Abstract
Background
With evolution of medicine, radiation therapy and surgical methods, cancer care has improved the quality of life for patients with improved survival and functional status in patients with skeletal metastasis. The most common site of skeletal metastases from other primary malignant neoplasms is the spine, hence, understanding the epidemiology of metastatic spine disease and its presentation is essential for developing a diagnostic and treatment strategy which eventually results in optimum care to reduce disease-related morbidity.
Purpose
With this review article we intend to describe an evidence-based review on the presentation, diagnosis and treatment of metastatic spinal disease.
Methods
We reviewed the current available literature on management of spinal metastasis and have described a step wise evaluation and management strategy of metastatic spine disease.
Conclusion
The present review article addresses various aspects and related controversies related to evaluation, staging and treatment options in the management of spinal metastasis.
Keywords: Spine metastasis, Radiotherapy, SRS, Back pain
Introduction
The skeletal system is the third most affected site by metastatic cancer [1], with one-third of them located in the spine [2]. Approximately 10% of all malignant tumor cases develop symptomatic spinal metastasis. Most common primary tumors causing spinal metastasis include breast, prostate and lung and thyroid cancer [2]. The thoracic spine (70%) is the most common affected location, followed by lumbar (20%) and cervical spine (10%) (depending on location, they are categorized as intradural or extradural. Extradural lesions account for 90–95% of spinal metastases [3]. Spinal metastatic disease treatment options are decided on various factors, i.e., host factors, disease pathology and burden of disease. Previously patients with spinal metastatic disease would hardly live for months after diagnosis of the metastasis, advent of newer targeted therapies, immunotherapy and systemic therapies for tumors such as melanoma and renal cell carcinoma, prognosis of many tumors has improved in terms of mean survival time from months to years.
There are approximately 180,000 new cases of metastatic involvement of the vertebral column annually in the USA, up to one-third of all patients with cancer develop metastases to the spinal column. Hence it is customary that over all prevalence of spinal metastasis will rise and large amount of resources will be required to provide essential clinical care for these patients. The appropriate treatment for an individual patient requires a multidisciplinary team input which includes an orthopaedic oncologist, spine surgeon, radiologist, radiation oncologist, medical oncologist and other healthcare personnel. It is important that health care personnel especially orthopaedic oncology surgeons, spine surgeons and neurosurgeons are updated with recent trends in the management of spinal metastasis.
With only 10–20% of patients with spinal metastasis surviving beyond 2 years from point of diagnosis, Goals in the management of patients with spinal metastases are essentially palliative. It includes pain control, preservation or restoration of neurological function, maintenance of spinal stability all in a setting of durable local tumor control and to be able to maintain health related quality of life (HRQOL). Apart from surgery, the evolution of spine stereotactic radio surgery has greatly improved local control rates [4]. The present review article addresses various aspect and related controversies related to evaluation, staging and treatment options.
Etiopathogenesis
Multiple theories have been postulated to explain the routes of spread of metastatic disease to the spinal column. Transmission through the arterial route is the most common method of transmission owing to the extensive blood supply along the vertebral column, or it could spread via the batsons plexus which is a valveless system of veins which connects the thoracic and pelvic vessels to the basivertebral veins, tumor cells may spread via the cerebrospinal fluid or it could be a direct extension from an adjacent organs, e.g., pancoasts tumor [5].
Clinical Presentation
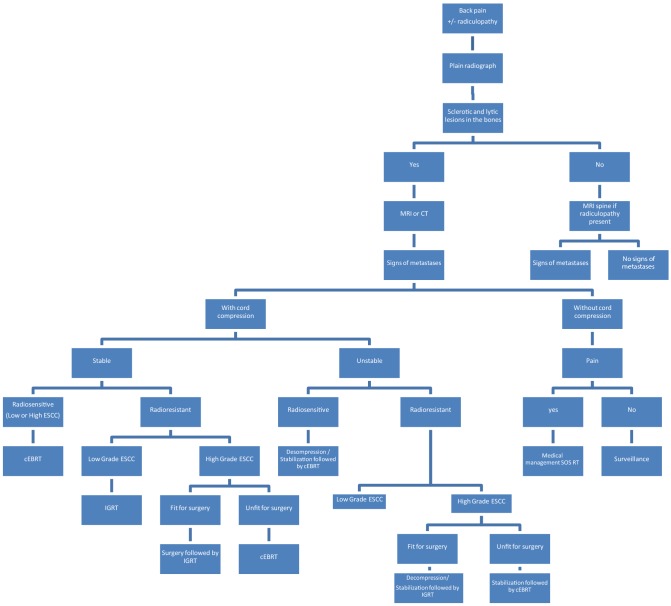
Back pain is the most common symptom and usually precedes the development of neurological symptoms. Pain can be due to proliferation of tumor cells in the vertebral body leading to damage of thick cortical bone and stretching of the periosteum (biological) or due to pressure on the spinal cord and nerve roots by disease-causing radiculopathy (radicular) or because of mechanical causes like pathological fracture resulting in acute pain, spinal instability with/without compression of the spinal cord. Pain due to biological causes is predominantly nocturnal and usually reduces with activity and responds to low-dose steroids. Mechanical pain resulting due to structural instability usually increases with spinal mobility. Radicular pain occurs during weight bearing and tends to be relieved by lying down.
Neurological deficit—patients may present with neurological deficits which could be sensory or motor or both. Based on the location of spinal involvement and severity of compression, the presenting symptoms vary, from numbness to paralysis to urinary and bowel incontinence. The degree of cord compression may vary and patient may present with monoparesis to a frank paraplegia. If a patient presents with painless urinary retention, it suggests a neurological cause. Isolated loss of bowel and bladder function without any motor or sensory symptoms is seen when there is involvement of the conus medullaris and in sacral tumors. Loss of autonomic function is usually a late finding when other segments are involved. Rarely spinal metastatic disease may present as atypical chest pain or with symptoms of cardiac chest pain which occurs due to infiltration of the thoracic nerve roots by disease [6].
Evaluation
Correct diagnosis of neurologic symptoms is necessary for deciding on appropriate treatment. Clinical, radiological and biochemical investigations are paramount to diagnose and assess the severity of metastatic spinal disease. Plain radiographs are quick and readily available, however, all metastatic lesions may not be apparent on plain radiographs, thus reducing their sensitivity. More than 50% of trabecular bone should be destroyed to become evident on X-ray [5] and often when the pedicle is found to be absent it is called the winking owl sign [2, 7].
MRI is the gold standard for evaluation of spinal metastasis and has the highest sensitivity and specificity in the detection of spinal metastases (98.5% vs. 98.9%) with an accuracy of 98.7% [2] Details of the extra osseous extent of disease, marrow infiltration, neural compression and status of other vertebral levels are seen well on an MRI. In a review by Avrahami et al. [8], 52% of cases who tested negative on bone scan had tumor on MRI hence highlighting the importance of MRI in evaluation of spinal metastasis.
Bone scan (99mTc-MDP) has an ability to screen entire skeleton in a single image and detects multiple skeletal sites of disease [5] it is helpful in locating a more accessible site for tissue diagnosis in unknown primaries. Bone scans show poor sensitivity for diseases with osteolytic lesions [8] and has high false-positive rates due to a variety of causes like trauma, inflammation, degenerative diseases and benign disorders of spine. Though used rarely, computed tomography can be utilized in planning for detection of severity and stabilization of mechanical instability. Positron emission tomography (PET) scan detects regions of increased uptake using tagged molecules. The usual radionuclides used are 18F-Fluoride and FDG. Its sensitivity for detecting bone metastasis ranges between 62 and 100% with specificity of 96 to 100% [9]. Flouride (F18) pet acts a marker of skeletal remodeling, making it a sensitive method for assessment of bone and bone marrow metastases.
Fluoro deoxy glucose (FDG) targets regions with increased metabolic activity either in soft tissues or in the skeleton. It signals regions of infectious, inflammatory and neoplastic activity hence could be non specific. It has additional benefit for detecting primary lesions in unknown primary. It can be used as a one-stop modality for overall evaluation of primary tumor and distant skeletal and visceral metastasis.
Biochemical investigations are important for the diagnosis of primary tumor pathology. Few commonly used tumor markers are Serum electrophoresis and Beta-2-microglobulin for Multiple myeloma, CA 19.9 for Pancreatic, gall bladder, bile duct tumors, CA 125 for Ovarian cancer, PSA for Prostate cancer, CA 15.3/CA 27.29 for Breast cancer, CEA for Colorectal and breast cancer and calcitonin for medullary thyroid cancer as such role of biochemical markers for spinal metastasis is limited.
Biopsy
Histopathological confirmation through a core biopsy is mandatory in all patients with unknown primary lesions, patients with inconclusive initial metastatic evaluation and in suspicion of infection [10]. Core needle biopsy can be performed via image-guided modalities like fluoroscopy or computerized tomography (CT scan) or open biopsy. Fine needle aspiration cytology (FNAC) may be adequate if the primary tumor has already been identified. In those with spinal cord compression, tissue analysis to evaluate tumor sensitivity to radiation therapy is needed [10].
Emergency surgical decompression and obtaining tissue samples for histopathology can be performed in the setting of acute and rapid progressive neurological deficits. Histology of the tumor plays a vital role in determining the treatment modality chosen. It will help decide if the patient needs hormone therapy, chemotherapy, radiotherapy, surgical management or any combination of strategies. For, e.g. hematological malignancies, round cell sarcoma, choriocarcinoma and small cell lung carcinomas and display sensitivity to radiotherapy, hence rarely require surgical decompression. Ca breast is sensitive to conventional external beam radiation (EBRT), colon and non small cell carcinomas are moderately sensitive. Sarcomas, renal carcinomas and melanomas and are radioresistant tumors, i.e., radio-resistant tumors with a moderate to high degree of cord compression generally require surgical decompression and radiosensitive tumors with high-grade cord compression can be treated with radiotherapy alone.
Management
Goals in the management of patients with spinal metastases are essentially palliative. It includes pain control, preservation or restoration of neurological function, maintenance of spinal stability all in a setting of durable local tumor control and to be able to maintain health related quality of life (HRQOL) [11].
The treatment strategies for the management of spinal metastasis may be subdivided as:
Medical management
Radiotherapy
Surgery
Medical Management
Medical management essentially involves administration of chemotherapeutic agents and other drugs to that improve the quality of life.
Other drugs includes:
Analgesic agents
Steroids
Bisphosphonates
Denosumab
Hormonal therapy
Pain being the most common debilitating factor in patients with spine metastasis, treating it is of paramount importance. Medications such as non-steroidal anti-inflammatory, opioids and steroids help to reduce biological pain. With advances in drug therapy, more effective routes of pain relief are currently available namely buccal, sublingual and intranasal preparations of fentanyl. In cases where there is uncontrollable pain despite high dosages of opioids, unacceptable side effects of analgesics, infiltration of disease around a neural plexus intrathecal infusions (epidural/intrathecal) though at a higher dosage rates play a significant role in alleviating pain [12, 13]. Therapeutic nerve blocks help reduce cancer related pain by blocking nerve transmission, these could be either damaging the affected nerve root (neurolytic) or not damaging the nerve root (non neurolytic). Pain due to instability requires surgical fixation. Steroids can also be used in cases with neurological impairment secondary to tumor compression. Steroids can be used to reduce spinal cord oedema and improve clinical symptoms. Dexamethasone stabilizes or improves neurologic status in patients by reducing vasogenic edema in acute spinal cord compression. Controversy exists as to optimal dose of dexamethasone in spinal metastasis [14]. Dexamethasone dose ranges from 16 mg/day in divided doses (moderate dose) to 96 mg/day in divided doses (high) with a loading dose of 10–100 mg. These doses are most effective when given within 12 h of the malignant spinal cord compression. As per current guidelines fewer complications are noted with as initial 10 mg intravenous bolus of dexamethasone followed by 16 mg PO QD when compared to 100 mg bolus dose and 96 mg QD. Higher doses lead to more complications such as gastrointestinal hemorrhage, hyperglycemia, intestinal perforation and AVN of hip [15].
Biphosphonates inhibit osteoclastic activity, suppress bone resorption, zolendronic acid is the most commonly used biphosphonate and are effective in the treatment of malignancy-associated hypercalcemia. Bisphosphonates have also shown to reduce or delay skeletal events, such as pathologic fractures [16] hence useful in patients with multiple myeloma and osteolytic metastases [17]. Febrile reactions, myalgias, thrombophlebitis, lymphopenia, neutropenia, and hypocalcemia are the most significant side effects seen and rarely ocular complications (uveitis) and jaw osteonecrosis [18]. Denosumab, a fully human monoclonal antibody, also plays an important role in delaying skeletal related events (SRE), its role was studied extensively in three large randomized controlled trials which included diseases of the breast, prostate, multiple myeloma and other solid tumors. Denosumab showed promising results in significantly reducing treatment related osteoporosis in breast and prostate cancer and was also superior to zolendronic acid in prevention or delaying SRE [11].
Chemotherapy is administered for systemic and local control of primary tumor. Chemotherapy plays a significant role in management of chemosensitive pathologies like malignant lymphoma, myeloma, Ewing’s sarcoma (PNET), and osteogenic sarcoma and germ cell tumors. It is helpful in both primary spinal tumors as well as metastatic spine disease. Long-term local controls can be achieved with histology-specific chemotherapeutic agents and helps in reducing morbidity.
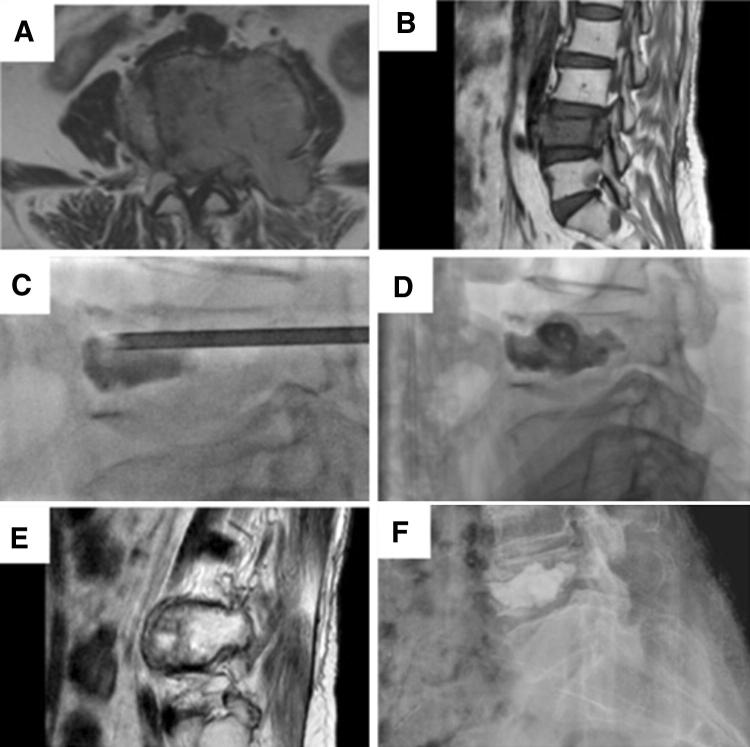
Hormonal therapy—malignancies like carcinoma of prostate and breast may be helpful in treating with conservative modality of therapy instead of any surgical intervention. Hormonal therapy is the treatment of choice for metastasis resulting from prostate cancer. This combination of hormonal therapy and ERT should be recognized as an important treatment option and it should be initiated as soon as possible [13] (Fig. 1).
Fig. 1.
Metastatic carcinoma of breast. a, b Magnetic resonance imaging sections showing cord compression. Managed with palliative radiotherapy and vertebroplasty (c, d). e, f Two years follow-up as noted on magnetic resonance imaging (e) and plain radiographs (f)
Radiotherapy
Radiotherapy (RT) plays an important role in the management of metastatic spinal cord disease. Its timely use may eliminate the need for surgical intervention in radiosensitive tumors in patients with a poor medical condition with limited life expectancy or in patients who present immediately after the onset of paraplegia. Radiotherapy is effective in cases with a radiosensitive tumor and the cause for the dural compression is a soft tissue component (Figs. 2, 3).
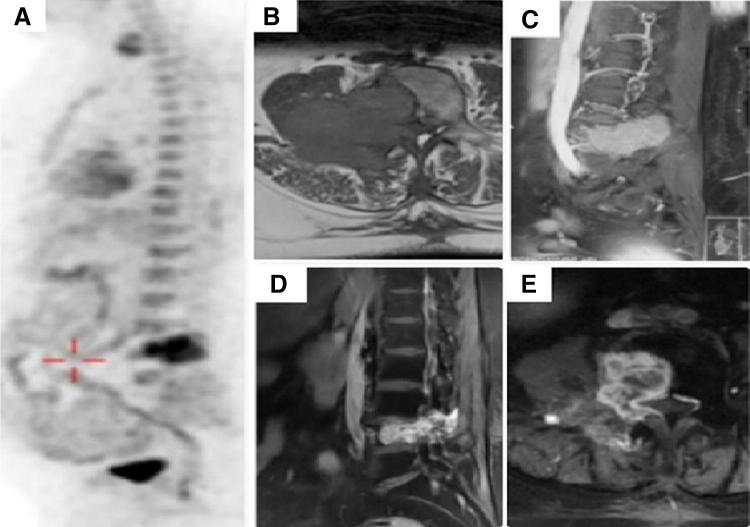
Fig. 2.
Metastatic carcinoma of papillary thyroid with spine metastasis at L4 vertebra. a FDG-PET showing solitary site of metastases. b, c Magnetic resonance imaging B (axial), c (sagittal) sections showing cord compression. d, e Follow-up images post radioactive iodine (RAI), angioembolization and radiotherapy
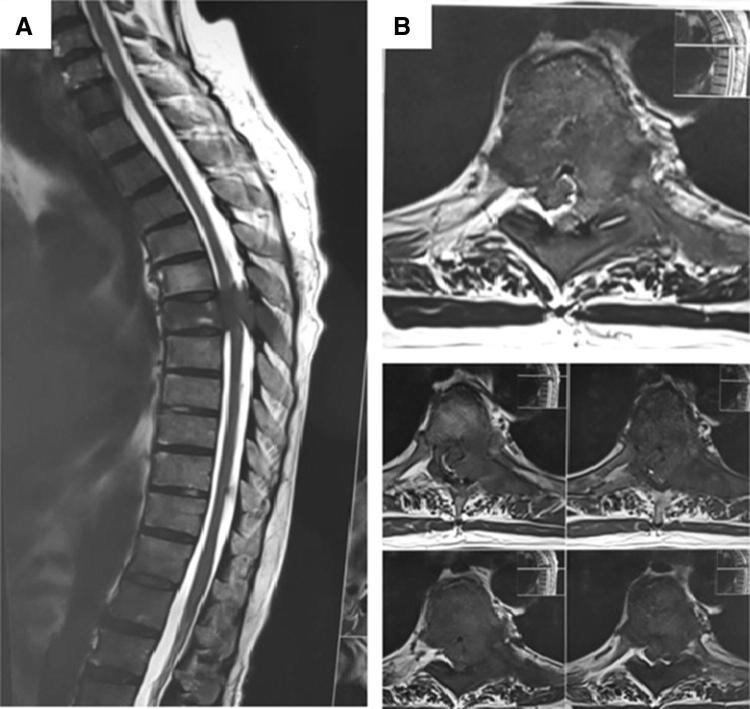
Fig. 3.
Metastatic carcinoma of lung. a, b Magnetic resonance imaging showing sagittal and coronal sections with cord compression due to soft tissue component. Treated with radiotherapy
Radiotherapy in spinal metastasis is delivered using conventional external beam radiation (cEBRT, e.g. 30 Gy in 10 fractions) and stereotactic radio surgery (SRS, e.g., 16–24 Gy single fraction or 24–30 Gy in 3–5 fractions) [19, 20]. These recommended radiation doses spare normal tissues and are ablative to the tumor tissue.
In a retrospective analysis of 235 patients, Gilbert et al. [21] evaluated the efficacy of radiotherapy alone versus surgery followed by radiotherapy. The analysis was based on the preoperative functional status and radio sensitivity of the tumor. Results showed that overall rate of postoperative ambulation in the surgery and radiotherapy group was 46% compared to 49% for the radiotherapy alone group. This study shifted the trend of managing radiosensitive tumors causing spinal cord compression with radiotherapy alone (Fig. 1).
Radiotherapy administration can be done as follows:
Radiosensitive tumors—conventional external beam radiation (cEBRT) cEBRT can be used for any degree of ESCC if it is a radiosensitive tumor [20]. E.g.: lymphoma, seminoma, myeloma [22] and solid ones such as breast, prostate, ovarian, and neuroendocrine carcinomas [22].
Radioresistant tumor without spinal cord compression—SRS radioresistant tumors, i.e., sarcoma, melanoma, thyroid, non-small cell lung carcinomas (Fig. 3), hepatocellular, renal and colon [22] respond poorly to EBRT. SRS has demonstrated greater than 90 percent response rates when used as a definitive modality of treatment in patients with minimal or no spinal cord compression [1]. Response to SRS is histology-independent unlike those seen with cEBRT. Guckenberger et al. [23] reported a multi-institutional retrospective review of 301 patients with 2-year local control rates at 83.9%.
Radioresistant tumors with high-grade spinal cord compression—surgery ± SRS Patchell et al. conducted a prospective randomized trial comparing surgery followed by radiotherapy to radiotherapy alone [24]. In cases of high-grade spinal cord compression with tumor histology known to be a radio-resistant tumor, surgery is the treatment recommended which could range from purely a spinal decompression with laminectomy to stabilization of the spine to preserve or restore neurological. The standard RT treatment for palliation of spinal metastases ranges from 300 to 3000 cGy daily. Ideally when radiotherapy is being contemplated, a single posterior field targeting the involved segment of vertebrae with one to two levels above and below is recommended. The limiting factor in radiotherapy to the spinal cord or cauda equina is the optimal tolerated dose which is lower than other areas of the body.
Recent advances in radiation delivery such as intensity-modulated radiation therapy (IMRT), three-dimensional conformal radiation therapy (3D-CRT), Intraoperative radiation therapy (IORT), we can deliver high tumoral dose of radiation with less RT associated effects to surrounding tissues and potentially smaller fields compared to EBRT.
Surgery
Indications for surgery in spinal metastases are broadly divided as:
Primary surgery
Spinal instability
Circumferential epidural tumor (Fig. 4)
Radio-resistant tumors (e.g. sarcoma, renal cell carcinoma, colon, lung).
Occult primary tumor
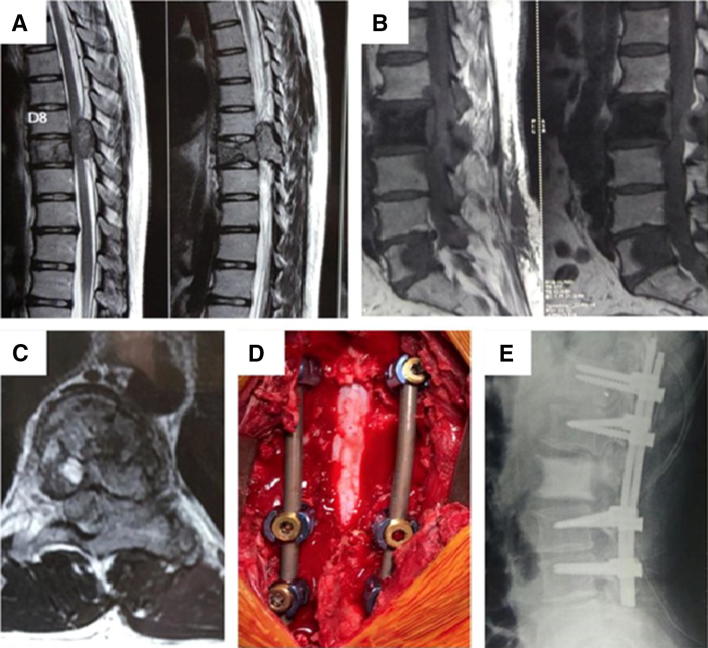
Fig. 4.
Metastatic carcinoma of prostrate. a, b Sagittal sections showing multilevel disease in vertebrae. c Axial image showing cord compression at D9 vertebrae. d Intra-operative image post spinal decompression and posterior stabilization. e Post operative radiograph
Post-treatment surgery (radiotherapy/chemotherapy)
Worsening neurological symptoms.
Progression of tumor with high-grade spinal cord compression.
Residual tumor post radiotherapy/chemotherapy (e.g. Ewing’s sarcoma, germ cell tumor).
Pre-requisites before deciding on surgical intervention for a patient with metastatic spinal disease
Life expectancy of the patient.
The need to improve function and to limit pain.
The need for complete local control.
The possibility of associating adjuvant treatments to improve the efficacy of the treatment, reducing morbidity.
Surgical strategy for spinal metastases (Table 3) consists of three prognostic factors [25]:
Grade of malignancy
Visceral metastases
Bone metastases
Table 3.
SINS scoring system
| SINS—spinal instability neoplastic score | |
|---|---|
| Location | |
| Junctional (Occiput–C2, C7–T2, T11–L1, L5–S1) | 3 |
| Mobile spine (C3–C6, L2–L4) | 2 |
| Semi-rigid (T3–T10) | 1 |
| Rigid (S2–S5) | 0 |
| Pain relief with recumbency and/or pain with movement/loading of the spine | |
| Yes | 3 |
| No (occasional pain but not mechanical) | 1 |
| Pain free lesion | 0 |
| Bone lesion | |
| Lytic | 2 |
| Mixed (lytic/blastic) | 1 |
| Blastic | 0 |
| Radiographic spinal alignment | |
| Subluxation/translation present | 4 |
| De novo deformity (kyphosis/scoliosis) | 2 |
| Normal alignment | 0 |
| Vertebral body collapse | |
| >50% collapse | 3 |
| <50% collapse | 2 |
| No collapse with > 50% body involved | 1 |
| None of the above | 0 |
| Posterolateral involvement of the spinal elements (facets, pedicle or CV joint fracture or replacement with tumor) | |
| Bilateral | 3 |
| Unilateral | 1 |
| None of the above | 0 |
| Score 0–6: No surgical consultation is required. Score of 7–18: Surgical consultation is advisable | |
Instability, evaluated by the SINS, is an indication for surgery. To warrant a surgical intervention the general consensus is that the survival should be more than 3 months Scoring systems such as Tokuhashi help in calculating the life expectancy. Modern spinal instrumentation techniques help improve neurological function, reduce pain caused due spinal instability [10].
Posterior decompression using a laminectomy is sufficient for cases with posterior epidural cord compression after ruling out no bony cause for the compression. If there is progressive kyphosis and worsening neurological symptoms, then instrumentation is indicated. Improved surgical outcomes have been obtained with advances in surgical approaches. These approaches include anterior, transcavitary, posterolateral and transpedicular. The decision on what approach to take is primarily based on surgeon familiarity with the approach, the location of the tumor and the type of reconstruction planned. [26–28].
In terms of tumor resection, approach depends classically on the location of the tumor, i.e. for vertebral body tumors, anterior paraspinal or epidural tumors, ideal approach would be the anterior transcavitary. Autologous bone graft with cage, bone cement (methyl methacrylate) and steinmann pins with addition of an anterior locking plate. For tumors located in the thoracic and lumbar spine and for cases with three column involvement the posterolateral approach is ideal. Reconstruction is often achieved with posterior spinal instrumentation and reinforced using an anterior strut, with steinmann pins and bone cement.
Final outcome after surgery depends on preoperative neurologic and functional status and favorable tumor histology to radiation. Klekamp et al. [29] showed that 96% of patients who were mobile prior to surgery continued to do so 3 months later in comparison to only 22% of patients who were non ambulatory prior to surgery who regained ambulation for the same duration. These results were similar to patients treated with radiotherapy alone as described by Maranzano et al. [30]. Pretreatment neurologic function is one of the strongest predictors of post treatment neurologic recovery along with the type of the tumor. Another important factor that could guide prognosis in spinal cord compression would be onset of symptoms in a case of epidural spinal cord compression, i.e. likelihood of being ambulatory after treatment is higher among patients whose motor deficits developed more slowly (over longer than 2 weeks versus less than 1 week prior to therapy).
Minimal Invasive Surgical (MIS) Technique
Surgical decompression has drawbacks such as delays in receiving postoperative radiotherapy/chemotherapy in addition to the huge cost which is always a deciding factor in decision making in economically backward societies [31]. To reduce the morbidity of invasive open surgical procedures, minimally invasive approaches are being developed. It reduces operation time, blood loss, hospital stay, surgical complication rates which leads to lower morbidity for the patient. Minimal invasive surgical techniques including percutaneous vertebroplasty (PVP), percutaneous kyphoplasty (PKP), radiofrequency ablation (RFA), cryoablation, and transarterial embolization [32].
PVP or PKP are useful in painful pathologic compression fractures without gross spinal instability or significant posterior element involvement [33, 34] In PVP, bone cement is injected through a minimal incision into the fractured site. In PKP, a balloon is inserted into the fractured site, followed by inflation-deflation to create a cavity into which the filler material is injected, and the balloon is taken out prior to cement injection [6]. PMMA, the most commonly used among numerous cement formulations, provides additional reinforcement to the vertebral body. Mende et al. [10] performed a systematic review of literature which resulted in a strong recommendation for the use of PVP or PKP in the setting of symptomatic osteolytic tumors.
Radiofrequency ablation is also used in the treatment of spinal metastases. RFA uses an electromagnetic current with 300–500 kHz frequency to trigger molecular friction movements, which is guided by imaging and results in temperature increase and diffusion in the tumor. The temperature should rise higher than 70 °C in order to achieve irreversible cell death by coagulation necrosis of the cell proteins [35]. Palussiere et al. [35] showed that 70–90% of patients with metastases experience considerable relief after RFA. And if the treatment fails, it can be offered again [32]. The indications for this technique are similar to open surgery, but they are limited by the experience of the surgeon. In a review done by Zuozhang Yang et al. [36] found that improvement in pain and neurological dysfunction is similar in MIS and open surgery group with open surgery associated with major complications and lower survival rates.
Tools to Help Decision Making for Treatment Strategy in MSCC
They are:
NOMS
Tokohashi scoring system
Spinal instability neoplastic score (SINS)
Tomita score
NOMS
In the recent past a new scoring system called ‘NOMS” has been developed that evaluate four factors namely: neurologic, oncologic, mechanical instability and systemic disease [37]. The ASIA score or the Frankel scale can also be used [38]. Performance status is typically assessed using the Eastern Cooperative Oncology Group (ECOG) score. There are various management protocol and nomograms.
NOMS decision frame work is described below (Table 1):
Table 1.
NOMS scoring
| Neurologic | Oncologic | Mechanical | Systemic | Decision |
|---|---|---|---|---|
| Low-grade ESCC/no myelopathy | Radiosensitive | Stable | cEBRT | |
| Low-grade ESCC/no myelopathy | Radiosensitive | Unstable | Stabilization* followed by cEBRT | |
| High-grade ESCC +/myelopathy | Radiosensitive | Stable | cEBRT | |
| High-grade ESCC +/myelopathy | Radiosensitive | Unstable | Stabilization* followed by cEBRT | |
| Low-grade ESCC/no myelopathy | Radioresistant | Stable | IGRT | |
| Low-grade ESCC/no myelopathy | Radioresistant | Unstable | Stabilization* followed by IGRT | |
| High-grade ESCC +/myelopathy | Radioresistant | Stable | Able to tolerate surgery | Decompression/stabilization followed by IGRT |
| High-grade ESCC +/myelopathy | Radioresistant | Stable | Unable to tolerate surgery | cEBRT |
| High-grade ESCC +/myelopathy | Radioresistant | Unstable | Able to tolerate surgery | Decompression/stabilization followed by IGRT |
| High-grade ESCC +/myelopathy | Radioresistant | Unstable | Unable to tolerate surgery | Stabilization* followed by cEBRT |
Tokuhashi Scoring System
Tokuhashi scoring system [39] (Table 2) can be used to predict survival and in-turn aid the decision making process.
Table 2.
Tokohashi scoring
| Tokuhashi scoring system for the prognosis of metastatic spine tumors | |
|---|---|
| Characteristics | Score |
| General condition (performance status) | |
| Poor (10–40%) | 0 |
| Moderate (50–70%) | 1 |
| Good (PS 80–100%) | 2 |
| No. of extraspinal bone metastasis foci | |
| >3 | 0 |
| 1–2 | 1 |
| 0 | 2 |
| No of metastasis in the vertebral body | |
| >3 | 0 |
| 2 | 1 |
| 1 | 2 |
| Metastasis to the major internal organs | |
| Unremovable | 0 |
| Removable | 1 |
| No metastasis | 2 |
| Primary site of cancer | |
| Lung, OGS, stomach, bladder, oesophagus, pancreas | |
| Liver, gallbladder, unidentified | |
| Other | |
| Kidney, uterus | |
| Rectum | |
| Thyroid, breast, prostate, carcinoid tumor | |
| Palsy | |
| Complete (Frankel A,B) | 0 |
| Incomplete (Frankel C,D) | 1 |
| None (Frankel E) | 2 |
|
Criteria of predicted prognosis: Total score (TS) 0–6 < 6 months, TS 9–11 = 6 months to 1 year, TS 12–15 = > 1 year | |
Spinal Instability Neoplastic Score: SINS
In cases where the spine is unstable, the spinal instability neoplastic score (SINS) (Table 3) is used for assessment.
Tomita Score (Table 4)
Table 4.
Tomita scoring
| Prognostic scoring system | |||
|---|---|---|---|
| Factor | Primary tumor | Mets to vital organs | Bone mets |
| Point | |||
| 1 | Slow | No mets | Isolated |
| 2 | Moderate | Controllable | Multiple |
| 3 | Rapid | Uncontrollable | |
|
Minimum requirement:
| |||
| Total score | Life expectancy | Treatment aim | Surgery |
|---|---|---|---|
| 2 | 2 years < | Long-term local control | Enbloc excision |
| 3 | |||
| 4 | |||
| 5 | 1–2 years | Middle-term local control | Debulking |
| 6 | |||
| 7 | 6–12 months | Short-term palliation | Palliative decomposition |
| 8 | |||
| 9 | < 3 months | Terminal care | No surgical treatment |
| 10 |
| Points | Growth of tumor | Site |
|---|---|---|
| 1 | Slow growing | Breast |
| Thyroid | ||
| Prostate | ||
| Testicular | ||
| 2 | Moderate growth | Renal |
| Uterus | ||
| Ovarian | ||
| Colorectal | ||
| 3 | Rapid growth | Lung |
| Stomach | ||
| Oesophagus | ||
| Nasopharyngeal | ||
| Hepatocellular | ||
| Pancreas | ||
| Bladder | ||
| Melanoma | ||
| Sarcoma | ||
| Primary unknown metastasis | ||
| Other rare cancers |
Conclusion
This review article was written to aid understanding in the management of spinal metastasis. Spinal metastases management requires a multidisciplinary approach. The SINS helps in the evaluation of instability of the spine. The NOMS algorithm has objectively summarized the ideal treatment modality for managing metastasis. Radiotherapy still remains the mainstay of therapy for both radiosensitive and radio-resistant histologies especially with development of cEBRT and SRS. Surgery plays a role in those with instability or in cases with severe compression. Recent improvements in minimal invasive modalities of surgery and advances in medical line of management, good quality of life can be provided to patients with metastatic spinal cord disease and cord compression. With continued advances in the field of medicine, the drive to improve the quality of life for these patients is never ending.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard statement
This is a review article. The article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all the patients whose imaging has been published.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Vineet Kurisunkal, Email: vineetkurisunkal@gmail.com.
Ashish Gulia, Email: aashishgulia@gmail.com.
Srinath Gupta, Email: srigups@gmail.com.
References
- 1.Hosono N, Yonenobu K, Fuji T, Ebara S, Yamashita K, Ono K. Orthopaedic management of spinal metastases. Clinical Orthopaedics and Related Research. 1995;312:148–159. [PubMed] [Google Scholar]
- 2.Greenlee RT, Murray T, Bolden S, Wingo PA. Cancer statistics, 2000. CA: A Cancer Journal for Clinicians. 2000;50(1):7–33. doi: 10.3322/canjclin.50.1.7. [DOI] [PubMed] [Google Scholar]
- 3.Gerzten PC, Welsch WC. Current surgical managment of metastatic spinal disease. Oncology (Huntingt) 2000;14:1013–1014. [PubMed] [Google Scholar]
- 4.Joaquim AF, Ghizoni E, Tedeschi H, Pereira EB, Giacomini LA. Stereotactic radiosurgery for spinal metastases: A literature review. Einstein (São Paulo) 2013;11(2):247–255. doi: 10.1590/s1679-45082013000200020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenthal DI. Radiologic diagnosis of bone metastases. Cancer. 1997;80(8):1595–1607. doi: 10.1002/(SICI)1097-0142(19971015)80:8+<1595::AID-CNCR10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Yimin Y, Zhiwei R, Wei M, Jha R. Current status of percutaneous vertebroplasty and percutaneous kyphoplasty--a review. Medical Science Monitor. 2013;19:826–836. doi: 10.12659/MSM.889479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buhmann (Kirchoff) S, Becker C, Duerr HR, Reiser M, Baur-Melnyk A. Detection of osseous metastases of the spine: Comparison of high resolution multi-detector-CT with MRI. European Journal of Radiology. 2009;69(3):567–573. doi: 10.1016/j.ejrad.2007.11.03. [DOI] [PubMed] [Google Scholar]
- 8.Avrahami E, Tadmor R, Daily O, Hadar H. Early MR demonstration of spinal metastases in patients with normal radiographs and CT and radionuclide bone scans. Journal of Computer Assisted Tomography. 1989;13(4):598–602. doi: 10.1097/00004728-198907000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. Journal of Clinical Oncology. 2004;22:2942–2953. doi: 10.1200/JCO.2004.08.181. [DOI] [PubMed] [Google Scholar]
- 10.Mendel E, Bourekas E, Gerszten P, Golan JD. Percutaneous techniques in the treatment of spine tumors: What are the diagnostic and therapeutic indications and outcomes? Spine. 2009;34(22 Suppl):S93–S100. doi: 10.1097/brs.0b013e3181b77895. [DOI] [PubMed] [Google Scholar]
- 11.Kumar A, Weber MH, Gokaslan Z, Wolinsky JP, Schmidt M, Rhines L, et al. Metastatic spinal cord compression and steroid treatment. Clinical Spine Surgery. 2017;30(4):156–163. doi: 10.1097/BSD.0000000000000528. [DOI] [PubMed] [Google Scholar]
- 12.Perrin RG, Laxton AW. Metastatic spine disease: Epidemiology, pathophysiology, evaluation of patients. Neurosurgery Clinics of North America. 2004;15(4):365–373. doi: 10.1016/j.nec.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Kato S, Hozumi T, Yamakawa K, Higashikawa A, Goto T, Kondo T, et al. Hormonal therapy with external radiation therapy for metastatic spinal cord compression from newly diagnosed prostate cancer. Journal of Orthopaedic Science. 2013;18(5):819–825. doi: 10.1007/s00776-013-0409-y. [DOI] [PubMed] [Google Scholar]
- 14.Bucholtz JD. Metastatic epidural spinal cord compression. Seminars in Oncology Nursing. 1999;15(3):150–159. doi: 10.1016/s0749-2081(99)80002-3. [DOI] [PubMed] [Google Scholar]
- 15.Maranzano E, Latini P, Beneventi S, et al. Radiotherapy without steroids in selected metastatic spinal cord compression patients. American Journal of Clinical Oncology. 1996;19:179–183. doi: 10.1097/00000421-199604000-00018. [DOI] [PubMed] [Google Scholar]
- 16.Hortobagyi GN, Theriault RL, Lipton A, et al. Long-term prevention of skeletal complications of metastatic breast cancer with pamidronate. Protocal 19 aredia breast cancer study group. Journal of Clinical Oncology. 1998;16:2038–2044. doi: 10.1200/JCO.1998.16.6.2038. [DOI] [PubMed] [Google Scholar]
- 17.Berenson JR, Lichtenstein A, Porter L, et al. Efficacy of pamidronate in reducing skeletal events in patients with advanced multiple myeloma. New England Journal of Medicine. 1996;334:488–493. doi: 10.1056/NEJM199602223340802. [DOI] [PubMed] [Google Scholar]
- 18.Coukell AJ, Markham A. Pamidronate. A review of its use in the management of osteolytic bone metastases, tumour-induced hypercalcaemia and Paget’s disease of the bone. Drugs and Aging. 1998;12:149–168. doi: 10.2165/00002512-199812020-00007. [DOI] [PubMed] [Google Scholar]
- 19.Laufer I, Iorgulescu JB, Chapman T, Lis E, Shi W, Zhang Z, et al. Local disease control for spinal metastases following “separation surgery” and adjuvant hypofractionated or high-dose single-fraction stereotactic radiosurgery: Outcome analysis in 186 patients. Journal of Neurosurgery: Spine. 2013;18(3):207–214. doi: 10.3171/2012.11.spine12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Findlay GFG. Adverse effects of the management of malignant spinal cord compression. Journal of Neurology, Neurosurgery and Psychiatry. 1984;47:761–768. doi: 10.1136/jnnp.47.8.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gilbert RW, Kim JH, Posner JB. Epidural spinal cord compression, metastatic tumour: Diagnosis and treatment. Annals of Neurology. 1978;3:40–51. doi: 10.1002/ana.410030107. [DOI] [PubMed] [Google Scholar]
- 22.Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: Clinical experience in 500 cases from a single institution. Spine. 2007;32(2):193–199. doi: 10.1097/01.brs.0000251863.76595.a2. [DOI] [PubMed] [Google Scholar]
- 23.Guckenberger M, Mantel F, Gerszten PC, Flickinger JC, Sahgal A, Létourneau D, et al. Safety and efficacy of stereotactic body radiotherapy as primary treatment for vertebral metastases: A multi-institutional analysis. Radiation Oncology. 2014;9(1):226. doi: 10.1186/s13014-014-0226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patchell RA, Tibbs PA, Regine WF, Payne R, Saris S, Kryscio RJ, Mohiuddin M, Young B. Direct decompressive surgical resection in the treatment of spinal cord compression caused by metastatic cancer: A randomised trial. Lancet. 2005;366(9486):643–648. doi: 10.1016/S0140-6736(05)66954-1. [DOI] [PubMed] [Google Scholar]
- 25.Tomita K, Kawahara K, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine. 2001;26:298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 26.Hosono N, Yonenobu K, Fuji T, et al. Vertebral body replacement with ceramic prosthesis for metastatic spinal tumors. Spine. 1995;20:2454–2462. doi: 10.1097/00007632-199511001-00015. [DOI] [PubMed] [Google Scholar]
- 27.Kostuik JP. Anterior spinal cord decompression for lesions of the thoracic and lumbar spine, techniques, new methods of internal fixation results. Spine. 1983;8:512–531. doi: 10.1097/00007632-198307000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Perrin RG, McBroom RJ. Anterior versus posterior decompression for symptomatic spinal metastasis. Canadian Journal of Neurological Sciences. 1987;14:75–80. doi: 10.1017/S0317167100026871. [DOI] [PubMed] [Google Scholar]
- 29.Klekamp J, Samii H. Surgical results for spinal metastases. Acta Neurochirurgica (Wien) 1998;140:957–967. doi: 10.1007/s007010050199. [DOI] [PubMed] [Google Scholar]
- 30.Maranzano E, et al. 8 Gy single-dose radiotherapy is effective in metastatic spinal cord compression: Results of a phase III randomized multicentre Italian trial. Radiotherapy and Oncology. 2009;93(2):174–179. doi: 10.1016/j.radonc.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Arrigo RT, Kalanithi P, Cheng I, Alamin T, Carragee EJ, Mindea SA, Park J, Boakye M. Predictors of survival after surgical treatment of spinal metastasis. Neurosurgery. 2011;68(3):674–681. doi: 10.1227/NEU.0b013e318207780c. [DOI] [PubMed] [Google Scholar]
- 32.Salapura V, Jeromel M. Minimally invasive (percutaneous) treatment of metastatic spinal and extraspinal disease—A review. Acta Clinica Croatica. 2014;53(1):44–54. [PubMed] [Google Scholar]
- 33.Burton AW, Mendel E. Vertebroplasty and kyphoplasty. Pain Physician. 2003;6(3):335–341. [PubMed] [Google Scholar]
- 34.Bartolozzi B, Nozzoli C, Pandolfo C, Antonioli E, Guizzardi G, Morichi R, Bosi A. Percutaneous vertebroplasty and kyphoplasty in patients with multiple myeloma. European Journal of Haematology. 2006;76(2):180–181. doi: 10.1111/j.1600-0609.2005.00573.x. [DOI] [PubMed] [Google Scholar]
- 35.Palussiere J, Pellerin-Guignard A, Descat E, Cornelis F, Dixmerias F. Radiofrequency ablation of bone tumours. Diagnostic and Interventional Imaging. 2012;93(9):660–664. doi: 10.1016/j.diii.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Yang Z, et al. Minimal access versus open spinal surgery in treating painful spine metastasis: A systematic review. World Journal of Surgical Oncology. 2015;13:68. doi: 10.1186/s12957-015-0468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laufer I, Rubin DG, Lis E, Cox BW, Stubblefield MD, Yamada Y, et al. The NOMS framework: Approach to the treatment of spinal metastatic tumors. Oncologist. 2013;18(6):744–751. doi: 10.1634/theoncologist.2012-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.International standards for neurological and functional classifications of spinal cord injury. American Spinal Injury Association, Chicago, IL, 1982 (Revised 1996) [DOI] [PubMed]
- 39.Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine. 2005;30(19):2186–2191. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]