Abstract
Background
Dogs infected with canine parvovirus (CPV) have compromised intestinal epithelial barrier integrity. Production of D‐lactate by enteric bacteria may directly reflect disease severity or contribute to metabolic acid‐base status in these dogs.
Hypothesis
Serum D‐lactate concentration will be increased in CPV dogs compared to healthy controls and correlate with markers of disease severity and acid‐base status.
Animals
Dogs with CPV undergoing treatment (n = 40) and healthy control dogs (n = 9).
Methods
Prospective observational study. Dogs with CPV had a baseline and daily CBC, venous blood gas with serum electrolyte concentrations, composite clinical severity score, and serum D‐lactate concentration performed. A single serum D‐lactate measurement was obtained from healthy control dogs.
Results
The CPV dogs had a higher D‐lactate concentration (mean ± SD) of 469 ± 173 μM compared to controls, 306 ± 45 μM (P < .001). There was no difference in baseline D‐lactate concentrations for CPV survivors (474 ± 28 μM), versus nonsurvivors (424 ± 116 μM; P = .70). D‐lactate concentration decreased over the first 4 days of treatment (−9.6 μM/d; P = .46). Dogs hospitalized for <4 days had lower baseline D‐lactate concentrations compared to those hospitalized ≥4 days (400 ± 178 μM versus 520 ± 152 μM; P = .03). No sustained correlation over time between serum D‐lactate concentration and clinical severity score or recorded acid‐base results.
Conclusions and Clinical Importance
Serum D‐lactate concentrations are higher in dogs with CPV compared to healthy controls but do not appear to be clinically relevant. No relationship identified between serum D‐lactate concentrations and markers of CPV disease severity, acid‐base status, or outcome.
Keywords: acidosis, D‐lactic acid, parvovirus
Abbreviations
- CPV
canine parvovirus
- D‐LDH
D‐lactate dehydrogenase
- HPLC
high‐performance liquid chromatography
- SIRS
systemic inflammatory response syndrome
- TS
total solids
1. INTRODUCTION
Destruction of intestinal crypt cells during infection with canine parvovirus (CPV) causes sloughing of the protective mucosal layer and hemorrhagic enteritis.1 Bacterial translocation across the intestinal epithelial barrier in CPV dogs may result in endotoxemia, development of systemic inflammatory response syndrome (SIRS) and sepsis.2 Identifying compromised epithelial barrier integrity or evidence of bacteremia in CPV dogs largely is based upon clinical suspicion, but measurement of bacterial metabolic byproducts may provide further insight into these complicating disease processes.3, 4, 5
One such byproduct of bacterial metabolism is D‐lactate, which is produced during anaerobic fermentation of carbohydrates within the intestinal tract. D‐lactate is a stereoisomer of L‐lactate that cannot be measured by current point‐of‐care testing methods. Mammals produce minimal amounts of D‐lactate endogenously via the methylglyoxal pathway, and therefore increased D‐lactate concentrations in mammalian blood are considered specific to bacterial origin.6, 7 In humans with short bowel syndrome, excess carbohydrates undergo bacterial fermentation into organic acids within the colon, thereby lowering colonic pH to create a favorable environment for further proliferation of acid‐resistant D‐lactate producing bacterial species such as Lactobacillus and Streptococcus. The D‐lactate then is systemically absorbed via proton‐linked monocarboxylate transporters (MCT1, MCT2, MCT3 and MCT4).8, 9, 10 The presence of MCT1 transporters has been identified along the entire length of the canine intestine, with a greater concentration found in the basolateral membranes of the colonic epithelial cells.10
Clinical signs reported in people with increased blood concentrations of D‐lactate include ataxia, mental depression, and metabolic acidosis.8 Similar findings of D‐lactic acidosis have been reported in young ruminants as well as cats with gastrointestinal disease.11, 12, 13, 14, 15 Increased D‐lactate concentrations could be present in CPV dogs considering the destruction of the protective intestinal epithelium, resultant maldigestion, risk of bacterial translocation, and the ability for systemic absorption of increased D‐lactate via MCT1 transporters in the colon.
A previous study examining D‐lactate concentrations in CPV dogs at hospital admission and 24 hours later identified no difference in D‐lactate concentrations when compared to healthy control dogs.16 One limitation of that study was an inability to determine trends in D‐lactate during hospitalization. Additionally, when using reference intervals established by numerous studies across monogastric and ruminant species, D‐lactate concentrations in both healthy and CPV dogs equated to concentrations high enough to be considered as being within an abnormal range.11, 12, 13, 14, 15, 17, 18 Because of these irregularities, additional information regarding the clinical relevance of D‐lactate in the pathogenesis of CPV is warranted.
Our study aimed to examine D‐lactate concentrations in dogs with CPV as well as its relationship to other measures of disease severity and acid‐base status. We hypothesized (1) that dogs with CPV would have increased concentrations of serum D‐lactate when compared to healthy age‐matched controls, (2) that D‐lactate concentrations would correlate with clinical severity scoring and the presence of SIRS, and (3) that D‐lactate concentrations would decrease during treatment for CPV.
2. MATERIALS AND METHODS
We conducted a prospective observational study at a university teaching hospital enrolling client‐owned dogs with naturally acquired CPV infection included as part of a larger clinical study comparing 2 protocol treatment options.19 Dogs were considered eligible for study inclusion if they had never received a CPV vaccination, were demonstrating clinical signs consistent with CPV (ie, lethargy, vomiting, and diarrhea or some combination of these), tested positive for CPV using an ELISA (SNAP Parvo Test, Idexx, Westbrook, Maine), had not received any treatment at another veterinary facility, and informed owner consent was obtained. All levels of clinical severity relating to CPV infection were considered eligible for study inclusion. Dogs were excluded from the study if they had identifiable comorbidities upon hospital presentation that could confound outcome (eg, intussusception, concurrent infection), had received prior treatment at another veterinary facility, or displayed a temperament that would affect study participation. Owners were financially responsible for the initial examination fee and ELISA CPV test. Any costs thereafter related to supplies, treatment, and diagnostic testing were covered by the study. The protocol used was approved by the Institutional Animal Use and Care Committee before study initiation.
Baseline data obtained from each dog included age, sex, breed, duration of clinical signs before hospital presentation, pertinent medical history, baseline vital signs (rectal temperature, pulse rate, and respiratory rate), body weight (kg), physical examination findings, PCV, and total solids (TS). A baseline clinical severity score using a previous composite scoring system,21 hydration status (% dehydration), and baseline pain score also were assigned to each patient (Data [Link], [Link]) by the authors.20, 21, 22
All dogs were hospitalized and supportive treatment for their disease was determined based on their assignment within a larger randomized, prospective study comparing an inpatient versus outpatient CPV treatment protocol.19 Both protocol groups had IV catheters placed and received goal‐directed fluid resuscitation at admission using an isotonic crystalloid (Normosol‐R, Abbott Laboratories, North Chicago, Illinois), with dextrose (Hospira Inc, Lake Forest, Illinois) supplementation as indicated by the initial venous blood gas and electrolyte results. After fluid resuscitation, the inpatient group had an IV catheter maintained throughout hospitalization for delivery of isotonic crystalloid fluids (120 mL/kg/d IV) with potassium chloride (APP Pharmaceuticals, Schaumburg, Illinois) supplementation, maropitant citrate (1 mg/kg IV q24h; Cerenia, Zoetis Inc, Kalamazoo, Michigan), cefoxitin (22 mg/kg IV q8h; Apotex Corporation, Weston, Florida), and dextrose supplementation as indicated. The outpatient group remained in‐hospital for the study under the care of the investigators and trained veterinary personnel same as did the inpatient group, but their treatments were modified to reflect what an owner potentially could accomplish at home if necessary. The outpatient group had their IV catheters removed after fluid resuscitation, and each dog was given 8 mg/kg cefovecin sodium (Convenia, Zoetis Inc) SC once. Ongoing treatment for the outpatient group included SC fluids (Normosol‐R, 30 mL/kg q6h), maropitant citrate (1 mg/kg SC q24h), as well as PO potassium (Tumil‐K, Virbac Animal Health, Fort Worth, Texas) and dextrose (Karo syrup, ACH Food Companies Inc, Memphis, Tennessee) supplementation as indicated. Dogs in both groups were syringe fed Hill's a/d (1 mL/kg q6h; Hill's Pet Nutrition, Topeka, Kansas) and provided buprenorphine (0.02 mg/kg IV or SC; Reckitt Benckiser Pharmaceuticals Inc, Richmond, Virginia) as needed for abdominal discomfort. Ondansetron (0.5 mg/kg IV or SC; West‐Ward, Eatontown, New Jersey) was given to any dog with vomiting that was refractory to maropitant. Additional medications could be provided during treatment at the primary clinician's discretion and were recorded in the medical record.
Blood samples were obtained at study admission and then daily at the same time for each day of hospitalization. Clinical criteria recorded at the time of blood sampling included vital signs (rectal temperature, pulse rate, and respiratory rate), clinical severity score (0‐12), pain score (0‐10), hydration status and the presence of SIRS (yes/no) based on established criteria.22 A CBC (Advia 120 Hematology System, Siemens Healthcare Diagnostics Inc, Newark, Delaware), venous blood gases and electrolyte concentrations (pH, anion gap, base excess, and L‐lactate, bicarbonate, sodium, chloride, potassium, and glucose concentrations; ABL 800 Flex Blood Gas Analyzer, Radiometer, Bronshoj, Denmark), PCV/TS, and serum D‐lactate concentration were determined on each sample. A fecal sample also was collected for double centrifugal fecal flotation using Sheather's sugar solution (Jorgenson Labs, Loveland, Colorado).
Serum for D‐lactate quantification was immediately transferred to a microcentrifuge tube and stored at −80°C within 30 minutes to prevent further glycolytic activity until sample analysis could be completed. Determination of D‐lactate concentrations was performed using a commercially available colorimetric assay kit (D‐Lactate Colorimetric Assay Kit; Catalog #K667‐100, BioVision Inc, Milpitas, California). The assay relies on oxidation of D‐lactate by D‐lactate dehydrogenase to generate a proportional color change in the sample (ƛmax = 450 nm) that then can be measured by a plate reader (Biotek Synergy HT plate reader; 3/23/2015 Bioteck Instruments Inc, Winooski, Vermont) to determine the results for each sample. A D‐lactate standard curve with an R 2 > 0.99 was established for each corresponding plate utilizing the D‐lactate standard prepared in serial dilution according to the manufacturer recommendations. Samples were assayed in triplicate, and the average result was compared to the standard curve for acceptability and inclusion. To remove any potential effect of hemolysis and background interference on sample analysis, serum samples were individually combined with only the D‐lactate buffer at the same dilution as the fully analyzed sample and evaluated by the plate reader to determine the background concentration. Those subsequent background concentrations then were subtracted from the original assay result for each corresponding sample to determine the final D‐lactate concentration (μM).
Dogs were considered ready for hospital discharge once vomiting had resolved, they were rehydrated and drinking voluntarily, voluntary appetite had returned, and CBC results indicated a rebound from their neutrophil nadir. Data recorded at hospital discharge included survival (yes/no) and duration of hospitalization (days).
Serum was collected from healthy age‐matched controls to compare serum D‐lactate concentrations using similar methodology. Nine healthy dogs were recruited from hospital staff members for a single blood draw. Health status was determined based upon the provided medical history and physical examination findings. Blood was collected by venipuncture and transferred to a sterile tube for centrifugation and serum collection. Serum was transferred immediately to a microcentrifuge tube and stored at −80°C until analysis could be performed following the same protocol as for the CPV samples.
2.1. Statistical methods
Statistical analysis of the data was performed using SAS (SAS Version 9.4, SAS Inc, Cary, North Carolina) and GraphPad Prism (GraphPad Prism Version 6.04, GraphPad Software Inc, San Diego, California). Normality testing on data sets was performed using the Shapiro‐Wilk test and QQ plotting. Comparison of daily serum D‐lactate concentrations between treatment groups (inpatient versus outpatient), and presence of SIRS (yes/no) was done using pooled and Satterthwaite t testing depending on equality of variance results. A Welch t test was used to analyze baseline results between survivor and nonsurvivor CPV dogs, and CPV and control dogs separately. Correlation of daily D‐lactate concentrations to their corresponding daily CBC results, venous blood gas results, PCV, TS, rectal temperature, pulse rate, respiratory rate, pain score, and clinical severity score was performed for each day individually using either Pearson or Spearman correlation testing depending on normality of data distribution. Linear regression and goodness‐of‐fit tests also were performed on these results in relation to D‐lactate. Linear regression models with least squares means and solution for fixed effects were used for analyzing D‐lactate concentrations over Days 0‐4 of hospitalization in study dogs. Linear regression models with mixed means modeling and solution for fixed effects were used to compare D‐lactate between survivors and nonsurvivors over Days 0‐4 of hospitalization. Nonparametric t tests were used to assess significance in D‐lactate concentrations on the individual day that dogs reached a segmented neutrophil nadir of 0.0 × 103/μL versus those that reached a nadir >0.0 × 103/μL. Nonparametric t tests also were used to compare baseline D‐lactate concentrations of dogs that were hospitalized <4 days versus ≥4 days. All tests were evaluated at a .05 significance level.
3. RESULTS
Forty total dogs with naturally occurring CPV infection were hospitalized and treated supportively for their disease. The total CPV population consisted of 20 intact males, 19 intact females, and 1 castrated male. The healthy control group included 4 intact males, 4 intact females, and 1 castrated male. The median age of the CPV group was 4.0 months (range, 1.5‐30 months) and the control group was 5.0 months (range, 2.8‐8.2 months; P = .40). The median body weight for the CPV group was 3.9 kg (range, 0.89‐21 kg) and 8.8 kg (range, 3.6‐25.2 kg) for the control group (P = .02).
Several different breeds were represented in the study with most dogs being mixed breed. Dog breeds were categorized into overall groups based upon their predominant breed conformational characteristics. Breeds represented in the CPV group included Chihuahua (9/40, 22.5%), unknown mixed (8/40, 20%), Pit Bull (5/40, 12.5%), Miniature Poodle (3/40, 7.5%), Pug (2/40, 5%), Maltese (2/40, 5%), Labrador Retriever (2/40, 5%), and 1 each of Border Collie, Australian Heeler, Boxer, Miniature Pinscher, Great Dane, Standard Poodle, and Siberian Husky (1/40, 2.5%). The control group included Dachshund (2/9, 22%), Rhodesian Ridgeback (1/9, 11%), Dalmatian (1/9, 11%) Labrador Retriever (1/9, 11%), German Shepherd (1/9, 11%), Golden Doodle (1/9, 11%), and unknown mixed (1/9, 11%) breed.
The median duration of clinical signs before presentation for the CPV group was 1.25 days (range, 1‐4 days), which showed a significant negative correlation to baseline D‐lactate concentrations (r = −0.3475, P = .03). Baseline data for measured variables in CPV dogs is presented in Table 1. In the CPV group, 5/40 dogs (12.5%) either died suddenly of their disease or were euthanized because of imminent cardiopulmonary arrest. The remaining 35/40 dogs (87.5%) survived to discharge and all 40 dogs were included in the results. Baseline D‐lactate concentrations for survivors (474 ± 28 μM) and nonsurvivors (424 ± 116 μM) did not differ significantly (P = .70), and linear regression analysis indicated that D‐lactate concentration slopes did not differ over time between the 2 groups (P = .80). A significant difference was found between mean baseline D‐lactate concentrations in CPV dogs (469 ± 173 μM) compared to healthy controls (306 ± 45 μM; P < .01; Figure 1).
Table 1.
Baseline results (Day 0) for 40 CPV dogs as well as correlation and linear regression with baseline serum D‐lactate measurement. Correlation r values are Pearson correlations except for (*) which indicates Spearman correlation rho. Values in bold indicate statistical significance (P < .05)
| Measured variable (with reference ranges) | Mean | Median | D‐lactate correlation r value | D‐lactate correlation P value | Coefficient of determination (goodness of fit) |
|---|---|---|---|---|---|
| Temperature [°C (°F)] | 38.6 ± 0.78 (101.4 ± 1.42) | 38.6 (37.3‐39.7) (101.5 [98.5‐104.7]) | 0.24 | .14 | 0.057 |
| Pulse (beats per minute) | 138.9 ± 5.2 | 140 (80‐200) | 0.43 | .005 | 0.188 |
| Respiration (breaths per minute) | 47.7 ± 15.2 | 49 (24‐80) | 0.44 | .005 | 0.190 |
| Dehydration (%) | 6.88 ± 1.65 | 7.0 (4‐10) | −0.11* | .49 | 0.015 |
| Clinical Severity Score (0‐12) | 6.98 ± 1.86 | 7.0 (3‐11) | −0.20 | .22 | 0.040 |
| Pain score (0‐10) | 2.9 ± 1.3 | 3.0 (0‐5) | −0.39* | .01 | 0.149 |
| Packed cell volume (%) | 47.2 ± 8.9 | 45 (31‐67) | −0.008 | .96 | <0.001 |
| Total solids (g/dL) | 5.4 ± 0.8 | 5.4 (4.0‐7.6) | −0.04 | .79 | 0.002 |
| Total nucleated cell count (4.5‐15.0 × 103/μL) | 7.4 ± 4.7 | 6.4 (0.4‐18.2) | 0.14 | .38 | 0.020 |
| Segmented neutrophils (2.6‐11 × 103/μL) | 5.9 ± 4.5 | 4.4 (0.0‐16.2) | 0.22* | .16 | 0.029 |
| Bands (0.0‐0.2 × 103/μL) | 0.15 ± 0.30 | 0.0 (0.0‐1.4) | −0.13* | .44 | 0.018 |
| Lymphocytes (1‐4.8 × 103/μL) | 0.94 ± 0.53 | 0.8 (0.3‐2.3) | −0.11* | .50 | 0.006 |
| Monocytes (0.2‐1.0 × 103/μL) | 0.34 ± 0.31 | 0.3 (0.0‐1.4) | 0.13* | .41 | 0.003 |
| Platelets (200‐500 × 103/μL) | 345.2 ± 117.4 | 325 (132‐584) | 0.24 | .13 | 0.059 |
| pH (7.33‐7.45) | 7.349 ± 0.07 | 7.360 (7.195‐7.670) | −0.21* | .18 | 0.034 |
| HCO3− (15‐24 mEq /L) | 19.8 ± 3.46 | 19.5 (13.7‐29.9) | −0.37 | .02 | 0.136 |
| Base excess | −5.02 ± 3.6 | −5.1 (−13.6 to 3.8) | −0.35 | .03 | 0.121 |
| Anion gap (13‐22 mEq/L) | 16.1 ± 2.791 | 15.9 (6.9‐21.00) | 0.07 | .69 | 0.004 |
| Sodium (142‐152 mEq/L) | 138.7 ± 3.5 | 138.5 (130‐145) | 0.02 | .91 | <0.001 |
| Chloride (110‐122 mEq/L) | 106.3 ± 3.8 | 138.5 (93‐112) | 0.29* | .07 | 0.101 |
| Potassium (3.5‐5.2 mEq/L) | 3.56 ± 0.46 | 3.65 (2‐4.2) | 0.20* | .22 | 0.057 |
| Glucose (75‐130 mg/dL) | 106.6 ± 29.2 | 112 (29‐165) | 0.18* | .26 | 0.009 |
| L‐lactate (0.2‐1.44 mmol/L) | 1.783 ± 0.67 | 1.6 (0.9‐3.4) | 0.34* | .03 | 0.269 |
Figure 1.
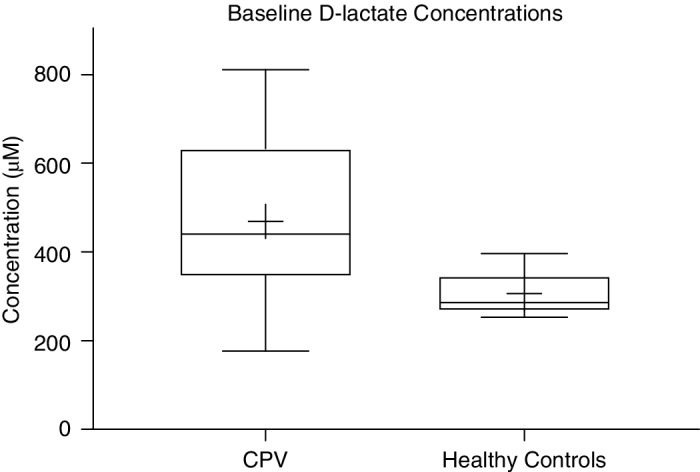
Box‐and‐whisker plot comparison of mean and SD baseline D‐lactate concentrations between CPV dogs (469 ± 173 μM) and healthy controls (306 ± 45 μM). The two lines outside the box indicate the minimum and maximum values, the ends of the box represent the upper and lower quartiles, and the horizontal line inside the box is the median value and the plus mark represents the mean value for each group
The median duration of hospitalization for CPV dogs was 4 days (range, 0‐11 days) and significant positive correlation was found between baseline D‐lactate concentrations and length of hospitalization (r = 0.36, P = .02). Further examination of that variable indicated that a difference in baseline D‐lactate concentrations (P = .03) was present in dogs hospitalized <4 days (n = 17; mean, 400 ± 179 μM) versus those hospitalized ≥4 days (n = 23; mean, 521 ± 152 μM). Linear regression of D‐lactate concentrations for the CPV group over time showed no significant change during Days 0‐4 of hospitalization (P = .46), but a daily change of −9.6 μM/d occurred. The numbers of CPV dogs hospitalized on each day of Days 0‐4 were 40, 38, 36, 34, and 23, respectively (Figure 2).
Figure 2.
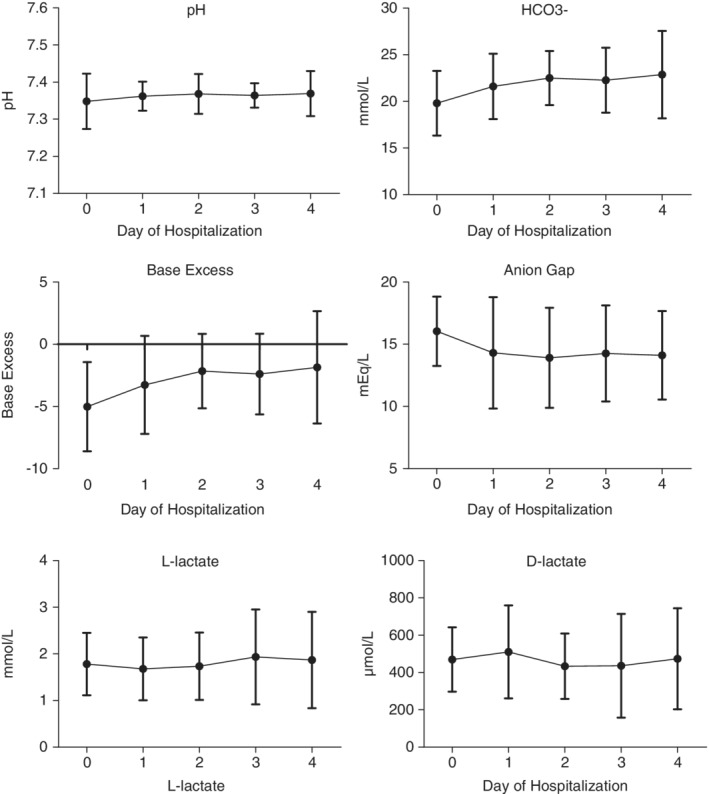
Mean and SD of venous blood gas results over time during Days 0‐4 of hospitalization
Of the 40 CPV dogs, each treatment group (inpatient versus outpatient protocol) within the larger study had 20 dogs randomly assigned. Overall and over time, D‐lactate concentrations did not differ significantly between the 2 treatment groups during hospitalization, except for Day 3 when the mean concentration was higher in the outpatient group (540 ± 313 μM) compared to the inpatient group (346 ± 214 μM; P = .04).
Significant correlation with Day 0 baseline D‐lactate concentrations was present for a variety of measured baseline variables including pain score (rho = −0.39, P = .01), pulse rate (r = 0.43, P < .01), respiratory rate (r = 0.43, P < .01), bicarbonate concentration (r = −0.37, P = .02), base excess (r = −0.43, P < .01), and L‐lactate concentration (rho = 0.34, P = .03; Table 1). During the first 4 days of hospitalization, other markers of acid‐base status intermittently correlated with serum D‐lactate concentration (Table 2). No significant findings were found when comparing D‐lactate concentrations to baseline and daily CBC results. No significant difference was found in baseline D‐lactate concentration for dogs that reached a segmented neutrophil nadir of 0.0 × 103/μL and those that reached a nadir >0.0 × 103/μL (P = .46). No significant association between daily D‐lactate concentrations and whether a dog met SIRS criteria, clinical severity score, or hydration status was observed either.
Table 2.
Mean and SD for acid‐base results with correlation and linear regression to D‐lactate concentrations across Days 0‐4 of hospitalization. Correlation r values are Pearson correlation except for (*) which indicates Spearman correlation rho. R‐squared is equal to the goodness of fit. Values in bold indicate statistical significance (P < .05)
| Variable | Day 0 | Day 1 | Day 2 | Day 3 | Day 4 |
|---|---|---|---|---|---|
| pH | |||||
| Mean ± SD | 7.348 ± 0.07 | 7.362 ± 0.04 | 7.368 ± 0.05 | 7.364 ± 0.03 | 7.369 ± 0.06 |
| r value | −0.21* | −0.28 | −0.23* | −0.17 | −0.40 |
| R‐squared | 0.034 | 0.077 | 0.153 | 0.029 | 0.164 |
| P value | .18 | .09 | .17 | .34 | .05 |
| Bicarbonate (HCO3‐) | |||||
| Mean ± SD | 19.81 ± 3.46 | 21.61 ± 3.50 | 22.51 ± 2.91 | 22.28 ± 3.49 | 22.87 ± 4.69 |
| r value | −0.37 | −0.02 | −0.22* | 0.05* | −0.05* |
| R‐squared | 0.136 | <0.001 | 0.022 | <0.001 | 0.004 |
| P value | .02 | .89 | .20 | .77 | .82 |
| Anion gap | |||||
| Mean ± SD | 16.04 ± 2.80 | 14.30 ± 4.48 | 13.91 ± 4.02 | 14.26 ± 3.87 | 14.11 ± 3.56 |
| r value | 0.07 | 0.02* | 0.45* | 0.22 | −0.23 |
| R‐squared | 0.004 | 0.002 | 0.144 | 0.050 | 0.053 |
| P value | .69 | .91 | <.01 | .2 | .29 |
| Base excess | |||||
| Mean ± SD | −5.02 ± 3.60 | −3.27 ± 3.94 | −2.16 ± 2.98 | −2.40 ± 3.25 | −1.85 ± 4.52 |
| r value | −0.35 | −0.20* | −0.29 | −0.15* | −0.22 |
| R‐squared | 0.121 | 0.015 | 0.085 | 0.001 | 0.007 |
| P value | .03 | .23 | .08 | .40 | .31 |
| L‐lactate | |||||
| Mean ± SD | 1.78 ± 0.67 | 1.68 ± 0.67 | 1.74 ± 0.73 | 1.94 ± 1.02 | 1.87 ± 1.03 |
| r value | 0.34 | 0.03* | 0.34 | 0.52* | 0.27* |
| R‐squared | 0.269 | 0.008 | 0.108 | 0.259 | 0.057 |
| P value | .03 | .84 | .05 | <.01 | .23 |
| Sodium | |||||
| Mean ± SD | 138.7 ± 3.50 | 138.9 ± 3.93 | 139.1 ± 3.37 | 139.6 ± 3.64 | 140.2 ± 3.99 |
| r value | 0.02 | −0.10* | 0.46* | −0.04 | −0.32 |
| R‐squared | <0.001 | 0.001 | 0.212 | 0.002 | 0.106 |
| P value | .91 | .56 | <.01 | .80 | .13 |
| Chloride | |||||
| Mean ± SD | 106.3 ± 3.78 | 106.9 ± 5.43 | 106.6 ± 3.89 | 107.1 ± 4.18 | 107.3 ± 5.03 |
| r value | 0.29 | 0.08* | 0.14 | −0.25 | −0.01* |
| R‐squared | 0.101 | <0.001 | 0.019 | 0.064 | 0.027 |
| P value | .07 | .66 | .42 | .16 | .95 |
| Potassium | |||||
| Mean ± SD | 3.56 ± 0.46 | 3.66 ± 0.61 | 3.89 ± 0.52 | 4.01 ± 0.62 | 4.10 ± 0.73 |
| r value | 0.20* | 0.20* | −0.04 | −0.06 | −0.25 |
| R‐squared | 0.022 | 0.039 | 0.001 | 0.006 | 0.063 |
| P value | .07 | .24 | .82 | .67 | .13 |
4. DISCUSSION
We evaluated daily serum D‐lactate concentrations in CPV dogs from hospital admission through discharge and compared those concentrations to clinicopathologic variables and markers of disease severity. Serum D‐lactate concentrations did not show sustained correlation over time with any of the measured variables, although weak correlations were identified at hospital admission and during the first 4 days of treatment. Serum D‐lactate concentration was not significantly associated with outcome (survival versus death), leukocyte numbers, or the presence or absence of SIRS. Aside from potential insight into the duration of hospitalization, these findings suggest that serum D‐lactate would not serve as a useful marker when managing CPV infection or prognosticating outcomes, especially when compared to other effective measures in CPV infection such as serum thyroxine or cortisol concentrations or leukocyte changes.23, 24, 25
Our study identified a significant difference in baseline D‐lactate concentrations when comparing CPV dogs (469 ± 173 μM) to healthy control dogs (306 ± 45 μM), which mirrors the findings of many other studies in human and veterinary medicine that identified differences between healthy and diseased patients.5, 11, 12, 13, 14, 15, 26, 27 The previous D‐lactate study in CPV dogs obtained higher concentrations for both CPV and control dogs when compared to other species, and CPV dogs actually had lower D‐lactate concentrations (2350 ± 2760 μM) when compared to healthy controls (2690 ± 1830 μM).16 This difference was not statistically significant. Seven of the 40 CPV dogs in the our study did have baseline D‐lactate concentrations less than the mean concentration for healthy controls, indicating that individual variation could be a contributing factor to possibly explain the difference between the 2 studies.
Although D‐lactic acidosis has proven to be a clinically relevant disease process in ruminants, its role in gastrointestinal diseases of monogastric species is not well established. The median D‐lactate concentration obtained in our population of CPV dogs (440 μM; range, 180‐810 μM) was consistent with the concentrations observed in cats with gastrointestinal disease (360 μM; range, 40‐8330 μM).11 Concentrations found in healthy dogs in our study (290 μM; range, 250‐400 μM) as well as healthy cats (220 μM; range, 40‐870 μM), are close to the normal D‐lactate concentrations reported in humans (250‐350 μM).11, 28
We documented steady serum D‐lactate concentrations in CPV dogs over the first 4 days of treatment. A decrease in D‐lactate concentration was observed over time (−9 μM/d), although this change was neither significant nor related to clinical treatment or timing of hospital discharge, which is different when compared to increased D‐lactate concentrations in ruminant species. Mammals lack the ability to convert D‐lactate to pyruvate using D‐lactate dehydrogenase, and instead rely on D‐α‐hydroxy‐acid‐dehydrogenase (D‐2‐HDH) for this process. The L‐lactate will competitively bind and be preferentially converted before D‐lactate by this mechanism and increased L‐lactate concentrations in CPV dogs may further delay metabolism of D‐lactate.8 Median L‐lactate concentrations during Days 0‐4 of hospitalization were above reference range in our current study and significant positive correlation between D‐lactate and L‐lactate concentrations was observed on Days 0 and 3.
Differences in treatment could affect D‐lactate concentration in CPV dogs, but the true impact of different treatment protocols may not have been fully evaluated in our study because of small sample size. Fluids containing lactate as a buffer (eg, lactated Ringer's solution) were not used in our study to avoid confounding D‐lactate measurements.6, 29 The dogs enrolled in our study were part of a larger prospective study comparing 2 separate treatment protocols (inpatient versus outpatient) for CPV in a hospitalized setting. Statistical analysis did not identify a repeatable difference in serum D‐lactate concentration between the groups throughout hospitalization, and therefore the groups were pooled into a single group for further analysis. Bias could have been introduced when evaluating clinical severity score, hydration status, and pain status of patients by multiple veterinary personnel using the same standardized criteria, but it is unlikely to have impacted the overall analysis given the lack of correlation of numerous other variables to D‐lactate.
Our study utilized a colorimetric assay involving enzymatic oxidation of D‐lactate to determine serum concentrations, whereas most veterinary studies have measured D‐lactate using high‐performance liquid chromatography (HPLC). Hemolysis may cause interference in D‐lactate measurement when using a colorimetric assay. This possibility was taken into consideration by combining serum samples and D‐lactate buffer at appropriate dilutions without the enzyme or substrate, and then analyzing the sample using the same plate reader. The result obtained from those readings was subtracted from the original enzyme reaction sample result to determine a final concentration. A limitation of our study is that the assay results were not validated using HPLC. The use of colorimetric assays however is commonplace in human medicine and in other studies of mammals with results that are comparable to HPLC measurements.18, 29, 30, 31, 32, 33
A recent study using the modified strong ion model in CPV dogs confirmed that acid‐base disturbances in this population are multifactorial, with chloride playing an important role in acid‐base changes.34 Our study identified intermittent correlation of D‐lactate to multiple acid‐base markers, most notably at admission, but no consistent trends were appreciated throughout hospitalization. Comparison of D‐lactate to other results such as albumin, phosphorus, and magnesium may show correlation, but our study suggests the clinical impact of any such correlation likely would be negligible.35
In conclusion, we found a significant difference in baseline serum D‐lactate concentrations in CPV dogs compared to healthy controls. Baseline concentrations provided potential information related to length of hospitalization (<4 versus ≥4 days), but otherwise no overall clinical relevance was identified between the 2 groups. The D‐lactate concentrations did not correlate with clinical severity score or the presence of SIRS in CPV dogs. Furthermore, D‐lactate did appear to contribute to the acid‐base status of CPV dogs before treatment and had intermittent correlation with results during treatment. The D‐lactate concentrations decreased over time but no significance was found with these changes. Measuring D‐lactate concentrations in CPV dogs is difficult to achieve in a clinical setting and our findings do not support that measurements would impact overall prognosis or treatment plans.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Cefovecin sodium.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
The Colorado State University IACUC committee program was utilized to review and approve study protocol before implementation.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
Supporting information
Data 1: Clinical Severity Score Criteria
Data 2: Internally modified Visual Analogue Scale (VAS) pain score criteria
ACKNOWLEDGMENTS
The authors acknowledge the contribution of Jim zumBrennen of the Colorado State University Statistics Department. This research was presented as a platform abstract presentation at the 2013 International Veterinary Emergency Critical Care Symposium, San Diego CA, and in poster form at the 2015 Colorado State University College of Veterinary Medicine and Biomedical Sciences Research Day, Fort Collins, CO.
Venn EC, Barnes AJ, Hansen RJ, Boscan PL, Twedt DC, Sullivan LA. Serum D‐lactate concentrations in dogs with parvoviral enteritis. J Vet Intern Med. 2020;34:691–699. 10.1111/jvim.15688
REFERENCES
- 1. Decaro N, Buonavoglia C. Canine parvovirus‐a review of epidemiological and diagnostic aspects, with emphasis on type 2c. Vet Microbiol. 2012;155(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Goddard A, Leisewitz AL. Canine parvovirus. Vet Clin North Am Small Anim Pract. 2010;40(6):1041‐1053. [DOI] [PubMed] [Google Scholar]
- 3. Smith SM, Eng RHK, Campos JM, Chmel H. D‐lactic acid measurements in the diagnosis of bacterial infections. J Clin Microbiol. 1989;27(3):385‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith SM. D‐lactic acid production as a monitor of the effectiveness of antimicrobial agents. Antimicrob Agents Chemother. 1991;35(2):237‐241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ewaschuk JB, Naylor JM, Zello GA. D‐lactate in human and ruminant metabolism. J Nutr. 2005;135(7):1619‐1625. [DOI] [PubMed] [Google Scholar]
- 6. Allen SE, Holm JL. Lactate: physiology and clinical utility. J Vet Emerg Crit Care. 2008;18(2):123‐132. [Google Scholar]
- 7. Karagiannis M. Lactate measurement as an indicator of perfusion. Vet Compendium. 2006;28:287‐298.8. [Google Scholar]
- 8. Petersen C. D‐lactic acidosis. Nutr Clin Pract. 2005;20(6):634‐645. [DOI] [PubMed] [Google Scholar]
- 9. Adeva‐Andany M, Lopez‐Ojen M, Funcasta‐Calderon R, et al. Comprehensive review of lactate metabolism in human health. Mitochondrion. 2014;17:76‐100. [DOI] [PubMed] [Google Scholar]
- 10. Shimoyama Y, Kirat D, Akihara Y, et al. Expression of monocarboxylate transporter 1 (MCT1) in the dog intestine. J Vet Med Sci. 2007;69(6):599‐604. [DOI] [PubMed] [Google Scholar]
- 11. Packer RA, Moore GE, Chang CY, et al. Serum D‐lactate concentrations in cats with gastrointestinal disease. J Vet Intern Med. 2012;26:905‐910. [DOI] [PubMed] [Google Scholar]
- 12. Packer RA, Cohn LA, Wohlstadter DR, et al. D‐lactic acidosis secondary to exocrine pancreatic insufficiency in a cat. J Vet Intern Med. 2005;19:106‐110. [DOI] [PubMed] [Google Scholar]
- 13. Ewaschuk JB, Naylor JM, Palmer R, Whiting SJ, Zello GA. D‐lactate production and excretion in diarrheic calves. J Vet Intern Med. 2004;18:744‐747. [DOI] [PubMed] [Google Scholar]
- 14. Bleul U, Schwantag S, Stocker H, et al. Floppy kid syndrome caused by D‐lactic acidosis in goat kids. J Vet Intern Med. 2006;20(4):1003‐1008. [DOI] [PubMed] [Google Scholar]
- 15. Angell JW, Jones G, Grove‐White DH, Jones E, Higgins RJ, Otter A. A prospective on farm cohort study investigating the epidemiology and pathophysiology of drunken lamb syndrome. Vet Rec. 2013;172(6):154. [DOI] [PubMed] [Google Scholar]
- 16. Nappert G, Dunphy E, Ruben D, Mann FA. Determination of serum organic acids in puppies with naturally acquired parvoviral enteritis. Can J Vet Res. 2002;66:15‐18. [PMC free article] [PubMed] [Google Scholar]
- 17. Omole OO, Brocks DR, Nappert G, Naylor JM, Zello GA. High‐performance liquid chromatographic assay (±)‐lactic acid and its enantiomers in calf serum. J Chromatogr B. 1999;727:23‐29. [DOI] [PubMed] [Google Scholar]
- 18. Omole OO, Nappert G, Naylor JM, Zello GA. Both L‐ and D‐lactate contribute to metabolic acidosis in diarrheic calves. J Nutr. 2001;131(8):2128‐2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Venn EC, Preisner K, Boscan PL, Twedt DC, Sullivan LA. Evaluation of an outpatient protocol in the treatment of canine parvoviral enteritis. J Vet Emerg Crit Care. 2017;27:52‐65. [DOI] [PubMed] [Google Scholar]
- 20. Mohr AJ, Leisewitz AL, Jacobson LS, Steiner JM, Ruaux CG, Williams DA. Effect of early enteral nutrition on intestinal permeability, intestinal protein loss, and outcome in dogs with severe parvoviral enteritis. J Vet Intern Med. 2003;17(6):791‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. DiBartola SP, Bateman S. Introduction to fluid therapy In: Winkel A, Stringer S, eds. Fluid, Electrolyte, and Acid‐Base Disorders in Small Animal Practice. 3rd ed. St. Louis, MO: Saunders Elsevier; 2006:330‐344. [Google Scholar]
- 22. Butler AL. Goal directed therapy in small animal critical illness. Vet Clin North Am Small Anim Pract. 2011;41:817‐838. [DOI] [PubMed] [Google Scholar]
- 23. Schoeman JP, Herrtage ME. Serum thyrotropin, thyroxine and free thyroxine concentrations as predictors of mortality in critically ill puppies with parvovirus infection: a model for human pediatric critical illness? Microbes Infect. 2008;10(2):203‐207. [DOI] [PubMed] [Google Scholar]
- 24. Schoeman JP, Goddard A, Leisewitz AL. Biomarkers in canine parvovirus enteritis. N Z Vet J. 2013;61(4):217‐222. [DOI] [PubMed] [Google Scholar]
- 25. Goddard A, Leisewitz AL, Christopher MM, Duncan NM, Becker PJ. Prognostic usefulness of blood leukocyte changes in canine parvoviral enteritis. J Vet Intern Med. 2008;22(2):309‐316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wiecek S, Chudek J, Wos H, et al. Serum level of D‐lactate in patients with cystic fibrosis: preliminary data. Dis Markers. 2018;2018:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nielsen C, Kirkegard J, Erlandsen E. D‐lactate is a valid biomarker of intesitinal ischemia induced by abdominal compartment syndrome. J Surg Res. 2014;194(4):400‐404. [DOI] [PubMed] [Google Scholar]
- 28. Takahashi K, Terashima H, Kohno K, Ohkohchi N. A stand‐alone synbiotic treatment for the prevention of D‐lactic acidosis in short bowel syndrome. Int Surg. 2013;98(2):110‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vail DM, Ogilvie GK, Fettman MJ, Wheeler SL. Exacerbation of hyperlactatemia by infusion of lactated ringer's solution in dogs with lymphoma. J Vet Intern Med. 1990;4(5):228‐232. [DOI] [PubMed] [Google Scholar]
- 30. Goldgof M, Xiao C, Chanturiya T, Jou W, Gavrilova O, Reitman ML. The chemical uncoupler 2,4‐dinitrophenol (DNP) protects against diet‐induced obesity and improves energy homeostasis in mice at thermoneutrality. J Biol Chem. 2014;289(28):19341‐19350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marti R, Segura RM, Surinach JM, et al. Determination of D‐lactate by enzymatic methods in biological fluids: study of interferences. Clin Chem. 1997;43(6):1010‐1015. [PubMed] [Google Scholar]
- 32. Talasniemi JP, Pennanen S, Savolainen H, Niskanen L, Liesivuori J. Analytical investigation: assay of D‐lactate in diabetic plasma and urine. Clin Biochem. 2008;41(13):1099‐1103. [DOI] [PubMed] [Google Scholar]
- 33. Buttery JE, Pannali PR. Colorimetric measurement of D(‐)lactate in plasma. Clin Biochem. 1987;20:237‐239. [DOI] [PubMed] [Google Scholar]
- 34. Burchell RK, Schoeman JP, Leisewitz AL. The central role of chloride in the metabolic acid‐base changes in canine parvoviral enteritis. Vet J. 2014;200:152‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaae J, de Morais HA. Anion gap and strong ion gap: a quick reference. Vet Clin North Am Small Anim Pract. 2008;38(3):443‐447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data 1: Clinical Severity Score Criteria
Data 2: Internally modified Visual Analogue Scale (VAS) pain score criteria


