Abstract
HIF-1α, an essential transcription factor under hypoxic condition, is indispensable for chondrocytes during skeletal development but its expression and roles in articular chondrocytes are yet to be revealed. We examined HIF-1α protein expression and the hypoxic condition during mouse osteoarthritis (OA) development using state of the art hypoxic probes and found that its expression decreased as OA progressed, coinciding with the change in hypoxic conditions in articular cartilage. Gain- and loss-of-function of HIF-1α in cell culture experiments showed that HIF-1α suppressed catabolic genes such as Mmp13 and Hif2a. We confirmed these anticatabolic effects by measuring glycosaminoglycan release from wild type and conditional knock-out mice femoral heads cultured ex vivo. We went on to surgically induce OA in mice with chondrocyte-specific deletion of Hif1a and found that the development of OA was exacerbated. Increased expression of catabolic factors and activation of NF-κB signalling was clearly evident in the knock-out mice. By microarray analysis, C1qtnf3 was identified as a downstream molecule of HIF-1α, and experiments showed it exerted anti-catabolic effects through suppression of NF-κB. We conclude that HIF-1α has an anti-catabolic function in the maintenance of articular cartilage through suppression of NF-κB signalling.
Subject terms: Mechanisms of disease, Osteoarthritis
Introduction
Advancement in the field of musculoskeletal research has resulted in unveiling molecular mechanisms of diseases such as osteoporosis, osteoarthritis (OA) and rheumatoid arthritis (RA). These have led to inventions of evolutionary treatment and the effects have significantly decreased the number of patients requiring surgical treatment in RA. OA is a multifactorial entity caused by mechanical stress and inflammation, so to reproduce the mechanism experimentally was challenging. However, since the introduction of murine surgical knee OA models, understanding of the molecular mechanisms and signalling pathways of the disease has accelerated. Matrix metalloproteinase-13 (Mmp3), nuclear factor kappa B (NF-κB), a disintegrin-like and metallopeptidase with thrombospondin type 1 motif 5 (Adamts5) and hypoxia-inducible factor 2-alpha (HIF-2α) are the representative catabolic factors revealed to date1–10. Among them, HIF-2α is an essential transcription factor in the pathophysiology of OA. HIF-2α is a member of HIF regulatory α-subunit proteins11 and is actually abundantly expressed in intermediate and deep layers of osteoarthritic cartilage8. HIF-2α is a direct transcriptional target of NF-κB, and its expression is dependent on the activation of NF-κB signalling by various stimulations8,10. Increased HIF-2α protein further induces various catabolic factors including Mmp13, which subsequently accelerate cartilage degeneration8.
Besides HIF-2α, HIF-1α is a representative member of the HIF family. HIF-1α proteins only exist under hypoxic conditions, and exert cyto-protective effects. Both HIF proteins share approximately 50% amino acid homology12; however, in contrast to HIF-2α, HIF-1α plays essential roles in development of cartilage, which is avascular and exists under hypoxic condition13. During limb formation, HIF-1α is dispensable for condensation of limb bud mesenchyme, but required for early chondrocyte differentiation14. HIF-1α is also indispensable for normal joint development, and chondrocyte survival in the growth plate13,14. In spite of these previous findings of HIF-1α in differentiating chondrocytes, the roles of HIF-1α and the association of HIF-1α and HIF-2α in articular cartilage have not been fully understood.
Considering the anabolic roles of HIF-1α in epiphyseal chondrocytes during skeletal development, we hypothesized that HIF-1α may exert some effects against HIF-2α and be involved in homeostasis of articular cartilage or OA development. The present study aimed to reveal roles of HIF-1α in cartilage degeneration, particularly in association with the catabolic effects of the NF-κB - HIF-2α pathways. Here, we examined expression of HIF-1α protein during articular cartilage degeneration, and its roles by mouse primary chondrocytes, femoral head culture, and surgical OA model using HIF-1α conditional knockout mice. We further analysed molecular mechanisms underlying the effects of HIF-1α by a microarray analysis and in vitro experiments.
Results
Expression of HIF-1α protein during OA development
We initially examined expression of HIF-1α protein in mouse surgically-induced OA models. In 8-week-old wild-type (WT) mouse knee joint, abundant expression of HIF-1α protein was detected in the articular cartilage (Fig. 1A). The rate of HIF-1α-positive cells decreased from 4 to 8 weeks after OA induction (Fig. 1A,B). Expression of HIF-1α protein in 18-month-old cartilage was similar to that of 4 weeks after OA induction (Fig. 1A,B). To examine alteration of oxygen concentration in articular cartilage with OA development, we used an Azo-based fluorescent hypoxic probe MAR15. We first tested the MAR using mouse primary chondrocytes. The cells on a culture plate were treated with the MAR, and sealed with a round cover glass (Supplementary Fig. S1A). After one hour culture, the signal intensity was detected around the centre of the cover glass (Supplementary Fig. S1B), indicating that the MAR functioned properly in chondrocytes. We then injected the MAR into WT mice knee joints before the OA surgery, 4 and 8 weeks after the surgery, and sacrificed them after five hours. The fluorescent signal was clearly detected in the surface of articular cartilage and menisci of the normal knee joints (Fig. 1C). However, the signal was decreased at 4 weeks, and hardly detected at 8 weeks after the surgery (Fig. 1C). These data indicate that loss of hypoxic condition and decreased expression of HIF-1α protein may be associated with OA development.
Figure 1.
Expression of HIF-1α protein during OA development. (A) Safranin O staining and immunofluorescence of HIF-1α in mouse knee cartilage. From top to bottom, 8 weeks old (8 w.o.: before OA surgery), 2, 4, 6, and 8 weeks after surgery, and 18 months old (18 m.o.) without surgical induction. Inset boxes in middle immunofluorescence images indicate the regions of high magnification. Scale bars, 100 µm and 50 µm for low and high magnification images, respectively. (B) The percentage of HIF-1α positive cells in the immunofluorescence. *P < 0.05 vs 0 w. (C) Fluorescent images of the hypoxic probe in mouse knee cartilage before surgery (0 w), 4 and 8 weeks after surgery. The mice were sacrificed five hours after intraarticular injection of the hypoxic probe, and analysed by frozen section technique. Inset boxes in Safranin O staining indicate the regions of fluorescent images of the hypoxic probe. Scale bars, 200 µm.
Regulation of catabolic genes by HIF-1α
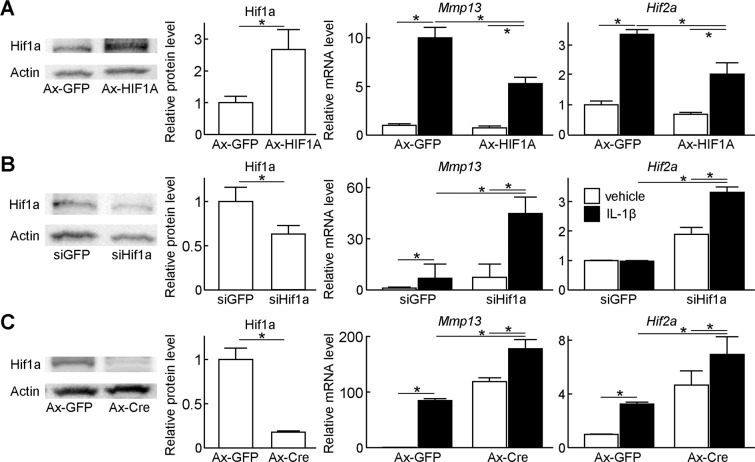
We next investigated association of chondrocyte catabolism and HIF-1α by cell culture experiments. For a gain-of-function study, we transduced adenoviral vectors of HIF1A or GFP into WT mouse primary chondrocytes (Fig. 2A). In GFP control cells, IL-1β treatment markedly induced Mmp13, a representative catabolic enzyme for cartilage matrix; however, in HIF-1α overexpressing cells, the induction of Mmp13 was significantly suppressed (Fig. 2A). Hif2a, a potent upstream factor of Mmp13, was also increased by IL-1β, but suppressed by HIF-1α overexpression (Fig. 2A). For a loss-of-function study, we transfected siRNA against Hif1a or GFP into WT mouse primary chondrocytes. Although the knockdown efficiency of Hif1a protein was approximately 50%, induction of Mmp13 and Hif2a by IL-1β treatment was significantly enhanced in Hif1a-suppressed cells compared with those in control cells (Fig. 2B). We further transduced adenoviral vectors of Cre recombinase or GFP into primary chondrocytes derived from Hif1afl/fl mice. Hif1a protein level in Cre-transduced cells was decreased to 17.7% of that in GFP-transduced control cells (Fig. 2C). Subsequently, induction of Mmp13 and Hif2a by IL-1β treatment was significantly enhanced in Hif1a-suppressed cells as well (Fig. 2C).
Figure 2.
Regulation of catabolic genes by HIF-1α. (A) Protein levels of Hif1a, and mRNA levels of Mmp13, and Hif2a in WT mouse primary chondrocytes transduced with GFP or HIF1A adenoviral vectors under the hypoxic condition (3% O2). GFP or HIF1A was transduced at a multiplicity of infection (MOI) of 100. The cells were treated with or without 10 ng/mL IL-1β for 2 days. Bars show the mean ± SD of three samples per group. *P < 0.05. (B) Protein levels of Hif1a, and mRNA levels of Mmp13, and Hif2a in WT mouse primary chondrocytes transfected with siRNA against GFP or Hif1a under the hypoxic condition. The cells were treated with or without 10 ng/mL IL-1β for 2 days. Bars show the mean ± SD of three samples per group. *P < 0.05. (C) Protein levels of Hif1a, and mRNA levels of Mmp13, and Hif2a in Hif1afl/fl primary chondrocytes transduced with GFP or Cre adenoviral vectors under the hypoxic condition. GFP or Cre was transduced at a MOI of 100. The cells were treated with or without 10 ng/mL IL-1β for 2 days. Bars show the mean ± SD of three samples per group. *P < 0.05.
Regulation of cartilage degradation by HIF-1α
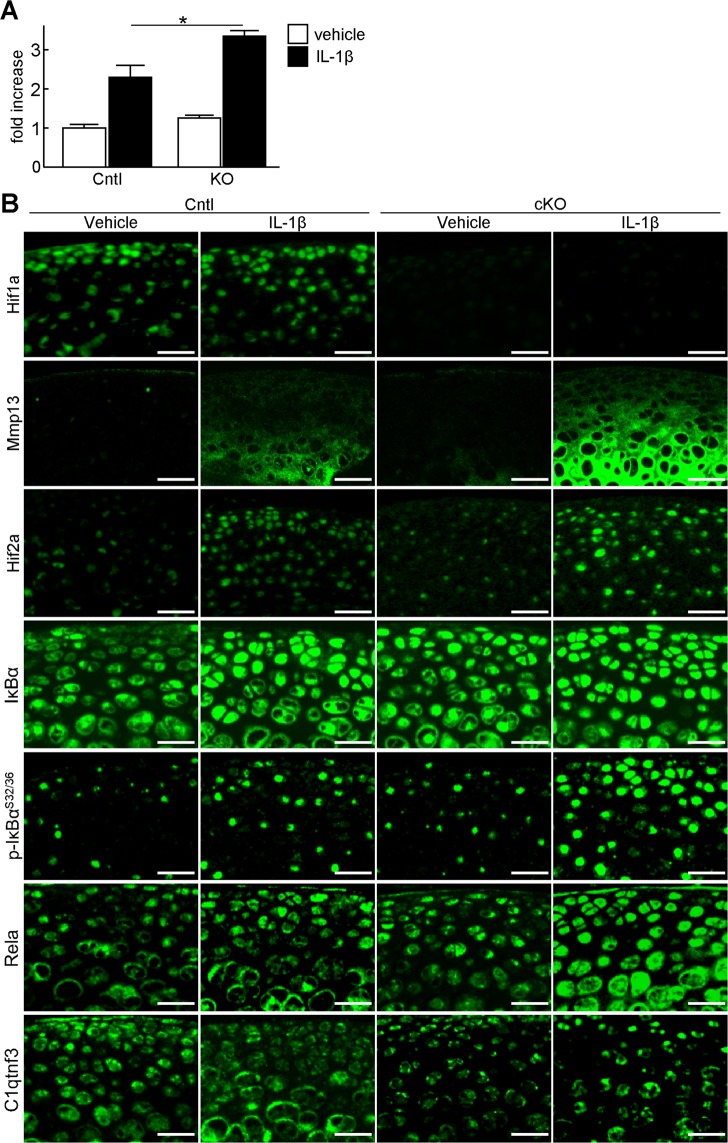
To examine whether HIF-1α is involved in cartilage degradation through regulation of HIF-2α and Mmp13, we performed organ culture of mouse femoral heads. We obtained femoral heads from Col2a1-CreERT2; Hif1afl/fl (KO) and Hif1afl/fl (Cntl) mice after tamoxifen treatment, and cultured them with or without IL-1β under hypoxia. The increased amount of glycosaminoglycans released into the medium by IL-1β treatment was further enhanced in the KO femoral head (Fig. 3A). Immunofluorescence using cartilage tissues of these femoral heads showed Mmp13 expression to be markedly enhanced in the KO femoral head treated with IL-1β (Fig. 3B). Since the NF-kB-HIF-2α pathway is a potent regulator of Mmp13 in OA development8,10, we analysed expression of the NF-kB-related proteins. In the KO femoral head treated with IL-1β, increase of Rela and phosphorylated IκBα proteins, and nuclear translocation of Rela were more prominent, indicating the enhanced activity of NF-κB signalling pathway (Fig. 3B). The subsequent increase of HIF-2α was also observed (Fig. 3B).
Figure 3.
Regulation of cartilage degradation by HIF-1α. (A) Amount of glycosaminoglycans released into the medium determined by the dimethylmethylene blue assay during 3 days culture of Hif1afl/fl (Cntl) and Col2a1-CreERT2;Hif1afl/fl (KO) femoral heads treated with or without 10 ng/mL IL-1β under the hypoxic condition (3% O2). Tamoxifen induction was performed three days before sacrifice. Bars show the mean ± SD of three samples per group. *P < 0.05. (B) Immunofluorescence of Mmp13, Hif2a, and Hif1a in the Cntl and KO femoral head cartilage after 3-day culture with or without 10 ng/mL IL-1β. Scale bars, 50 µm.
Regulation of OA development by HIF-1α
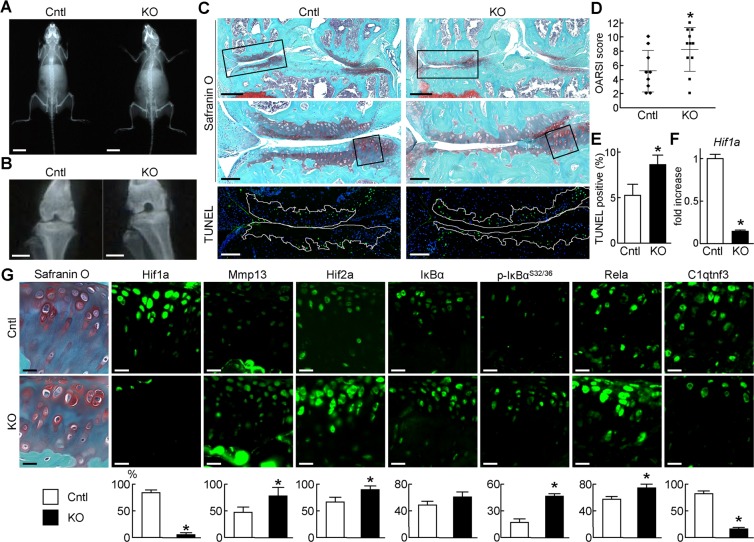
To further elucidate roles of HIF-1α in mouse articular cartilage, we examined OA development of KO and Cntl mice. Tamoxifen induction was performed at 7 weeks. Since the skeletal development was not affected at 8 weeks (Fig. 4A), we created the surgical OA model using KO and Cntl mice. Cartilage degeneration was accelerated in KO knee joints 8 weeks after the surgery, and quantification by the OARSI scoring system confirmed the difference to be statistically significant (Fig. 4B–D). Meanwhile, osteoarthritic changes were not observed in 16-week-old KO and Cntl knee joints without OA surgery (Supplementary Fig. S2). TUNEL staining showed enhanced apoptosis of chondrocyte in KO cartilage (Fig. 4C,E). Efficient knockdown of Hif1a in cartilage was confirmed by qPCR and immunofluorescence (Fig. 4F,G). Similar to the results of organ culture, Mmp13 and Hif2a were markedly enhanced by Hif1a deletion (Fig. 4G). Immunofluorescence of phosphorylated IκBα and Rela indicated the activation of NF-κB signalling as well (Fig. 4G). Similar expression patterns of marker proteins were observed in 16-week-old mice knee joints without OA surgery (Supplementary Fig. S3). All these data indicate that HIF-1α suppresses the activation of the NF-κB - Hif2a pathway.
Figure 4.
Regulation of OA development by HIF-1α. Plain radiographs of the entire bodies at 8 weeks of age (A), and knee joints 8 weeks after OA surgery (B) of Cntl and cKO littermates. Scale bars, 10 mm and 1 mm, respectively. (C) Safranin O and TUNEL staining’s of Cntl and KO knee joints at 8 weeks after OA surgery (n = 9 and 11, respectively). Tamoxifen induction was performed at 7 weeks. Inset boxes in top images indicate the regions of high magnification Safranin O and TUNEL images. Inset boxes in middle images indicate the regions of immunofluorescence in (G). White dotted lines in TUNEL images indicate the cartilage area. Scale bars, 400 µm and 100 µm, respectively. (D) OARSI (Osteoarthritis Research Society International) score of OA development. Lines show the mean ± SD. *P < 0.05. (E) Rate of TUNEL-positive cells in the cartilage area. Bars show the mean ± SD. *P < 0.05. (F) mRNA levels of Hif1a in articular cartilage tissues of Cntl and KO. Bars show the mean ± SD. *P < 0.05. (G) Immunofluorescence of Hif1a, Mmp13, Hif2a, IκBα, Ser 32 and 36 dual phosphorylated IκBα, Rela, and C1qtnf3. Scale bars, 20 µm. The percentage of positive cells in the immunofluorescence is shown below. *P < 0.05.
C1qtnf3 mediates downregulation of NF-κB signalling by HIF-1α
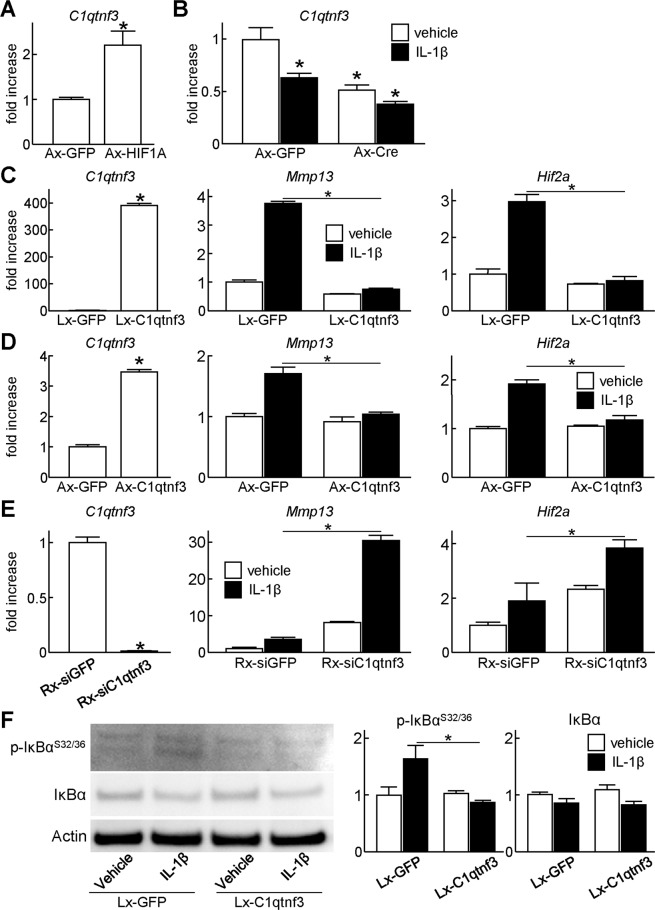
To investigate how HIF-1α suppresses NF-κB signalling, we performed a microarray analysis using primary Hif1afl/fl chondrocytes transduced with adenovirus of GFP or Cre. Lists of upregulated and downregulated genes by Hif1a suppression were shown in Supplementary Tables S1 and S2. We further narrowed down NF-κB-related genes by Gene Ontology analysis (Supplementary Tables S3 and S4), and focused on C1q and tumour necrosis factor related protein 3 (C1qtnf3, also known as CTRP3, or CORS-26) among them. C1qtnf3 is a secreted protein structurally closely related to adiponectin, containing an N-terminal collagenous region and a carboxyterminal complement factor C1q globular domain16. C1qtnf3 has been known as an anti-inflammatory secreted protein17, and a recent study showed that collagen-induced arthritis is exacerbated in C1qtnf3 null mice18. Considering these previous findings, we hypothesized that HIF-1α suppresses NF-κB signalling through C1qtnf3 induction. We first analysed how C1qtnf3 expression was altered by HIF-1α. We found that mRNA level of C1qtnf3 was significantly increased in the HIF-1α overexpressing cells (Fig. 5A). In contrast, C1qtnf3 was decreased by Hif1a knockdown in primary Hif1afl/fl chondrocytes, femoral head explants, and articular cartilage of knee joints (Figs. 3B, 4G, 5B, Supplementary Fig. S3). Intriguingly, C1qtnf3 expression was also decreased by the IL-1β treatment (Figs. 3B, 5B).
Figure 5.
Suppression of catabolic gene expression by C1qtnf3. (A) mRNA levels of C1qtnf3 in WT mouse primary chondrocytes transduced with GFP or HIF1A adenoviral vectors under the hypoxic condition (3% O2). GFP or HIF1A was transduced at a multiplicity of infection (MOI) of 100. (B) mRNA levels of C1qtnf3 in Hif1afl/fl primary chondrocytes transduced with GFP or Cre adenoviral vectors under the hypoxic condition. GFP or Cre was transduced at a MOI of 100. The cells were treated with or without 10 ng/mL IL-1β for 2 days. (C) mRNA levels of C1qtnf3, Mmp13, and Hif2a in ATDC5 cells transfected via lentivirus with GFP or C1qtnf3. The cells were treated with or without 10 ng/mL IL-1β for 2 days. (D) mRNA levels of C1qtnf3, Mmp13, and Hif2a in WT mouse primary chondrocytes transduced with GFP or C1qtnf3 adenoviral vectors at a MOI of 10. The cells were treated with or without 10 ng/mL IL-1β for 2 days. (E) mRNA levels of C1qtnf3, Mmp13, and Hif2a in ATDC5 cells transfected via retrovirus with siRNA against GFP or C1qtnf3. The cells were treated with or without 10 ng/mL IL-1β for 2 days. (F) Protein levels of IκBα, and Ser 32 and 36 dual phosphorylated IκBα in ATDC5 cells transfected via lentivirus with GFP or C1qtnf3. The cells were treated with or without 10 ng/mL IL-1β for 24 hours. In all panels, bars show the mean ± SD of three samples per group. *P < 0.05.
We then analysed involvement of C1qtnf3 in downregulation of NF-κB signalling and Mmp13 by HIF-1α. We lentivirally generated stable lines of ATDC5 which overexpress GFP or C1qtnf3 dependent on doxycycline induction (Fig. 5C). Expression of Mmp13 and Hif2a were increased by IL-1β treatment in GFP-overexpressing cells, while they were suppressed in C1qtnf3-overexpressing cells (Fig. 5C). Similar results were observed in primary chondrocytes in which C1qtnf3 was overexpressed by adenoviral infection (Fig. 5D). We further generated stable lines of ATDC5 which overexpress siRNA against GFP or C1qtnf3 by retroviral vectors (Fig. 5E). Endogenous C1qtnf3 expression was efficiently suppressed by stable expression of siRNA (Fig. 5E). Notably, increase of Mmp13 and Hif2a by IL-1β treatment was enhanced by C1qtnf3 suppression (Fig. 5E). We finally examined phosphorylation of IκBα by western blotting (Fig. 5F). Phosphorylated IκBα was upregulated in GFP-overexpressing ATDC5 cells treated with IL-1β; however, it was not increased in C1qtnf3-overexpressing ATDC5 cells by IL-1β treatment (Fig. 5F).
Discussion
The present study demonstrated that HIF-1α, highly expressed in normal articular cartilage, exerts protective effects against cartilage degeneration and osteoarthritis development. Expression of HIF-2α and activation of NF-κB signalling are negatively regulated by HIF-1α. We further identified C1qtnf3 as a mediator of these anti-catabolic roles of HIF-1α by the microarray analysis. The present data indicate the essential roles of HIF-1α in articular cartilage, which are consistent with a recent study by Bouaziz et al.19. They have reported that HIF-1α inhibits OA development through suppression of Wnt canonical pathway19. HIF-1α knockout leads to increase of Mmp13 and activation of Wnt canonical signalling19. They further displayed the direct interaction of HIF-1α and β-catenin, which attenuated transcription factor complex containing β-catenin19. Since both up- and down-regulation of Wnt canonical pathway result in acceleration of OA development, its activity should be tightly regulated in articular chondrocytes3,7. Considering that HIF-2α enhances the activity of Wnt canonical pathway by interacting β-catenin20, balance of HIF-1α and HIF-2α is likely to be the key regulator of Wnt canonical pathway in chondrocytes. Additionally, the present data have demonstrated that HIF-1α suppresses NF-κB signalling, a potent upstream of HIF-2α. Taken together, the anabolic factor HIF-1α and the catabolic factor HIF-2α are complicatedly associated with Wnt and NF-κB signalling pathways.
Under conditions of normoxia, HIF-1α is virtually undetectable due to its rapid degradation through the ubiquitin-proteasome pathway, and has a short half-life (~five minutes)21,22. However, when cells are exposed to hypoxia (i.e., 1% O2), its half-life is increased to approximately thirty minutes, and exerts cyto-protective effects21,22. HIF-1α lability is mediated by the oxygen-dependent degradation domain (ODD)21. Although a similar ODD exists in HIF-2α21, HIF-2α protein is highly expressed in vascularized tissues11,23. The Azo-based fluorescent hypoxic probe indicates that normal articular cartilage is hypoxic, but the oxygen concentration increases along with the OA development (Fig. 1C). The present data is compatible with the previous findings using pimonidazole hydrochloride19. Articular cartilage is a large avascular tissue, and is regarded to be nourished from synovial fluid produced by synovial membranes, and partially from subchondral bone by diffusion. In OA joints, synovial membranes usually become hyperplasia, and subchondral bone displays sclerosis. Hypervascularity of synovial membranes may increase the oxygen concentration of synovial fluid, and lead to HIF-1α degradation, which subsequently results in cartilage degeneration through enhanced chondrocyte apoptosis and activation of NF-κB and Wnt canonical signalling pathways.
The roles of hypoxia and HIF proteins in OA have been widely studied using mice models and human surgical samples. A series of previous studies show that HIF-1α induces anabolic genes such as COL2A1 and aggrecan, and plays essential roles in chondrocyte survival24. Additionally, Thoms et al. reported that hypoxic conditions suppress degradation of human articular cartilage in explant culture25. They showed that HIF-1α mediates suppression of the cartilage catabolism under hypoxic conditions, while HIF-2α induces sex determining region Y-box 9 (SOX9), a master transcription factor for chondrogenesis25. Although the roles of HIF-2α in human cartilage are still controversial, the hypoxic conditions and HIF-1α protein certainly contribute to cartilage homeostasis.
The previous studies showed severe impairment of joint formation and skeletal growth by HIF-1α deletion in limb bud mesenchyme, and enhanced apoptosis of growth plate chondrocytes in chondrocyte-specific HIF-1α deletion13,14. Interestingly, spontaneous OA changes were not obvious in 16-week-old chondrocyte-specific HIF-1α knockout mice which had received tamoxifen injection at 7 weeks (Supplementary Fig. S2). Zhang et al. recently showed that when chondrocyte death was induced prior to surgical OA mice models, articular cartilage damage decreased compared to those with intact chondrocytes26. They suggested that chondrocyte catabolism, not cell death, contributed to OA development by surgical induction26. Considering this theory, HIF-1α deletion in adult articular chondrocytes may not immediately lead to cartilage damage through enhanced apoptosis in the natural course, but may accelerate OA by surgical induction through suppression of anti-catabolism. Meanwhile, the decrease of HIF-1α protein level with aging was rather moderate, compared with that in OA progression by surgical induction (Fig. 1A,B). Further studies are necessary to determine roles of HIF-1α in OA development with aging.
The present study revealed that C1qtnf3, known as anti-inflammatory secreted protein, is induced by HIF-1α, and suppresses NF-κB signalling. C1qtnf3 inhibits LPS-induced inflammatory cytokine production from human adipocytes, monocytes, and fibroblasts17,27,28. C1qtnf3 null mice are fertile, and were born in the expected Mendelian ratios, and grow up normally18. In spite of no abnormalities in healthy conditions, arthritis incidence and severity were higher in the C1qtnf3 null mice when they were treated with type II collagen and complete Freund’s adjuvant18. Considering that NF-κB signalling and HIF-2α are deeply involved in rheumatoid arthritis as well as OA29–32, C1qtnf3 may regulate the inflammatory arthritis through suppressing the NF-κB - HIF-2α axis, similar to the present findings. On the other hand, molecular mechanisms underlying suppression of NF-κB by C1qtnf3 is still unrevealed. A recent report shows that C1qtnf3 regulates expression of fibroblast growth factor receptor-1 (FGFR1) and FGFR3, and further alters phosphorylation of phosphatidylinositol 3-kinase (PI3K) and Akt33. C1qtnf3 may contribute to articular cartilage homeostasis through various kinds of pathways, other than NF-κB. Recently, Fernández-Torres et al. reported single nucleotide polymorphisms (SNPs) which are involved both in knee OA development and the HIF-1α signaling pathway34. They displayed SNPs of AKT2, IGF1, COL2A1, and GSK3B34, indicating that HIF-1α may affect OA pathogenesis through various signalling pathways such as insulin growth factor 1 (IGF1) – insulin receptor substrate (IRS) pathway, phosphoinositide 3-kinase (PI3K)–AKT pathway, mammallian target of rapamycin (mTOR), and canonical Wnt pathway, as well as NF-κB.
In conclusion, we demonstrated that HIF-1α is involved in chondrocyte survival in articular cartilage and maintains articular cartilage homeostasis through suppression of the NF-κB - HIF-2α axis. We further identified that C1qtnf3 mediates the cartilage-protective effects of HIF-1α as its downstream molecule. The present data may provide a clue to development of novel therapeutics against OA.
Methods
Mice
All experiments were performed according to protocols approved by the Animal Care and Use Committee of The University of Tokyo. In each experiment, we compared the genotypes of littermates maintained in a C57BL/6 J background. Col2a1-CreERT2 mice were obtained from Dr. Di Chen35. Mice carrying the loxP-flanked conditional alleles of HIF-1α in C57BL/6 background were obtained from Dr. Randall S Johnson36,37. Col2a1-CreERT2 mice were mated with Hif1afl/fl mice to generate Col2a1-CreERT2:Hif1afl/+ and these were mated to Hif1afl/fl mice to generate Col2a1-CreERT2:Hif1afl/fl mice.
Osteoarthritis experiments
Osteoarthritis was induced by resection of the medial collateral ligament and medial meniscus to Hif1afl/fl (n = 10) and Col2a1-CreERT2:Hif1afl/fl (n = 10) littermates at 8 weeks of age under general anaesthetics38. Tamoxifen (100 µg per gram of body weight) was injected intraperiosteally to the mice 1 week prior to surgery for 5 consecutive days. The progression of osteoarthritis in the mice were analysed 8 weeks after surgery.
Histological analyses
For hypoxic probe experiments, we prepared frozen sections according to Kawamoto’s film method39,40. In brief, the mice were sacrificed by cervical dislocation and fixed immediately with 4% paraformaldehyde to terminate the hypoxic reaction. The knee was taken out and placed into hexane with dry ice, and embedded in SCEM compound (Section-lab, Hiroshima, Japan). We prepared 4-μm sections using Cryofilm Type IIC (Section-lab) in the sagittal plane of the medial joint space using a Leica cryostat, and stained with Safranin-O according to the standard protocol. Images of the Safranin-O staining and hypoxic probes were captured and analysed by BZ-X710 microscope system (KEYENCE, Osaka, Japan).
To analyse the progression of osteoarthritis, we used paraffin sections. The mouse knee samples were fixed in 4% paraformaldehyde followed by decalcification in ethylenediaminetetraacetic acid at 37 °C for 5 days and embedded in paraffin. 4 µm coronal sections were stained with Safranin O and evaluated using the Osteoarthritis Research Society International (OARSI) scoring system for the medial compartment. For immunohistochemistry, we incubated the sections with antibodies to HIF1A (1:100; NB100–105, Novus Biologicals), MMP13 (1:100; MAB8887, Merck Millipore), HIF2A (1:100; sc-28706, Santa Cruz Biotechnology)), IκBα (1:100; sc-371, Santa Cruz Biotechnology), p-IκBαS32/36 (1:100; sc-101713, Santa Cruz Biotechnology), Rela (1:100; #8242, Cell Signalling Technology), C1qtnf3 (1:100; ab36870, Abcam).
For TUNEL assay, we used an in situ Cell Death Detection Kit (Roche) and the number of positive cells in the articular cartilage area were counted using analyser software of BZ-X710 microscope system (Keyence).
Hypoxic probe
Azo-based fluorescent probe, mono-azo rhodamine (MAR), was developed as previously described15. To test the sensitivity of the probe in vitro, we cultured primary articular chondrocytes under a cover glass to create an oxygen gradient i.e. oxygen concentration decreases towards the centre. The probe was tested at 1 mM, the concentration used for in vivo experiments. We used osteoarthritis model mice to evaluate the hypoxic condition in osteoarthritic cartilage. We performed surgery on 8week-old mice and compared the extent of hypoxia at 0, 4, 8 weeks after surgery by injecting 0.1 ml of 1 mM hypoxic probe 4 hours before sacrifice. To deliver the probe consistently, we incised the skin and exposed the knee before injection and sutured the skin all under general anaesthetics.
Cell cultures
Primary articular chondrocytes were isolated from 6-day-old neonatal mice as described previously41. Isolated chondrocytes were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin (PS) under the normoxic condition (20% O2, 5% CO2). For hypoxic culture experiments, the hypoxic condition (3% O2, 5% CO2) was prepared using nitrogen gas. The mouse chondrogenic cell line ATDC5 (Riken Cell Bank, Tsukuba, Japan) was cultured in DMEM/F12(1:1) with 5% FBS.
Construction of expression vectors
We amplified the coding sequences of Hif1a and C1qtnf3 from mouse complimentary DNA. These were cloned into pCMV-HA (Clontech). Adenoviral vectors for GFP, Cre, Hif1a and C1qtnf3 were created by the AdenoX Expression System (Clontech). The Tet-On 3G system was used to generate GFP, Hif1a, C1qtnf3 expressing ATDC5 cells introduced via lentiviruses. We transfected si-Hif1a (MSS205124, Invitrogen) and its negative control (#12935200, Invitrogen) using lipofectamine (Invitrogen). piGENEmU6 system (iGENE Therapeutics) was used to construct siRNA vector for the mouse Hif1a gene targeting 5′-gcatgaggacgtagaggaagt-3′ (nucleotides 732–752), and the siRNA cassette was transferred into pMx vectors for retrovirus vectors as previously described42. Each construct was verified by DNA sequencing.
RT-qPCR
Total RNA was isolated with an RNeasy Mini Kit (Qiagen). One µg of RNA was of total RNA was reverse transcribed with QuantiTect Reverse Transcription (Qiagen) to prepare single-stranded cDNA. Real time RT- PCR was performed with a Thermal Cycler Dice (Takara) using FastStart Universal SYBR Green Master Mix (Roche) according to the manufacturer’s instructions. The mRNA copy numbers for each gene was calculated using a standard curve generated by serially diluted plasmids contains PCR amplicons synthesized with ZERO Blunt II TOPO Cloning kit (Invitrogen). The copy number was normalized to rodent total RNA (Thermo Fisher Scientific) with mouse RNA polymerase II as the internal control. Primer sequences for real-time RT-PCR are shown in Supplementary Table S5.
Western blotting
Cells were lysed in M-PER (Thermo-Fisher Scientific) supplemented with COMPLETE protease inhibitor mixture (Roche). Total protein was quantified using the BCA Protein Assay Kit (Pierce). Equal amounts of protein were subjected to SDS-PAGE using 7.5% to 15% Tris-Glycine gradient gels, and blotted onto PVDF membranes (Bio-Rad Laboratories, Inc.). After blocking with 5% skimmed milk, membranes were incubated with primary antibodies against HIF-1α (1:1000, NB100–134, Novus Biologicals), IκBα (1:200; sc-371, Santa Cruz Biotechnology), p- IκBαS32/36 (1:200; sc-101713, Santa Cruz Biotechnology) or Actin (1:1000, AC-74, Sigma-Aldrich) in Can Get Signal solution (Toyobo). The membranes were then incubated with HRP-conjugated secondary antibody (Promega), and immunoreactive bands were visualized with ECL plus (GE Healthcare) according to the manufacturer’s instructions. Original images of the immunoblots were shown in Supplementary Fig. S4. For quantification of bands, densitometry analysis was performed using ImageJ software (National Institutes of Health).
Proteoglycan release assay
Proteoglycan release assay was performed as reported previously43. Tamoxifen (100 µg per gram of body weight) was injected intraperiosteally to 2-week-old Col2a1-CreERT2:Hif1afl/fl and Hif1afl/fl mice and the femoral heads were collected after 1 week. These were cultured for three days in DMEM with or without IL-1β under the hypoxic condition (3%). The amount of proteoglycan released in the medium was measured by a colorimetric assay using dimethylmethlene blue.
Microarray analysis
Total RNA was extracted from primary chondrocytes obtained from Hif1afl/fl mice transduced with adenovirus expressing GFP or Cre. Harvested RNA was processed into cDNA and hybridized against SurePrint G3 Human Gene Expression 8 × 60 K v3 microarray chips (Agilent Technologies), following the manufacturer’s instructions. The raw data were deposited in the Gene Expression Omnibus (www.ncbi.nlm.nih.gov/geo/) under accession no. GSE106965.
Statistical analyses
To assess the statistical significance of experimental data, we used a two-tailed unpaired Student’s t test in comparison between two groups, and post hoc testing using Tukey’s method in comparison between multiple groups. P values less than 0.05 were considered significant.
Supplementary information
Acknowledgements
We thank Dr. Di Chen and Dr. Randall S Johnson for generously providing Col2a1-CreERT2 and Hif1a-flox mice, respectively. We thank J. Sugita, R. Yamaguchi, K. Kaneko and H. Kawahara for technical assistance. This study was supported by JSPS KAKENHI Grant Numbers (23689065, 15H06170, 17K10996, 19H05654, 19H05565, 19K22677, 19K09568).
Author contributions
T.S. designed the research; K.O., D.M., Y.Ma., H.N., Y.Mu., F.Y., S.C., Y.T., H.K., H.S., W.P. and T.S. performed the reseach; K.O., F.Y., N.T., K.H., T.N., S.T. and T.S. analysed the data: K.O. and T.S. wrote the manuscript. All authors have read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Keita Okada and Daisuke Mori.
Supplementary information
is available for this paper at 10.1038/s41598-020-62463-4.
References
- 1.Glasson SS, et al. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 2.Stanton H, et al. ADAMTS5 is the major aggrecanase in mouse cartilage in vivo and in vitro. Nature. 2005;434:648–652. doi: 10.1038/nature03417. [DOI] [PubMed] [Google Scholar]
- 3.Zhu M, et al. Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 2008;58:2053–2064. doi: 10.1002/art.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Echtermeyer F, et al. Syndecan-4 regulates ADAMTS-5 activation and cartilage breakdown in osteoarthritis. Nat Med. 2009;15:1072–1076. doi: 10.1038/nm.1998. [DOI] [PubMed] [Google Scholar]
- 5.Lin AC, et al. Modulating hedgehog signaling can attenuate the severity of osteoarthritis. Nat Med. 2009;15:1421–1425. doi: 10.1038/nm.2055. [DOI] [PubMed] [Google Scholar]
- 6.Little CB, et al. Matrix metalloproteinase 13-deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. 2009;60:3723–3733. doi: 10.1002/art.25002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhu M, et al. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24:12–21. doi: 10.1359/jbmr.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saito T, et al. Transcriptional regulation of endochondral ossification by HIF-2alpha during skeletal growth and osteoarthritis development. Nat Med. 2010;16:678–686. doi: 10.1038/nm.2146. [DOI] [PubMed] [Google Scholar]
- 9.Yang S, et al. Hypoxia-inducible factor-2alpha is a catabolic regulator of osteoarthritic cartilage destruction. Nat Med. 2010;16:687–693. doi: 10.1038/nm.2153. [DOI] [PubMed] [Google Scholar]
- 10.Kobayashi H, et al. Biphasic regulation of chondrocytes by Rela through induction of anti-apoptotic and catabolic target genes. Nat Commun. 2016;7:13336. doi: 10.1038/ncomms13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–1991. [PubMed] [Google Scholar]
- 12.Tian H, Hammer RE, Matsumoto AM, Russell DW, McKnight SL. The hypoxia-responsive transcription factor EPAS1 is essential for catecholamine homeostasis and protection against heart failure during embryonic development. Genes Dev. 1998;12:3320–3324. doi: 10.1101/gad.12.21.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schipani E, et al. Hypoxia in cartilage: HIF-1alpha is essential for chondrocyte growth arrest and survival. Genes Dev. 2001;15:2865–2876. doi: 10.1101/gad.934301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Provot S, et al. Hif-1alpha regulates differentiation of limb bud mesenchyme and joint development. J Cell Biol. 2007;177:451–464. doi: 10.1083/jcb.200612023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Piao W, et al. Development of azo-based fluorescent probes to detect different levels of hypoxia. Angew Chem Int Ed Engl. 2013;52:13028–13032. doi: 10.1002/anie.201305784. [DOI] [PubMed] [Google Scholar]
- 16.Schaffler A, Weigert J, Neumeier M, Scholmerich J, Buechler C. Regulation and function of collagenous repeat containing sequence of 26-kDa protein gene product “cartonectin”. Obesity (Silver Spring) 2007;15:303–313. doi: 10.1038/oby.2007.566. [DOI] [PubMed] [Google Scholar]
- 17.Weigert J, et al. The adiponectin paralog CORS-26 has anti-inflammatory properties and is produced by human monocytic cells. FEBS Lett. 2005;579:5565–5570. doi: 10.1016/j.febslet.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 18.Murayama MA, et al. CTRP3 plays an important role in the development of collagen-induced arthritis in mice. Biochem Biophys Res Commun. 2014;443:42–48. doi: 10.1016/j.bbrc.2013.11.040. [DOI] [PubMed] [Google Scholar]
- 19.Bouaziz W, et al. Interaction of HIF1alpha and beta-catenin inhibits matrix metalloproteinase 13 expression and prevents cartilage damage in mice. Proc Natl Acad Sci USA. 2016;113:5453–5458. doi: 10.1073/pnas.1514854113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Choi H, Chun YS, Kim TY, Park JW. HIF-2alpha enhances beta-catenin/TCF-driven transcription by interacting with beta-catenin. Cancer Res. 2010;70:10101–10111. doi: 10.1158/0008-5472.CAN-10-0505. [DOI] [PubMed] [Google Scholar]
- 21.Huang LE, Gu J, Schau M, Bunn HF. Regulation of hypoxia-inducible factor 1alpha is mediated by an O2-dependent degradation domain via the ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 1998;95:7987–7992. doi: 10.1073/pnas.95.14.7987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fedele AO, Whitelaw ML, Peet DJ. Regulation of gene expression by the hypoxia-inducible factors. Mol Interv. 2002;2:229–243. doi: 10.1124/mi.2.4.229. [DOI] [PubMed] [Google Scholar]
- 23.Stewart AJ, Houston B, Farquharson C. Elevated expression of hypoxia inducible factor-2alpha in terminally differentiating growth plate chondrocytes. J Cell Physiol. 2006;206:435–440. doi: 10.1002/jcp.20481. [DOI] [PubMed] [Google Scholar]
- 24.Zhang FJ, Luo W, Lei GH. Role of HIF-1alpha and HIF-2alpha in osteoarthritis. Joint Bone Spine. 2015;82:144–147. doi: 10.1016/j.jbspin.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Thoms BL, Dudek KA, Lafont JE, Murphy CL. Hypoxia promotes the production and inhibits the destruction of human articular cartilage. Arthritis Rheum. 2013;65:1302–1312. doi: 10.1002/art.37867. [DOI] [PubMed] [Google Scholar]
- 26.Zhang M, et al. Induced superficial chondrocyte death reduces catabolic cartilage damage in murine posttraumatic osteoarthritis. J Clin Invest. 2016;126:2893–2902. doi: 10.1172/JCI83676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kopp A, et al. C1q/TNF-related protein-3 represents a novel and endogenous lipopolysaccharide antagonist of the adipose tissue. Endocrinology. 2010;151:5267–5278. doi: 10.1210/en.2010-0571. [DOI] [PubMed] [Google Scholar]
- 28.Hofmann C, et al. C1q/TNF-related protein-3 (CTRP-3) is secreted by visceral adipose tissue and exerts antiinflammatory and antifibrotic effects in primary human colonic fibroblasts. Inflamm Bowel Dis. 2011;17:2462–2471. doi: 10.1002/ibd.21647. [DOI] [PubMed] [Google Scholar]
- 29.Alghasham A, Rasheed Z. Therapeutic targets for rheumatoid arthritis: Progress and promises. Autoimmunity. 2014;47:77–94. doi: 10.3109/08916934.2013.873413. [DOI] [PubMed] [Google Scholar]
- 30.Ryu JH, et al. Hypoxia-inducible factor-2alpha is an essential catabolic regulator of inflammatory rheumatoid arthritis. Plos Biol. 2014;12:e1001881. doi: 10.1371/journal.pbio.1001881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hua S, Dias TH. Hypoxia-Inducible Factor (HIF) as a Target for Novel Therapies in Rheumatoid Arthritis. Front Pharmacol. 2016;7:184. doi: 10.3389/fphar.2016.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park, M. H. & Hong, J. T. Roles of NF-kappaB in Cancer and Inflammatory Diseases and Their Therapeutic Approaches. Cells5 (2016). [DOI] [PMC free article] [PubMed]
- 33.Huang Y, Wan G, Tao J. C1q/TNF-related protein-3 exerts the chondroprotective effects in IL-1beta-treated SW1353 cells by regulating the FGFR1 signaling. Biomed Pharmacother. 2017;85:41–46. doi: 10.1016/j.biopha.2016.11.128. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Torres J, et al. Impact of the gene-gene interactions related to the HIF-1alpha signaling pathway with the knee osteoarthritis development. Clin Rheumatol. 2019;38:2897–2907. doi: 10.1007/s10067-019-04635-w. [DOI] [PubMed] [Google Scholar]
- 35.Zhu M, Chen M, Lichtler AC, O’Keefe RJ, Chen D. Tamoxifen-inducible Cre-recombination in articular chondrocytes of adult Col2a1-CreER(T2) transgenic mice. Osteoarthritis Cartilage. 2008;16:129–130. doi: 10.1016/j.joca.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–3015. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryan HE, et al. Hypoxia-inducible factor-1alpha is a positive factor in solid tumor growth. Cancer Res. 2000;60:4010–4015. [PubMed] [Google Scholar]
- 38.Kamekura S, et al. Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthritis Cartilage. 2005;13:632–641. doi: 10.1016/j.joca.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Kawamoto T, Kawamoto K. Preparation of thin frozen sections from nonfixed and undecalcified hard tissues using Kawamot’s film method (2012) Methods Mol Biol. 2014;1130:149–164. doi: 10.1007/978-1-62703-989-5_11. [DOI] [PubMed] [Google Scholar]
- 40.Mori Y, Chung UI, Tanaka S, Saito T. Determination of differential gene expression profiles in superficial and deeper zones of mature rat articular cartilage using RNA sequencing of laser microdissected tissue specimens. Biomed Res. 2014;35:263–270. doi: 10.2220/biomedres.35.263. [DOI] [PubMed] [Google Scholar]
- 41.Gosset M, Berenbaum F, Thirion S, Jacques C. Primary culture and phenotyping of murine chondrocytes. Nat Protoc. 2008;3:1253–1260. doi: 10.1038/nprot.2008.95. [DOI] [PubMed] [Google Scholar]
- 42.Saito T, Ikeda T, Nakamura K, Chung UI, Kawaguchi H. S100A1 and S100B, transcriptional targets of SOX trio, inhibit terminal differentiation of chondrocytes. EMBO Rep. 2007;8:504–509. doi: 10.1038/sj.embor.7400934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stanton H, et al. Investigating ADAMTS-mediated aggrecanolysis in mouse cartilage. Nat Protoc. 2011;6:388–404. doi: 10.1038/nprot.2010.179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.