-5F and fRPB2-7cR primers (Liu et al.)59 (Table S2). PCR reaction mixtures (25 µl) consisted of 1 µl genomic DNA (~100 ng), 1 µl forward and reverse primers (10 pM), and 12.5 µl Premix Taq (TaKaRa Biotechnology Ltd., Japan), and 10.5 µl PCR quality water. PCR reaction programs were an initial denaturation at 94 °C for 3 min, followed by 30 cycles of denaturation (94 °C for 30 s), annealing (56 °C (ITS) and 55 °C (RPB2) for 30 s), extension (72 °C for 1 min) and a final extension at 72 °C for 5 min. PCR products analysis and purification, sequencing and phylogenetic analysis were made as described previously7,12.
Cell suspension culture
Callus of C. avellana (ecotype Gerd Ashkorat) was produced from seed cotyledons on MS medium60 supplemented with 2 mg l−1 2, 4-D and 0.2 mg l−1 BAP, and 8 g l−1 agar agar6. CSC of C. avellana was obtained as described by Salehi et al.6,7,10,12.
Elicitor preparation
CE and CF elicitors were prepared as described previously7. The isolation of cell wall of strain YEF33 was performed as described by Prados‐Rosales et al.61, with minor modifications. Seven-day-old mycelia of strain YEF33 grown in potato dextrose broth (PDB) medium on a gyratory shaker at 110 rpm in darkness at 25 °C were harvested by filtration and rinsed three times with double distilled water. Then the freeze-dried mycelia were crushed in liquid nitrogen, and soaked in a buffer containing 10 mM Tris–HCl with pH 7.5, 5 mM dithiothreitol, and 1 mM phenylmethanesulfonyl fluoride (PMSF), and mixed thoroughly. The suspension was partitioned into a cell wall portion (pellet) and a soluble cytoplasmic portion (supernatant) by centrifuging at 10,000 g for 15 min. Then, fungal cell wall pellet was washed five times with deionized water supplemented with 1 M NaCl and 1 mM PMSF, and then washed five times with ice-cold water supplemented with 1 mM PMSF. Finally, the crushed cell wall was soaked in deionized water including 1% (v/v) acetic acid (1 mg ml−1), mixed well, and incubated at 50 °C for 2 h. Then the mixture was filtered through 0.22 μm cellulose acetate syringe filters and designated as cell wall (CW).
Elicitation experiment
For elicitation, 1.5 ± 0.1 g of C. avellana cells (fresh mass) was cultured in 100 ml flasks having 30 mL MS medium supplemented with 2 mg l−1 2,4-D and 0.2 mg l−1 BAP and then elicited with 50 mM MBCD, either individually or a combined treatment with fungal elicitors (CE, CF, CW and chitin). It is noteworthy that MBCD was added to culture medium before autoclaving.
Based on the preliminary experiment, four concentrations (1, 2.5, 5 and 10% (v/v)) of fungal elicitors “CE, CF, CW and chitin”, and also mid (day 13) and late (day 17) log phase were elected for adding fungal elicitors. Control received an equal volume of water (for CE)/ PDB (for CF)/ water including 1% (v/v) acetic acid (for CW and chitin). The growth curve of C. avellana cells has been given elsewhere6.
Cell growth measurement
Cell growth was defined by the measurement of cell dry weight (DW). Cell biomass was separated from culture medium by the filtration (Whatman No. 1) and rinsed with distilled water for eliminating the residual medium, afterward freeze-dried to constant weight by a vacuum-freeze drier.
Quantification of paclitaxel
C. avellana cells were separated from culture medium by a filter paper (Whatman No. 1). Extracellular and intracellular paclitaxel were extracted from the cells and culture medium using a procedure described by Salehi et al.6,7,12. Filtering all samples was performed by 0.22 µm cellulose acetate syringe filters before HPLC analysis. Paclitaxel in the samples were analyzed by HPLC (Waters, USA) with a C18 analysis column (MachereyeNagel EC 250/4.6 Nucleodur). Each sample (20 µl) was injected and detected at 230 nm using a UV detector. The mobile phase was methanol: water (80:20 v/v) at a flow rate of 1.0 ml/min. The quantification of paclitaxel was based on an external standard of genuine paclitaxel (Sigma) (Figs. S2 and S3).
Statistical analysis
The experiment was conducted as factorial based on a complete randomized block design (CRBD). The factorial arrangement of the treatments consisted of four factors containing MBCD with two levels (0 and 50 mM), elicitor type with 7 levels (CE, CF, CW, chitin, water, PDB and water including 1% (v/v) acetic acid), the concentration level of elicitor with four levels (1, 2.5, 5 and 10% (v/v)) and elicitor-adding time with two levels (mid and late log phase), given 112 treatments.
The experiment was conducted in triplicate. The normality and equal variance hypotheses were met and conventional parametric statistics were applied for the analysis. The data was analyzed using analysis of variance (ANOVA) and mean comparisons were performed by least significant difference (LSD) using SAS (SAS 9.1) and SPSS (SPSS 15.0). Term “significant” indicates the differences for P < 0.05. GraphPad Prism (GraphPad Prism 5) software was used for making graphs.
Declarations
All authors approve Ethics and consent for participation and publication.
All authors of the manuscript have read and agreed to its content and are accountable for all aspects of the accuracy and integrity of the manuscript in accordance with ICMJE criteria
That the article is original, has not already been published in a journal, and is not currently under consideration by another journal.
Supplementary information
Acknowledgements
Authors gratefully acknowledge Research Deputy of Tarbiat Modares University, Tehran for financial support of this research project. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
S. Farhadi carried out all experiments and analyses, and wrote the manuscript. A. Moieni directed the research. N. Safaie directed the sections related to fungus. M.S. Sabet and M. Salehi advised the experiments. M. Salehi helped in the experiments and writing the manuscript. All authors read and approved the final manuscript.
Data availability
The dataset supporting the conclusions of this article is included in the article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-62196-4.
References
- 1.Cragg GM, Schepartz SA, Suffness M, Grever MR. The taxol supply crisis. New NCI policies for handling the large-scale production of novel natural product anticancer and anti-HIV agents. Journal of natural products. 1993;56:1657–1668. doi: 10.1021/np50100a001. [DOI] [PubMed] [Google Scholar]
- 2.Perdue, R. E. search for plant sources of anticancer drugs. Morris Arboretum Bull (1969).
- 3.Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT. Plant antitumor agents. VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. Journal of the American Chemical Society. 1971;93:2325–2327. doi: 10.1021/ja00738a045. [DOI] [PubMed] [Google Scholar]
- 4.Weaver BA. How Taxol/paclitaxel kills cancer cells. Molecular biology of the cell. 2014;25:2677–2681. doi: 10.1111/j.1467-7652.2011.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walsh V, Goodman J. Cancer chemotherapy, biodiversity, public and private property: the case of the anti-cancer drug Taxol. Social science & medicine. 1999;49:1215–1225. doi: 10.1016/s0277-9536(99)00161-6. [DOI] [PubMed] [Google Scholar]
- 6.Salehi M, Moieni A, Safaie N. A novel medium for enhancing callus growth of hazel (Corylus avellana L.) Scientific reports. 2017;7:15598. doi: 10.1038/s41598-017-15703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salehi M, Moieni A, Safaie N, Farhadi S. Elicitors derived from endophytic fungi Chaetomium globosum and Paraconiothyrium brasiliense enhance paclitaxel production in Corylus avellana cell suspension culture. Plant Cell, Tissue and Organ Culture (PCTOC) 2019;136:161–171. doi: 10.1007/s11240-018-1503-9. [DOI] [Google Scholar]
- 8.Espinosa-Leal CA, Puente-Garza CA, García-Lara S. In vitro plant tissue culture: means for production of biological active compounds. Planta. 2018;248:1–18. doi: 10.1007/s00425-018-2910-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bestoso F, et al. In vitro cell cultures obtained from different explants of Corylus avellana produce Taxol and taxanes. BMC biotechnology. 2006;6:45. doi: 10.1186/1472-6750-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salehi M, Moieni A, Safaie N, Farhadi S. New synergistic co-culture of Corylus avellana cells and Epicoccum nigrum for paclitaxel production. Journal of industrial microbiology & biotechnology. 2019;46:613–623. doi: 10.1007/s10295-019-02148-8. [DOI] [PubMed] [Google Scholar]
- 11.Ottaggio L, et al. Taxanes from shells and leaves of Corylus avellana. Journal of natural products. 2007;71:58–60. doi: 10.1021/np0704046. [DOI] [PubMed] [Google Scholar]
- 12.Salehi M, Moieni A, Safaie N. Elicitors derived from hazel (Corylus avellana L.) cell suspension culture enhance growth and paclitaxel production of Epicoccum nigrum. Scientific reports. 2018;8:12053. doi: 10.1038/s41598-018-29762-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman A. Bioprospecting for taxol in angiosperm plant extracts-using high performance liquid chromatography thermospray mass spectroscopy to detect the anticancer agent and its related metabolites in Filbert trees. Spectroscopy. 1998;13:22–32. [Google Scholar]
- 14.Service RF. Hazel trees offer new source of cancer drug. Science (New York, NY) 2000;288:27. doi: 10.1126/science.288.5463.27a. [DOI] [PubMed] [Google Scholar]
- 15.Rahpeyma SA, Moieni A, Jalali Javaran M. Paclitaxel production is enhanced in suspension cultured hazel (Corylus avellana L.) cells by using a combination of sugar, precursor, and elicitor. Engineering in Life Sciences. 2015;15:234–242. doi: 10.1002/elsc.201400115. [DOI] [Google Scholar]
- 16.Miele M, Mumot AM, Zappa A, Romano P, Ottaggio L. Hazel and other sources of paclitaxel and related compounds. Phytochemistry reviews. 2012;11:211–225. doi: 10.1007/s11101-012-9234-8. [DOI] [Google Scholar]
- 17.Gallego A, et al. Assessing factors that affect the growth of Corylus avellana cell suspension cultures: a statistical approach. In Vitro Cellular & Developmental Biology-Plant. 2015;51:530–538. doi: 10.1007/s11627-015-9693-x. [DOI] [Google Scholar]
- 18.Gallego A, et al. Taxol from Corylus avellana: paving the way for a new source of this anti-cancer drug. Plant Cell, Tissue and Organ Culture (PCTOC) 2017;129:1–16. doi: 10.1007/s11240-016-1164-5. [DOI] [Google Scholar]
- 19.Smetanska, I. Production of secondary metabolites using plant cell cultures. In: Food biotechnology (eds. Stahl, U., Donalies, U. E. & Nevoigt, E.) 187–228, 10.1007/10_2008_103 (Springer, 2008). [DOI] [PubMed]
- 20.Salehi M, Karimzadeh G, Naghavi MR. Synergistic effect of coronatine and sorbitol on artemisinin production in cell suspension culture of Artemisia annua L. cv. Anamed. Plant Cell, Tissue and Organ Culture (PCTOC) 2019;137:587–597. doi: 10.1007/s11240-019-01593-8. [DOI] [Google Scholar]
- 21.Salehi M, Karimzadeh G, Naghavi MR, Badi HN, Monfared SR. Expression of artemisinin biosynthesis and trichome formation genes in five Artemisia species. Industrial crops and products. 2018;112:130–140. doi: 10.1016/j.indcrop.2017.11.002. [DOI] [Google Scholar]
- 22.Salehi M, Karimzadeh G, Naghavi MR, Badi HN, Monfared SR. Expression of key genes affecting artemisinin content in five Artemisia species. Scientific reports. 2018;8:12659. doi: 10.1038/s41598-018-31079-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salehi M, Naghavi MR, Bahmankar M. A review of Ferula species: Biochemical characteristics, pharmaceutical and industrial applications, and suggestions for biotechnologists. Industrial Crops and Products. 2019;139:111511. doi: 10.1016/j.indcrop.2019.111511. [DOI] [Google Scholar]
- 24.Naik P, Al-Khayri J. Impact of abiotic elicitors on in vitro production of plant secondary metabolites: a review. J Adv Res Biotech. 2016;1:7. doi: 10.15226/2475-4714/1/2/00102. [DOI] [Google Scholar]
- 25.Yuan Y-J, Li C, Hu Z-D, Wu J-C, Zeng A-P. Fungal elicitor-induced cell apoptosis in suspension cultures of Taxus chinensis var. mairei for taxol production. Process Biochemistry. 2002;38:193–198. doi: 10.1016/S0032-9592(02)00071-7. [DOI] [Google Scholar]
- 26.Newman M-A, Sundelin T, Nielsen JT, Erbs G. MAMP (microbe-associated molecular pattern) triggered immunity in plants. Frontiers in plant science. 2013;4:139. doi: 10.3389/fpls.2013.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geoghegan I, Steinberg G, Gurr S. The role of the fungal cell wall in the infection of plants. Trends in microbiology. 2017;25:957–967. doi: 10.1016/j.tim.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 28.Jones JD, Dangl JL. The plant immune system. nature. 2006;444:323. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu T, et al. Two LysM receptor molecules, CEBiP and OsCERK1, cooperatively regulate chitin elicitor signaling in rice. The Plant Journal. 2010;64:204–214. doi: 10.1111/j.1365-313X.2010.04324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shibuya N, Minami E. Oligosaccharide signalling for defence responses in plant. Physiological and Molecular Plant Pathology. 2001;59:223–233. doi: 10.1006/pmpp.2001.0364. [DOI] [Google Scholar]
- 31.Orlita A, et al. Application of chitin and chitosan as elicitors of coumarins and furoquinolone alkaloids in Ruta graveolens L.(common rue) Biotechnology and Applied Biochemistry. 2008;51:91–96. doi: 10.1042/BA20070200. [DOI] [PubMed] [Google Scholar]
- 32.Simic SG, et al. Polysaccharide elicitors enhance phenylpropanoid and naphtodianthrone production in cell suspension cultures of Hypericum perforatum. Plant Cell, Tissue and Organ Culture (PCTOC) 2015;122:649–663. doi: 10.1007/s11240-015-0798-z. [DOI] [Google Scholar]
- 33.Malerba M, Cerana R. Chitosan effects on plant systems. International Journal of Molecular Sciences. 2016;17:996. doi: 10.3390/ijms17070996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fesel PH, Zuccaroauthor A. β-glucan: Crucial component of the fungal cell wall and elusive MAMP in plants. Fungal Genetics and Biology. 2015;90:53–60. doi: 10.1016/j.fgb.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Tashackori H, Sharifi M, Chashmi NA, Behmanesh M, Safaie N. Piriformospora indica cell wall modulates gene expression and metabolite profile in Linum album hairy roots. Planta. 2018;248:1289–1306. doi: 10.1007/s00425-018-2973-z. [DOI] [PubMed] [Google Scholar]
- 36.Ryan CA, Bishop PD, Graham JS, Broadway RM, Duffey SS. Plant and fungal cell wall fragments activate expression of proteinase inhibitor genes for plant defense. Journal of chemical ecology. 1986;12:1025–1036. doi: 10.1007/BF01638994. [DOI] [PubMed] [Google Scholar]
- 37.Zhang CH, Mei XG, Liu L, Yu LJ. Enhanced paclitaxel production induced by the combination of elicitors in cell suspension cultures of Taxus chinensis. Biotechnology Letters. 2000;22:1561–1564. doi: 10.1023/A:1005684901329. [DOI] [Google Scholar]
- 38.Rezaei A, Ghanati F, Behmanesh M, Mokhtari-Dizaji M. Ultrasound-potentiated salicylic acid–induced physiological effects and production of taxol in hazelnut (Corylus avellana L.) cell culture. Ultrasound in medicine &. biology. 2011;37(11):1938–1947. doi: 10.1016/j.ultrasmedbio.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 39.Bru R, Sellés S, Casado-Vela J, Belchí-Navarro S, Pedreño MA. Modified cyclodextrins are chemically defined glucan inducers of defense responses in grapevine cell cultures. Journal of Agricultural and Food Chemistry. 2006;54:65–71. doi: 10.1021/jf051485j. [DOI] [PubMed] [Google Scholar]
- 40.Ramirez-Estrada K, et al. Changes in gene transcription and taxane production in elicited cell cultures of Taxus×media and Taxus globosa. Phytochemistry. 2015;117:174–184. doi: 10.1016/j.phytochem.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Ramirez-Estrada K, et al. Elicitation, an effective strategy for the biotechnological production of bioactive high-added value compounds in plant cell factories. Molecules. 2016;21:182. doi: 10.3390/molecules21020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sabater-Jara AB, et al. Synergistic effect of cyclodextrins and methyl jasmonate on taxane production in Taxus×media cell cultures. Plant biotechnology journal. 2014;12:1075–1084. doi: 10.1111/pbi.12214. [DOI] [PubMed] [Google Scholar]
- 43.Kashani K, Javaran MJ, Sabet MS, Moieni A. Identification of rate-limiting enzymes involved in paclitaxel biosynthesis pathway affected by coronatine and methyl-β-cyclodextrin in Taxus baccata L. cell suspension cultures. DARU. Journal of Pharmaceutical Sciences. 2018;26:129–142. doi: 10.1007/s40199-018-0217-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cortinas M-N, et al. First record of Colletogloeopsis zuluense comb. nov., causing a stem canker of Eucalyptus in China. Mycological research. 2006;110:229–236. doi: 10.1016/j.mycres.2005.08.012. [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Wu J, Mei X. Enhancement of taxol production and excretion in Taxus chinensis cell culture by fungal elicitation and medium renewal. Applied microbiology and biotechnology. 2001;55:404–410. doi: 10.1007/s002530000567. [DOI] [PubMed] [Google Scholar]
- 46.Expósito O, et al. Effect of taxol feeding on taxol and related taxane production in Taxus baccata suspension cultures. New biotechnology. 2009;25:252–259. doi: 10.1016/j.nbt.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 47.Wilson SA, Roberts SC. Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant biotechnology journal. 2012;10:249–268. doi: 10.1111/j.1467-7652.2011.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Belchí-Navarro S, Almagro L, Lijavetzky D, Bru R, Pedreño MA. Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and methyljasmonate. Plant cell reports. 2012;31:81–89. doi: 10.1007/s00299-011-1141-8. [DOI] [PubMed] [Google Scholar]
- 49.Somssich IE, Hahlbrock K. Pathogen defence in plants—a paradigm of biological complexity. Trends in plant Science. 1998;3:86–90. doi: 10.1016/S1360-1385(98)01199-6. [DOI] [Google Scholar]
- 50.Zhao J, Davis LC, Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnology advances. 2005;23:283–333. doi: 10.1016/j.biotechadv.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 51.Barrett LG, Heil M. Unifying concepts and mechanisms in the specificity of plant–enemy interactions. Trends in plant science. 2012;17:282–292. doi: 10.1016/j.tplants.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 52.Ghanati F, Safari M, Hajnorouzi A. Partial clarifcation of signaling pathway of taxanes increase biosynthesis by low intensity ultrasound treatment in hazel (Corylus avellana) cells. South African Journal of Botany. 2015;96:65–70. doi: 10.1016/j.sajb.2014.10.012. [DOI] [Google Scholar]
- 53.Jamshidi M, Ghanati F, Rezaei A, Bemani E. Change of antioxidant enzymes activity of hazel (Corylus avellana L.) cells by AgNPs. Cytotechnology. 2014;68(3):525–530. doi: 10.1007/s10616-014-9808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gallego A, et al. Development of a hazel cell culture-based paclitaxel and baccatin III production process on a benchtop scale. Journal of Biotechnology. 2015;195:93–102. doi: 10.1016/j.jbiotec.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 55.Rezaei, A., Ghanati, F., Behmanesh, M., Safari, M. & Sharafi, Y. Synergistic accumulative effect of salicylic acid and dibutyl phthalate on paclitaxel production in Corylus avellana cell culture. Journal of Stress Physiology & Biochemistry9(1), 157–168, http://research.shahed.ac.ir/WSR/WebPages/Report/PaperView.aspx?PaperID=6983 (2013).
- 56.Bemani E, Ghanati F, Yousefzadeh, Boroujeni L, Khatami F. Antioxidant activity, total phenolics and taxol contents response of hazel (Corylus avellana L.) cells to benzoic acid and cinnamic Acid. Notulae Botanicae Horti Agrobotanici ClujNapoca. 2012;40(1):69–73. doi: 10.15835/nbha4017404. [DOI] [Google Scholar]
- 57.Strobel G, et al. Taxol from Pestalotiopsis microspora, an endophytic fungus of Taxus wallachiana. Microbiology. 1996;142:435–440. doi: 10.1099/13500872-142-2-435. [DOI] [PubMed] [Google Scholar]
- 58.White T.J., Bruns T., Lee S., Taylor J. PCR Protocols. 1990. AMPLIFICATION AND DIRECT SEQUENCING OF FUNGAL RIBOSOMAL RNA GENES FOR PHYLOGENETICS; pp. 315–322. [Google Scholar]
- 59.Liu YJ, Whelen S, Hall BD. Phylogenetic relationships among ascomycetes: evidence from an RNA Polymerase II subunit. Molecular Biology and Evolution. 1999;16:1799–1808. doi: 10.1093/oxfordjournals.molbev.a026092. [DOI] [PubMed] [Google Scholar]
- 60.Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia plantarum. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 61.Prados-Rosales R, Luque‐Garcia JL, Martínez-López R, Gil C, Di Pietro A. The Fusarium oxysporum cell wall proteome under adhesion-inducing conditions. Proteomics. 2009;9:4755–4769. doi: 10.1002/pmic.200800950. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting the conclusions of this article is included in the article.